Successful implementation of the National Radiation Oncology Registry should improve the quality of care for patients with cancer treated with radiation.
Abstract
The National Radiation Oncology Registry (NROR), sponsored by the Radiation Oncology Institute and the American Society for Radiation Oncology, is designed to collect standardized information on cancer care delivery among patients treated with radiotherapy in the United States and will focus on patients with prostate cancer. Stakeholders were engaged through a forum that emphasized the need for patient-centered outcomes, minimal data burden, and maximal connectivity to existing registries and databases. An electronic infrastructure is under development to provide connectivity across radiation oncology and hospital information systems. The NROR Gateway features automatic abstraction as well as aggregation of treatment and outcome data. The prostate cancer data dictionary provides standardized elements in four domains: facility, physician, patient, and treatment. The pilot phase will consist of clinical centers chosen to provide a representative mix of radiation treatment modalities, facility types, population-based settings, and regional locations. The initial set of radiation practice metrics includes physician board certification and maintenance, ordering of staging scans, active surveillance discussion, dose prescriptions for low-risk/high-risk disease, radiation fields for low-risk/high-risk disease, image-guided radiation therapy use, androgen deprivation therapy use, post-brachytherapy implant computed tomography dosimetry, collection of toxicity assessments, and longitudinal patient follow-up. The NROR pilot study will provide the framework for expansion to a nationwide electronic registry for radiation oncology.
Introduction
Patient registries have emerged as important tools for collection of data on the quality, safety, effectiveness, and value of medical therapies.1 Examples of successful registries include the American College of Cardiology Registries,2 the Society of Thoracic Surgeons National Database,3 the National Oncologic PET Registry,4 and the Quality Oncology Practice Initiative by the American Society for Clinical Oncology.5 A radiation oncology–specific national registry comprising standardized aggregated data on therapies and technologies used to treat specific types of cancers and their outcomes would yield invaluable quality improvement potential by focusing on best practices, treatment effectiveness, and practice patterns of care as provided by radiation oncologists.
The National Radiation Oncology Registry (NROR) is a national collaborative initiative between the Radiation Oncology Institute and the American Society for Radiation Oncology. The overarching mission of the NROR is to improve the care of patients with cancer by capturing reliable information on treatment delivery and health outcomes.6 Prostate cancer (PCa) has been selected for the NROR pilot as a model disease site given its high incidence, multiple management options, potential public health and economic implications, and its identification by the Institute of Medicine as the area of oncology most in need of comparative-effectiveness research.7 A group of committed volunteers has provided expert input at every stage of development. As the regulatory sponsor, the Radiation Oncology Institute provides leadership at the national level, promoting the NROR and its goals within the radiation oncology community.
The most significant accomplishments to date include the finalization of a standardized set of data elements for the prostate pilot; the development of an information technology infrastructure to support the efficient collection, aggregation, and analysis of clinical data; and the establishment of a standardized set of radiation practice metrics.
Registry Development
Stakeholder Engagement
To build consensus for the goals of the NROR through stakeholder engagement, a Stakeholder Forum was held on April 19, 2012. Participants included representatives from the American Society for Radiation Oncology and other professional societies, physicians and physicists, patient advocates, participating vendors, the federal government, payers, and other registries (Appendix Table A1, online only). The Stakeholder Forum emphasized the need for minimal data collection burden, maximal connectivity to existing registries and databases, and patient-centered outcomes. A strong foundation for long-term engagement among participants and the organizations and constituencies they represent was established.
Electronic Infrastructure
We have developed the detailed functional specifications for a software platform to fulfill the NROR mission of collecting patient-specific radiotherapy treatment and outcome data electronically in close collaboration with radiotherapy information system vendors. A unique feature of the NROR will be the automatic abstraction and aggregation of treatment and outcome data from electronic medical records, patient portals, oncology information systems, and radiation therapy treatment planning systems.
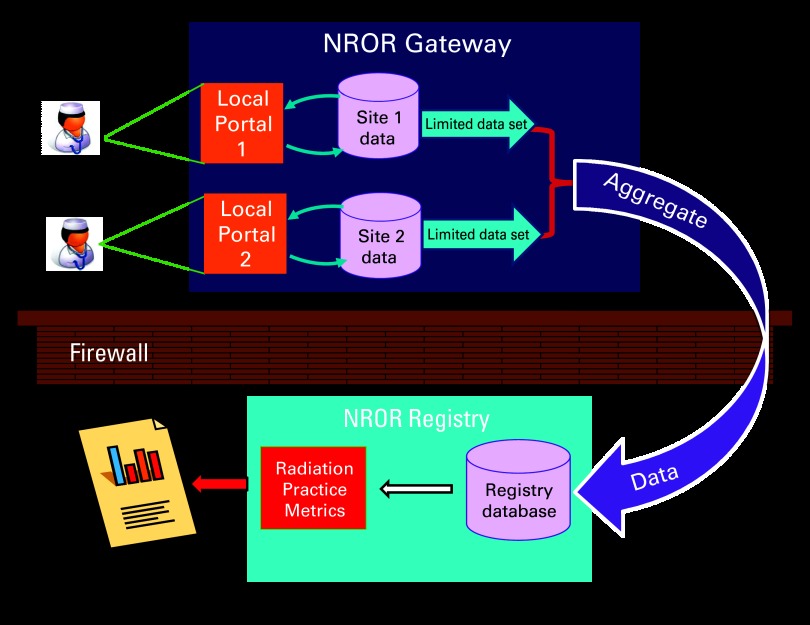
Clinical data will be submitted from multiple clinical sites and undergo quality review consisting of source document verification at the site before the data are uploaded into the NROR database to be aggregated and subsequently analyzed. The registry database fulfills all security and privacy considerations and maintains redundancy to minimize data loss, as illustrated in Figure 1. A gateway web application (NROR Gateway) performs the initial data aggregation for each institution, supporting manual data entry and automated transfers via open web services application programming interfaces. Deidentified, quality assured data are submitted from the Gateway via web services and stored in a relational database optimized for analytics. The decoupling of the data collection from the analytic repository facilitates a hybrid system of both electronic and manual data entry that can support all clinics regardless of their level of electronic medical record implementation.
Figure 1.
National Radiation Oncology Registry (NROR) electronic infrastructure. Personal health information will be collected at the sites and used for quality assurance at the local level, but only a limited data set will be transferred to the registry database for aggregation and analysis.
Prostate Data Dictionary
The NROR Prostate Data Dictionary comprises data elements derived from authoritative sources in radiation oncology, including the Radiation Therapy Oncology Group radiotherapy trials, Cancer of the Prostate Strategic Urologic Research Endeavor,8 the Quantitative Analysis of Normal Tissue Effects in the Clinic review of radiotherapy toxicity,9 the Quality Research in Radiation Oncology Patterns of Care studies,10 the National Cancer Institute Common Toxicity Criteria, Agency for Healthcare Research and Quality processes of care elements, and the American College of Radiology facility descriptors. When possible, an effort was made to adhere to established data standards.
The Data Dictionary has been reduced to the essential elements needed for the pilot in PCa through review by PCa health services and technical experts. The data elements are organized into a comprehensive taxonomy for the collection of facility, physician, patient, disease, treatment, and outcomes data, as illustrated in Figure 2. The Data Dictionary can be downloaded at http://www.roinstitute.org/NROR/Documents/DDV2.pdf.
Figure 2.
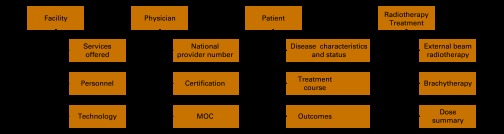
National Radiation Oncology Registry data dictionary hierarchy. Abbreviation: MOC, maintenance of certification.
Pilot in PCa
More than 80 clinical centers indicated willingness to participate in the PCa pilot by completing a comprehensive online questionnaire. The pilot phase will consist of clinical centers chosen to provide a representative mix of radiation treatment modalities, facility types, settings, regional locations, and PCa patient volumes selected through an objective, blinded, and transparent process. The NROR is being rolled out in phases, with the first phase consisting of seven beta testing sites that will rigorously test the electronic infrastructure and develop efficient workflows to minimize the burden of data collection and submission at the pilot sites. The beta testing phase began in December 2012 and will continue through the middle of 2013. After the beta testing phase, the NROR will grow to a total of approximately 30 pilot sites in 2013, with planned expansion to additional radiation facilities in 2014 and additional diseases in 2015. This phased approach will provide an opportunity for incremental improvements and implementation of lessons learned along the way.
Radiation Practice Metrics
We have developed a set of top 10 radiation oncology–specific quality measures for PCa treatment known as the radiation practice metrics (RPMs), which will serve as benchmarks for quality improvement. Sources for the RPMs include National Comprehensive Cancer Network Clinical Practice Guidelines, European Association of Urology Guidelines, American College of Radiology Appropriateness Criteria, American Urological Association Prostate Guidelines, Radiation Therapy Oncology Group Consensus Panel Atlas, American Brachytherapy Society consensus guidelines, Transatlantic Consensus Group, the National Quality Forum, Quality Oncology Practice Initiative, and the National Cancer Institute Cancer Therapy Evaluation Program.
RPM benchmarking reports will be provided to participating sites on a regular and timely basis. The initial list of RPMs includes physician board certification and maintenance,11 ordering of staging scans,12–15 active surveillance discussion,12,16,17 dose prescriptions for low-risk/high-risk disease,12,18–20 radiation fields for low-risk/high-risk disease,18,19,21,22 image-guided radiation therapy use,17–19,21,23 androgen deprivation therapy use,12–14,16,20 postbrachytherapy implant computed tomography dosimetry,24,25 collection of toxicity assessments,26 and longitudinal patient follow-up.12,20,27,28 Over time, the NROR registry will attempt to validate the relationship of these measures to patient-centered health outcomes. Table 1 lists RPMs and their sources.
Table 1.
RPMs
| Metric | Source |
|---|---|
| Physician board certification and maintenance status | American Board of Radiology Maintenance of Certification |
| Ordering of staging scans: bone, CT, etc | EUA Guidelines, QOPI, NCCN Clinical Practice Guidelines, NQF |
| Active surveillance discussion | Transatlantic Consensus Group, EAU Guidelines, AUA Guidelines |
| Dose prescriptions for low-risk/high-risk disease | ACR Appropriateness Criteria, EAU Guidelines |
| Radiation fields for low-risk/high-risk disease | RTOG Consensus Panel Atlas, ACR Appropriateness Criteria |
| Image-guided radiation therapy utilization | Transatlantic Consensus Group, ACR Appropriateness Criteria |
| Androgen deprivation therapy utilization | EAU Guidelines, AUA Guidelines, NCCN Clinical Practice Guidelines, NQF |
| Postbrachytherapy implant CT dosimetry | ABS Consensus Guidelines, ACR Appropriateness Criteria |
| Collection of toxicity assessments | NCI CTEP Common Terminology Criteria for Adverse Events 4.0 |
| Longitudinal patient follow-up | EUA Guidelines, ACR Appropriateness Criteria |
Abbreviations: ABS, American Brachytherapy Society; ACR, American College of Radiology; AUA, American Urological Association; CT, computed tomography; CTEP, Cancer Therapy Evaluation Program; EUA, European Association of Urology; NCCN, National Comprehensive Cancer Network; NCI, National Cancer Institute; NQF, National Quality Forum; QOPI, Quality Oncology Practice Initiative; RPM, radiation practice metric; RTOG, Radiation Therapy Oncology Group.
Regulatory
To streamline the review/approval process and minimize the regulatory burden on the sites, we selected a central institutional review board, New England Institutional Review Board. After consultation with both the New England Institutional Review Board and other registries, we developed a regulatory policy using an opt-out informed consent: an informational handout describing the pilot and providing the option to refuse participation will be distributed to eligible patients. Participating sites will sign a participation agreement governing data sharing in compliance with the Health Insurance Portability and Accountability Act and the Health Information Technology for Economic and Clinical Health Act.
Discussion
The overall goal of the NROR is to improve the quality of cancer care through real-world and near–real-time assessment of care delivery and outcomes. We have established a pilot registry using a consensus-based set of PCa data elements as a model. The NROR pilot will provide the framework for expansion to a national electronic registry for radiation oncology in the United States. Benchmarking across institutions will promote rapid learning and accountable cancer care that is anticipated to benefit patients. The electronic infrastructure will facilitate population-based examination of patient-level dosimetric and treatment data, transforming efforts to improve quality and safety in radiation oncology.
With its great potential, the NROR still faces several challenges, including developing incentives for broad participation, identification of sources of sustainable funding, establishing links to other registries, implementation of the patient portal for patient-reported outcomes, and further streamlining of the data extraction process to maximize data collection while minimizing burden on participating sites. Successful implementation of the NROR should improve the quality of care for patients with cancer treated with radiation.
Acknowledgment
The Radiation Oncology Institute (ROI) National Radiation Oncology Registry (NROR) is funded through the ROI, the American Society for Radiation Oncology (ASTRO), and the Federal Share of program income earned by Massachusetts General Hospital on Grant No. C06 CA059267.
The NROR pilot was presented in two posters and a discussion (General Session IV) at the inaugural American Society for Clinical Oncology Quality Care Symposium in San Diego, CA, November 30-December 1, 2012.
We thank Cheryl Reinhardt, Maryam Mojarrad, Jatinder Palta, Bruce Curran, Warren Suh, Carl Heller, and Roman Tsyvine. We thank the ROI and ASTRO for supporting this initiative; Bogardus Medical Systems, Varian Medical Systems, and Elekta for contributing valuable time; the small group of beta testing sites for providing their time and expertise; HealthCare IT for their technical advice, design, and development of the NROR electronic infrastructure; the Center for Medical Technology Policy for organizing and bringing together key stakeholders for this project; all the stakeholders including representatives from payer groups, the federal government, industry, professional organizations, and patient advocates for their shared insights and support.
Appendix
Table A1.
National Radiation Oncology Registry Stakeholder Forum Participating Organizations
| Organization | Constituency |
|---|---|
| Accuray | Industry |
| Agency for Healthcare Research and Quality | Federal government |
| American Association for Physicists in Medicine | Professional society |
| American Board of Radiology | Professional society |
| American College of Cardiology/American Heart Association Task Force on Clinical Data Standards | Other registries |
| American College of Radiology | Professional society |
| American Medical Association | Professional society |
| American Society for Radiation Oncology | Professional society |
| American Society of Clinical Oncology | Other registries |
| American Urological Association | Professional society |
| Blue Cross Blue Shield Association | Payer |
| Center for Medical Technology Policy | Nonprofit |
| Centers for Medicare and Medicaid Services | Federal government |
| Christiana Care | Payer |
| Duke University | Physician/physicist |
| Elekta | Industry |
| Food and Drug Administration | Federal government |
| Friends of Cancer Research | Patient advocate |
| HealthCare IT | Vendor |
| IBA Group | Industry |
| Johns Hopkins University | Physician/physicist |
| Massachusetts General Hospital | Physician/physicist |
| Medical College of Wisconsin | Physician/physicist |
| Mevion | Industry |
| National Cancer Institute | Federal government |
| OncoChart | Industry |
| Procure | Industry |
| Radiation Oncology Institute | Professional society |
| Siemens | Industry |
| St Luke's Hospital of Kansas City | Physician/physicist |
| UnitedHealthcare | Payer |
| University of California Los Angeles | Payer |
| University of Florida | Physician/physicist |
| University of Michigan | Physician/physicist |
| University of Oklahoma | Physician/physicist |
| University of Pennsylvania | Physician/physicist |
| Us, Too, Prostate Cancer Support Group | Patient advocate |
| Valley Radiotherapy Associates Medical Group | Physician/physicist |
| Vanderbilt School of Medicine | Physician/physicist |
| Varian | Industry |
| Washington University | Physician/physicist |
| WellPoint | Payer |
| 21st Century Oncology | Physician group |
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Jason A. Efstathiou, Radiation Oncology Institute (U); C. Bob Bogardus, Radiation Oncology Institute (U), Bogardus Medical Systems (C); Jeffrey Carlin, Bogardus Medical Systems (C); Dave Eggert, Elekta AB (C); Benedick A. Fraass, Radiation Oncology Institute (U); Joel Goldwein, Elekta (C); Ken Hotz, Varian Medical Systems (C); Colleen A. Lawton, American Society for Radiation Oncology (U), Elsevier (C); Sasa Mutic, Radiation Oncology Institute (U); Christopher M. Rose, Radiation Oncology Institute (U), Terry Wall, Radiation Oncology Institute (U); Anthony L. Zietman, American Society for Radiation Oncology (U); Peter E. Gabriel, Radiation Oncology Institute (U); Justin E. Bekelman, Radiation Oncology Institute (U) Consultant or Advisory Role: None Stock Ownership: Ken Hotz, Varian Medical Systems Honoraria: Benedick A. Fraass, Varian Medical Systems Research Funding: Todd R. McNutt, Philips Healthcare, Elekta; Margie Hunt, Varian Medical Systems; Charles Mayo, Varian Medical Systems; Wolfgang Tomé, Philips Radiation Oncology Systems Expert Testimony: None Other Remuneration: None
Author Contributions
Conception and design: All authors
Administrative support: Deborah S. Nassif
Provision of study materials or patients: Jason A. Efstathiou, Deborah S. Nassif, Todd R. McNutt, Walter Bosch, Ron C. Chen, Henry Chou, Joel Goldwein, Karen E. Hoffman, Margie Hunt, Colleen A.F. Lawton, Charles Mayo, Jeff M. Michalski, Sasa Mutic, Louis Potters, Howard M. Sandler, Gregory Sharp, Wolfgang Tomé, Phuoc Tran, Anthony L. Zietman, Peter E. Gabriel, Justin E. Bekelman
Collection and assembly of data: Jason A. Efstathiou, Deborah S. Nassif, Todd R. McNutt, Walter Bosch, Ron C. Chen, Henry Chou, Joel Goldwein, Karen E. Hoffman, Margie Hunt, Colleen A.F. Lawton, Charles Mayo, Jeff M. Michalski, Sasa Mutic, Louis Potters, Howard M. Sandler, Gregory Sharp, Wolfgang Tomé, Phuoc Tran, Anthony L. Zietman, Peter E. Gabriel, Justin E. Bekelman
Data analysis and interpretation: Jason A. Efstathiou, Deborah S. Nassif, Todd R. McNutt, Peter E. Gabriel, Justin E. Bekelman
Manuscript writing: Jason A. Efstathiou, Deborah S. Nassif, Todd R. McNutt, Ronald C. Chen, Joel Goldwein, Karen E. Hoffman, Margie Hunt, Colleen A.F. Lawton, Christopher M. Rose, Phuoc T. Tran, Anthony L. Zietman, Peter E. Gabriel, Justin E. Bekelman
Final approval of manuscript: All authors
References
- 1.Asher AL, McGirt MJ, Glassman SD, et al. Regulatory considerations for prospective patient care registries: Lessons learned from the National Neurosurgery Quality and Outcomes Database. Neurosurg Focus. 2013;34:E5. doi: 10.3171/2012.10.FOCUS12300. [DOI] [PubMed] [Google Scholar]
- 2.Messenger JC, Ho KK, Young CH, et al. The National Cardiovascular Data Registry (NCDR) data quality brief: The NCDR data quality program in 2012. J Am Coll Cardiol. 2012;60:1484–1488. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Shahian DM, Jacobs JP, Edwards FH, et al. The Society of Thoracic Surgeons National Database. Heart. doi: 10.1136/heartjnl-2012-303456. [epub ahead of print on Jan 18, 2013] [DOI] [PubMed] [Google Scholar]
- 4.Tunis S, Whicher D. The National Oncologic PET Registry: Lessons learned for coverage with evidence development. J Am Coll Radiol. 2009;6:360–365. doi: 10.1016/j.jacr.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Neuss MN, Desch CE, McNiff KK, et al. A process for measuring the quality of cancer care: The Quality Oncology Practice Initiative. J Clin Oncol. 2005;23:6233–6239. doi: 10.1200/JCO.2005.05.948. [DOI] [PubMed] [Google Scholar]
- 6.Palta JR, Efstathiou JA, Bekelman JE, et al. Developing a national radiation oncology registry: From acorns to oaks. Pract Radiat Oncol. 2011;2:10–17. doi: 10.1016/j.prro.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Committee on Comparative Effectiveness Research Prioritization Board on Health Care Services of the Institute of Medicine. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: National Academies Press; 2009. [Google Scholar]
- 8.Cooperberg MR, Broering JM, Litwin MS, et al. The contemporary management of prostate cancer in the United States: Lessons from the cancer of the prostate strategic urologic research endeavor (CapSURE), a national disease registry. J Urol. 2004;171:1393–1401. doi: 10.1097/01.ju.0000107247.81471.06. [DOI] [PubMed] [Google Scholar]
- 9.Jackson A, Marks LB, Bentzen SM, et al. The lessons of QUANTEC: Recommendations for reporting and gathering data on dose-volume dependencies of treatment outcome. Int J Radiat Oncol Biol Phys. 2010;76(suppl):S155–S160. doi: 10.1016/j.ijrobp.2009.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson JF, Owen J. Quality research in radiation oncology: A self-improvement initiative 30 years ahead of its time? J Am Coll Radiol. 2005;2:1001–1007. doi: 10.1016/j.jacr.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Kun LE, Haffty BG, Bosma J, et al. American Board of Radiology Maintenance of Certification. Part IV: Practice quality improvement for radiation oncology. Int J Radiat Oncol Biol Phys. 2007;68:7–12. doi: 10.1016/j.ijrobp.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 12.Heidenreich A, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part 1: Screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology prostate cancer: Prostate cancer 2012, version 1.2013. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 14.National Quality Forum. NQF endorsed quality measures. http://www.qualityforum.org/QPS/
- 15.American Society of Clinical Oncology. QOPI summary of measures: Spring 2013. http://qopi.asco.org/Documents/QOPISpring13MeasuresSummary_002.pdf.
- 16.Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed HU, Akin O, Coleman JA, et al. Transatlantic Consensus Group on active surveillance and focal therapy for prostate cancer. BJU Int. 2012;109:1636–1647. doi: 10.1111/j.1464-410X.2011.10633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michalski JM, Roach M, 3rd, Merrick G, et al. ACR appropriateness criteria on external beam radiation therapy treatment planning for clinically localized prostate cancer expert panel on radiation oncology–prostate. Int J Radiat Oncol Biol Phys. 2009;74:667–672. doi: 10.1016/j.ijrobp.2008.12.073. [DOI] [PubMed] [Google Scholar]
- 19.Moran BJ, DeRose P, Hsu IC, et al. ACR Appropriateness Criteria definitive external beam irradiation in stage T1 and T2 prostate cancer. Am J Clin Oncol. 2011;34:636–647. doi: 10.1097/COC.0b013e3182354a65. [DOI] [PubMed] [Google Scholar]
- 20.Mottet N, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer: Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011;59:572–583. doi: 10.1016/j.eururo.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 21.Boehmer D, Maingon P, Poortmans P, et al. Guidelines for primary radiotherapy of patients with prostate cancer. Radiother Oncol. 2006;79:259–269. doi: 10.1016/j.radonc.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Gay HA, Barthold HJ, O'Meara E, et al. Pelvic normal tissue contouring guidelines for radiation therapy: A Radiation Therapy Oncology Group consensus panel atlas. Int J Radiat Oncol Biol Phys. 2012;83:e353–e362. doi: 10.1016/j.ijrobp.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simmons MN, Berglund RK, Jones JS. A practical guide to prostate cancer diagnosis and management. Cleve Clin J Med. 2011;78:321–331. doi: 10.3949/ccjm.78a.10104. [DOI] [PubMed] [Google Scholar]
- 24.Yamada Y, Rogers L, Demanes DJ, et al. American Brachytherapy Society consensus guidelines for high-dose-rate prostate brachytherapy. Brachytherapy. 2012;11:20–32. doi: 10.1016/j.brachy.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Expert Panel on Radiation-Prostate. Frank SJ, Arterbery VE, et al. American College of Radiology Appropriateness Criteria permanent source brachytherapy for prostate cancer. Brachytherapy. 2011;10:357–362. doi: 10.1016/j.brachy.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 26.National Cancer Insitutute. Common Terminology Criteria for Adverse Events (CTCAE), 2010, version 4.03. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf.
- 27.Casalino DD, Remer EM, Arellano RS, et al. ACR Appropriateness Criteria posttreatment follow-up of prostate cancer. J Am Coll Radiol. 2011;8:863–871. doi: 10.1016/j.jacr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 28.McIntosh HM, Neal RD, Rose P, et al. Follow-up care for men with prostate cancer and the role of primary care: A systematic review of international guidelines. Br J Cancer. 2009;100:1852–1860. doi: 10.1038/sj.bjc.6605080. [DOI] [PMC free article] [PubMed] [Google Scholar]