Abstract
Context:
In congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency, a strong genotype-phenotype correlation exists in childhood. However, similar data in adults are lacking.
Objective:
The objective of the study was to test whether the severity of disease-causing CYP21A2 mutations influences the treatment and health status in adults with CAH.
Research Design and Methods:
We analyzed the genotype in correlation with treatment and health status in 153 adults with CAH from the United Kingdom Congenital adrenal Hyperplasia Adult Study Executive cohort.
Results:
CYP21A2 mutations were distributed similarly to previously reported case series. In 7 patients a mutation was identified on only 1 allele. Novel mutations were detected on 1.7% of alleles (5 of 306). Rare mutations were found on 2.3% of alleles (7 of 306). For further analysis, patients were categorized into CYP21A2 mutation groups according to predicted residual enzyme function: null (n = 34), A (n = 42), B (n = 36), C (n = 34), and D (n = 7). Daily glucocorticoid dose was highest in group null and lowest in group C. Fludrocortisone was used more frequently in patients with more severe genotypes. Except for lower female height in group B, no statistically significant associations between genotype and clinical parameters were found. Androgens, blood pressure, lipids, blood glucose, and homeostasis model assessment of insulin resistance were not different between groups. Subjective health status was similarly impaired across groups.
Conclusions:
In adults with classic CAH and women with nonclassic CAH, there was a weak association between genotype and treatment, but health outcomes were not associated with genotype. The underrepresentation of males with nonclassic CAH may reflect that milder genotypes result in a milder condition that is neither diagnosed nor followed up in adulthood. Overall, our results suggest that the impaired health status of adults with CAH coming to medical attention is acquired rather than genetically determined and therefore could potentially be improved through modification of treatment.
Congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency (21OHD) is an autosomal recessive disorder and one of the most frequent inborn conditions. Inactivating mutations in the 21-hydroxylase (CYP21A2) gene account for approximately 95% of cases (1–3). Steroid 21-hydroxylase deficiency is clinically classified into classic CAH, comprising the salt-wasting (SW) and the simple virilizing (SV) forms, and nonclassic CAH. The classic form occurs in a frequency of approximately 1 in 10 000 to 1 in 15 000 in the general population, whereas the nonclassic form is more frequent with an estimated incidence of about 1 in 1000 (1). The classic form of 21-hydroxylase deficiency is characterized by androgen excess, glucocorticoid deficiency, and, in two thirds of CAH patients, mineralocorticoid deficiency (1, 2).
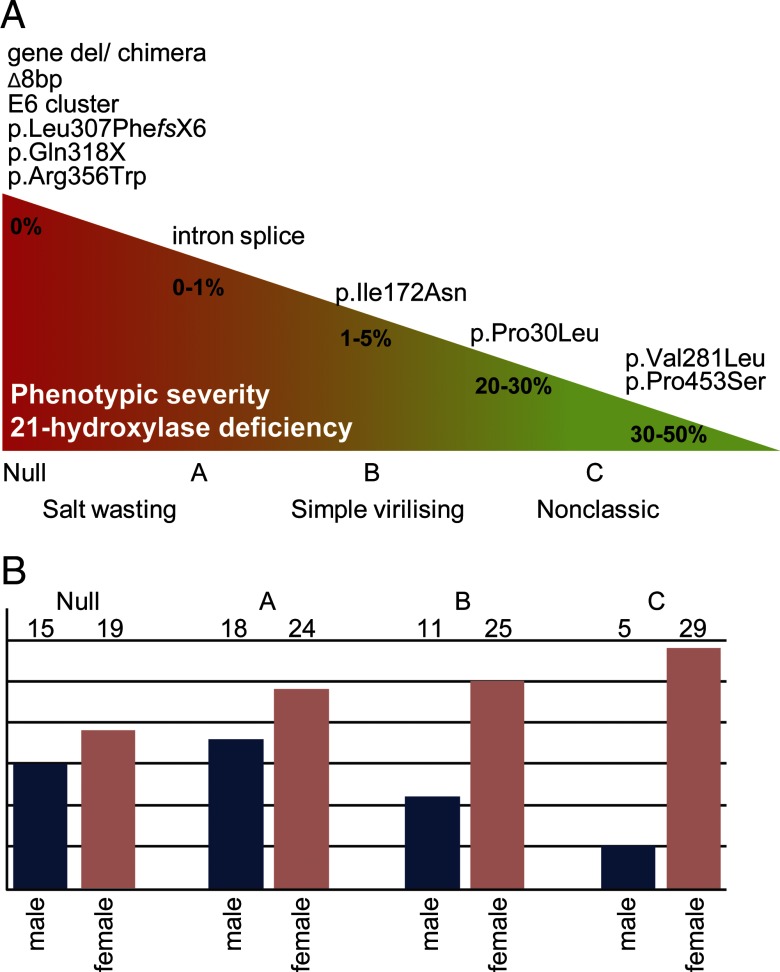
In more than 95% of patients with 21OHD, common CYP21A2 mutations are detected. Approximately 20%–25% of mutant alleles in 21OHD are CYP21A2 gene deletions and CYP21A1P/CYP21A2 chimeric genes (traditionally termed large gene conversions) caused by unequal crossing-over events within the RCCX locus (4). The transfer of genetic material from the CYP21A1P pseudogene into the CYP21A2 gene accounts for about 70%–75% of CYP21A2 disease-causing mutations, including 7 point mutations, a deletion of 8 base pairs in exon 3, and a cluster of 3 point mutations in exon 6 (1, 5). In addition, more than 100 pseudogene-independent mutations are listed by the Human Cytochrome P450 allele Nomenclature Committee (http://www.cypalleles.ki.se/cyp21.htm). CYP21A2 genotypes have been categorized into different mutation groups based on their in vitro 21-hydroxylase activity; using these categories, a strong genotype-phenotype correlation has been established with high positive predictive value for salt wasting (5) and impairment of adrenomedullary function (6) (Figure 1A).
Figure 1.
Definition of mutation groups and their distribution in the cohort. A, Genotype-phenotype correlations in CAH due to 21-hydroxylase deficiency based on in vitro CYP21A2 activity. Mutation groups null and A are associated with the SW form of 21OHD, group B with the SV form, and group C with the nonclassic (NC) form. E6 cluster refers to the p.Ile236Asn, p.Val237Glu, and p.Met239Leu mutation cluster at exon 6; intron splice refers to the c.293–13A/C>G mutation (other names: i2G, I2G, IVS2–13A/C>G); Δ8bp refers to the p.Gly110ValfsX21 mutation. B, Patient cohort according to CYP21A2 mutation group and gender.
In recent years, increasing attention has been paid to long-term health problems in adults with CAH (7–9). Our recent cohort study [United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE)] of 203 adults with congenital adrenal hyperplasia demonstrated their suboptimal health status with a poor metabolic profile, impaired fertility, and reduced quality of life (QOL) (10), but the cause for these poor outcomes remains to be elucidated. We hypothesized that the severity of the disease-causing CYP21A2 mutation might influence the outcome and health status in adults with CAH. To this end we have carried out a comprehensive genotype-phenotype analysis in the UK CaHASE cohort.
Patients and Methods
Patients were recruited from 17 centers into this nationwide study as previously described (10). The study protocol was approved by West Midlands Multicentre Research Ethics Committee (MREC/03/7/086) and registered with www.ClinicalTrials.gov (NCT00749593). All patients were 18 years old or older and had a confirmed diagnosis of CAH. The recruitment started August 2004 and ended July 2007. Written informed consent was obtained from all participants.
General procedures
All participants were seen at the center routinely responsible for their clinical care in the morning after fasting overnight and taking their regular medication. After medical history and physical examination, fasting blood samples were taken. Thereafter patients were served breakfast and psychometric questionnaires were completed. The physical examination included recording of blood pressure (BP) (3 seated and 1 standing, separated by 5 minutes), height, weight, waist circumference, presence and degree of stretch marks, and in females hirsutism and in males testicular volume (orchidometer) and presence of masses.
The following biochemical parameters were assessed: renal and liver function, fasting plasma glucose, serum insulin, cholesterol (total, low density lipoprotein, and high-density lipoprotein), triglycerides, plasma renin activity, and steroid hormones including 17-hydroxyprogesterone (17OHP), androstenedione, testosterone, and SHBG. The median (interquartile range) for the time from intake of last glucocorticoid dose until blood sampling was 3.75 (2.25–12.5) hours. All biochemical measurements were performed at local laboratories. The results were recorded as below, within, or above the local reference range, apart from 17OHP, androstenedione, and plasma renin, for which absolute values were compared with previously recommended target ranges for patients with CAH and adrenal insufficiency (1, 5). All laboratories participate in the United Kingdom National External Quality Assessment Service scheme for quality control of steroid immunoassays. Serum insulin was measured in a single laboratory using an ultrasensitive ELISA (DRG Instruments, Marburg, Germany) and used for calculation of homeostasis model assessment of insulin resistance (HOMA-IR). Psychometric evaluation was carried out using self-administered questionnaires, including 2 validated questionnaires for the assessment of subjective health status [36 item short form health survey (SF-36)].
Mutation analysis, mutation groups, and in vitro analysis of CYP21A2 mutations
DNA was available for mutation analysis of the CYP21A2 gene in 153 of 203 patients from the UK CaHASE cohort (10). CYP21A2 gene deletion and chimeric genes were detected using a commercially available multiplex-ligation probe amplification strategy following the manufacturer's protocol (mrc-Holland, Amsterdam, The Netherlands). Pseudogene-derived CYP21A2 point mutations were detected by targeted multiplex minisequencing after allele-specific PCR amplification of the CYP21A2 gene analysis as described previously (11). Sequence variants were designated according to Human Genome Variation Society recommendations (www.hgvs.org/rec.html) using the reference sequences GenBank NC_000006 (genomic DNA) and Gen-Bank NP_000491.4 (protein).
If the multiplex-ligation probe amplification analysis showed complex rearrangements, a second published PCR amplification method (12) was used to avoid nonamplification of the CYP21A2 gene and misinterpretation of the results, which was also followed by multiplex minisequencing. If no common mutations were detected, direct DNA sequencing of the entire CYP21A2 gene was performed (13).
Patients were categorized into established CYP21A2 mutation groups according to their genotype, with the less severe mutation determining the group: null (mutations predicting absent in vitro activity), A (intron 2 splice site mutation), B (mutations such as the p.IIe172Asn mutation and mutations with 1%–10% in vitro residual enzyme activity), C (mutations such as p.Pro30Leu, p.Val281Leu, and p.Pro453Ser or greater than 20%–30% in vitro 21-hydroxylase activity) (14–16) (Figure 1A).
To allocate patients to appropriate mutation groups, the enzymatic activity of novel or rare CYP21A2 missense mutations without published residual 21-hydroxylase activity was analyzed using a previously published in vitro expression assay measuring the conversion of progesterone to 11-deoxycorticosterone in five independent triplicates (17).
Statistical analysis
Patients were stratified according to their genotypes into mutation groups. Continuous variables were analyzed using ANOVA, with post hoc comparisons between groups by Tukey's test. When the assumptions of ANOVA were not met, Kruskal-Wallis tests were used, with comparisons between groups using the post hoc pairwise comparisons in SPSS 19 (IBM SPSS Inc, Chicago, Illinois). Rates were compared using Fisher's exact tests. Where significant differences were detected, post hoc comparisons between groups were made, with the critical P value Bonferroni corrected to account for the effect of multiple comparisons. These analyses were performed using SPSS version 19.0.0 (IBM SPSS Inc), with P < .05 or the corresponding Bonferroni-adjusted P value when multiple comparisons are made, deemed significant.
Reference data for SF-36 scores were obtained from John Brazier (University of Sheffield, Sheffield, United Kingdom) including a representative random sample of 14 430 subjects aged between 18 and 79 years from the UK population. We randomly selected 20 (SF-36) sex- and age-matched controls for every CAH patient from the respective reference samples for comparison. Score values from patients and controls were transformed into age-adjusted (decade) and sex-adjusted z-scores, with subsequent comparison by the Mann-Whitney U test. These analyses were performed using Stata version 10 (College Station, Texas) and SPSS version 18.0 (SPSS). Statistical significance was assumed when P < .05.
Results
Molecular genetic analysis
CYP21A2 mutation analysis was performed in 153 patients [median age 35 years (range 18–69 years); 103 females, 50 males]. The distribution of mutations in our cohort is similar to population studies analyzing the allele frequency of CYP21A2 mutations in CAH patients from published cases series (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). In 7 patients a mutation was identified on only 1 allele. Novel mutations were detected on 1.7% of alleles (5 of 306) and rare mutations were detected on 2.3% of alleles (7 of 306). Mutations were classified according to their published in vitro activity; for novel variants and mutations without published activities activity was measured using in vitro expression assays (17). The 5 novel mutations included 1 missense mutation (g.752C>T, p.R124C), 1 nonsense mutation (g.803C>T, p.Q141X), 1 splice site mutation (IVS9 +1G>C), and 2 frame shift mutations (g.2047delG, p.V334fxX28; g.2674dupC, p.R483PfsX40). The p.R124C mutation had a residual activity for the conversion of progesterone to deoxycorticosterone of 16% (±0.6 SEM) and was classified as a mutation group B variant. The previously published L308F mutation showed a residual activity of approximately 3% (3.2% ±0.6 SEM) for 21-hydroxylation of progesterone and was categorized into mutation group B, which is in line with a previous prediction that this variant should lead to moderate functional impairment as it was found in a patient with simple virilizing CAH (18). The previously published variant p.R435C (19) had a residual activity of approximately 7% (6.5% ± 0.9 SEM) for the conversion of progesterone to deoxycorticosterone.
Patients were categorized into CYP21A2 mutation groups for further analysis: null 34 (19 females, 15 males), A 42 (24 females, 18 males), B 36 (25 females, 11 males), C 34 (29 females, 5 males), and D 7 (6 females, 1 male) (Figure 1B). The 7 patients in mutation group D were excluded from the further data analysis because this group comprised heterozygous patients in whom the phenotype prediction is not possible.
Anthropometric and metabolic data
The age distribution was similar between mutation groups except for all and female patients in mutation group B, who were slightly older than patients in mutation group null (Table 1). Anthropometric measures did not differ between mutation groups in either sex, with the exception of women in mutation group B having a lower final height than females in other genotype groups.
Table 1.
Anthropometric Measurements and Blood Pressure
| Gender | Mutation Group |
Significance of Mutation Group (ANOVA) | ||||
|---|---|---|---|---|---|---|
| Null | A | B | C | |||
| Age, ya | All | 27.98 (25.65–30.52) | 32.04 (29.15–35.22) | 39.42 (34.95–44.46) | 33.79 (30.37–37.60) | <.001b |
| Male | 26.31 (22.75–30.41) | 31.33 (26.31–37.30) | 35.70 (28.81–44.24) | 28.75 (18.00–45.91) | .123 | |
| Female | 29.37 (26.25–32.87) | 32.59 (29.09–36.51) | 41.18 (35.37–47.93) | 34.74 (31.11–38.81) | .003b | |
| Height, cmc | All | 160.76 (7.11) | 159.50 (9.52) | 154.49 (9.99) | 158.50 (8.03) | .021b |
| Male | 166.20 (5.38) | 163.72 (9.04) | 165.20 (8.59) | 161.80 (10.64) | .698 | |
| Female | 156.4 (5.11) | 156.33 (8.76) | 150.20 (6.84) | 157.93 (7.58) | .001b | |
| Body mass index, kg/m2a | All | 28.08 (26.13–30.17) | 31.32 (28.99–33.83) | 29.73 (27.65–31.96) | 30.07 (27.88–32.43) | .211 |
| Male | 28.43 (24.90–32.46) | 29.99 (27.36–32.88) | 27.71 (24.30–31.61) | 28.64 (24.38–24.90) | .760 | |
| Female | 27.81 (25.48–30.35) | 32.25 (28.68–36.50) | 30.57 (27.94–33.45) | 30.32 (27.79–33.08) | .221 | |
| Waist circumference, cma | All | 90.81 (85.96–95.94) | 98.65 (93.40–104.20) | 92.81 (87.67–98.26) | 92.82 (87.37–98.60) | .162 |
| Male | 91.61 (84.06–99.84) | 99.86 (92.32–108.03) | 89.44 (80.26–99.67) | 89.69 (68.59–117.29) | .250 | |
| Female | 90.20 (83.36–97.60) | 97.76 (90.20–105.97) | 94.06 (87.63–100.97) | 93.39 (87.59–99.57) | .516 | |
| Sitting systolic BP, mm Hgc | All | 119.25 (13.71) | 122.11 (15.96) | 123.82 (17.19) | 119.08 (14.57) | .508 |
| Male | 122.10 (14.37) | 124.91 (19.03) | 122.79 (15.12) | 117.13 (11.65) | .817 | |
| Female | 117.16 (13.19) | 119.93 (13.11) | 124.28 (18.31) | 119.41 (15.16) | .458 | |
| Sitting diastolic BP, mm Hgc | All | 75.26 (8.23) | 75.91 (10.55) | 76.42 (9.77) | 74.76 (9.58) | .894 |
| Male | 74.74 (8.07) | 75.96 (9.55) | 76.18 (10.73) | 73.87 (6.08) | .948 | |
| Female | 75.65 (8.56) | 75.87 (11.49) | 76.55 (9.55) | 74.92 (10.14) | .948 | |
Data presented as geometric mean (95% confidence interval) after log transformation.
Significance of Tukey's paired comparisons, significant at the 5% level; age, all: null vs B, < 0.001; A vs B, 0.018; age, female: null vs B, 0.002; A vs B, 0.036; height, all: null vs B, 0.018; height, female: null vs B, 0.029, A vs B, 0.021, B vs C, 0.001.
Data presented as mean (SD).
There were no differences between mutation groups in systolic or diastolic BP (Table 1), blood glucose concentrations or HOMA-IR, or lipid profiles (Table 2).
Table 2.
Blood Glucose and Lipids
| Gender | Mutation Group |
Significance of Mutation Group (ANOVA) | ||||
|---|---|---|---|---|---|---|
| Null | A | B | C | |||
| Glucosea | All | 4.60 (4.30–5.20) | 4.55 (4.20–5.00) | 4.50 (4.20–5.00) | 4.75 (4.50–5.10) | .351a |
| Median (quartiles) | Male | 4.90 (4.30–5.20) | 4.60 (4.10–4.83) | 4.70 (4.50–5.30) | 4.80 (4.20–6.15) | .476a |
| Female | 4.50 (4.20–5.05) | 4.50 (4.25–5.08) | 4.30 (4.00–4.70) | 4.70 (4.50–5.10) | .127a | |
| HOMA-IRb | All | 1.71 (1.19–2.45) | 1.71 (1.35–2.16) | 1.54 (1.16–2.05) | 1.63 (1.26–2.10) | .937 |
| Male | 1.89 (0.74–4.83) | 1.71 (1.10–2.64) | 1.63 (0.82–3.20) | 2.89 (1.07–7.80) | .801 | |
| Female | 1.61 (1.12–2.31) | 1.70 (1.27–2.28) | 1.50 (1.09–2.05) | 1.52 (1.16–2.00) | .916 | |
| Cholesterol | All | 5.09 (1.26) | 4.94 (1.21) | 5.28 (1.16) | 5.14 (1.16) | .676 |
| Mean (±SD) | Male | 4.63 (0.88) | 4.46 (0.90) | 4.77 (0.91) | 4.50 (1.47) | .867 |
| Female | 5.50 (1.42) | 5.28 (1.30) | 5.49 (1.21) | 5.27 (1.07) | .876 | |
| HDLb | All | 1.43 (1.29–1.59) | 1.53 (1.38–1.70) | 1.59 (1.46–1.74) | 1.54 (1.37–1.72) | .532 |
| Male | 1.27 (1.08–1.49) | 1.32 (1.16–1.51) | 1.50 (1.21–1.86) | 1.22 (0.97–1.54) | .401 | |
| Female | 1.58 (1.40–1.79) | 1.72 (1.48–2.01) | 1.63 (1.48–1.79) | 1.61 (1.42–1.83) | .792 | |
| LDLb | All | 2.87 (2.55–3.23) | 2.73 (2.46–3.02) | 2.96 (2.61–3.36) | 2.96 (2.66–3.29) | .663 |
| Male | 2.64 (2.17–3.22) | 2.47 (2.09–2.91) | 2.86 (2.50–3.26) | 2.70 (1.25–5.84) | .740 | |
| Female | 3.09 (2.65–3.60) | 2.96 (2.59–3.38) | 3.00 (2.51–3.59) | 3.01 (2.73–3.31) | .981 | |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein. Analyses performed using 1-way ANOVA/Tukey's paired comparisons unless stated otherwise.
Kruskall-Wallis test.
Data presented as geometric mean (95% confidence interval) after log transformation.
Steroid replacement therapy and quality of biochemical control
Patients with null genotypes received higher hydrocortisone doses than patients in mutation group C. We also observed a trend toward higher doses of prednisolone and dexamethasone in patients with predicted SW-CAH (groups null and A) than in the patients in groups associated with SV-CAH (group B) or nonclassic CAH (group C) (Table 3).
Table 3.
Glucocorticoid and Mineralocorticoid Treatment
| Mutation Group |
Significance of Mutation Group (ANOVA) | Significance of Tukey's Paired Comparisons |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Null | A | B | C | Null vs A | Null vs B | Null vs C | A vs B | A vs C | B vs C | ||
| Hydrocortisone only | |||||||||||
| n (%) | 10 (29.4%) | 10 (23.8%) | 9 (25.0%) | 7 (20.6%) | .864 | ||||||
| Median dose (range), mg/d | 30 (15–30) | 25 (15–30) | 30 (20–33) | 20 (10–35) | .021a | .178 | 1.000 | .023a | 1.000 | 1.000 | .343 |
| Median dose per BSA (range), mg/m2 · d | 17.3 (8.1–30.8) | 14.3 (7.9–16.8) | 15.7 (10.7–20.3) | 11.1 (4.5–20.8) | .043a | .321 | 1.000 | .053 | 1.000 | 1.000 | .375 |
| Prednisolone only | |||||||||||
| n (%) | 12 (35.3%) | 15 (35.7%) | 16 (44.4%) | 17 (50.0%) | .529 | ||||||
| Median dose (range), mg/d | 6.8 (5–9) | 7.5 (4–10) | 5.5 (3–10) | 5 (1–8) | .124 | ||||||
| Median dose per BSA (range), mg/m2 · d | 3.7 (2.6–4.9) | 3.5 (1.9–6.0) | 3.2 (1.2–4.9) | 3.0 (0.4–4.5) | .324 | ||||||
| Dexamethasone only | |||||||||||
| n (%) | 6 (17.6%) | 11 (26.2%) | 10 (27.8%) | 6 (17.6%) | .629 | ||||||
| Median dose (range), mg/d | 0.50 (0.25–0.75) | 0.50 (0.25–0.75) | 0.35 (0.25–0.75) | 0.25 (0.25–0.50) | .176 | ||||||
| Median dose per BSA (range), mg/m2 · d | 0.30 (0.13–0.49) | 0.24 (0.11–0.47) | 0.21 (0.13–0.46) | 0.14 (0.11–0.25) | .246 | ||||||
| Combination of glucocorticoids, n (%) | 6 (17.6%) | 6 (14.3%) | 1 (2.8%) | 3 (8.8%) | .171 | ||||||
| Reverse circadian glucocorticoid administration, n (%) | 17 (50.0%) | 19 (45.2%) | 17 (47.2%) | 14 (41.2%) | .914 | ||||||
| Fludrocortisone | |||||||||||
| n (%) | 32 (94.1%) | 33 (78.6%) | 14 (38.9%) | 10 (29.4%) | <.001a | .098 | <.001b | <.001b | <.001b | <.001b | .457 |
| Median dose (range), μg/d | 163 (100–500) | 100 (50–200) | 100 (50–200) | 100 (50–500) | .015a | .134 | .044a | .155 | 1.000 | 1.000 | 1.000 |
| Median dose per BSA (range), μg/m2 · d | 94 (51–231) | 64 (27–134) | 63 (30–121) | 57 (27–219) | .016a | .058 | .270 | .074 | 1.000 | 1.000 | 1.000 |
Significant at the 5% level.
Significant at the Bonferroni-adjusted level of 1.67%.
Biochemical disease control did not differ between mutation groups. Only 16% of patients with null mutations (5 of 31), 7% of patients with group A mutations (3 of 41), 12% of patients with group B mutations (4 of 34), and 29% of patients with group C mutations (9 of 31) had 17OHP concentrations in the target range (12–36 nmol/L). All other patients had either suppressed or increased 17OHP concentrations, which did not correlate with the underlying genotype (Supplemental Figure 1A). Compared with 17OHP, a higher percentage of patients achieved normal values for androstenedione (3–11 nmol/L), again with no differences between mutation groups: 19% of females and 42% of males in group null; 44% of females and 44% of males in group A; 26% of females and 20% of males in group B; and 41% of females in group C (Supplemental Figure 1, B and C). Most men (85%) in all mutation groups had normal testosterone concentrations across CYP21A2 mutation groups. Only half of the women had normal testosterone concentrations (53%), with 23.9% having suppressed (24% of null; 14% of A, 44% of B, and 15% of C) and 23% increased (29% of null, 23% of A, 17% of B, and 23% of C) testosterone values.
Fludrocortisone was used more frequently and in higher doses in patients with the more severe genotypes. However, due to the small sample size and large number of comparisons being made, there was insufficient statistical power to detect the pairs of groups between which significant differences occurred (Table 3). Only 8% of patients with null mutations had plasma renin levels in the upper reference range, with 79% of patients having renin levels above the reference range indicative of underreplacement. In addition, 26.7% of patients in group A had a renin in the upper reference range, whereas half of the patients in group B were found in the reference range (Supplemental Table 2). Overtreatment, defined by renin concentrations either in the lower reference range or suppressed, was only rarely observed throughout mutation groups.
Fertility
Menarche occurred spontaneously in more than 80% of women (94% of null, 87% of A, 82% of B, and 94% of C). The age of menarche was not different between mutation groups [null, 14.5 years (9–25 years); A, 14.4 years (10–20 years); B, 14.2 years (9–22 years); and C, 13 years (9–22)].
Only 29% of women reported having ever been pregnant. Importantly, 59% of females reported having never attempted to become pregnant. A clear difference was found between patients with more severe CAH (groups null and A) and patients with simple virilizing and nonclassic CAH (groups B and C). Approximately 80% of women in mutation groups null and A had never tried to have children (Supplemental Table 3). In contrast, more than half of patients in group B had tried to have children and 36% of patients with group B mutations had been pregnant. About 60% of females with group C mutations had tried to be, and about half of all patients had been, pregnant in this group. There was a clear age dependency. Most women younger than 30 years had never tried to get pregnant, whereas a significantly higher proportion of older women when trying achieved by a higher success rate of pregnancies (Supplemental Table 3).
Most men in all mutations groups did not have children (87% of null, 77% of A, 90% of B), and of these, 79% had never tried to have children (73% of null, 91% of A, 78% of B). The data set was insufficient to analyze any relation to testicular adrenal rest tumors.
Subjective health status
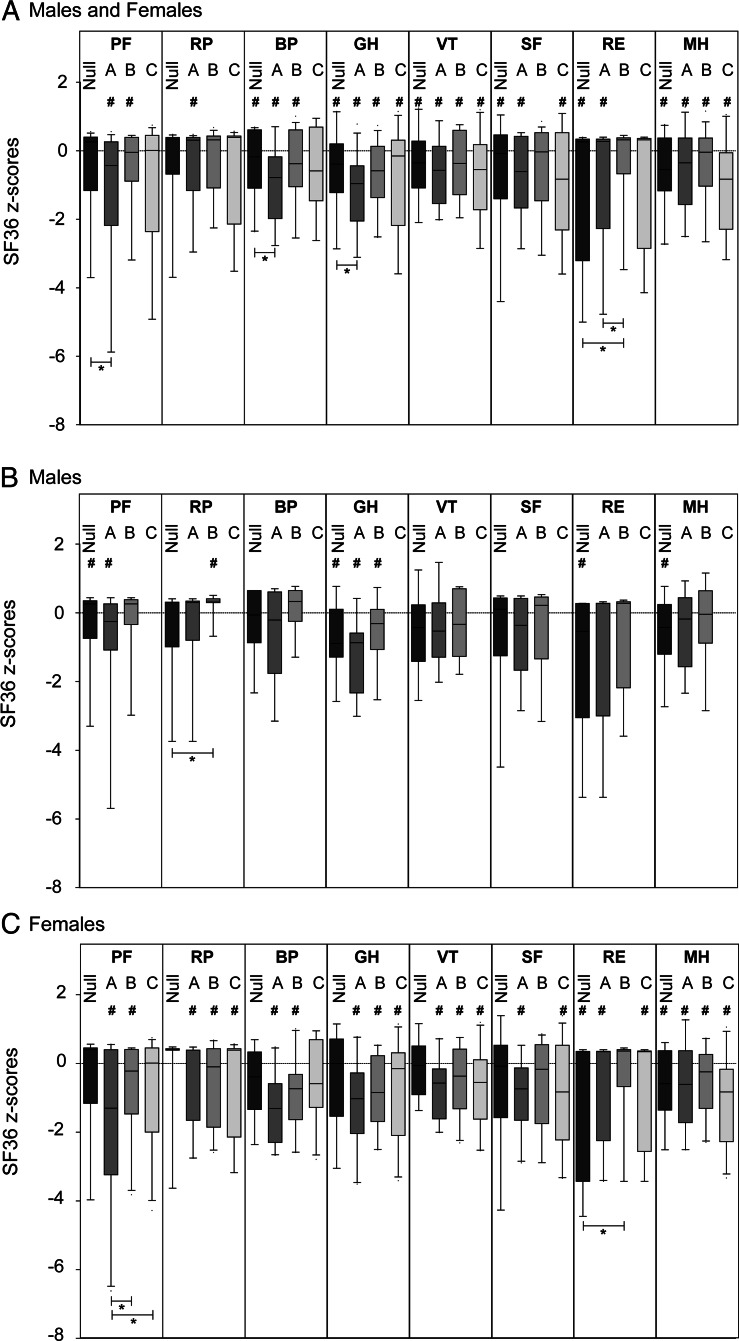
We previously demonstrated significantly impaired subjective health status in our CAH patients in comparison with healthy controls (10). Here we found no correlation between genotype and subjective health status (Figure 2). Subjective health status was similarly impaired in male and female patients (Figure 2C) and in patients younger and older than 30 years.
Figure 2.
Subjective health status according to SF-36 dimension scores for different mutation groups (panel A): null [n = 27; 13 females (f), 14 males (m)]; A (n = 35; 20 f, 15 m); B (n = 34; 22 f, 12 m); C (n = 27; 25 f, 2 m). Panels B and C depict the same analysis for males (B) and females (C) in a separate fashion. Results for the males in mutation group C are not shown due to the small sample size (n = 5). #, Significant difference (P < .05) between patients and normal controls; *, difference between mutation groups at a significance level of P < .05. SF-36 dimensions: BP, bodily pain; GH, general health; MH, mental health; PF, physical functioning; RE, role limitations due to emotional problems; RP, role limitations due to physical problems; SF, social functioning; VT, vitality.
Discussion
The CYP21A2 genotype has not been correlated previously with health outcomes in adults with CAH, except in relation to outcome of surgical procedures (20) and gender typical behavior (21). In children, there is a correlation between genotype and renal salt loss (5) and impairment of adrenomedullary function (6). The correlation between virilization of the external genitalia in 46,XX individuals and the CYP21A2 genotype has been variably reported, with more severe CYP21A2 gene mutations tending to be associated with more severe virilization (14–16). Here we investigated whether outcome measures in adults with CAH related to the severity of the genotype.
Our CAH patient cohort is genetically representative because we found the same distribution pattern of genotypes previously described in studies analyzing the spectrum of CYP21A2 mutations in CAH. We, like others, observed a reduced number of men, in particular those with a milder genotype (16, 22, 23).
We observed that higher glucocorticoid doses tended to be used in patients with more severe mutations, which was significantly different only in patients on hydrocortisone replacement. The lack of statistical significance might well be caused by the small sample size and heterogeneity of glucocorticoid use, which included hydrocortisone, prednisolone, and dexamethasone. However, the possibility remains that either substantially different glucocorticoid doses are not required in patients with contrasting genotypes or that the treatment might not be well adjusted to individual requirements. In all mutation groups, only a low percentage of patients had parameters of hormonal control in the desired target ranges (Supplemental Figure 1).
It seems that in general, patients were often treated with insufficient doses of mineralocorticoids. Importantly, patients with all disease severities may benefit from renin measurement to assess whether they benefit from tailored mineralocorticoid replacement. This is supported by our findings in a few patients within mutation group C, which is normally associated with nonclassic CAH. However, several patients had increased renin levels at least 1-fold above the normal range and were not on fludrocortisone (Supplemental Table 2).
The strong correlation between renal salt loss phenotype and genotype in children (5) is reflected in our findings that use of fludrocortisone and fludrocortisone dose correlated with the underlying genotype (Supplemental Table 2). The use of mineralocorticoids in patients with mutation group C can be explained by the fact that even some patient with nonclassic CAH may have subclinical signs of salt loss (24). Thus, the overall correlation between genotype and salt loss in adults with CAH seems to be similar to children, in whom a higher degree of subclinical and clinical salt loss is observed in individuals with the more severe genotype.
Variable outcomes have been described for most cardiovascular and metabolic parameters in adult patients with CAH. Higher prevalence of obesity and lower levels of insulin sensitivity in comparison with the general population are commonly reported (7–9). Our previous report on the UK CaHASE cohort illustrated suboptimal outcome and several health problems in adults with CAH (10). However, we did not find any relation between the underlying genotype and the metabolic outcome in our patient cohort. This may well be explained, as outlined above, by a significant proportion of patients receiving suboptimal management with glucocorticoids and mineralocorticoids.
The age of menarche did not differ between mutation groups, suggesting a similar course of pubertal development in females. Most women with null and A mutations in our study had never tried to have children, which is a similar finding to reports from Sweden (25). In contrast to the Swedish study, we observed 1 patient with null mutations, who had 1 child. An increasing number of children were born in patients with less severe genotypes and females with milder genotypes had tried for fertility more commonly (Supplemental Table 3). Using our data set, we are able to report only on fertility, but importantly, some recent studies established almost normal fecundity in women with CAH who wish to have children (25, 26). We did not assess whether ovulation frequency differed between patients with different genotypes. However, factors other than hormonal control may well play an important role in decreased fertility in women with CAH (27). Unfortunately, we did not explore sexual orientation or behavior in our patients, which would have provided insights into the effect of the underlying genotype on potential differences in cerebral masculinization caused by androgen effects on the female brain. Others have described higher rates of homosexual and bisexual orientation in women with CAH with increasing frequency in those with severe underlying genotypes (21).
Patients from all mutation groups showed impaired subjective heath status compared with normal controls for most items. However, outcome between patients from different mutation groups did not differ, and subjective health status was similarly impaired when comparing men and women and patients younger and older than 30 years of age. Comparing the different disease severities suggests that impaired subjective health status is caused by exogenous factors such as treatment or noncompliance rather than by predetermined genetic factors. Chronic conditions are generally associated with several difficulties potentially affecting QOL. Impaired subjective health status has been described in our UK CaHASE cohort (10). This impairment was similar to if not greater than previously observed in patients with adrenal insufficiency on current standard replacement therapy (28). The situation in CAH appears to be more complex because CAH features not only adrenal insufficiency but also the additional problem of androgen excess. It is, however, unclear why different studies on subjective health status in CAH have led to variable results. Interestingly, most studies applying the SF-36 showed an impaired subjective health status (10, 29, 30). However, adults with CAH in the multicenter study from the United Kingdom had a worse subjective health status than controls and patients with adrenal insufficiency (10), whereas a study including 2 specialized centers from Germany showed only mild impairment compared with control and better QOL than patients with adrenal insufficiency (30). Other studies including adult CAH patients did find normal QOL (21, 31) or even better QOL in CAH patients compared with normal controls (32). Differences in health care provision may account for such differences, but clear evidence for such assumptions is lacking. In a recent paper on an American cohort of CAH patients, similar findings to ours were described, with most patients showing suboptimal biochemical control of CAH (33). In conclusion, most current health problems in adult CAH patients with 21OHD under medical follow-up do not correlate with the severity of the underlying genetic CYP21A2 genotype. This was observed at an equal level in both sexes and all mutation severity groups. The only exemption is men with nonclassic CAH, who were underrepresented in the current study. The most likely common nongenetic influences on health in different patients are the prescribed steroid hormone replacement regimen and general health care provision. Thus, the future improvement of care provision requires improved individualized treatment strategies tailored to the needs of individual patients. It is possible that differences between different CYP21A2 mutation groups will be unmasked consequent to future improvements of health care provision for adult patients with CAH.
Supplementary Material
Acknowledgments
This study was registered with clinical trial registration number ClinicalTrials.gov NCT00749593.
This work was supported by the WellcomeTrust (Clinician Scientist Fellowship GR079865MA to N.K.) and the Medical Research Council United Kingdom (Programme Grant 0900567 to W.A.). CaHASE gratefully acknowledges support from the Clinical Endocrinology Trust (United Kingdom Registered Charity Number 288679) and the Society for Endocrinology.
Disclosure Summary: R.J.R. is a founding director and equity holder in Diurnal Ltd, which is developing new hydrocortisone preparations for patients with CAH. All other authors have nothing to disclose.
Footnotes
- BP
- Blood pressure
- CAH
- congenital adrenal hyperplasia
- CaHASE
- Congenital adrenal Hyperplasia Adult Study Executive
- HOMA-IR
- homeostasis model assessment of insulin resistance
- 21OHD
- 21-hydroxylase deficiency
- 17OHP
- 17-hydroxyprogesterone
- QOL
- quality of life
- SF-36
- 36-item short form health survey
- SV
- simple virilizing
- SW
- salt wasting.
References
- 1. White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. 2000;21:245–291 [DOI] [PubMed] [Google Scholar]
- 2. Krone N, Dhir V, Ivison HE, Arlt W. Congenital adrenal hyperplasia and P450 oxidoreductase deficiency. Clin Endocrinol (Oxf). 2007;66:162–172 [DOI] [PubMed] [Google Scholar]
- 3. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koppens PF, Hoogenboezem T, Degenhart HJ. Carriership of a defective tenascin-X gene in steroid 21-hydroxylase deficiency patients: TNXB-TNXA hybrids in apparent large-scale gene conversions. Hum Mol Genet. 2002;11:2581–2590 [DOI] [PubMed] [Google Scholar]
- 5. Krone N, Arlt W. Genetics of congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab. 2009;23:181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Charmandari E, Eisenhofer G, Mehlinger SL, et al. Adrenomedullary function may predict phenotype and genotype in classic 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2002;87:3031–3037 [DOI] [PubMed] [Google Scholar]
- 7. Ogilvie CM, Crouch NS, Rumsby G, Creighton SM, Liao LM, Conway GS. Congenital adrenal hyperplasia in adults: a review of medical, surgical and psychological issues. Clin Endocrinol (Oxf). 2006;64:2–11 [DOI] [PubMed] [Google Scholar]
- 8. Arlt W, Krone N. Adult consequences of congenital adrenal hyperplasia. Horm Res. 2007;68:158–164 [DOI] [PubMed] [Google Scholar]
- 9. Reisch N, Arlt W, Krone N. Health problems in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res Paediatr. 2011;76:73–85 [DOI] [PubMed] [Google Scholar]
- 10. Arlt W, Willis DS, Wild SH, et al. Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J Clin Endocrinol Metab. 2010;95:5110–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krone N, Braun A, Weinert S, et al. Multiplex minisequencing of the 21-hydroxylase gene as a rapid strategy to confirm congenital adrenal hyperplasia. Clin Chem. 2002;48:818–825 [PubMed] [Google Scholar]
- 12. Day DJ, Speiser PW, Schulze E, et al. Identification of non-amplifying CYP21 genes when using PCR-based diagnosis of 21-hydroxylase deficiency in congenital adrenal hyperplasia (CAH) affected pedigrees. Hum Mol Genet. 1996;5:2039–2048 [DOI] [PubMed] [Google Scholar]
- 13. Krone N, Roscher AA, Schwarz HP, Braun A. Comprehensive analytical strategy for mutation screening in 21-hydroxylase deficiency. Clin Chem. 1998;44:2075–2082 [PubMed] [Google Scholar]
- 14. Speiser PW, Dupont J, Zhu D, et al. Disease expression and molecular genotype in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Invest. 1992;90:584–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wedell A, Thilen A, Ritzen EM, Stengler B, Luthman H. Mutational spectrum of the steroid 21-hydroxylase gene in Sweden: implications for genetic diagnosis and association with disease manifestation. J Clin Endocrinol Metab. 1994;78:1145–1152 [DOI] [PubMed] [Google Scholar]
- 16. Krone N, Braun A, Roscher AA, Knorr D, Schwarz HP. Predicting phenotype in steroid 21-hydroxylase deficiency? Comprehensive genotyping in 155 unrelated, well defined patients from southern Germany. J Clin Endocrinol Metab. 2000;85:1059–1065 [DOI] [PubMed] [Google Scholar]
- 17. Grischuk Y, Rubtsov P, Riepe FG, et al. Four novel missense mutations in the CYP21A2 gene detected in Russian patients suffering from the classical form of congenital adrenal hyperplasia: identification, functional characterization, and structural analysis. J Clin Endocrinol Metab. 2006;91:4976–4980 [DOI] [PubMed] [Google Scholar]
- 18. Robins T, Carlsson J, Sunnerhagen M, Wedell A, Persson B. Molecular model of human CYP21 based on mammalian CYP2C5: structural features correlate with clinical severity of mutations causing congenital adrenal hyperplasia. Mol Endocrinol. 2006;20:2946–2964 [DOI] [PubMed] [Google Scholar]
- 19. Deneux C, Tardy V, Dib A, et al. Phenotype-genotype correlation in 56 women with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2001;86:207–213 [DOI] [PubMed] [Google Scholar]
- 20. Nordenskjold A, Holmdahl G, Frisen L, et al. Type of mutation and surgical procedure affect long-term quality of life for women with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2008;93:380–386 [DOI] [PubMed] [Google Scholar]
- 21. Frisen L, Nordenstrom A, Falhammar H, et al. Gender role behavior, sexuality, and psychosocial adaptation in women with congenital adrenal hyperplasia due to CYP21A2 deficiency. J Clin Endocrinol Metab. 2009;94:3432–3439 [DOI] [PubMed] [Google Scholar]
- 22. Finkielstain GP, Chen W, Mehta SP, et al. Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2011;96:E161–E172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marino R, Ramirez P, Galeano J, et al. Steroid 21-hydroxylase gene mutational spectrum in 454 Argentinean patients: genotype-phenotype correlation in a large cohort of patients with congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2011;75:427–435 [DOI] [PubMed] [Google Scholar]
- 24. Nimkarn S, Lin-Su K, Berglind N, Wilson RC, New MI. Aldosterone-to-renin ratio as a marker for disease severity in 21-hydroxylase deficiency congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2007;92:137–142 [DOI] [PubMed] [Google Scholar]
- 25. Hagenfeldt K, Janson PO, Holmdahl G, et al. Fertility and pregnancy outcome in women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Hum Reprod. 2008;23:1607–1613 [DOI] [PubMed] [Google Scholar]
- 26. Casteras A, De Silva P, Rumsby G, Conway GS. Reassessing fecundity in women with classical congenital adrenal hyperplasia (CAH): normal pregnancy rate but reduced fertility rate. Clin Endocrinol (Oxf). 2009;70:833–837 [DOI] [PubMed] [Google Scholar]
- 27. Meyer-Bahlburg HF. What causes low rates of child-bearing in congenital adrenal hyperplasia? J Clin Endocrinol Metab. 1999;84:1844–1847 [DOI] [PubMed] [Google Scholar]
- 28. Hahner S, Loeffler M, Fassnacht M, et al. Impaired subjective health status in 256 patients with adrenal insufficiency on standard therapy based on cross-sectional analysis. J Clin Endocrinol Metab. 2007;92:3912–3922 [DOI] [PubMed] [Google Scholar]
- 29. Nermoen I, Husebye E, Svartberg J, Lovas K. Subjective health status in men and women with congenital adrenal hyperplasia: a population-based survey in Norway. Eur J Endocrinol. 2010;453–459 [DOI] [PubMed] [Google Scholar]
- 30. Reisch N, Hahner S, Bleicken B, et al. Quality of life is less impaired in adults with congenital adrenal hyperplasia because of 21-hydroxylase deficiency than in patients with primary adrenal insufficiency. Clin Endocrinol (Oxf). 2011;74:166–173 [DOI] [PubMed] [Google Scholar]
- 31. Kuhnle U, Bullinger M, Schwarz HP. The quality of life in adult female patients with congenital adrenal hyperplasia: a comprehensive study of the impact of genital malformations and chronic disease on female patients life. Eur J Pediatr. 1995;154:708–716 [DOI] [PubMed] [Google Scholar]
- 32. Jaaskelainen, Voutilainen R. Long-term outcome of classical 21-hydroxylase deficiency: diagnosis, complications and quality of life. Acta Paediatr. 2000;89:183–187 [DOI] [PubMed] [Google Scholar]
- 33. Finkielstain GP, Kim MS, Sinaii N, et al. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2012;97:4429–4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.