Abstract
Background
Contingency management (CM) and significant other involvement (SO) were evaluated as strategies to enhance treatment retention, medication compliance, and outcome for naltrexone treatment of opioid dependence.
Methods
One hundred twenty-seven recently detoxified opioid-dependent individuals were randomly assigned to 1 of 3 conditions delivered for 12 weeks: (1) standard naltrexone treatment, given 3 times a week; (2) naltrexone treatment plus contingency management (CM), with delivery of vouchers contingent on naltrexone compliance and drug-free urine specimens; or (3) naltrexone treatment, CM, plus significant other involvement (SO), where a family member was invited to participate in up to 6 family counseling sessions. Principal outcomes were retention in treatment, compliance with naltrexone therapy, and number of drug-free urine specimens.
Results
First, CM was associated with significant improvements in treatment retention (7.4 vs 5.6 weeks; P=.05) and in reduction in opioid use (19 vs 14 opioid-free urine specimens; P=.04) compared with standard naltrexone treatment. Second, assignment to SO did not significantly improve retention, compliance, or substance abuse outcomes compared with CM. Significant effects for the SO condition over CM on retention, compliance, and drug use outcomes were seen only for the subgroup who attended at least 1 family counseling session. The SO condition was associated with significant (P=.02) improvements in family functioning.
Conclusion
Behavioral therapies, such as CM, can be targeted to address weaknesses of specific pharmacotherapies, such as noncompliance, and thus can play a substantial role in broadening the utility of available pharmacotherapies.
In the treatment of opioid dependence, naltrexone, an opioid antagonist that blocks the subjective effects of opioids, has tremendous potential. Relative to methadone hydrochloride and other maintenance therapies, naltrexone is nonaddicting, has a benign adverse effect profile, and can be prescribed without concerns about diversion (eg, naltrexone is rarely traded in the illicit drug market).1 Moreover, naltrexone is not subject to the restrictive regulatory requirements associated with methadone and levomethadyl acetate and hence can be delivered in a range of settings, which may make it more attractive to those opioid-dependent individuals who would not enter traditional drug abuse treatment programs. Furthermore, naltrexone may be less costly, in terms of demands on professional time and patient time, than the near-daily clinic visits required for methadone maintenance therapy.2 Also important are the behavioral aspects of naltrexone, as un-reinforced opioid use allows extinction of relationships between drug cues, craving, and drug use.
Naltrexone has not, despite its many advantages, fulfilled its promise. Naltrexone remains comparatively rare and underused vs methadone maintenance.2 This is in large part due to problems with attrition and noncompliance, particularly during the induction phase, during which on average 40% of patients drop out during the first month of treatment and 60% drop out by 3 months.1 Another factor in reducing naltrexone’s appeal to patients is that it eliminates, without replacing, the powerful reinforcing effects of opioids. Thus, despite its tremendous potential, naltrexone has been overshadowed by methadone, and research on naltrexone has decreased considerably, with the exception of evaluating its utility among select populations (eg, professionals or individuals mandated to treatment).1,3
Combined behavior therapy and pharmacotherapy is considered the optimal strategy for many psychiatric disorders, including substance dependence.4,5 However, systematic research identifying the most effective strategies for combining behavioral and pharmacological treatments has been infrequent. An important, but rarely evaluated, strategy is to apply specific behavioral therapies to directly targeted weaknesses of pharmacotherapies. For example, to compensate for naltrexone’s lack of pharmacological reward, contingency management (CM)6 could be used to reward and thus enhance naltrexone compliance. Similarly, significant other involvement (SO) in treatment could be used to provide incentives for retention and encouragement to persist with treatment despite protracted opioid withdrawal symptoms.
SUBJECTS AND METHODS.
SUBJECTS
The subjects were 127 individuals seeking treatment for opioid dependence who completed outpatient detoxification (95% of subjects) offered through the Central Medical Unit of The APT Foundation in New Haven, Conn, or inpatient detoxification at other facilities. All subjects met current DSM-IV criteria for opioid dependence, as confirmed by Structured Clinical Interview for DSM-IV7 interviews conducted by the master’s-level project director (D.A.E.) (reliability was established in earlier projects and supported through recalibration interviews). Individuals excluded were those who (1) had significant medical conditions, such as abnormal liver function or active hepatitis, or any other condition that would contraindicate naltrexone treatment; (2) did not have a significant other willing to participate in treatment; (3) met the DSM-IV criteria for lifetime schizophrenia or bipolar disorder; or (4) had been involved in substance abuse treatment within the past 3 months.
Of 315 individuals screened, 27 did not meet the eligibility criteria (4 because of medical reasons, 4 because they did not meet the criteria for opioid dependence, 6 because they could not identify a significant other, 3 because of psychological problems, 6 because of current drug treatment, 3 because of imminent incarceration, and 1 because the individual lived too far from the clinic to meet the 3 times a week medication schedule). Eleven individuals dropped out during the pretreatment evaluation process. The 277 individuals who met the eligibility criteria were offered an outpatient detoxification, using clonidine or clonidine-naltrexone detoxification protocols described in previous reports,8,9 or were referred for an inpatient detoxification at another facility in New Haven. Of these individuals, 61 dropped out before initiating detoxification, 48 did not complete detoxification, and 41 completed detoxification but did not return to the clinic for randomization.
The 127 individuals who were randomized were compared with the 150 eligible individuals not randomized on sex, race, employment status, marital status, educational level, treatment history, and severity and chronicity of substance use. The subjects who did not initiate naltrexone treatment were significantly different from the randomized sample only on sex (there were more women in the non-randomized group [χ2=4.63, P=.03]) and number of previous detoxifications (1.50 vs 0.95; F=4.5, P=.04).
TREATMENTS
Following the completion of detoxification, baseline assessment, and provision of written informed consent, subjects were randomly assigned, using an urn randomization program, to 1 of 3 conditions.
Standard Naltrexone Treatment
This treatment, which was delivered to subjects in all 3 conditions, included naltrexone treatment, 3 times a week (Monday, 100 mg; Wednesday, 100 mg; and Friday, 150 mg), under the supervision of a research nurse (D.A.E.). Urine specimens were collected 3 times a week, coinciding with medication visits. In addition, weekly group therapy sessions, consisting of manual-guided CBT,10 were co-led by a master’s-level counselor and a nurse practitioner who received weekly supervision.
Voucher-Based CM
In addition to standard naltrexone, as previously described, subjects in this group received vouchers redeemable for goods and services contingent on targeted behaviors. The voucher system developed by Higgins and colleagues6,11 was adapted to directly address naltrexone’s weaknesses. We hypothesized that if reinforcement was provided only for naltrexone ingestion, cocaine and other drug use that would not be subject to behavioral contingencies might remain problematic or even increase. Therefore, vouchers were provided for 2 target behaviors on 2 independent reinforcement “tracks”: (1) naltrexone ingestion and (2) submission of drug-negative urine specimens. Thus, the first time a subject submitted a drug-free urine specimen, the subject earned the equivalent of $0.80, and the value of vouchers for each consecutive drug-negative urine specimen thereafter increased by $0.40. Similarly, the first time the subject took naltrexone, the subject also earned the equivalent of $0.80, and the value of the vouchers also increased by $0.40 for each consecutive ingestion of naltrexone thereafter. Failure to submit a urine specimen, specimens that tested positive for any illicit drug, or missing a naltrexone visit reset the value of the vouchers for that track back to the starting point ($0.80), from which point the value could escalate again according to the same schedule. Subjects who complied perfectly with the naltrexone regimen and whose urine screen results were all negative could earn a maximum of $561 worth of items during the 12-week treatment. As in the Higgins system,6,11 money was not given directly to subjects. Instead, vouchers were redeemed for items consistent with a drug-free lifestyle (eg, gift certificates for food and clothing or purchasing robes for singing in a church choir).
Significant Other Involvement
Subjects assigned to this condition, in addition to standard naltrexone treatment and CM, as previously described, were offered up to 6 additional family sessions with a non–substance-abusing parent, spouse, child, sibling, or close friend of the subject’s choice. These sessions were manual guided, adapted from the guidelines described by Budney and Hig-gins6 for reciprocal relationship counseling, and delivered by a master’s-level social worker. The goals of the sessions included (1) educating the significant other regarding opioid dependence and ways he or she could support the subject in complying with treatment and remaining abstinent and (2) identifying strategies for enhancing relationships with significant others. This approach was intended to be consistent with and to capitalize on the availability of vouchers to develop supportive interpersonal relationships as alternatives to drug use. Subjects were thus encouraged in the family sessions to redeem vouchers for goods and services that might strengthen relationships with others (eg, going fishing with a parent or hosting a child’s birthday party).
ASSESSMENT
Subjects were assessed immediately before randomization, weekly during treatment, and at the end of the 12-week course of treatment, at which time the CM and SO components were terminated and subjects were transferred to the naltrexone maintenance program of the Substance Abuse Treatment Unit of the Connecticut Mental Health Center of Yale University. Primary outcome measures were (1) compliance with naltrexone treatment (number of times naltrexone was ingested over 12 weeks), (2) frequency of opioid use (self-reported days of opioid use and percentage of opioid-free urine specimens during treatment), and (3) frequency of cocaine use (self-reported days of cocaine use and percentage of cocaine-free urine specimens during treatment). Primary outcomes were assessed using the Substance Abuse Calendar, which is similar to the Form 9012 and collects information on treatment involvement, medication compliance, and substance use on a day-by-day basis and, thus, allows for a flexible continuous evaluation of outcome with minimization of missing data. Secondary outcomes included psychosocial functioning, as assessed by the Addiction Severity Index,13 and human immunodeficiency virus risk behaviors, using the Risk Assessment Battery.14 The Structured Clinical Interview for DSM-IV7 was used to evaluate current and lifetime psychiatric disorders in the sample.
The OnTrak TesTcup (Roche Diagnostics Corp, Indianapolis, Ind) was used to evaluate each urine specimen for the presence of metabolites of opioids, cocaine, and benzodiazepines. This allowed for rapid feedback to subjects regarding urine toxicology results, minimizing the delay usually associated with obtaining urinalysis results from a commercial laboratory. Of 2130 urine specimens collected from all subjects during the treatment phase of the study, 96.1% were consistent with the subjects’ self-reports of opioid use. Of the 83 urine specimens that were inconsistent with self-reports, 16 (0.8% of the total) indicated no opioid use when the subjects reported they had used opioids and 67 (3.1% of the total) indicated recent opioid use when the subjects denied recent use. For cocaine use, 93.1% of the specimens collected were consistent with the subjects’ reports of recent cocaine use (1.0% indicated no recent use when the subjects reported use, and 5.9% indicated recent cocaine use when the subjects denied use). In addition, there were no significant (P=.26) differences between groups in the number of missing urine specimens.
DATA ANALYSES
The principal analytic strategies were analysis of covariance (for aggregate data, such as number of sessions completed) and random regression models (for data collected weekly, such as frequency of use by week) for the primary outcome variables, with 2 orthogonal contrasts: (1) CM contrast, to evaluate the efficacy of CM, the 2 groups that received CM were compared with the group that received standard naltrexone (CM and SO plus CM vs standard naltrexone); and (2) SO contrast, to evaluate the efficacy of adding SO to CM, the SO plus CM group was compared with the CM group (SO plus CM vs CM).
Analyses were conducted on the intent-to-treat sample, that is, all subjects randomized. For all analyses, the α level was .05 and tests were 2-tailed. In cases in which a subject dropped out of treatment, the subject was followed up and interviewed at the 12-week point. Thus, of the 72 subjects who dropped out, 62 (86%) were interviewed; of the 5 subjects who were randomized but did not initiate treatment, 2 were interviewed.
The present study evaluates CM and SO as treatments for recently detoxified opioid addicts taking naltrexone as maintenance therapy. Three approaches were evaluated: (1) standard naltrexone, which included naltrexone taken 3 times per week plus weekly cognitive-behavioral group therapy (CBT); (2) standard naltrexone plus CM, with reinforcement for naltrexone compliance and abstinence; and (3) standard naltrexone, CM, plus SO. The following research questions will be addressed: (1) Does adding CM to standard naltrexone treatment enhance naltrexone compliance and outcome? (2) Does adding SO to CM further enhance naltrexone compliance and outcome? (3) Are component-specific effects of treatment detectable? We hypothesized that those assigned to the SO intervention would report improved family/social functioning relative to subjects who did not receive SO.
RESULTS
SAMPLE DESCRIPTION
As shown in Table 1, the 127 subjects randomized to treatment were predominantly young (mean age, 32), male (76%), white (77%), and unemployed (51%). Most (65%) were single or divorced, and 81% had completed high school. The subjects had substantial legal histories, with a mean of 6 previous arrests. Most (55%) reported they had been treated for drug use previously, and 23% reported previous methadone maintenance therapy. Subjects reported using heroin a mean of 21 of the 28 days before detoxification, with a mean of 3 “bags” per day. The groups differed significantly on baseline intensity of opioid use (number of bags per day). Because this variable was significantly (P<.05) correlated with outcome, all outcome analyses included baseline intensity of opioid use as a covariate.
Table 1.
Baseline Demographic, Substance Use, and Treatment History Data by Treatment Group*
| Variable | Treatment Condition†
|
F or χ2 | df | P | |||
|---|---|---|---|---|---|---|---|
| Standard Naltrexone (n = 44) | CM (n = 35) | SO + CM (n = 48) | Total (N = 127) | ||||
| Demographic characteristics | |||||||
| Age, y‡ | 33.0 (7.1) | 32.1 (9.6) | 32.2 (7.8) | 32.4 (8.1) | 0.16 | 2,124 | .85 |
| Female sex | 11 (25) | 7 (21) | 13 (27) | 31 (24) | 0.40 | 2 | .82 |
| Race | |||||||
| African American | 7 (16) | 3 (9) | 8 (17) | 18 (14.4) | … | … | … |
| Hispanic | 3 (7) | 1 (3) | 5 (10) | 9 (7) | … | … | … |
| White | 34 (77) | 29 (88) | 35 (73) | 98 (77) | 2.95 | 2 | .57§ |
| Unemployed | 20 (46) | 20 (59) | 25 (51) | 65 (51) | 1.30 | 2 | .50 |
| On public assistance | 12 (28) | 3 (9) | 7 (15) | 22 (17) | 5.00 | 2 | .07 |
| On probation or parole | 10 (23) | 6 (18) | 6 (13) | 22 (17) | 1.80 | 2 | .40 |
| Education | |||||||
| Less than high school | 10 (23) | 8 (23) | 6 (12) | 24 (19) | … | … | … |
| High school or GED | 16 (33) | 17 (50) | 25 (51) | 58 (46) | … | … | … |
| Some college or a higher degree | 18 (41) | 9 (26) | 19 (37) | 45 (35) | 4.32 | 2 | .36 |
| Current drug use‡ | |||||||
| No. of “bags” of heroin/d | 2.6 (1.5) | 2.7 (1.4) | 3.4 (1.7) | 2.9 (1.6) | 3.30 | 2,124 | .03 |
| Use in the past 28 d, d | |||||||
| Opioids | 20.1 (7.3) | 21.4 (5.8) | 20.7 (7.1) | 20.6 (6.9) | 0.35 | 2,119 | .71 |
| Cocaine | 5.4 (8.8) | 3.4 (7.3) | 4.4 (6.5) | 4.5 (7.6) | 0.61 | 2,120 | .54 |
| Alcohol | 4.4 (6.7) | 4.7 (7.3) | 4.7 (7.1) | 4.6 (7.1) | 0.02 | 2,121 | .97 |
| Regular use, y | |||||||
| Opioids | 1.7 (5.2) | 2.2 (5.2) | 1.0 (3.0) | 1.6 (4.5) | 0.72 | 2,124 | .48 |
| Cocaine | 3.4 (4.6) | 3.8 (5.1) | 3.6 (4.6) | 3.6 (4.7) | 0.08 | 2,118 | .92 |
| Treatment history | |||||||
| Any previous substance abuse treatment | 26 (59) | 18 (51) | 24 (50) | 68 (54) | 1.00 | 2 | .60 |
| Any previous methadone maintenance treatment | 10 (23) | 9 (26) | 9 (19) | 28 (22) | 0.82 | 2 | .66 |
| Any previous naltrexone treatment | 5 (11) | 4 (11) | 9 (19) | 18 (14) | 1.10 | 2 | .56 |
| Previous opioid detoxifications‡ | 1.0 (1.9) | 1.2 (2.7) | 0.79 (1.7) | 0.96 (2.1) | 0.34 | 2,120 | .70 |
| Lifetime psychiatric diagnoses (those meeting lifetime DSM-IV criteria)|| | |||||||
| Cocaine dependence | 15 (44) | 18 (62) | 22 (58) | 55 (54) | 2.30 | 2 | .31 |
| Alcohol dependence | 11 (32) | 12 (41) | 14 (37) | 37 (36) | 0.55 | 2 | .75 |
| Affective disorder | 5 (15) | 1 (3) | 5 (13) | 11 (10) | 2.30 | 2 | .30 |
| Anxiety disorder | 2 (6) | 1 (3) | 5 (13) | 8 (7) | 2.40 | 2 | .29 |
| Antisocial personality disorder | 5 (15) | 6 (21) | 4 (11) | 15 (15) | 1.20 | 2 | .52 |
Standard naltrexone indicates thrice weekly naltrexone maintenance plus weekly cognitive-behavioral coping skills groups; CM, contingency management in addition to standard naltrexone plus thrice weekly naltrexone; SO + CM, significant other involvement in addition to CM, CBT, plus thrice weekly naltrexone; and GED, general equivalency diploma.
Data are given as the number (percentage) of subjects in each group unless otherwise indicated.
Data are given as mean (SD).
Excludes the 2 subjects who identified themselves as “other.”
n = 101 because some subjects failed to complete the interview.
ATTRITION AND COMPLIANCE
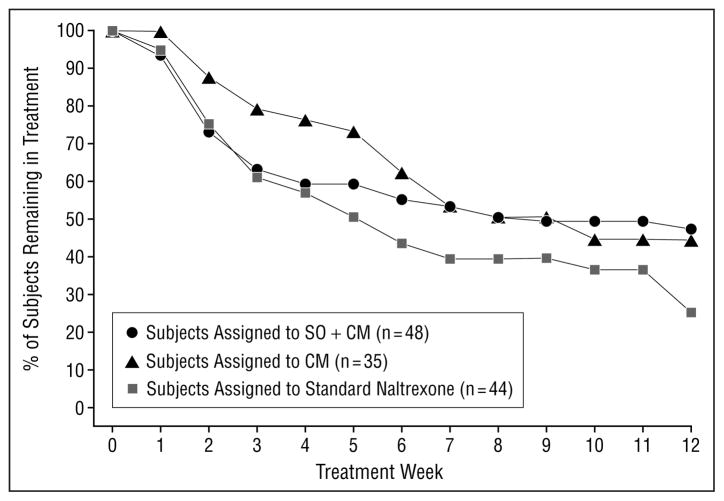
Of the 127 subjects randomized, 122 initiated treatment. The mean number of treatment weeks completed was 7.1 (SD, 4.7). Ten subjects were removed from the treatment protocol: 3 were removed because of discomfort associated with naltrexone, 3 because of clinical deterioration (continued high levels of intravenous drug use), and 1 because of medical complications of the human immunodeficiency virus; 1 was administratively discharged; and 2 moved from the area. One subject died of an accidental overdose 1 month after he had successfully completed the study and had been transferred to a long-term naltrexone maintenance program. Subjects assigned to the 2 CM groups earned a mean of $189 (SD, $220) in vouchers. Subjects assigned to the SO plus CM condition completed a mean of 2.6 (SD, 2.6) significant other sessions (Figure 1).
Figure 1.
Retention by week by treatment group (N=127). All subjects were taking naltrexone 3 times a week as maintenance therapy. CM indicates contingency management; SO+CM, significant other involvement and CM.
As shown in Table 2, retention was significantly higher in the 2 groups assigned to CM compared with the standard naltrexone group, but significant differences between the SO plus CM and the CM groups were not seen, suggesting no additional benefit of SO in addition to CM. Rates of treatment completion were highest in the SO plus CM group (47%), followed by the CM (42.9%) and standard naltrexone (25.6%) groups. Similarly, naltrexone compliance was higher in both CM groups (although this effect fell just short of statistical significance), with no additional benefit for SO plus CM compared with CM alone.
Table 2.
Treatment Retention and Primary Outcomes by Group for the 127 Subjects in the Intention-to-Treat Sample*
| Outcome Variables | Treatment Condition†
|
CM Contrast‡
|
SO Contrast‡
|
||||
|---|---|---|---|---|---|---|---|
| Standard Naltrexone (n = 44) | CM (n = 35) | SO + CM (n = 48) | t | P | t | P | |
| Primary | |||||||
| No. of weeks in treatment | 5.6 (4.5) | 7.4 (4.4) | 7.4 (5.1) | 2.0 | .05 | 0.1 | .96 |
| No. of naltrexone doses | 14.2 (12.4) | 17.8 (13.4) | 19.4 (15.4) | 1.9 | .06 | 0.2 | .83 |
| No. of drug-free urine specimens during treatment | 8.9 (12.0) | 13.6 (13.6) | 16.7 (15.1) | 2.3 | .02 | 0.9 | .35 |
| Secondary | |||||||
| No. of opiate-free urine specimens | 13.5 (12.0) | 18.9 (13.7) | 20.2 (15.5) | 2.1 | .04 | 0.7 | .48 |
| No. of cocaine-free urine specimens | 12.2 (12.6) | 16.0 (13.5) | 18.5 (15.0) | 1.9 | .06 | 0.8 | .44 |
| Drug-free urine specimens, % | 45.2 (39.3) | 57.4 (39.1) | 59.7 (39.7) | 1.8 | .08 | 0.3 | .77 |
| Maximum consecutive abstinence, d | |||||||
| Opioids | 37.7 (32.8) | 49.1 (32.7) | 53.4 (36.5) | 2.0 | .05 | 0.5 | .60 |
| Cocaine | 37.1 (32.4) | 45.0 (32.3) | 51.7 (35.4) | 1.7 | .09 | 0.9 | .39 |
| Days abstinent, % | |||||||
| Opioids | 79.8 (25.5) | 87.5 (20.9) | 89.0 (20.3) | 1.9 | .06 | 0.3 | .77 |
| Cocaine | 82.6 (23.0) | 84.3 (24.5) | 88.6 (14.9) | 0.9 | .34 | 0.9 | .37 |
Data are the results of an analysis of variance. df = 2,124. Abbreviations are explained in the first footnote to Table 1.
Data are given as adjusted mean (SE). Treatment groups are explained in the second footnote to Table 1.
The CM and SO contrasts are described in the “Data Analyses” subsection of the “Subjects and Methods” section.
EFFECTS ON SUBSTANCE USE
In the intention-to-treat sample, for most substance use outcome variables assessed, subjects assigned to the 2 CM groups had improved outcomes compared with the group assigned to standard naltrexone, with little additional benefit associated with the SO condition. For example, subjects assigned to either CM group had significantly more mean days of abstinence from opioids, significantly longer periods of consecutive abstinence from opioids, a significantly higher total number of opioid-negative urine specimens, and a higher percentage of opioid-negative urine specimens compared with those in the standard naltrexone group. However, none of the SO contrasts were significant, again suggesting little additional benefit of adding SO to CM.
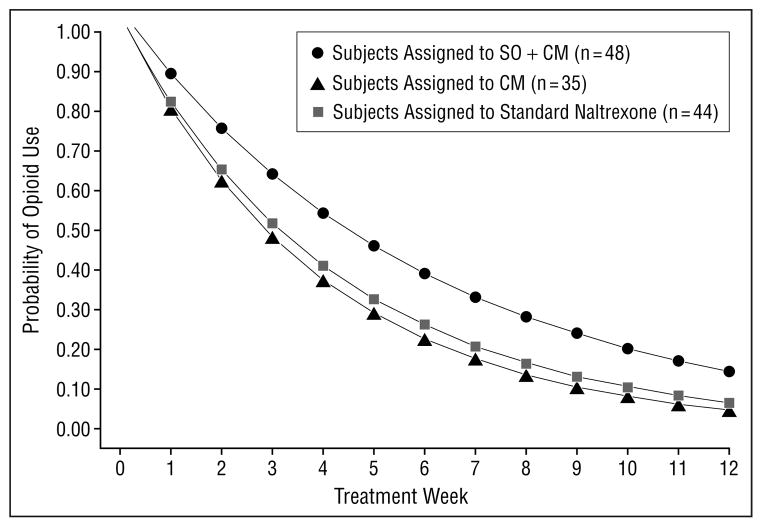
As shown in Figure 2, random regression analyses indicated significant effects for the CM by time contrast (z=−2.63, P=.008), suggesting subjects assigned to the 2 CM groups made greater reductions in their frequency of opioid use over time compared with those assigned to the standard naltrexone group. Because the model presupposes linearity, and 100% of subjects reported using opioids weekly at baseline and less than 10% of those remaining in treatment reported opioid use in the last 4 weeks of treatment, the estimated slope, or rate of change, is modeled accordingly. An additional model that included additional post-attrition data from subjects who dropped out of treatment was consistent with these findings.
Figure 2.
Probability of opioid use by week by treatment group (N=127), result of random regression analyses, using a linear model. All subjects were taking naltrexone 3 times a week as maintenance therapy. CM indicates contingency management; SO+CM, significant other involvement and CM.
Effects of treatment on cocaine use also favored both groups assigned to the CM condition compared with those assigned to standard naltrexone, with little additional benefit for SO. That is, there were trends favoring CM for mean total number of days of abstinence from cocaine and for maximum days of abstinence from cocaine and number of cocaine-negative urine specimens. Effects on alcohol use followed a similar trend, but none of the effects were significant.
Regarding treatment effects on human immunodeficiency virus risk behaviors, there was a significant reduction in frequency of drug-related risk behaviors over time across groups (time effect t=2.7, P=.007); however, neither of the treatment contrasts were significant, indicating no differential effect of CM or SO compared with standard naltrexone on drug risk behaviors. Effects for time or treatment group on frequency of sexual risk behaviors were not significant.
TREATMENT SPECIFICITY
To explore whether the study treatments affected theoretically relevant outcomes, we evaluated whether SO differentially improved family functioning. There was a significant contrast by time effect on the family/social composite score of the Addiction Severity Index13 for the SO contrast (z=2.30, P=.02), suggesting a greater reduction in family problems over time for subjects assigned to the SO plus CM group compared with those assigned to the CM group. In addition, exploratory analyses comparing the SO plus CM group with the other 2 groups also indicated a significant contrast by time effect (z=−2.4, P=.02), suggesting that the beneficial effect of SO on family functioning was not due to the influence of CM.
TREATMENT-EXPOSED SUBGROUPS AND OUTCOME
Although confounded with attrition and compliance, analyses evaluating effects of treatment exposure can be valuable in several respects. Participation of significant others in treatment requires special efforts on the part of the subject to identify an appropriate non–substance-abusing family member and encourage this person to become involved in treatment. Thus, we repeated our principal analyses using the subgroup (30 [62%]) of subjects assigned to the SO plus CM condition who attended 1 or more family sessions. These analyses suggested statistically significant effects for SO plus CM compared with CM on weeks of treatment (t=2.61, P=.01), number of naltrexone doses (t=2.66, P=.009), percentage of opioid-free urine toxicology screens (t=2.28, P=.03), and maximum days of abstinence from opioids (t=2.47, P=.02) and cocaine (t=2.81, P=.006). However, these analyses are confounded with treatment retention; only the effect on number of clean urine specimens was statistically significant after controlling for treatment retention by adding number of treatment weeks as a covariate.
COMMENT
This evaluation of CM and SO to target retention, compliance, and drug use in naltrexone treatment of opioid dependence suggested the following. First, CM significantly improved treatment retention, naltrexone compliance, and opioid use relative to standard naltrexone treatment. Second, assignment to family therapy sessions in addition to CM did not significantly improve retention, compliance, or drug use outcomes compared with CM, but did improve family functioning. Third, exploratory analyses suggested significant effects on virtually all outcomes for the subgroup of subjects who attended at least 1 session with SO. Overall, these findings underscore the value of combining behavioral and pharmacological interventions and point to the promise of systematically targeting behavioral interventions to address specific weaknesses of particular pharmacotherapies.
These findings also add to strong empirical support for each of the therapies evaluated herein. Although a recent meta-analysis15 of family therapy in the treatment of substance dependence indicated a robust effect size for family therapy, family therapy has infrequently been used as a strategy to enhance compliance with pharmacotherapy. The present study involved a comparatively challenging “test” of this approach, because the study design evaluated whether SO conferred additional benefits over CM. That the involvement of significant others in treatment greatly improved outcomes compared with CM for the subgroup of subjects who attended at least 1 session with a family member underlines the promise of this intervention, as does the finding that SO differentially improved family functioning.
Adding SO as a component of treatment dramatically increases treatment complexity, as the patient must be willing to have a family member participate in treatment and that individual must be willing to attend. In this study, ability to participate in family therapy was not a significant obstacle to enrollment, as only 6 individuals screened could not identify a significant other who was willing to participate. Nevertheless, it was difficult for some subjects to engage their significant others in the family sessions, given that subjects had often become estranged from family and friends after years of drug dependence or the subject’s drug use had resulted in serious negative consequences for the family. Thus, just as outcome for pharmacotherapies has been shown to be dependent on compliance,16,17 it should be acknowledged that outcome for behavioral therapies is generally best among patients who make at least minimal efforts to comply.
The study results also add to strong levels of empirical support for CM. A recent meta-analysis18 estimated an effect size of 0.25 for CM in the context of methadone maintenance treatment, the basis of which was the landmark series of studies by Stitzer and colleagues.19 Those studies demonstrated that behavioral incentives, such as increases in methadone dose and take-home doses, could be used to reduce cocaine abuse and other problems frequently seen with methadone maintenance programs. However, because those studies used reinforcers that occur naturally in the context of methadone maintenance treatment, their feasibility outside of methadone maintenance settings had been limited. Thus, a significant advantage of the highly flexible voucher-based CM approach developed by Higgins and colleagues6,11 is that it can be used in many settings and used to target a wide range of behaviors, including medication compliance, as demonstrated herein.
The findings supporting CM are consistent with the recent report by Preston et al,20 which suggested improved retention and compliance with naltrexone when patients were reinforced for compliance. However, while results of the present study suggested significant differences in drug use by treatment condition, the study by Preston et al did not. This may have occurred because the study by Preston et al targeted naltrexone compliance only, whereas the present study targeted compliance and abstinence.
It is also notable that naltrexone was found to be safe with this comparatively large sample, with 3 reports of minor adverse events and 1 death due to overdose after a subject stopped taking naltrexone during the follow-up phase of the study. This is in contrast, for example, to a recent report21 of 13 overdoses and 4 deaths due to overdose in a sample of 81 detoxified opioid addicts treated with comparable doses of naltrexone.
Limitations of the present study include the generalizability of the sample. Because individuals who were not willing to take naltrexone after detoxification were excluded, results may not generalize to opioid addicts seeking detoxification only or those seeking agonist therapies. Second, many subjects did not complete detoxification or return to the clinic following detoxification. Although the rates of dropout during detoxification (22%) and of failure to initiate naltrexone treatment after detoxification (24%) compare favorably with those of previous studies15,16 in this setting, attrition during the detoxification phase is an acknowledged drawback of naltrexone treatment and one that limits its feasibility relative to agonist approaches. Whether introduction of CM or SO in the detoxification phase might further improve retention and, hence, the viability of naltrexone is an area worthy of future investigation. Similarly, despite the effects of the treatments evaluated herein on retention, attrition remained high overall, and there was limited power for some analyses. Finally, only 1 therapist delivered the SO intervention; thus, this introduced a possible therapist or therapy confound and it is not possible to determine if effects of this condition were associated with this treatment or with it being delivered by this particular therapist.
A major implication of these findings is that behavioral therapies can play a substantial role in broadening the utility of available pharmacotherapies. Despite the many advantages of naltrexone and the importance of increasing the availability of treatment for opioid addicts, naltrexone maintenance programs remain rare. The compliance problems associated with naltrexone have rendered it a highly specific treatment primarily used with a few special populations (eg, opioid-abusing physicians and other professionals) for whom special contingencies (eg, loss of licensure or employment) can be leveraged to monitor and, thus, enhance naltrexone compliance. Accordingly, it is significant that the present study involved a population of predominantly unemployed “street addicts” with substantial prior involvement in drug abuse treatment and the legal system.
That behavioral therapies can be used to make effective pharmacotherapies available to a wider proportion of substance abusers is a point that has considerable implications beyond making naltrexone a more viable treatment option. Our arsenal of effective pharmacotherapies for substance use and other psychiatric disorders is not unsubstantial, but it has been demonstrated repeatedly that the effectiveness of these agents is undermined by significant problems with compliance. Thus, capitalizing on behavioral approaches to enhance pharmacotherapy outcomes has important implications for improving outcomes among the many psychiatric patients for whom outcome is particularly compromised by compliance issues, including the more highly impaired subgroups (eg, patients with dual diagnoses and those with personality disorders). This also suggests that ongoing efforts to develop antagonist treatments for cocaine and other substance use disorders should be informed by the history of naltrexone. Those agents, unless delivered with a potent behavioral therapy, are likely to be marginalized in much the same way naltrexone has been.
Acknowledgments
This study was supported by grants P50-DA0924, K05-DA00457 (Dr Carroll), and KO5-DA00089 (Dr Rounsaville) from the National Institute on Drug Abuse, Rockville, Md.
We thank Sister Maureen Lewis, MD, Art Woodard, MSW, Monica Canning-Ball, Carol Eggers, APRN, Susan Henry, RN, Theresa Babuscio, Sister Janet Constantino, APRN, Roseann Bisighini, Lynn Gordon, RN, MPA, Mark Hayes, MSW, and the staff of the Central Medical Unit of The APT Foundation, New Haven, for their contributions.
References
- 1.Greenstein RA, Fudala PJ, O’Brien CP. Alternative pharmacotherapies for opiate addiction. In: Lowinsohn JH, Ruiz P, Millman RB, Langrod JG, editors. Comprehensive Text-book of Substance Abuse. 3. New York, NY: Williams & Wilkins; 1997. pp. 415–425. [Google Scholar]
- 2.Rounsaville BJ. Can psychotherapy rescue naltrexone treatment of opioid addiction? In: Onken LS, Blaine JD, editors. Potentiating the Efficacy of Medications. Rockville, Md: National Institute on Drug Abuse; 1995. pp. 37–52. NIDA Research Monograph 105. [PubMed] [Google Scholar]
- 3.Cornish JW, Metzger D, Woody GE, Wilson D, McLellan AT, Vandergrift B, O’Brien CP. Naltrexone pharmacotherapy for opioid dependent federal probationers. J Subst Abuse Treat. 1997;14:529–534. doi: 10.1016/s0740-5472(97)00020-2. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278:66–70. doi: 10.1126/science.278.5335.66. [DOI] [PubMed] [Google Scholar]
- 5.McLellan AT, Arndt IO, Metzger DS, Woody GE, O’Brien CP. The effects of psychosocial services in substance abuse treatment. JAMA. 1993;269:1953–1959. [PubMed] [Google Scholar]
- 6.Budney AJ, Higgins ST. A Community Reinforcement Plus Vouchers Approach: Treating Cocaine Addiction. Rockville, Md: National Institute on Drug Abuse; 1998. [Google Scholar]
- 7.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV, Patient Edition. Washington, DC: APA Press Inc; 1995. [Google Scholar]
- 8.O’Connor PG, Waugh ME, Carroll KM, Rounsaville BJ, Diakogiannis IA, Schottenfeld RS. Primary care–based ambulatory opioid detoxification: the results of a clinical trial. J Gen Intern Med. 1995;10:255–260. doi: 10.1007/BF02599882. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor PG, Carroll KM, Shi JM, Schottenfeld RS, Kosten TR, Rounsaville BJ. A randomized trial of three methods of primary care–based outpatient opioid detoxification. Ann Intern Med. 1997;127:526–530. doi: 10.7326/0003-4819-127-7-199710010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Carroll KM. A Cognitive-Behavioral Approach: Treating Cocaine Addiction. Rockville, Md: National Institute on Drug Abuse; 1998. [Google Scholar]
- 11.Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, Fenwick JW. A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- 12.Miller WR, Del Boca FK. Measurement of drinking behavior using the Form 90 family of instruments. J Stud Alcohol Suppl. 1994;12:112–118. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- 13.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argerious M. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 14.Navaline HA, Snider EC, Petro CJ, Tobin D, Metzger D, Alterman AI, Woody GE. Preparation for AIDS vaccine trials: an automated version of the Risk Assessment Battery. AIDS Res Hum Retroviruses. 1994;10(suppl):S281–S291. [PubMed] [Google Scholar]
- 15.Stanton MD, Shadish WR. Outcome, attrition, and family-couples treatment for drug abuse: a meta-analysis and review of the controlled, comparative studies. Psychol Bull. 1997;122:170–191. doi: 10.1037/0033-2909.122.2.170. [DOI] [PubMed] [Google Scholar]
- 16.Volpicelli JR, Rhines KC, Rhines JS, Volpicelli LA, Alterman AI, O’Brien CP. Naltrexone and alcohol dependence: role of subject compliance. Arch Gen Psychiatry. 1997;54:737–742. doi: 10.1001/archpsyc.1997.01830200071010. [DOI] [PubMed] [Google Scholar]
- 17.Fuller RK, Branchey L, Brightwell DR. Disulfiram treatment for alcoholism: a Veterans Administration cooperative study. JAMA. 1986;256:1449–1455. [PubMed] [Google Scholar]
- 18.Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone treatment: a meta-analysis. Drug Alcohol Depend. 2000;58:55–66. doi: 10.1016/s0376-8716(99)00068-x. [DOI] [PubMed] [Google Scholar]
- 19.Stitzer ML, Iguchi MY, Felch LJ. Contingent take-home incentive: effects on drug use of methadone maintenance patients. J Consult Clin Psychol. 1992;60:927–934. doi: 10.1037//0022-006x.60.6.927. [DOI] [PubMed] [Google Scholar]
- 20.Preston KL, Silverman K, Umbricht A, DeJesus A, Montoya ID, Schuster CR. Improvement in naltrexone treatment compliance with contingency management. Drug Alcohol Depend. 1999;54:127–135. doi: 10.1016/s0376-8716(98)00152-5. [DOI] [PubMed] [Google Scholar]
- 21.Miotto K, McCann MJ, Rawson RA, Frosch D, Ling W. Overdose, suicide attempts and death among a cohort of naltrexone-treated opioid addicts. Drug Alcohol Depend. 1997;45:131–134. doi: 10.1016/s0376-8716(97)01348-3. [DOI] [PubMed] [Google Scholar]