Abstract
Purpose
To determine the prognostic significance of histologic type in radiation-associated soft tissue sarcomas (RASs) and determine whether RASs are associated with an inferior prognosis compared with sporadic soft tissue sarcomas (STSs).
Patients and Methods
One hundred thirty primary RASs were identified from 7,649 STS patients from 1982 to 2007. Multivariate analysis of clinicopathologic factors for disease-specific survival (DSS) was performed for RASs, and a multivariate analysis of radiation exposure was also performed for RASs and sporadic sarcomas. A matched-cohort analysis was performed for radiation-associated and sporadic malignant fibrous histiocytoma (MFH).
Results
Most RASs were high grade (83%), deep (87%), and truncal (61.5%). The median interval between radiation therapy and RAS development was 10 years (range, 1.3 to 74 years), which varied significantly by histologic type (P = .003). The 5-year DSS was 58%, and independent predictors were size > 5 cm, margin positivity, and histologic type. Multivariate analysis of histologic types of primary, high-grade radiation-associated and sporadic STSs showed that RAS was associated with a worse DSS (hazard ratio, 1.7; range, 1.1 to 2.4; P = .007). For pleomorphic MFH—the most common RAS type—the 5-year DSS was 44% versus 66% in a matched cohort of sporadic MFH patients (P = .07). DSS was significantly worse in primary RAS malignant peripheral nerve sheath tumors (MPNSTs) compared with unmatched sporadic MPNSTs (P = .001).
Conclusion
Histologic type, margin status, and tumor size are the most important independent predictors of DSS in patients with RASs. DSS in patients with primary RAS is significantly worse compared with sporadic STS independent of sarcoma histologic type.
INTRODUCTION
Radiation therapy (RT) is increasingly used as a primary curative modality in many solid tumors including laryngeal, esophageal, cervical, and prostate cancers. Adjuvant RT is also widely administered to limit the extent of surgical resection, prevent local recurrence, and improve functional and cosmetic outcome in breast, rectal, and musculoskeletal tumors. Approximately 60% of all patients with cancer will receive RT during the course of their disease.1 Its use is associated with toxicity, such as impaired wound healing, anastomotic breakdown, fibrosis, and joint stiffness. However, an ominous sequela that manifests years after therapy is the development of a secondary malignancy. Soft tissue sarcomas (STSs) are one of the most common types of radiation-associated tumors in the general population2–5 and in individuals with cancer susceptibility syndromes. For instance, patients with retinoblastoma mutations have a 36% cumulative incidence over 50 years of developing sarcoma in previously irradiated tissue.6
Previous reports from our institution7,8 demonstrated that radiation-associated soft tissue sarcomas (RASs) are predominately high-grade tumors that are difficult to completely resect, with an R0 (negative) resection rate of 54% in our most recent series. The 5-year overall survival in this cohort of surgically resected patients was 41%.8 It has been suggested that RASs may represent a subgroup of tumors associated with poor prognosis.9–11 Interestingly, a recent study did not find an inferior prognosis in radiation-associated bone sarcoma12; however, this question remains unanswered for STSs. The goal of this study was to determine the prognostic significance of histologic type in RASs and determine whether RASs are associated with an inferior prognosis compared with sporadic STSs.
PATIENTS AND METHODS
Between July 1, 1982, and December 31, 2007, 7,649 adult patients treated at Memorial Sloan-Kettering Cancer Center (MSKCC) were identified from a prospective STS database. There were 199 patients (2.5%) identified with RASs, which were defined as (1) history of radiation exposure at least 6 months before the development of sarcoma, (2) occurrence of sarcoma within the radiation field, and (3) pathologic confirmation of a sarcoma that was histologically different from the primary cancer.13,14 One hundred thirty of these 199 patients presented with primary RASs and had no evidence of metastasis at presentation. Histologic review was performed by dedicated sarcoma pathologists (C.R.A. and M.A.E.), with molecular confirmation of known translocations (synovial, Ewing sarcoma). Myxofibrosarcoma (MYXF), a myxoid variant of malignant fibrous histiocytoma (MFH), comprises a spectrum of malignant fibroblastic lesions with variably myxoid stroma (at least 10%), pleomorphism, and a distinctive curvilinear vascular pattern. Pleomorphic MFH represents a pleomorphic sarcoma showing fibroblastic/myofibroblastic differentiation.
Clinicopathologic data included age at diagnosis, sex, histologic type, tumor depth, grade, site, size, margin status, indication for RT, radiation dose (Gy), and use of concomitant chemotherapy. Tumor depth and grade were defined as previously reported.15 Histologic type was divided into six main categories for statistical analysis: leiomyosarcoma, fibrosarcoma/MYFX, angiosarcoma, pleomorphic MFH, malignant peripheral nerve sheath tumor (MPNST), and other. Sites of disease were defined as (1) extremity (upper and lower extremity), (2) abdomen or retroperitoneum (abdomen/RP), and (3) trunk (chest wall, proximal extremity/groin, thoracic, head and neck). Tumor size was recorded as the largest dimension and was also stratified as ≤ 5 cm or > 5 cm. Margins of resection were defined as R0 (negative), R1 (microscopically positive), and R2 (grossly positive).
The primary end point of the analysis was disease-specific survival (DSS), defined as time from date of surgery to date of death as a result of disease or complication. The influence of clinicopathologic features on DSS was analyzed using the Kaplan-Meier method and the log-rank test in the univariate setting and using the Cox proportional hazard regression analysis in the multivariate setting. A P value < .05 was considered significant.
To determine prognostic significance of radiation exposure, we performed a multivariate analysis using all patients presenting to MSKCC with primary, resectable, high-grade, radiation-associated, and sporadic STSs with the following histologic types: leiomyosarcoma, fibrosarcoma/MYXF, angiosarcoma, pleomorphic MFH, and MPNST. Clinicopathologic variables included in the model were RAS versus sporadic STS, age, sex, tumor site, size, depth, margin, and histologic type (leiomyosarcoma, fibrosarcoma/MYXF, angiosarcoma, pleomorphic MFH, MPNST). A matched-cohort study was performed only for patients with pleomorphic MFH, since inadequate sample size precluded matching for the other less common histologic types. A subset of sporadic patients with primary, resectable, high-grade pleomorphic MFH was selected using the R software and the Matching library16 to match the RAS pleomorphic MFH patients on the basis of six prognostic variables (age, sex, and tumor size, site, depth, and margin status).17
RESULTS
Patient, Tumor, and Treatment Characteristics
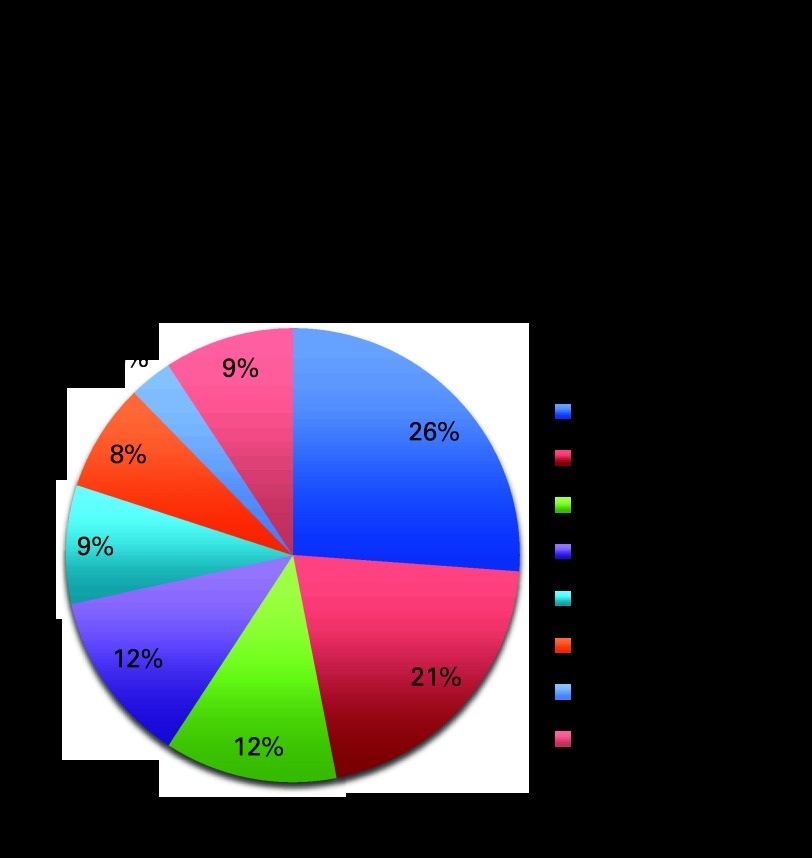
A total of 130 patients with primary RAS were identified. Patient characteristics are summarized in Table 1. The median age was 58.5 years (range, 18 to 86 years), and age at the time of treatment with radiation was 45 years (range, 0.5 to 81 years). Most RASs were high grade (83%) and deep (87%). Truncal site predominated (61.5%), and 69% of patients underwent an R0 resection. The most common histologic type was pleomorphic MFH (26%) followed by angiosarcoma (21%), leiomyosarcoma (12%), fibrosarcoma (12%), MPNST (9%), MYXF (8%), and liposarcoma (3%; Fig 1).
Table 1.
Clinicopathologic and Treatment Characteristics in 130 Patients With Primary Radiation-Associated Sarcomas
| Characteristic | No. | % of Total |
|---|---|---|
| Age, years | ||
| Median | 58.5 | |
| Range | 18-86 | |
| Sex | ||
| Female | 75 | 58 |
| Male | 55 | 42 |
| Grade | ||
| High | 108 | 83 |
| Low | 22 | 17 |
| Tumor size, cm | ||
| Median | 5.7 | |
| Range | 0.5-28 | |
| Tumor depth | ||
| Deep | 113 | 87 |
| Superficial | 17 | 13 |
| Tumor site | ||
| Extremity | 28 | 21.5 |
| Abdominal/RP | 22 | 17 |
| Trunk | 80 | 61.5 |
| Tumor margin | ||
| Negative (R0) | 90 | 69 |
| Positive micro (R1) | 31 | 24 |
| Positive gross (R2) | 9 | 7 |
| Latency, years | ||
| Median | 10 | |
| Range | 1.3-74 | |
| Radiation dose, Gy | ||
| Median | 54 | |
| Range | 20-160 | |
| Chemotherapy for primary tumor | ||
| Yes | 46 | 35 |
| No | 84 | 65 |
Abbreviation: RP, retroperitoneum.
Fig 1.
Histology of primary radiation-associated sarcomas (n = 130). MFH, malignant fibrous histiocytoma; MPNST, malignant peripheral nerve sheath tumor.
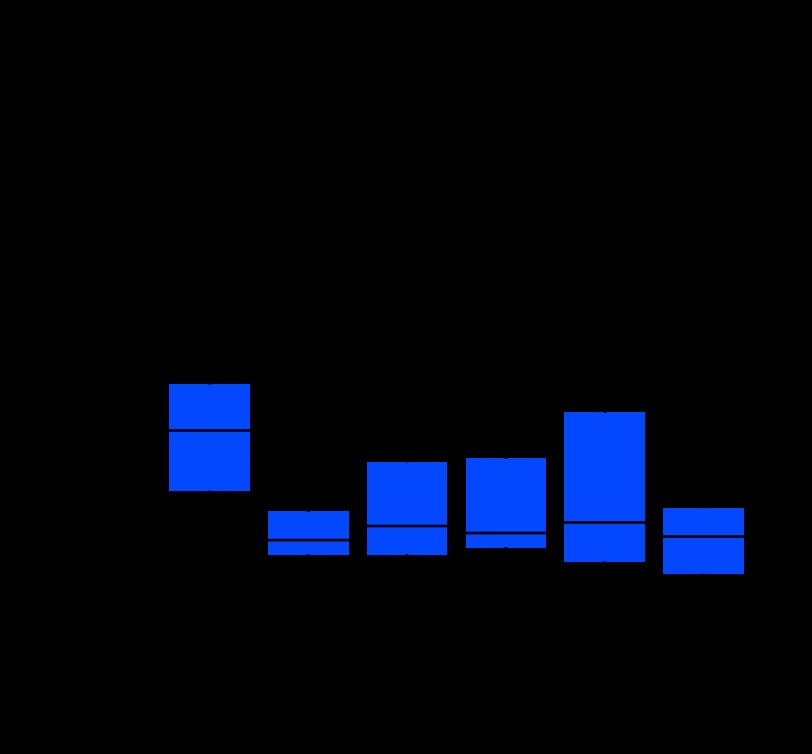
The median interval between radiation and the development of sarcoma was 10 years (range, 1.3 to 74 years; Table 1). The majority of patients (98%) received external beam radiation therapy, with a median dose of 54 Gy (range, 20 to 160 Gy). Most patients (65%) did not receive concurrent chemotherapy for their primary cancer (Table 1). The most common indication for radiation therapy was the management of breast cancer (34%; Appendix Table A1, online only). Interestingly, the latency of RAS varied by histologic type, with the shortest latency observed in liposarcoma (median, 4.3 years; range, 3 to 17 years; data not shown) and the longest latency observed in leiomyosarcoma (median, 23.5 years; range, 7.0 to 74.0 years), which was statistically significant (P = .003; Appendix Fig A1, online only). Furthermore, eight RASs developed within 1 to 3 years after RT (data not shown), and although the development of RAS within 3 years of treatment is controversial, we found that the STS histologic types that arose support the development of early onset RAS (n = 3 for pleomorphic MFH; n = 1 each of extra-osseous osteosarcoma, liposarcoma, chondrosarcoma, Ewing sarcoma, and fibrosarcoma).
For the 130 primary RAS patients, 65% were treated solely with surgical resection, 18% received neoadjuvant/adjuvant chemotherapy, and 22% were treated with neoadjuvant/adjuvant radiotherapy. In contrast, for the 1,173 primary, high-grade, sporadic STS patients encompassing six histologic types, 43% were treated solely with surgical resection, 21% received neoadjuvant/adjuvant chemotherapy, and 49% were treated with neoadjuvant/adjuvant radiotherapy. Thus, neoadjuvant/adjuvant radiation was used 50% less frequently for the treatment of RAS patients compared with sporadic STS patients. There was no difference in use of chemotherapy between RAS and sporadic STS patients.
Survival Analysis
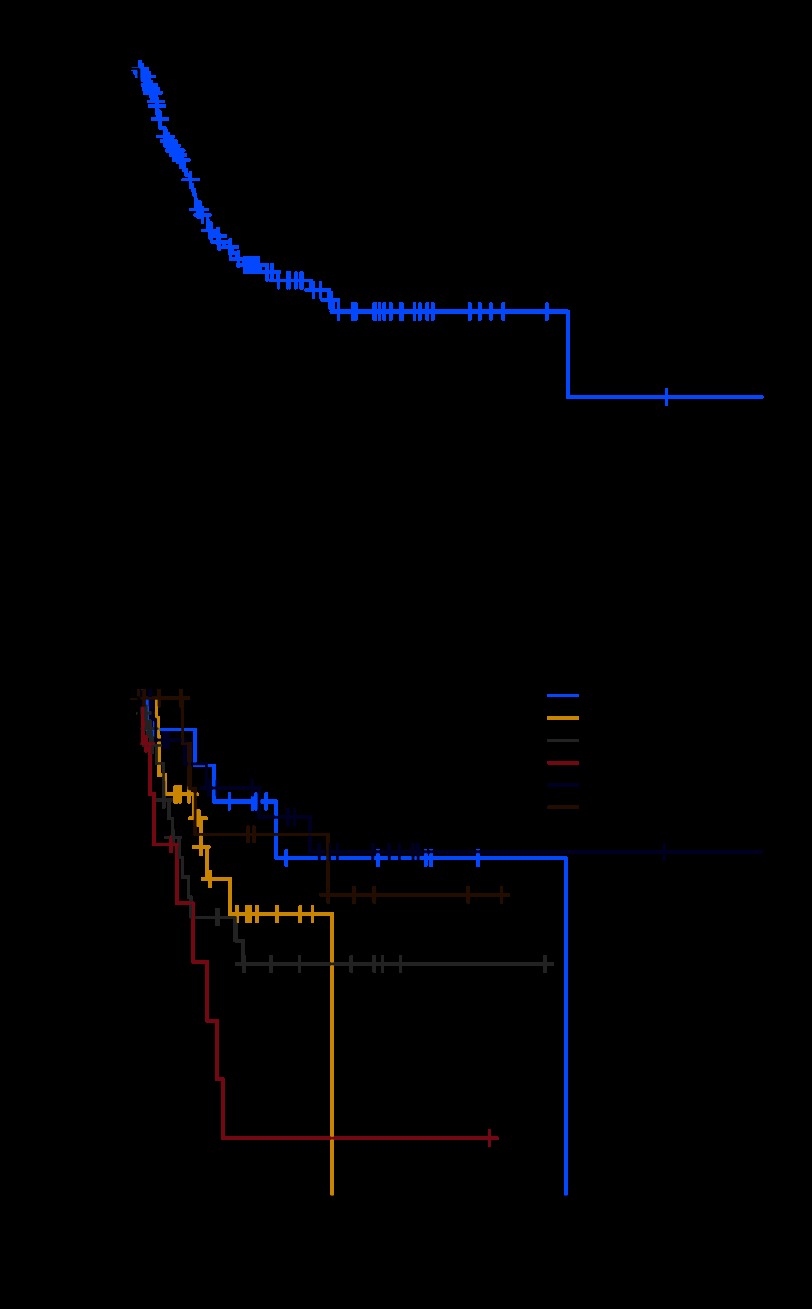
Sixty-four of the 130 patients were alive, with a median follow-up of 26.7 months (range, 0.4 to 263 months). The 1-year, 3-year, and 5-year DSS rates were 85%, 63%, and 58%, respectively, and the median DSS was 13.8 years (Fig 2A). Histologic type was a statistically significant predictor of DSS (P = .004; Fig 2B). The worst outcome was seen in MPNST RAS patients (5-year DSS, 12%), whereas fibrosarcoma/MYXF had the best prognosis (5-year DSS, 76%; Table 2), and pleomorphic MFH and angiosarcoma had a 5-year DSS of 47% and 57%, respectively.
Fig 2.
(A) Disease-specific survival (DSS) for resected primary radiation-associated sarcomas (RASs) and (B) subtype-specific DSS for resected primary RASs (P = .004). LMS, leiomyosarcoma; AS, angiosarcoma; MFH, malignant fibrous histiocytoma; MPNST, malignant peripheral nerve sheath tumor; FS/MYXF, fibrosarcoma or myxofibrosarcoma.
Table 2.
Analysis of DSS in Patients With Primary Radiation-Associated Sarcoma
| Prognostic Factor | No. of Patients | 5-Year DSS (%) | Univariate P | Multivariate P | Hazard Ratio | 95% CI |
|---|---|---|---|---|---|---|
| Age, years | ||||||
| ≤ 60 | 69 | 58 | .961 | — | ||
| > 60 | 61 | 59 | ||||
| Sex | ||||||
| Female | 75 | 57 | .699 | — | ||
| Male | 55 | 60 | ||||
| Grade | ||||||
| High | 108 | 49 | < .001 | — | ||
| Low | 22 | 90 | ||||
| Tumor size, cm* | ||||||
| ≤ 5 | 51 | 80 | < .001 | |||
| > 5 | 75 | .001 | 3.9 | 1.8 to 8.4 | ||
| Tumor depth | ||||||
| Deep | 113 | 54 | .135 | — | ||
| Superficial | 17 | 82 | ||||
| Tumor site | ||||||
| Extremity | 28 | 55 | .744 | — | ||
| Abdominal/RP | 22 | 56 | ||||
| Trunk | 80 | 59 | ||||
| Tumor margin | ||||||
| Negative | 90 | 68 | < .001 | |||
| Micro/gross positive | 40 | < 10 | .039 | 2.0 | 1.0 to 3.8 | |
| Histologic type† | ||||||
| LMS | 16 | 68 | .004 | |||
| FS/MYXF | 26 | 76 | .539 | 1.6 | 0.4 to 7.3 | |
| Angiosarcoma | 27 | 57 | .155 | 2.6 | 0.7 to 9.7 | |
| MFH | 34 | 47 | .025 | 4.3 | 1.2 to 15.2 | |
| MPNST | 11 | 12 | .005 | 7.5 | 1.9 to 30.6 | |
| Other | 16 | 73 | .174 | 2.9 | 0.6 to 13.2 |
Abbreviations: DSS, disease-specific survival; RP, retroperitoneum; LMS, leiomyosarcoma; FS/MYXF, fibrosarcoma or myxofibrosarcoma; MFH, malignant fibrous histiocytoma; MPNST, malignant peripheral nerve sheath tumor.
Size unknown in four patients.
Compared with LMS in multivariate analysis.
Multivariate analysis to identify prognostic factors that are important for DSS in high-grade RAS patients is provided in Table 2. The independent predictors of DSS were tumor size > 5 cm (hazard ratio [HR], 3.9; 95% CI, 1.8 to 8.4; P = .001), microscopic or grossly positive margin (HR, 2.0; 95% CI, 1.0 to 3.8; P = .039), pleomorphic MFH (HR, 4.3; 95% CI, 1.2 to 15.2; P = .025), and MPNST (HR, 7.5; 95% CI, 1.9 to 30.6; P = .005). On multivariate analysis fibrosarcoma/MYXF and angiosarcoma had DSS that was not significantly different from that of leiomyosarcoma after controlling for other prognostic factors.
The results from a multivariate DSS analysis using all patients presenting to MSKCC with primary, resectable, high-grade RASs and sporadic STSs with the histologic types leiomyosarcoma, fibrosarcoma/MYXF, angiosarcoma, pleomorphic MFH, and MPNST are given in Table 3. RAS was associated with a worse DSS compared with sporadic STS (HR, 1.7; 95% CI, 1.1 to 2.4; P = .007), despite adjusting for other independent predictors of DSS such as age, histologic type, and tumor size, site, depth, and margin status.
Table 3.
Multivariate Analysis of Disease-Specific Survival in Primary, High-Grade RAS and Sporadic STS, Adjusting for Histologic Type and Other Prognostic Variables
| Factor | P | HR | 95% CI |
|---|---|---|---|
| RAS | |||
| No | |||
| Yes | .007 | 1.7 | 1.1 to 2.4 |
| Age | .004 | 1.0 | 1.0 to 1.0 |
| Sex | |||
| Female | |||
| Male | .100 | 1.2 | 1.0 to 1.5 |
| Tumor site | |||
| Extremity | |||
| Trunk | .033 | 1.4 | 1.1 to 2.7 |
| Abdomen | .947 | 1.0 | 0.7 to 1.4 |
| Tumor size, cm | |||
| ≤ 5 | |||
| > 5 | < .001 | 3.0 | 2.3 to 4.1 |
| Tumor depth (deep) | .005 | 1.7 | 1.2 to 2.4 |
| Tumor margin | |||
| R0 | |||
| R1/R2 | .001 | 1.5 | 1.2 to 2.0 |
| Histologic type | |||
| LMS | |||
| AS | .380 | 1.2 | 0.8 to 2.0 |
| MFH | .375 | 0.9 | 0.7 to 1.2 |
| MPNST | .023 | 1.5 | 1.1 to 2.3 |
| FS/MYXF | .001 | 0.6 | 0.4 to 0.8 |
Abbreviations: RAS, radiation-associated sarcoma; STS, soft tissue sarcoma; HR, hazard ratio; LMS, leiomyosarcoma; AS, angiosarcoma; MFH, malignant fibrous histiocytoma; MPNST, malignant peripheral nerve sheath tumor; FS/MYXF, fibrosarcoma or myxofibrosarcoma.
RAS Versus Sporadic Pleomorphic MFH: A Matched-Cohort Analysis
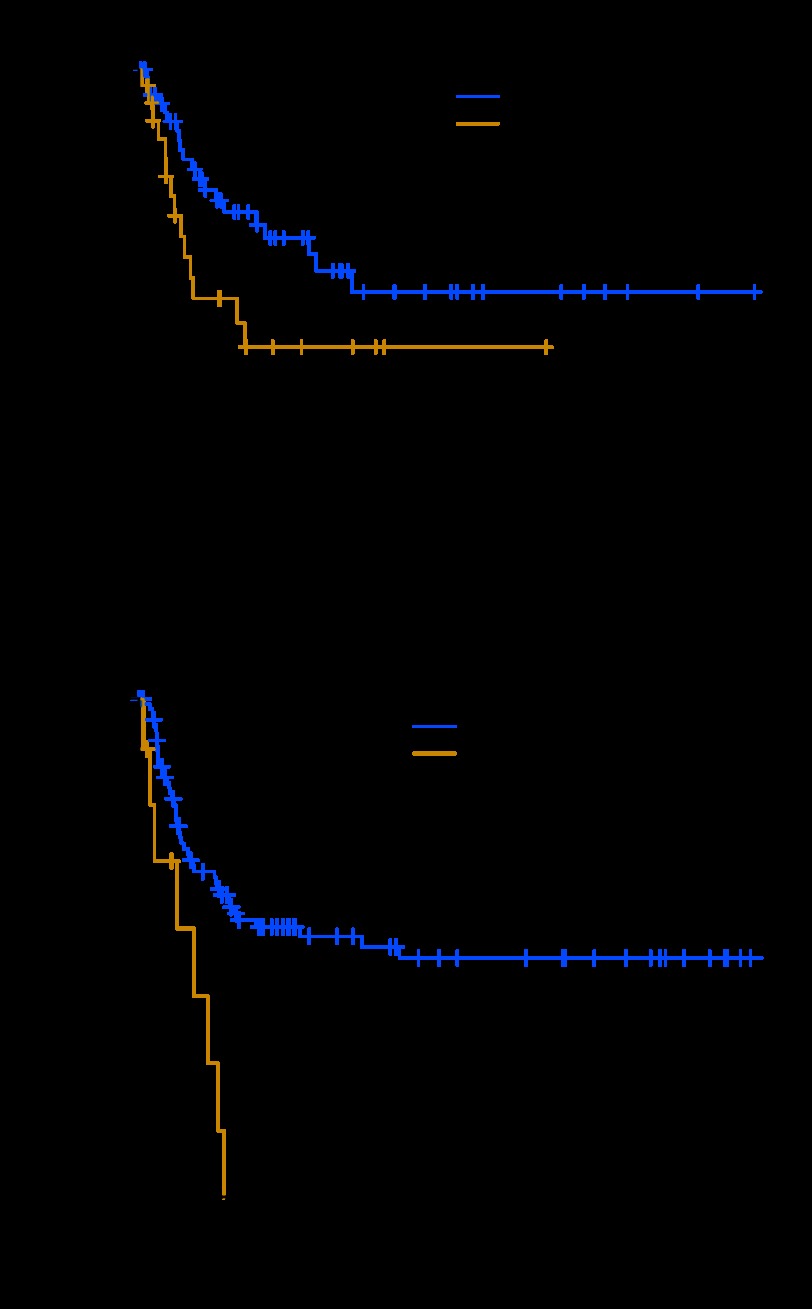
To determine whether RAS MFH was associated with the same prognosis as sporadic MFH, a matched-cohort analysis was performed using known prognostic factors from our postoperative sarcoma nomogram.17 We identified 402 sporadic MFH patients who had high-grade, primary, and resectable tumors. On further matching (age, sex, and tumor site, size, depth, and margin), a final group of 62 sporadic MFH patients (matching ratio 1:2) was used to compare DSS with that of 31 RAS MFH patients (Fig 3A). The 5-year DSS for the RAS MFH group was 44%, compared with a 5-year DSS of 66% for the matched sporadic MFH patients, which approached significance (P = .07).
Fig 3.
Disease-specific survival (DSS) for radiation-associated sarcomas (RASs) compared with sporadic soft tissue sarcomas. (A) Patients with RAS malignant fibrous histiocytoma (MFH) have inferior DSS compared with sporadic MFH patients; case mix with a 1:2 ratio (P = .070). (B) DSS for high-grade RAS malignant peripheral nerve sheath tumor (MPNST) patients is inferior to that for primary sporadic MPNST patients (P = .001).
RAS Versus Sporadic MPNST
We were not able to successfully generate a matched cohort for the MPNST RAS group. However, their dismal outcome is quite striking (Fig 3B), and thus we performed a preliminary analysis to compare our 10 high-grade RAS MPNST patients with 102 primary high-grade sporadic MPNST patients. The 5-year DSS was 0% and 54% for radiation-associated and sporadic high-grade primary MPNST, respectively (P = .001; Fig 3B). The RAS MPNST were smaller tumors (median, 6.5 cm; range, 2 to 10.5 cm v control group median, 9 cm; range, 0.5 to 40 cm), and they were predominantly seen in the truncal distribution (45% v 35% in the sporadic patients). RAS MPNSTs were found in a younger patient population (median age, 31 years; range, 25 to 58 years) versus sporadic MPNST patients (median age, 38 years; range, 16 to 87 years) with a 50% (RAS) R0 resection rate versus 73% (sporadic; P = .16). Finally, the majority of patients with RAS MPNST were radiated for lymphoma (9 of 11 [82%] comprising 9 of 24 patients radiated for lymphoma). We also repeated the survival analysis reported in this section treating death due to other causes as a competing risk, and the results remain similar.
DISCUSSION
In this study, we show that histologic type, margin status, and tumor size are independently prognostic for DSS in RAS. MFH and MPNST RAS were associated with worse outcomes compared with leiomyosarcoma RAS. Outcome in RAS is most favorable for leiomyosarcoma, fibrosarcoma, and MYXF histologic types. RAS was associated with a 1.7-fold worse DSS compared with sporadic STS in a multivariate analysis that adjusted for the five most common histologic types as well as age and tumor site, size, depth, and margin status. Patients with RAS MFH had a reduced DSS compared with patients with sporadic MFH.
To the best of our knowledge, this study is the first to suggest that RAS is associated with an inferior prognosis compared with sporadic STS. Our data confirm the clinical impression that sarcomas that arise in radiation fields likely do worse.9–11 Our findings contrast a recent report12 of RAS bone sarcomas that were found to have the same prognosis as sporadic sarcomas; however, this study may be limited by small case numbers (24 patients, three institutions) or by underlying biologic differences between STS and bone RAS tumors.
The majority of RAS angiosarcoma patients were initially treated for breast cancer (81%). Angiosarcomas were predominately high-grade (96%) or truncal (93%) tumors that had a median size of 5.2 cm (range, 0.5 to 11.5 cm). Since they are often multifocal,19these factors likely contribute to the increased rate of incomplete resection (22%) seen in this study. Strikingly, 38% of all angiosarcomas at our institution were RAS, in keeping with an M.D. Anderson Cancer Center study that also reported that 42% (23 of 55) of all breast angiosarcomas were radiation associated.20 Our findings of worse outcomes in patients with RASs compared with sporadic STSs supports the notion that RAS angiosarcomas have an inferior prognosis.3,21,22
At our institution, neoadjuvant/adjuvant chemotherapy was used at similar rates in sporadic and RAS patients; however, radiation therapy was used 50% less frequently in RAS patients because of concerns about toxicity. The mainstay of therapy for RAS at our institution remains wide surgical resection with an aggressive approach to achieve negative margins. For RAS histologic types such as angiosarcoma that typically present with advanced local or multifocal disease, neoadjuvant chemotherapy before surgical resection is often recommended to improve local control and potentially eradicate subclinical metastatic disease.
We also report that latency of RAS STSs varied significantly by histologic type (P = .003; Appendix Fig A1), with the shortest latency seen in liposarcoma and the longest in leiomyosarcoma. In comparison, recent series12,23 of bone RAS have been observed to have a median onset of 16 to 17 years (ranges, 3 to 48 and 4 to 55 years), contrary to the initial report by Cahan et al13 that bone tumors arose more rapidly (median, 10 years; range, 7 to 22 years). We treated eight cases of high-grade STS that occurred 1 to 3 years after RT. We believe the definition of radiation-associated sarcoma should be modified to include the development of RAS as soon as 6 months after radiation therapy, in contrast to previous pathologic definitions.13,24
Patients who undergo radiation therapy for many leukemias, lymphomas, and solid tumors other than STS (eg, lung, esophageal, breast, stomach, bladder, and rectal), should be monitored for RAS. RT was recently shown to have a beneficial impact on DSS in early-stage breast cancer patients, because a large recent meta-analysis reported that the avoidance of local recurrence demonstrated a survival advantage.25 However, the merits of RT must be tempered with toxicity, especially in tumors where there is a marginal survival benefit, as demonstrated in phase III randomized controlled trials.26,27 In a population-based, retrospective Surveillance, Epidemiology and End Results (SEER) database review, Huang et al3 reviewed the data on 194,798 women treated for breast cancer between 1973 and 1995. Although the follow-up period may have been inadequate to reflect the incidence of RAS, they demonstrated a 16-fold increase in angiosarcoma in RT patients versus controls and a two-fold increase in all STSs in RT patients. Furthermore, the authors calculated a crude 5-year survival rate of 32% with a median survival of 25 months.
The increased use of diagnostic computed tomography scans, which expose patients to higher doses of ionizing radiation than conventional x-ray, has generated concerns about future public health risk.28 Although a clear dose-response relationship for radiation-associated malignancies is not established, it is generally accepted that carcinomas arise in tissues exposed to lower doses, whereas sarcomas are induced in heavily radiated tissues in or close to the radiation fields.29 Furthermore, advances in the delivery of RT, specifically the use of intensity-modulated radiation therapy (IMRT), involves the use of more fields and consequently exposes more normal tissue to low-dose RT.30 To deliver the specified dose to the isocenter of the tumor, IMRT requires the accelerator to be energized longer, which results in higher radiation doses. Therefore, it has been estimated that the incidence of RASs may increase by 0.5% with IMRT.30
RASs are a rare but important group of STS, and understanding their biology may have clinical ramifications. The histologic type of RAS is an important prognostic factor for outcome. We have shown that RASs are associated with inferior survival compared with sporadic STSs, even if one adjusts for known prognostic factors such as histologic type, size, age, margin status, and site. It is unclear whether treatment should be modified in RAS versus sporadic STS. Since the majority of cancer patients receive RT, it is critical that clinicians are aware of the possible development of RASs, which can occur decades after RT. Biopsy of any masses that develop in or adjacent to previous radiation fields should be routinely performed. Future studies examining the genetics of RAS may illuminate the mechanisms responsible for sarcomagenesis and how they can be targeted to improve outcome.
Appendix
Fig A1.
Latency of resected primary radiation-associated sarcomas varies with histologic type. Boxplots represent distribution of latency where the median is represented by a solid line. LMS, leiomyosarcoma; AS, angiosarcoma; MFH, malignant fibrous histiocytoma; MPNST, malignant peripheral nerve sheath tumor; FS/MYXF, fibrosarcoma or myxofibrosarcoma.
Table A1.
Indication for Radiation
| Cancer Type | No. of Patients | % of Total |
|---|---|---|
| Breast | 44 | 34 |
| Genitourinary | 22 | 17 |
| Lymphoma/leukemia | 24 | 18 |
| Head and neck | 9 | 7 |
| Gynecologic | 6 | 5 |
| Sarcoma | 5 | 4 |
| Other* | 20 | 15 |
Other: CNS cancer (4), genetic predisposition (5), GI tract cancer (3), lung cancer (2), benign tumor (3), skin cancer (2), unknown (1).
Footnotes
Supported by Soft Tissue Sarcoma Program Project Grant No. P01 CA 047179 (L.-X.Q., C.R.A., M.F.B., and S.S.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Rebecca A. Gladdy, Samuel Singer
Financial support: Samuel Singer
Administrative support: Samuel Singer
Provision of study materials or patients: Murray F. Brennan, Samuel Singer
Collection and assembly of data: Rebecca A. Gladdy, Nicole Moraco, Mark A. Edgar, Samuel Singer
Data analysis and interpretation: Rebecca A. Gladdy, Li-Xuan Qin, Mark A. Edgar, Cristina R. Antonescu, Kaled M. Alektiar, Murray F. Brennan, Samuel Singer
Manuscript writing: Rebecca A. Gladdy, Li-Xuan Qin, Samuel Singer
Final approval of manuscript: Rebecca A. Gladdy, Murray F. Brennan, Samuel Singer
REFERENCES
- 1.Perez CA, Brady LW, editors. Principles and Practice of Radiation Oncology. ed 5. Philadelphia, PA: Lippincott Williams and Wilkins; 2004. [Google Scholar]
- 2.Kirova YM, Gambotti L, De Rycke Y, et al. Risk of second malignancies after adjuvant radiotherapy for breast cancer: A large-scale, single-institution review. Int J Radiat Oncol Biol Phys. 2007;68:359–363. doi: 10.1016/j.ijrobp.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Huang J, Mackillop WJ. Increased risk of soft tissue sarcoma after radiotherapy in women with breast carcinoma. Cancer. 2001;92:172–180. doi: 10.1002/1097-0142(20010701)92:1<172::aid-cncr1306>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 4.Henderson TO, Whitton J, Stovall M, et al. Secondary sarcomas in childhood cancer survivors: A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2007;99:300–308. doi: 10.1093/jnci/djk052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Travis LB, Fossa SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: Focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354–1365. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- 6.Kleinerman RA, Tucker MA, Tarone RE, et al. Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: An extended follow-up. J Clin Oncol. 2005;23:2272–2279. doi: 10.1200/JCO.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 7.Brady MS, Gaynor JJ, Brennan MF. Radiation-associated sarcoma of bone and soft tissue. Arch Surg. 1992;127:1379–1385. doi: 10.1001/archsurg.1992.01420120013002. [DOI] [PubMed] [Google Scholar]
- 8.Cha C, Antonescu CR, Quan ML, et al. Long-term results with resection of radiation-induced soft tissue sarcomas. Ann Surg. 2004;239:903–909. doi: 10.1097/01.sla.0000128686.51815.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson E, Neugut AI, Wylie P. Clinical aspects of postirradiation sarcomas. J Natl Cancer Inst. 1988;80:233–240. doi: 10.1093/jnci/80.4.233. [DOI] [PubMed] [Google Scholar]
- 10.Wiklund TA, Blomqvist CP, Raty J, et al. Postirradiation sarcoma: Analysis of a nationwide cancer registry material. Cancer. 1991;68:524–531. doi: 10.1002/1097-0142(19910801)68:3<524::aid-cncr2820680313>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 11.Laskin WB, Silverman TA, Enzinger FM. Postradiation soft tissue sarcomas: An analysis of 53 cases. Cancer. 1988;62:2330–2340. doi: 10.1002/1097-0142(19881201)62:11<2330::aid-cncr2820621113>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Shaheen M, Deheshi BM, Riad S, et al. Prognosis of radiation-induced bone sarcoma is similar to primary osteosarcoma. Clin Orthop Relat Res. 2006;450:76–81. doi: 10.1097/01.blo.0000229315.58878.c1. [DOI] [PubMed] [Google Scholar]
- 13.Cahan WG, Woodard HQ, Higinbotham NL, et al. Sarcoma arising in irradiated bone: Report of eleven cases—1948. Cancer. 1998;82:8–34. doi: 10.1002/(sici)1097-0142(19980101)82:1<8::aid-cncr3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.Huvos AG, Woodard HQ, Cahan WG, et al. Postradiation osteogenic sarcoma of bone and soft tissues: A clinicopathologic study of 66 patients. Cancer. 1985;55:1244–1255. doi: 10.1002/1097-0142(19850315)55:6<1244::aid-cncr2820550617>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Hajdu SI, Shiu MH, Brennan MF. The role of the pathologist in the management of soft tissue sarcomas. World J Surg. 1988;12:326–331. doi: 10.1007/BF01655665. [DOI] [PubMed] [Google Scholar]
- 16.Sekhon JS. Multivariate and propensity score matching software with automated balance optimization: The matching package for R. J Statist Software. http://sekhon.berkeley.edu/papers/MatchingJSS.pdf.
- 17.Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20:791–796. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- 18. Reference deleted.
- 19.Fineberg S, Rosen PP. Cutaneous angiosarcoma and atypical vascular lesions of the skin and breast after radiation therapy for breast carcinoma. Am J Clin Pathol. 1994;102:757–763. doi: 10.1093/ajcp/102.6.757. [DOI] [PubMed] [Google Scholar]
- 20.Vorburger SA, Xing Y, Hunt KK, et al. Angiosarcoma of the breast. Cancer. 2005;104:2682–2688. doi: 10.1002/cncr.21531. [DOI] [PubMed] [Google Scholar]
- 21.Abraham JA, Hornicek FJ, Kaufman AM, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14:1953–1967. doi: 10.1245/s10434-006-9335-y. [DOI] [PubMed] [Google Scholar]
- 22.Fury MG, Antonescu CR, Van Zee KJ, et al. A 14-year retrospective review of angiosarcoma: Clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005;11:241–247. doi: 10.1097/00130404-200505000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Inoue YZ, Frassica FJ, Sim FH, et al. Clinicopathologic features and treatment of postirradiation sarcoma of bone and soft tissue. J Surg Oncol. 2000;75:42–50. doi: 10.1002/1096-9098(200009)75:1<42::aid-jso8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 24.Arlen M, Higinbotham NL, Huvos AG, et al. Radiation-induced sarcoma of bone. Cancer. 1971;28:1087–1099. doi: 10.1002/1097-0142(1971)28:5<1087::aid-cncr2820280502>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 25.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 26.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 27.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 28.Brenner DJ, Hall EJ. Computed tomography: An increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 29.Kuttesch JF, Jr, Wexler LH, Marcus RB, et al. Second malignancies after Ewing's sarcoma: Radiation dose-dependency of secondary sarcomas. J Clin Oncol. 1996;14:2818–2825. doi: 10.1200/JCO.1996.14.10.2818. [DOI] [PubMed] [Google Scholar]
- 30.Hall EJ, Wuu CS. Radiation-induced second cancers: The impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56:83–88. doi: 10.1016/s0360-3016(03)00073-7. [DOI] [PubMed] [Google Scholar]