Abstract
Purpose
The purpose of cancer staging systems is to accurately predict patient prognosis. The outcome of advanced hepatocellular carcinoma (HCC) depends on both the cancer stage and the extent of liver dysfunction. Many staging systems that include both aspects have been developed. It remains unknown, however, which of these systems is optimal for predicting patient survival.
Patients and Methods
Patients with advanced HCC treated over a 5-year period at Memorial Sloan-Kettering Cancer Center were identified from an electronic medical record database. Patients with sufficient data for utilization in all staging systems were included. TNM sixth edition, Okuda, Barcelona Clinic Liver Cancer (BCLC), Cancer of the Liver Italian Program (CLIP), Chinese University Prognostic Index (CUPI), Japan Integrated Staging (JIS), and Groupe d'Etude et de Traitement du Carcinome Hepatocellulaire (GETCH) systems were ranked on the basis of their accuracy at predicting survival by using concordance index (c-index). Other independent prognostic variables were also identified.
Results
Overall, 187 eligible patients were identified and were staged by using the seven staging systems. CLIP, CUPI, and GETCH were the three top-ranking staging systems. BCLC and TNM sixth edition lacked any meaningful prognostic discrimination. Performance status, AST, abdominal pain, and esophageal varices improved the discriminatory ability of CLIP.
Conclusion
In our selected patient population, CLIP, CUPI, and GETCH were the most informative staging systems in predicting survival in patients with advanced HCC. Prospective validation is required to determine if they can be accurately used to stratify patients in clinical trials and to direct the appropriate need for systemic therapy versus best supportive care. BCLC and TNM sixth edition were not helpful in predicting survival outcome, and their use is not supported by our data.
INTRODUCTION
In contrast to other cancers, the prognosis and treatment options for patients with hepatocellular carcinoma (HCC) depend not only on the tumor stage but also on the extent of liver dysfunction.1 A staging system is needed to help predict survival outcome and may be helpful for deciding optimal medical care, especially in this era of complexity of added choice for the treatment of advanced HCC,2 for which best supportive care may be a more appropriate therapeutic approach in the more advanced cases. Additionally, there is a continued need to appropriately interpret data from clinical trials in patients with advanced HCC. Identification of relevant prognostic factors for both the liver cancer and liver function has led to the development of staging systems that include both. Reported staging systems for HCC include TNM sixth edition,3 Okuda,4 Barcelona Clinic Liver Cancer (BCLC),5 Cancer of the Liver Italian Program (CLIP),6,7 Chinese University Prognostic Index (CUPI),8 Japan Integrated Staging Score (JIS score),9,10 and the Groupe d'Etude et de Traitement du Carcinome Hepatocellulaire Prognostic classification (GETCH).11 The utility of most of these staging systems in predicting survival in patients with advanced HCC seen by medical oncologists for consideration of systemic therapy is often limited. The characteristics of patients with advanced disease might significantly differ from a population of patients balanced between early and advanced disease, for which the available staging systems were originally developed. Whether any one staging system is more informative in patients with advanced HCC, and whether additional variables not included in these systems have prognostic significance, is unknown.
Objectives
The aim of this analysis was to compare the accuracy of each staging system in predicting survival for patients with advanced HCC evaluated by medical oncologists for consideration of systemic therapy. This analysis was also designed to identify independent prognostic factors for patients with advanced HCC and to determine if the utility of the staging system identified as the best for the population of advanced HCC could be improved by the inclusion of additional prognostic factors identified in a multivariate analysis.
PATIENTS AND METHODS
Patients
We retrospectively identified patients with HCC (with International Classification of Diseases code 155.0 for primary liver cancer) who were evaluated by medical oncologists at Memorial Sloan-Kettering Cancer Center. Patients were included if they had confirmed pathology or had fulfilled the European Association for the Study of the Liver12 radiologic criteria of two imaging studies (ultrasound, computed tomography [CT], or magnetic resonance imaging [MRI]) showing a focal lesion greater than 2 cm with arterial hypervascularization or the combined criteria of one imaging study showing a focal lesion greater than 2 cm with arterial hypervascularization and an α-fetoprotein (AFP) level greater than 400 ng/mL. A baseline evaluation that included clinical examination, laboratory studies, and imaging studies (ie, CT or MRI) was required. The baseline was defined as the time of the first evaluation for advanced HCC. Advanced HCC was defined as a liver tumor not eligible for local therapies given the extent of disease or liver tumors that recurred after local therapies. Intrahepatic recurrence after local treatment was considered metastatic disease.13 Typically, such patients are considered candidates for bland embolization and are treated by hepatobiliary surgeons and interventional radiologists.14 However, on progression of disease or ineligibility for continued embolization, those patients are referred to medical oncologists for consideration of systemic therapy, clinical trials, or best supportive care, similar to those with advanced disease.
Patients were excluded if there were missing data required for classifying patients in any of the seven staging systems or if there was no follow-up information. Patients with a diagnosis or history of other concurrent malignancies other than treated skin basal cell carcinoma were also excluded.
Data Collection
Institutional review board approval was obtained to retrieve data from the electronic charts needed to stage patients in all seven staging systems. These included demographics, risk factors for developing HCC, clinical data including but not limited to performance status, parameters of liver cirrhosis manifestations (eg, ascites and encephalopathy). For patients in whom only Karnofsky performance status (KPS) was recorded, Eastern Cooperative Oncology Group performance status (ECOG PS) was deducted on the basis of best evidence available, as follows15: ECOG 0 = KPS 100%, ECOG 1 = KPS 80% to 90%, ECOG 2 = 60% to 70%, ECOG 3 = KPS 40% to 50%, and ECOG 4 = KPS 10% to 30%. Laboratory data, including different cirrhosis parameters and AFP, were captured. Bilirubin references of the CUPI and GETCH scores were converted from μmol/L to mg/dL by using a metric conversion calculator. For patients on oral anticoagulation, prothrombin time/international normalized ratio values were obtained at the time anticoagulation was interrupted for some reason, including for liver biopsy. The Child-Pugh score was calculated from obtained clinical and laboratory data. TNM fifth edition scores were generated to calculate the CUPI scores. All previous therapies received were recorded. Tumor characteristics that were reported were number of lesions, diameter of largest lesion, extent of disease, lobar involvement, vascular invasion, portal vein thrombosis, organ invasion, nodes status, and metastatic disease status; these were retrospectively recorded from the radiology report, with the exception of tumor extent, that was prospectively determined by review of the available baseline CT and/or MRI of the liver by a medical oncologist.
Staging
Collected data was used to restage all patients. This included all patients assessed by the TNM sixth edition (2006), Okuda, BCLC, CLIP, CUPI, JIS, and GETCH systems.
Statistical Analysis
Overall survival was estimated by using the Kaplan-Meier method from the date of initial evaluation for advanced HCC to the date of death or last follow-up. Survival analysis was stratified according to prognostic categories for each of the seven staging systems. Differences in survival among their prognostic strata were tested by using the log-rank test. Independent prognostic factors were identified through stepwise selection in a Cox regression model. Added variables that significantly related to survival in univariate Cox models (P < .1) were subsequently included in a multivariate model.
Ranking of staging systems was done by using the concordance index (c-index), which measures the capacity of the different staging systems to discriminate patients with different outcomes: the higher the c- index, the more informative the model is about a patient's outcome. The c-indices of the different staging systems were compared by using bootstrap16 and by applying random resampling to test the inferences we generated.
Those additional pertinent prognostic variables previously identified were then added to the top-ranked staging system. A new c-index was calculated to quantify the improvement. The c-index of the resulting model was internally validated by using bootstrap to quantify the improvement.
RESULTS
Between July 1, 2001 and June 30, 2006, 513 patients with HCC were seen by medical oncologists at Memorial Sloan-Kettering Cancer Center; of these, 326 patients either did not fulfill the inclusion and exclusion criteria or had incomplete or no follow-up. The remaining 187 patients were staged by using each of the seven different staging systems discussed in the Methods section. Their baseline characteristics are listed in Table 1. Median follow-up was 13.4 months. Of the 187 patients, 137 (73%) had died at the time of the final analysis (May 29, 2007). Of note, 94% of referred patients had advanced tumor stages (32% stage III and 62% stage IV on the basis of TNM sixth edition) with preserved liver function status (66% Child-Pugh A). Despite the advanced tumor stage, 31% of patients were asymptomatic at presentation. At the time of evaluation, 29% of patients entered onto a clinical trial, and 33% received chemotherapy outside of a clinical trial.
Table 1.
Baseline Demographic and Clinical Characteristics of Patients With Advanced HCC
| Characteristic | Patients (N = 187) |
|---|---|
| Age, years | |
| Median | 64 |
| Range | 24-88 |
| Sex, % | |
| Male | 75 |
| Female | 25 |
| Ethnicity, % | |
| White | 63 |
| African American | 12 |
| Asian | 20 |
| Hispanic | 4 |
| Unknown | 1 |
| Cirrhosis, % | |
| Yes | 55 |
| No | 45 |
| Etiology, % | |
| Hepatitis C | 30 |
| Hepatitis B | 33 |
| Alcohol | 30 |
| Other | 14 |
| None | 24 |
| Symptoms, % | |
| Present | 78 |
| Absent | 22 |
| ECOG PS, % | |
| 0-1 | 80 |
| 2-3 | 20 |
| Abdominal pain, % | |
| Yes | 34 |
| No | 66 |
| Weight loss, % | |
| Yes | 33 |
| No | 67 |
| Ascites, % | |
| Yes | 18 |
| No | 82 |
| Encephalopathy, % | |
| Yes | 2 |
| No | 98 |
| Esophageal varices, % | |
| Yes | 21 |
| No | 73 |
| Laboratory values, medians | |
| Total bilirubin, mg/dL | 1.1 |
| Albumin, g/dL | 3.2 |
| PT | 1.06 |
| Platelets, K/μL | 179 |
| Alkaline phosphatase, U/L | 146 |
| AST, U/L | 72 |
| ALT, U/L | 45 |
| AFP, ng/mL | 355 |
| Total bilirubin, mg/dL | 1.1 |
| Albumin, g/dL | 3.2 |
| Liver function by Child-Pugh stage, % | |
| A | 67 |
| B | 29 |
| C | 4 |
| Tumor characteristic, % | |
| No. of liver tumors | |
| 0 | 6 |
| 1-5 | 39 |
| > 5 | 55 |
| Diameter of largest lesion, cm | 6.7 |
| Bilobar disease | |
| Yes | 70 |
| No | 30 |
| Extent | |
| ≤ 50% | 70 |
| > 50% | 30 |
| Vascular invasion | |
| Yes | 36 |
| No | 64 |
| Portal vein thromboses | |
| Yes | 30 |
| No | 70 |
| Organ invasion | |
| Yes | 4 |
| No | 96 |
| T | |
| 0-2 | 36 |
| 3-4 | 64 |
| N | |
| Yes | 37 |
| No | 63 |
| M | |
| Yes | 60 |
| No | 40 |
| Previous treatment, % | |
| No. of previous treatments | |
| 0 | 43 |
| 1 | 32 |
| ≥ 2 | 25 |
| Surgery | 25 |
| Embolization | 34 |
| Ablation | 14 |
| Chemoembolization | 6 |
| Chemotherapy | 4 |
| Transplantation | 1 |
| Treatment offered, % | |
| Clinical trial | 29 |
| Chemotherapy | 33 |
| Best supportive care | 38 |
| Staging system | |
| TNM* | |
| 1-2 | 6 |
| 2 | 32 |
| 3 | 63 |
| Okuda stage | |
| 1 | 49 |
| 2 | 40 |
| 3 | 11 |
| BCLC | 1 |
| A | 1 |
| B | 1 |
| C | 85 |
| D | 13 |
| CLIP score | 9 |
| 0 | 9 |
| 1 | 19 |
| 2 | 31 |
| 3 | 23 |
| 4 | 12 |
| 5 | 6 |
| CUPI | 38 |
| Low | 38 |
| Intermediate | 50 |
| High | 12 |
| JIS score | 0 |
| 0 | 0 |
| 1 | 2 |
| 2 | 8 |
| 3 | 64 |
| 4 | 22 |
| 5 | 3 |
| GETCH | 15 |
| Low | 15 |
| Intermediate | 71 |
| High | 14 |
Abbreviations: HCC, hepatocellular carcinoma; ECOG PS, Eastern Cooperative Oncology Group performance status; PT, prothrombin time; AFP, α-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CLIP, Cancer of the Liver Italian Program; CUPI, Chinese University Prognostic Index; JIS, Japan Integrated Staging; GETCH, Groupe d'Etude et de Traitement du Carcinome Hepatocellulaire.
Sixth edition.
Evaluation of Staging Systems
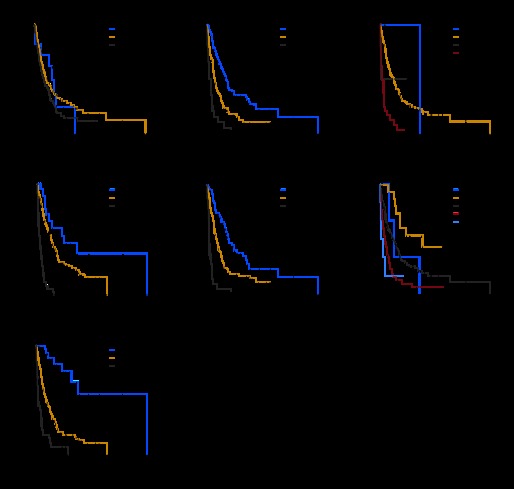
Kaplan-Meier curves were generated for each of all staging systems except TNM fifth edition. Results are summarized in Figure 1. The Kaplan-Meier curves showed clear different prognostic strata for CLIP, CUPI, GETCH, and Okuda, with high statistical significance (log-rank P < .001 in all cases). Although some overlapping of survival curves is observed for JIS and BCLC, overall the difference in survival among different prognostic strata is also highly statistically significant (log-rank P < .001 in both cases). Only the TNM sixth edition could not demonstrate survival difference among its prognostic strata (log-rank P = .18 for TNM sixth edition).
Fig 1.
Kaplan-Meier survival curves for patients with advanced hepatocellular carcinoma by staging system: (A) TNM 6th edition, (B) Okuda, (C) Barcelona Clinic Liver Cancer, (D) Cancer of the Liver Italian Program, (E) Chinese University Prognostic Index, (F) Japan Integrated Staging score, and (G) Groupe d'Etude et de Traitement du Carcinome Hepatocellulaire.
Comparison of Staging Systems
The comparison of the prognostic ability of the different staging systems was done by using the c-index (Table 2). On the basis of the c-index, CLIP (0.69; 95% CI, 0.65 to 0.73), CUPI (0.67; 95% CI, 0.63 to 0.71), and GETCH (0.66; 95% CI, 0.62 to 0.70) were the top three ranking staging systems, and there was no statistically significant difference among each other (CLIP compared with CUPI, P = .26; CLIP compared with GETCH, P = .17; and CUPI compared with GETCH, P = .7). There was a borderline difference between prognostic ability of Okuda (c-index, 0.65; 95% CI, 0.61 to 0.70) compared with CLIP (P = .06). JIS (c-index, 0.63), BCLC (c-index, 0.58), and TNM sixth edition (c-index, 0.47) were all significantly less valuable than CLIP (P < .004).
Table 2.
Ranking of Staging Systems in Patients With Advanced HCC By Using C-Index
| Rank | System | C-Index | 95% CI |
|---|---|---|---|
| 1 | CLIP | 0.69 | 0.65 to 0.73 |
| 2 | CUPI | 0.67 | 0.63 to 0.71 |
| 3 | GETCH | 0.66 | 0.62 to 0.70 |
| 4 | Okuda | 0.65 | 0.61 to 0.70 |
| 5 | JIS | 0.63 | 0.59 to 0.67 |
| 6 | BCLC | 0.58 | 0.55 to 0.62 |
| 7 | TNM6 | 0.47 | 0.42 to 0.52 |
Abbreviations: HCC, hepatocellular carcinoma; c-index, concordance index; CLIP, Cancer of the Liver Italian Program; CUPI, Chinese University Prognostic Index; GETCH, Groupe d'Etude et de Traitement du Carcinome Hepatocellulaire; JIS, Japan Integrated Staging; BCLC, Barcelona Clinic Liver Cancer; TNM6, TNM staging, sixth edition.
Prognostic Factors
Independent prognostic factors for overall survival identified through multivariate analysis are reported in Table 3. The most significant of those prognostic factors that are not regularly used as part of the different staging systems are performance status, the presence of abdominal pain, and the presence of esophageal varices. Identified laboratory prognostic factors include elevated alkaline phosphatase, AST, and AFP levels.
Table 3.
Independent Prognostic Factors for Overall Survival in Patients With Advanced HCC According to Multivariate Analysis
| Variable | Hazard Ratio for Death | 95% CI | P |
|---|---|---|---|
| No. of liver lesions, 1 v > 1 | 0.25 | 0.089 to 0.720 | .009 |
| Bilobar disease, yes v no | 1.76 | 1.08 to 2.784 | .0166 |
| Abdominal pain, yes v no | 2.29 | 1.551 to 3.39 | < .001 |
| Esophageal varices, yes v no | 1.61 | 1.069 to 2.437 | .0227 |
| ECOG PS, 2-3 v 0-1 | 2.95 | 1.868 to 4.666 | < .001 |
| Child Pugh stage, B v A | 1.88 | 1.243 to 2.832 | .0028 |
| Alkaline phosphatase | 1.96 | 1.007 to 3.817 | .04 |
| AFP | 1.24 | 1.106 to 1.393 | < .001 |
| AST | 3.47 | 1.799 to 6.686 | .002 |
Abbreviations: HCC, hepatocellular carcinoma; ECOG PS, Eastern Cooperative Oncology Group performance status; AFP, α-fetoprotein.
Improvement of Staging Systems
Addition of the independent prognostic variables of performance status, AST, abdominal pain, and presence of esophageal varices improved the discriminatory ability of CLIP with a new c-index of 0.76 compared with 0.69 (bootstrap validated).
DISCUSSION
Medical oncologists assess patients with advanced HCC. Of particular impact for these patients is information pertaining not only to tumor stage but also to the extent of liver dysfunction.1 Unlike other cancers, a TNM staging seems insufficient, and multiple other comprehensive prognostic staging systems have been developed. There have been different views to the validity of certain staging systems reflected by the multidisciplinary approach to the management of HCC. Yet it remains unknown which staging system is most informative in patients with advanced HCC in regard to survival outcome and direction of care. As new therapies continue to emerge for HCC, identifying patients who are suited for such therapies versus best supportive care is essential. The interpretation of clinical trials in advanced HCC may also be dependent on which staging system was used. It is also unclear whether additional variables not considered in these systems have prognostic significance.
In our study, we attempted to help define which staging system should be used for the evaluation of patients with advanced HCC who are referred to medical oncology specialists. We retrospectively identified and then compared seven different staging systems in terms of their prognostic abilities by using c-index. We showed that the purely anatomic TNM sixth edition was not useful for predicting overall survival. BCLC has been viewed “as the standard classification that is used for trial design and clinical management of patients with HCC” on the basis of a commentary report,17p699 supported by two prospective validation studies that had a limited number of patients with metastatic disease,18–19 which was the subject of this study. BCLC is the most comprehensive staging system available. It is designed with an ability to factor therapeutic choices for patients at different stages of disease. However, it did not score well in our exercise, because we were studying a specific niche of patients with advanced disease who fall under the single C category in BCLC, which limits any discriminatory abilities. BCLC is currently under revision, and a substratification of category C is possible.20
CLIP, CUPI, and GETCH staging systems were the most informative regarding survival outcome. CLIP is a commonly used staging system for patients with HCC. The staging system was validated in a population of patients treated prospectively in a clinical trial that tested tamoxifen therapy in HCC that had underlying hepatitis C as the main etiology. Five prognostic strata were defined according to a score derived from the analysis of variables related to cirrhosis (Child-Pugh score), tumor morphology, AFP level, and portal vein thrombosis, as detailed in Appendix Table A1 (online only). The higher the score, the worse the outcome was. A patient with limited metastatic disease exemplified by, for example, a few lesions in the lung but with a CLIP score of 6 would have a median survival of a few weeks and may be recommended best supportive care rather than systemic therapy.
CUPI was developed in a population of patients with HCC who predominantly had hepatitis B.8 CUPI parameters include bilirubin, ascites, AFP, alkaline phosphatase, the tumor extent as defined by the TNM staging system, and the absence or presence of clinical symptoms on presentation. CUPI was the only prognostic system, other than BCLC, to introduce a clinical assessment variable. CUPI is strengthened by the weighted scores for each parameter that were derived from the regression coefficients of various factors (Appendix Table A2, online only).
GETCH11 is based on assessment of total bilirubin, KPS, AFP, alkaline phosphatase, and portal vein thrombosis (Appendix Table A3, online only). The points from Appendix Table A3 range from 0 to 11; in patients with HCC, group A includes those at a low risk of death (score = 0); group B indicates an intermediate risk of death (score = 1 to 5); and group C indicates a high risk of death (score ≥ 6). Similar to CUPI, GETCH is powered by the portal vein thrombosis parameter, a critical prognostic factor for HCC.
We independently examined the prognostic importance of independent clinical, pathologic, and laboratory variables. Novel prognostic variables were identified as follows: performance status, abdominal pain, AST level, and esophageal varices. Performance status is a universal prognostic variable that has a clear impact on outcome, abdominal pain may be a manifestation of tumor extent, and the presence of esophageal varices is an indirect measure of the presence of portal hypertension; all are viewed as important prognostic factors of HCC. The presence of an elevated AST level shows one of the highest hazard ratios for poor outcome. Of note, in patients with chronic hepatitis and cirrhosis, an increase in AST/ALT ratio is associated with progressive liver functional impairment.21,22 Those added variables helped improve the discriminatory ability of the CLIP, one of the three top-ranked models in this retrospective analysis.
Our effort is obviously limited by the retrospective nature of the analysis and the single-institution experience. Our future goal is to help evaluate our hypothesis in a prospective fashion as part of a prospective clinical trial. We were also unable to explain the lack of any meaningful difference between CLIP, CUPI, and GETCH; this lack of difference may be real or limited by the retrospective analysis of the study, or it may be influenced proportionally by the equal contribution of the patients with hepatitis B virus, hepatitis C virus, and alcohol risk factors to the database (Table 1).
A comparison of four staging systems in two French clinical trials in patients with advanced HCC has recently been reported.22 The authors compared Okuda, CLIP, and BCLC, staging systems that use different statistical tools. CLIP was reported to be the most adaptive scoring system in the setting of advanced disease.
Other multiple studies comparing staging systems in hepatocellular carcinoma have been conducted and have reported different ranking of staging systems.18,23–27 However, these studies included patients with multiple stages of HCC, whereas our studied population included patients with advanced disease that is not amenable to curative or local therapies. This observation highlights once again that, for different stages of the natural history of a disease, the relevance of certain prognostic factors and the usefulness of staging systems might vary. Patients with advanced HCC who present to a medical oncologist for consideration of systemic therapy have distinct clinical characteristics, tumor extent, and residual liver function.
In summary, CLIP, CUPI, and GETCH were the most informative staging systems in predicting survival in patients with advanced HCC. Prospective validation is required to determine if these staging systems can be accurately used to stratify patients in clinical trials and to help direct medical care. BCLC has limited discriminatory abilities in this population, and we do not recommend its use in this setting. Ongoing revisions of the BCLC with respect to this patient population may change this observation in the future. TNM sixth edition was not helpful, mostly because of a lack of any prognostic parameters of liver dysfunction.
Appendix
Table A1.
Cancer of the Liver Italian Program
| Parameter | Point |
||
|---|---|---|---|
| 0 | 1 | 2 | |
| Child-Pugh score | A | B | C |
| Tumor | Uninodular | Multinodular | Massive |
| Morphology | Extension ≤ 50% | Extension ≤ 50% | Extension > 50% |
| Portal vein thrombosis | No | Yes | |
| AFP,ng/dL | < 400 | ≥ 400 | |
Abbreviation: AFP, α-fetoprotein.
Table A2.
Chinese University Prognostic Index
| Parameter | Weight (CUPI Score) |
|---|---|
| Bilirubin, μmol/L | |
| < 34 | 0 |
| 34-51 | 3 |
| ≥ 52 | 4 |
| Ascites present | 3 |
| Alkaline phosphatase ≥ 200 IU/L | 3 |
| TNM stage* | |
| I and II | −3 |
| IIIA and IIIB | −1 |
| IVA and IVB | 0 |
| AFP ≥ 500 ng/mL | 2 |
| No disease symptoms on presentation | −4 |
| Risk group score | |
| Low | −7 to 1 |
| Intermediate | 2 to 7 |
| High | 8 to 12 |
Abbreviation: AFP, α-fetoprotein.
According to American Joint Commission on Cancer fifth edition.
Table A3.
Groupe d'Etude et de Traitement du Carcinome Hepatocellulaire
| Factor | Points |
|||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Karnofsky index, % | ≥ 80 | < 80 | ||
| Serum bilirubin, μmol/L | < 50 | ≥ 50 | ||
| Serum alkaline phosphatase, ULN | < 2 | ≥ 2 | ||
| Serum AFP, μg/L | < 35 | ≥ 35 | ||
| Portal obstruction | No | Yes | ||
Abbreviations: ULN, upper limit of normal; AFP, α-fetoprotein.
Footnotes
Presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2007, and the Gastrointestinal Meeting of the American Society of Clinical Oncology, Orlando, FL, January 25-27, 2008.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Fidel David Huitzil Melendez, Marinela Capanu, Eileen M. O'Reilly, Austin Duffy, Ghassan K. Abou-Alfa
Provision of study materials or patients: Fidel David Huitzil Melendez, Eileen M. O'Reilly, Austin Duffy, Leonard L. Saltz, Ghassan K. Abou-Alfa
Collection and assembly of data: Fidel David Huitzil Melendez, Bolorsukh Gansukh, Ghassan K. Abou-Alfa
Data analysis and interpretation: Fidel David Huitzil Melendez, Marinela Capanu, Eileen M. O'Reilly, Austin Duffy, Bolorsukh Gansukh, Leonard L. Saltz, Ghassan K. Abou-Alfa
Manuscript writing: Fidel David Huitzil Melendez, Marinela Capanu, Eileen M. O'Reilly, Leonard L. Saltz, Ghassan K. Abou-Alfa
Final approval of manuscript: Fidel David Huitzil Melendez, Marinela Capanu, Eileen M. O'Reilly, Austin Duffy, Bolorsukh Gansukh, Leonard L. Saltz, Ghassan K. Abou-Alfa
REFERENCES
- 1.Abou-Alfa GK. Hepatocellular carcinoma: Molecular biology and therapy. Semin Oncol. 2006;33(suppl 11):S79–S83. doi: 10.1053/j.seminoncol.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abou-Alfa GK. Selection of patients with hepatocellular carcinoma for sorafenib. J Natl Compr Canc Netw. 2009;7:397–403. doi: 10.6004/jnccn.2009.0028. [DOI] [PubMed] [Google Scholar]
- 3.Lei HJ, Chau GY, Lui WY, et al. Prognostic value and clinical relevance of the 6th Edition 2002 American Joint Committee on Cancer staging system in patients with resectable hepatocellular carcinoma. J Am Coll Surg. 2006;203:426–435. doi: 10.1016/j.jamcollsurg.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 4.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment: Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Sem Liv Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 6.A new prognostic system for hepatocellular carcinoma. A retrospective study of 435 patients—The Cancer of the Liver Italian Program (CLIP) inves-tigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 7.Prospective validation of the CLIP score. A new prognostic system for patients with cirrhosis and hepatocellular carcinoma—The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology. 2000;31:840–845. doi: 10.1053/he.2000.5628. [DOI] [PubMed] [Google Scholar]
- 8.Leung TW, Tang AM, Zee B, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: A study based on 926 patients. Cancer. 2002;94:1760–1769. doi: 10.1002/cncr.10384. [DOI] [PubMed] [Google Scholar]
- 9.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): Its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score) J Gastroenterol. 2003;38:207–215. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 10.Kudo M, Chung H, Haji S, et al. Validation of a new prognostic staging system for hepatocellular carcinoma: The JIS score compared with the CLIP score. Hepatology. 2004;40:1396–1405. doi: 10.1002/hep.20486. [DOI] [PubMed] [Google Scholar]
- 11.Chevret S, Trinchet JC, Mathieu D, et al. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma: Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire. J Hepatol. 1999;31:133–141. doi: 10.1016/s0168-8278(99)80173-1. [DOI] [PubMed] [Google Scholar]
- 12.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma: Conclusions of the Barcelona-2000 EASL conference—European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 13.Cha C, Fong Y, Jarnagin WR, et al. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg. 2003;197:753–758. doi: 10.1016/j.jamcollsurg.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Covey AM, Maluccio MA, Schubert J, et al. Particle embolization of recurrent hepatocellular carcinoma after hepatectomy. Cancer. 2006;106:2181–2189. doi: 10.1002/cncr.21883. [DOI] [PubMed] [Google Scholar]
- 15.Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: A prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32A:1135–1141. doi: 10.1016/0959-8049(95)00664-8. [DOI] [PubMed] [Google Scholar]
- 16.Riffenburgh RH. Statistics in Medicine. ed 2. Burlington, MA: Elsevier Academic Press; 2006. p. xli. 622. [Google Scholar]
- 17.Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 18.Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: Comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707–716. doi: 10.1002/hep.20636. [DOI] [PubMed] [Google Scholar]
- 19.Cillo U, Vitale A, Grigoletto F, et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol. 2006;44:723–731. doi: 10.1016/j.jhep.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Llovet JM. Statement at the State of the Clinical Science Meeting: Hepatocellular Carcinoma (HCC). Presented at the State of Clinical Science Meeting; December 12-13, 2008; Bethesda, MD. [Google Scholar]
- 21.Giannini E, Botta F, Fasoli A, et al. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Dig Dis Sci. 1999;44:1249–1253. doi: 10.1023/a:1026609231094. [DOI] [PubMed] [Google Scholar]
- 22.Giannini E, Risso D, Botta F, et al. Validity and clinical utility of the aspartate aminotransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease. Arch Intern Med. 2003;163:218–224. doi: 10.1001/archinte.163.2.218. [DOI] [PubMed] [Google Scholar]
- 23.Collette S, Bonnetain F, Paoletti X, et al. Prognosis of advanced hepatocellular carcinoma: Comparison of three staging systems in two French clinical trials. Ann Oncol. 2008;19:1117–1126. doi: 10.1093/annonc/mdn030. [DOI] [PubMed] [Google Scholar]
- 24.Guglielmi A, Ruzzenente A, Pachera S, et al. Comparison of seven staging systems in cirrhotic patients with hepatocellular carcinoma in a cohort of patients who underwent radiofrequency ablation with complete response. Am J Gastroenterol. 2008;103:597–604. doi: 10.1111/j.1572-0241.2007.01604.x. [DOI] [PubMed] [Google Scholar]
- 25.Kondo K, Chijiiwa K, Nagano M, et al. Comparison of seven prognostic staging systems in patients who undergo hepatectomy for hepatocellular carcinoma. Hepatogastroenterology. 2007;54:1534–1538. [PubMed] [Google Scholar]
- 26.Grieco A, Pompili M, Caminiti G, et al. Prognostic factors for survival in patients with early-intermediate hepatocellular carcinoma undergoing non-surgical therapy: Comparison of Okuda, CLIP, and BCLC staging systems in a single Italian centre. Gut. 2005;54:411–418. doi: 10.1136/gut.2004.048124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cillo U, Bassanello M, Vitale A, et al. The critical issue of hepatocellular carcinoma prognostic classification: Which is the best tool available? J Hepatol. 2004;40:124–131. doi: 10.1016/j.jhep.2003.09.027. [DOI] [PubMed] [Google Scholar]