Abstract
Mutations in connexin 46 are associated with congenital cataracts. The purpose of this project was to characterize cellular and functional properties of two congenital cataract-associated mutations located in the NH2 terminus of connexin 46: Cx46D3Y and Cx46L11S, which we found localized to gap junctional plaques like wild-type Cx46 in transfected HeLa cells. Dual two-microelectrode-voltage-clamp studies of Xenopus oocyte pairs injected with wild-type or mutant rat Cx46 showed that oocyte pairs injected with D3Y or L11S cRNA failed to induce gap junctional coupling, whereas oocyte pairs injected with Cx46 showed high levels of coupling. D3Y, but not L11S, functionally paired with wild-type Cx46. To determine whether coexpression of D3Y or L11S affected the junctional conductance produced by wild-type lens connexins, we studied pairs of oocytes coinjected with equal amounts of mutant and wild-type connexin cRNA. Expression of D3Y or L11S almost completely abolished gap junctional coupling induced by Cx46. In contrast, expression of D3Y or L11S failed to inhibit junctional conductance induced by Cx50. To examine effects of the D3Y and L11S mutations on hemichannel activity, hemichannel currents were measured in connexin cRNA-injected oocytes. Oocytes expressing D3Y exhibited reduced hemichannel activity as well as alterations in voltage gating and charge selectivity while oocytes expressing L11S showed no hemichannel activity. Moreover, coexpression of mutant with wild-type Cx50 or Cx46 gave rise to hemichannels with distinct electrophysiological properties, suggesting that the mutant connexins were forming heteromeric channels with wild-type connexins. These data suggest D3Y and L11S cause cataracts by similar but not identical mechanisms.
Keywords: connexin, gap junction, cataract, intercellular communication
gap junctional channels are intercellular channels that allow the passage of ions and other small molecules between neighboring cells. They play a vital role in synchronizing the activity of cells and in maintaining metabolic homeostasis. Gap junctions form by the docking of two connexons or hemichannels, each of which is formed by the oligomerization of six transmembrane proteins called connexins (Cx). In addition to forming gap junctional channels, undocked connexons are present in the nonjunctional plasma membrane as hemichannels and can mediate the transfer of ions and other small molecules such as ATP between the cytosol and extracellular environment.
Connexins are a family of closely related proteins with 21 human members (40). All of the connexins are thought to have a similar membrane topology consisting of four transmembrane α- helices (TM1–4), two extracellular loops (E1 and E2), and one cytoplasmic loop (IL), with both the amino terminus (NT) and carboxyl terminus (CT) located on the cytoplasmic side of the membrane (4). Functional and structural studies demonstrate that NT along with E1 and TM1 contribute to the pore lining region of the hemichannel (8, 17, 22, 28, 37).
Mutations in connexins have been associated with multiple human genetic diseases including congenital cataracts (Cx50 and Cx46) (reviewed in Refs. 27 and 43). Several mechanisms have been proposed to account for the role of these mutations in the development of disease: 1) inability to form functional gap junctions; 2) alterations in the gating and permeability properties of gap junctions; 3) dominant negative effects on other connexins; 4) aberrant hemichannel activity; and 5) mistrafficking leading to cytoplasmic accumulation of protein. With a few exceptions (20, 29, 34), most of the cataract-associated connexin mutations that have been studied are unable to form gap junctional plaques when expressed by themselves and show mistrafficking (2–4, 7, 19, 24, 25, 32, 42). Some of them also act as dominant negative inhibitors of wild-type connexin activity. The goal of the present project was to characterize the functional and cellular effects of two congenital cataract mutations, D3Y(1) and L11S(13), which are located in the NH2 terminus of Cx46. We report that although both mutations of Cx46 form gap junctional plaques in HeLa cells, their gap junctions are not functional and their hemichannels exhibit distinct electrophysiological properties.1
METHODS
Generation of constructs.
Mutations were introduced into wild-type rat Cx46 cDNA in a SP64T vector with QuikChange Site-Directed Mutagenesis Kit (Stratagene) by using PCR primers that amplified the whole plasmid. The primers used were as follows: D3Y, sense 5′-cgg gag caa tgg gct act gga gct tcc tgg gg-3′ and antisense 5′-ccc cag gaa gct cca gta gcc cat tgc tcc cg-3′; L11S, sense 5′-cct ggg gcg gct gtc gga gaa tgc gca gga g-3′ and antisense 5′-ctc ctg cgc att ctc cga cag ccg ccc cag g-3′. The coding regions of all amplified constructs were fully sequenced at the Cancer Research Center DNA Sequencing Facility of the University of Chicago (Chicago, IL) to confirm the absence of additional unwanted mutations.
Cell culture, transfection, and immunofluorescence localization.
HeLa cells were grown on collagen type I-coated glass coverslips (BD BioSciences, San Jose, CA) at a density of 1.8 × 105 per 35-mm dish. At 2 days postplating, cells were transfected with Cx46, D3Y, or L11S and cotransfected with a GFP plasmid (to assess transfection efficiency) using Lipofectamine 2000 Transfection Reagent (Invitrogen, Carlsbad, CA). Two days later, cells on coverslips were fixed in iced methanol at −20°C for 10 min and stored at −4°C prior to immunostaining. Cell samples were re-equilibrated in phosphate-buffered saline (PBS) preincubated in 30% normal donkey serum for 25 min at room temperature and incubated in rabbit IgG anti-Cx46 (Alpha Diagnostics) for 2 1/2 h at 37°C. Six washes in PBS over 30 min were followed by incubation in DyLight594-conjugated anti-rabbit IgG (Jackson ImmunoResearch) for 1 h at 37°C. After six washes in PBS over 45 min, immunostained specimens were mounted in Prolong Gold antifade reagent with DAPI (Invitrogen) and left overnight in the dark at room temperature. Specimens were observed and images were captured with a Leica DMRB microscope equipped with a Hamamatsu CCD camera and Openlab imaging software (Improvision, Coventry, England).
Expression of connexins in Xenopus oocytes.
Connexin cRNAs were synthesized using the mMessage mMachine in vitro transcription kit (Ambion, Austin, TX) according to the manufacturer's instructions. The amount of cRNA was quantitated by measuring the absorbance at 260 nm.
Adult female Xenopus laevis frogs were anesthetized with tricaine and a partial ovariectomy was performed in accordance with protocols approved by the Rosalind Franklin University Animal Care and Use Committee. The oocytes were manually defolliculated after treating them with collagenase IA (Worthington Biochemical, Lakewood, NJ). Stage V and VI oocytes were selected and pressure-injected using a Nanoject variable microinjection apparatus (model mo. 3-000-203, Drummond Scientific, Broomal, PA) with 36.8 nl of 0.5–600 ng/μl of connexin cRNA and 5 ng/36.8 nl of oligonucleotide antisense to mRNA for Xenopus Cx38. The oocytes were incubated overnight at 18°C in L-15 (GIBCO-Invitrogen, Carlsbad, CA) containing 2 mM CaCl2 before the electrophysiological experiments were performed.
Electrophysiological measurements and data analysis.
For measurement of gap junctional coupling, connexin cRNA-injected oocytes were devitellinized and paired as previously described (9). Double two-microelectrode voltage-clamp experiments were performed using a GeneClamp 500 (Molecular Devices, Sunnyvale, CA) and a TEV-200A voltage clamp amplifier (Dagan, MN) as previously described (42).
Hemichannel currents were recorded from single oocytes using a GeneClamp 500 two-electrode voltage-clamp amplifier (Molecular Devices). A bath clamp was used to minimize the effect of the voltage drop across the bath grounding electrode. The standard external bath solution contained (in mM) 88 choline Cl, 1 KCl, 2.4 NaHCO3, 1 MgCl2, and 15 HEPES, pH 7.6 to which different concentrations of calcium were added. For the charge selectivity experiments, the external solutions contained 89 mM NaCl or 89, 44.5, or 9.8 mM KCl, 1 mM MgCl2, and 5 mM HEPES (pH 7.4). Mannitol was used to adjust the osmolarity to ∼200 mosM. Leakage correction was performed by subtracting current traces recorded in the presence of 2 mM extracellular calcium concentration ([Ca2+]o; to block connexin hemichannel currents) from current traces recorded using the same voltage-clamp protocol in the absence of external calcium.
To determine reversal potential, a ramp protocol was used. In this protocol, the cell was voltage clamped to 30 mV for 1.9 s to activate the hemichannel current and then a 500 ms ramp from 30 to −40 mV was applied. An identical ramp was applied in the absence of a prepulse. The voltage at which the two current-voltage (I-V) curves intersected was taken to be the reversal potential of the hemichannel current.
Pulse generation and data acquisition were performed using a PC computer equipped with PCLAMP9 software and a Digidata 1322A data acquisition system (Molecular Devices). All experiments were performed at room temperature (20–22°C).
Homology modeling.
A homology-based model of rat Cx46 was constructed using the X-ray crystallographic structure of human Cx26 [Protein Data Bank entry 2ZW3 (17)] as a template. Modeling was carried out using Molecular Operating Environment (version 2011.10; Chemical Computing Group, Montreal, Canada). The resulting model was checked to ensure that no irregularities occurred in the Ramachandran plot, bond lengths, bond angles, and dihedral angles.
Models of the D3Y and L11S mutants were constructed starting from the rat Cx46 model. Side chains were modified, and conformational searches were carried out for the modified side chains as well as for residues that interact with the modified residue.
RESULTS
The ability of wild-type and mutant Cx46 to form gap junction plaques was assessed by immunofluorescence microscopy in transiently transfected HeLa cells (Fig. 1). As expected, Cx46 formed large gap junctional plaques at regions of cell-to-cell apposition. D3Y and L11S formed plaques that were similar in size to those formed by Cx46, indicating that both mutations were correctly targeted.
Fig. 1.
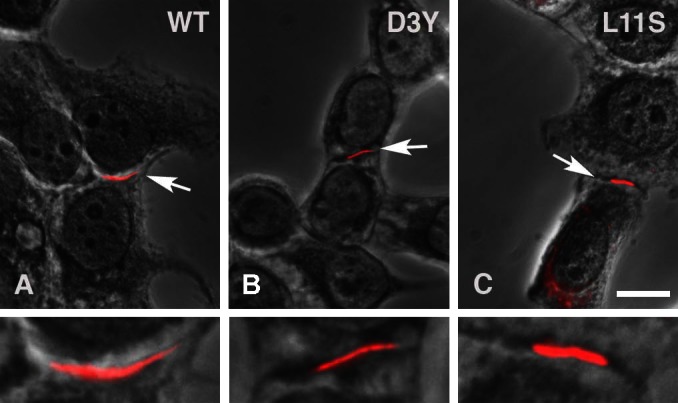
Wild-type (WT) and mutant connexin 46 (Cx46) form gap junction plaques. Immunolocalization with Cx46 antibodies shows that wild-type Cx46 (A), Cx46D3Y (B), and Cx46L11S (C) all form gap junctions in transfected HeLa cells along the plasma membrane between expressing cells. White arrows indicate gap junctions immunostained red, which are shown enlarged in the bottom panel. Calibration bar, 10 μm.
Effect of D3Y and L11S on gap junctional function.
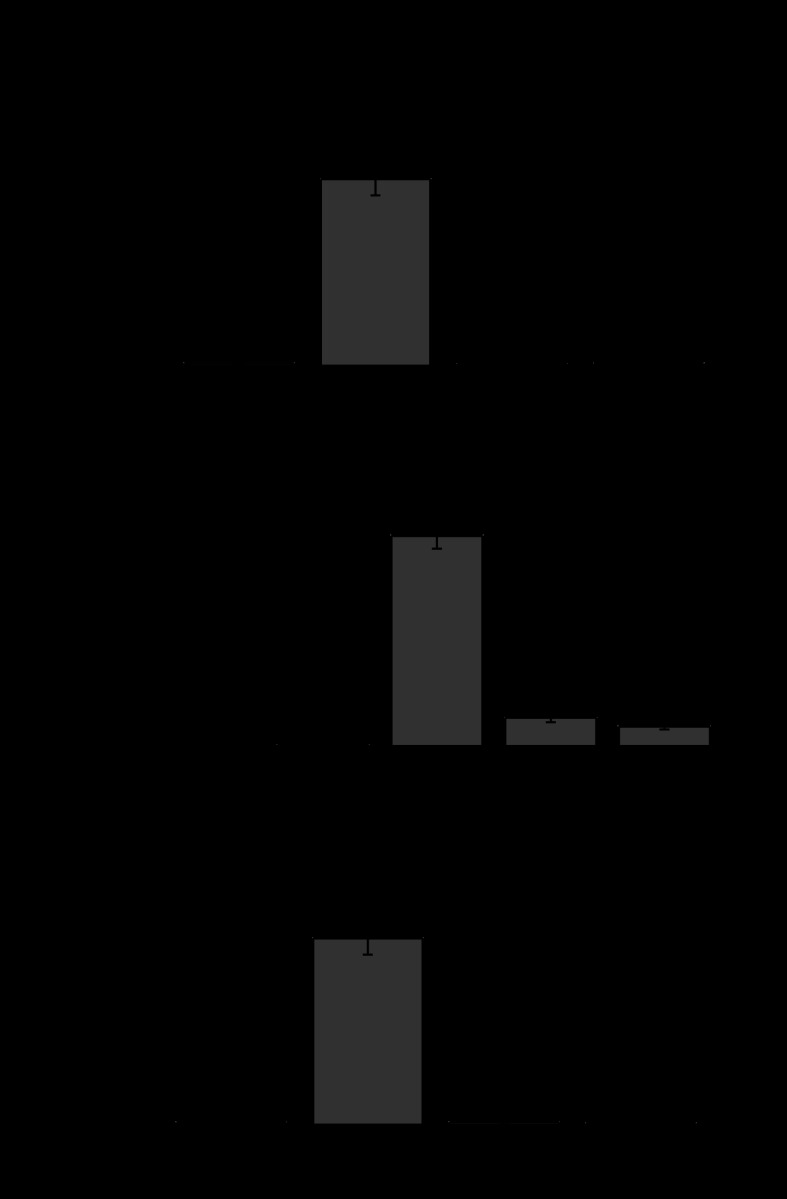
The capacity of D3Y and L11S to form functional gap junctional channels in Xenopus oocyte pairs was tested using a double two-electrode voltage-clamp technique. The results of these experiments are summarized in Fig. 2A. Homotypically paired oocytes injected with mutant cRNA showed no gap junctional coupling above background control levels, whereas homotypic oocyte pairs injected with Cx46 cRNA showed high levels of coupling. However, analysis of heterotypic pairings between D3Y- and Cx46- or Cx50-expressing oocytes revealed that D3Y was capable of forming heterotypic channels with mean junctional conductance levels significantly higher than background (Fig. 2B). In contrast, L11S-expressing oocytes did not induce significant coupling when paired heterotypically with Cx46- or Cx50- expressing oocytes (Fig. 2C).
Fig. 2.
D3Y and L11S fail to induce gap junctional coupling. A–C: bar graphs showing the junctional conductances (means ± SE) in pairs of Xenopus oocytes expressing Cx46, D3Y, and L11S or heterotypic combinations of wild-type and mutant lens connexins as determined by double whole cell voltage clamp. The total amount of cRNA injected into each oocyte was held constant. All oocytes were injected with antisense oligonucleotides (AS) to block endogenous Cx38 junctional currents. The number of pairs is indicated in parentheses. *P < 0.001 compared with Cx46-injected oocyte pairs; **P < 0.001 compared with heterotypic Cx50/Cx46 pairs.
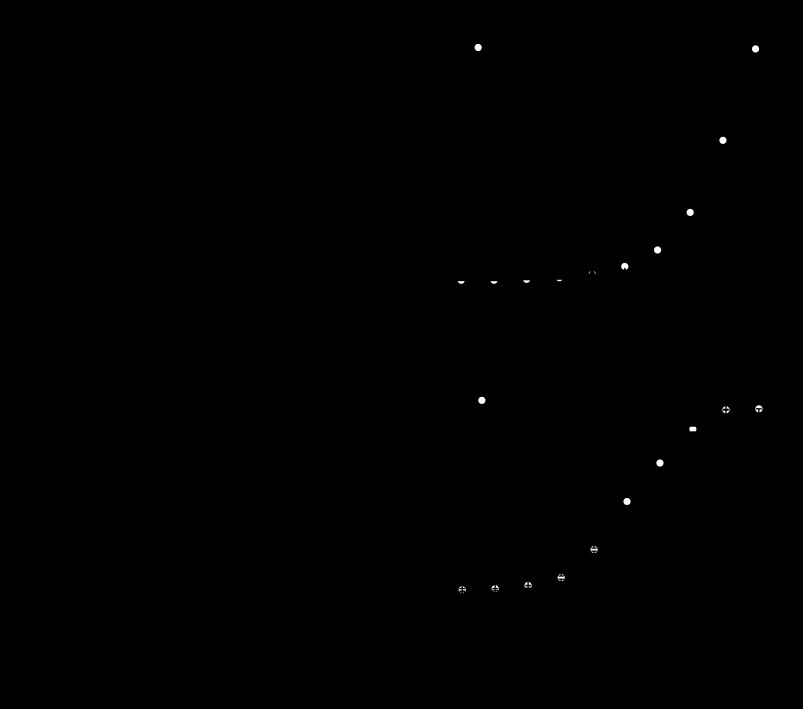
Figure 3 shows typical families of gap junctional current traces and plots of normalized steady-state junctional conductance (Gj) versus junctional voltage (Vj) for heterotypic Cx46/Cx50, Cx46/D3Y, and Cx50/D3Y pairs. Only oocyte pairs that displayed junctional conductances <11 μS were used for analysis of voltage-gating properties. Heterotypic Cx46/Cx50 pairs exhibited a junctional current that decayed slowly over time at transjunctional potentials >20 mV. The normalized Gj-Vj relationship was nearly symmetrical as previously reported (38). In contrast, heterotypic Cx46/D3Y and Cx50/D3Y pairs showed markedly asymmetric voltage-gating behavior. The channels were partially closed at a junctional potential of 0 mV. Application of positive junctional potentials (relative to the D3Y-expressing oocyte) caused the channels to activate, while application of negative junctional potentials resulted in channel closure.
Fig. 3.
Heterotypic Cx46/D3Y and Cx50/D3Y gap junctional channels show asymmetrical transjunctional voltage (Vj) gating properties. A–C: representative families of junctional current traces recorded from oocyte pairs expressing Cx46/Cx50 (A), Cx46/D3Y (B), or Cx50/D3Y (C). D–F: plots of normalized steady-state junctional conductance (Gjss) vs. transjunctional voltage for Cx46/Cx50 (D; n = 4), Cx46/D3Y (E; n = 6), and Cx50/D3Y (F; n = 6). Positive and negative Vj refers to depolarization and hyperpolarization, respectively, of the cell indicated on the right side of the pairing designation. Points represent means ± SE.
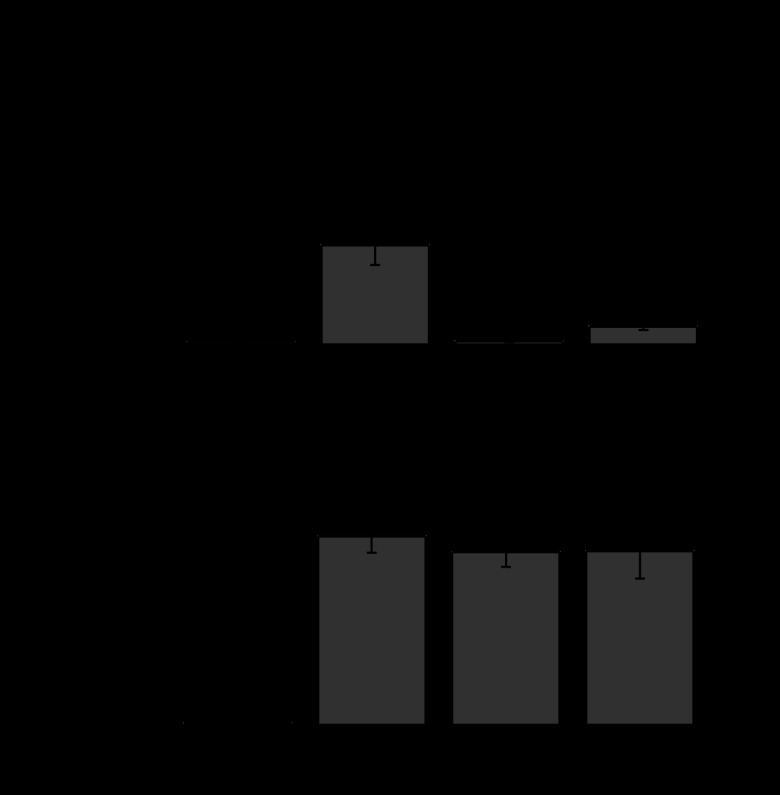
To determine whether coexpression of D3Y or L11S affected the junctional conductance produced by Cx46, we tested pairs of oocytes coinjected with equal amounts of mutant and wild-type Cx46 cRNA. Coexpression of D3Y or L11S almost completely abolished gap junctional coupling induced by Cx46 (Fig. 4A). These results suggest that both D3Y and L11S act as strong dominant negative inhibitors of Cx46. In contrast, coexpression of D3Y or L11S with equal amounts of Cx50 had no effect on the junctional conductances induced by Cx50 (Fig. 4B).
Fig. 4.
D3Y and L11S act as strong dominant negative inhibitors of wild-type Cx46 but not wild-type Cx50 gap junctional channels. Bar graphs show the junctional conductances (means ± SE) in pairs of Xenopus oocytes expressing Cx46, Cx50, or a 1:1 mixture of wild-type and mutant connexins as determined by double whole cell voltage clamp. The amount of Cx46 or Cx50 injected into each oocyte was held constant. The number of pairs is indicated in parentheses. *P < 0.001 compared with Cx46-injected cell pairs; **P < 0.001 compared with AS-injected cell pairs.
Effect of the D3Y and L11S mutations on hemichannel properties.
The effect of the D3Y and L11S mutations on hemichannel properties was tested in single Xenopus oocytes using a conventional two-electrode voltage-clamp technique. Oocytes injected with D3Y cRNA exhibited greatly reduced hemichannel activity compared with oocytes injected with similar amounts of Cx46 (data not shown). No detectable hemichannel activity above background was observed in L11S cRNA-injected oocytes.
To increase the magnitude of the D3Y currents, the amount of injected D3Y cRNA was increased to a level 100 times greater than that required to induce high levels of hemichannel activity in Cx46 cRNA-injected oocytes. Figure 5 compares families of hemichannel currents and corresponding steady-state I-V and conductance-voltage (G-V) curves recorded from Cx46 or D3Y cRNA-injected oocytes in the presence of zero added external calcium. Both Cx46 and D3Y cRNA-injected oocytes exhibited hemichannel currents that activated on application of depolarizing voltage-clamp steps. However, the steady-state G-V curve for D3Y was shifted to more positive potentials and its slope was reduced compared with that of Cx46. In addition, unlike Cx46, which partially inactivated at large positive potentials, D3Y hemichannel currents showed no inactivation, suggesting that the D3Y mutation was disrupting or reversing the polarity of the Vj gating of Cx46. Alternatively, the loss of gating at positive potentials could be due to a rightward shift in voltage sensitivity.
Fig. 5.
Cells expressing D3Y show alterations in hemichannel gating. A–C: representative examples of hemichannel currents in single oocytes injected with D3Y (A) or Cx46 (B) cRNA or no RNA (AS, C). The bath solution contained choline chloride Ringer with zero added calcium. The hemichannel currents were recorded in response to a series of voltage-clamp steps from +80 mV to −100 mV in decrements of 20 mV followed by a short pulse to −80 mV. Holding potential = −10 mV. Data were corrected for leakage. Dashed line represents zero current. D: steady-state current-voltage relationships for oocytes injected with cRNA for D3Y (○), Cx46 (■), or AS alone (▲) using the paradigm described in A. E: conductance-voltage relationships for D3Y (○, n = 6) and Cx46 (■, n = 6). Initial amplitudes of tail currents were measured at −80 mV after 20-s pulses to different potentials, normalized to the maximum amplitude of the tail current, and plotted as a function of prepulse potential.
Another effect of the D3Y mutation was to reverse the direction of instantaneous current rectification. This can be observed in Fig. 5, A and B, as a marked increase in the ratio between the activated outward current and the initial inward tail current elicited in response to an 8-s voltage-clamp step to 40 mV followed by a hyperpolarizing step to −80 mV. This ratio was 1.77 for oocytes expressing D3Y and 0.545 for oocytes expressing Cx46 (Table 1). The reversal in the direction of current rectification suggests that the D3Y mutation may be altering charge selectivity.
Table 1.
Cx46D3Y shows alterations in current rectification
| Cell 1 | Iss (40 mV)/Itail(−80 mV) | n |
|---|---|---|
| Cx46 | 0.545 ± 0.079 | 6 |
| Cx46 + Cx50 | 0.307 ± 0.13* | 5 |
| D3Y | 1.77 ± 0.0924* | 3 |
| D3Y + Cx46 | 1.27 ± 0.1985* | 4 |
| D3Y + Cx50 | 0.677 ± 0.0627† | 6 |
| L11S + Cx46 | 0.545 ± 0.087 | 4 |
| L11S + Cx50 | 0.157 ± 0.0297‡ | 5 |
Values are means ± SD. Cx, connexin; Iss, steady-state current; Itail, tail current.
P < 0.005 compared with Cx46 cRNA-injected oocytes;
P < 0.005 compared with Cx46 + Cx50 cRNA-injected oocytes;
P < 0.016 compared with Cx46 + Cx50 cRNA-injected oocytes.
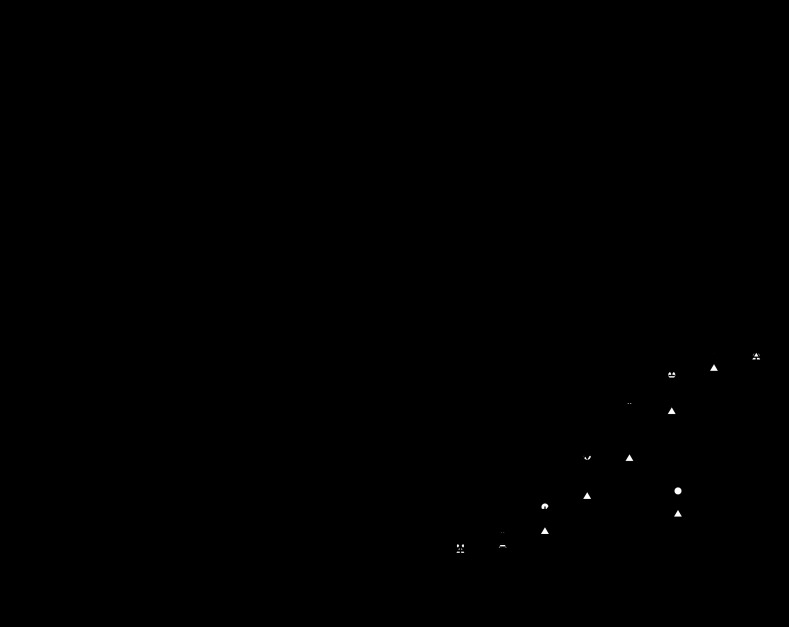
Using a voltage-clamp ramp protocol, the measured reversal potential, Vrev, of wild-type Cx46 in 89 mM NaCl solution was −13.3 ± 0.6 mV (n = 3). Total replacement of NaCl with KCl caused Vrev to shift to −6.9 ± 0.92 mV (n = 3). In contrast, D3Y had a Vrev of −15.5 ± 0.4 (n = 3) and −16.3 ± 0.74 (n = 3) mV in NaCl and KCl solutions, respectively. To investigate the effect of the D3Y on charge selectivity, reversal potential values were measured as a function of external KCl concentration (Fig. 6). The reversal potential, Vrev, of the Cx46 hemichannel current shifted to more positive values with increasing KCl concentration, indicating that the Cx46 channel is primarily permeable to cations as previously reported (35). In contrast, Vrev of the D3Y current shifted to more negative values with increasing KCl concentration, indicating that the D3Y channel is more permeable to anions than cations. The data could be described by the Goldman-Hodgkin-Katz (GHK) constant field equation with permeability ratios of 1:0.5:0.4 for K+:Na+:Cl−. In contrast, Vrev of the D3Y current shifted to more negative values with increasing KCl concentration, indicating that the D3Y channel is more permeable to anions than cations. The direction and amplitude of the shifts in Vrev could be described by the GHK constant field equation with permeability ratios of 1:0.5:2.8 for K+:Na+:Cl−.
Fig. 6.
Summary of the reversal potentials (Vrev) of Cx46 (A) and D3Y (B) in external solutions containing different KCl concentrations. Symbols represent means of measured reversal potentials ± SE (n = 3–8). The solid lines were calculated according to the Goldman-Hodgkin-Katz (GHK) equation. The dashed lines show the predictions for ideally cationic and anionic conductances. The intracellular permeant ion activity was assumed to be 93 mM for K+, 9.4 mM for Na+, and 35 mM for Cl− (12). Vrev was determined using a ramp protocol as described in methods.
The effect of coexpressing mutant with wild-type Cx46 on the gating properties of Cx46 hemichannel currents is shown in Fig. 7. Oocytes injected with a 1:1 mixture of D3Y or L11S and Cx46 cRNA developed hemichannel currents that were approximately half the size of hemichannel currents recorded from oocytes injected with similar concentrations of Cx46 alone. Comparison of the steady-state G-V curve for (D3Y+Cx46) to that of Cx46 shows that coexpression of D3Y with wild-type Cx46 caused a large shift in the G-V curve to more positive potentials and reduced the amount of instantaneous inward current rectification (Table 1). In contrast, coexpression of L11S with Cx46 resulted in only a small positive shift in the G-V curve and no change in current rectification.
Fig. 7.
Oocytes coinjected with D3Y and Cx46 induce large hemichannel currents with novel voltage gating properties. A and C: representative examples of hemichannel currents in single oocytes injected with D3Y + Cx46 (A) or L11S + Cx46 (C) cRNA. The bath solution contained choline chloride Ringer with zero added calcium. The hemichannel currents were recorded in response to a series of voltage-clamp steps from +80 mV to −100 mV in decrements of 20 mV followed by a short pulse to −80 mV. Holding potential = −10 mV. Data were corrected for leakage. Dashed line represents zero current. B and D: conductance-voltage relationships for D3Y + Cx46 (n = 4; B) and L11S + Cx46 (n = 4; D). Initial amplitudes of tail currents were measured at −80 mV after 20-s pulses to different potentials, normalized to the maximum amplitude of the tail current, and plotted as a function of prepulse potential. For comparison, the conductance-voltage curve for Cx46 (dashed line) is shown.
Figure 8 shows the effect of coexpressing D3Y or L11S with Cx50. Like Cx50, (D3Y+Cx50) and (L11S+Cx50) hemichannels had a high probability of opening near 0 mV and closed at large positive or negative potentials. However, the rate and extent of channel closure differed. (D3Y+Cx50) hemichannel currents deactivated faster and more completely than Cx50 or (L11S+Cx50) hemichannel currents at negative potentials. At large positive potentials, (D3Y+Cx50) hemichannels exhibited a biphasic response consisting of a rapid inactivation phase followed by a slower activation phase. In addition, mixing D3Y with Cx50 altered the degree of instantaneous rectification (Table 1), suggesting that the ionic permeability properties of the mixed channels may be different from those of Cx50.
Fig. 8.
Heteromeric (D3Y + Cx50) and (L11S + Cx50) hemichannels demonstrate altered gating properties. A–C: representative examples of hemichannel currents in single oocytes injected with Cx50 (A) or L11S + Cx50 (B) or D3Y + Cx50 (C) cRNA. The bath solution contained choline chloride Ringer with zero added calcium. The hemichannel currents were recorded in response to a series of voltage-clamp steps from +80 mV to −100 mV in decrements of 20 mV followed by a short pulse to −80 mV. Holding potential = −10 mV. Data were corrected for leakage. Dashed line represents zero current. D: conductance-voltage relationships for Cx50 (■ squares, n = 7), Cx50 + Cx46 (○, n = 6), D3Y + Cx50 (△, n = 5), L11S + Cx50 (▼, n = 4). Initial amplitudes of tail currents were measured at −80 mV after 20-s pulses to different potentials, normalized to the maximum amplitude of the tail current, and then plotted as a function of prepulse potential.
DISCUSSION
We have examined the effects of two cataract-associated mutations located in the NH2 terminus of Cx46: Cx46D3Y and Cx46L11S. Our results show that both of these mutations generated on a rat Cx46 backbone are able to oligomerize and traffic to the plasma membrane properly in transfected HeLa cells. However, they fail to form functional intercellular channels in Xenopus oocytes when paired homotypically. In addition, D3Y and L11S act as strong dominant negative inhibitors of wild-type Cx46 but not wild-type Cx50 gap junctional channels. Single oocytes expressing D3Y exhibit reduced hemichannel activity as well as alterations in voltage gating and charge selectivity, while oocytes expressing L11S show no hemichannel activity.
We chose to use rat Cx46 instead of its human counterpart because it generated higher levels of functional expression in oocytes than did human Cx46. Consequently, we were able to perform a more detailed functional analysis of the effects of the mutations than would have been otherwise possible. Comparison of the amino acid sequence of rat and human Cx46 shows that the NH2-terminal half of rat Cx46 is identical to human Cx46 up to amino acid residue 114, with the exception of a single conservative amino acid change at residue 43. This region of the protein corresponds to the NH2 terminus (NT), the first and second transmembrane domains (TM1, TM2), and the first extracellular domain (E1). Structural and functional studies have shown that the NH2-terminal half of Cx46 contains the main determinants for single-channel conductance, permeability, and voltage-dependent gating, with NT, TM1, and E1 forming the pore funnel (14, 15, 31, 33, 36). Therefore, we would expect rat and human Cx46 to have similar functional properties and predict point mutations within the NT of rat and human Cx46 to have analogous effects on channel function. In agreement with these structural predictions, biophysical studies of rat and human Cx46 have shown both connexins to form functional hemichannels that exhibit similar voltage-gating properties and calcium sensitivities (10, 11).
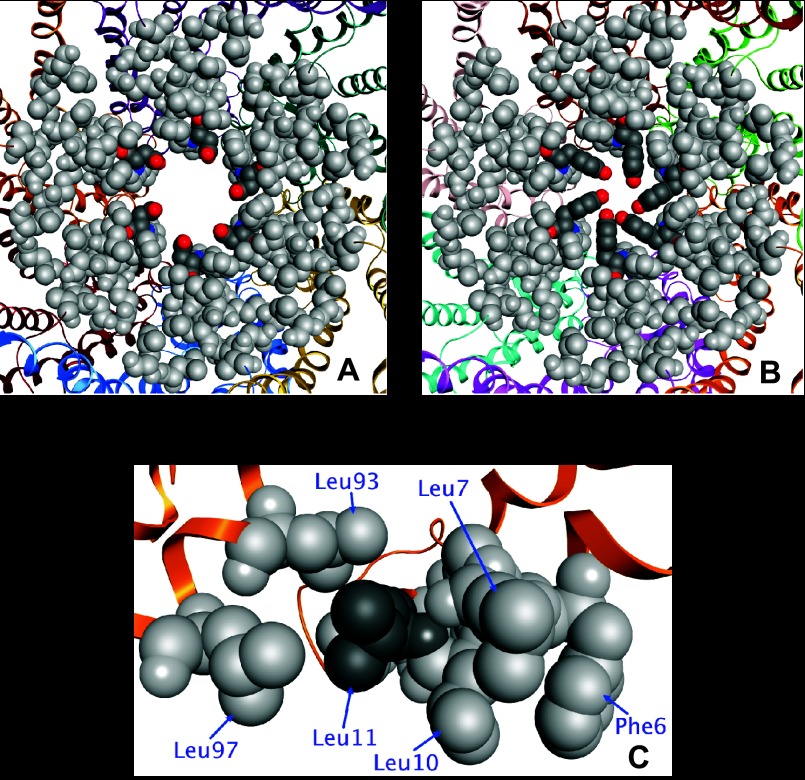
Structural studies on Cx26 have shown that the NH2 terminus bends into the pore and forms a funnel(17). We developed a homology model of rat Cx46 based on the crystal structure of the Cx26 gap junction to examine the effects of these mutations. The aspartic acid at residue 3 forms a ring of negatively charged side chains at the narrowest part of the hemichannel (Fig. 9). In the D3Y mutant, the narrowest part of the channel is predicted to be occluded by the larger tyrosine rings resulting in closure or partial closure of the channel in the absence of an applied voltage consistent with our experimental results. In contrast, Leu-11 sits at the center of a hydrophobic cluster surrounded by Phe-6, Leu-7, Leu-10, Leu-93, and Leu-97 (Fig. 9C). Mutation of Leu-11 to a serine would disrupt this cluster, and may easily lead to large-scale reorganization of this region and cause the experimentally observed loss of channel function. Since this hydrophobic cluster forms a portion of the interface between two long helices within each monomer, and between helices on adjacent monomers, the serine mutation could also have long-range effects on the conformation of the hemichannel.
Fig. 9.
A and B: effect of substituting tyrosine for Asp-3 in the homology-modeled structure of Cx46. Asp-3 forms a ring of negatively charged side chains at the narrowest part of the hemichannel in wild-type Cx46 (A). In the D3Y mutant, the narrowest part of the channel is occluded by the larger tyrosine rings (B). Residues 1–10 and 12–14 are shown as light gray space filling structures; residue 3 is shown as dark gray space filling structures, with oxygen in red and nitrogen in blue. All hydrogens are omitted for clarity. C: effect of substituting serine for Leu-11 in the homology-modeled structure of Cx46. Leu-11 sits at the center of a hydrophobic cluster, surrounded by Phe-6, Leu-7, Leu-10, Leu-93, and Leu-97. Mutation of Leu-11 to a serine would disrupt this cluster, and may easily lead to large-scale reorganization of this region. Since this cluster forms a portion of the interface between two long helices within each monomer, and between helices on adjacent monomers, the serine mutation could also have long-range effects on the conformation of the hemichannel.
Gating properties and charge selectivity of D3Y.
Electrophysiological studies suggest that connexin hemichannels and gap junctional channels have two gating mechanisms that can close the channels (21, 36, 37). One gating mechanism, termed the Vj gate, is associated with rapid gating transitions from the main open state to long-lasting substates. The polarity of Vj gating depends on charged amino acids in the amino terminus. This gate is proposed to be responsible for the closure of gap junctions to a residual conductance state during prolonged application of a transjunctional voltage gradient. The second voltage-gating mechanism closes the channels only at negative potentials. This gating mechanism is associated with very slow transitions from the main open state to the fully closed state. It is often referred to as “loop gating” because it resembles gating transitions that occur during docking and is thought to involve conformational changes in extracellular loops that can be modulated by external divalent cations (6, 11, 26, 31, 35, 36).
Previous studies have shown that the residue corresponding to D3 in Cx46 plays an important role in determining the polarity of fast Vj gating in Cx26 and Cx32 channels (21, 37). In addition, mutagenesis studies on Cx50 and Cx46 have shown that replacing the negatively charged aspartic acid at position 3 with a neutral asparagine disrupts hemichannel closure at positive potentials, reduces single channel conductance by 70%, and increases the number of full channel closures throughout the voltage range of ±70 mV (31). Recently, Xin et al. (41) reported a similar reduction in single-channel conductance and increase in transitions between the open and the fully closed state for a D-to-E substitution at residue 3 in Cx50. In this study, we show that substitution of a polar tyrosine at residue 3 for the negatively charged aspartic acid present in wild-type Cx46 results in a rightward shift in the activation curve and appears to disrupt hemichannel closure at positive potentials. In another recent report, Schlingmann et al. (29) found that this same D3Y substitution in human wild-type Cx46 resulted in the lack of voltage-sensitive hemichannels when expressed in Xenopus oocytes. The macroscopic gating properties of Cx46D3Y hemichannels resemble those previously reported for Cx46D3N. Furthermore, our studies on heterotypic Cx46/D3Y or Cx50/D3Y pairs revealed that the mutant can form functional gap junctional channels with markedly asymmetrical gating properties that resemble those previously reported for Cx46D3N/Cx46 and Cx50D3N/Cx50 gap junctions (31). At negative Vj relative to the D3Y-expressing cell, the gap junctional channels are mostly closed. Application of positive Vj results in channel opening. The voltage-gating properties of the heterotypic gap junctions show a remarkable resemblance to those of the nonjunctional D3Y hemichannel currents, indicating that the negative D3Y gate is mostly responsible for Vj gating regardless of whether D3Y is paired with Cx50 or Cx46. The lack of contribution of wild-type connexons to the gating properties of the heterotypic gap junctional channels suggests that the D3Y hemichannel may have a significantly lower unitary conductance than the wild-type hemichannels. Under these conditions, more of the Vj drop would occur across the D3Y hemichannel, resulting in an apparent increase in the Vj sensitivity of the D3Y hemichannel and a reduction in the Vj sensitivity of the wild-type hemichannel. A similar mechanism was first proposed to account for the voltage-gating properties of heterotypic gap junctions containing Cx45 (5). The complete absence of coupling in homotypic D3Y/D3Y oocyte pairs can be explained by the gating properties of D3Y hemichannels as follows. In the absence of a Vj difference, both D3Y hemichannels will be mostly closed. The probability of opening the gap junctional channels will be equal to the product of the probability of opening each component hemichannel. Even if a Vj difference is applied across the junction resulting in activation of the D3Y hemichannel on the positive side of the junction, the hemichannel on the negative side will remain closed. A similar mechanism has been proposed to account for the loss of function of the deafness-associated Cx26 mutation, M34T (30).
Another effect of D3Y was to alter the charge selectivity and current rectification of Cx46 hemichannels. Substitution of tyrosine for aspartic acid at position 3 resulted in a switch from cation to anion permeability and reversed the direction of current rectification from inward to outward. These results suggest that D3 contributes a negative charge to the pore lining that acts to concentrate cations near the entry of the channel and thereby increases the selectivity of the channel for cations. The large effect of the D-to-Y substitution can be accounted for by a localized narrowing of the pore in the vicinity of D3, which serves to increase the concentration of fixed negative charges and mobile cations. This interpretation is supported by the results of homology modeling studies that predict that D3 forms a ring of negatively charged side chains at the narrowest part of the channel. Previous modeling and experimental studies on Cx26 and Cx32*43E1 indicate that the charged residue corresponding to D3 in Cx46 may play a similar role in determining charge selectivity in other connexin hemichannels (16, 23).
Interactions between mutant and wild-type lens connexins.
Our results suggest that D3Y and L11S act as strong dominant negative inhibitors of wild-type Cx46 but not wild-type Cx50 gap junctional coupling when coexpressed in Xenopus oocytes. These differences may be explained by alterations in gating properties. Heteromeric (D3Y+Cx46) and (L11S+Cx46) hemichannels have a significantly reduced probability of channel opening at Vm ≤ 0 mV compared with Cx46 hemichannels, Thus, in agreement with our experimental results, coexpression of D3Y or L11S with Cx46 would be expected to inhibit Cx46 gap junctional channel activity. Alternative explanations for the marked reduction in coupling in heteromeric (D3Y+Cx46) and (L11S+Cx46) cRNA-injected oocyte pairs include the following: reduction in unitary gap junctional conductance; inability of the channels to dock properly; and inability of the channels to open after docking. In addition to modifying gating, coexpression of D3Y with Cx46 or Cx50 altered the degree of instantaneous current rectification. These alterations in current rectification indicate that the mixed channels may have altered permaselectivity properties, potentially resulting in metabolic imbalance and contributing to the formation of cataracts in the lens.
Mechanism of cataract formation.
The lens has been proposed to have an internal microcirculation system that delivers nutrients to and removes waste products from its core (18). Gap junctions are key components of this system, serving to interconnect all of the cells of the lens. Another critical component of this system is a sodium leak conductance that allows sodium to enter fiber cells from the extracellular spaces. The molecular identity of the sodium leak conductance remains unclear. One possibility is that it is due to connexin hemichannels. Since the D3Y and L11S mutations cause loss and/or reduction of hemichannel activity as well as gap junctional channel activity, they could potentially disrupt circulating current flow in the lens by either or both of these mechanisms, resulting in metabolic imbalance and the development of cataracts. Our data also suggest that the connexin mutations could interact with wild-type Cx50 to form mixed gap junctional channels and hemichannels with altered permaselectivity properties that potentially contribute to the formation of cataracts as well. Differences in the gating or permeability properties of mixed channels formed by wild-type lens connexins and D3Y or L11S may also explain the phenotypic differences in cataract type observed in people who inherit these mutations.
GRANTS
This work was supported by National Institutes of Health, National Eye Institute Grant RO1 EY-10589 (to L. Ebihara).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.-J.T. and L.E. conception and design of research; J.-J.T., B.C.S., A.L., D.E.W., and B.M.V. performed the experiments; J.-J.T., D.E.W., B.M.V., and L.E. analyzed the data; J.-J.T., D.E.W., B.M.V., and L.E. interpreted the results of the experiments; J.-J.T., B.C.S., A.L., D.E.W., B.M.V., and L.E. approved the final version of the manuscript; B.C.S., D.E.W., B.M.V., and L.E. edited and revised the manuscript; D.E.W., B.M.V., and L.E. prepared the figures; L.E. drafted the manuscript.
Footnotes
This article is the topic of an Editorial Focus by Thomas W. White (39).
REFERENCES
- 1. Addison PK, Berry V, Holden KR, Espinal D, Rivera B, Su H, Srivastava AK, Bhattacharya SS. A novel mutation in the connexin 46 gene (GJA3) causes autosomal dominant zonular pulverulent cataract in a Hispanic family. Mol Vis 12: 791–795, 2006 [PubMed] [Google Scholar]
- 2. Arora A, Minogue PJ, Liu X, Addison PK, Russel-Eggitt I, Webster AR, Hunt DM, Ebihara L, Beyer EC, Berthoud VM, Moore AT. A novel connexin50 mutation associated with congenital nuclear pulverulent cataracts. J Med Genet 45: 155–160, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arora A, Minogue PJ, Liu X, Reddy MA, Ainsworth JR, Bhattacharya SS, Webster AR, Hunt DM, Ebihara L, Moore AT, Beyer EC, Berthoud VM. A novel GJA8 mutation is associated with autosomal dominant lamellar pulverulent cataract: further evidence for gap junction dysfunction in human cataract. J Med Genet 43: e2, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berthoud VM, Minogue PJ, Guo J, Williamson EK, Xu X, Ebihara L, Beyer EC. Loss of function and impaired degradation of a cataract-associated mutant connexin50. Eur J Cell Biol 82: 209–221, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bukauskas FF, Angele AB, Verselis VK, Bennett MV. Coupling asymmetry of heterotypic connexin 45/connexin 43-EGFP gap junctions: properties of fast and slow gating mechanisms. Proc Natl Acad Sci USA 99: 7113–7118, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bukauskas FF, Elfgang C, Willecke K, Weingart R. Biophysical properties of gap junction channels formed by mouse connexin40 in induced pairs of transfected human HeLa cells. Biophys J 68: 2289–2298, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DeRosa AM, Xia CH, Gong X, White TW. The cataract-inducing S50P mutation in Cx50 dominantly alters the channel gating of wild-type lens connexins. J Cell Sci 120: 4107–4116, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Dong L, Liu X, Li H, Vertel BM, Ebihara L. Role of the N-terminus in permeability of chicken connexin45.6 gap junctional channels. J Physiol 576: 787–799, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ebihara L. Expression of gap junction proteins in Xenopus oocyte pairs. In: Ion Channels, edited by Rudy B, Iverson LE. San Diego, CA: Academic, 1992, p. 376–380 [DOI] [PubMed] [Google Scholar]
- 10. Ebihara L, Liu X, Pal JD. Effect of external magnesium and calcium on human connexin46 hemichannels. Biophys J 84: 277–286, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ebihara L, Steiner E. Properties of a nonjunctional current expressed from a rat connexin46 cDNA in Xenopus oocytes. J Gen Physiol 102: 59–74, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gunzel D, Kucharski LM, Kehres DG, Romero MF, Maguire ME. The MgtC virulence factor of Salmonella enterica serovar Typhimurium activates Na+,K+-ATPase. J Bacteriol 188: 5586–5594, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hansen L, Yao W, Eiberg H, Funding M, Riise R, Kjaer KW, Hejtmancik JF, Rosenberg T. The congenital “ant-egg” cataract phenotype is caused by a missense mutation in connexin46. Mol Vis 12: 1033–1039, 2006 [PubMed] [Google Scholar]
- 14. Kronengold J, Srinivas M, Verselis VK. The N-terminal half of the connexin protein contains the core elements of the pore and voltage gates. J Membr Biol 245: 453–463, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kronengold J, Trexler EB, Bukauskas FF, Bargiello TA, Verselis VK. Single-channel SCAM identifies pore-lining residues in the first extracellular loop and first transmembrane domains of Cx46 hemichannels. J Gen Physiol 122: 389–405, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kwon T, Harris AL, Rossi A, Bargiello TA. Molecular dynamics simulations of the Cx26 hemichannel: evaluation of structural models with Brownian dynamics. J Gen Physiol 138: 475–493, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature 458: 597–602, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Mathias RT, Kistler J, Donaldson P. The lens circulation. J Membr Biol 216: 1–16, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Minogue PJ, Liu X, Ebihara L, Beyer EC, Berthoud VM. An aberrant sequence in a connexin46 mutant underlies congenital cataracts. J Biol Chem 280: 40788–40795, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Minogue PJ, Tong JJ, Arora A, Russell-Eggitt I, Hunt DM, Moore AT, Ebihara L, Beyer EC, Berthoud VM. A mutant connexin50 with enhanced hemichannel function leads to cell death. Invest Ophthalmol Vis Sci 50: 5837–5845, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oh S, Abrams CK, Verselis VK, Bargiello TA. Stoichiometry of transjunctional voltage-gating polarity reversal by a negative charge substitution in the amino terminus of a connexin32 chimera. J Gen Physiol 116: 13–31, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oh S, Rubin JB, Bennett MV, Verselis VK, Bargiello TA. Molecular determinants of electrical rectification of single channel conductance in gap junctions formed by connexins 26 and 32. J Gen Physiol 114: 339–364, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oh S, Verselis VK, Bargiello TA. Charges dispersed over the permeation pathway determine the charge selectivity and conductance of a Cx32 chimeric hemichannel. J Physiol 586: 2445–2461, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pal JD, Berthoud VM, Beyer EC, Mackay D, Shiels A, Ebihara L. Molecular mechanism underlying a Cx50-linked congenital cataract. Am J Physiol Cell Physiol 276: C1443–C1446, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Pal JD, Liu X, Mackay D, Shiels A, Berthoud VM, Beyer EC, Ebihara L. Connexin46 mutations linked to congenital cataract show loss of gap junction channel function. Am J Physiol Cell Physiol 279: C596–C602, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Pfahnl A, Dahl G. Gating of Cx46 gap junction hemichannels by calcium and voltage. Pflügers Arch 437: 345–353, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Pfenniger A, Wohlwend A, Kwak BR. Mutations in connexin genes and disease. Eur J Clin Invest 41: 103–116, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Purnick PE, Benjamin DC, Verselis VK, Bargiello TA, Dowd TL. Structure of the amino terminus of a gap junction protein. Arch Biochem Biophys 381: 181–190, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Schlingmann B, Schadzek P, Busko S, Heisterkamp A, Ngezahayo A. Cataract-associated D3Y mutation of human connexin46 (hCx46) increases the dye coupling of gap junction channels and suppresses the voltage sensitivity of hemichannels. J Bioenerg Biomembr 44: 607–614, 2012 [DOI] [PubMed] [Google Scholar]
- 30. Skerrett IM, Di WL, Kasperek EM, Kelsell DP, Nicholson BJ. Aberrant gating, but a normal expression pattern, underlies the recessive phenotype of the deafness mutant Connexin26M34T. FASEB J 18: 860–862, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Srinivas M, Kronengold J, Bukauskas FF, Bargiello T, Verselis V. Correlative studies of gating in Cx46 and Cx50 hemichannels and gap junction channels. Biophys J 88: 1725–1739, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thomas BC, Minogue PJ, Valiunas V, Kanaporis G, Brink PR, Berthoud VM, Beyer EC. Cataracts are caused by alterations of a critical N-terminal positive charge in connexin50. Invest Ophthalmol Vis Sci 49: 2549–2556, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tong JJ, Ebihara L. Structural determinants for the differences in voltage gating of chicken Cx56 and Cx45.6 gap-junctional hemichannels. Biophys J 91: 2142–2154, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tong JJ, Minogue PJ, Guo W, Chen TL, Beyer EC, Berthoud VM, Ebihara L. Different consequences of cataract-associated mutations at adjacent positions in the first extracellular boundary of connexin50. Am J Physiol Cell Physiol 300: C1055–C1064, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trexler EB, Bennett MV, Bargiello TA, Verselis VK. Voltage gating and permeation in a gap junction hemichannel. Proc Natl Acad Sci USA 93: 5836–5841, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Trexler EB, Bukauskas FF, Kronengold J, Bargiello TA, Verselis VK. The first extracellular loop domain is a major determinant of charge selectivity in connexin46 channels. Biophys J 79: 3036–3051, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Verselis VK, Ginter CS, Bargiello TA. Opposite voltage gating polarities of two closely related connexins. Nature 368: 348–351, 1994 [DOI] [PubMed] [Google Scholar]
- 38. White TW, Bruzzone R, Wolfram S, Paul DL, Goodenough DA. Selective interactions among the multiple connexin proteins expressed in the vertebrate lens: the second extracellular domain is a determinant of compatibility between connexins. J Cell Biol 125: 879–892, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. White TW. Is half of a lens gap junction channel better than none? Focus on “Properties of two cataract-associated mutations located in the NH2 terminus of connexin 46.” Am J Physiol Cell Physiol (February 7, 2013). doi:10.1152/ajpcell.00035.2013 [DOI] [PubMed] [Google Scholar]
- 40. Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem 383: 725–737, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Xin L, Nakagawa S, Tsukihara T, Bai D. Aspartic acid residue D3 critically determines Cx50 gap junction channel transjunctional voltage-dependent gating and unitary conductance. Biophys J 102: 1022–1031, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu X, Ebihara L. Characterization of a mouse Cx50 mutation associated with the No2 mouse cataract. Invest Ophthalmol Vis Sci 40: 1844–1850, 1999 [PubMed] [Google Scholar]
- 43. Zoidl G, Dermietzel R. Gap junctions in inherited human disease. Pflügers Arch 460: 451–466, 2010 [DOI] [PubMed] [Google Scholar]