Abstract
Epidermal growth factor receptor (EGFR)-mediated signaling is required for optimal intestinal wound healing. Since n-3 polyunsaturated fatty acids (PUFA), specifically docosahexaenoic acid (DHA), alter EGFR signaling and suppress downstream activation of key signaling pathways, we hypothesized that DHA would be detrimental to the process of intestinal wound healing. Using a mouse immortalized colonocyte model, DHA uniquely reduced EGFR ligand-induced receptor activation, whereas DHA and its metabolic precursor eicosapentaenoic acid (EPA) reduced wound-induced EGFR transactivation compared with control (no fatty acid or linoleic acid). Under wounding conditions, the suppression of EGFR activation was associated with a reduction in downstream activation of cytoskeletal remodeling proteins (PLCγ1, Rac1, and Cdc42). Subsequently, DHA and EPA reduced cell migration in response to wounding. Mice were fed a corn oil-, DHA-, or EPA-enriched diet prior to intestinal wounding (2.5% dextran sodium sulfate for 5 days followed by termination after 0, 3, or 6 days of recovery). Mortality was increased in EPA-fed mice and colonic histological injury scores were increased in EPA- and DHA-fed mice compared with corn oil-fed (control) mice. Although kinetics of colonic EGFR activation and downstream signaling (PLCγ1, Rac1, and Cdc42) were delayed by both n-3 PUFA, colonic repair was increased in EPA- relative to DHA-fed mice. These results indicate that, during the early response to intestinal wounding, DHA and EPA uniquely delay the activation of key wound-healing processes in the colon. This effect is mediated, at least in part, via suppression of EGFR-mediated signaling and downstream cytoskeletal remodeling.
Keywords: colonic wounding, epithelial restitution, epidermal growth factor receptor, n-3 polyunsaturated fatty acids
intestinal wound healing requires a delicate balance of migration, proliferation, and differentiation of epithelial cells (36). The first step of wound healing is epithelial restitution, in which epithelial cells adjacent to the wound rapidly migrate into the wounded area (11, 12, 48). Restitution begins quickly, within minutes to hours, following injury, and this process has been shown to be independent of cell proliferation (11, 48). The next step of wound healing, which begins hours to days after injury, requires proliferation of the mucosal epithelium to increase the number of enterocytes available to repopulate the injured area (11, 26). The final stage of wound healing requires maturation and differentiation of epithelial cells to restore and maintain intestinal function (48).
Many pathways that are hijacked by cancer cells are integral to the process of wound healing. Importantly, EGF receptor (EGFR) signaling is involved in all the stages of intestinal wound healing. It has been clearly demonstrated that treatment with EGFR ligands, including EGF, heparin-binding EGF, and transforming growth factor (TGF)-α, promotes intestinal wound healing (15, 17). Additionally, in response to a wounding event, EGFR becomes activated. This activation is regulated in part by G protein-coupled receptors, which signal to activate matrix metalloproteinases (MMPs) (55). Multiple downstream signaling pathways from EGFR are central in regulating distinct stages of wound healing. Restitution requires extensive reorganization of the actin cytoskeleton through activation of Rho family small GTPases, including Rac1 and Cdc42 (19, 23). Induction of Rac1 and Cdc42 facilitates actin polymerization (13, 43). It has been clearly demonstrated that EGFR activates Rac1 and Cdc42 through multiple downstream signaling cascades, including phosphatidylinositol 3-kinase and Src (12, 16), and EGFR-mediated activation of these mediators is imperative for wound healing. PLCγ1 is also a downstream signaling partner of EGFR that mediates intestinal epithelial cell migration (30, 41). PLCγ1 and Rac1 directly interact to coregulate cytoskeletal remodeling and cell migration in response to EGF (30).
We previously demonstrated that a dietary long-chain n-3 PUFA, docosahexaenoic acid (DHA), suppresses EGFR signaling in mouse colonocytes (50). By altering the plasma membrane localization of EGFR, DHA increased receptor phosphorylation but paradoxically suppressed downstream activation of Ras, ERK1/2, and STAT3. DHA has also been shown to exert similar effects on EGFR-dependent activation of Ras and ERK1/2 activation in several cancer cell lines (42). On the basis of the central role of EGFR-mediated signaling in wound healing, we hypothesized that DHA would be detrimental with respect to intestinal wound healing. This postulate is supported by a recent study conducted by our laboratory demonstrating that mice fed a diet enriched in fish oil containing DHA and its precursor eicosapentaenoic acid (EPA) exhibited reduced overall survival and increased wounding following treatment with dextran sodium sulfate (DSS) (27). An additional study conducted in humans demonstrated that wound healing of the skin is delayed in patients dosed with a combination EPA and DHA (33). Conversely, some studies have demonstrated a beneficial effect of n-3 PUFA in response to injury (18, 25). These conflicting data warrant further investigation into the mechanisms by which n-3 PUFA modulate wound healing.
MATERIALS AND METHODS
Cell culture.
Conditionally immortalized young adult mouse colonic (YAMC) cells were obtained from R. H. Whitehead (Ludwig Cancer Institute, Melbourne, Australia). YAMC cells (passages 12–17) were cultured under permissive conditions, 33°C and 5% CO2, in RPMI 1640 medium (Mediatech, Manassas, VA) supplemented with 5% FBS (Hyclone, Logan, UT), 2 mM GlutaMAX (GIBCO, Grand Island, NY), 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml selenious acid (Collaborative Biomedical Products, Bedford, MA), and 5 IU/ml murine IFNγ (Roche Applied Science, Mannheim, Germany). Select cultures were treated for 72 h with 50 μM fatty acid [DHA, linoleic acid (LA, 18:2n-6), or EPA; NuChek, Elysian, MN] complexed with bovine fatty acid-free serum albumin (BSA; Roche Applied Science). In select cultures, for the final 16–18 h, complete medium was replaced with low-serum (0.5% FBS) medium. Cells were then stimulated with 0–25 ng/ml recombinant mouse EGF (Sigma, St. Louis, MO) and harvested. For preparation of whole cell extracts of wounded cells, cells were grown to confluence in 150-mm dishes. Monolayers were cross-scraped 25 times in all directions (horizontal, vertical, and diagonals; 100 scrapes total) with a 1-ml pipette tip, as previously described (14). Monolayers were then gently washed three times with ice-cold PBS and harvested.
Western blotting.
For Western blotting, cells were homogenized in ice-cold homogenization buffer (50 mM Tris·HCl, pH 7.2, 250 mM sucrose, 2 mM EDTA, 1 mM EGTA, 50 mM sodium fluoride, 100 mM sodium orthovanadate, 1% Triton X-100, 100 μM activated sodium orthovanadate, 10 mM β-mercaptoethanol, and protease inhibitor cocktail), as previously described (10). After homogenization, lysates were sheared using a 29-gauge needle, incubated on ice for 30 min, and centrifuged at 16,000 g for 20 min. The supernatant was collected, and protein concentration was assessed using Pierce Coomassie Plus Protein assay (Thermo Fisher Scientific, Rockford, IL). Lysates were treated with 1× pyronin sample buffer and subjected to SDS-PAGE in precast 4–20% Tris-glycine mini gels (Invitrogen, Carlsbad, CA). After electrophoresis, proteins were electroblotted onto a polyvinylidene difluoride membrane with the use of a Hoefer Mighty Small Transphor unit at 400 mA for 90 min. After transfer, the membrane was incubated in 5% IgG-free BSA (Roche Applied Science) and 0.1% Tween 20 in Tris-buffered saline (TBST) at room temperature for 1 h with shaking and then with primary antibody diluted in 5% BSA in TBST at 4°C overnight with shaking. Membranes were washed with TBST and incubated with peroxidase-conjugated secondary antibody according to the manufacturer's instructions. Bands were developed using Pierce SuperSignal West Femto maximum-sensitivity substrate. Blots were scanned using a Fluor-S Max MultiImager system (Bio-Rad, Hercules, CA). Bands were quantified using Quantity One software (Bio-Rad). Monoclonal rabbit anti-EGFR (catalog no. 2646), anti-phosphorylated (Tyr1068) EGFR (catalog no. 3777), anti-PLCγ1 (catalog no. 2822), and anti-phosphorylated (Tyr783) PLCγ1 (catalog no. 2821) were purchased from Cell Signaling. Peroxidase-conjugated goat anti-rabbit IgG was purchased from Kirkegaard and Perry Laboratories (Gaithersburg, MD).
Small Rho GTPase activity assay.
Activation of Cdc42 and Rac1 was assessed using kits from Cytoskeleton (Denver, CO). Samples for these assays were harvested as described above using the lysis buffer provided with the kits and supplemented with protease and phosphatase inhibitors (Sigma). Activation of Cdc42 and Rac1 was analyzed using G-LISA Cdc42 and Rac1 Activation Assay Biochem kits, respectively, in the colorimetric format. The assays were performed using 25 μg of protein according to the manufacturer's instructions. Absorbance was measured on a microplate reader (SpectraMax 190, Molecular Devices, Sunnyvale, CA).
Scratch assay.
YAMC cells in T-75 flasks were untreated or treated with 50 μM fatty acid (LA, DHA, or EPA) for 24 h. Cells were then trypsinized and seeded at a density of 1 × 105 cells/ml into 35-mm glass-bottom dishes. Cells were cultured for an additional 48 h in the presence of fatty acid and serum-starved (0.5% FBS) for the final 16–18 h. The serum-starved cells were washed once with PBS and scratched using a sterile P-200 pipette tip. Cells were then washed twice with PBS and incubated with serum-free medium supplemented with 25 ng/ml EGF. Cells were then imaged with a ×10 Plan Fluor phase objective on a Nikon (Tokyo, Japan) TiE inverted microscope equipped with a Perfect Focus system to maintain focus over time and an incubation chamber at 33°C with 5% CO2. Images were taken with a Photometrics CoolSNAP HQ2, 14-bit, 20-MHz, monochrome cooled charge-coupled device camera. Image acquisition and analysis were performed using NIS Elements AR software (Nikon). Images were acquired every 15 min for 24 h to observe wound healing. Wound healing was quantified by counting the number of cells that infiltrated the wounded area at 12 and 24 h following the wounding event.
Transwell migration assay.
Cell migration was assessed using the QCM 24-well colorimetric cell migration assay (Millipore, Billerica, MA). YAMC cells were seeded into T-75 flasks and untreated or treated with fatty acid (LA, DHA, or EPA) for 72 h. For the final 16–18 h of treatment, cells were serum-starved (0.5% FBS). Cells were then trypsinized and seeded onto inserts at a density of 0.5 × 106 cells/ml in serum-free medium. The inserts, composed of membranes with 8-μm pores through which cells can migrate, were set into wells containing medium supplemented with 25 ng/ml EGF, and the membrane of the insert was placed into contact with the medium. Cultures were incubated for 12 h at 33°C and 5% CO2 and allowed to migrate through the membrane. After incubation, cells on the top side of the insert were thoroughly removed, and the migration insert was stained according to kit instructions. Subsequently, the stain was eluted, and dye mixture was added to a 96-well plate. The optical density was measured at 560 nm on a microplate reader (SpectraMax 190, Molecular Devices).
Bioenergetic profile.
Cellular bioenergetic profiles were measured using an extracellular flux analyzer (model XF24, Seahorse Bioscience, North Billerica, MA). YAMC cells were seeded at a density of 3.0 × 104 cells/well onto 24-well cell culture plates (catalog no. XF, Seahorse Bioscience) and cultured overnight in complete medium at 33°C and 5% CO2. Cells were then placed into unbuffered assay medium (DMEM supplemented with 11 mM glucose, 2 mM glutamine, and 1 mM pyruvate) and incubated in a CO2-free environment at 33°C for 1 h. After incubation, the 24-well culture plates were transferred to the extracellular flux analyzer. Hydrated cartridges containing mitochondrial mediators, oligomycin (10 μmol/l), carbonylcyanide p-trifluoromethoxyphenylhydrazone (3 μmol/l), and rotenone (1 μmol/l) were added. The compounds were injected at timed intervals into the wells (final concentration of mitochondrial mediators in each well was 1/10th of the initial concentration), and oxygen consumption rate was monitored continuously.
Animals.
Male C57BL/6 wild-type mice aged 12–15 wk were purchased from Jackson Laboratories. All procedures followed guidelines approved by the US Public Health Service and were approved by the Institutional Animal Care and Use Committee at Texas A & M University. Mice were maintained under barrier conditions and initially consumed a Teklad commercial mouse nonpurified diet (Harlan, Indianapolis, IN) ad libitum. Subsequently, mice were divided into groups and, for 15–21 days, fed one of three isocaloric semipurified diets that were adequate in all nutrients but differed in their lipid compositions: 3% corn oil (CO) control diet (Dyets, Bethlehem, PA), DHA-enriched (1% DHA + 2% CO) diet, or EPA-enriched (1% EPA + 2% CO) diet. Highly purified lipid sources were utilized, i.e., DHA ethyl ester (>70% pure; Incromega DHA700E SR, Bioriginal Food and Science, Saskatchewan, SK, Canada) and EPA-free fatty acid (>95% pure; SLA Pharma, Watford, UK). Diet composition (g/kg diet) was as follows: 440 sucrose, 200 casein, 220 cornstarch, 3 dl-methionine, 35 AIN-76 salt mix, 10 AIN-76 mineral mix, 2 choline chloride, and 60 cellulose (Bio-Serv) + 30 CO (CO diet), 10 DHA + 20 CO (DHA diet), or 10 EPA + 20 CO (EPA diet). Diets were replaced daily. Animals were fed the experimental diets for 10 days prior to treatment with 2.5% DSS (36,000–50,000 mol wt; MP Biomedicals, Santa Ana, CA) in the drinking water for 5 days. Some animals suffered adverse symptoms due to the nature of disease progression [inflammatory bowel disease (IBD)] that merited euthanasia. The mice were monitored for lack of fecal pellets, as well as overt appearance of distress. Any animal showing signs of physical distress was removed to an individual cage and monitored. Animals that did not groom, displayed lack of mobility, had multiple days of rectal bleeding, or did not produce enough fecal pellets were terminated. After DSS treatment, mice were allowed to recover for 0, 3, or 6 days. At 2 h before termination, a subset of mice were injected with 2′-deoxy-5-ethynyl uridine (EdU) for assessment of cell proliferation, as previously described (32). Mice were terminated using CO2 asphyxiation. The colon was removed and cut longitudinally in half. One half of the colon was Swiss-rolled, fixed in 4% paraformaldehyde, and embedded in paraffin, and cross sections were stained with hematoxylin-eosin and analyzed in a blinded manner by a board-certified pathologist (B. Weeks). Epithelial injury (score of 0–3) or inflammation (i.e., immune cell infiltration, score of 0–3) was scored in accordance with criteria reported elsewhere (27). Additionally, epithelial repair (score of 0–3) was scored according to the following criteria: no histological evidence of unexpected epithelial regeneration or repair was assigned a score of 0; minimal-to-mild histological evidence of increased epithelial regeneration/repair (e.g., increased number of mitotic figures in crypts and mild elongation of crypts) was assigned a score of 1; mild-to-moderate histological evidence of epithelial repair (e.g., crypt elongation and mild tortuosity, increased mitotic index, mild multifocal flattening/squamification of mucosa adjacent to and extending into areas of injury, and evidence of fibroplasia in the subjacent submucosa) was assigned a score of 2; moderate-to-marked evidence of epithelial repair (e.g., extensive crypt elongation with tortuosity and squamification of mucosa in regions adjacent to and extending to injury, marked fibroplasia within the submucosa, or high mitotic index in residual crypt epithelium) was assigned a score of 3. Unstained cross sections were additionally analyzed for proliferation using the Click-IT EdU kit (Invitrogen) or for apoptosis using the TACS 2 TdT-mediated dUTP nick-end labeling assay (Trevigen, Gaithersburg, MD) according to the manufacturer's instructions. Colonic mucosal scrapings were collected from the other longitudinal half of the colon and used for RNA or protein analyses. For protein isolation, mucosal scrapings were combined with lysis buffer from the Rac1 activation assay buffer. Lysates were sheared by passage through a 27-gauge needle, incubated on ice for 30 min, and then centrifuged at 16,000 g for 20 min. Supernatants were collected, and protein concentration was determined using the Coomassie Protein Plus assay (Pierce).
Colonic mucosal mRNA expression.
RNA was isolated from colonic mucosal scrapings (n = 4 per dietary group no-DSS controls or n = 6–8 per dietary group at all recovery times post-DSS) using the RNA 4-PCR kit (Life Technologies, Carlsbad, CA). Real-time RT-PCR was used to quantify mRNA expression, and amplification was performed using TaqMan Universal PCR master mix (Life Technologies), as described previously (34). TaqMan gene expression kits (Life Technologies) were used for amplification, namely, IL-1β (Mm00434228_m1), IL-6 (Mm00446190_m1), IFNγ (Mm01168134_m1), TNFα (Mm00443260_g1), CCL2 [monocyte chemoattractant protein 1 (MCP-1), Mm00441242_m1], IL-10 (Mm00439614_m1), TGFβ1 (Mm01178820_m1), IL-4 (Mm00445259_m1), IL-13 (Mm00434204_m1), and IL-22 (Mm01226722_g1). Amplification of mRNA (fluorescence) was recorded over 40 cycles, and the corresponding cycle numbers (Ct) were used to calculate mRNA expression as follows: 2(40 − Ct) Target gene expression was normalized to ribosomal 18S expression (Mm03928990_g1).
Fatty acid analysis.
For assessment of dietary lipid incorporation into membrane phospholipids, lipids were extracted from scraped colonic mucosa with 2:1 (vol/vol) chloroform-methanol. Total phospholipids were subsequently separated by thin-layer chromatography with 90:8:1:0.8 (vol/vol/vol/vol) chloroform-methanol-acetic acid-water. After transesterification, fatty acid methyl esters were quantified by capillary gas chromatography-mass spectrometry (45).
Statistics.
The effect of independent variables (treatment effects) was assessed using one-way ANOVA, and differences among means were evaluated using Tukey's post hoc test of contrast or least-squares means. The effect of two independent variables was assessed using two-way ANOVA. Data sets not exhibiting a normal distribution were subjected to the Kruskal-Wallis test (χ2 approximation) followed, if justified, by the statistical probability outcome using Wilcoxon's two-sample test. P < 0.05 was considered statistically significant.
RESULTS
Wounding-induced EGFR transactivation.
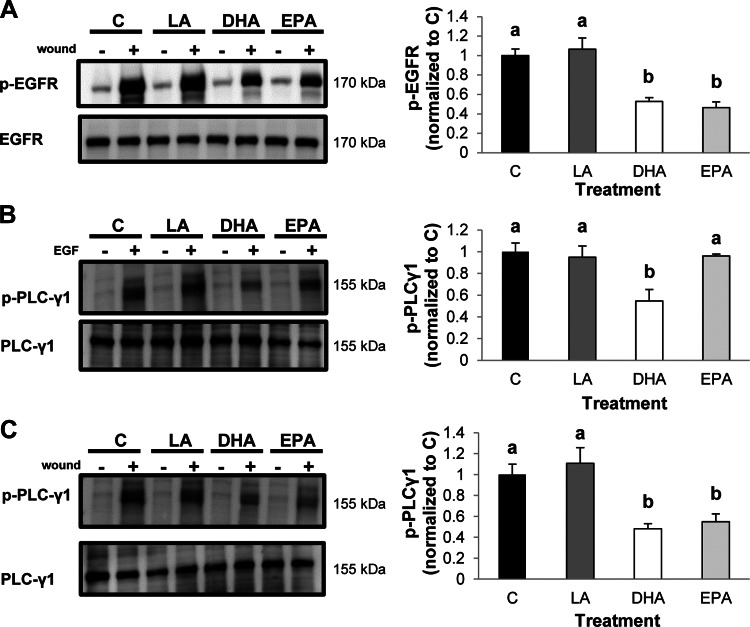
We previously showed that EGFR phosphorylation in response to direct stimulation with ligand is increased by DHA (50). However, other long-chain PUFA, including LA and EPA, did not recapitulate this same effect. To expand on these observations, we evaluated the effects of fatty acids on EGFR activation in response to injury. It has been reported that EGFR is transactivated in response to wounding (14). Therefore, we utilized a technique to wound colonic epithelial cells by extensively scratching a confluent monolayer of colonocytes. As expected, wounding strongly induced phosphorylation of EGFR in untreated control and LA-treated cells (Fig. 1A). Treatment of cells with DHA or EPA, however, reduced the injury-induced transactivation of EGFR. This observation is noteworthy, because DHA promotes ligand-induced EGFR phosphorylation, whereas EPA has no effect on phosphorylation of the receptor (50), indicating that n-3 PUFA may modulate EGFR activation in a context-specific manner.
Fig. 1.
Omega-3 polyunsaturated fatty acids (n-3 PUFA) suppress EGF receptor (EGFR)-dependent signaling in response to injury. Young adult mouse colonic (YAMC) cells were untreated [control (C)] or treated with 50 μM fatty acid [linoleic acid (LA), docosahexaenoic acid (DHA), or eicosapentaenoic acid (EPA)] for 72 h. For the final 16–18 h, cells were serum-starved (0.5% FBS); then they were subjected to extensive wounding (A and C) or stimulation with 25 ng/ml EGF (B). Subsequently, cells were incubated for 2 min and harvested, and cell lysates were collected. Total cell lysates were analyzed by Western blotting for phosphorylated (p-EGFR) and total EGFR (A) or phosphorylated (p- PLCγ1) and total PLCγ1 (B and C). Left: representative immunoblots. Two independent experiments were conducted. Right: quantification of band volumes using Quantity One software. Values are means ± SE (n = 4). At least 2 independent assays were conducted. Statistical difference (P < 0.05), as indicated by different letters (a and b), was measured using 1-way ANOVA and Tukey's test of contrast.
Activation of downstream signaling in response to EGF or injury.
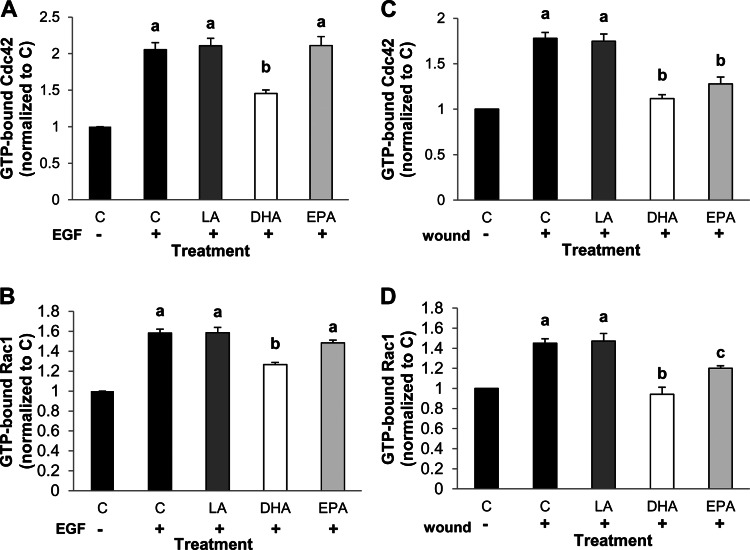
EGFR signaling is required to activate many important downstream mediators that stimulate cellular events required for wound healing. For example, EGFR-mediated activation of PLCγ1, Rac1, and Cdc42 has been shown to be integral in colonic wound healing (12, 16, 30). Therefore, we assessed activation of each of these signaling mediators in response to direct stimulation with an EGFR-specific ligand, EGF, or in response to injury. Initially, YAMC cells were stimulated with EGF or by wounding, and PLCγ1 activation status was assessed. Phosphorylated (Tyr783) PLCγ1 was quantified, because phosphorylation at Tyr783 is directly mediated by binding of PLCγ1 to EGFR (28). Addition of EGF enhanced phosphorylation of PLCγ1 in all treatment groups. However, PLCγ1 activation in DHA-treated cells was significantly reduced relative to all other treatment groups (Fig. 1B). This is consistent with our previous observation that DHA reduces EGF-stimulated activation of ERK1/2 and STAT3 (50). In contrast, EPA treatment had no effect on EGF-stimulated PLCγ1 compared with control or LA-treated cells. Alternatively, treatment with EPA or DHA inhibited injury-induced activation of PLCγ1 (Fig. 1C), which is consistent with the observation that DHA and EPA reduced injury-induced EGFR phosphorylation (Fig. 1A). Subsequently, we assessed EGF-induced activation of Rac1 and Cdc42 with an assay that specifically recognizes the activated, GTP-bound forms of these molecules. Incubation with EGF induced activation of Cdc42 to a similar extent in control and LA- and EPA-treated cells (Fig. 2A). Conversely, treatment with DHA significantly reduced EGF-stimulated activation of Cdc42. Furthermore, whereas Rac1 activation was equally induced in control and LA- and EPA-treated cells, DHA significantly reduced activation of Rac1 in response to EGF (Fig. 2B). Additionally, we assessed activation of Cdc42 and Rac1 in response to injury. DHA and EPA significantly reduced activation of Cdc42 relative to control and LA-treated cells (Fig. 2C). Similarly, compared with control cells, DHA and EPA decreased injury-mediated activation of Rac1 (Fig. 2D). Additionally, Rac1 activation was suppressed to a greater extent by DHA than EPA. These data indicate that DHA reduces ligand-stimulated EGFR signaling, whereas DHA and EPA reduce injury-induced EGFR signaling.
Fig. 2.
n-3 PUFA suppress injury-induced activation of Cdc42 and Rac1. YAMC cells were untreated (control) or treated with 50 μM fatty acid (LA, DHA, or EPA) for 72 h. For the final 16–18 h, cells were serum-starved (0.5% FBS); then they were left unstimulated or stimulated by treatment with 25 ng/ml EGF (A and B) or by extensive wounding (C and D). After stimulation, cells were incubated for 2 min and harvested, and cell lysates were collected. Total cell lysates were analyzed by G-LISA for active GTP-bound Cdc42 (A and C) or Rac1 (B and D). Representative data are presented as means ± SE (n = 6) for stimulated samples. Two independent assays were conducted. Statistical difference (P < 0.05), as indicated by different letters (a–c), was measured using 1-way ANOVA and Tukey's test of contrast.
Scratch and migration assays.
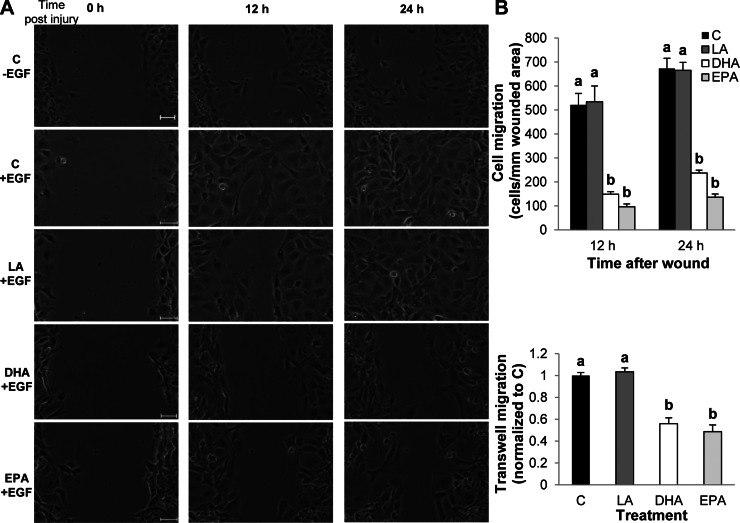
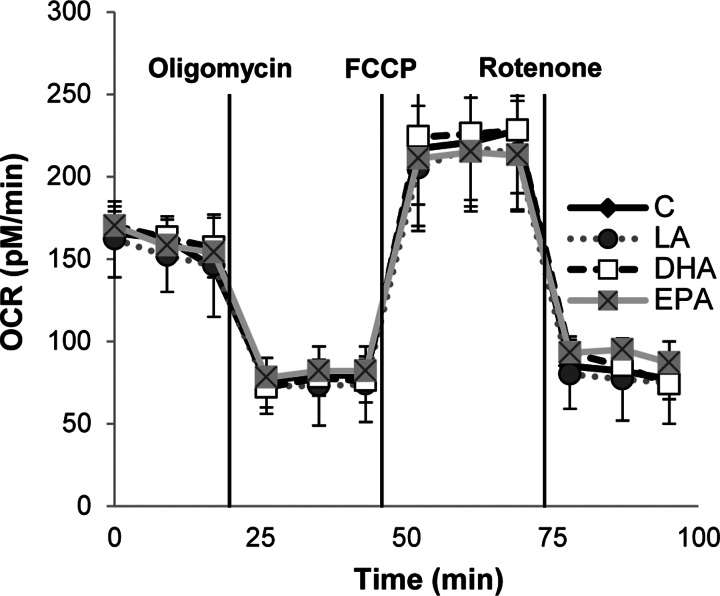
On the basis of our initial observation that DHA and EPA impede wound-induced EGFR signaling, we utilized a functional assay to assess wound healing. Cells were grown to confluency, and monolayers were subsequently scrape-wounded. Then the cells were treated with EGF, and the wound was imaged every 15 min for 24 h. Wound healing was measured by counting the number of cells that had infiltrated the wounded area at 12 and 24 h after the wounding event. Typically, unstimulated (no EGF) cells required >24 h for complete wound closure (Fig. 3A). In contrast, EGF stimulated wound healing, and complete wound closure was observed by 24 h in control and LA-treated cells. However, EGF-mediated wound healing of DHA- and EPA-treated cells was significantly delayed. Cell migration was additionally assessed using a transwell migration assay. EGF was utilized to stimulate cell migration through a membrane with 8-μm pores over a 12-h incubation period. A similar extent of migration was observed in control and LA-treated cells (Fig. 3B). However, treatment with DHA or EPA significantly reduced cell migration. To ensure that the reduction in wound healing was not due to a nonspecific effect on cell viability, mitochondrial respiration was assessed (Fig. 4). Fatty acid treatment did not influence oxygen consumption rates compared with control untreated cells, indicating that DHA and EPA did not impact cell viability.
Fig. 3.
n-3 PUFA impede colonocyte wound healing. YAMC cells were untreated (control) or treated with 50 μM fatty acid (LA, DHA, or EPA) for 72 h. For the final 16–18 h, cells were serum-starved (0.5% FBS). A: cells were then scratched with a p-200 pipette tip and stimulated with 25 ng/ml EGF. Cell migration into the wounded area was imaged every 15 min on a Nikon TiE inverted microscope for 24 h and quantified by counting the number of cells that migrated into the wounded area at 12 and 24 h after the initial wound. Four independent experiments were conducted, and representative images are presented. Scale bars, 50 μm. B: after serum starvation, cells were trypsinized and counted, and an equal number of cells were seeded into the top chamber of a 2-chamber well in serum-free medium. The bottom chamber was filled with medium supplemented with 25 ng/ml EGF. Cells were incubated for 12 h and allowed to migrate through the membrane between the chambers. Cells were then stained, and cells remaining in the top chamber were removed. Dye was eluted from the stained cells that had migrated through the membrane, and optical density of the dye/elution mixture was measured on a spectrophotometer. Two independent experiments were conducted. Values are means ± SE (n = 6). Statistical difference (P < 0.05), as indicated by unique letters (a and b), was measured using 1-way ANOVA and Tukey's test of contrast.
Fig. 4.
n-3 PUFA have no effect on mitochondrial respiration. YAMC cells were untreated (control) or treated with 50 μM fatty acid (LA, DHA, or EPA) for 72 h. Cells were seeded into Seahorse XF 24-well cell culture plates for the final 16 h, and cellular bioenergetics were analyzed using the Seahorse XF24 Extracellular Flux Analyzer. Oxygen consumption rates (OCR) were analyzed continuously under basal conditions and following addition of a mitochondrial mediator: oligomycin (2 μmol/l), carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP, 4 μmol/l), or rotenone (4 μmol/l). Values (means ± SE) are normalized to cell count (n = 5). No statistical significance (P > 0.05) was observed on analysis with 1-way ANOVA and Tukey's test of contrast.
Survival rates in vivo.
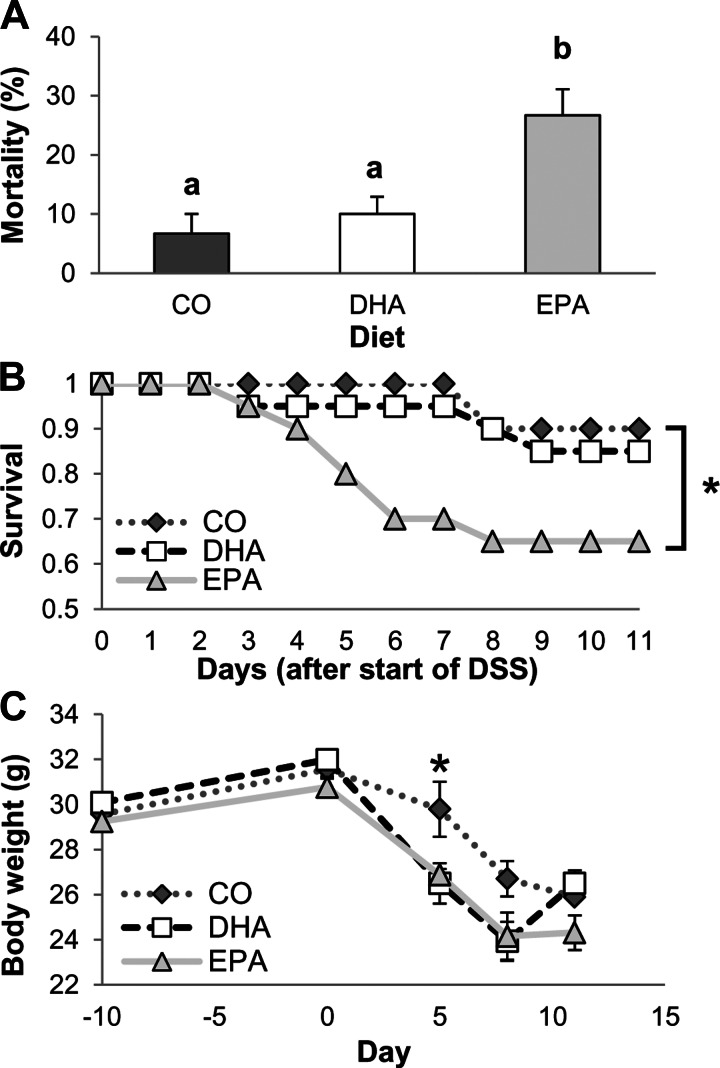
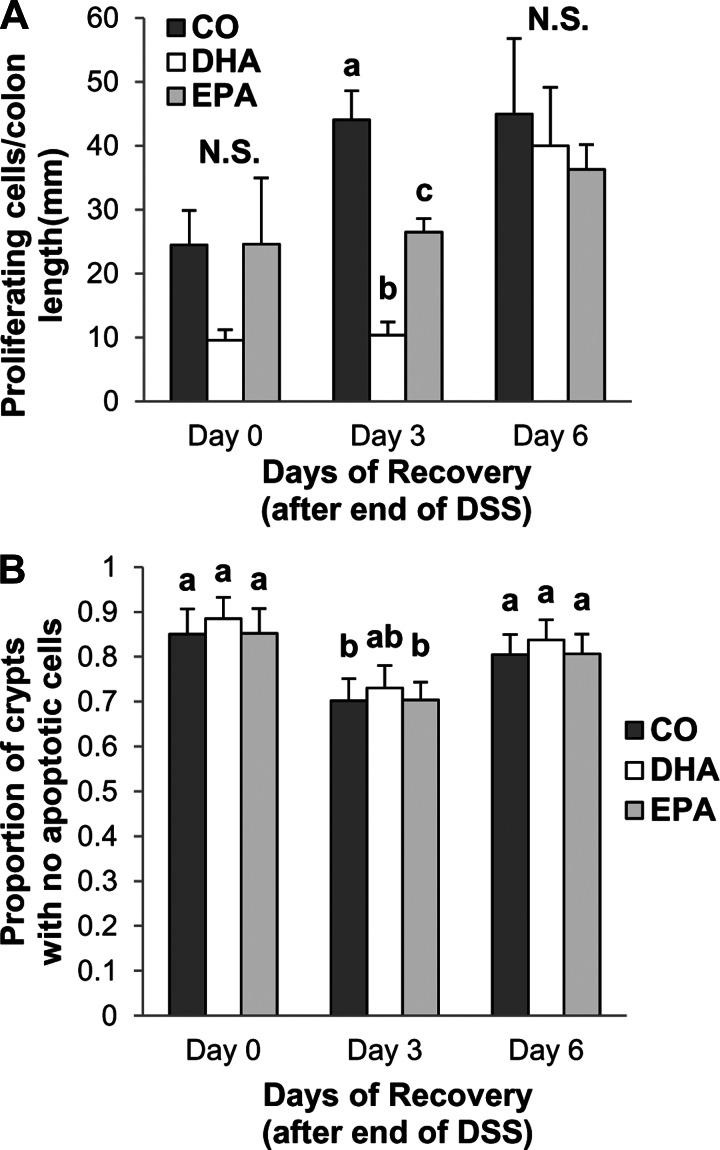
On the basis of the in vitro observations, we assessed whether the dietary lipid source can alter the sequelae associated with acute wound healing in an animal model. Mice were fed a diet enriched in CO, purified EPA, or DHA for 10 days and then exposed for 5 days to 2.5% DSS in the drinking water. DSS is a wounding agent that is often utilized to induce a form of colitis that presents clinical and histological features similar to human IBDs (29, 54). After DSS treatment, mice were allowed no recovery period (0 days) or allowed to recover for 3 or 6 days. Mice were maintained on the experimental diets throughout the study period, and the colonic mucosa phospholipid composition was assessed following the 6-day recovery period (Table 1). Arachidonic acid (20:4 n-6) levels were significantly lower in mucosa from DHA- and EPA- than CO-fed mice. Additionally, DHA-fed mice had significantly enriched levels of DHA and intermediate levels of EPA, whereas EPA-fed mice had the highest levels of EPA and docosapentaenoic acid (22:5 n-3) and intermediate levels of DHA. Supplementation of humans with DHA at 1.6 g/day has been reported to result in a fourfold enrichment of DHA in the serum (9), which is similar to the enrichment observed in the colonic mucosa of the DHA-fed mice. The highest mortality was associated with the EPA-fed mice (Fig. 5A). Additionally, over the 6-day recovery period, EPA significantly reduced survival compared with the CO diet (Fig. 5B). The DHA-enriched diet, however, did not reduce overall survival compared with the CO diet. In addition, the DHA- and EPA-fed mice lost more weight than the CO-fed mice (Fig. 5C). Interestingly, the DHA-fed mice began to regain weight at days 3 and 6 of recovery, whereas mice fed the other diets did not. Assessment of colonocyte proliferation revealed that, immediately (day 0) and at day 3 after DSS treatment, proliferation in the colon was significantly reduced in DHA- compared with EPA- and CO-fed animals (Fig. 6A). Additionally, at day 3 of recovery, proliferation was significantly less in EPA- than CO-fed mice. By day 6 of recovery, all treatment groups displayed similar levels of colonic proliferation. We also analyzed apoptosis, but we observed no significant difference between diet groups (Fig. 6B). However, apoptosis was significantly higher in CO- and EPA-fed mice at day 3 of recovery than at days 0 and 6 of recovery. This result is intriguing, since EGFR is a major regulator of proliferation.
Table 1.
Colonic mucosal fatty acid composition
| Diet Group |
|||
|---|---|---|---|
| Fatty Acid | CO | DHA | EPA |
| 14:0 | 1.45 ± 0.20 | 1.36 ± 0.15 | 1.41 ± 0.12 |
| 16:0 | 26.35 ± 0.79 | 26.13 ± 0.74 | 24.88 ± 0.40 |
| 16:1 n-7 | 2.53 ± 0.61 | 2.45 ± 0.28 | 2.53 ± 0.18 |
| 18:0 | 16.35 ± 1.40 | 19.07 ± 0.99 | 17.75 ± 0.58 |
| 18:1 n-9 | 17.87 ± 1.63 | 16.03 ± 0.49 | 16.13 ± 0.57 |
| 18:1 n-7 | 3.49 ± 0.46 | 3.34 ± 0.28 | 3.11 ± 0.39 |
| 18:2 n-6 | 17.74 ± 1.84 | 15.08 ± 1.77 | 14.31 ± 1.15 |
| 20:3 n-6 | 2.91 ± 0.52 | 2.79 ± 0.43 | 3.25 ± 0.15 |
| 20:4 n-6 | 9.24 ± 0.86† | 5.60 ± 0.71* | 5.58 ± 0.29* |
| 20:5 n-3 | 0.43 ± 0.12* | 2.91 ± 0.14† | 6.44 ± 0.55‡ |
| 22:5 n-3 | 0.00 ± 0.00* | 0.18 ± 0.02* | 1.02 ± 0.12† |
| 22:6 n-3 | 1.63 ± 0.19* | 5.05 ± 0.38‡ | 3.58 ± 0.19† |
Values (means ± SE) are expressed on a weight percent basis (g/100 g fatty acid); n = 5 mice per diet group.
CO, corn oil (control); DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
For each fatty acid within each diet group, values not sharing a symbol (*, †, ‡) differ (P ≤ 0.05).
Fig. 5.
Dietary DHA and EPA alter survival of dextran sodium sulfate (DSS)-treated mice. Mice were fed a diet with corn oil (CO) as the sole lipid source or a diet enriched with purified DHA or EPA before and after exposure to 2.5% DSS for 5 days. Mice were then allowed to recover from DSS for 0, 3, or 6 days. A: overall mortality of mice at all time points. Values are means ± SE (n = 65–75 mice per diet). B: Kaplan-Meier curve showing survival of animals that were allowed to recover for 6 days. Values are means ± SE (n = 20 mice per diet). *P ≤ 0.05 (by log-rank test). C: initial (day −10) body weight and body weight at the beginning of DSS treatment (day 0), at the end of DSS treatment (day 5), and following recovery (days 8 and 11). Average body weight was calculated and graphed over time for each diet group (n = 25–75) at each time point. Statistical significance (P ≤ 0.05) between dietary groups, as indicated by different letters (a and b), was determined using a 1-way ANOVA and Tukey's test of contrast.
Fig. 6.
Dietary DHA and EPA alter colonocyte proliferation, but not apoptosis. Mice were fed a diet containing CO as the sole lipid source or a diet enriched with purified DHA or EPA before and after exposure to 2.5% DSS for 5 days. Mice were allowed to recover from DSS for 0, 3, or 6 days. A: cell proliferation was assessed by 2′-deoxy-5-ethynyl uridine (EdU) incorporation. Values are means ± SE (n = 4–5 mice per diet per time point). Statistical significance (P ≤ 0.05) between dietary groups, as indicated by different letters (a–c), was determined using 1-way ANOVA and Tukey's test of contrast. NS, not significant. B: colonocyte apoptosis was assessed by TdT-mediated dUTP nick-end labeling assay. Values are means ± SE (n = 3 mice per diet per time point). Statistical significance (P ≤ 0.05) was tested by 2-way ANOVA. No significant effect of diet on apoptosis was observed.
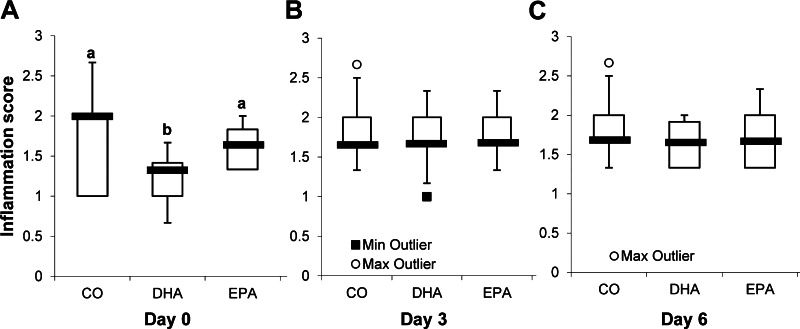
Injury and repair scores.
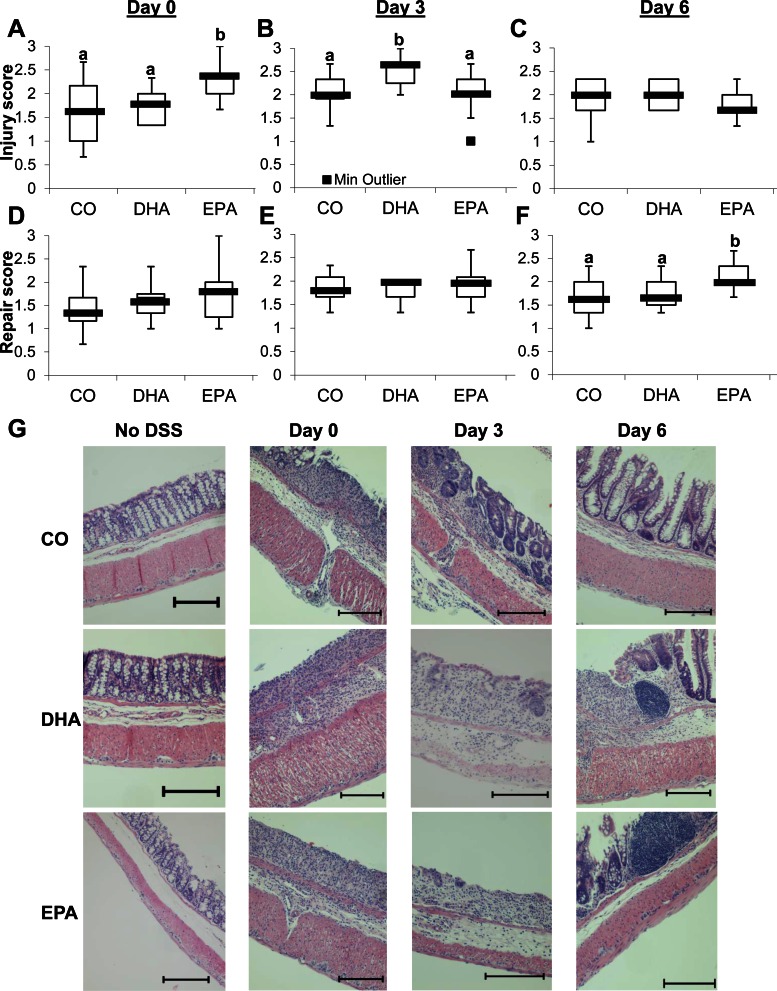
The degree of colonic injury (score 0–3) and repair (score 0–3) was graded in a blinded manner by a board-certified pathologist (B. Weeks). For all dietary groups, no significant difference was observed in injury or repair in non-DSS-exposed mice (data not shown). At day 0 of recovery, EPA-fed mice exhibited significantly higher levels of injury than DHA- or CO-fed animals (Fig. 7A). Additionally, DHA-fed mice displayed the highest level of injury at day 3 of recovery (Fig. 7B). From day 0 to day 3 of recovery, injury of EPA-fed mice declined, whereas injury of DHA-fed mice increased (Fig. 7, A and B). Colonic injury was reduced in all diet groups between days 3 and 6 of recovery (Fig. 7C). No significant differences in mucosal repair were observed between groups at day 0 or 3 of recovery (Fig. 7, D and E). Significant increase in injury in EPA- and DHA-fed mice at days 0 and 3 suggests that activation of repair mechanisms is hindered. At day 6, however, EPA-fed mice exhibited significantly higher levels of repair than DHA- or CO-fed animals (Fig. 7F). Representative images of colon sections from mice fed each diet are shown in Fig. 7G. Overall, the data indicate that DHA and EPA negatively impact colonic wounding and repair following an acute wounding event.
Fig. 7.
Dietary DHA and EPA exacerbate colonic injury in mice on exposure to DSS. Mice were fed a diet with CO as the sole lipid source or a diet enriched with purified DHA or EPA for 10 days. Mice were continued on the respective diets and exposed to 2.5% DSS for 5 days and allowed to recover from DSS for 0, 3, or 6 days. Longitudinal halves of the colon were Swiss-rolled, fixed with 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin-eosin. Sections were analyzed, a score of 0–3 for injury (A–C) or repair (D–F) in the distal, middle, and proximal colon was assigned, and the average score for the entire colon was quantified from these 3 scores. Box plots show scores from 11–15 mice per diet. Median score is represented by thick black line on the box plot. Statistical significance between dietary groups, as indicated by different letters (a and b), was assessed by Kruskall-Wallis test followed by, if justified, the statistical probability outcome (P ≤ 0.05) using Wilcoxon 2-sample testing. G: representative images of hematoxylin-eosin-stained colon sections from mice fed each diet and unexposed (no DSS) or allowed to recover for 0, 3, or 6 days after 5 days of exposure to 2.5% DSS. Scale bars, 500 μm.
EGFR activation and signaling in mice.
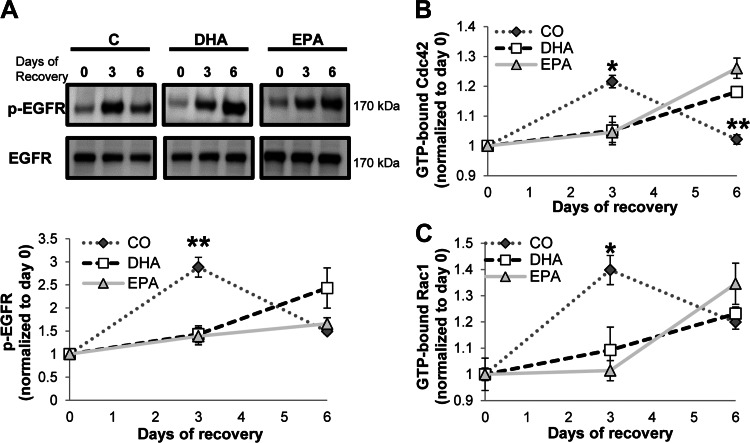
For the purpose of assessing EGFR activation status in mice fed each of the experimental diets, EGFR phosphorylation status was measured in lysates derived from scraped colonic mucosa. Specifically, EGFR phosphorylation was measured at days 0, 3, and 6 of recovery from DSS exposure. EGFR phosphorylation peaked at day 3 of recovery in CO-fed mice and decreased by day 6 (Fig. 8A). Activation of EGFR was significantly higher in CO- than DHA- or EPA-fed mice at day 3 of recovery. In the DHA- and EPA-fed animals, peak EGFR phosphorylation was observed on day 6 of recovery, with EGFR phosphorylation progressively increasing from day 0 to day 6. This suggests that regulation of EGFR activation is altered by dietary lipids.
Fig. 8.
Dietary lipids modulate the pattern of activation of EGFR, Ccd42, and Rac1. Mice were fed a diet with CO as the sole lipid source or a diet enriched with purified DHA or EPA for 10 days. Mice were continued on the diet and exposed to 2.5% DSS for 5 days and allowed to recover from DSS for 0, 3, or 6 days. Whole cell lysates were isolated from scraped colonic mucosa. A: lysates were probed by Western blotting for total and phosphorylated EGFR (top). Bottom: quantification of band volume. Values (means ± SE) are normalized to day 0 of recovery (n = 6). B: lysates were analyzed by G-LISA for GTP-bound Cdc42. Two independent assays were conducted. Values are means ± SE (n = 6–8). C: lysates were analyzed by G-LISA for GTP-bound Rac1. *P ≤ 0.05; **P ≤ 0.001 (by 1-way ANOVA and Tukey's test of contrast).
After observing diet-induced differences in EGFR phosphorylation, we compared downstream activation of Rac1 and Cdc42. Consistent with EGFR phosphorylation, Cdc42 activation peaked at day 3 of recovery in CO-fed mice and then decreased by day 6 of recovery (Fig. 8B). In contrast, Cdc42 activation continued to increase in DHA- and EPA-fed mice throughout the recovery period. Activation of Cdc42 was significantly higher in CO- than DHA- or EPA-fed mice at day 3 of recovery but was significantly lower at day 6. A similar overall pattern was observed with respect to activation of Rac1 (Fig. 8C). Rac1 activation was significantly higher in CO- than DHA- or EPA-fed mice at day 3 of recovery, but no significant difference was observed at day 6. The data further indicate that EGFR signaling is modified by DHA and EPA.
Colonic mucosal inflammation.
Since exposure to acute DSS elicits an intestinal inflammatory response, colonic inflammation, e.g., histological changes in immune cell infiltration and colonic mucosal cytokine mRNA expression, was assessed. The degree of colonic inflammation (score 0–3) was graded in a blinded manner by a board-certified pathologist (B. Weeks). At day 0 of recovery, inflammation scores were significantly lower for DHA- than EPA- or CO-fed mice (Fig. 9A). No significant differences were observed between the dietary groups at day 3 or 6 of recovery (Fig. 9, B and C). Additionally, no significant difference in colonic inflammation was observed between dietary groups in non-DSS-exposed mice (data not shown). Next, the inflammatory response during the recovery phase following DSS exposure was tracked by analysis of expression of proinflammatory mediators (IFNγ, IL-1β, TNFα, IL-6, and MCP-1), anti-inflammatory mediators (IL-10), and cytokines related to wound healing (IL-4, TGFβ1, IL-13, and IL-22) (Table 2). For all genes assessed, there was no difference between dietary groups in terms of basal mRNA expression in non-DSS-exposed mice (P > 0.05), with the exception of MCP-1, where basal expression was upregulated in EPA- and DHA- compared with CO-fed mice. After DSS exposure (day 0 of recovery), mRNA expression of IFNγ and TNFα was reduced in EPA- compared with CO- and DHA-fed mice (P < 0.05). After day 3 of recovery, MCP-1 and TNFα mRNA expression was reduced in EPA- and DHA- compared with CO-fed mice (P = 0.03 and P = 0.004, respectively). Gene expression of the inflammatory cytokines IFNγ and IL-6 was reduced only by the EPA-enriched diet (P < 0.05), whereas only the DHA-enriched diet reduced mRNA expression of IL-1β (P = 0.03) compared with the CO diet. After day 6 of recovery, the EPA- and DHA-enriched diets reduced mRNA expression of IL-6 and IL-1β compared with the CO diet (P < 0.05), whereas expression levels of the inflammatory cytokines/chemokines IFNγ, TNFα, and MCP-1 did not differ between dietary groups (P > 0.05). Gene expression of IL-4, TGFβ1, and IL-10 in mice from either n-3 PUFA-enriched diet did not differ from that in mice fed the CO diet at any recovery time after DSS (P > 0.05). Interestingly, mRNA expression of IL-13, which promotes wounding by inducing apoptosis and delaying epithelial restitution (1, 21, 44), was increased in the DHA-fed mice after days 0 and 3 of recovery from DSS (P = 0.034 and P = 0.015, respectively), whereas mRNA levels did not differ between the EPA- and CO-fed mice. After day 6 of recovery, mRNA expression of IL-13 was reduced in the EPA- and DHA- compared with the CO-fed mice (P = 0.018). Conversely, colonic mRNA expression of IL-22, which promotes epithelial cell restitution and intestinal barrier integrity (5), was increased in the DHA- and EPA- compared with CO-fed mice at all time points after DSS (P < 0.05). The data demonstrate that DHA and EPA alter colonic inflammation following injury.
Fig. 9.
DHA reduces colonic inflammation in DSS-treated mice. Mice were fed a diet with CO as the sole lipid source or a diet enriched with purified DHA or EPA for 10 days. Mice were then continued on the diet and exposed to 2.5% DSS for 5 days and subsequently allowed to recover from DSS for 0 (A), 3 (B), or 6 (C) days. Longitudinal halves of the colon were Swiss-rolled, fixed with 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin-eosin. Sections were analyzed, a score of 0–3 for inflammation in the distal, middle, and proximal colon was assigned, and the average score for the entire colon was quantified from these 3 scores. Box plots show scores from 11–15 mice per diet. Median score is represented by thick black line on the box plot. Statistical significance (P ≤ 0.05) between dietary groups, as indicated by different letters (a and b) was assessed by Kruskall-Wallis test followed, if justified, by the statistical probability outcome using Wilcoxon's 2-sample testing.
Table 2.
Colonic mucosal mRNA expression normalized to ribosomal 18S
| DSS |
||||
|---|---|---|---|---|
| No DSS | Day 0 of recovery | Day 3 of recovery | Day 6 of recovery | |
| IFNγ | ||||
| CO | 0.05 ± 0.01 | 4.32 ± 0.95* | 3.35 ± 1.02* | 0.09 ± 0.07 |
| DHA | 0.06 ± 0.007 | 2.63 ± 0.73* | 1.05 ± 0.23* | 0.08 ± 0.66 |
| EPA | 0.04 ± 0.01 | 1.84 ± 0.55† | 0.46 ± 0.30† | 0.12 ± 0.18 |
| P | 0.07 | 0.05 | 0.026 | 0.60 |
| IL-1β | ||||
| CO | 0.83 ± 0.06 | 68.05 ± 16.96 | 101.03 ± 28.29* | 70.97 ± 16.04* |
| DHA | 0.63 ± 0.12 | 85.53 ± 15.15 | 14.19 ± 11.79† | 28.98 ± 7.67† |
| EPA | 0.63 ± 0.06 | 72.15 ± 35.74 | 30.34 ± 10.70*† | 29.57 ± 7.80† |
| P | 0.18 | 0.86 | 0.030 | 0.01 |
| TNFα | ||||
| CO | 9.33 ± 1.51 | 127.64 ± 22.79* | 20.49 ± 2.76* | 2.14 ± 0.38 |
| DHA | 6.03 ± 1.33 | 126.56 ± 25.88* | 8.73 ± 0.85† | 1.69 ± 0.27 |
| EPA | 5.24 ± 1.34 | 65.65 ± 8.27† | 12.29 ± 1.80† | 2.04 ± 0.49 |
| P | 0.17 | 0.04 | 0.004 | 0.53 |
| IL-6 | ||||
| CO | 0.01 ± 0.002 | 8.30 ± 1.22 | 1.13 ± 0.02* | 9.92 ± 0.04* |
| DHA | 0.01 ± 0.001 | 9.71 ± 2.32 | 0.89 ± 0.02* | 3.74 ± 0.06† |
| EPA | 0.01 ± 0.002 | 7.79 ± 0.02 | 0.78 ± 0.09† | 2.64 ± 0.04† |
| P | 0.19 | 0.82 | 0.05 | 0.02 |
| MCP-1 | ||||
| CO | 0.01 ± 0.01* | 24.15 ± 7.12*† | 7.83 ± 2.25* | 0.17 ± 0.08 |
| DHA | 0.09 ± 0.01† | 42.33 ± 6.93* | 2.91 ± 1.63† | 0.18 ± 0.17 |
| EPA | 0.10 ± 0.03† | 17.62 ± 6.13† | 3.10 ± 0.34† | 0.15 ± 0.06 |
| P | 0.01 | 0.05 | 0.03 | 0.92 |
| IL-4 | ||||
| CO | 0.004 ± 0.002 | 0.10 ± 0.06 | 0.02 ± 0.06 | 0.14 ± 0.12 |
| DHA | 0.004 ± 0.001 | 0.10 ± 0.03 | 0.02 ± 0.03 | 0.23 ± 0.17 |
| EPA | 0.002 ± 0.001 | 0.21 ± 0.13 | 0.04 ± 0.01 | 0.13 ± 0.06 |
| P | 0.6501 | 0.30 | 0.49 | 0.7592 |
| IL-10 | ||||
| CO | 0.01 ± 0.003 | 2.00 ± 0.49 | 0.01 ± 0.03*† | 0.04 ± 0.01 |
| DHA | 0.01 ± 0.001 | 3.50 ± 0.88 | 0.29 ± 0.19* | 0.04 ± 0.01 |
| EPA | 0.01 ± 0.002 | 3.55 ± 1.74 | 0.07 ± 0.04† | 0.05 ± 0.01 |
| P | 0.22 | 0.18 | 0.05 | 0.61 |
| TGFβ1 | ||||
| CO | 8.91 ± 0.86 | 39.47 ± 9.66 | 11.78 ± 2.23 | 15.29 ± 3.61 |
| DHA | 8.12 ± 0.82 | 54.94 ± 8.81 | 10.90 ± 2.24 | 16.21 ± 3.45 |
| EPA | 12.47 ± 1.97 | 47.08 ± 10.96 | 9.64 ± 0.68 | 18.57 ± 3.86 |
| P | 0.10 | 0.44 | 0.71 | 0.73 |
| IL-13 | ||||
| CO | 0.01 ± 0.003 | 0.43 ± 0.02* | 0.06 ± 0.01* | 0.10 ± 0.003* |
| DHA | 0.01 ± 0.002 | 1.55 ± 0.35† | 0.21 ± 0.06† | 0.02 ± 0.005† |
| EPA | 0.01 ± 0.002 | 0.94 ± 0.34* | 0.10 ± 0.02* | 0.02 ± 0.002† |
| P | 0.46 | 0.03 | 0.01 | 0.02 |
| IL-22 | ||||
| CO | 0.43 ± 0.40* | 0.06 ± 0.12* | 0.003 ± 0.02* | |
| DHA | 1.56 ± 1.32† | 0.21 ± 0.01† | 0.011 ± 0.02† | |
| EPA | 0.94 ± 2.03† | 0.10 ± 0.05* | 0.013 ± 0.03† | |
| P | 0.03 | 0.01 | 0.04 | |
Values are means ± SE; n = 4–8 mice per diet group.
DSS, dextran sodium sulfate; MCP-1, monocyte chemoattractant protein 1; TGFβ1, transforming growth factor-β1.
For each gene within each treatment stage, values not sharing a symbol (*, †) differ (P ≤ 0.05).
DISCUSSION
To our knowledge, this is the first examination of the differential effects of DHA and EPA on EGFR signaling within the context of wound healing. There is an abundance of interest in this field, especially since many Americans take fish oil supplements because of their well-established health benefits (52). Interestingly, our data indicate that n-3 PUFA may not always promote optimal health, which may explain some of the inconsistencies in the literature.
DHA and EPA reduced wound-induced transactivation of EGFR (Fig. 1A). Multiple signaling pathways are known to modulate transactivation of EGFR. For example, PGE2 mediates activation of MMPs, which cleave EGFR ligands from the plasma membrane (2, 38, 39). It is well established that DHA and EPA reduce production of PGE2 (6, 49), which could dampen transactivation of EGFR. Similarly, Src has been shown to mediate activation of MMPs to induce activation of EGFR (39, 53). Interestingly, Src signaling has been shown to be facilitated by lipid rafts (22), and n-3 PUFA incorporation into raft mesodomains perturbs downstream signaling events (7, 8, 46). It is likely, therefore, that the effect of DHA and EPA on the activation of EGFR could be caused by inhibition of one of these mechanisms. Clearly, further work is required to pinpoint the mechanism by which n-3 PUFA inhibit transactivation of EGFR in response to injury.
Next, we observed that, in response to ligand (i.e., EGF), DHA, but not EPA, inhibited EGFR signaling through PLCγ1, Rac1, and Cdc42 (Figs. 1 and 2). This is consistent with our previous observations that DHA suppresses EGFR signal transduction (50). In contrast, in response to wounding, EPA and DHA reduced activation of PLCγ1, Rac1, and Cdc42 (Figs. 1 and 2), which is consistent with the observation that EPA and DHA inhibit wound-induced transactivation of EGFR. In addition to these mediators, we previously showed that n-3 PUFA reduce activation of ERK1/2 and STAT3 (35, 50). All these factors are integral in mediating wound healing, which further suggests that n-3 PUFA could be unfavorable for recovery from injury.
With respect to the analysis of wound healing and cell migration in vitro, EGF enhanced wound healing in control and LA-treated cells (Fig. 3A). However, EGF-mediated wound healing was strongly impeded by treatment with DHA or EPA. Similar results were observed for cell migration (Fig. 3B). It is intriguing that although EPA did not impair ligand-induced EGFR signaling, it did impair EGF-induced biological responses. This suggests that the effects of EPA on wound healing and migration may be partially independent of EGFR. Additional studies are needed to clarify the role of EPA in EGF-induced wound healing. Interestingly, EGF-induced cytoskeletal remodeling has been shown to be mediated by products of 5-lipoxygenase and cyclooxygenase pathways (40). DHA and EPA compete with arachidonic acid as substrates for these enzymes, which could affect cytoskeletal remodeling and cell migration in a manner independent of activation of EGFR signaling mediators.
Our in vivo results further demonstrate that DHA and EPA alter wound healing, in part by modifying EGFR-mediated signaling events. Consistent with the in vitro effect of EPA on wound healing, EPA-fed animals displayed the lowest survival levels (Fig. 5, A and B). Additionally, body weight loss in response to DSS treatment tended to be greater in DHA- and EPA- than CO-fed mice (Fig. 5C). Furthermore, DHA-fed mice displayed significant reductions in cell proliferation during the early stages (days 0 and 3) of recovery (Fig. 6A), whereas no effect of diet was observed on apoptosis (Fig. 6B). Collectively, these data suggest that early wound-healing events are delayed by DHA and EPA. Upon analysis of colonic injury, we observed that the levels of injury were highest at day 0 of recovery in EPA-fed mice (Fig. 7A) and subsequently declined progressively to day 6 (Fig. 7, B and C). However, in DHA-fed mice, injury largely increased from day 0 to day 3, suggesting that colonic repair was impeded. Upon assessment of downstream signaling, peak activation of EGFR and downstream signaling mediators was delayed in animals fed EPA or DHA (Fig. 8). This suggests that the CO-fed mice recover more quickly than the n-3 PUFA-fed mice. Although it is premature to conclude that the perturbations in downstream signaling are only due to changes in EGFR regulation, the data clearly demonstrate differential regulation of important wound-healing signaling events in n-3 PUFA- compared with CO-fed mice.
Because of the well-documented effects of DHA and EPA on inflammation, we additionally assessed the degree of colonic inflammation and gene expression of inflammatory mediators to further understand how the colonic mucosal microenvironment is impacted by dietary lipids. DHA significantly reduced the inflammation histological score at day 0 of recovery compared with CO- and EPA-fed mice (Fig. 9A), which is indicative of reduced immune cell infiltration into the colonic mucosa. In contrast, on day 0, EPA-fed mice exhibited increased colonic injury scores (Fig. 7A). At day 0 of recovery, colonic mRNA expression of IFNγ and TNFα was reduced in EPA- compared with CO- and DHA-fed mice (Table 2). The anti-inflammatory effects of EPA and DHA were apparent at later stages of DSS recovery, as both n-3 PUFA reduced mRNA expression of MCP-1, TNFα, IL-6, and IL-1β (Table 2). In the early stages of recovery, mRNA expression of IL-13, a cytokine that antagonizes epithelial restitution (21, 44), was upregulated in DHA-fed mice and may additionally contribute to the increased injury scores observed at day 3 of recovery in these mice (Fig. 7B). Collectively, the response to an intestinal wounding event is a complicated process representing the sum of multiple inputs that promote or inhibit the process of epithelial restitution. Further studies are required to determine the mechanisms through which n-3 PUFA affect these pathways.
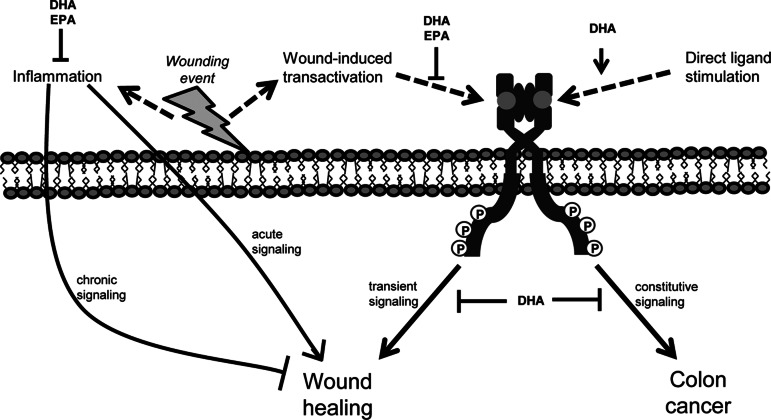
Although it remains unclear how DHA and EPA alter EGFR activation in colonic wound healing, we present cogent data indicating that these bioactive lipids present a barrier to wound healing due to altered mucosal cell signaling. Several decades of research indicate that DHA and EPA suppress inflammatory mediators in experimental models and clinical trials (6). Although early immune-mediated events are required for wound healing, prolonged inflammation can be detrimental to this process. DHA and EPA can be metabolized into a number of lipid mediators, including resolvins, protectins, and maresins, which facilitate resolution of inflammation (3). These lipid mediators have varying biological activities, which may mediate the effects of EPA and DHA. In this study, we observed that the DHA-fed animals started to gain weight between days 3 and 6 of recovery, whereas the EPA- and CO-fed animals did not. This suggests that DHA may facilitate events that expedite the later stages of wound healing, perhaps by resolving inflammation. We have presented a putative model depicting the complicated role that n-3 PUFA play in regulating EGFR signaling and inflammation and the effects they may have on wound healing and colon carcinogenesis (Fig. 10).
Fig. 10.
Representation of the complex role of DHA and EPA in wound healing. Transient EGFR signaling is important for cellular functions that promote wound healing. However, constitutive activation of EGFR signaling can facilitate malignant transformation of the colon. n-3 PUFA alter EGFR signaling, but DHA and EPA function through different mechanisms. DHA, but not EPA, increases ligand-induced activation of EGFR but inhibits signal transduction. Alternatively, DHA and EPA impede transactivation of EGFR stimulated by injury. Furthermore, DHA and EPA have well-documented anti-inflammatory activities, and inflammation can stimulate and exacerbate wound healing.
Findings from our study are particularly relevant to individuals who repeatedly experience colonic wounding, e.g., IBD. These patients undergo multiple cycles of active disease and recovery. They have low expression of EGFR ligands (37), and current work suggests that supplementation with EGF elicits a positive response in these patients (47). Conflicting data suggest a beneficial role (4, 18, 25), no effect, or even a detrimental role (20, 24, 31) for n-3 PUFA in IBD. A systematic review and meta-analysis of human studies indicated no clear beneficial role for n-3 PUFA in IBD patients (51). The data presented here suggest that the inhibition of EGFR signaling by n-3 PUFA could delay wound healing in these patients. However, the anti-inflammatory effects of n-3 PUFA may be beneficial during some disease stages, e.g., resolution of chronic inflammation. Future work should focus on determining optimal timing and dosing of n-3 PUFA to elicit the beneficial effects of these dietary lipids while avoiding the detrimental effects.
In summary, we propose that the inhibitory effects of DHA and EPA on injury-induced transactivation of EGFR likely contribute to delayed wound healing. This provides a potential mechanism to explain why n-3 PUFA do not always facilitate recovery and remission in IBD patients.
GRANTS
This work was supported by National Cancer Institute Grants CA-59034, CA-129444, and CA-168312.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.F.T., J.M.M., and R.S.C. are responsible for conception and design of the research; H.F.T., J.M.M., Y.-Y.F., and E.S.C. performed the experiments; H.F.T., J.M.M., and B.W. analyzed the data; H.F.T. and J.M.M. interpreted the results of the experiments; H.F.T. prepared the figures; H.F.T. drafted the manuscript; H.F.T., J.M.M., and R.S.C. edited and revised the manuscript; H.F.T., J.M.M., Y.-Y.F., E.S.C., B.W., and R.S.C. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Justin Slagel (SLA Pharma, Whatford, UK) for purified dietary EPA and Croda (Edison, NJ) for purified DHA. We also thank Laurie Davidson for help with the animal study and technical assistance, Gonzalo Rivera for expert advice, and Roger Zho and Jennifer Goldsby for assistance with statistical analyses.
REFERENCES
- 1. Ahdieh M, Vandenbos T, Youakim A. Lung epithelial barrier function and wound healing are decreased by IL-4 and IL-13 and enhanced by IFN-γ. Am J Physiol Cell Physiol 281: C2029–C2038, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Al-Salihi MA, Ulmer SC, Doan T, Nelson CD, Crotty T, Prescott SM, Stafforini DM, Topham MK. Cyclooxygenase-2 transactivates the epidermal growth factor receptor through specific E-prostanoid receptors and tumor necrosis factor-α converting enzyme. Cell Signal 19: 1956–1963, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bannenberg GL. Therapeutic applicability of anti-inflammatory and proresolving polyunsaturated fatty acid-derived lipid mediators. Sci World J 10: 676–712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belluzzi A, Brignola C, Campieri M, Pera A, Boschi S, Miglioli M. Effect of an enteric-coated fish-oil preparation on relapses in Crohn's disease. N Engl J Med 334: 1557–1560, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A, Leclair S, Herrmann K, Seiderer J, Ochsenkuhn T, Goke B, Auernhammer CJ, Dambacher J. IL-22 is increased in active Crohn's disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol 290: G827–G838, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Calder PC. Polyunsaturated fatty acids and inflammation. Biochem Soc Trans 33: 423–427, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Chapkin RS, McMurray DN, Davidson LA, Patil BS, Fan YY, Lupton JR. Bioactive dietary long-chain fatty acids: emerging mechanisms of action. Br J Nutr 100: 1152–1157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chapkin RS, Wang N, Fan YY, Lupton JR, Prior IA. Docosahexaenoic acid alters the size and distribution of cell surface microdomains. Biochim Biophys Acta 1778: 466–471, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Conquer JA, Holub BJ. Supplementation with an algae source of docosahexaenoic acid increases (n-3) fatty acid status and alters selected risk factors for heart disease in vegetarian subjects. J Nutr 126: 3032–3039, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Davidson LA, Lupton JR, Jiang YH, Chapkin RS. Carcinogen and dietary lipid regulate Ras expression and localization in rat colon without affecting farnesylation kinetics. Carcinogenesis 20: 785–791, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Dignass AU, Podolsky DK. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor-β. Gastroenterology 105: 1323–1332, 1993 [DOI] [PubMed] [Google Scholar]
- 12. Dise RS, Frey MR, Whitehead RH, Polk DB. Epidermal growth factor stimulates Rac activation through Src and phosphatidylinositol 3-kinase to promote colonic epithelial cell migration. Am J Physiol Gastrointest Liver Physiol 294: G276–G285, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Eden S, Rohatgi R, Podtelejnikov AV, Mann M, Kirschner MW. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature 418: 790–793, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Egan LJ, de Lecea A, Lehrman ED, Myhre GM, Eckmann L, Kagnoff MF. Nuclear factor-κB activation promotes restitution of wounded intestinal epithelial monolayers. Am J Physiol Cell Physiol 285: C1028–C1035, 2003 [DOI] [PubMed] [Google Scholar]
- 15. El-Assal ON, Besner GE. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology 129: 609–625, 2005 [DOI] [PubMed] [Google Scholar]
- 16. El-Sibai M, Nalbant P, Pang H, Flinn RJ, Sarmiento C, Macaluso F, Cammer M, Condeelis JS, Hahn KM, Backer JM. Cdc42 is required for EGF-stimulated protrusion and motility in MTLn3 carcinoma cells. J Cell Sci 120: 3465–3474, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goke M, Kanai M, Lynch-Devaney K, Podolsky DK. Rapid mitogen-activated protein kinase activation by transforming growth factor-α in wounded rat intestinal epithelial cells. Gastroenterology 114: 697–705, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Gravaghi C, La Perle KM, Ogrodwski P, Kang JX, Quimby F, Lipkin M, Lamprecht SA. Cox-2 expression, PGE2 and cytokines production are inhibited by endogenously synthesized n-3 PUFAs in inflamed colon of fat-1 mice. J Nutr Biochem 22: 360–365, 2011 [DOI] [PubMed] [Google Scholar]
- 19. Hall A. Rho GTPases and the actin cytoskeleton. Science 279: 509–514, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Hawthorne AB, Daneshmend TK, Hawkey CJ, Belluzzi A, Everitt SJ, Holmes GK, Malkinson C, Shaheen MZ, Willars JE. Treatment of ulcerative colitis with fish oil supplementation: a prospective 12 month randomised controlled trial. Gut 33: 922–928, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 129: 550–564, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Holzer RG, Park EJ, Li N, Tran H, Chen M, Choi C, Solinas G, Karin M. Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell 147: 173–184, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hopkins AM, Pineda AA, Winfree LM, Brown GT, Laukoetter MG, Nusrat A. Organized migration of epithelial cells requires control of adhesion and protrusion through Rho kinase effectors. Am J Physiol Gastrointest Liver Physiol 292: G806–G817, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol 106: 563–573, 2011 [DOI] [PubMed] [Google Scholar]
- 25. Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A, Serhan CN, Kang JX. Transgenic mice rich in endogenous ω-3 fatty acids are protected from colitis. Proc Natl Acad Sci USA 103: 11276–11281, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iizuka M, Konno S. Wound healing of intestinal epithelial cells. World J Gastroenterol 17: 2161–2171, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jia Q, Lupton JR, Smith R, Weeks BR, Callaway E, Davidson LA, Kim W, Fan YY, Yang P, Newman RA, Kang JX, McMurray DN, Chapkin RS. Reduced colitis-associated colon cancer in Fat-1 (n-3 fatty acid desaturase) transgenic mice. Cancer Res 68: 3985–3991, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim JW, Sim SS, Kim UH, Nishibe S, Wahl MI, Carpenter G, Rhee SG. Tyrosine residues in bovine phospholipase C-γ phosphorylated by the epidermal growth factor receptor in vitro. J Biol Chem 265: 3940–3943, 1990 [PubMed] [Google Scholar]
- 29. Laroui H, Ingersoll SA, Liu HC, Baker MT, Ayyadurai S, Charania MA, Laroui F, Yan Y, Sitaraman SV, Merlin D. Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLos One 7: e32084, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li S, Wang Q, Wang Y, Chen X, Wang Z. PLC-γ1 and Rac1 coregulate EGF-induced cytoskeleton remodeling and cell migration. Mol Endocrinol 23: 901–913, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lorenz R, Weber PC, Szimnau P, Heldwein W, Strasser T, Loeschke K. Supplementation with n-3 fatty acids from fish oil in chronic inflammatory bowel disease—a randomized, placebo-controlled, double-blind cross-over trial. J Intern Med Suppl 731: 225–232, 1989 [DOI] [PubMed] [Google Scholar]
- 32. Ma DW, Finnell RH, Davidson LA, Callaway ES, Spiegelstein O, Piedrahita JA, Salbaum JM, Kappen C, Weeks BR, James J, Bozinov D, Lupton JR, Chapkin RS. Folate transport gene inactivation in mice increases sensitivity to colon carcinogenesis. Cancer Res 65: 887–897, 2005 [PMC free article] [PubMed] [Google Scholar]
- 33. McDaniel JC, Belury M, Ahijevych K, Blakely W. Omega-3 fatty acids effect on wound healing. Wound repair and regeneration. Wound Repair Regen 16: 337–345, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Monk JM, Jia Q, Callaway E, Weeks B, Alaniz RC, McMurray DN, Chapkin RS. Th17 cell accumulation is decreased during chronic experimental colitis by (n-3) PUFA in Fat-1 mice. J Nutr 142: 117–124, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Monk JM, Kim W, Callaway E, Turk HF, Foreman JE, Peters JM, He W, Weeks B, Alaniz RC, McMurray DN, Chapkin RS. Immunomodulatory action of dietary fish oil and targeted deletion of intestinal epithelial cell PPARδ in inflammation-induced colon carcinogenesis. Am J Physiol Gastrointest Liver Physiol 302: G153–G167, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moyer RA, Wendt MK, Johanesen PA, Turner JR, Dwinell MB. Rho activation regulates CXCL12 chemokine stimulated actin rearrangement and restitution in model intestinal epithelia. Lab Invest 87: 807–817, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oikonomou KA, Kapsoritakis AN, Kapsoritaki AI, Manolakis AC, Tsiopoulos FD, Germenis AE, Potamianos SP. Downregulation of serum epidermal growth factor in patients with inflammatory bowel disease. Is there a link with mucosal damage? Growth Factors 28: 461–466, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Oshima H, Popivanova BK, Oguma K, Kong D, Ishikawa TO, Oshima M. Activation of epidermal growth factor receptor signaling by the prostaglandin E2 receptor EP4 pathway during gastric tumorigenesis. Cancer Sci 102: 713–719, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med 8: 289–293, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Peppelenbosch MP, Tertoolen LG, Hage WJ, de Laat SW. Epidermal growth factor-induced actin remodeling is regulated by 5-lipoxygenase and cyclooxygenase products. Cell 74: 565–575, 1993 [DOI] [PubMed] [Google Scholar]
- 41. Polk DB. Epidermal growth factor receptor-stimulated intestinal epithelial cell migration requires phospholipase C activity. Gastroenterology 114: 493–502, 1998 [DOI] [PubMed] [Google Scholar]
- 42. Rogers KR, Kikawa KD, Mouradian M, Hernandez K, McKinnon KM, Ahwah SM, Pardini RS. Docosahexaenoic acid alters epidermal growth factor receptor-related signaling by disrupting its lipid raft association. Carcinogenesis 31: 1523–1530, 2010 [DOI] [PubMed] [Google Scholar]
- 43. Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97: 221–231, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Schulzke JD, Ploeger S, Amasheh M, Fromm A, Zeissig S, Troeger H, Richter J, Bojarski C, Schumann M, Fromm M. Epithelial tight junctions in intestinal inflammation. Ann NY Acad Sci 1165: 294–300, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Seo J, Barhoumi R, Johnson AE, Lupton JR, Chapkin RS. Docosahexaenoic acid selectively inhibits plasma membrane targeting of lipidated proteins. FASEB J 20: 770–772, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Siddiqui RA, Harvey KA, Zaloga GP, Stillwell W. Modulation of lipid rafts by omega-3 fatty acids in inflammation and cancer: implications for use of lipids during nutrition support. Nutr Clin Pract 22: 74–88, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Sinha A, Nightingale J, West KP, Berlanga-Acosta J, Playford RJ. Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. N Engl J Med 349: 350–357, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Sturm A, Dignass AU. Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol 14: 348–353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Trebble TM, Wootton SA, Miles EA, Mullee M, Arden NK, Ballinger AB, Stroud MA, Burdge GC, Calder PC. Prostaglandin E2 production and T cell function after fish-oil supplementation: response to antioxidant cosupplementation. Am J Clin Nutr 78: 376–382, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Turk HF, Barhoumi R, Chapkin RS. Alteration of EGFR spatiotemporal dynamics suppresses signal transduction. PLos One 7: e39682, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Turner D, Shah PS, Steinhart AH, Zlotkin S, Griffiths AM. Maintenance of remission in inflammatory bowel disease using omega-3 fatty acids (fish oil): a systematic review and meta-analyses. Inflamm Bowel Dis 17: 336–345, 2011 [DOI] [PubMed] [Google Scholar]
- 52. Wu CH, Wang CC, Kennedy J. Changes in herb and dietary supplement use in the US adult population: a comparison of the 2002 and 2007 National Health Interview Surveys. Clin Ther 33: 1749–1758, 2011 [DOI] [PubMed] [Google Scholar]
- 53. Xu KP, Yin J, Yu FS. SRC-family tyrosine kinases in wound- and ligand-induced epidermal growth factor receptor activation in human corneal epithelial cells. Invest Ophthalmol Vis Sci 47: 2832–2839, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yan Y, Kolachala V, Dalmasso G, Nguyen H, Laroui H, Sitaraman SV, Merlin D. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLos One 4: e6073, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yin J, Yu FS. ERK1/2 mediate wounding- and G-protein-coupled receptor ligands-induced EGFR activation via regulating ADAM17 and HB-EGF shedding. Invest Ophthalmol Vis Sci 50: 132–139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]