Abstract
Background:
Complex bidirectional relationships have been described between body weight, thyroid function, and risk of thyroid disorders, including thyroid autoimmunity. We used a life-course approach to examine the potential association of childhood or adult body weight with the risk of thyroid autoimmunity and other thyroid disorders at age 60–64 years in a large population-based birth cohort study.
Methods:
In the UK Medical Research Council 1946 British Birth Cohort study, at age 60–64 years, 1277 women and 1185 men (78% of the target sample) responded to a postal questionnaire, which included questions on thyroid disease and thyroid medication. Circulating antithyroid peroxidase antibodies, free T4, and TSH concentrations were measured in 1057 women and 997 men at a subsequent clinic visit. Birth weight was recorded, and height and weight were measured at ages 2, 4, 6, 7, 11, 15 years and also repeatedly in adulthood.
Results:
At age 60–64 years, 10.9% of women (139 of 1277) and 2.3% of men (27 of 1185) reported they were taking T4, and 11.5% of women (122 of 1057) and 3.3% of men (33 of 997) had positive anti-TPO antibodies (>100 IU/mL), consistent with thyroid autoimmunity. Among women, both T4 use and positive anti-TPO antibodies at age 60–64 years were positively associated with childhood body weight, childhood overweight, and adult body mass index. Childhood weight gain between 0 and 14 years of age was positively associated with later T4 use (odds ratio 1.21, 95% confidence interval 1.03–1.42) and positive anti-TPO antibodies (1.21, 1.00–1.47). Women who were overweight or obese at age 14 years (127 of 972) had a higher risk of later positive anti-TPO antibodies (2.05, 1.12–3.76). In men and women without any thyroid disorders, serum free T4 concentrations were inversely associated with concurrent body mass index (P = .002).
Conclusions:
Childhood weight gain and childhood overweight conferred an increased susceptibility to later hypothyroidism and thyroid autoimmunity, particularly in women.
A complex bidirectional interrelationship between body weight and thyroid function is recognized (1). Thyroid hormones increase thermogenesis and energy expenditure, and overt hyperthyroidism typically leads to weight loss and overt hypothyroidism leads to weight gain (2). There is also evidence for the opposite causal direction, ie, that changes in body weight may alter thyroid function. For example, in 1 study obese children had higher serum TSH and free T3 concentrations than lean children, and these concentrations declined in those children who lost weight (3). Similarly, obese adults often have isolated hyperthyrotropinemia (4), and weight loss after gastric banding leads to a reduction in free T3 and a rise in free T4 concentrations (5).
However, findings from studies that compare thyroid function between obese and normal-weight individuals are inconsistent (6), and their findings often differ from those in the general population. For example, in 1 large population-based study, body mass index (BMI) was inversely associated with serum free T4 concentrations (7), which is consistent with the expected physiological actions of T4 on energy expenditure. This discordance between reports could be explained by the possibility that changes in weight can alter thyroid function through several different mechanisms (8). In addition to proposals that the adipocytokine leptin promotes TSH secretion (9) and that adiposity influences the activity of the deiodinase enzymes D1 and D2 resulting in a higher free T3 to free T4 ratio (5), a recent study reported that obese adults had a 2-fold higher risk of thyroid autoimmunity compared with normal-weight controls (10).
Thyroid autoimmunity has a complex pathogenesis. Various environmental determinants have been identified, including nutritional factors (11). For example, iodine overload, or even modest increases in iodine intake, increase the risk for thyroid autoimmunity and overt thyroid dysfunction (12). Of interest, the genetic susceptibility loci for thyroid autoimmunity include many immunoregulatory genes that confer susceptibility to other autoimmune diseases, such as type 1 diabetes, multiple sclerosis (MS), and rheumatoid arthritis (13), and such common disease pathways may explain the observed clustering of autoimmune diseases (14). Rapid weight gain and obesity may contribute to the pathogenesis of autoimmune disorders (15). A recent meta-analysis identified a consistent prospective association between childhood BMI and subsequent type 1 diabetes, with obese children having a 2-fold higher risk of diabetes (16). Similarly, in separate large US and Swedish studies, obese adolescents had a 2-fold higher risk of developing MS (17, 18). The association between obesity and thyroid autoimmunity has so far been described in only 1 cross-sectional study in adults (10).
To examine the prospective influences of childhood and adult body weight on the risk of hypothyroidism and thyroid autoimmunity, in a large UK birth cohort study, we collected data on the reported use of thyroid medication and measured circulating antithyroid peroxidase (anti-TPO) antibodies at age 60–64 years and related these values to weight, BMI, and height across the life course. We also measured circulating free T4 and TSH concentrations at age 60–64 years and related these values to concurrent BMI.
Materials and Methods
Study design and data collection
The UK Medical Research Council National Survey of Health and Development is a prospective cohort of 2547 women and 2815 men, a socially stratified sample of all births that took place in England, Scotland, and Wales during 1 week in 1946 (19). Birth weight was extracted from medical records within 6 weeks of delivery, and heights and weights were measured by trained personnel at the ages of 2, 4, 6, 7, 11, 15, 36, 43, 53, and 60–64 years and were self-reported at the ages of 20 and 26 years. The study received Multi-Centre Research Ethics Committee approval, and written informed consent was given by the participants.
At age 60–64 years, 3163 participants were asked to complete a postal questionnaire, which included questions on thyroid disease and the use of thyroid medications (20). The remaining study members in the original cohort had died (n = 717), lived abroad (n = 567), had been untraced for more than 10 years (n = 319), or had previously withdrawn from the study (n = 595) (Stafford et al, manuscript under review). Of the target sample of 3163, 78% (1277 women and 1185 men) responded to this questionnaire; primary care physicians were subsequently contacted by questionnaire to validate positive self-reports of thyroid disease, including the age at first diagnosis. In instances in which there was missing age at first diagnosis of thyroid disease, responses were supplemented by similar information collected by nurse interview in 1999.
A fasting venous blood sample was collected at a research clinic or home visit at age 60–64 years on 2143 study members [75% of those sent invitations between 2 months and 2 years after the postal questionnaire (Stafford et al, manuscript under review)]. Thyroid function was analyzed at the Department of Clinical Biochemistry, Addenbrooke's Hospital, Cambridge, United Kingdom, according to the study's Duty of Care protocol (20). Serum TSH and free T4 were measured by chemiluminescent immunoassay (Advia Centaur; Bayer Diagnostics, Newbury, United Kingdom). Any sample with abnormal values of free T4 or TSH was also analyzed for free T3 concentrations, by DELFIA (PerkinElmer, Buckinghamshire, United Kingdom) up to August 2009 and then by Advia Centaur using an analogous time-resolved fluorescence method. Circulating titers of thyroid peroxidase (TPO) antibodies were measured by chemiluminescent immunoassay (LUMI-test anti-TPO; Brahms, Berlin, Germany). For TSH, coefficients of variation (CVs) were 5.4%, 4.1%, and 4.3% at 0.5, 6.6, and 42.1 mU/L, respectively. For free T4, CVs were 7.6% and 5.6% at 9.7 and 24.1 pmol/L, respectively. For free T3, CVs were 3.0% and 2.8% at 4.4 and 9.1 pmol/L, respectively. The manufacturer's normal range for TSH was 0.4–5.5 mIU/L and for free T4, 10–19.8 pmol/L. The normal range for free T3 was 3.0–7.5 pmol/L for samples analyzed by DELFIA (PerkinElmer) and 3.5–6.5 pmol/L by Advia Centaur (Bayer). Positive anti-TPO antibodies were defined as values greater than 100 IU/mL.
Calculations
Among those study members without known thyroid disease, we identified previously unsuspected overt and subclinical thyroid dysfunction using the following established and reported criteria (21): 1) overt hyperthyroidism (low TSH with raised free T4 and free T3 or raised free T3 alone); 2) subclinical hyperthyroidism (low TSH with normal free T4 and free T3); 3) euthyroid status (normal range TSH and free T4); 4) subclinical hypothyroidism (high TSH with normal free T4); or 5) overt hypothyroidism (high TSH with low free T4).
BMI was calculated as weight (kilograms) divided by height squared (meters squared). Measures of BMI, weight, and height were converted into SD scores (SDS) using internally generated, sex-specific growth charts, which is preferable for historical cohorts, and were constructed using the LMS method (22). Overweight or obesity at age 14 years was defined as BMI greater than the 90th centile according to sex-specific internal references. Childhood weight gain between 0 and 14 years was calculated as weight SDS at 14 years minus weight SDS at birth.
Statistical analysis
Comparisons between groups were tested by χ2 tests for categorical outcomes and ANOVA for continuous outcomes. Because of observed large differences in the prevalence of thyroid disorders between men and women, sex-specific analyses were performed to compare height, weight, BMI, and thyroid function test values between those with and without positive anti-TPO antibodies and the use of T4. Due to the very small numbers taking antithyroid medication (2 men and 3 women), we compared those taking T4 with those not taking any thyroid medication. The odds ratios for thyroid disorders by childhood weight gain between 0 and 14 years and for childhood overweight or obesity at 14 years were calculated using logistic regression, separately by sex. Childhood weight gain was also adjusted for the intercepts (birth weight and height at 2 years) and for height gain at 2–14 years. In final logistic regression models for risk of thyroid disorders, childhood weight at 14 years was added to models that included adult weight or BMI at 60–64 years.
To investigate the potential physiological relationships between thyroid function and concurrent BMI, we restricted further analyses to those participants without any thyroid disorders (ie, those not taking any thyroid medication, with negative anti-TPO antibodies and being biochemically euthyroid). Normal-range free T4 and TSH values were categorized into sex-specific quartiles, and these were related to BMI at 60–64 years using linear regression.
Results
Reported thyroid disorders
The use of T4 at age 60–64 years was more common in women (10.9%; 139 of 1277) than in men (2.3%; 27 of 1185; χ2, P < .0001). A further 0.2% (n = 3) of women and 0.2% (n = 2) of men reported taking antithyroid medication. Of those participants whose primary care physicians provided information, use of T4 to treat hypothyroidism was confirmed in 94 of 95 women (98.9%) and in 18 of 18 men (100%). Information on age at first diagnosis of hypothyroidism was available for 124 of 139 women and 23 of 27 men taking T4; age at first diagnosis was younger than 20 years in 1 woman (at age 2 years), 20–30 years in 1 woman, and older than 30 years in the remainder.
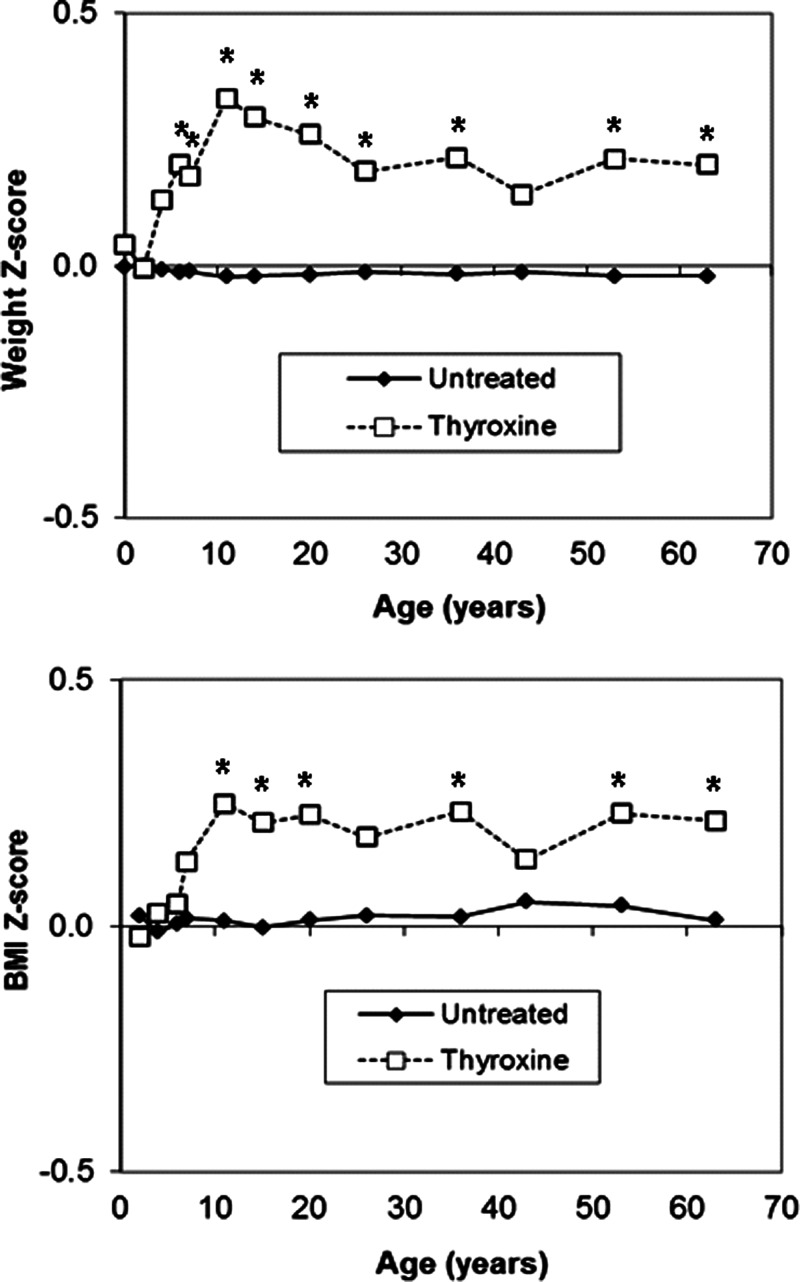
Women who reported taking T4 had higher body weight (P = .03) and higher BMI (P = .04; Table 1) at 60–64 years than women not taking any thyroid medication; in men no such differences were seen (both P > .3). Omission of the 1 woman with nonvalidated use of T4, and the 1 woman with a diagnosis of hypothyroidism before age 20 years did not substantially change the results (data not shown). When examining their previous body measurements, no differences were seen with birth weight (P = .6), but women taking T4 had higher body weights at ages 6, 7, 11, 15, 20, 26, 36, and 53 years and higher BMI at ages 11, 15, 20, 36, and 53 years, compared with other women (Figure 1) (see Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Table 1.
Body Size and Thyroid Function Test Results at Age 60–64 Years by Self-Reported Use of T4 Medication
| No Thyroid Medication | Taking T4 | Student's t Test (P Value) | |
|---|---|---|---|
| Women | |||
| BMI, kg/m2 | |||
| Mean | 27.8 ± 5.3 | 28.9 ± 5.4 | .04 |
| n | 947 | 110 | |
| Weight, kg | |||
| Mean | 72.8 ± 14.7 | 75.9 ± 14.8 | .03 |
| n | 948 | 110 | |
| Height, cm | |||
| Mean | 1.62 ± 0.06 | 1.62 ± 0.06 | .4 |
| n | 949 | 110 | |
| Free T4, pmol/L | |||
| Mean | 14.4 ± 2.6 | 17.3 ± 3.5 | <.001 |
| n | 876 | 102 | |
| TSH, mIU/L | |||
| Mean | 3.1 ± 5.5 | 2.6 ± 3.6 | <.001 |
| n | 874 | 102 | |
| Men | |||
| BMI, kg/m2 | |||
| Mean | 27.9 ± 4.1 | 26.9 ± 4.2 | .3 |
| n | 940 | 21 | |
| Weight, kg | |||
| Mean | 85.3 ± 13.5 | 83.1 ± 13.1 | .4 |
| n | 940 | 22 | |
| Height, cm | |||
| Mean | 1.75 ± 0.07 | 1.75 ± 0.08 | .8 |
| n | 941 | 21 | |
| Free T4, pmol/L | |||
| Mean | 14.6 ± 2.2 | 16.5 ± 3.4 | <.001 |
| n | 892 | 20 | |
| TSH, mIU/L | |||
| Mean | 2.3 ± 3.1 | 4.3 ± 10.5 | .9 |
| n | 885 | 20 |
Mean ± SD are displayed. These data exclude 3 women and 2 men who were taking antithyroid medication.
Figure 1.
Mean weight and BMI Z-scores from birth to age 60–64 years in women taking T4 and untreated women. *P < .05.
Women taking T4 had higher circulating free T4 concentrations and lower TSH concentrations than untreated women (Table 1), which reduces the likelihood of inadequate thyroid replacement as a cause of their higher body weight and BMI.
Thyroid autoimmunity
At age 60–64 years, the prevalence of positive anti-TPO antibodies (>100 IU/mL) was more common in women (11.5%; 122 of 1057) than in men (3.3%; 33 of 997; P < .0001). Among those taking T4, the prevalence of positive anti-TPO antibodies was similar in women (38.6%; 39 of 101) and in men (35.0%; 7 of 20). Accordingly, among those with positive anti-TPO antibodies, the prevalence of T4 use was similar in women (32.0%; 39 of 122) and in men (21.2%; 7 of 33).
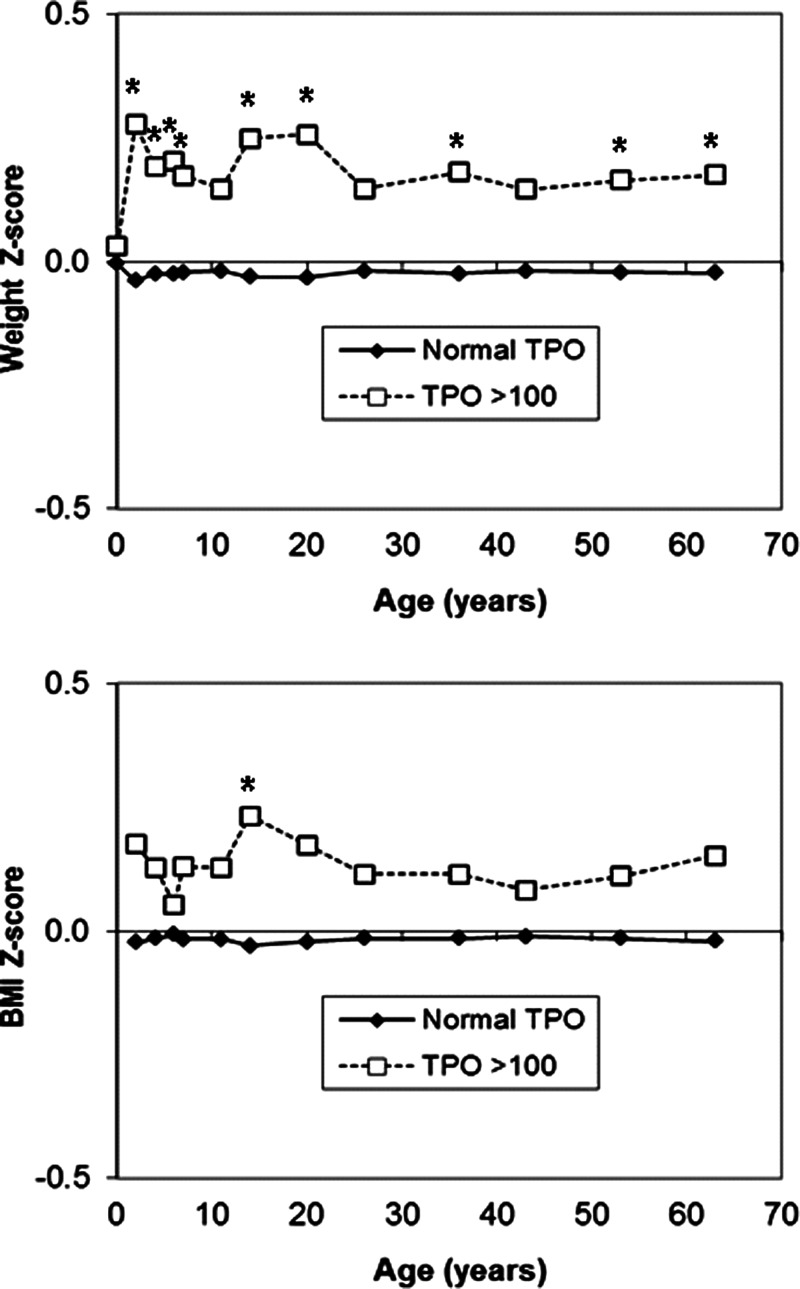
Women with positive anti-TPO antibodies had higher body weight (P = .04) and tended to have higher BMI (P = .08) than other women; in men no such differences were seen (Table 2). Similar trends to higher BMI and body weight by anti-TPO antibody positivity were seen among those women not taking any thyroid medication (BMI: P = .07; weight: P = .02, data not shown). When examining their previous body measurements, no differences were seen with birth weight (P = .7), but women with positive anti-TPO antibodies had higher body weights at ages 2, 4, 6, 7, 15, 20, 36, and 53 years and higher BMI at age 14 years (Figure 2 and Supplemental Table 2).
Table 2.
Body Size and Thyroid Function Test Results at Age 60–64 Years by Anti-TPO Antibody Levels
| Anti TPO <100 IU/mL | Anti TPO >100 IU/mL | Student's t Test (P Value) | |
|---|---|---|---|
| Women | |||
| BMI, kg/m2 | |||
| Mean | 27.7 ± 5.2 | 28.5 ± 5.7 | .08 |
| N | 931 | 122 | |
| Weight, kg | |||
| Mean | 72.4 ± 14.5 | 75.3 ± 15.6 | .04 |
| N | 932 | 122 | |
| Height, cm | |||
| Mean | 1.62 ± 0.06 | 1.62 ± 0.06 | .2 |
| N | 933 | 122 | |
| Free T4, pmol/La | |||
| Mean | 14.5 ± 2.2 | 13.8 ± 6.0 | .01 |
| N | 870 | 83 | |
| TSH, mIU/La | |||
| Mean | 2.6 ± 2.2 | 7.8 ± 16.0 | <.001 |
| N | 869 | 82 | |
| Men | |||
| BMI, kg/m2 | |||
| Mean | 27.9 ± 4.1 | 28.0 ± 4.8 | .9 |
| N | 959 | 33 | |
| Weight, kg | |||
| Mean | 85.3 ± 13.4 | 86.5 ± 15.1 | .6 |
| N | 959 | 33 | |
| Height, cm | |||
| Mean | 1.75 ± 0.07 | 1.76 ± 0.06 | .4 |
| N | 960 | 33 | |
| Free T4, pmol/La | |||
| Mean | 14.6 ± 2.2 | 12.8 ± 2.6 | <.001 |
| N | 948 | 26 | |
| TSH, mIU/La | |||
| Mean | 2.1 ± 1.2 | 7.8 ± 15.8 | <.001 |
| n | 941 | 26 |
Mean ± SD are displayed. Anti-TPO antibody levels greater than 100 IU/mL were taken as indicative of thyroid autoimmunity.
Free T4 and TSH analyses were restricted to study members not taking thyroid medications.
Figure 2.
Mean weight and BMI Z-scores from birth to age 60–64 years in women with high (>100 IU/mL) vs negative anti-TPO antibodies. *P < .05.
Among those not taking any thyroid medication, men and women with positive anti-TPO antibodies had lower serum free T4 and higher TSH concentrations than those with negative anti-TPO antibodies (Table 2).
Childhood weight gain and overweight
Independent of childhood height gain, each +1 SDS in childhood weight gain between 0 and 14 years was associated with higher risks for T4 use [odds ratio (OR) 1.42, 95% confidence interval (CI) 1.09–1.84] and positive anti-TPO antibodies (OR 1.71, 95% CI 1.23–2.37) at 60–64 years in women but not in men (Table 3). Accordingly, women who were overweight or obese at age 14 years (127 of 972) had a trend to higher risk for T4 use (OR 1.36, 95% CI 0.80–2.31) and a higher risk of positive anti-TPO antibodies (OR 2.05, 95% CI 1.12–3.76). Men who were overweight or obese at age 14 years (137 of 924) had a higher risk of T4 use at 60–64 years (OR 3.50, 95% CI 1.16–10.61) but no difference in risk of positive anti-TPO antibodies (OR 1.16, 95% CI 0.38–3.51) (Table 3). After adjustment for childhood weight at age 14 years, adult weight and BMI at 60–64 years were no longer associated with T4 use or positive anti-TPO antibodies in men or women (all P > .1, data not shown).
Table 3.
Odds Ratios for Thyroid Disorders in Men and Women at Age 60–64 Years by Childhood Weight Gain, Height Gain and Weight Status at Age 14 Years
| Women | Men | |
|---|---|---|
| Use of thyroxine | ||
| Weight gain 0–14 ya | 1.42, 1.09–1.84 | 1.32, 0.60–2.86 |
| Height gain 2–14 ya | 1.07, 0.82–1.41 | 0.98, 0.45–2.11 |
| Overweight or Obese at 14 yb | 1.36, 0.80–2.31 | 3.50, 1.16–10.61 |
| High anti-TPO antibodies (>100 IU/ml) | ||
| Weight gain 0–14 ya | 1.71, 1.23–2.37 | 0.97, 0.46–2.03 |
| Height gain 2–14 ya | 1.00, 0.73–1.39 | 1.17, 0.57–2.40 |
| Overweight or Obese at 14 yb | 2.05, 1.12–3.76 | 1.16, 0.38–3.51 |
Statistically significant (P < .05) associations are highlighted in bold type.
Odds ratios (95% CI) are shown per +1 U gain in weight SDS between 0–14 yr, and per +1 U gain in height SDS between 2–14 yr. Models included both weight gain and height gain, and were also adjusted for birth weight SDS and height SDS at age 2 yr.
Odds ratios (95% CI) are shown for overweight or obesity at age 14 yr (defined as BMI >90th centile according to sex-specific internal references) vs. all others.
Thyroid function tests
At age 60–64 years, thyroid function test values were used to categorize study subjects not taking any thyroid medication into categories of previously unsuspected overt or subclinical thyroid dysfunction using established criteria (Table 4) (21). Fewer women (91.1%; 874 of 959) were categorized as biochemically euthyroid than men (97.5%; 953 of 977). Having positive anti-TPO antibodies was associated with a higher risk of overt or subclinical hypothyroidism in women (OR 8.6, 95% CI 5.0–14.9) and in men (OR 21.3, 95% CI 7.3–61.7).
Table 4.
Prevalence of Undiagnosed Thyroid Disorders at Age 60–64 Years By Sex
| Categorya | Women | Men | Total |
|---|---|---|---|
| Overt hyperthyroidism | 4 (0.4%) | 1 (0.1%) | 5 (0.3%) |
| Subclinical hyperthyroidism | 7 (0.7%) | 4 (0.4%) | 11 (0.6%) |
| Euthyroid | 874 (91.1%) | 953 (97.5%) | 1827 (94.4%) |
| Subclinical hypothyroidism | 65 (6.8%) | 18 (1.8%) | 83 (4.3%) |
| Overt hypothyroidism | 9 (0.9%) | 1 (0.1%) | 10 (0.5%) |
| Total | 959 | 977 | 1936 |
Based on categories described by Wilson et al (21).
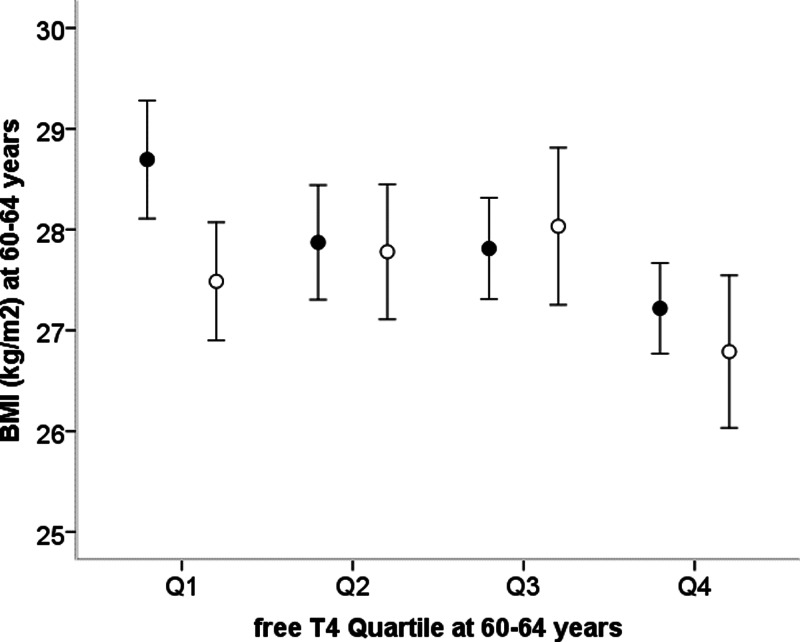
Among those men (n = 906) and women (n = 806) without any thyroid disorders (ie, not taking any thyroid medication, negative anti-TPO antibodies, and euthyroid thyroid function test values), higher free T4 quartile was associated with lower concurrent BMI (linear trend, P = .002, adjusted for sex; Figure 3). Within each sex, those in the highest free T4 quartile had lower BMI than those in any of the 3 lower quartiles (mean ± SD in women, 26.8 ± 5.4 vs 27.7 ± 5.1 kg/m2; P = .03; in men, 27.3 ± 3.4 vs 28.2 ± 4.3, P = .004).
Figure 3.
Mean ± 95% confidence intervals for BMI at age 60–64 years by quartiles of circulating free T4 concentration in women (open circles) and men (solid circles) without any thyroid disorders.
There was no association between TSH quartile and concurrent BMI in women (linear trend, P = .3) or in men (linear trend, P = .6) separately or combined (linear trend, P = .3, adjusted for sex).
Discussion
In a large prospective birth cohort study, we found evidence for both pathological and physiological links between body weight and thyroid function. Women taking T4 and women with positive anti-TPO antibodies at age 60–64 years were heavier as children and as adults than other women, they had faster weight gain in childhood independent of height gain, and they were more likely to have been overweight or obese as children. Separately, in men and women without any detectable thyroid disorders, we identified an inverse relationship between serum free T4 concentrations and concurrent BMI, which is consistent with previous studies (7) and also consistent with the expected physiological influence of T4 on energy expenditure (2).
Strengths and limitations
The main strengths of the study are its representative nature and its long-term prospective design, which allowed robust assessment of the timing of childhood weight gain on later thyroid outcomes. Furthermore, we found consistent associations between childhood weight status or weight gain and both self-reported T4 use and objectively assessed thyroid antibody status.
Limitations of our study include the lack of measures of thyroid autoantibodies or thyroid function prior to 60–64 years. Autoimmune hypothyroidism is the most common cause of hypothyroidism in this age group in the United Kingdom (23), yet less than 40% of study members taking T4 had positive anti-TPO antibodies, probably reflecting the decline in thyroid antibody titers after T4 treatment and time from diagnosis (24). Repeated measures of anti-TPO antibody levels during adult life may have confirmed more study members with thyroid autoimmunity but whose antibody levels regressed spontaneously or after T4 treatment. Further follow-up measurements would also have allowed us to assess the prospective influence of free T4 and TSH concentrations on adult body weight. T4 use was based on self-reported information and used as a proxy for the diagnosis of hypothyroidism; in more than two thirds of these cases, we ascertained information from primary care physicians who provided validation in all but 1 case. Our analyses relating to clinical outcomes were restricted to hypothyroidism because there were few cases of hyperthyroidism. We found inconsistent associations in men, which likely reflects the lower prevalence of autoimmune thyroid disease in men and therefore reduced power, although there may be other explanations such as possible differences in pathogenesis between men and women.
Comparison with other studies
The prevalences of T4 use, high anti-TPO antibodies, and previously undiagnosed overt or subclinical hypothyroidism were higher in women than in men, as expected, and were broadly similar to those reported in other population-based studies. In a large Danish survey, the prevalence of detectable anti-TPO antibodies (>30 U/mL) in women and men aged 60–65 years was 21.7% and 7.3%, respectively, and was higher in areas with moderate vs mild iodine deficiency (25). In a slightly older UK population (mean age 73 years) without known thyroid disease prior to biochemical screening, 4.1% of women and 2.4% of men had subclinical or overt hypothyroidism (21).
Among biochemically euthyroid study members with negative anti-TPO antibodies, we found no association between serum TSH concentration and BMI. Some, but not all, studies have reported positive associations between TSH concentrations and adiposity among euthyroid subjects (26). However, such associations may reflect adiposity driving a compensatory higher TSH secretion and hence higher thyroid hormone production, or higher serum TSH being a marker of relative thyroid hormone deficiency.
Previous smaller studies have suggested that thyroid function and thyroid disorders in women may have developmental origins. In a UK birth cohort study of 303 women (27), in those who had been bottle fed, serum free T4 concentrations at age 60–71 years fell and serum TSH concentrations rose with increasing birth weight. In a Finnish birth cohort study of 293 women (28), those women (n = 20) with adult-onset hypothyroidism had lower birth weight and shorter birth length, and they remained shorter in early childhood and thinner during later childhood; at age 61 years, they were still shorter but had no differences in BMI than the other women, findings that are inconsistent with our larger study. Similarly, an initial observation of positive anti-TPO antibodies in lower-birth-weight twins was not confirmed in a larger cohort (29) or in our current analysis; however, the lack of data on gestational age is a further limitation of our study. In contrast, we found that weight gain after birth was positively associated with later T4 use and positive thyroid autoantibodies in women.
Implications for the origins of thyroid autoimmunity
The mechanisms linking greater childhood weight gain and childhood overweight to hypothyroidism and thyroid autoimmunity in later life are unclear. They could include specific actions of adiposity or diet on thyroid hormone production or presentation of thyroid antigens to the immune system. Nutritional factors implicated in the risk of thyroid autoimmunity include iodine overload or supplementation and deficiencies of selenium, zinc, vitamin B12, and vitamin D (11), and these may be more prevalent in some overweight children. Alternatively, childhood weight gain and overweight may increase the risk of autoimmune disorders in general because similar positive associations with childhood weight status have been identified with the risk of other autoimmune diseases, such as type 1 diabetes (16, 30) and MS (17, 18). The potential effect sizes of childhood overweight or obesity on these outcomes are substantial, with approximately 2-fold higher relative risks for type 1 diabetes, MS, and thyroid autoimmunity. These autoimmune disorders share a number of genetic susceptibility loci involved in immune function (13), and obesity is associated with chronic low inflammation (31). Therefore, rapid childhood weight gain and adiposity could potentially trigger common pathogenic mechanisms to autoimmune disease, possibly by exacerbating specific inflammatory responses or by the actions of adipocytokines on reducing immune tolerance (15).
We did not have information on a family history of thyroid disease and autoimmunity to examine whether these associations might reflect a common genetic mechanism; however, there is yet no evidence of overlap between the genetic variants for autoimmune disease (13) and those for obesity (32). We suggest that future analyses could use genetic factors for obesity as instrumental variables to examine the causal relationship between weight gain and autoimmunity.
Conclusions
We identified novel associations between childhood weight gain, or childhood overweight, and later thyroid autoimmunity and hypothyroidism, particularly in women. These novel findings require confirmation in other studies, but they provide increasing support for the recommendation that finding mild thyroid dysfunction in overweight and obese individuals should not lead automatically to treatment but should prompt careful clinical and biochemical evaluation (1). Furthermore, we propose that a common mechanism links rapid childhood weight gain to the pathogenesis of autoimmune disorders in general, including thyroid autoimmunity. Although the prevention of childhood overweight is an established public health target, the identification of specific inflammatory or immune system pathways linking childhood adiposity to autoimmunity may allow potential future intervention in those at high risk of autoimmune disorders.
Supplementary Material
Acknowledgments
We thank the National Survey of Health and Development study members as well as the staff involved in data collection for this cohort over the last 65 years.
This work was supported by the UK Medical Research Council Grants U120063239, U123092720, and U105960371.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- anti-TPO
- antithyroid peroxidase
- BMI
- body mass index
- CI
- confidence interval
- CV
- coefficient of variation
- MS
- multiple sclerosis
- OR
- odds ratio
- SDS
- SD score.
References
- 1. Rotondi M, Magri F, Chiovato L. Thyroid and obesity: not a one-way interaction. J Clin Endocrinol Metab. 2011;96:344–346 [DOI] [PubMed] [Google Scholar]
- 2. Danforth E, Jr, Burger A. The role of thyroid hormones in the control of energy expenditure. Clin Endocrinol Metab. 1984;13:581–595 [DOI] [PubMed] [Google Scholar]
- 3. Reinehr T, de Sousa G, Andler W. Hyperthyrotropinemia in obese children is reversible after weight loss and is not related to lipids. J Clin Endocrinol Metab. 2006;91:3088–3091 [DOI] [PubMed] [Google Scholar]
- 4. Rotondi M, Leporati P, La Manna A, et al. Raised serum TSH levels in patients with morbid obesity: is it enough to diagnose subclinical hypothyroidism? Eur J Endocrinol. 2009;160:403–408 [DOI] [PubMed] [Google Scholar]
- 5. Dall'Asta C, Paganelli M, Morabito A, et al. Weight loss through gastric banding: effects on TSH and thyroid hormones in obese subjects with normal thyroid function. Obesity (Silver Spring). 2010;18:854–857 [DOI] [PubMed] [Google Scholar]
- 6. Biondi B. Thyroid and obesity: an intriguing relationship. J Clin Endocrinol Metab. 2010;95:3614–3617 [DOI] [PubMed] [Google Scholar]
- 7. Knudsen N, Laurberg P, Rasmussen LB, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90:4019–4024 [DOI] [PubMed] [Google Scholar]
- 8. Reinehr T. Obesity and thyroid function. Mol Cell Endocrinol. 2010;316:165–171 [DOI] [PubMed] [Google Scholar]
- 9. Mantzoros CS, Ozata M, Negrao AB, et al. Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: evidence for possible partial TSH regulation by leptin in humans. J Clin Endocrinol Metab. 2001;86:3284–3291 [DOI] [PubMed] [Google Scholar]
- 10. Marzullo P, Minocci A, Tagliaferri MA, et al. Investigations of thyroid hormones and antibodies in obesity: leptin levels are associated with thyroid autoimmunity independent of bioanthropometric, hormonal, and weight-related determinants. J Clin Endocrinol Metab. 2010;95:3965–3972 [DOI] [PubMed] [Google Scholar]
- 11. Duntas LH. Environmental factors and thyroid autoimmunity. Ann Endocrinol (Paris). 2011;72:108–113 [DOI] [PubMed] [Google Scholar]
- 12. Laurberg P, Cerqueira C, Ovesen L, et al. Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res Clin Endocrinol Metab. 2010;24:13–27 [DOI] [PubMed] [Google Scholar]
- 13. Brand OJ, Gough SC. Immunogenetic mechanisms leading to thyroid autoimmunity: recent advances in identifying susceptibility genes and regions. Curr Genomics. 2011;12:526–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boelaert K, Newby PR, Simmonds MJ, et al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med 2010;123:183.e181–e189 [DOI] [PubMed] [Google Scholar]
- 15. Hersoug LG, Linneberg A. The link between the epidemics of obesity and allergic diseases: does obesity induce decreased immune tolerance? Allergy. 2007;62:1205–1213 [DOI] [PubMed] [Google Scholar]
- 16. Verbeeten KC, Elks CE, Daneman D, Ong KK. Association between childhood obesity and subsequent type 1 diabetes: a systematic review and meta-analysis. Diabet Med. 2011;28:10–18 [DOI] [PubMed] [Google Scholar]
- 17. Munger KL, Chitnis T, Ascherio A. Body size and risk of MS in two cohorts of US women. Neurology. 2009;73:1543–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hedstrom AK, Olsson T, Alfredsson L. High body mass index before age 20 is associated with increased risk for multiple sclerosis in both men and women. Mult Scler. 2012;18:1334–1336 [DOI] [PubMed] [Google Scholar]
- 19. Wadsworth M, Kuh D, Richards M, Hardy R. Cohort profile: the 1946 National Birth Cohort (MRC National Survey of Health and Development). Int J Epidemiol. 2006;35:49–54 [DOI] [PubMed] [Google Scholar]
- 20. Kuh D, Pierce M, Adams J, et al. Cohort profile: updating the cohort profile for the MRC National Survey of Health and Development: a new clinic-based data collection for ageing research. Int J Epidemiol. 2011;40:e1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilson S, Parle JV, Roberts LM, et al. Prevalence of subclinical thyroid dysfunction and its relation to socioeconomic deprivation in the elderly: a community-based cross-sectional survey. J Clin Endocrinol Metab. 2006;91:4809–4816 [DOI] [PubMed] [Google Scholar]
- 22. Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11:1305–1319 [DOI] [PubMed] [Google Scholar]
- 23. Roberts CG, Ladenson PW. Hypothyroidism. Lancet. 2004;363:793–803 [DOI] [PubMed] [Google Scholar]
- 24. Rieu M, Richard A, Rosilio M, et al. Effects of thyroid status on thyroid autoimmunity expression in euthyroid and hypothyroid patients with Hashimoto's thyroiditis. Clin Endocrinol (Oxf). 1994;40:529–535 [DOI] [PubMed] [Google Scholar]
- 25. Pedersen IB, Knudsen N, Jorgensen T, Perrild H, Ovesen L, Laurberg P. Thyroid peroxidase and thyroglobulin autoantibodies in a large survey of populations with mild and moderate iodine deficiency. Clin Endocrinol (Oxf). 2003;58:36–42 [DOI] [PubMed] [Google Scholar]
- 26. de Moura Souza A, Sichieri R. Association between serum TSH concentration within the normal range and adiposity. Eur J Endocrinol. 2011;165:11–15 [DOI] [PubMed] [Google Scholar]
- 27. Phillips DI, Barker DJ, Osmond C. Infant feeding, fetal growth and adult thyroid function. Acta Endocrinol (Copenh). 1993;129:134–138 [DOI] [PubMed] [Google Scholar]
- 28. Kajantie E, Phillips DI, Osmond C, Barker DJ, Forsen T, Eriksson JG. Spontaneous hypothyroidism in adult women is predicted by small body size at birth and during childhood. J Clin Endocrinol Metab. 2006;91:4953–4956 [DOI] [PubMed] [Google Scholar]
- 29. Brix TH, Hansen PS, Rudbeck AB, et al. Low birth weight is not associated with thyroid autoimmunity: a population-based twin study. J Clin Endocrinol Metab. 2006;91:3499–3502 [DOI] [PubMed] [Google Scholar]
- 30. Harder T, Roepke K, Diller N, Stechling Y, Dudenhausen JW, Plagemann A. Birth weight, early weight gain, and subsequent risk of type 1 diabetes: systematic review and meta-analysis. Am J Epidemiol. 2009;169:1428–1436 [DOI] [PubMed] [Google Scholar]
- 31. Calder PC, Ahluwalia N, Brouns F, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Brit J Nutr. 2011;106(suppl 3:S5–S78 [DOI] [PubMed] [Google Scholar]
- 32. Speliotes EK, Willer CJ, Berndt SI, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.