Abstract
Background
Hypoglycaemic events can be a serious complication of insulin therapy in Type 1 diabetes mellitus. Severe hypoglycaemic exposure can lead to episodic memory impairments, including anterograde amnesia. However, relatively little is known regarding the long-term impact of severe hypoglycaemia on brain structure in Type 1 diabetes mellitus. The goals of the present study were to gain a greater understanding of the long-term effects of severe hypoglycaemia exposure on brain structure and the neural correlates of memory impairments in Type 1 diabetes mellitus.
Case report
Regional grey and white matter volume and total white matter lesion volume were quantified in an individual with long-standing hypoglycaemia-induced anterograde amnesia and compared with age- and gender-matched healthy control subjects. Our patient has significant reductions in grey matter volume in the hippocampus, thalamus and pallidum, and significant reductions in white matter volume in the splenium, isthmus of the cingulate and cerebellum. He also has a significantly larger total white matter lesion volume than control subjects.
Conclusion
This case study highlights the potential of hypoglycaemia for permanent deleterious effects on brain structure and memory function. Our results suggest that subcortical grey matter, periventricular white matter and posterior white matter may be most susceptible to injury from hypoglycaemia exposure, and that structural damage to the hippocampus and isthmus of the cingulate may play a central role in hypoglycaemia-induced memory impairments.
Introduction
Hypoglycaemic events can be a serious complication of insulin therapy in Type 1 diabetes mellitus [1]. Severe hypoglycaemia can lead to acute and chronic decrements in cognition, with memory function being particularly vulnerable [2,3]. For example, anterograde amnesia, the inability to form new memories of events, has been reported following severe hypoglycaemia in Type 1 diabetes [4–6]. However, relatively little is known regarding the long-term impact of severe hypoglycaemia on brain structure in Type 1 diabetes. In cross-sectional research in adolescents and adults, some studies have not found significant alterations in brain structure associated with severe hypoglycaemia exposure [7,8]. Others have reported reductions in grey matter volume in the left posterior cerebellum [9], and the thalamus [10] and uncus [9] bilaterally. Clinical magnetic resonance imaging (MRI) scans administered within hours of severe hypoglycaemia have revealed abnormal signals in the hippocampus [4–6], thalamus [11], basal ganglia [12], cortical grey matter [4,5,11–13], splenium [14,15], internal capsule [13,15], centrum semiovale [11] and corona radiata [13]. Short-term clinical follow-up suggests that some of these signal abnormalities may lessen or even resolve over time [4,6,13,14], but longitudinal MRI and cognitive data are scarce. No quantitative structural analyses have compared these cases with age- and gender-matched control subjects, potentially missing important effects.
The present study quantified regional brain structure abnormalities in an individual with a history of multiple severe hypoglycaemic events, including one that resulted in permanent anterograde amnesia. Our goals were to examine the long-term effects of hypoglycaemia exposure on brain structure and the neural correlates of memory impairments in Type 1 diabetes.
Clinical history
Our patient was diagnosed with Type 1 diabetes at age 2 years. He experienced multiple episodes of severe hypoglycaemia (defined by American Diabetes Association criteria [16]) before he was 10 years of age, including one that resulted in transient right-sided upper extremity paralysis and confusion. In 1991, at 24 years of age, he was taken to the emergency department after having been found to be drowsy and disorientated. He was given an infusion of 50% dextrose in the ambulance. His blood glucose had recovered to 17.1 mmol/l at arrival in the emergency department. A neurological examination administered in the emergency department was normal except for disorientation, retrograde amnesia for recent events and profound anterograde amnesia. No structural abnormalities were noted on neuroradiological readings of a clinical computed tomography (CT) scan administered in the emergency department nor a clinical MRI exam that included proton density, T2-weighted and T1-weighted (with and without contrast) sequences administered the day after his hospital admission. His electroencephalogram (EEG) was also normal during his hospitalization. From an extensive medical diagnostic examination and neuropsychological testing, he was diagnosed with amnesia resulting from a hypoglycaemic episode. Between 1991 and 2010, he had at least four additional episodes of severe hypoglycaemia. However, his exposure to hyperglycaemia has been relatively minimal, as documented by his average HbA1c from 2000 to 2010 of 56 mmol/mol (7.3%) [range 46 mmol/mol (6.4%) to 70 mmol/mol (8.6%)] and the absence of episodes of diabetic ketoacidosis. In 2010, at age 43 years, no signs or symptoms of retinopathy, nephropathy or peripheral neuropathy were detected in the history or physical examination, and his vitamin B12 levels were within the normal range. However, an electrocardiogram revealed a borderline R-R variability ratio of 1.18, suggesting that he may have been in the beginning stages of cardiovascular autonomic neuropathy. He also had a score of 21 on the Beck Depression Inventory II, suggesting that he had a moderate level of depressive symptoms.
Neuropsychological assessments
Our patient’s intelligence quotient (IQ) and memory performance were assessed in 1991 and 2010, and were compared with a normative database. He was given the Wechsler Adult Intelligence Scale—Revised (WAIS-R), Wechsler Memory Scale—Revised (WMS-R), California Verbal Learning Test (CVLT) and Rey Complex Figure Test (RCFT; 2010 only).
Control subjects
Archival MRI data from 20 men (mean age 43 years, range 30–54 years) were used in volumetric analyses. They were screened for traumatic brain injuries and neurological and psychiatric conditions.
MRI data acquisition
The MRI examination included T1-weighted magnetization-prepared rapid acquisition with gradient echo (MP-RAGE) (1× 1× 1-mm sagittal slices, TR = 2400, TE = 3.16, TI = 1000, flip angle = 8, frames = 176; patient and control subjects), T2-weighted (1× 1× 1-mm sagittal slices, TR = 3200, TE = 455, flip angle = 120, frames = 176; patient only) and fluid attenuated inversion recovery (FLAIR) (0.86× 0.86× 3-mm transverse slices, TR = 9190, TE = 94, TI = 2500, flip angle = 150, frames = 42; patient only) scans acquired on a 3-Tesla Siemens Trio scanner (XXXX, XXXX).
Automated calculation of grey and white matter volume
FreeSurfer (v5.0; http://surfer.nmr.mgh.harvard.edu/) was used to calculate raw regional subcortical grey, gyral grey, subcortical white and gyral white matter volumes from high-resolution T1-weighted images of control subjects and our patient from 2010. Raw regional volumes were totalled across hemispheres and divided by intracranial volume to account for variability in head size. Total white matter lesion burden was calculated with FreeSurfer by dividing the raw total volume of hypointense regions within white matter on the T1-weighted images by intracranial volume.
Statistical analyses
Crawford and Howell’s [17] modified t-test was used to compare our patient’s brain measurements with those of the control subjects. For a priori region of interest (grey matter: hippocampus, thalamus, caudate, putamen and pallidum; white matter: splenium) and total white matter lesion volume analyses, we set statistical significance at P < 0.05, one-tailed. We hypothesized that our patient’s grey and white matter volumes in these regions of interest would be smaller and that his total white matter lesion volume would be greater than those of the control subjects. For exploratory regions, significance was set at P < 0.05, two-tailed. Research procedures were approved by the Human Studies Committees of University of Missouri – St Louis and Washington University.
Results
Neuropsychological findings (Table 1)
Table 1.
Neuropsychological assessments of our patient’s cognitive function
| Testing session | ||||
|---|---|---|---|---|
| Autumn 1991 | Spring 2010 | |||
| Score | Percentile | Score | Percentile | |
| Wechsler Adult Intelligence Scale— Revised (WAIS-R) | ||||
| Verbal IQ | 116 | 86 | 119 | 90 |
| Performance IQ | 83 | 13 | 109 | 73 |
| Full-scale IQ | 101 | 53 | 117 | 87 |
| Wechsler Memory Scale—Revised (WMS-R) | ||||
| General memory | < 50 | < 0.1 | 96 | 39 |
| Verbal memory | < 50 | < 0.1 | 96 | 39 |
| Visual memory | < 50 | < 0.1 | 103 | 58 |
| Attention/concentration | 93 | 32 | 123 | 94 |
| Delayed recall | < 50 | < 0.1 | 65 | 1 |
| California Verbal Learning Test (CVLT) | ||||
| Trial 1 | 5 | 2 | 6 | 16 |
| Trial 5 | 7 | < 0.1 | 11 | 16 |
| List B | 4 | 2 | 8 | 85 |
| Short delayed recall | 0 | < 0.1 | 7 | 2 |
| Short cued recall | 0 | < 0.1 | 9 | 2 |
| Long delayed recall | 0 | < 0.1 | 11 | 50 |
| Long cued recall | 0 | < 0.1 | 12 | 50 |
| Recognition | 1 | < 0.1 | 16 | 85 |
| Rey Complex Figure Test (RCFT)* | ||||
| Copy | — | — | 36/36 | > 16 |
| Short delayed recall | — | — | 17.5/36 | 21 |
| Long delayed recall | — | — | 15/36 | 7 |
Not administered during autumn of 1991.
Our patient’s full-scale IQ score in 2010 was within the high average range. At his initial assessment, his memory scores were below the 5th percentile, indicating severe deficits in immediate recall, delayed recall and recognition. He performed better on memory tests in 2010, particularly within the recognition memory domain, suggesting some recovery over time. However, his general memory and delayed recall scores on the Wechsler Memory Scale—Revised were still more than 20 standard scale points below his full-scale IQ, consistent with anterograde amnesia [18].
Neuroradiological assessment of our patient’s MRI scans (Fig. 1)
Figure 1.
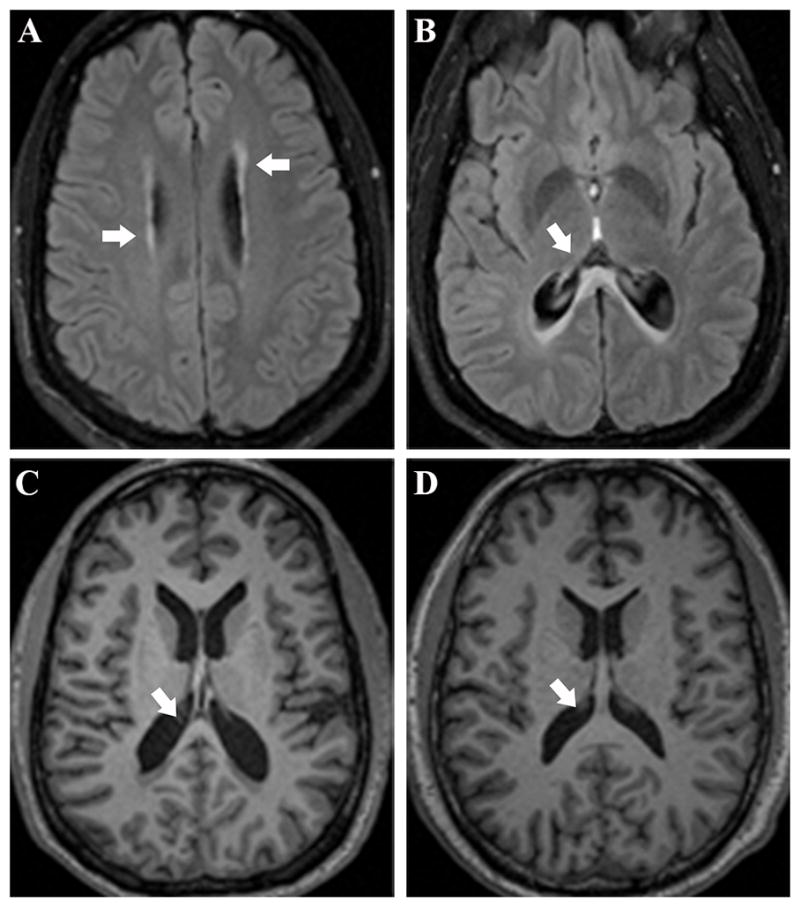
(a) Axial fluid attenuated inversion recovery (FLAIR) image of our patient demonstrates hyperintense signal in the periventricular white matter. (b) Axial FLAIR image of our patient demonstrates hyperintense signal in the splenium. (c) Magnetization-prepared rapid acquisition with gradient echo (MP-RAGE) image showing marked atrophy in our patient’s splenium. (d) MP-RAGE image showing the splenium of a 42-year-old healthy control subject.
Clinical readings of our patient’s 2010 MRI scans by a board-certified neuroradiologist (JSS) revealed a thin rim of hyperintense signal in the periventricular white matter on the FLAIR. There was also hyperintense signal on the FLAIR and T2, and hypointense signal on the T1, in the splenium of the corpus callosum, which extended into adjacent white matter.
Quantitative comparisons of grey and white matter volumes (Tables 2 and 3)
Table 2.
Regional grey matter volume of our patient and our control subjects
| Region | Control subjects (%) | Patient (%) | t | P | z† | |
|---|---|---|---|---|---|---|
| Mean | SD | |||||
| A priori regions of interest | ||||||
| Hippocampus* | 0.56 | 0.05 | 0.42 | −2.55 | 0.010 | −2.61 |
| Thalamus* | 0.91 | 0.06 | 0.76 | −2.55 | 0.010 | −2.61 |
| Caudate | 0.47 | 0.06 | 0.38 | −1.50 | 0.075 | −1.54 |
| Putamen | 0.73 | 0.06 | 0.63 | −1.68 | 0.055 | −1.72 |
| Pallidum* | 0.22 | 0.02 | 0.18 | −2.36 | 0.014 | −2.42 |
| Exploratory regions of interest | ||||||
| Superior frontal | 2.93 | 0.40 | 3.09 | 0.40 | 0.696 | 0.41 |
| Precentral | 1.76 | 0.24 | 1.96 | 0.84 | 0.410 | 0.86 |
| Caudal middle frontal | 0.81 | 0.12 | 0.94 | 1.02 | 0.320 | 1.05 |
| Rostral middle frontal | 2.06 | 0.30 | 2.04 | −0.07 | 0.943 | −0.07 |
| Pars opercularis | 0.58 | 0.09 | 0.47 | −1.13 | 0.274 | −1.15 |
| Pars triangularis | 0.50 | 0.09 | 0.49 | −0.08 | 0.941 | −0.08 |
| Pars orbitalis | 0.29 | 0.04 | 0.27 | −0.37 | 0.719 | −0.37 |
| Lateral orbital frontal | 0.94 | 0.11 | 0.96 | 0.17 | 0.868 | 0.17 |
| Medial orbital frontal | 0.67 | 0.10 | 0.62 | −0.47 | 0.645 | −0.48 |
| Insula | 0.93 | 0.14 | 0.85 | −0.50 | 0.620 | −0.52 |
| Rostral anterior cingulate | 0.32 | 0.07 | 0.36 | 0.47 | 0.642 | 0.48 |
| Caudal anterior cingulate | 0.26 | 0.07 | 0.23 | −0.41 | 0.683 | −0.43 |
| Posterior cingulate | 0.43 | 0.08 | 0.38 | −0.64 | 0.528 | −0.66 |
| Postcentral | 1.25 | 0.21 | 1.21 | −0.20 | 0.846 | −0.20 |
| Paracentral | 0.48 | 0.08 | 0.59 | 1.35 | 0.193 | 1.38 |
| Superior parietal | 1.82 | 0.25 | 2.09 | 1.05 | 0.307 | 1.08 |
| Inferior parietal | 1.82 | 0.29 | 1.79 | −0.09 | 0.933 | −0.09 |
| Supramarginal | 1.44 | 0.19 | 1.55 | 0.59 | 0.563 | 0.60 |
| Precuneus | 1.34 | 0.18 | 1.32 | −0.12 | 0.904 | −0.13 |
| Isthmus of the cingulate | 0.36 | 0.05 | 0.31 | −1.03 | 0.314 | −1.06 |
| Transverse temporal | 0.15 | 0.04 | 0.16 | 0.38 | 0.707 | 0.39 |
| Superior temporal | 1.50 | 0.19 | 1.45 | −0.26 | 0.801 | −0.26 |
| Banks superior temporal sulcus | 0.31 | 0.04 | 0.31 | −0.05 | 0.959 | −0.05 |
| Middle temporal | 1.47 | 0.19 | 1.37 | −0.48 | 0.637 | −0.49 |
| Inferior temporal | 1.40 | 0.24 | 1.60 | 0.83 | 0.419 | 0.85 |
| Entorhinal | 0.27 | 0.05 | 0.29 | 0.48 | 0.640 | 0.49 |
| Parahippocampal | 0.30 | 0.06 | 0.33 | 0.45 | 0.658 | 0.46 |
| Fusiform | 1.37 | 0.18 | 1.66 | 1.56 | 0.137 | 1.59 |
| Lingual | 0.92 | 0.12 | 0.79 | −1.07 | 0.297 | −1.10 |
| Pericalcarine | 0.29 | 0.06 | 0.22 | −1.09 | 0.290 | −1.12 |
| Cuneus | 0.40 | 0.06 | 0.38 | −0.33 | 0.748 | −0.33 |
| Lateral occipital | 1.63 | 0.18 | 1.46 | −0.97 | 0.344 | −0.99 |
| Cerebellum | 6.66 | 0.63 | 5.95 | −1.09 | 0.289 | −1.12 |
Regional volumes are expressed as percentages of intracranial volume.
P < 0.05.
z-score effect size estimate.
Table 3.
Regional white matter volume of our patient and our control subjects
| Region | Controls (%) | Patient (%) | t | p | z‡ | |
|---|---|---|---|---|---|---|
| Mean | SD | |||||
| A priori region of interest | ||||||
| Splenium* | 0.06 | 0.01 | 0.03 | −2.13 | 0.023 | −2.18 |
| Exploratory regions of interest | ||||||
| Anterior corpus callosum | 0.06 | 0.01 | 0.06 | 0.25 | 0.808 | 0.25 |
| Mid anterior corpus callosum | 0.03 | 0.01 | 0.03 | −0.21 | 0.835 | −0.22 |
| Central corpus callosum | 0.03 | 0.01 | 0.02 | −1.43 | 0.169 | −1.47 |
| Mid posterior corpus callosum | 0.03 | 0.01 | 0.02 | −1.65 | 0.115 | −1.69 |
| Superior frontal | 2.44 | 0.17 | 2.49 | 0.34 | 0.737 | 0.35 |
| Precentral | 1.82 | 0.17 | 1.85 | 0.20 | 0.840 | 0.21 |
| Caudal middle frontal | 0.81 | 0.09 | 0.92 | 1.18 | 0.255 | 1.20 |
| Rostral middle frontal | 1.74 | 0.14 | 1.59 | −1.02 | 0.320 | −1.05 |
| Pars opercularis | 0.46 | 0.06 | 0.41 | −0.97 | 0.345 | −0.99 |
| Pars triangularis | 0.41 | 0.04 | 0.42 | 0.26 | 0.795 | 0.27 |
| Pars orbitalis | 0.14 | 0.01 | 0.11 | −1.55 | 0.138 | −1.59 |
| Lateral orbital frontal | 0.87 | 0.06 | 0.79 | −1.31 | 0.207 | −1.34 |
| Medial orbital frontal | 0.50 | 0.06 | 0.44 | −0.97 | 0.347 | −0.99 |
| Insula | 1.22 | 0.09 | 1.19 | −0.36 | 0.726 | −0.36 |
| Rostral anterior cingulate | 0.33 | 0.04 | 0.34 | 0.27 | 0.787 | 0.28 |
| Caudal anterior cingulate | 0.39 | 0.05 | 0.33 | −1.13 | 0.271 | −1.16 |
| Posterior cingulate | 0.58 | 0.04 | 0.51 | −1.74 | 0.098 | −1.78 |
| Postcentral | 0.98 | 0.11 | 0.85 | −1.14 | 0.267 | −1.17 |
| Paracentral | 0.55 | 0.06 | 0.52 | −0.50 | 0.621 | −0.51 |
| Superior parietal | 1.64 | 0.11 | 1.67 | 0.25 | 0.804 | 0.26 |
| Inferior parietal | 1.43 | 0.16 | 1.29 | −0.88 | 0.392 | −0.90 |
| Supramarginal | 1.17 | 0.14 | 1.30 | 0.92 | 0.371 | 0.94 |
| Precuneus | 1.26 | 0.10 | 1.21 | −0.41 | 0.690 | −0.42 |
| Isthmus of the cingulate† | 0.47 | 0.04 | 0.32 | −3.25 | 0.004 | −3.33 |
| Transverse temporal | 0.09 | 0.01 | 0.09 | 0.38 | 0.707 | 0.39 |
| Superior temporal | 0.97 | 0.08 | 0.95 | −0.24 | 0.815 | −0.24 |
| Banks superior temporal sulcus | 0.36 | 0.07 | 0.25 | −1.58 | 0.132 | −1.62 |
| Middle temporal | 0.78 | 0.09 | 0.80 | 0.20 | 0.841 | 0.21 |
| Inferior temporal | 0.82 | 0.09 | 0.88 | 0.73 | 0.475 | 0.75 |
| Entorhinal | 0.11 | 0.03 | 0.13 | 0.43 | 0.675 | 0.44 |
| Parahippocampal | 0.23 | 0.03 | 0.20 | −1.09 | 0.289 | −1.12 |
| Fusiform | 0.93 | 0.10 | 0.95 | 0.21 | 0.834 | 0.22 |
| Lingual | 0.74 | 0.08 | 0.58 | −2.06 | 0.054 | −2.11 |
| Pericalcarine | 0.47 | 0.09 | 0.29 | −1.92 | 0.070 | −1.97 |
| Cuneus | 0.34 | 0.05 | 0.25 | −1.69 | 0.107 | −1.74 |
| Lateral occipital | 1.28 | 0.15 | 1.02 | −1.67 | 0.112 | −1.71 |
| Cerebellum† | 2.18 | 0.19 | 1.53 | −3.36 | 0.003 | −3.45 |
Regional volumes are expressed as percentages of intracranial volume.
P < 0.05,
P < 0.01.
z-score effect size estimate.
A priori region of interest analyses revealed that our patient has significantly reduced grey matter volume in the hippocampus, thalamus and pallidum compared with control subjects. He also has significantly reduced white matter volume in the splenium. Exploratory analyses revealed no significant differences between cortical or cerebellar grey matter volumes of our patient and the control subjects. However, his white matter volume in the isthmus of the cingulate and cerebellum is more than three standard deviations lower than that of the control subjects. In addition, his total white matter lesion volume is significantly larger than that of the control subjects (control subjects’ mean = 0.09%, SD = 0.03%; patient = 0.25%; t = 5.17, P = 0.000, z = 5.29), likely driven by periventricular white matter lesions.
Discussion
This study investigated the long-term effects of hypoglycaemia exposure on brain structure and the neural correlates of hypoglycaemia-induced memory impairments in a rare case of hypoglycaemia-induced anterograde amnesia. Given the limitations of case studies, and the lack of a control group with Type 1 diabetes with matched hyperglycaemia exposure but no hypoglycaemia exposure, we cannot make strong conclusions about the causal relationships among our patient’s hypoglycaemia exposure, volumetric changes and memory deficits. For example, we cannot rule out the possibility that hyperglycaemia exposure or glycaemic variability may have contributed to some of the effects noted. However, our patient’s lack of retinopathy, nephropathy or peripheral neuropathy symptoms, and his available HbA1c records, suggest that the alterations in brain structure observed in our patient likely resulted from his significant hypoglycaemia exposure.
Overall, our results suggest that hypoglycaemia can be associated with permanent reductions in subcortical grey matter volume, and that the hippocampus, thalamus and globus pallidus are among the brain regions most susceptible. The hippocampus plays a critical role in episodic (event) memory [19] and therefore alterations in hippocampal function are likely a major contributor to episodic memory impairments following hypoglycaemia. White matter surrounding the ventricles and in posterior brain regions, such as the splenium, isthmus of the cingulate and cerebellum, may also be susceptible to permanent injury from severe hypoglycaemia. Interestingly, anterograde amnesia has been reported following lesions to the isthmus of the cingulate (i.e. the retrosplenial region) [20], suggesting that this region could also play a role in hypoglycaemia-induced memory impairments. Overall, these results suggest that quantitative statistical analyses of high-resolution MRI scans in case studies are more sensitive than neuroradiological reads in identifying regional anomalies. This quantitative approach allows the detection of relatively subtle volumetric changes associated with critical case characteristics, which has heuristic value for planning future prospective group studies.
Acknowledgments
Funding sources
This work was supported by the Diabetes and Research Training Center at Washington University in St Louis and the National Institutes of Health (NIDDK DK64832 and UL1 RR024992).
The authors thank our patient and his family and friends for their support, and Deanna Barch, Alan Anticevic, Denise Head and The Charles F. and Joanne Knight Alzheimer’s Disease Research Center at Washington University in St Louis for providing control brain imaging data. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Footnotes
Competing interests
None declared.
References
- 1.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Hershey T, Perantie DC, Warren SL, Zimmerman EC, Sadler M, White NH. Frequency and timing of severe hypoglycemia affects spatial memory in children with type 1 diabetes. Diabetes Care. 2005;28:2372–2377. doi: 10.2337/diacare.28.10.2372. [DOI] [PubMed] [Google Scholar]
- 3.Blasetti A, Chiuri RM, Tocco AM, Di Giulio C, Mattei PA, Ballone E, et al. The effect of recurrent severe hypoglycemia on cognitive performance in children with type 1 diabetes: a meta-analysis. J Child Neurol. 2011;26:1383–1391. doi: 10.1177/0883073811406730. [DOI] [PubMed] [Google Scholar]
- 4.Chalmers J, Risk MTA, Kean DM, Grant R, Ashworth B, Campbell IW. Severe amnesia after hypoglycemia: clinical, psychometric, and magnetic resonance imaging correlations. Diabetes Care. 1991;14:922–925. doi: 10.2337/diacare.14.10.922. [DOI] [PubMed] [Google Scholar]
- 5.Boeve BF, Bell DG, Noseworthy JH. Bilateral temporal lobe MRI changes in uncomplicated hypoglycemic coma. Can J Neurol Sci. 1995;22:56–58. doi: 10.1017/s031716710004052x. [DOI] [PubMed] [Google Scholar]
- 6.Holemans X, Dupuis M, Misson N, Vanderijst JF. Reversible amnesia in a Type 1 diabetic patient and bilateral hippocampal lesions on magnetic resonance imaging (MRI) Diabet Med. 2001;18:761–763. doi: 10.1046/j.1464-5491.2001.00481.x. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson SC, Blane A, Perros P, McCrimmon RJ, Best JJK, Wardlaw J, et al. Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes. 2003;52:149–156. doi: 10.2337/diabetes.52.1.149. [DOI] [PubMed] [Google Scholar]
- 8.Kodl CT, Franc DT, Rao JP, Anderson FS, Thomas W, Mueller BA, et al. Diffusion tensor imaging identifies deficits in white matter microstructure in subjects with type 1 diabetes that correlate with reduced neurocognitive function. Diabetes. 2008;57:3083–3089. doi: 10.2337/db08-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musen G, Lyoo IK, Sparks CR, Weinger K, Hwang J, Ryan CM, et al. Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes. 2006;55:326–333. doi: 10.2337/diabetes.55.02.06.db05-0520. [DOI] [PubMed] [Google Scholar]
- 10.Northam EA, Rankins D, Lin A, Wellard RM, Pell GS, Finch SJ, et al. Central nervous system function in youth with type 1 diabetes 12 years after disease onset. Diabetes Care. 2009;32:445–450. doi: 10.2337/dc08-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho SJ, Minn YK, Kwon KH. Severe hypoglycemia and vulnerability of the brain. Arch Neurol. 2006;63:138. doi: 10.1001/archneur.63.1.138. [DOI] [PubMed] [Google Scholar]
- 12.Jung SL, Kim BS, Lee KS, Yoon KH, Byun JY. Magnetic resonance imaging and diffusion-weighted imaging changes after hypoglycemic coma. J Neuroimaging. 2005;15:193–196. doi: 10.1177/1051228405274533. [DOI] [PubMed] [Google Scholar]
- 13.Aoki T, Sato T, Hasegawa K, Ishizaki R, Saiki M. Reversible hyperintensity lesion on diffusion-weighted MRI in hypoglycemic coma. Neurology. 2004;63:392–393. doi: 10.1212/01.wnl.0000130181.05016.68. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Choi JY, Koh SB, Lee Y. Reversible splenial abnormality in hypoglycemic encephalopathy. Neuroradiology. 2007;49:217–222. doi: 10.1007/s00234-006-0184-y. [DOI] [PubMed] [Google Scholar]
- 15.Taguchi Y, Kamiyama H, Kubo M, Horie Y. Internal capsule and splenial lesions in hypoglycemic hemiparesis. Intern Med. 2011;50:533–534. doi: 10.2169/internalmedicine.50.4756. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association Workgroup on Hypoglycemia. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association workgroup on hypoglycemia. Diabetes Care. 2005;28:1245–1249. doi: 10.2337/diacare.28.5.1245. [DOI] [PubMed] [Google Scholar]
- 17.Crawford JR, Howell DC. Comparing an individual’s test score against norms derived from small samples. Clin Neuropsychol. 1998;12:482–486. [Google Scholar]
- 18.Squire LR, Shimamura AP. Characterizing amnesic patients for neurobehavioral study. Behav Neurosci. 1986;100:866–877. doi: 10.1037//0735-7044.100.6.866. [DOI] [PubMed] [Google Scholar]
- 19.Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010;35:86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maeshima S, Ozaki F, Masuo O, Yamaga H, Okita R, Moriwaki H. Memory impairment and spatial disorientation following a left retrosplenial lesion. J Clin Neurosci. 2001;8:450–451. doi: 10.1054/jocn.2000.0801. [DOI] [PubMed] [Google Scholar]


