Abstract
Objective. In OA, there is enhanced expression of pro-inflammatory cytokines such as IL-1β in the affected joint. Delphinidin, an anthocyanidin found in pigmented fruits and vegetables, has been shown to possess anti-inflammatory and antioxidant properties. In the present study we determined whether delphinidin would inhibit the IL-1β-induced activation of NF-κB in human chondrocytes and determined the mechanism of its action.
Methods. PGE2 levels and activation of NF-κB p65 in human OA chondrocytes were determined by ELISA-based assays. Protein expression of cyclo-oxygenase-2 (COX-2) and phosphorylation of kinases was determined by western immunoblotting. Expression level of mRNAs was determined by TaqMan assays.
Results. Delphinidin inhibited IL-1β-induced expression of COX-2 and production of PGE2 in human chondrocytes. Delphinidin also inhibited IL-1β-mediated phosphorylation of IL-1 receptor-associated kinase-1Ser376, phosphorylation of IKKα/β, expression of IKKβ, degradation of IκBα, and activation and nuclear translocation of NF-κB/p65. Phosphorylation of TGF-β-activated kinase 1 was not observed but NF-κB-inducing kinase (NIK) was phosphorylated and phosphorylation of NIK was blocked by delphinidin in IL-1β-treated human chondrocytes.
Conclusion. These data identify delphinidin as a novel inhibitor of IL-1β-induced production of cartilage-degrading molecule PGE2 via inhibition of COX-2 expression and provide new insight into the mechanism of its action. Our results also identify inhibition of IRAK1Ser376 phosphorylation by delphinidin in IL-1β-induced activation of NF-κB in human chondrocytes. Given the important role played by IL-1β-induced NF-κB activation, COX-2 expression and PGE2 production in OA, our results may have important implications for the development of novel therapeutic strategies for the prevention/treatment of OA.
Keywords: osteoarthritis, IL-1β, delphinidin, chondrocytes, NF-κB, COX-2, PGE2
Introduction
Progressive cartilage breakdown is the main feature of OA, which results from an imbalance in the anabolic and catabolic activities in chondrocytes that help in remodelling and maintaining the extracellular matrix (ECM) of cartilage. Synovial inflammation has now emerged as one of the most important factors that lead to dysregulation of normal chondrocyte function [1]. Inflammatory cytokines such as IL-1β, TNF-α and IL-6, secreted from inflamed synovium, are major mediators of disturbed chondrocyte function and cartilage degeneration.
In articular cartilage chondrocytes are the principal cell type, which are embedded in a network of collagen fibres and proteoglycans [2]. IL-1β suppresses the expression of type II collagen [3, 4] and aggrecan [5, 6], two principal constituents of cartilage ECM, by chondrocytes and also stimulates the synthesis of several proteolytic enzymes by chondrocytes including aggrecanases, MMPs—MMP-1, MMP-3 and MMP-13—which play an important role in cartilage degradation [7, 8]. Stimulation with IL-1β results in up-regulation of iNOS and cyclo-oxygenase-2 (COX-2) in chondrocytes and leads to the release of nitric oxide (NO) and PGE2. High-level production of NO and reactive oxygen species [9] and the down-regulation of antioxidant enzyme expression by IL-1β induces the oxidative stress in chondrocytes [10, 11] and probably contributes to the degradation of articular cartilage in OA. These catabolic effects of IL-1β are exerted through the activation of several signalling pathways including jun-N-terminal kinases (JNK), p-38 mitogen-activated protein kinase (MAPK) and the activation of transcription factor NF-κB. NF-κB is the master regulator of expression of several genes involved in inflammation, immune response and apoptosis. Due to its central role in the regulation of genes involved in the pathogenesis of OA, including iNOS and COX-2, NF-κB pathway has been identified as an important target of therapeutic strategies aimed at the treatment of OA [12]. Recent studies suggest that some phytochemicals may prevent cartilage degradation by inhibiting the production of catabolic mediators in OA.
Delphinidin (2-(3,4,5-trihydroxyphenyl)chromenylium-3,5,7-triol) is an active ingredient of pomegranate and other pigmented fruits [13, 14]. Recently, delphinidin was reported to exert anti-tumour activity [13], anti-inflammatory activity and inhibition of NF-κB [15, 16]. The aim of the present study was to investigate whether delphinidin will block the IL-1β-mediated expression of COX-2 and production of PGE2—known to play important roles in the pathogenesis of OA—in human chondrocytes. For these studies we used monolayer cultures of human primary OA chondrocytes to determine the effect of delphinidin on IL-1β-induced activation of NF-κB and the expression of COX-2 and production of PGE2. Our results demonstrate that treatment with delphinidin suppressed the IL-1β-induced expression of COX-2 and production of PGE2 in human OA chondrocytes. We also demonstrate for the first time that delphinidin blocks the activation of NF-κB by suppressing the activation of upstream kinases NF-κB-inducing kinase (NIK) and IL-1 receptor-associated kinase-1 (IRAK1) in human OA chondrocytes. Furthermore, OA chondrocytes with diminished NIK and IRAK1Ser376 phosphorylation also inhibited phosphorylation of IKKβSer177 and of IκBαSer32 and reduced levels of IKKβ mRNA and protein expression. These findings suggest a novel mechanism that may contribute to OA prevention/treatment by delphinidin or compounds derived from it.
Materials and methods
Reagents
Media and reagents for cell culture were purchased either from HyClone Laboratories (Logan, UT, USA) or from Invitrogen (Carlsbad, CA, USA). Primocin was purchased from InvivoGen (San Diego, CA, USA). Pronase and collagenase were purchased from Roche Diagnostics (Indianapolis, IN, USA). Recombinant human IL-1β and TNF-α were purchased from R&D Systems (St Paul, MN, USA). Primary antibodies against IκB, phospho-IκB, IKKβ, Phospho-NIK, IRAK-1 and β-actin were from Santa Cruz Biotechnologies (Santa Cruz, CA, USA). Primary antibodies against IKKα, phospho-IKKα/β and NIK were from Cell Signaling Techonology (Beverly, MA, USA). Antibody against P-IRAK-1 was from Pierce Biotechnology (Rockford, IL, USA). IRDye 600 and 800 conjugated secondary antibodies were purchased from LI-COR Biosciences (Lincoln, NE, USA). Delphinidin chloride was purchased from Extrasynthese (Extrasynthese SA, Lyon, Nord-Genay, France). BAY 11-7082 was from Calbiochem (EMD Millipore, Rockland, MA, USA).
Human samples and preparation of primary human chondrocytes
The study was reviewed and approved by the Institutional Review Board of MetroHealth Medical Center, Cleveland, OH, USA, as exempt and that no informed consent was needed. Discarded and de-identified femoral head samples were collected from the patients who underwent total joint replacement surgery at MetroHealth Medical Center, Cleveland, OH, USA. Cartilage was resected from macroscopically unaffected areas (no staining with India ink, smooth cartilage). Human chondrocytes were prepared from cartilage pieces by sequential digestion with pronase (1 mg/ml) for 1 h followed by collagenase (1 mg/ml) overnight essentially as described previously [17–19].
Treatment of OA chondrocytes with delphinidin, BAY 11-7082, TNF-α and IL-1β
OA chondrocytes (1 × 106/ml) were allowed to grow in DMEM 90% and fetal calf serum (FCS) 10% supplemented with penicillin (100 U/ml) and streptomycin (100 µg/ml) for 2–3 days after plating and only primary (unpassaged) chondrocytes were used in the experiments. At about 80% confluence, OA chondrocytes were serum starved for 12–15 h and were then treated with delphinidin or BAY 11-7082 for 2 h followed by treatment with human recombinant IL-1β or TNF-α for different times. Fifty µg/ml delphinidin was used to study the NF-κB pathway and IKKβ expression while 10 µg/ml delphinidin for 24 h was used for COX-2 expression and PGE2 production as higher amounts used for 24 h were toxic for the cells. Chondrocytes not treated with delphinidin or IL-1β or TNF-α served as controls. All experiments were completed within 4–5 days after plating the cells to avoid dedifferentiation of chondrocytes.
Western immunoblotting
Immunoblot analysis was performed essentially as described previously [17, 18]. Briefly, after treatments, chondrocytes were washed once with ice-cold PBS and were lysed in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris–HCl, pH 7.5; 150 mM NaCl; 1% IGEPAL, 4 mM EDTA, 0.1% sodium deoxycholate; 10 mM Na4P2O7, 10 mM NaF, 2 mM Na3VO4, 1 mM phenylmethylsulphonyl fluoride (PMSF), 1 μg/ml leupeptin, 1 μg/ml aprotinin) in the dishes on ice for 30 min. The cell lysate was cleared of cell debris by centrifugation at 18 000 g at 4°C for 10 min. Total protein concentration was estimated using DC protein assay kit (BioRad Laboratories, Hercules, CA, USA). Equal amounts of total proteins (25 µg/lane) were resolved by SDS–PAGE and transferred to nitrocellulose membrane (LI-COR Biosciences, Lincoln, NE, USA). The membranes were blocked using Odyssey blocking buffer (LI-COR Biosciences) for 1 h at room temperature. Blocked membranes were incubated overnight at 4°C with primary antibodies diluted in the blocking buffer. Membranes were washed and then incubated with secondary antibodies for 1 h at room temperature followed by another wash and the immunoreactive proteins were visualized using the Odyssey infrared imaging system (LI-COR Biosciences).
RNA isolation and real-time PCR analysis of gene expression
Total RNA was isolated using Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA, USA) on Qiacube automated sample prep platform (Qiagen) according to the manufacturer’s protocol. Total RNA concentration was determined by absorbance at 260 nm using the Eppendorf spectrophotometer. cDNA synthesis was performed using the QuantiTect reverse transcription kit (Qiagen) using 500 ng of total RNA according to the instructions provided with the kit. Two microlitres from a 20 µl cDNA synthesis reaction was used for TaqMan assays using the StepOne real-time PCR system (Applied Biosystems/Life Technologies Corp, Carlsbad, CA, USA). Relative quantification was performed using ΔΔCT method with β-actin as internal control.
Measurement of PGE2 production
Human chondrocytes were plated in six-well dishes and grown to 80% confluence in 2 ml of growth medium, treated with delphinidin for 2 h before treatment with IL-1β and then harvested 24 h later. The amount of PGE2 released into the medium was measured using the PGE2 enzyme immunoassay kit essentially according to the instructions of the manufacturer (Oxford Biomedical Research, Oxford, MI, USA).
Measurement of NF-κB p65 activation by ELISA
Activation of NF-κB p65 and DNA-binding activity assay was performed using Trans-AM ELISA kit (Active Motif, Carlsbad, CA, USA) according to the manufacturer’s protocol. Chondrocytes were treated as described above for the indicated times and nuclear extracts were prepared using the nuclear extract kit (Active Motif, Carlsbad, CA, USA). After clearing by centrifugation, 5 µg of the nuclear extract were added to the 96-well plates coated with oligonucleotide containing an NF-κB consensus binding site and incubated at room temperature for 1 h. Wells were washed and primary antibody was added to the wells to bind the target protein in the extract. After incubation for 1 h, the antibody was removed, and 100 μl of HRP-conjugated secondary antibody was added to the wells and incubated for 1 h. After thorough washing, 100 μl of developing solution was added, incubated for 2–10 min and then 100 μl of stop solution was added. The absorbance at 450 nm was determined using the EnSpire 2300 multilabel plate reader (PerkinElmer, Waltham, MA, USA).
Statistical analysis
Each experiment was repeated on primary chondrocytes obtained from at least three donors (n = 3) to ensure reproducibility of the data. All the experiments were performed in duplicate. The blots shown are representative of at least three blots with similar results. Values shown are mean ± s.d. and were compared using two-tailed Student’s t-test. A P < 0.05 was considered significant. Data were plotted using the Origin 8.1 software (OriginLab Corporation, Northamton, MA, USA).
Results
Delphinidin inhibits IL-1β-induced expression of COX-2 mRNA and protein and the production of PGE2 in OA chondrocytes
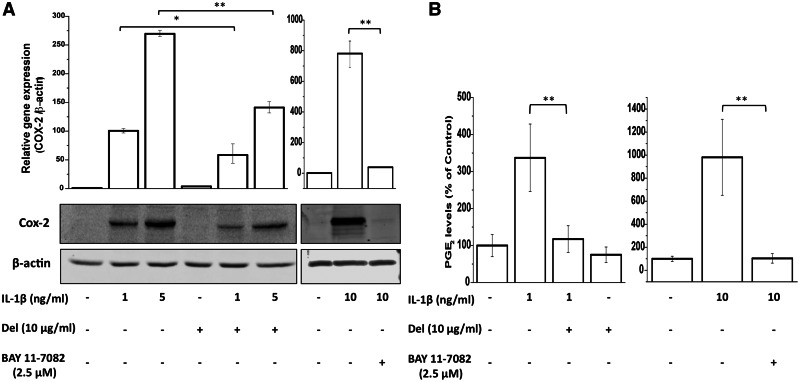
Over-expression of COX-2, a key mediator of inflammation, and its product, PGE2 are associated with cartilage degradation and pain in OA. We determined whether delphinidin has any effect on IL-1β-induced COX-2 expression in OA chondrocytes. OA chondrocytes were first treated with delphinidin (10 µg/ml) for 2 h and then stimulated with IL-1β (1 and 5 ng/ml) for 24 h. OA chondrocytes not treated with delphinidin or IL-1β served as controls. At the termination of the experiment, chondrocytes were harvested and half of the chondrocytes were used for the preparation of cell lysates and the remaining half were used for the extraction of total RNA. Gene and protein expression levels were determined by TaqMan assays and immunoblot analysis, respectively. As shown previously [20], in IL-1β-treated OA chondrocytes gene expression of COX-2 mRNAs was induced in a dose-dependent manner and was several hundred fold higher than in control OA chondrocytes at the highest concentration of IL-1β used (Fig. 1A). Interestingly, OA chondrocytes treated with delphinidin showed significantly suppressed levels of COX-2 mRNA even at the highest dose of IL-1β used in these studies (>50% reduction, P < 0.01; Fig. 1A). This suppression of gene expression was also reflected in reduced protein levels of COX-2 in OA chondrocytes despite the presence of IL-1β (Fig. 1A). The production of PGE2, which increased following treatment with IL-1β, was also suppressed by delphinidin treatment (Fig. 1B). These results indicated that delphinidin could effectively repress IL-1β-induced COX-2 mRNA and protein expression and PGE2 production in OA chondrocytes. Involvement of NF-κB in transcriptional regulation of COX-2 gene expression is well documented [21, 22]. To investigate whether delphinidin had its inhibitory effect on COX-2 expression and PGE2 production via suppression of NF-κB activity, we used a small molecule inhibitor of NF-κB BAY 11-7082. IL-1β stimulated COX-2 expression, both at mRNA and protein levels, as well as PGE2 production by human chondrocytes was significantly inhibited upon treatment with BAY 11-7082 (Fig. 1A and 1B).
Fig. 1.
Inhibition of IL-1β-induced expression of COX-2 (A) and PGE2 levels (B) by delphinidin.
Serum-starved primary human chondrocytes were pre-treated with delphinidin (10 µg/ml) and BAY 11-7082 (2.5 µM) for 2 h and then treated with IL-1β for 24 h. Total RNA was isolated and real-time analysis was performed using TaqMan assay for COX-2. β-actin was used as endogenous control. Protein expression was investigated by immunoblotting using antibodies against COX-2 (Cell Signaling Technologies). β-actin was used as a control for equal loading. PGE2 levels in the culture supernatant were measured using the PGE2 EIA kit (Oxford Biomedical Research). Data were plotted using Origin 8.1 software. Results of real-time PCR and PGE2 EIA were averaged from three separate experiments (n = 3) each performed in duplicate and are presented as relative gene expression ±s.d. within parentheses (real-time) and percentage of untreated control with s.d. within parentheses (PGE2 EIA) (*P < 0.05, **P < 0.01). Protein expression data are presented as representative of three blots.
Delphinidin suppresses IL-1β-induced activation and DNA binding activity of NF-κB p65 in OA chondrocytes
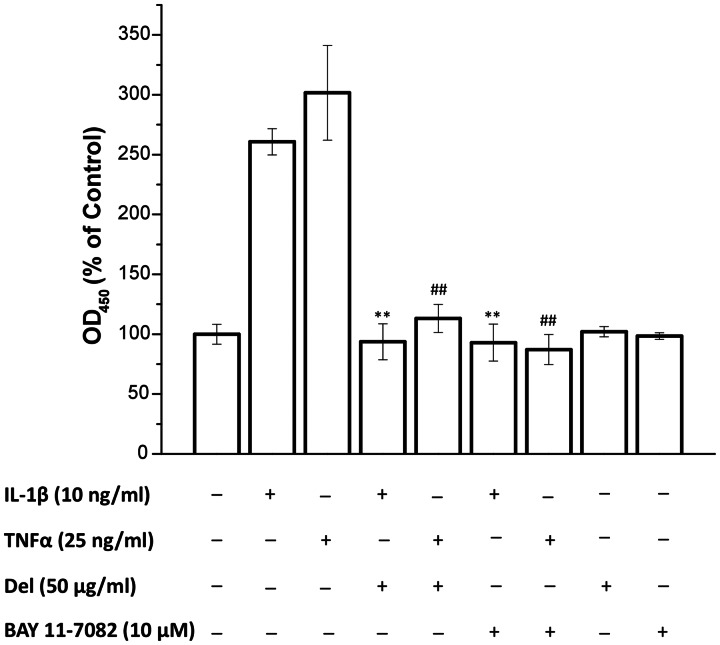
We next measured the effect of delphinidin treatment on IL-1β and TNF-α-stimulated activation of NF-κB-p65 in OA chondrocytes. In these studies, OA chondrocytes were pre-treated with delphinidin (50 µg/ml) and BAY 11-7082 for 2 h followed by treatment with IL-1β (10 ng/ml) and TNF-α for 30 min. Activation and DNA-binding activity of NF-κB-p65 was assayed by a highly specific ELISA. There was an intense activation of NF-κB p65 after 30 min of treatment with IL-1β and TNF-α as compared with control OA chondrocytes (Fig. 2). Activation and DNA binding activity of NF-κB was significantly inhibited in OA chondrocytes pre-treated with delphinidin and BAY 11-7082 (Fig. 2; P < 0.01). OA chondrocytes treated with delphinidin and BAY 11-7082 alone did not show any significant activation of NF-κB-p65 above the basal level detected in controls. These results suggest that delphinidin may exert its COX-2 suppressive effect through the inhibition of NF-κB in OA chondrocytes.
Fig. 2.
Inhibition of IL-1β-induced DNA-binding activity of NF-κB p65 in human chondrocytes by delphinidin.
Cells were pre-treated with delphinidin (50 µg/ml) or BAY 11-7082 (10 µM) for 2 h followed by treatment with IL-1β for 30 min. After the treatments, cells were washed with ice-cold PBS and nuclear extracts were prepared using nuclear extract kit (Active Motif). Five micrograms of the cleared nuclear extracts were used for the ELISA-based p65 DNA-binding activity assay using Trans-AM kit (Active Motif). Results from three separate experiments (n = 3) performed in duplicate were averaged and were plotted using Origin 8.1 software. Data are expressed as a percentage of untreated control with s.d. within parentheses (**P < 0.01 compared with sample treated with IL-1β only; ##P < 0.01 compared with sample treated with TNF-α only).
IL-1β-induced degradation of IκBα was inhibited by delphinidin in OA chondrocytes
In the quiescent state, IκB binds to NF-κB in the cytoplasm and prevents its activation and translocation to the nucleus. In response to stimulation with IL-1β and other stimuli, IκBα is phosphorylated at Ser32 by the IKK complex leading to its ubiquitination and proteasomal degradation, which unmasks the nuclear localization sequence resulting in the release of NF-κB protein, which is then translocated to the nucleus. To understand the mechanism of inhibition of NF-κB in OA chondrocytes by delphinidin (Fig. 2), we evaluated the effect of delphinidin on the phosphorylation and degradation of IκBα in response to stimulation with IL-1β. Stimulation with IL-1β alone caused an intense phosphorylation of IκBα at Ser32 within 5 min of treatment followed by rapid degradation of IκBα (Fig. 3). When OA chondrocytes were pre-treated with delphinidin IL-1β-induced phosphorylation of IκBα at Ser32 and its subsequent degradation were completely blocked (P < 0.05) (Fig. 3). As IκBα is phosphorylated at Ser32 by the upstream IKK serine–threonine kinase complex, we also determined the effect of delphinidin on the activation of IKK in IL-1β-stimulated OA chondrocytes and discovered that IL-1β-induced phosphorylation of IKKα/β was also completely blocked by delphinidin in OA chondrocytes (Fig. 3).
Fig. 3.
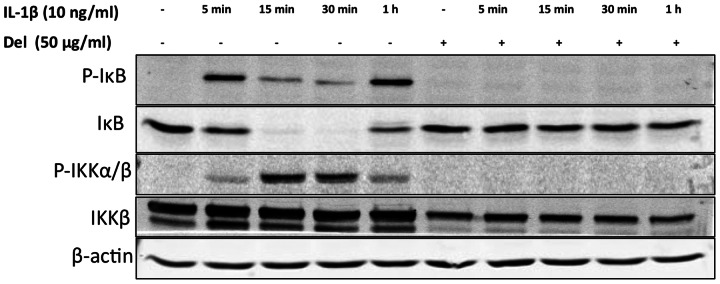
Delphinidin inhibited the IL-1β-induced phosphorylation of IκB and IKKα/β in human chondrocytes.
Primary human chondrocytes were pre-treated with delphinidin (50 µg/ml) for 2 h and then treated with IL-1β (10 ng/ml) for indicated times. Cells were washed with ice-cold PBS and cell lysates were prepared using RIPA buffer. Twenty-five micrograms of total cell lysates were used for western immunoblotting employing the standard methods. Each blot is a representative of at least three blots performed using samples from three donors.
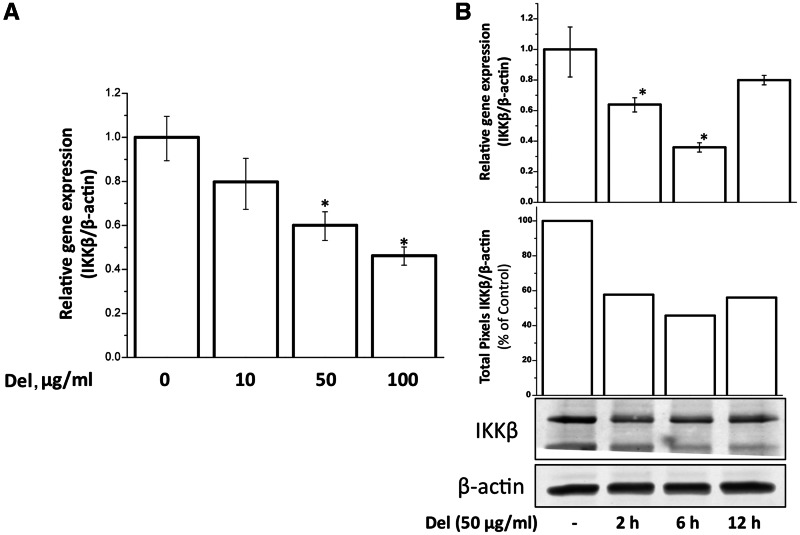
Delphinidin down-regulated the protein and mRNA expressions of IKKβ
Importantly, when we checked for the total IKKβ protein, we consistently found a decreased level in cells that were treated with delphinidin (Fig. 3). We then investigated whether this was at transcriptional level and indeed found a decrease in mRNA levels upon treatment of cells with delphinidin for 2 h in a dose-dependent manner (10–100 µg/ml) (Fig. 4A). To check the time course of this effect, we treated the cells with 50 µg/ml delphindin for 2, 6 and 12 h and then assayed the mRNA and protein levels. We found a decrease of up to 40% both at mRNA and protein level within 2 h of treatment, which reached to its maximum at 6 h. At 12 h there was slight increase in expression but that was still less than untreated control (Fig. 4B).
Fig. 4.
Delphinidin inhibited IKKβ expression in a dose- and time-dependent manner.
(A) Human chondrocytes were treated with indicated doses of delphinidin for 2 h. Total RNA was isolated and subjected to real-time analysis using TaqMan assay for IKKβ. β-actin was used as an endogenous control. Results are represented as mean of relative gene expression compared with untreated control with s.d. within parentheses (*P < 0.05). (B) Human chondrocytes were treated with 50 µg/ml delphinidin for indicated times. mRNA and protein levels were assayed by the methods described above. Protein bands were digitized using UnScan-It software (Silk Scientific, Orem, UT, USA) and total pixels were plotted as percentage of untreated control after normalizing with corresponding bands on β-actin blot. The presented blot is representative of three separate blots using cartilage samples from three donors.
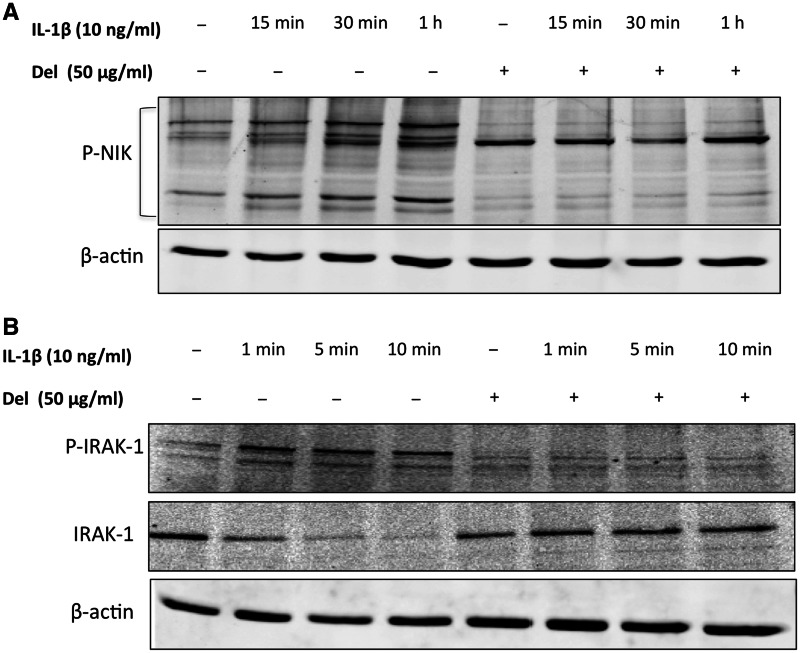
Delphinidin inhibited the intrinsic as well as IL-1β-induced phosphoryaltion of NIK in primary human chondrocytes
After stimulation with IL-1β a sequential series of events takes place which leads to the activation of NF-κB. In this step-wise process of cellular signalling activation of NF-κB can be controlled by NIK, a member of the MAP3K family [23]. NIK forms a complex with and phosphorylates IKKα and IKKβ, which then phosphorylate IκBα at Ser32 leading to its degradation and the translocation of NF-κB to the nucleus [24–26]. To determine if the observed inhibition of IKK phosphorylation was due to the inhibition of the upstream kinase, we analysed the activation of NIK in OA chondrocytes stimulated with IL-1β. Our results showed active phosphorylation of NIK in OA chondrocytes stimulated with IL-1β, which was inhibited in OA chondrocytes pre-treated with delphinidin (Fig. 5A). This data indicated that delphinidin inhibited the IL-1β-induced activation of NF-κB in OA chondrocytes by suppressing the activation of a kinase upstream of NIK in OA chondrocytes.
Fig. 5.
Delphinidin inhibited IL-1β-induced phosphorylation of NIK and phosphorylation as well as degradation of IRAK1.
(A) Primary human chondrocytes from OA patients were pre-treated with delphinidin (50 µg/ml) for 2 h followed by treatment with IL-1β (10 ng/ml) for indicated times. Chondrocytes were lysed in RIPA buffer and total protein was resolved by SDS–PAGE followed by transfer to nitrocellulose membrane. Blots were probed with phospho-NIK antibody and developed using standard methods. β-actin was used as control for equal loading. The presented blot is representative of three separate blots using samples from three donors. (B) Primary human chondrocytes from OA patients were pre-treated with delphinidin (50 µg/ml) for 2 h followed by treatment with IL-1β (10 ng/ml) for indicated times and subjected to western blot analysis. The presented blot is representative of three separate blots using samples from three donors. The band detected with the antibody against phospho form of IRAK1 ran much higher than the unphosphorylated form suggesting modification of the protein upon treatment with IL-1β. This band was not observed when cells were treated with delphinidin.
Delphinidin inhibited the IL-1β-induced phosphorylation and degradation of IRAK1 in OA chondrocytes
The most proximal kinase to IL-1 R is the IRAK1, which is a serine/threonine-specific kinase. IRAK1 undergoes autophosphorylation shortly after stimulation with IL-1β and the subsequent series of events involve its dissociation from the IL-1RI complex, its ubiquitination and association with two membrane-bound proteins: TAB2 and TRAF6. The resulting IRAK–TRAF6–TAB2 complex is then released into the cytoplasm where it activates protein kinases TGF-β-activated kinase 1 (TAK1), NIK, IKKs and the stress-activated kinases [27, 28]. To determine whether delphinidin interferes with the activation of IRAK1 we analysed the phosphorylation and degradation of IRAK1 in OA chondrocytes treated with IL-1β and delphinidin. Our results demonstrate that stimulation with IL-1β resulted in the phosphorylation of IRAK1Ser376, which was then degraded rapidly (Fig. 5B). IL-1β-induced phosphorylation of IRAK1Ser376 and its degradation was blocked by delphinidin in OA chondrocytes. Taken together these results suggested that the inhibition of IRAK1 by delphinidin leads to the suppression of downstream kinases involved in NF-κB activation, resulting in the inhibition of NF-κB activity and expression of COX-2 in OA chondrocytes. These are unique findings and have not been reported previously.
Discussion
Focal degradation of ECM is a hallmark of OA, which is a late-onset disease of the articulating joints. IL-1β is the apical cytokine in several inflammatory cascades and is one of the most important cytokines in OA where it plays a key role in cartilage catabolism by up-regulating the expression of several mediators of cartilage degradation [1, 29]. IL-1β is also capable of inhibiting the production of cartilage-specific macromolecules, including type II collagen, through modulation of the transcription factors Sp1 and Sp3 [3]. Inhibition of IL-1β has been shown to result in amelioration of OA-like pathology in animal models and the role of IL-1 in OA pathogenesis is further substantiated by studies in IL-1-deficient mice [30, 31]. Elevated levels of IL-1β in the SF from OA joints have been described and it has been shown that IL-1β stimulates the expression of iNOS, COX-2, MMPs and aggrecanases in chondrocytes [reviewed in 29]. High levels of COX-2 result in excessive production of PGE2, which is a crucial molecule in a variety of physiological and pathological conditions including cartilage degradation. IL-1β induces the activation of the nuclear transcription factor NF-κB, p38-MAP kinases, JNK and extracellular regulated MAP kinase 1/2 (ERK1/2) pathways within minutes. Signalling occurs via the 213 amino acid-long cytoplasmic domain of IL-1RI leading to the activation of IκB kinase complex resulting in the phosphorylation and degradation of IκBα and the nuclear translocation of NF-κB [30, 32, 33]. Therefore, these sequential cellular signalling pathways offer a therapeutic opportunity to prevent cartilage degradation in OA. In search of new therapeutic and preventive agents that can inhibit cartilage matrix degradation and, thus, progress of OA, we found that in vitro treatment of human primary chondrocytes with delphinidin inhibited IL-1β-induced expression of COX-2 as well as secretion of PGE2 into the culture medium (Fig. 1). However, delphinidin did not show any effect on IL-1β-induced over-expression of aggrecanases ADAMTS-4 and -5 (data not shown).
Dietary polyphenols and extracts rich in polyphenols exhibit protective effects against a number of chronic diseases. Previous studies have shown that anthocyanins are potent antioxidants and suppress oxidative stress-induced apoptosis and tumour cell invasion and migration [34, 35]. NF-κB pathway is an important target for the treatment of inflammatory diseases including RA and OA [reviewed in 12]. Several cytokines responsible for cartilage degeneration in OA, including IL-1β and TNF-α, act through the activation of NF-κB pathway. NF-κB is a master regulator, which controls the expression of several genes involved in inflammatory response including COX-2 [36, 37]. Therefore, there is a continuing effort to find the ways to pharmacologically modulate this pathway with little or no toxicity for treating OA. In the present study, we show a strong inhibitory effect of delphinidin on mRNA and protein expression of COX-2 via suppression of NF-κB activation in OA chondrocytes (Figs 1 and 2).
Catabolic role of IL-1β in cartilage degeneration during OA is well established [38]. Elevated level of IL-1β causes increased degradation of the cartilage resulting in the release of matrix constituents such as proteoglycan and collagen. This may be the consequence of an increase in expression and activity of COX-2 resulting in the production of a potent effecter molecule, namely, PGE2. In the present study, we found that there was very low or no expression of COX-2 in unstimulated OA chondrocytes, but this was increased several hundred fold in response to IL-1β treatment. Delphinidin has previously been shown to inhibit the expression of UV-induced expression of COX-2 in cancer cells [16]. In the present study, we found that delphinidin is also a potent inhibitor of IL-1β-induced increase in COX-2 expression and the production of PGE2 in human chondrocytes. This effect was similar to the effect of BAY 11-7082, an established small molecule inhibitor of NF-κB (Fig. 1).
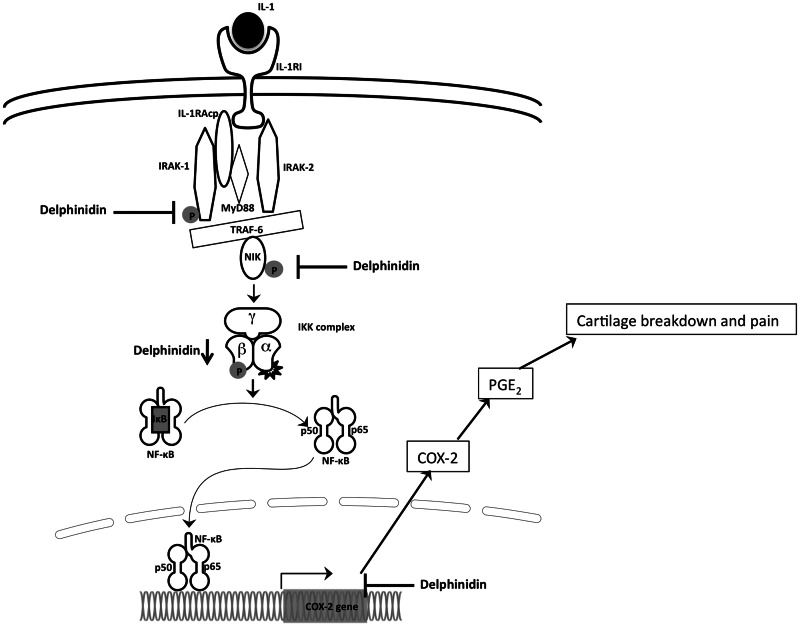
To further understand the mechanism, we investigated the site of delphinidin’s action within the NF-κB pathway. IL-1β activates NF-κB through the canonical pathway. Upon binding of IL-1β to its receptor, IL-1RI, a multi-protein complex containing IL-1RI, IL-1RAcp, IRAK1, IRAK2 and MyD88 is formed. TRAF6 is then recruited to the complex and activates Tak-1 and NIK. Results presented here show a novel mechanism of inhibition of NF-κB activation by delphinidin by inhibiting the phosphorylation of IRAK1 (Fig. 5) and by down-regulating the expression of IKKβ, both at protein and mRNA levels (Figs 3 and 4). Delphinidin did not interfere with IL-1 binding to IL-1 R as in a bioassay no effect on IL-1-induced IL-2 production by a T-cell line was observed (data not shown). We cannot rule out the possibility of delphinidin’s interference with interaction of MyD88 and other members of the receptor complex, but that needs further investigation.
The IKK complex is a major checkpoint in the NF-κB pathway. Many signalling pathways that activate NF-κB merge at IKK complex including the signalling induced by inflammatory cytokines such as TNF-α and IL-1β, endotoxins, viral infection, double-stranded RNA and UV irradiation [39]. Multiple upstream kinases have been reported as IKK kinases including TAK-1 and NIK [28, 40]. We did not observe the phosphorylation of TAK-1 in IL-1β-stimulated human chondrocytes (data not shown), but NIK was phosphorylated in these cells. This suggests that in human chondrocytes IL-1β-mediated activation of NF-κB proceeds via activation of NIK. Activated NIK phosphorylates IKKβ in this complex, which then phosphorylates two adjacent serines of IκB leading to its ubiquitination and proteosomal degradation. In addition to phosphorylating IκB, IKKβ has inhibitory autophosphyraltion activity resulting in a feedback effect [41]. Given the central role played by IKKβ in regulation of NF-κB pathway, IKKβ is an important target for pharmacological modulation of NF-κB pathway, particularly for the treatment of diseases with an inflammatory component such as OA and RA. In a rat model with adjuvant-induced arthritis, dominant-negative IKKβ inhibited the NF-κB activation resulting in suppression of cytokine-induced IL-6, IL-8 and ICAM-1 expressions [42]. Some small-molecule inhibitors of IKKβ activity, for example, SC-514 and BMS-345541, have also been demonstrated to have modifying effects on arthritis in vitro and in vivo [43, 44]. Similar to our results, Vidya Priyadarsini et al. [45] recently showed a down-regulation of IKKβ at protein level in HeLa cells treated with a flavonoid, quercetin. It will be interesting to further investigate the mechanism of down-regulation of IKKβ expression by delphinidin.
The first line of treatment option for OA includes acetaminophen and NSAIDs [46]. However, both these drugs have their side effects. While prolonged use of NSAIDs causes gastrointestinal ulcers [47] and cardiovascular adverse events [48], use of acetaminophen is associated with hepatotoxicity. Due to these limitations, use of natural health products or nutraceuticals to treat OA is on the rise [49]. Polyphenols or plant extracts rich in polyphenols are the most widely studied natural health products with relevance to OA. Positive effects of resveratrol from grapes, curcumin from turmeric and EGCG from green tea have been reported [49, 50]. A number of natural products including curcumin and resveratrol have been particularly studied as modulators of NF-κB pathway [51]. Delphinidin, one of the major anthocyanidin class of polyphenols, is abundantly present in coloured flowers and fruits including grape and pomegranate, and has been shown to exhibit strong antioxidant activity [52]. We recently reported the inhibitory effect of pomegranate fruit extract (PE), a rich source of anthocyanidins including delphinidin, on IL-1β-induced activation of MKK-3, p38alpha-MAPK and transcription factor RUNX-2 in human OA chondrocytes [18]. We also showed the bioavailability and protective effects of PE constituents on the joints in CIA mouse model [53]. Results of our present study revealed that delphinidin, a constituent of PE, may act as a potent chondroprotective agent providing a potential novel option for the treatment of OA.
In summary, we are reporting for the first time that delphinidin can inhibit the phosphorylation and activation of IRAK1 in IL-1β-stimulated human chondrocytes. This could block the activation of downstream signalling events and the activation of NF-κB (Fig. 6). Our data also show a potent inhibitory effect of delphinidin on IL-1β-induced production of cartilage-degrading molecule PGE2 via suppressing IL-1β-induced expression of COX-2 mRNA and protein in human OA chondrocytes (Fig. 6). These findings are of importance to understand the chondroprotective mechanisms and clinical applications of delphinidin.
Fig. 6.
Schematic of mechanism of action of delphinidin on NF-κB pathway and COX-2 expression in human chondrocytes.
Upon binding of IL-1 to IL-1RI a multiprotein complex involving IL-1RI/IRAK1/IRAK2/IL-1RAcp/MyD88 is formed. TRAF6 is recruited to the complex and activates NIK, which in turn activates the IKK complex. Activated IKK complex phosphorylates IκB, which is degraded by proteosome resulting in the release and translocation of NF-κB to the nucleus and subsequent transcription of COX-2. Elevated level of COX-2 result in production and release of PGE2 from the chondrocytes. Delphinidin blocks the process by inhibiting the phosphorylation of IRAK and NIK as well as by inhibiting the expression of IKKβ resulting in the inhibition of COX-2 expression and production of PGE2.
Acknowledgements
A.H. carried out the experimental work, data collection, interpretation of results and manuscript drafting. D.C. carried out the experimental work. T.M.H. conceived of the study, its design, coordinated, data interpretation and manuscript drafting. All authors have read and approved the final manuscript.
Funding: This work was supported by the National Institute of Health/National Center for Complimentary and Alternative Medicine grants RO1-AT-003267; RO1-AT-005520; and R21-AT-504615 to T.M.H. Financial support from the MetroHealth Medical Center, Cleveland, OH, USA is also gratefully acknowledged.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Loeser RF. Molecular mechanisms of cartilage destruction: mechanics, inflammatory mediators, and aging collide. Arthritis Rheum. 2006;54:1357–60. doi: 10.1002/art.21813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–34. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 3.Chadjichristos C, Ghayor C, Kypriotou M, et al. Sp1 and Sp3 transcription factors mediate interleukin-1 beta down-regulation of human type II collagen gene expression in articular chondrocytes. J Biol Chem. 2003;278:39762–72. doi: 10.1074/jbc.M303541200. [DOI] [PubMed] [Google Scholar]
- 4.Shakibaei M, Schulze-Tanzil G, John T, Mobasheri A. Curcumin protects human chondrocytes from IL-1beta-induced inhibition of collagen type II and beta1-integrin expression and activation of caspase-3: an immunomorphological study. Ann Anat. 2005;187:487–97. doi: 10.1016/j.aanat.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Stöve J, Huch K, Günther KP, Scharf HP. Interleukin-1beta induces different gene expression of stromelysin, aggrecan and tumor-necrosis-factor-stimulated gene 6 in human osteoarthritic chondrocytes in vitro. Pathobiology. 2000;68:144–9. doi: 10.1159/000055915. [DOI] [PubMed] [Google Scholar]
- 6.Nietfeld JJ, Wilbrink B, Den Otter W, Huber J, Huber-Bruning O. The effect of human interleukin 1 on proteoglycan metabolism in human and porcine cartilage explants. J Rheumatol. 1990;17:818–26. [PubMed] [Google Scholar]
- 7.Bondeson J, Wainwright S, Hughes C, Caterson B. The regulation of the ADAMTS4 and ADAMTS5 aggrecanases in osteoarthritis: a review. Clin Exp Rheumatol. 2008;26:139–45. [PubMed] [Google Scholar]
- 8.Lefebvre V, Peeters-Joris C, Vaes G. Modulation by interleukin 1 and tumor necrosis factor alpha of production of collagenase, tissue inhibitor of metalloproteinases and collagen types in differentiated and dedifferentiated articular chondrocytes. Biochim Biophys Acta. 1990;1052:366–78. doi: 10.1016/0167-4889(90)90145-4. [DOI] [PubMed] [Google Scholar]
- 9.Afonso V, Champy R, Mitrovic D, Collin P, Lomri A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 2007;74:324–9. doi: 10.1016/j.jbspin.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Mathy-Hartert M, Hogge L, Sanchez C, Deby-Dupont G, Crielaard JM, Henrotin Y. Interleukin-1beta and interleukin-6 disturb the antioxidant enzyme system in bovine chondrocytes: a possible explanation for oxidative stress generation. Osteoarthritis Cartilage. 2008;16:756–63. doi: 10.1016/j.joca.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Scott JL, Gabrielides C, Davidson RK, et al. Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann Rheum Dis. 2010;69:1502–10. doi: 10.1136/ard.2009.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roman-Blas JA, Jimenez SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14:839–48. doi: 10.1016/j.joca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Afaq F, Syed DN, Malik A, et al. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J Invest Dermatol. 2007;127:222–32. doi: 10.1038/sj.jid.5700510. [DOI] [PubMed] [Google Scholar]
- 14.Seong AR, Yoo JY, Choi K, et al. Delphinidin, a specific inhibitor of histone acetyltransferase, suppresses inflammatory signaling via prevention of NF-κB acetylation in fibroblast-like synoviocyte MH7A cells. Biochem Biophys Res Commun. 2011;410:581–6. doi: 10.1016/j.bbrc.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 15.Hafeez BB, Siddiqui IA, Asim M, et al. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: involvement of nuclear factor-kappaB signaling. Cancer Res. 2008;68:8564–72. doi: 10.1158/0008-5472.CAN-08-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon JY, Lee KW, Kim JE, et al. Delphinidin suppresses ultraviolet B-induced cyclooxygenases-2 expression through inhibition of MAPKK4 and PI-3 kinase. Carcinogenesis. 2009;30:1932–40. doi: 10.1093/carcin/bgp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasheed Z, Anbazhagan AN, Akhtar N, Ramamurthy S, Voss FR, Haqqi TM. Green tea polyphenol epigallocatechin-3-gallate inhibits advanced glycation end product-induced expression of tumor necrosis factor-alpha and matrix metalloproteinase-13 in human chondrocytes. Arthritis Res Ther. 2009;11:R71. doi: 10.1186/ar2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasheed Z, Akhtar N, Haqqi TM. Pomegranate extract inhibits the interleukin-1β-induced activation of MKK-3, p38α-MAPK and transcription factor RUNX-2 in human osteoarthritis chondrocytes. Arthritis Res Ther. 2010;12:R195. doi: 10.1186/ar3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akhtar N, Rasheed Z, Ramamurthy S, Anbazhagan AN, Voss FR, Haqqi TM. MicroRNA-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthritis Rheum. 2010;62:1361–71. doi: 10.1002/art.27329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyons-Giordano B, Pratta MA, Galbraith W, Davis GL, Arner EC. Interleukin-1 differentially modulates chondrocyte expression of cyclooxygenase-2 and phospholipase A2. Exp Cell Res. 1993;206:58–62. doi: 10.1006/excr.1993.1120. [DOI] [PubMed] [Google Scholar]
- 21.Newton R, Kuitert LM, Bergmann M, Adcock IM, Barnes PJ. Evidence for involvement of NF-kappaB in the transcriptional control of COX-2 gene expression by IL-1beta. Biochem Biophys Res Commun. 1997;237:28–32. doi: 10.1006/bbrc.1997.7064. [DOI] [PubMed] [Google Scholar]
- 22.Lianxu C, Hongti J, Changlong Y. NF-kappaBp65-specific siRNA inhibits expression of genes of COX-2, NOS-2 and MMP-9 in rat IL-1beta-induced and TNF-alpha-induced chondrocytes. Osteoarthritis Cartilage. 2006;14:367–76. doi: 10.1016/j.joca.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 23.O'Neill LA, Greene C. Signal transduction pathways activated by the IL-1 receptor family: ancient signaling machinery in mammals, insects, and plants. J Leukoc Biol. 1998;63:650–7. [PubMed] [Google Scholar]
- 24.Regnier CH, Song HY, Gao X, Goeddel DV, Cao Z, Rothe M. Identification and characterization of an IkappaB kinase. Cell. 1997;90:373–83. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 25.Woronicz JD, Gao X, Cao Z, Rothe M, Goeddel DV. IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK. Science. 1997;278:866–9. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 26.Ling L, Cao Z, Goeddel DV. NF-kappaB-inducing kinase activates IKK-alpha by phosphorylation of Ser-176. Proc Natl Acad Sci USA. 1998;95:3792–7. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janssens S, Beyaert R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol Cell. 2003;11:293–302. doi: 10.1016/s1097-2765(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 28.Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–4. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 29.Pujol JP, Chadjichristos C, Legendre F, et al. Interleukin-1 and transforming growth factor-beta 1 as crucial factors in osteoarthritic cartilage metabolism. Connect Tissue Res. 2008;49:293–7. doi: 10.1080/03008200802148355. [DOI] [PubMed] [Google Scholar]
- 30.Blom AB, van der Kraan PM, van den Berg WB. Cytokine targeting in osteoarthritis. Curr Drug Targets. 2007;8:283–92. doi: 10.2174/138945007779940179. [DOI] [PubMed] [Google Scholar]
- 31.Lotz M. Cytokines in cartilage injury and repair. Clin Orthop Relat Res. 2001;(391 Suppl):S108–15. doi: 10.1097/00003086-200110001-00011. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh S, Hayden MS. Celebrating 25 years of NF-κB research. Immunol Rev. 2012;246:5–13. doi: 10.1111/j.1600-065X.2012.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shih PH, Yeh CT, Yen GC. Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J Agric Food Chem. 2007;55:9427–35. doi: 10.1021/jf071933i. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Vareed SK, Nair MG. Human tumor cell growth inhibition by nontoxic anthocyanidins, the pigments in fruits and vegetables. Life Sci. 2005;76:1465–72. doi: 10.1016/j.lfs.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto K, Arakawa T, Ueda N, Yamamoto S. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J Biol Chem. 1995;270:31315–20. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- 37.Taylor BS, de Vera ME, Ganster RW, et al. Multiple NF- kappaB enhancer elements regulate cytokine induction of the human inducible nitric oxide synthase gene. J Biol Chem. 1998;273:15148–56. doi: 10.1074/jbc.273.24.15148. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi M, Squires GR, Mousa A, et al. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52:128–35. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- 39.Schmid JA, Birbach A. IkappaB kinase beta (IKKbeta/IKK2/IKBKB)—a key molecule in signaling to the transcription factor NF-kappaB. Cytokine Growth Factor Rev. 2008;19:157–65. doi: 10.1016/j.cytogfr.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–51. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 41.Prajapati S, Verma U, Yamamoto Y, Kwak YT, Gaynor RB. Protein phosphatase 2Cbeta association with the IkappaB kinase complex is involved in regulating NF-kappaB activity. J Biol Chem. 2004;279:1739–46. doi: 10.1074/jbc.M306273200. [DOI] [PubMed] [Google Scholar]
- 42.Aupperle K, Bennett B, Han Z, Boyle D, Manning A, Firestein G. NF-kB regulation by IkB kinase-2 in rheumatoid arthritis synoviocytes. J Immunol. 2001;166:2705–11. doi: 10.4049/jimmunol.166.4.2705. [DOI] [PubMed] [Google Scholar]
- 43.Blackwell NM, Sembi P, Newson JS, Lawrence T, Gilroy DW, Kabouridis PS. Reduced infiltration and increased apoptosis of leukocytes at sites of inflammation by systemic administration of a membrane-permeable IkappaBalpha repressor. Arthritis Rheum. 2004;50:2675–84. doi: 10.1002/art.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kishore N, Sommers C, Mathialagan S, et al. A selective IKK-2 inhibitor blocks NF-kappa B-dependent gene expression in interleukin-1 beta-stimulated synovial fibroblasts. J Biol Chem. 2003;278:32861–71. doi: 10.1074/jbc.M211439200. [DOI] [PubMed] [Google Scholar]
- 45.Vidya Priyadarsini R, Senthil Murugan R, Maitreyi S, Ramalingam K, Karunagaran D, Nagini S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur J Pharmacol. 2010;649:84–91. doi: 10.1016/j.ejphar.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 46.Seed SM, Dunican KC, Lynch AM. Treatment options for osteoarthritis: considerations for older adults. Hosp Pract. 2011;39:62–73. doi: 10.3810/hp.2011.02.375. [DOI] [PubMed] [Google Scholar]
- 47.Bjordal JM, Ljunggren AE, Klovning A, Slørdal L. Non-steroidal anti inflammatory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: meta-analysis of randomised placebo controlled trials. BMJ. 2004;329:1317. doi: 10.1136/bmj.38273.626655.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006;296:1633–44. doi: 10.1001/jama.296.13.jrv60011. [DOI] [PubMed] [Google Scholar]
- 49.Khalifé S, Zafarullah M. Molecular targets of natural health products in arthritis. Arthritis Res Ther. 2011;13:102. doi: 10.1186/ar3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henrotin Y, Lambert C, Couchourel D, Ripoll C, Chiotelli E. Nutraceuticals: do they represent a new era in the management of osteoarthritis?—a narrative review from the lessons taken with five products. Osteoarthritis Cartilage. 2011;19:1–21. doi: 10.1016/j.joca.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 51.Bremner P, Heinrich M. Natural products as targeted modulators of the nuclear factor-kappaB pathway. J Pharm Pharmacol. 2002;54:453–72. doi: 10.1211/0022357021778637. [DOI] [PubMed] [Google Scholar]
- 52.Noda Y, Kaneyuki T, Mori A, Packer L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: delphinidin, cyanidin, and pelargonidin. J Agric Food Chem. 2002;50:166–71. doi: 10.1021/jf0108765. [DOI] [PubMed] [Google Scholar]
- 53.Shukla M, Gupta K, Rasheed Z, Khan KA, Haqqi TM. Bioavailable constituents/metabolites of pomegranate (Punica granatum L) preferentially inhibit COX2 activity ex vivo and IL-1beta-induced PGE2 production in human chondrocytes in vitro. J Inflamm. 2008;5:9. doi: 10.1186/1476-9255-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]