Abstract
Objective. IA steroid injections (IASIs) have been shown to relieve pain in knee OA and are widely used in clinical practice. There is, however, evidence of some variation in response. Knowledge of predictors of response could aid in the selection of patients for this therapy. The aim of this systematic review was to determine factors associated with response to IASI in knee OA.
Methods. Medline, Embase, AMED, CINAHL, Web of Science and Cochrane Central Registers for Controlled Trials up to January 2012 were searched with additional hand searches of relevant articles. Studies included were those that involved adults diagnosed with knee OA in whom IASIs were administered and factors that predicted treatment response were investigated.
Results. Eleven publications meeting these criteria were reviewed and relevant information extracted. It was not possible to pool the results because of the different predictors studied, variable outcome measures, different criteria for symptom change and missing data. Given the relative paucity of data and small heterogeneously designed studies, it was difficult to identify predictors of response. Data from individual publications, although not consistent across studies, suggest that the presence of effusion, withdrawal of fluid from the knee, severity of disease, absence of synovitis, injection delivery under US guidance and greater symptoms at baseline may all improve the likelihood of response to IASI.
Conclusion. Further larger-scale studies using standardized methods are required to characterize predictors of response and should focus on synovitis, effusion, pain and structural severity of disease. Such data would help in better targeting therapy to those most likely to benefit.
Keywords: osteoarthritis of the knee, clinical trials, treatment response, predictors of response, systematic review
Introduction
OA is the most common chronic joint disease worldwide. IA steroid injection (IASI) has been widely used in the management of symptomatic knee OA, one of the most commonly affected joints. There is evidence of short-term benefit of IASI to provide pain relief for up to 3–4 weeks [1–18]. However, there is disagreement on the long-term benefit of therapy [2, 19].
Data from the published trials indicate, however, that there is significant variation in both the magnitude and duration of response to steroid injections. As an example, the magnitude of pain improvement measured using a visual analogue scale (VAS) on a 0–100 scale varied between a mean change of 16.2–35.7 mm [8, 11, 14, 20–22], while the duration of pain relief varied between 1 and 8 weeks [4, 5, 8, 11, 14, 20, 21]. The reason for variation in response is unclear, but may be related to disease factors, treatment or patient-related factors. If factors consistently associated with response to steroids could be identified, steroid injections might be better targeted to those most likely to respond. We undertook a systematic review of the published literature to determine whether there are patient-, treatment- or disease-related factors that predict either the magnitude or duration of response to IASI in knee OA.
Materials and methods
Search strategies
Publications that contained reports of factors that may predict response from IASI in knee OA were identified from searching six databases up to January 2012: Medline (from 1948 onwards), Embase (1974 onwards), AMED (1985 onwards), Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus (1937 onwards), Web of Science (1950 onwards) and Cochrane Central Registers for Controlled Trials. The search was conducted with close reference to the users’ guides for undertaking electronic searches of the medical literature [23]. There were no language restrictions. The databases were searched individually for all possible terms and combinations of terms to accommodate differences in the search engines. All medical subject heading (MeSH) searches were explored when possible. The key terms used in combination (AND) were knee osteoarthritis, intra-articular (IA), corticosteroids, injection, trials and predictors. The reference lists of all identified papers were scanned and, in addition, the contents pages of Arthritis and Rheumatism, Annals of the Rheumatic Diseases and Arthritis Care and Research for the past 10 years were hand-searched for other relevant publications.
Study selection criteria
Publications considered were those that included adults with knee OA based on the ACR clinical classification criteria for OA [24] or based on the study having stated patients had knee OA from detailed clinical and/or radiographic assessment of the knee joint. Also, we included studies where IASIs were administered and factors that predicted treatment response investigated. These factors included the presence of effusion, clinical synovitis, synovial hypertrophy, severity of knee OA (based on radiographic grade), presence of knee pain, knee range of movement, muscle strength, stiffness, local tenderness, heat, duration of symptoms and functional, anxiety and depression indices. Outcome measures considered important for evaluating clinical predictors of steroid injections were improvement in pain. Studies could be either clinical trials in which predictors of response were presented or observational studies that included information on predictors of response.
Quality assessment
The quality of randomized controlled trials (RCTs) included was graded using the Jadad score [25], a validated and widely used assessment tool [26]. It comprises a maximum of 5 points, where a point each is awarded if a trial uses randomization, double blinding and provides appropriate and correct description of the randomization and the double blinding, and gives a description of drop-outs or withdrawal of participants. The Consolidated Standards of Reporting Trials (CONSORT) statement was referred to when deciding if the randomization and double-blinding procedures were appropriate to score the relevant points [27, 28]. We considered trials as of low quality when scores were ≤2, whereas trials scoring >2 were deemed to be of high quality [27]. Concealment to treatment allocation and the generation of allocation schedules were assessed using tools developed by Schulz et al. [28] Allocation concealment was scored as adequate or inadequate, or unclear if there was insufficient information to make the judgement. For observational studies, ‘Strengthening the Reporting of Observational Studies in Epidemiology’ (STROBE) guidelines were used to assess quality [29], with close reference to further elaboration and explanation of the criteria given in another publication of the STROBE statement [30]. The STROBE statement contains a checklist of 22 items that cover the appropriateness of the study’s title, aims, methodology, and adequateness of abstract, results and discussion. Two reviewers (N.M. and M.J.C.) independently assessed and scored the publications and reached consensus in two cases of disagreement.
Data extraction
Relevant information from the papers was extracted and presented in tabular form. Because of heterogeneity in the various predictors and outcome variables, it was not possible to pool data from the different studies. For each predictor of interest, the study result was classified as positive (statistically significant increased likelihood of either intensity or duration of response), null (no significant relation of predictor to response) or negative (predictor associated with significantly worse response).
Results
Search outcome
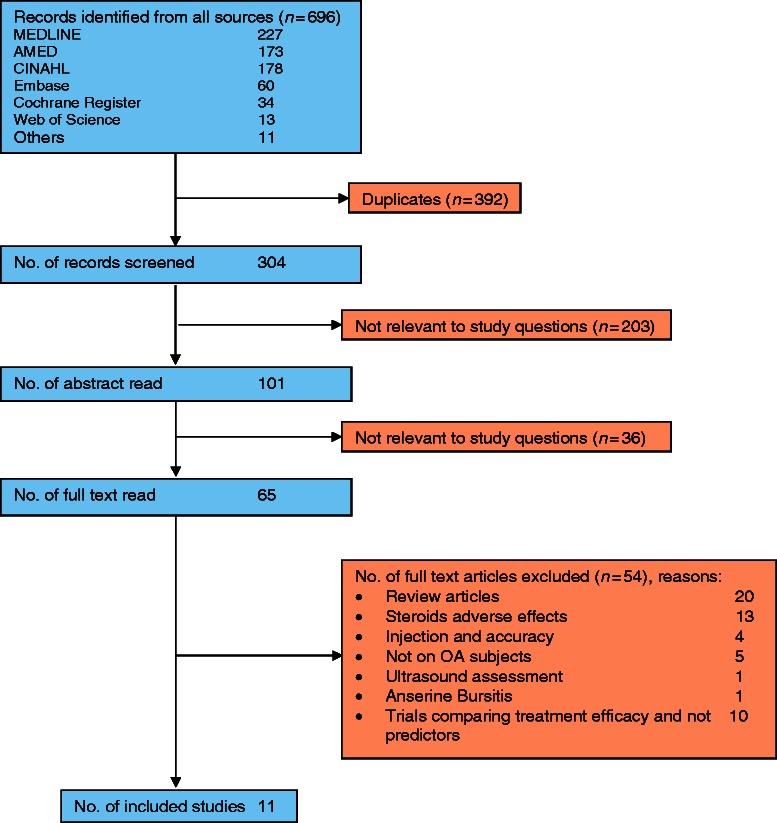
From all databases, 696 records were retrieved. Duplicates were eliminated, including those identified from reference lists of papers and content pages of selected journals (Fig. 1). Text words from 304 journal article titles were screened, and 203 failed to meet the required criteria. One hundred and one abstracts and a further 65 full-text articles were read for eligibility. Of the 65 full texts, 54 were rejected for failing to meet the required criteria or not having predictor data. Eleven publications met the inclusion criteria (Table 1).
Fig. 1.
Summary of search results.
Table 1.
Trials on predictors of response to IASI
| Reference | Setting | Design | Sample size | Mean age, years | Intervention | Follow-up, weeks | Outcome measures | Predictors | Assessment of predictors | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Arden et al. [33] | Southampton/Portsmouth, England | RCT, parallel, single blind | 79a | 67.7 | 40 mg TA/ 2 ml 1% lidocaine or tidal irrigation/ 15–20 ml lidocaine | 2, 4, 12, 26 | WOMAC | Effusion | Bulge sign, patellar tap test | Presence of clinically detectable effusion was associated with greater reduction in WOMAC pain score at 26 weeks (P = 0.04) |
| Radiographic grade | KL 0–4 | KL grade 2 (P = 0.015) and 3/4 (P = 0.012) was associated with less reduction in pain than KL 0/1 | ||||||||
| Chao et al. [4] | California, USA | RCT, parallel, double blind, placebo controlled | 34b | 65.3 | 40 mg TA | 4, 12 | WOMAC | Synovitis | Non-contrast US assessment of synovitis (synovial hypertrophy) | Absence of synovitis was a predictor of response leading to significant improvement in pain at 12 weeks (P = 0.03) |
| Effusion | Non-contrast US assessment of effusion ≥5 mm (present/absent) | Effusion was not a predictor of response | ||||||||
| Chavez-Chiang et al. [34] | New Mexico, USA | RCT (LMP vs ALJL), parallel | 83 |
|
80 mg TA | 2, 26 |
|
Injection approach (LMP, ALJL) | No differences between the two injection approaches, pain outcome at 2 weeks and 6 weeks, per cent responders, per cent non-responder, duration of effect and time to next procedure | |
| Dieppe et al. [5] | Bristol, Bath, England | (i) RCT, parallel, single blind, placebo controlled (ii) RCT, cross-over, single blind, placebo controlled | 12 (24 knees) | 63.5 | 20 mg TH | 1, 2, 4, 6 | Pain score (VAS) | Chondrocalcinosis
|
Radiological assessment
|
|
| 16 (24 knees) | 65 | Radiographic grade | Graded 1–4 | Radiographic grading did not predict response | ||||||
| Gaffney et al. [21] | London, England | RCT, parallel, single blind, placebo controlled | 42 | 66 | 20 mg TH | 1, 6 | Pain score (VAS) | Effusion | Bulge sign, patellar tap test | Clinical evidence of joint effusion (P < 0.05) and successful aspiration of SF (P < 0.01) led to better response to IASI at week 1 but not others |
| SF aspiration | Yes/no | |||||||||
| Duration of symptoms | Assessed, years | |||||||||
| Baseline pain | VAS | |||||||||
| 1-min walking distance | Measured | |||||||||
| Lower limb function | Modified HAQ | |||||||||
| Radiographic grade | Graded 0–9 | |||||||||
| SF volume | Volume of aspiration | |||||||||
| SF leucocyte | Count | |||||||||
| Jones and Doherty [8] | Nottingham, England | RCT, cross-over double blind, placebo controlled | 59 | 70.6 | 40 mg MPA | 3, 8 | 15% reduction in pain score (VAS) | Age | Only tenderness was linked with improved response (OR 1.80; 95% CI 1.03, 1.67) | |
| Gender | ||||||||||
| Knee ROM | Measurement, in degrees | |||||||||
| Morning stiffness | Duration, min | |||||||||
| Inactivity stiffness | Duration, min | |||||||||
| Local tenderness | Clinical assessment (graded 0–3) | |||||||||
| Local heat | Clinical assessment (present/absent) | |||||||||
| Synovial thickening | Clinical assessment (present/absent) | |||||||||
| Quadriceps strength | Measurement by strain gauge | |||||||||
| Anxiety/depression | HADS | |||||||||
| Function | HAQ | |||||||||
| Lower limb function | Lower limb HAQ | |||||||||
| SF aspiration | Yes/no | |||||||||
| Effusion | Clinical assessment (graded 0–3) | |||||||||
| Pendleton et al. [10] | Belfast, Ireland | Open trial | 86 | 65c | 40 mg MPA | 1, 6 | WOMAC | Baseline pain | WOMAC pain subscore | Higher baseline scores were associated with greater WOMAC reduction at week 1 (pain/function: P < 0.01; stiffness: P < 0.05) and week 6 (all: P < 0.01). |
| Baseline function | WOMAC function subscore | |||||||||
| Baseline stiffness | WOMAC stiffness subscore | |||||||||
| Synovitis | Non-contrast, PD, US assessment of synovial membrane graded 1–4. | Synovitis and effusion were not predictors of response | ||||||||
| Effusion | Non-contrast, PD, US assessment of effusion graded 1–4. | |||||||||
| Patellar tendonitis | Clinical assessment | Patellar tendonitis (P < 0.01) and presence of heat (P < 0.05) was associated with greater reduction in night pain | ||||||||
| Local heat | Clinical assessment (present/absent) | |||||||||
| Pyne et al. [32] | London, England | RCT, parallel, Double blind | 29 | 62.8 | 20 mg TH vs 40 mg MPA | 3, 8 | Pain score (VAS) | Effusion | Volume of fluid aspirated | Effusion was not a predictor of response in both treatment arms |
| 28 | 62.2 | |||||||||
| Shah and Wright [13] | Yorkshire, England | RCT (M vs IFP injection approach), parallel, single blind | 26 (36 knees) | –d | 50 mg HA | 4 | Patient-rated improvement (nil/slight/great) | Injection approach | M or IFP knee injections | Injection approach was not a predictor of response |
| SF aspiration | Yes/no | Aspiration of fluid using medial approach was not associated with improvement | ||||||||
| Sibbitt et al. [31] | America | RCT (blind vs US-guided injection), parallel | 92 | 61.9 | 80 mg TA/ 3 ml lidocaine | 2, 26 | Pain score (VAS) | Sonographic needle guidance | US | US-guided knee injection was associated with improved response, that is, 42% reduction in pain scores at 2 weeks (P < 0.03); 107% increase in the responder rate (P < 0.001); 52% reduction in the non-responder rate (P < 0.001); 36% increase in duration of symptom relief (P = 0.01); 22% less re-injection within 12 months (P = 0.03) |
| Responder rate (pain VAS <2) | ||||||||||
| Non-responder rate (pain VAS ≥2) | ||||||||||
| Duration of Sx-relief | ||||||||||
| Time to re-injection | ||||||||||
| No. of re-injections | ||||||||||
| Smith et al. [14] | Adelaide, South Australia | RCT, parallel, double blind, placebo controlled | 38 | 67.3 | 120 mg MPA preceded by arthroscopy | 4 | OARSI response criteria | Radiographic grade | KL 1–4 | Radiographic severity of OA was a predictor of response (RR 0.59, 95% CI 0.356, 0.996; P < 0.05). Duration of symptoms and arthroscopic grading of cartilage damage did not predict response |
| Duration of symptoms | Assessed, years | |||||||||
| Arthroscopic cartilage damage | Arthroscopic cartilage grading (0–24) |
aSeventy-nine recruited; data for 77 available for analysis at weeks 2 and 4, 73 at week 12 and 71 at week 26. bForty recruited; data for 34 available for primary analysis. cMedian age. dMean age presented for knees rather than subjects. HA: hydrocortisone acetate; LMP: lateral midpatellar; ALJL: anterolateral joint line; M: medial; IFP: infrapatelllar; PD: power Doppler; WCC: white cell count; ROM: range of movement; RR: relative risk.
Ten of the 11 studies were RCTs and 1 was an observational study. Only two trials [8, 31] had as a primary aim determination of predictors of response. The rest of the studies were trials that included evaluation of predictors of response as part of a secondary or post hoc analysis of the data. Some studies included only knee OA with effusion [5, 32] or those without effusion [31], while others included both [4, 8, 10, 13, 14, 21].
Participants in all the studies were followed for at least 4 weeks. Four looked at longer-term effects up to 6–12 months [14, 31, 33, 34]. Five of the 10 RCTs were placebo/controlled trials evaluating steroids such as methylprednisolone acetate (MPA), triamcinolone hexacetonide (TH) and triamcinolone acetonide (TA) against placebo injections. In these trials, only the steroid group’s response was used to identify factors predicting response. One RCT [32] compared treatment effects between two types of steroid, MPA and TH, another [33] compared treatment efficacy between tidal irrigation and IASI, two others [13, 34] compared injection techniques and one compared blind and US-guided knee injections [31].
When evaluating factors predicting response, outcomes used in the studies were measurement of pain improvement such as change in Western Ontario McMaster Universities index (WOMAC) pain scores [4, 10, 33], VAS pain [5, 8, 21, 31, 32, 34], patient-rated improvement [13] and Osteoarthritis Research Society International (OARSI) response criteria [14]. Table 2 summarizes the main predictor factors and the number of studies that had found null findings or positive or negative direction of response to IASI in knee OA.
Table 2.
Factors affecting treatment response
| Predictor factor | No. of studies showing positive effect | No. of studies showing no effect | No. of studies showing negative effect |
|---|---|---|---|
| Synovitis | 0 | 2a | 1 |
| Aspiration | 1 | 2 | 0 |
| Effusion | 2 | 4 | 0 |
| Severity of radiographic degeneration | 2 | 2 | 0 |
| Sonographic-guided injection | 1 | 0 | 0 |
| Worse pain | 1 | 1 | 0 |
| Worse stiffness | 1 | 1 | 0 |
| Worse knee function | 1 | 2 | 0 |
| Duration of symptom | 0 | 2 | 0 |
aOne study used clinical assessment of synovitis.
Data quality
Table 3 contains the Jadad and the allocation concealment scores of the 10 RCTs. Based on the Jadad scores, 5 of the 10 studies were high-quality trials. Based on the STROBE guidelines, the one observational study scored 14 points out of a maximum score of 22.
Table 3.
Quality evaluation of RCTs
| Study | Allocation concealment | Total Jadad score | Jadad scoring criteria |
||||
|---|---|---|---|---|---|---|---|
| Randomized | Double blind | Description of drop-outs or withdrawal | Randomization is described and appropriate | Double blinding is described and appropriate | |||
| Arden et al. [33] | Adequate | 3 | 1 | – | 1 | 1 | – |
| Chao et al. [4] | Adequate | 4 | 1 | 1 | 1 | – | 1 |
| Chavez-Chiang et al. [34] | Unclear | 1 | 1 | – | – | – | – |
| Dieppe et al. [5] | Unclear | 1 | 1 | – | – | – | – |
| Gaffney et al. [21] | Unclear | 2 | 1 | – | 1 | – | |
| Jones and Doherty [8] | Unclear | 3 | 1 | 1 | 1 | – | – |
| Pyne et al. [32] | Unclear | 4 | 1 | 1 | 1 | – | 1 |
| Shah and Wright [13] | Unclear | 1 | 1 | – | – | – | – |
| Sibbitt et al. [31] | Unclear | 2 | 1 | – | 1 | – | – |
| Smith et al. [14] | Adequate | 5 | 1 | 1 | 1 | 1 | 1 |
Predictors of response
Synovitis
Two studies evaluated synovitis using non-contrast sonography [4, 10], though only Pendleton et al. [10] used power Doppler US, a technique that is better at detecting synovitis [35] than the grey-scale mode. With the grey-scale mode it was assumed that synovial hypertrophy was synonymous with synovitis [4].
In their RCT, Chao et al. [4] found, among the 34 participants who received IASI, that the absence of synovitis, in comparison with its presence, was associated with a significant improvement in WOMAC pain subscale at 12 weeks. A single person interpreted the saved images and synovial hypertrophy was assessed at one location, the suprapatellar pouch. Pendleton et al. [10] used an observational study design to study the effect of US-assessed synovitis in 86 participants and found it did not predict response. In a third study based on clinician’s assessment, the presence of synovial thickening (present/absent) was not associated with a treatment response [8]. Thus the role of synovitis in predicting response was unclear.
Effusion
Six studies looked at whether the presence of effusion was associated with response (see Tables 1 and 2). Of these six studies, one found effusion, assessed by the presence of a positive bulge sign and presence of patellar tap, to be a predictor of improved response when pain was evaluated using a VAS (P < 0.01 at 1 week, P < 0.05 at 6 weeks) [21]. Although not influencing the magnitude of response, one other trial found the presence of effusion (either positive bulge or patellar test) increased the duration of response [33]. The former study involved SF aspiration prior to IASI, while the latter included injection of 2 ml of 1% lidocaine with the steroids. In four other trials, however, there was no association between effusion and symptom improvement [4, 8, 10, 32]. Again, prior arthrocentesis [8, 10, 32] within the injection protocol did not seem to influence subsequent outcomes.
Aspiration
Three of 11 studies evaluated whether aspiration of SF was associated with treatment response. Gaffney et al. [21] reported greater improvement in VAS for pain following successful SF aspiration (P < 0.01 at week 1). Jones and Doherty [8] and Shah and Wright [13], in contrast, failed to find an association between aspiration and treatment response.
Severity of disease
Radiographic severity of OA was found to be a predictor in two studies; the more severe the disease, the less likely the patient was to have an OARSI response [relative risk (RR) 0.59, 95% CI 0.36, 1.0; P < 0.05] [14] or to show significant change in WOMAC pain score (P < 0.02) [33]. Both these trials used Kellgren–Lawrence (KL) scores to grade the OA. In contrast, two other trials that used different OA scoring systems—Dieppe et al. [5] in their trial of 16 participants (grade 1–4; P-values not given) and Gaffney et al. [21] (scores 0–9; P-values not given)—did not find that radiographic OA grading predicted response. In a further study, arthroscopic grading of cartilage damage at the time of the steroid injection was not linked with response (P = 0.3) [14].
Sonographically guided injection
While studies including patients with a variety of rheumatic conditions exist, we found only one study of knee OA, and in that study sonographically guided injections when compared with blind injections led to a further 42% decrease in absolute pain VAS (P < 0.03) from baseline scores at 2 weeks, a 1.1-month longer duration of therapeutic effect (P < 0.01), 107% increase in responder rate defined by VAS <2 cm (P < 0.001) and 52% reduction in non-responder rate defined by VAS ≥2 cm (P < 0.001) [31]. However, pain outcomes at 6 months were similar whether these injections were performed blind or sonographically guided.
Other factors potentially affecting response
Local knee tenderness (using a scale ranging from 0 to 3) was linked with improved response in one study [odds ratio (OR) 1.80; 95% CI 1.03, 1.67] [8]. In one trial, higher baseline pain was associated with greater response to IASI [10] but not in another [21]. Greater baseline functional impairment was also associated with improved clinical response, with a higher score of WOMAC function demonstrating better response at 1 and 6 weeks in one trial [10], but two other studies found no association between functional scores prior to treatment and symptom improvement [8, 21]. Pendleton et al. [10] found a higher WOMAC stiffness score led to greater response at 1 and 6 weeks. However, in a different study, morning and inactivity stiffness was not associated with response to IASI [8]. The presence of patellar tendonitis and local heat was also associated with greater reduction in night pain [10]. However, Jones and Doherty [8] did not find that local heat predicted response.
In other studies, chondrocalcinosis [5, 21], crystals and SF cell counts [5], and SF leucocytes [21] were not linked with response to IASI. Duration of symptoms did not predict response [RR 0.91; P = 0.6] in two studies [14, 21].
One study of 59 participants evaluated multiple other potential predictors age, gender and knee range of movement did not predict response [8]. Maximum isometric quadriceps strength measured using a commercial strain gauge and levels of anxiety and depression [assessed using the Hospital Anxiety and Depression Scale (HADS)] were not linked to IASI response [8]. Neither was disability, assessed as composite and lower limb scores using the Stanford HAQ, associated with IASI response.
Two other studies investigated whether different injection sites and approaches influenced outcome from IASI [13, 34]. Shah and Wright [13] found no differences in the therapeutic response when 50 mg of hydrocortisone acetate was injected using the infrapatellar or medial knee approach. Similarly, no difference in outcome was observed when IASI was delivered using the lateral mid-patellar approach or through the anterolateral joint line performed with the knee flexed and needle angled towards the medial femoral condyle [34].
Discussion
IASI is commonly used to relieve symptoms of knee OA; however, factors that predict response are poorly characterized, making it difficult to select patients who are most likely to be successfully treated using this approach. While this systematic review uncovered inconsistent findings across studies, there were several features that were reported by one or more studies as enhancing the likelihood of IASI response.
Although the mechanism of the therapeutic effect of CSs in knee OA is unclear, it is likely related in part to their potent anti-inflammatory effect. In this context, it is perhaps surprising that there was no consistent link between synovitis or presence of effusion on outcome [4, 8, 10, 32]. Indeed, in one study, the absence of synovitis was linked with a beneficial effect [4]. The difference in the findings of the two studies that used US-assessed synovitis [4, 10] also raises questions about whether their findings were attributable to different patient characteristics and disease severity, different trial design or different criteria for defining responder status. The Pendleton et al. [10] study was the larger of the two and included power Doppler assessments of synovitis, suggesting that its null findings may be more generalizable.
Methodological limitations in relation to defining the predictor variables may be another explanation for inconsistencies across studies. To assess knee synovitis, direct visualization and measurement of synovitis through sonography or other imaging is preferable. In the case of effusion, unsuccessful aspiration may also not always indicate the absence of effusion [36, 37]. Needle placement outside the joint, loculated or highly viscous SF, obese knees and errors from injectors all affect the ability to aspirate fluid [37, 38]. Even when the needle has been successfully placed within the joint capsule, it can move into the synovium or fat pad, resulting in a dry tap [38]. Medial knee plica can also obstruct aspiration [38], and it has been reported that fluid may be inaccessible if present in low volume [37, 38]. In relation to SF, small effusion volumes may not be readily detectable during clinical assessment. When using US to assess the presence of knee effusion, SF volume <7 ml, which is equivalent to about 2 mm thickness, may not be discernible during scanning [39, 40]. In some knees, effusion may be localized in the suprapatellar pouch or the medial or lateral recesses of the knee [40, 41], hence restricting US assessment to only one region may result in false-negative findings.
Surprisingly, there were only a few studies that formally studied the effect of the severity of joint and cartilage degeneration in knee OA on treatment response. Trials that used KL grading of knee OA appeared to find positive findings [14, 33], while those that used other scoring systems had null results [5, 21]. Smith et al. [14] also did not find arthroscopic grading of cartilage damage associated with treatment response, despite the fact that the same trial found more severe disease, as assessed radiographically, to be associated with a worse response. This trial was an investigation of the effect of IASI given at the time of arthroscopy, where the adjunct treatment of arthroscopy could be a variable affecting outcome.
One major reason for null findings of studies is that most of the studies included in our review had too few subjects to be likely to detect significant risk factor effects, even if these effects were of clinical importance. We estimate that a sample size of 93 would be needed to ensure an 80% likelihood of detecting a factor with a prevalence of 50% to increase the odds of response to steroids 2-fold. Only 1 of the 11 studies had a sample size that was this large. The small-study bias could partially explain the conflicting results for the different predictors.
We are unable to evaluate whether the duration of follow-up in our studies accounts for some of the null findings in terms of predictors. The studies in general examined patients anywhere from 1 week to several weeks after injection, but a few studies looked at patients as late as 6 months after injection. In these latter studies, there were earlier evaluations and we focused on them, to be consistent.
Another consideration is whether the different steroids used in the trials partially explain the conflicting results for each outcome. Triamcinolone acetate, MPA and TH are said to share similar potency with similar recommended dosing [42]. Trials that have evaluated different steroid preparations in knee OA have not found significant differences among the various IA steroids [15, 32, 43–45]. However, one trial indicated that TH might act more quickly and could lead to a greater reduction in pain than MPA in the first 3 weeks after the injection [32]. The studies reviewed in this article of IASI predictors were primarily those using MPA, TH and TA (see Table 1) and differences in steroids should not have affected the results examining other predictors, although we cannot exclude the possibility that differences in doses across the studies would have affected the durability or intensity of steroid response. None of the studies we reviewed formally evaluated dose response for the commonly used IA steroid preparations in knee OA.
The sparse evidence for factors that may influence IASI reflected from this systematic review is partly because predictor factors are understudied. We could only identify 11 publications, of which many of them evaluated predictor factors as part of a secondary or post hoc analysis of the data. Secondly, predictor factors are poorly studied in trials. Many of the trials identified in this review were RCTs but the design of placebo/control comparisons of treatment effects of steroids means that evaluation of predictors of response to IASI can be made on one group only, the group that received the IASI, while the control/placebo group is disregarded. As evaluation of the predictor factors was now confined to the treatment group, this reduced further the sample size on many of the already small trials such that even if there is a predictor factor present, the power of the study would not be sufficient to detect it. This raises the question of whether RCT is the primary design for predictors of response. A longitudinal design such as observational studies, in contrast, may have allowed study of a wider spectrum of the disease and overcome some of the main constraints faced by RCTs.
To find additional studies, we expanded the search to trials that compared other agents such as hyaluronate with steroids in knee OA, but we were unable to find predictor studies on IASI among them. The use of Jadad scores may not provide the best evidence for quality [46] but our findings using scoring systems for individual items evaluating quality did not differ much from the Jadad scores, since many of these trials lacked aspects of methodological rigour.
Other potential predictors, including previous knee injections, BMI, knee joint misalignment, use of walking aids, presence of muscle atrophy and also socio-economic factors have not been investigated. Future studies should include sufficient numbers of patients to provide adequate power and a longitudinally designed large observational study may be more appropriate to study IA steroid predictor factors. There should be clear information about the methods used to determine putative predictors and also details about the intervention, including delivery of therapy and whether or not US was used. Standardized outcomes should be reported, including pain, stiffness and function.
In summary, to our knowledge this is the first systematic review that attempts to investigate factors that may predict response to IASI in knee OA. Because of heterogeneity (in exposures), it was not possible to pool data across studies. Data from individual publications, although not consistent across studies, indicated there could be a number of predictors of response to IASI, including effusion, withdrawal of fluid from the knee, absence of synovitis, delivering injections under US guidance, structural severity of disease and pain. Further studies using standardized methods of assessment are needed to confirm these predictor factors and to characterize treatment response to IASI in patients with knee OA. Such data will be of help in better targeting therapy to those most likely to benefit.

Acknowledgements
The authors would like to acknowledge Matthew Parkes, the Research into Osteoarthritis in Manchester (ROAM) statistician, for his statistical advice and support. N.M. is funded by a clinical doctoral fellowship award from the National Institute for Health Research.
Funding: This work was supported by a strategic award from Arthritis Research UK (grant number 18676) ISRCTN07329370.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Bellamy N, Campbell J, Welch V, Gee TL, Bourne R, Wells GA. Cochrane Database Syst Rev. 2009. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Advance Access published 15 April 2009, doi:10.1002/14651858.CD005328.pub2. [Google Scholar]
- 2.Bjordal JM, Klovning A, Ljunggren AE, Slordal L. Short-term efficacy of pharmacotherapeutic interventions in osteoarthritic knee pain: a meta-analysis of randomised placebo-controlled trials. Eur J Pain. 2007;11:125–38. doi: 10.1016/j.ejpain.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Cederlof S, Jonson G. Intraarticular prednisolone injection for osteoarthritis of the knee. A double blind test with placebo. Acta Chir Scand. 1966;132:532–7. [PubMed] [Google Scholar]
- 4.Chao J, Wu C, Sun B, et al. Inflammatory characteristics on ultrasound predict poorer longterm response to intraarticular corticosteroid injections in knee osteoarthritis. J Rheumatol. 2010;37:650–5. doi: 10.3899/jrheum.090575. [DOI] [PubMed] [Google Scholar]
- 5.Dieppe PA, Sathapatayavongs B, Jones HE, Bacon PA, Ring EF. Intra-articular steroids in osteoarthritis. Rheumatol Rehabil. 1980;19:212–7. doi: 10.1093/rheumatology/19.4.212. [DOI] [PubMed] [Google Scholar]
- 6.Godwin M, Dawes M. Intra-articular steroid injections for painful knees. Systematic review with meta-analysis. Can Fam Physician. 2004;50:241–8. [erratum appears in Can Fam Physician 2009;55:590] [PMC free article] [PubMed] [Google Scholar]
- 7.Hepper CT, Halvorson JJ, Duncan ST, Gregory AJM, Dunn WR, Spindler KP. The efficacy and duration of intra-articular corticosteroid injection for knee osteoarthritis: a systematic review of level I studies. J Am Acad Orthop Surg. 2009;17:638–46. doi: 10.5435/00124635-200910000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Jones A, Doherty M. Intra-articular corticosteroids are effective in osteoarthritis but there are no clinical predictors of response. Ann Rheum Dis. 1996;55:829–32. doi: 10.1136/ard.55.11.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirwan JR, Rankin E. Intra-articular therapy in osteoarthritis. Bailliere's Clin Rheumatol. 1997;11:769–94. doi: 10.1016/s0950-3579(97)80009-x. [DOI] [PubMed] [Google Scholar]
- 10.Pendleton A, Millar A, O'Kane D, Wright GD, Taggart AJ. Can sonography be used to predict the response to intra-articular corticosteroid injection in primary osteoarthritis of the knee? Scand J Rheumatol. 2008;37:395–7. doi: 10.1080/03009740802050738. [DOI] [PubMed] [Google Scholar]
- 11.Ravaud P, Moulinier L, Giraudeau B, et al. Effects of joint lavage and steroid injection in patients with osteoarthritis of the knee: results of a multicenter, randomized, controlled trial. Arthritis Rheum. 1999;42:475–82. doi: 10.1002/1529-0131(199904)42:3<475::AID-ANR12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 12.Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48:370–7. doi: 10.1002/art.10777. [DOI] [PubMed] [Google Scholar]
- 13.Shah KD, Wright V. Intra-articular hydrocortisone in osteo-arthrosis. Ann Rheum Dis. 1967;26:316–8. doi: 10.1136/ard.26.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith MD, Wetherall M, Darby T, et al. A randomized placebo-controlled trial of arthroscopic lavage versus lavage plus intra-articular corticosteroids in the management of symptomatic osteoarthritis of the knee. Rheumatology. 2003;42:1477–85. doi: 10.1093/rheumatology/keg398. [DOI] [PubMed] [Google Scholar]
- 15.Thorpe P. Intra-articular triamcinolone acetonide and methylprednisolone acetate in arthritis. Curr Ther Res. 1985;38:513–8. [Google Scholar]
- 16.Towheed TE, Hochberg MC. A systematic review of randomized controlled trials of pharmacological therapy in osteoarthritis of the knee, with an emphasis on trial methodology. Semin Arthritis Rheum. 1997;26:755–70. doi: 10.1016/s0049-0172(97)80043-1. [DOI] [PubMed] [Google Scholar]
- 17.Uthman I, Raynauld JP, Haraoui B. Intra-articular therapy in osteoarthritis. Postgrad Med J. 2003;79:449–53. doi: 10.1136/pmj.79.934.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476–99. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ. 2004;328:869–73. doi: 10.1136/bmj.38039.573970.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman DM, Moore ME. The efficacy of intraarticular steroids in osteoarthritis: a double-blind study. J Rheumatol. 1980;7:850–6. [PubMed] [Google Scholar]
- 21.Gaffney K, Ledingham J, Perry JD. Intra-articular triamcinolone hexacetonide in knee osteoarthritis: factors influencing the clinical response. Ann Rheum Dis. 1995;54:379–81. doi: 10.1136/ard.54.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller JH, White J, Norton TH. The value of intra-articular injections in osteoarthritis of the knee. J Bone Joint Surg Br. 1958;40-B:636–43. doi: 10.1302/0301-620X.40B4.636. [DOI] [PubMed] [Google Scholar]
- 23.Oxman AD, Sackett DL, Guyatt GH. Users' guides to the medical literature. I. How to get started. The Evidence-Based Medicine Working Group. JAMA. 1993;270:2093–5. [PubMed] [Google Scholar]
- 24.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 25.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 26.Olivo SA, Macedo LG, Gadotti IC, Fuentes J, Stanton T, Magee DJ. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. 2008;88:156–75. doi: 10.2522/ptj.20070147. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352:609–13. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- 28.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. JAMA. 1995;273:408–12. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 29.von EE, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–9. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Vandenbroucke JP, Elm Ev, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147:573–7. doi: 10.7326/0003-4819-147-8-200710160-00010-w1. [DOI] [PubMed] [Google Scholar]
- 31.Sibbitt WL, Jr, Band PA, Kettwich LG, Chavez-Chiang NR, Delea SL, Bankhurst AD. A randomized controlled trial evaluating the cost-effectiveness of sonographic guidance for intra-articular injection of the osteoarthritic knee. J Clin Rheumatol. 2011;17:409–15. doi: 10.1097/RHU.0b013e31823a49a4. [DOI] [PubMed] [Google Scholar]
- 32.Pyne D, Ioannou Y, Mootoo R, Bhanji A. Intra-articular steroids in knee osteoarthritis: a comparative study of triamcinolone hexacetonide and methylprednisolone acetate. Clin Rheumatol. 2004;23:116–20. doi: 10.1007/s10067-003-0841-z. [DOI] [PubMed] [Google Scholar]
- 33.Arden NK, Reading IC, Jordan KM, et al. A randomised controlled trial of tidal irrigation vs corticosteroid injection in knee osteoarthritis: the KIVIS Study. Osteoarthritis Cartilage. 2008;16:733–9. doi: 10.1016/j.joca.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Chavez-Chiang CE, Sibbitt WL, Jr, Band PA, Chavez-Chiang NR, Delea SL, Bankhurst AD. The highly accurate anteriolateral portal for injecting the knee. Sports Med Arthrosc Rehabil Ther Technol. 2011;3:6. doi: 10.1186/1758-2555-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walther M, Harms H, Krenn V, Radke S, Faehndrich TP, Gohlke F. Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2001;44:331–8. doi: 10.1002/1529-0131(200102)44:2<331::AID-ANR50>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 36.Jones A, Regan M, Ledingham J, Pattrick M, Manhire A, Doherty M. Importance of placement of intra-articular steroid injections. BMJ. 1993;307:1329–30. doi: 10.1136/bmj.307.6915.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qvistgaard E, Kristoffersen H, Terslev L, Danneskiold-Samsoe B, Torp-Pedersen S, Bliddal H. Guidance by ultrasound of intra-articular injections in the knee and hip joints. Osteoarthritis Cartilage. 2001;9:512–7. doi: 10.1053/joca.2001.0433. [DOI] [PubMed] [Google Scholar]
- 38.Roberts NW, Hayes CW, Breitbach SA, Owen J. Dry taps and what to do about them: a pictorial essay on failed arthrocentesis of the knee. Am J Med. 1996;100:461–4. doi: 10.1016/s0002-9343(97)89524-1. [DOI] [PubMed] [Google Scholar]
- 39.Delaunoy I, Feipel V, Appelboom T, Hauzeur JP. Sonography detection threshold for knee effusion. Clin Rheumatol. 2003;22:391–2. doi: 10.1007/s10067-003-0759-5. [DOI] [PubMed] [Google Scholar]
- 40.Hong BY, Lee JI, Kim HW, Cho YR, Lim SH, Ko YJ. Detectable threshold of knee effusion by ultrasonography in osteoarthritis patients. Am J Phys Med Rehabil. 2011;90:112–8. doi: 10.1097/PHM.0b013e3182017321. [DOI] [PubMed] [Google Scholar]
- 41.Roberts WN. Primer: pitfalls of aspiration and injection. Nat Clin Pract Rheum. 2007;3:464–72. doi: 10.1038/ncprheum0558. [DOI] [PubMed] [Google Scholar]
- 42.Schumacher HR, Chen LX. Injectable corticosteroids in treatment of arthritis of the knee. Am J Med. 2005;118:1208–14. doi: 10.1016/j.amjmed.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Popov VV, Boonchook NV, Apeniesheva NP. Treatment of gonarthrosis with intra-articular drug administration. Klinischeskaia Meditsina. 1989;67:104–8. [PubMed] [Google Scholar]
- 44.Valtonen EJ. Clinical comparison of triamcinolonehexacetonide and betamethasone in the treatment of osteoarthrosis of the knee-joint. Scand J Rheumatol Suppl. 1981;41:1–7. [PubMed] [Google Scholar]
- 45.Wright V, Chandler GN, Morison RA, Hartfall SJ. Intra-articular therapy in osteo-arthritis; comparison of hydrocortisone acetate and hydrocortisone tertiary-butylacetate. Ann Rheum Dis. 1960;19:257–61. doi: 10.1136/ard.19.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282:1054–60. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]