Abstract
Objective. Epidemiological studies have shown an association between OA and increased BMD. To explore the nature of this relationship, we examined whether the risk of OA is increased in individuals with high bone mass (HBM), in whom BMD is assumed to be elevated due to a primary genetic cause.
Methods. A total of 335 115 DXA scans were screened to identify HBM index cases (defined by DXA scan as an L1 Z-score of ≥+3.2 and total hip Z-score ≥+1.2, or total hip Z-score ≥+3.2 and L1 Z-score ≥+1.2). In relatives, the definition of HBM was L1 Z-score plus total hip Z-score ≥+3.2. Controls comprised unaffected relatives and spouses. Clinical indicators of OA were determined by structured assessment. Analyses used logistic regression adjusting for age, gender, BMI and social deprivation.
Results. A total of 353 HBM cases (mean age 61.7 years, 77% female) and 197 controls (mean age 54.1 years, 47% female) were included. Adjusted NSAID use was more prevalent in HBM cases versus controls [odds ratio (OR) 2.17 (95% CI 1.10, 4.28); P = 0.03]. The prevalence of joint replacement was higher in HBM cases (13.0%) than controls (4.1%), with an adjusted OR of 2.42 (95% CI 1.06, 5.56); P = 0.04. Adjusted prevalence of joint pain and knee crepitus did not differ between cases and controls.
Conclusion. HBM is associated with increased prevalence of joint replacement surgery and NSAID use compared with unaffected controls.
Keywords: osteoarthritis, high bone mass, bone mineral density, DXA, joint replacement
Introduction
OA is one of the 10 most disabling diseases in developed countries, with symptomatic OA estimated to affect 9.6% of men and 18% of women aged over 60 worldwide [1]. Rates of joint replacement surgery (for which the main indication is OA) are also increasing; in 2010, 81 979 knee replacements and 76 759 hip replacements were recorded within the National Joint Registry for England and Wales [2].
An inverse association between OA and osteoporosis is widely reported. Numerous studies have examined the relationship between BMD and OA, with several reviews [3–5]. The most studied joints are the hip and knee, with the majority of studies finding positive evidence of a relationship between OA at these joint sites and increased hip and/or lumbar spine BMD [6–9]. Higher BMD has also been reported in association with OA of the spine [10] and hand [8, 9, 11]. Studies have generally reported a difference in BMD between OA cases and controls no greater than 10% [6, 7, 9]. For example, a cross-sectional analysis of middle-aged women recruited from a general practice register in London reported an increase in lumbar spine BMD ranging from 3.0% to 9.3% and femoral neck BMD from 1.3% to 6.3% in women with radiographic OA compared with controls, depending on joint site [9]. Similarly, in an older Dutch population, femoral neck BMD was increased 3–8% in subjects with radiographic knee and hip OA compared with controls [7]. While earlier studies were mainly cross-sectional, recently several longitudinal studies have confirmed an increased incidence of radiographic knee OA associated with higher BMD [12–15].
However, opinion regarding the OA–BMD relationship lacks consensus. Although increased BMD is associated with an increased risk of incident OA, some longitudinal studies have reported an inverse relationship with progression of existing OA [13, 15]. Both increased [7] and decreased [10, 12] rates of bone loss over time have been reported in individuals with radiographic OA compared with controls. Furthermore, the vast majority of publications have used a radiographic definition of OA relying heavily on the presence of osteophytes. Several studies have observed a stronger relationship of BMD with osteophytosis than with joint space narrowing, the radiographic feature most indicative of cartilage loss [6, 11, 16]. Some authors have speculated that higher BMD may be associated specifically with osteophyte formation rather than true OA, possibly reflecting a bone-forming phenotype [3].
Alternatively, the observed OA–BMD association could be artefactual. Osteophytes and subchondral sclerosis at DXA sites (e.g. lumbar spine) could lead to falsely elevated BMD measurement. Or OA may be associated with increased bone size [17, 18], leading to overestimation of areal BMD where no true increase in volumetric BMD exists [18]. Therefore the true nature of the relationship between OA and BMD remains a topic of active research interest.
Several uncommon single gene disorders (e.g. sclerosteosis [19], LRP5 mutations [20]) are associated with markedly elevated BMD. However, we have recently reported that high bone mass (HBM) may, more commonly, be an incidental finding on routine DXA scanning; only half of cases (approximately) being explained by artefact [21]. This investigation has led to the establishment of a rare UK-based population of individuals with unexplained HBM; genetic evaluation is currently under way. Compared with unaffected family and spouse controls, individuals with HBM have several features suggestive of a mild skeletal dysplasia, including broad frame, mandible enlargement, increased shoe size and extra bone at tendon and ligament insertions [21].
In this study we aimed to investigate the presence of clinical indicators of OA in a population with unexplained high BMD of likely genetic origin. To achieve this, we compared the prevalence of several OA-related phenotypes, including a history of joint replacement surgery, between HBM cases and controls. The prevalence of joint replacement in older HBM cases (≥65 years) was also compared with general population joint replacement data from the Health Survey for England (HSE) 2005.
Methods
Recruitment and HBM definition
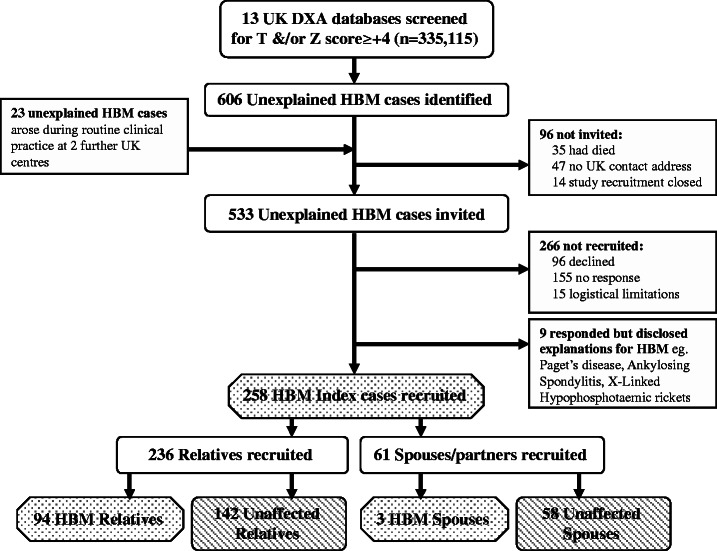
The HBM study is a UK-based multicentre observational study of adults with unexplained HBM. Full details of DXA database screening and participant recruitment have previously been reported [21] (Fig. 1). Briefly, 335 115 DXA scans from 13 UK DXA databases were initially screened for T- and/or Z-scores ≥+4 in the hip and/or lumbar spine. Potential cases were identified prospectively at two further centres. As part of the screening process, scans were visually inspected to exclude identifiable causes of raised BMD on DXA; 49.4% of inspected scans were excluded due to the presence of degenerative disease/OA/scoliosis. Index cases with unexplained HBM were recruited and asked to invite relatives and spouses to undergo DXA screening. DXA referral indications in the HBM population have previously been shown to be similar to that in the general population [21]. Recruitment ran from September 2008 to April 2010. Participants were excluded if under 18 years of age, pregnant or unable to provide informed consent. Written informed consent was obtained from all participants in line with the Declaration of Helsinki [22] and the study was approved by the Bath multicentre Research Ethics Committee (REC) and each NHS local REC.
Fig. 1.
Flow diagram summarizing the recruitment process for HBM index cases and then their relatives and spouses.
The definition of HBM in index cases was either L1 Z-score ≥+3.2 plus total hip Z-score ≥+1.2, or total hip Z-score ≥+3.2 plus L1 Z-score ≥+1.2. In first-degree relatives of HBM index cases, given established positive affection status within the family, the definition of HBM was a summed L1 Z-score plus total hip Z-score ≥+3.2. Spouses were classified according to the index definition. While no standard definition of HBM currently exists, a similar approach has previously been used for defining HBM [20], and this threshold was felt to most appropriately differentiate cases from controls within our study population [21]. The L1 lumbar vertebra was selected as, in contrast to lower lumbar levels, it was not found to be associated with the presence of lumbar spine OA assessed on DXA images [21]. Applying this definition, 41% of relatives screened were affected and thus combined with HBM index cases. Remaining first-degree relatives served as the control group, which was supplemented by unaffected spouses.
Clinical assessment of study subjects
Study participants attended their local centre for clinical assessment by a doctor or nurse. A structured interview was conducted using a standardized pro forma and, where possible, a clinical examination was performed. Previous orthopaedic operations (self-reported) were recorded and coded using the OPCS4 [Office of Population, Censuses and Surveys Classification of Surgical Operations and Procedures (4th revision)]. Joint replacement included OPCS4 codes W37–W58 inclusive. Hip replacements (W37–W39 and W46–W48) and knee replacements (W40–W42) were identified separately. A further three participants reported a history of hip resurfacing (n = 2) or bilateral partial hip replacement (n = 1), and these were included within the definition of hip replacement. Knee hemiarthroplasty was included within the definition of knee replacement (n = 2). A validation study was carried out for 29 of 55 self-reported hip and knee replacements where plain radiographs were available; we were able to validate 97% of self-reported joint replacements within this sample.
A history of joint pain was recorded, including site, duration and whether pain was ongoing. Two joint pain variables were then generated: (i) any joint pain, any site, ever, and (ii) joint pain for at least 1 month, still ongoing. A medication history was taken, including current use of NSAIDs. Where possible, a clinical examination for passive knee crepitus was carried out by a doctor and graded on a 4-point scale (0 = none, 1 = mild, 2 = moderate, 3 = severe), then dichotomized into none/mild versus moderate/severe.
Data were collected regarding relevant covariates including age, gender, BMI [calculated as weight (kilograms)/height (metres2)], menopausal status, self-reported use of oestrogen replacement therapy (ERT) and physical activity. A cumulative lifetime physical activity score (0–24) was derived from a questionnaire based on the best available evidence [23–25] and divided into five categories for analysis.
Socioeconomic position (SEP), another important potential confounder, was estimated using the Index of Multiple Deprivation (IMD); the use of area-based measures as proxies for individual level indicators is a recognized approach to estimating SEP in epidemiological studies [26]. As IMD cannot be directly compared between countries, and the majority of participants were resident in England rather than Wales, this variable was limited to English participants (n = 496). Participant postcodes were matched to Lower Super Output Areas using the Office of National Statistics Postcode Directory (August 2011) and used to obtain IMD ranks (English Indices of Deprivation 2010) [27]. These ranks were divided into quartiles compared with the whole of England (for descriptive statistics) and within our HBM study sample (for regression model).
Comparison of joint replacement in HBM with the general population
The HSE is an annual survey assessing the health of people living in England (since 1991); a stratified, random sampling strategy is used to select households for inclusion [28]. In 2005 [29] the survey focused on the health of older people and assessed adult joint replacement prevalence; more than 70% of eligible households took part. A total of 4269 individuals ≥65 years of age living in private households were included. As part of a structured interview, participants were asked about a history of joint replacement (yes/no), site [hip(s), knee(s) or other joint] and the indication for hip replacement only [arthritis, fracture, both arthritis and fracture, other reason (not specified)]. Hip replacements for which the indication was fracture alone were excluded; all other categories were included.
Statistical analysis
Descriptive statistics for HBM cases and controls are presented as mean (s.d.) for continuous and counts (%) for categorical data, with P values for between-group differences shown (t-test for continuous variables and χ2 test for categorical variables). In this case–control analysis, categorical variables were initially cross-tabulated in a contingency table and percentages were calculated. The χ2 test was used to assess the association between two categorical variables. Logistic regression was then used to assess the association between the binary case–control outcome and binary exposure (HBM). OA-related outcome variables were joint pain, NSAID use, knee crepitus and joint replacement. Age and gender were considered a priori confounders; other potential confounders were BMI, SEP, lifetime physical activity, menopausal status and ERT. Odds ratios (ORs) before and after adjustment are presented with 95% CIs. Joint replacement prevalence in our study population ≥65 years of age and the HSE 2005 is shown as unadjusted percentages. Data were managed using Microsoft Access (data entry checks; error rate <0.12%) and analysed using Stata release 11 statistical software (StataCorp, College Station, TX, USA).
Results
Participant characteristics
A total of 550 participants (353 HBM cases and 197 unaffected family controls) had complete data for key outcomes and covariates. All but three were Caucasian. On average, HBM cases were older than controls [mean age 61.7 years (range 18–89) versus 54.1 years (range 18–88)] and a greater proportion were female (76.5% versus 46.7%) (Table 1). Mean BMI was higher in HBM cases than controls (30.5 versus 28.0 kg/m2). As expected, mean BMD Z-scores for L1, total hip and their sum were much greater in HBM cases compared with controls. Participants were less deprived than the English average, with more than two-thirds of ranks within the upper two population quartiles for IMD; however, there was no discernible difference between cases and controls (Table 1).
Table 1.
Comparison of baseline characteristics in HBM cases and controls
| Descriptive characteristic | HBM cases | Controls | P valuea |
|---|---|---|---|
| Age (years), mean (s.d.) | 61.7 (13.8) | 54.1 (16.2) | <0.001 |
| BMI (kg/m2), mean (s.d.) | 30.5 (5.9) | 28.0 (4.8) | <0.001 |
| Sum hip and L1 Z-scores, mean (s.d.) | 6.96 (2.2) | 0.98 (1.8) | <0.001 |
| Max total hip Z-score (n = 529), mean (s.d.) | 3.00 (1.2) | 0.53 (0.9) | <0.001 |
| L1 Z-score (n = 542), mean (s.d.) | 3.92 (1.5) | 0.48 (1.2) | <0.001 |
| Female | 270 (76.5) | 92 (46.7) | <0.001 |
| Post-menopausal | 218 (82.9) | 48 (54.6) | <0.001 |
| Oestrogen replacement (ever) | 128 (52.7) | 15 (19.2) | <0.001 |
| Prior fractureb | 134 (38.0) | 90 (45.7) | 0.077 |
| Any joint replacement risk factor (not OA)c | 24 (6.8) | 3 (1.5) | 0.006 |
| English IMD quartiles (n = 496)d | |||
| 1 (most deprived) | 38 (11.9) | 18 (10.2) | |
| 2 | 68 (21.3) | 36 (20.3) | 0.909 |
| 3 | 100 (31.4) | 56 (31.6) | |
| 4 (least deprived) | 113 (35.4) | 67 (37.9) |
Results presented as n (%) unless otherwise indicated. n = 550 (i.e. 353 cases and 197 controls) except where stated. aP values shown from t-test (continuous variables) and χ2 test (categorical variables) comparing HBM cases with controls. bAny lifetime fracture regardless of mechanism. cIncludes RA (n = 17), AS (n = 2), SLE with joint involvement (n = 2), PsA (n = 5), steroid-induced avascular necrosis leading to hip replacement (n = 1). dQuartiles of IMD ranks compared with the whole of England (1–32 482), 1: most deprived.
OA phenotypes in HBM cases and controls
The unadjusted prevalence of joint pain was higher in cases than controls, using both definitions (Table 2). Likewise, the prevalence of current NSAID use was more than twofold higher among HBM cases than controls. Approximately 20% more HBM cases than controls had knee crepitus; this was unchanged when participants with knee replacements were excluded. Overall, 54 participants (9.8%) reported a prior joint replacement, and the (unadjusted) prevalence of prior joint replacement surgery at the hip, knee and overall was notably higher in HBM cases versus controls (Table 2). No reported joint replacements in either cases or controls were performed due to fracture. Only one participant had undergone a revision joint replacement.
Table 2.
Unadjusted prevalence of clinical OA indicators in HBM cases and controls
| n (550) | HBM cases, n (%) | Controls, n (%) | χ2 P value | |
|---|---|---|---|---|
| Joint pain | 536 | |||
| Ever, any site | 299 (86.9) | 151 (78.7) | 0.012 | |
| For months or years, still ongoing | 206 (59.9) | 103 (53.7) | 0.161 | |
| NSAID use (current) | 549 | 58 (16.5) | 13 (6.6) | 0.001 |
| Knee crepitus | ||||
| Moderate/severe | 408 | 155 (59.2) | 57 (39.0) | <0.001 |
| Excluding knee replacements | 388 | 143 (58.1) | 55 (38.7) | <0.001 |
| Joint replacement | 550 | |||
| Any jointa | 46 (13.0) | 8 (4.1) | 0.001 | |
| Hip | 22 (6.2) | 2 (1.0) | 0.004 | |
| Knee | 24 (6.8) | 7 (3.6) | 0.114 |
Frequencies and percentages (value in parentheses) are shown. aMostly hip and/or knee replacement except great toe joint replacement (n = 1), bilateral ankle replacement (n = 1) and patella resurfacing (n = 1).
Increased odds of joint pain and knee crepitus were observed in HBM cases compared with controls, which was fully explained by age and gender adjustment (Model 2, Table 3). Increased odds of NSAID use were also observed in HBM cases versus controls, which persisted after adjustment for age, gender and BMI (Model 3).
Table 3.
Logistic regression analysis of clinical OA characteristics in HBM compared with controls
| Clinical characteristic | Modela | OR | 95% CI | P value |
|---|---|---|---|---|
| Joint pain (ever, any site) (n = 536) | 1 | 1.80 | 1.13, 2.88 | 0.013 |
| 2 | 1.08 | 0.64, 1.84 | 0.767 | |
| 3 | 0.98 | 0.57, 1.68 | 0.944 | |
| NSAID use (current) (n = 549) | 1 | 2.79 | 1.49, 5.24 | 0.001 |
| 2 | 2.50 | 1.28, 4.87 | 0.007 | |
| 3 | 2.17 | 1.10, 4.28 | 0.026 | |
| Knee crepitus (n = 408) | 1 | 2.26 | 1.50, 3.42 | < 0.001 |
| 2 | 1.36 | 0.85, 2.20 | 0.202 | |
| 3 | 1.15 | 0.70, 1.89 | 0.572 |
aResults are shown unadjusted (Model 1), adjusted for age and gender (Model 2) and adjusted for age, gender and BMI (Model 3).
In analyses adjusted for age and gender HBM cases were more likely than controls to report a history of joint replacement at any site [OR 2.60 (1.15, 5.90), P = 0.02) and at the hip [OR 4.56 (1.02, 20.30), P = 0.05), whereas weak evidence of an increase in knee replacement was seen [OR 1.48 (0.59, 3.72), P = 0.40] (Model 2, Table 4). Further adjustment for BMI (Model 3) failed to attenuate these associations. In the subgroup with physical activity data, further adjustment for lifetime physical activity (n = 476) did not change the association between joint replacement and HBM [OR 2.57 (1.01, 6.56) with adjustment, OR 2.63 (1.03, 6.71) without adjustment, Model 3]. As only one pre-menopausal female reported a prior joint replacement, menopausal status was not considered a potential confounder in the analyses.
Table 4.
Stepwise logistic regression analysis of joint replacement variables in HBM cases compared with controls
| Outcome | n | Modela | OR | 95% CI | P value |
|---|---|---|---|---|---|
| Hip replacement | 550 | 1 | 6.48 | 1.51, 27.86 | 0.012 |
| 2 | 4.56 | 1.02, 20.30 | 0.046 | ||
| 3 | 4.79 | 1.07, 21.51 | 0.041 | ||
| Knee replacement | 550 | 1 | 1.98 | 0.84, 4.68 | 0.120 |
| 2 | 1.48 | 0.59, 3.72 | 0.402 | ||
| 3 | 1.23 | 0.48, 3.16 | 0.671 | ||
| Any joint replacement | 550 | 1 | 3.54 | 1.64, 7.66 | 0.001 |
| 2 | 2.60 | 1.15, 5.90 | 0.022 | ||
| 3 | 2.42 | 1.06, 5.56 | 0.037 | ||
| Any joint replacementb | 496 | 1 | 4.56 | 1.90, 10.93 | 0.001 |
| 2 | 3.33 | 1.33, 8.32 | 0.010 | ||
| 3 | 3.05 | 1.21, 7.71 | 0.019 | ||
| 4 | 3.20 | 1.26, 8.15 | 0.015 |
aResults are shown unadjusted (Model 1), adjusted for age and gender (Model 2), adjusted for age, gender and BMI (Model 3) and adjusted for age, gender, BMI and IMD (Model 4). bEnglish participants only.
A higher IMD quartile (indicating decreasing deprivation) was associated with a greater prevalence of any joint replacement (Supplementary Table 1, available at Rheumatology Online). However, additional adjustment for IMD did not attenuate the association between HBM case status and joint replacement history (Model 4, Table 4). Additional adjustment for a range of potentially relevant comorbidities, including diabetes, hypothyroidism, gout and ischaemic heart disease, did not materially alter point estimates for joint pain, NSAID use or joint replacement (data not shown).
Sensitivity analyses were performed as follows: (i) excluding participants with underlying conditions (other than OA) possibly predisposing to the need for joint replacement (see Table 1 legend) (n = 27): the direction and magnitude of effect was similar with an adjusted (Model 3) OR for total joint replacement of 2.28 (0.97, 5.32), hip replacement 4.24 (0.93, 19.38) and knee replacement 1.21 (0.46, 3.17); (ii) excluding those HBM cases with a very extreme phenotype (a Z-score > +6, n = 30): point estimates were not materially altered although CIs were widened; (iii) excluding participants with a prior joint replacement (n = 54) from the analyses for joint pain and NSAID use: this did not materially affect point estimates for pain, but the fully adjusted (Model 3) OR for NSAID use in cases versus controls increased to 2.95 (1.34, 6.47).
Prevalence of joint replacement in HBM versus the HSE 2005
The 2005 HSE survey on the health of older people included 4269 adults ≥65 years of age in whom data on self-reported prior joint replacement were available for 4263 subjects (mean age 74.5 years, range 65–100, 55.6% female). The prevalence of joint replacement in this group was compared with the prevalence of joint replacement in HBM cases and controls aged over 65, comprising 147 cases [mean age 73.8 years (range 65.1–89.8), 67.4% female] and 54 controls [mean age 73.7 years (range 65.4–88.4), 37% female] (Fig. 2). Notably, the prevalence of any joint, hip or knee replacement was similar in HBM controls and the HSE survey, whereas values were approximately twofold higher in HBM cases.
Fig. 2.
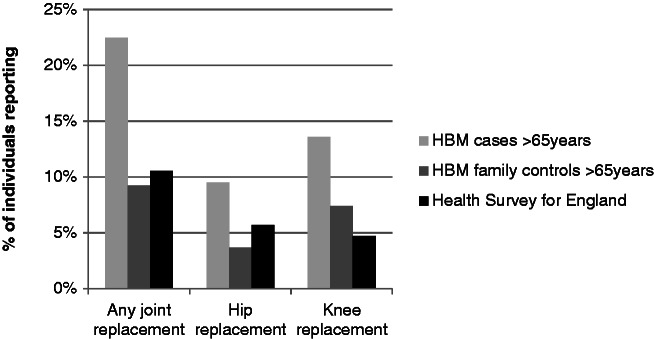
Comparison of joint replacement prevalence in HBM cases and controls with Health Survey for England 2005.
Data shown for HBM cases and controls >65 years only, n = 147 (HBM cases) and 54 (HBM controls). Health Survey for England (HSE), n = 4269 (any joint replacement), 4263 (knee replacement) and 4231 (hip replacement, excluding indication of fracture). Unadjusted prevalences are shown, no weighting applied.
Discussion
To our knowledge, this is the first study to look specifically at the prevalence of phenotypes associated with OA in a rare group of individuals with extreme HBM. Our finding of an increased prevalence of joint replacement and NSAID use in HBM cases compared with controls is consistent with previously reported associations between OA and higher BMD in unselected individuals [6–11]. However, in contrast to previous observational studies, the study of HBM patients, in whom BMD is assumed to be elevated before the onset of OA, represents a novel approach to examining causal pathways between BMD and OA. Our HBM population has a high prevalence of phenotypic characteristics indicating mild skeletal dysplasia [21], suggesting a genetic basis for their elevated BMD. Hence any increased OA risk may either represent a consequence of their HBM phenotype or arise due to genetic pleiotropy whereby BMD genes also influence OA risk.
Although no previous study has systematically examined OA phenotypes in a substantial population of HBM individuals, several case series have examined this relationship in individuals with single gene disorders associated with very high BMD, producing conflicting results. For example, early-onset OA has been associated with osteoclast underactivity in autosomal-dominant type II osteopetrosis (ADOII) by several authors [30, 31], including a female aged 16 years who required hip replacement [32]. Furthermore, a case series of 42 ADOII patients reported a hip OA prevalence of 27%, frequently requiring hip replacement [33], but without comparison with a control population. An increased prevalence of degenerative hip and knee arthritis has also been linked with ADOII [34]. In contrast, SOST mutations causing osteoblast overactivity have not been associated with degenerative arthritis to date [35]. In adults with activating LRP5 mutations causing HBM, hip pain [36], knee OA [37] and hip replacement [38] have all been reported in individual cases; however, a systematic assessment of OA in LRP5 HBM is currently lacking.
Mechanistically an HBM phenotype arising from increased osteoblast activity could favour increased periarticular bone formation, increasing subchondral BMD. Radin et al. [39] proposed a mechanical theory of OA initiation in which increased subchondral bone stiffness may result in shear stresses within the overlying articular cartilage, leading to cartilage damage. However, more recent observations suggest rather that OA periarticular bone is hypomineralized with reduced material density [40]. Other studies examining the presence of OA in skeletons have associated osteophytes with enthesophytes (bony outgrowths at the entheses) and eburnation [41]. These features were observed to be widespread in affected skeletons, suggesting a generalized tendency to form new bone; OA may therefore represent a systemic disorder of bone response [41]. In theory, this bone-forming phenotype could result in both acquisition of a higher peak bone mass (HBM) and abnormal response of periarticular bone to later mechanical stress leading to premature OA, particularly radiographic OA.
Alternatively, as mentioned above, genes determining BMD could also influence OA risk as a result of pleiotropy, involving a pathway independent of BMD. These could include direct effects on cartilage or on developmental processes that determine joint shape [42]. For example, the wnt signalling pathway has been implicated in OA (for a review, see [43]) as well as BMD regulation [20]. LRP5 is a key receptor in this pathway, and direct sequencing for mutations affecting exons 2, 3 and 4 of LRP5 has identified causative mutations in a small number of HBM subjects [44]. Wnt signalling is thought to be necessary for both synovial joint formation during skeletogenesis and ongoing joint homeostasis [43], and molecular products have been implicated in primary hip OA [45]. Furthermore, raised β-catenin (the effector molecule in the canonical wnt signalling pathway) levels are evident in knee cartilage samples in individuals undergoing joint replacement for OA [46]. However, genetic association studies of wnt pathway gene polymorphisms and OA have yielded conflicting results [47–49]. It is interesting to note that the increased prevalence of joint replacement in this HBM population appears to be driven mainly by hip replacement, possibly suggesting a causative mechanism more specific to this joint (e.g. by influencing hip shape). However, due to the small numbers of outcome events when joint replacements are further subdivided by site, this needs to be interpreted with caution.
Although case series suggest that of the established HBM-causing disorders, ADOII is most strongly associated with OA, underlying osteopetrosis is unlikely to explain the associations reported here, as detailed phenotypic examination (including assessment of clinical features, spine and femur radiographs and blood count measurement [21]) did not identify any such cases among our HBM study population.
Having taken into account confounding factors, we did not observe an association between HBM and joint pain, whereas an association was seen with NSAID use. This may reflect the fact that our joint pain definitions were relatively crude and did not consider symptom frequency or severity. Knee crepitus, while common, was strongly associated with age (and to a lesser extent gender), adjustment for which markedly attenuated the relationship with HBM.
In addition to limitations in our definition of pain, our finding of an association between HBM and joint replacement, but not joint pain, may reflect the fact that joint replacement is a more specific indicator of OA or indicates more severe disease. Joint replacement surgery indications lack consensus, although most guidelines consider symptoms and functional limitation to be of central importance [50]. However, in contrast, the multicentre European EUROHIP study [50] found that among patients undergoing hip replacement for OA, symptom severity was quite variable, whereas radiographic change was moderate or severe in the vast majority. Consistent with other reports [51], joint symptoms and radiographic OA features were poorly associated in EUROHIP, suggesting that X-ray appearances may have a significant influence on the decision to perform a joint replacement in individuals with pain [50]. As X-rays directly visualize bone only, it might be that an altered bone response in HBM individuals could lead to a more severe early radiographic appearance and hence a lower threshold for joint replacement despite similar levels of pain and symptoms; quantification of radiographic OA features in our HBM population is now planned.
Limitations
Although elevated BMD in HBM cases is likely to be genetically determined, it remains possible that confounding factors could explain our observed associations. The small number of participants reporting a prior joint replacement limited our ability to adjust for other potentially relevant factors such as ERT, which was more prevalent in HBM cases. However, most studies have suggested that ERT reduces OA risk [52], hence ERT would most likely negatively confound (i.e. strengthen) our observed associations. Unfortunately, data concerning participant age at the time of joint replacement and use of other analgesics, including paracetamol, were incomplete. Only a proportion of HBM index cases were able to contribute family/spouse controls, resulting in a control:case ratio of less than 1:1 and thus reducing the power of the study. An additional concern relating to our family-based recruitment strategy was the potential for joint replacements to cluster within families; however, only one family studied had more than one member with joint replacements (four individuals with hip and two with knee replacements), minimally influencing our findings.
Comparison of joint replacement prevalence in our HBM cases with the HSE 2005 population data was unadjusted and hence must be interpreted with caution. Although the age distribution was similar between the groups, the gender distribution varied, and BMI data for the HSE group were not available. SEP within HSE respondents might also be expected to be lower than in our study population and more similar to the UK population as a whole (although there is some suggestion of a bias towards higher SEP in the 71% of households that did respond to the survey, inferred from region and dwelling type) [28]. This difference could conceivably contribute to a lower number of joint replacements in HSE, given the previously observed relationship between SEP and joint replacement [53]. However, the similar prevalence of joint replacement in our family control group and the HSE provides some reassurance that the effect of this difference is likely to have been limited.
In conclusion, we have found evidence of an increased prevalence of joint replacement and NSAID use in our HBM cases compared with family and spouse controls. Comparison with general population data from the HSE 2005 provided further evidence that joint replacement prevalence may be increased in HBM. This study adds to the existing epidemiological evidence that increased BMD is a risk factor for OA. We next plan to explore the relationship between HBM and other OA phenotypes, including radiographic OA. It is also hoped that identifying genetic mutations responsible for HBM will provide novel insights into the genetic basis of OA.

Supplementary Material
Acknowledgements
We would like to thank all our study participants, the radiology staff at our collaborating centres and particularly staff at the Wellcome Trust Clinical Research Facility in Birmingham; Royal National Hospital for Rheumatic Diseases in Bath; Cambridge NIHR Biomedical Research Centre and Addenbrooke’s Wellcome Trust Clinical Research Facility, Bone Research Unit in Cardiff; Musculoskeletal Research Unit in Bristol; NIHR Bone Biomedical Research Unit in Sheffield and the Brocklehurst Centre for Metabolic Bone Disease in Hull. This study was supported by the Wellcome Trust and the NIHR CRN (portfolio number 5163); supporting CLRNs included Birmingham and the Black Country, London South, Norfolk and Suffolk, North and East Yorkshire and Northern Lincolnshire, South Yorkshire, Surrey and Sussex, West Anglia and Western.
We would also like to acknowledge other members of the UK DINAG consortium for assistance in setting up the local study centres, including Sue Steel (Hull and East Yorkshire Hospitals NHS Trust), Dr John Ayuk (University Hospitals Birmingham NHS Foundation Trust), Dr Ashok Bhalla (Royal National Hospital for Rheumatic Diseases NHS Foundation Trust), Dr Gavin Clunie (Ipswich Hospital NHS Trust), Professor Ignac Fogelman (Guy's and St Thomas’ NHS Foundation Trust and King’s College London), Dr Stuart Linton (Nevill Hall Hospital, Gwent), Professor Eugene McCloskey (Northern General Hospital and University of Sheffield), Dr Katie Moss (St George’s Healthcare NHS Trust, London), Dr Tom Palferman (Yeovil District Hospital), Dr Sam Panthakalam (East Sussex Hospitals NHS Trust, Eastbourne), Dr Ken Poole (Cambridge University Hospitals NHS Foundation Trust), Dr Mike Stone (Cardiff and Vale UHB) and Professor John Wass (Nuffield Orthopaedic Centre NHS Trust, Oxford).
Health Survey for England 2005 data: Depositor: National Centre for Social Research; Sponsor: Information Centre for Health and Social Care; Access: ESDS Government, UK Data Archive. Disclaimer statement: Although all efforts are made to ensure the quality of the materials, neither the original data creators, depositors or copyright holders, the funders of the data collections, nor the UK Data Archive bear any responsibility for the accuracy or comprehensiveness of these materials.
Funding: This work was supported by the Wellcome Trust (funded C.L.G. through a Wellcome Trust Clinical Research Training Fellowship, 080280/Z/06/Z), Arthritis Research UK (funded S.A.H. through a Clinical PhD Studentship, grant ref. 19580) and the NIHR CRN (portfolio number 5163).
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.World Health Organization. Chronic rheumatic conditions. http://www.who.int/chp/topics/rheumatic/en/ (13 August 2012, date last accessed) [Google Scholar]
- 2.National Joint Registry for England and Wales 8th Annual Report. 2011. www.njrcentre.org.uk (13 August 2012, date last accessed) [Google Scholar]
- 3.Lane NE, Nevitt MC. Osteoarthritis, bone mass, and fractures: how are they related? Arthritis Rheum. 2002;46:1–4. doi: 10.1002/1529-0131(200201)46:1<1::aid-art10068>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 4.Dequeker J, Aerssens J, Luyten FP. Osteoarthritis and osteoporosis: clinical and research evidence of inverse relationship. Aging Clin Exp Res. 2003;15:426–39. doi: 10.1007/BF03327364. [DOI] [PubMed] [Google Scholar]
- 5.Stewart A, Black AJ. Bone mineral density in osteoarthritis. Curr Opin Rheumatol. 2000;12:464–7. doi: 10.1097/00002281-200009000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Nevitt MC, Lane NE, Scott JC, et al. Radiographic osteoarthritis of the hip and bone mineral density. The Study of Osteoporotic Fractures Research Group. Arthritis Rheum. 1995;38:907–16. doi: 10.1002/art.1780380706. [DOI] [PubMed] [Google Scholar]
- 7.Burger H, van Daele PL, Odding E, et al. Association of radiographically evident osteoarthritis with higher bone mineral density and increased bone loss with age. The Rotterdam Study. Arthritis Rheum. 1996;39:81–6. doi: 10.1002/art.1780390111. [DOI] [PubMed] [Google Scholar]
- 8.Sowers MF, Hochberg M, Crabbe JP, et al. Association of bone mineral density and sex hormone levels with osteoarthritis of the hand and knee in premenopausal women. Am J Epidemiol. 1996;143:38–47. doi: 10.1093/oxfordjournals.aje.a008655. [DOI] [PubMed] [Google Scholar]
- 9.Hart DJ, Mootoosamy I, Doyle DV, et al. The relationship between osteoarthritis and osteoporosis in the general population: the Chingford Study. Ann Rheum Dis. 1994;53:158–62. doi: 10.1136/ard.53.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peel NF, Barrington NA, Blumsohn A, et al. Bone mineral density and bone turnover in spinal osteoarthrosis. Ann Rheum Dis. 1995;54:867–71. doi: 10.1136/ard.54.11.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcelli C, Favier F, Kotzki PO, et al. The relationship between osteoarthritis of the hands, bone mineral density, and osteoporotic fractures in elderly women. Osteoporos Int. 1995;5:382–8. doi: 10.1007/BF01622261. [DOI] [PubMed] [Google Scholar]
- 12.Sowers M, Lachance L, Jamadar D, et al. The associations of bone mineral density and bone turnover markers with osteoarthritis of the hand and knee in pre- and perimenopausal women. Arthritis Rheum. 1999;42:483–9. doi: 10.1002/1529-0131(199904)42:3<483::AID-ANR13>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Hannan MT, Chaisson CE, et al. Bone mineral density and risk of incident and progressive radiographic knee osteoarthritis in women: the Framingham Study. J Rheumatol. 2000;27:1032–7. [PubMed] [Google Scholar]
- 14.Nevitt MC, Zhang Y, Javaid MK, et al. High systemic bone mineral density increases the risk of incident knee OA and joint space narrowing, but not radiographic progression of existing knee OA: the MOST study. Ann Rheum Dis. 2010;69:163–8. doi: 10.1136/ard.2008.099531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart DJ, Cronin C, Daniels M, et al. The relationship of bone density and fracture to incident and progressive radiographic osteoarthritis of the knee: the Chingford Study. Arthritis Rheum. 2002;46:92–9. doi: 10.1002/1529-0131(200201)46:1<92::AID-ART10057>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Hannan MT, Anderson JJ, Zhang Y, et al. Bone mineral density and knee osteoarthritis in elderly men and women. The Framingham Study. Arthritis Rheum. 1993;36:1671–80. doi: 10.1002/art.1780361205. [DOI] [PubMed] [Google Scholar]
- 17.Arokoski JP, Arokoski MH, Jurvelin JS, et al. Increased bone mineral content and bone size in the femoral neck of men with hip osteoarthritis. Ann Rheum Dis. 2002;61:145–50. doi: 10.1136/ard.61.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdin-Mohamed M, Jameson K, Dennison EM, et al. Volumetric bone mineral density of the tibia is not increased in subjects with radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2009;17:174–7. doi: 10.1016/j.joca.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Zhang Y, Kang H, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–7. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 20.Little RD, Carulli JP, Del Mastro RG, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–9. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregson CL, Steel SA, O’Rourke KP, et al. ‘Sink or swim’: an evaluation of the clinical characteristics of individuals with high bone mass. Osteoporos Int. 2012;23:643–54. doi: 10.1007/s00198-011-1603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. 59th WMA General Assembly, Seoul. World Medical Association (2008) Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects.
- 23.Chasan-Taber L, Erickson JB, McBride JW, et al. Reproducibility of a self-administered lifetime physical activity questionnaire among female college alumnae. Am J Epidemiol. 2002;155:282–9. doi: 10.1093/aje/155.3.282. [DOI] [PubMed] [Google Scholar]
- 24.Kriska AM, Sandler RB, Cauley JA, et al. The assessment of historical physical activity and its relation to adult bone parameters. Am J Epidemiol. 1988;127:1053–63. doi: 10.1093/oxfordjournals.aje.a114881. [DOI] [PubMed] [Google Scholar]
- 25.Suleiman S, Nelson M. Validation in London of a physical activity questionnaire for use in a study of postmenopausal osteopaenia. J Epidemiol Commun Health. 1997;51:365–72. doi: 10.1136/jech.51.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galobardes B, Shaw M, Lawlor DA, et al. Indicators of socioeconomic position (part 2) J Epidemiol Commun Health. 2006;60:95–101. doi: 10.1136/jech.2004.028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The English Indices of Deprivation. 2010. Department for Communities and Local Government March 2011. https://www.gov.uk/government/publications/english-indices-of-deprivation-2010 (7 January 2013, date last accessed) [Google Scholar]
- 28.Craig R, Mindell J, editors. Health survey for England 2005. Vol. 5 Methodology and documentation. The Information Centre, 2007. https://catalogue.ic.nhs.uk/publications/public-health/surveys/heal-surv-heal-old-peo-eng-2005/heal-surv-heal-old-peo-eng-2005-meth.pdf (7 January 2013, date last accessed) [Google Scholar]
- 29.Health Survey for England. 2005. National Centre for Social Research, UK Data Archive, July 2011. http://www.esds.ac.uk/findingData/snDescription.asp?sn=5675 (7 January 2013, date last accessed) [Google Scholar]
- 30.Casden AM, Jaffe FF, Kastenbaum DM, et al. Osteoarthritis associated with osteopetrosis treated by total knee arthroplasty. Report of a case. Clin Orthop Relat Res. 1989;247:202–7. [PubMed] [Google Scholar]
- 31.Cameron HU, Dewar FP. Degenerative osteoarthritis associated with osteopetrosis. Clin Orthop Relat Res. 1977;127:148–9. [PubMed] [Google Scholar]
- 32.Matsuno T, Katayama N. Osteopetrosis and total hip arthroplasty. Report of two cases. Int Orthop. 1997;21:409–11. doi: 10.1007/s002640050196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benichou OD, Laredo JD, de Vernejoul MC. Type II autosomal dominant osteopetrosis (Albers-Schonberg disease): clinical and radiological manifestations in 42 patients. Bone. 2000;26:87–93. doi: 10.1016/s8756-3282(99)00244-6. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro F. Osteopetrosis. Current clinical considerations. Clin Orthop Relat Res. 1993:34–44. [PubMed] [Google Scholar]
- 35.Hamersma H, Gardner J, Beighton P. The natural history of sclerosteosis. Clin Genet. 2003;63:192–7. doi: 10.1034/j.1399-0004.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- 36.Rickels MR, Zhang X, Mumm S, et al. Oropharyngeal skeletal disease accompanying high bone mass and novel LRP5 mutation. J Bone Miner Res. 2005;20:878–85. doi: 10.1359/JBMR.041223. [DOI] [PubMed] [Google Scholar]
- 37.Balemans W, Devogelaer JP, Cleiren E, et al. Novel LRP5 missense mutation in a patient with a high bone mass phenotype results in decreased DKK1-mediated inhibition of Wnt signaling. J Bone Miner Res. 2007;22:708–16. doi: 10.1359/jbmr.070211. [DOI] [PubMed] [Google Scholar]
- 38.Whyte MP, Reinus WH, Mumm S. High-bone-mass disease and LRP5. N Engl J Med. 2004;350:2096–9. doi: 10.1056/NEJM200405133502017. author reply 2099. [DOI] [PubMed] [Google Scholar]
- 39.Radin EL, Paul IL, Rose RM. Role of mechanical factors in pathogenesis of primary osteoarthritis. Lancet. 1972;1:519–22. doi: 10.1016/s0140-6736(72)90179-1. [DOI] [PubMed] [Google Scholar]
- 40.Burr DB. Anatomy and physiology of the mineralized tissues: role in the pathogenesis of osteoarthrosis. Osteoarthritis Cartilage. 2004;12(Suppl A):S20–30. doi: 10.1016/j.joca.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 41.Rogers J, Shepstone L, Dieppe P. Is osteoarthritis a systemic disorder of bone? Arthritis Rheum. 2004;50:452–7. doi: 10.1002/art.20136. [DOI] [PubMed] [Google Scholar]
- 42.Baker-LePain JC, Lane NE. Relationship between joint shape and the development of osteoarthritis. Curr Opin Rheumatol. 2010;22:538–43. doi: 10.1097/BOR.0b013e32833d20ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luyten FP, Tylzanowski P, Lories RJ. Wnt signaling and osteoarthritis. Bone. 2009;44:522–7. doi: 10.1016/j.bone.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Duncan EL, Gregson CL, Addison K, et al. Mutations in LRP5 and SOST are a rare cause of high bone mass in the general population. Presented at the 36th European Symposium on Calcified Tissues, Vienna, Austria, 23–27 May 2009. [Google Scholar]
- 45.Kumarasinghe DD, Hopwood B, Kuliwaba JS, et al. An update on primary hip osteoarthritis including altered Wnt and TGF-beta associated gene expression from the bony component of the disease. Rheumatology (Oxford) 2011;50:2166–75. doi: 10.1093/rheumatology/ker291. [DOI] [PubMed] [Google Scholar]
- 46.Zhu M, Tang D, Wu Q, et al. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009;24:12–21. doi: 10.1359/JBMR.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerkhof JM, Uitterlinden AG, Valdes AM, et al. Radiographic osteoarthritis at three joint sites and FRZB, LRP5, and LRP6 polymorphisms in two population-based cohorts. Osteoarthritis Cartilage. 2008;16:1141–9. doi: 10.1016/j.joca.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Smith AJ, Gidley J, Sandy JR, et al. Haplotypes of the low-density lipoprotein receptor-related protein 5 (LRP5) gene: are they a risk factor in osteoarthritis? Osteoarthritis Cartilage. 2005;13:608–13. doi: 10.1016/j.joca.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 49.Loughlin J, Dowling B, Chapman K, et al. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc Natl Acad Sci USA. 2004;101:9757–62. doi: 10.1073/pnas.0403456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dieppe P, Judge A, Williams S, et al. Variations in the pre-operative status of patients coming to primary hip replacement for osteoarthritis in European orthopaedic centres. BMC Musculoskelet Disord. 2009;10:19. doi: 10.1186/1471-2474-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol. 2006;20:3–25. doi: 10.1016/j.berh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Dixon T, Shaw M, Ebrahim S, et al. Trends in hip and knee joint replacement: socioeconomic inequalities and projections of need. Ann Rheum Dis. 2004;63:825–30. doi: 10.1136/ard.2003.012724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.