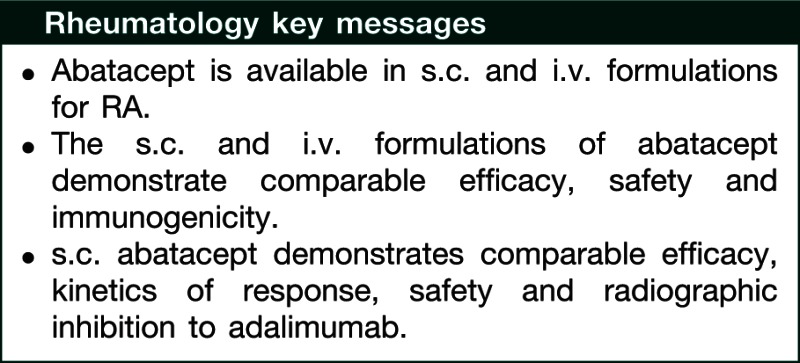
Abstract
The efficacy, safety and tolerability of i.v. abatacept are well established in patients with active RA. A s.c. abatacept formulation is now available in some countries. Here, we review clinical data for s.c. abatacept. Six trials are presented (Phase II dose-finding study, ACQUIRE, ALLOW, ACCOMPANY, ATTUNE and AMPLE) and issues important to both patients and clinicians are addressed. The primary focus assesses whether the i.v. and s.c. abatacept formulations have similar efficacy, including whether the recommended fixed dose of s.c. abatacept is comparable to the weight-tiered i.v. dosing and whether efficacy is sustained with long-term treatment. Safety and immunogenicity are also discussed, including the short- and long-term safety of s.c. abatacept, and whether immunogenicity is increased following a switch from i.v. to s.c. abatacept, after withdrawal or reintroduction of s.c. abatacept or in the absence of MTX. Year 1 data from the AMPLE study, comparing s.c. abatacept with the TNF antagonist adalimumab, are discussed. Although fewer patient-years of exposure are available for s.c. compared with i.v. abatacept, observations suggest that s.c. abatacept has a similar long-term efficacy to the i.v. formulation, improving the signs, symptoms, disease activity and physical function in patients with RA. With continued treatment, these improvements are maintained over time with high retention rates, similar to i.v. abatacept. s.c. abatacept is associated with low immunogenicity and short- and long-term safety that is consistent with i.v. abatacept. In addition, s.c. abatacept demonstrates comparable efficacy, kinetics of response, safety and radiographic inhibition to adalimumab.
Keywords: rheumatoid arthritis, abatacept, subcutaneous, biologic therapy
Introduction
Abatacept is a human fusion protein that selectively modulates naïve T cell activation [1], thus working upstream in the immune response and impacting early in the RA disease process. Abatacept is available as an i.v. formulation, administered monthly according to a weight-tiered dosing regimen. The efficacy profile of i.v. abatacept is well established in a range of patient populations, including MTX-naïve patients with early RA [2], MTX inadequate responders (MTX-IR) [3–6] and patients who have failed prior anti-TNF therapy [7, 8]. Integrated analyses of up to 7 years of treatment confirm that continued long-term use of i.v. abatacept does not lead to an increase in the incidence of infections, malignancies or autoimmune events over time [9]. A s.c. formulation of abatacept is now available in some countries, using a fixed-dose regimen of 125 mg weekly. As such, the clinical utility of s.c. abatacept is of interest and this review provides an overview of its efficacy, safety and tolerability.
Methods
A literature search was completed on 10 May 2012 using PubMed to identify publications reporting clinical data for all s.c. abatacept studies to date: Phase II dose-finding study (NCT00254293), ACQUIRE (NCT00559585), ALLOW (NCT00533897) and ATTUNE (NCT00663702). This search was restricted to the English language and used the following search terms: subcutaneous (or SC) abatacept (or Orencia, CTLA-4Ig). No date limits were applied. Congress abstract archives up to 2012 were also searched using the same criteria to identify further clinical data from these studies plus ACCOMPANY (NCT00547521) and AMPLE (NCT00929864) that have been presented at the ACR and European League Against Rheumatism (EULAR) meetings. Data from AMPLE and ACCOMPANY were published during the development of this manuscript and were added so that this review was as complete as possible.
s.c. abatacept clinical trial programme: an overview
Phase II dose-finding study
This Phase IIa, double-blind, randomized study was designed to assess the pharmacokinetics (PK), safety and immunogenicity of different dosing regimens of s.c. abatacept in patients with active RA despite prior treatment with MTX with or without one additional oral DMARD [10]. Patients (n = 68) were randomly assigned (3:1) to s.c. abatacept administered in five parallel groups, each with an i.v. loading dose of ∼10 mg/kg according to weight range, or placebo for 12 weeks. Abatacept dosing groups investigated both weight-tiered (75–200 mg) and fixed (125 mg) dosing [10].
Phase III studies
Key inclusion criteria, baseline demographics and clinical characteristics for all Phase III studies are described in Table 1.
Table 1.
Baseline demographics and clinical characteristics
| ACQUIRE [11] | ALLOWa [17] | ACCOMPANY [15] | ATTUNE [16] | AMPLE [19] | |||||
|---|---|---|---|---|---|---|---|---|---|
| Published inclusion criteria | ≥10 swollen joints, ≥12 tender joints, CRP ≥0.8 mg/dl | DAS28 (CRP) ≥3.2 and ≤5.1 | Global Assessment of Disease Activity VAS >20 | ≥4 years of treatment with i.v. abatacept in AIM or ATTAIN trial | ≤5 years disease duration, DAS28 (CRP) ≥3.2, history of anti-CCP or RF seropositivity, or elevated ESR or CRP | ||||
| Patient population | Active RA, MTX-IR | Active RA, MTX-IR | Active RA, IR to ≥1 DMARD (MTX-naïve or MTX-IR) | Active RA, MTX-IRb | Active RA, anti-TNF IRc | Active RA, MTX-IR, biologic-naïve | |||
| Treatment | s.c. abatacept + MTX | i.v. abatacept + MTX | s.c. abatacept + MTX | s.c. abatacept with/without MTX | s.c. abatacept | s.c. abatacept + MTX | s.c. adalimumab + MTX | ||
| Continuous | Withdrawal/ reintroduction | ||||||||
| n | 736 | 721 | 40 | 80 | 100 | 71 | 52 | 318 | 328 |
| Age, years | 49.9 (13.2) | 50.1 (12.6) | 48.9 (14.2) | 49.1 (12.8) | 54.0 (10.9) | 52.8 (13.8) | 56.5 (10.2) | 51.4 (12.6) | 51.0 (12.8) |
| Weight, kg | 72.0 (18.0) | 71.8 (17.6) | 67.9 (15.8) | 68.9 (14.7) | 83.1 (20.6) | NR | NR | 80.8 (20.3) | 80.1 (20.7) |
| Gender, % female | 84.4 | 80.4 | 85.0 | 83.8 | 75.0 | 83.1 | 80.8 | 81.4 | 82.3 |
| Ethnicity, % Caucasian | 74.7 | 74.5 | 95.0 | 93.8 | 76.0 | 98.6 | 90.4 | 80.8 | 78.0 |
| Disease duration, years | 7.6 (8.1) | 7.7 (7.8) | 7.4 (7.7) | 6.2 (5.8) | 10.1 (11.1) | NR | NR | 1.9 (1.4) | 1.7 (1.4) |
| DAS28 (CRP) | 6.2 (0.9) | 6.2 (0.8) | 4.8 (0.8) | 4.8 (0.8) | 5.4 (1.4) | 3.3 (1.3) | 3.6 (1.2) | 5.5 (1.1) | 5.5 (1.1) |
| HAQ-DI score | 1.7 (0.7) | 1.7 (0.7) | 1.4 (0.7) | 1.3 (0.7) | 1.4 (0.7) | 0.9 (0.7) | 1.0 (0.7) | 1.5 (0.7) | 1.5 (0.7) |
| Tender joints | 30.1 (14.1) | 29.1 (13.3) | 13.6 (7.7) | 14.6 (9.2) | 24.1 (16.2) | 8.8 (12.4) | 9.1 (12.8) | 25.4 (15.3) | 26.3 (15.8) |
| Swollen joints | 20.4 (9.6) | 19.4 (8.6) | 10.5 (5.4) | 10.6 (5.4) | 17.2 (12.1) | 4.3 (5.9) | 5.4 (6.4) | 15.8 (9.8) | 15.9 (10.0) |
| RF positive, % | 84.8 | 85.9 | 85.0 | 86.1 | 67.0 | NR | NR | 75.5 | 77.4 |
Data are mean (s.d.), unless otherwise stated. aData are presented for patients treated in period II; baseline is the start of period I. NR: not reported; VAS: visual analogue scale. bPatient recruited from the AIM study (Abatacept in Inadequate Responders to Methotrexate). cPatients recruited from the ATTAIN study (Abatacept Trial in Treatment of Anti-TNF Inadequate responders).
ACQUIRE
ACQUIRE was a Phase IIIb, double-blind, randomized, non-inferiority study comparing the efficacy, safety, PK and immunogenicity of s.c. and i.v. abatacept in 1457 MTX-IR patients with active RA [11–14]. Patients were randomized (1:1) to weekly s.c. abatacept [fixed dose 125 mg, with an i.v. loading dose on day 1 (∼10 mg/kg)] or monthly i.v. abatacept (∼10 mg/kg, on days 1, 15, 29 and every 28 days thereafter), plus background MTX, for 6 months [11–13]. All patients underwent standard PK profile analysis to determine serum anti-abatacept concentrations in pre-dose samples collected on day 1 and months 3 and 6 [11, 14]. After 6 months, patients could enter an open-label, long-term extension (LTE) period, during which all patients received s.c. abatacept (125 mg/week).
ACCOMPANY
ACCOMPANY was a 4-month, Phase IIIb, stratified (1:1), open-label study evaluating the immunogenicity, PK, efficacy and safety of s.c. abatacept with or without background MTX (n = 100) [15]. Patients stratified to s.c. abatacept monotherapy included MTX-naïve patients and patients who discontinued MTX due to inadequate response or intolerance. Patients stratified to s.c. abatacept plus MTX (combination) continued their current MTX dose [15]. Patients who completed the initial 4-month period could continue treatment with s.c. abatacept by entering an LTE.
ATTUNE
ATTUNE was a 12-month, Phase IIIb, open-label, single-arm study assessing the safety, immunogenicity and efficacy of switching from long-term i.v. (≥4 years) to s.c. abatacept in 123 patients with active RA who were MTX-IR or anti-TNF-IR [16]. Patients who were responding well to abatacept treatment, as evidenced by their baseline clinical characteristics, were transitioned from the open-label periods of two Phase III abatacept trials [AIM (NCT00048568) and ATTAIN (NCT00048581)] [16].
ALLOW
ALLOW was a Phase IIIb, double-blind, randomized study to determine the effects of withdrawal and reintroduction of s.c. abatacept on immunogenicity and safety [17, 18]. This study consisted of three 12-week treatment periods: period I (n = 167), consisting of an i.v. loading dose (∼10 mg/kg) on day 1, followed by weekly s.c. abatacept (125 mg); period II (n = 120) involving randomization (2:1) to s.c. placebo or s.c. abatacept and period III (n = 119), in which patients receiving s.c. abatacept continued treatment and patients on placebo were reintroduced to s.c. abatacept [17, 18]. Patients who completed period III could continue treatment by entering an open-label LTE at the end of month 9, while period I non-responders could enter the LTE at the end of month 3.
AMPLE
AMPLE is an ongoing, 2-year, Phase IIIb, randomized, head-to-head, non-inferiority study designed to compare two biologics according to standard of care and EULAR recommendations [19]. The main objective was to compare the efficacy and safety of s.c. abatacept and adalimumab, plus MTX, in 646 biologic-naïve patients with active RA. Patients were randomized (1:1) to s.c. abatacept (125 mg, with no i.v. loading dose) weekly or s.c. adalimumab (40 mg) every other week, in combination with MTX [19].
Fixed vs weight-tiered dosing
Contrary to the i.v. formulation, which is administered as a dose of ∼10 mg/kg/month according to a patient’s body weight, s.c. abatacept is administered as a fixed dose of 125 mg/week, regardless of weight. In the Phase II dose-finding study, fixed dosing achieved trough serum concentrations comparable to those for weight-tiered s.c. abatacept dosing, and similar to those seen with the i.v. dosing regimen (∼10 mg/kg/month) [10]. These findings are supported by data from the ACQUIRE study, in which weekly administration of s.c. abatacept elicited therapeutic trough concentrations in >90% of all patients across all body weights [14] and conferred comparable ACR20 response rates to those achieved with the i.v. abatacept regimen [11].
Clinical efficacy
Comparable efficacy of s.c. and i.v. abatacept
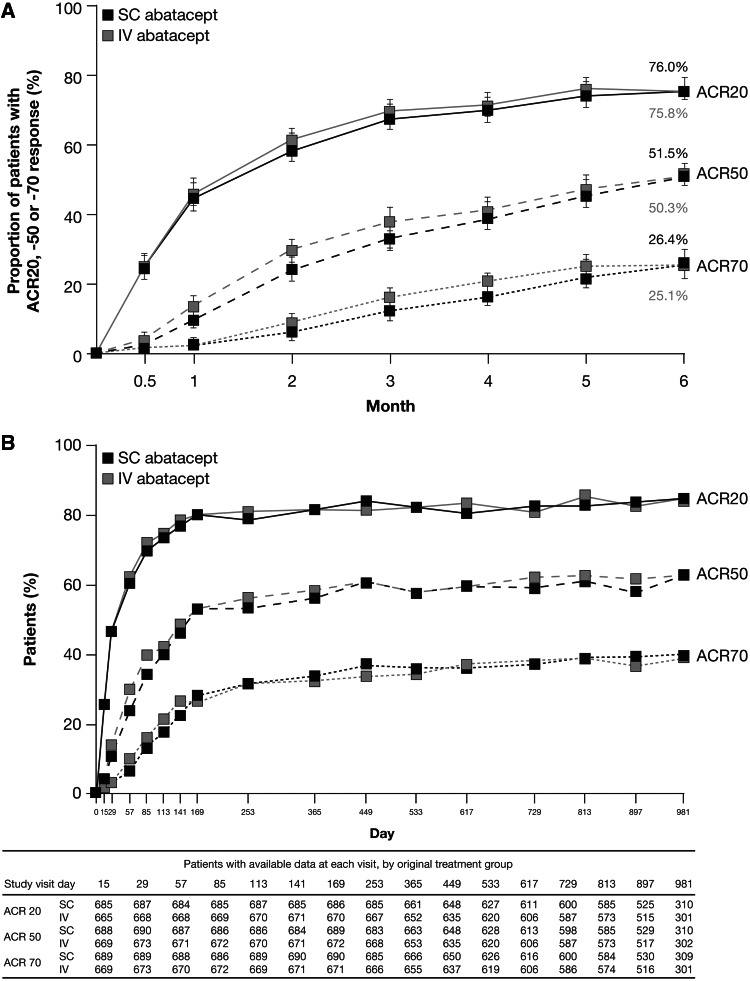
After 6 months of treatment in the ACQUIRE study, 76.0% of patients treated with s.c. abatacept and 75.8% of patients treated with i.v. abatacept in the per-protocol population experienced an ACR20 response. The primary endpoint was met, with an estimated difference between the two treatment groups in ACR20 response at month 6 of 0.3% (95% CI −4.2%, 4.8%; Fig. 1A), demonstrating that s.c. abatacept was non-inferior to i.v. abatacept. This analysis was supported by the intention-to-treat (ITT) analysis; the estimated difference between s.c. and i.v. abatacept was 0.5% (95% CI −4.0%, 4.9%) [11]. Improvements in disease activity and physical function were comparable between the s.c. and i.v. groups at month 6 (Table 2) [11].
Fig. 1.
Proportion of s.c. or i.v. abatacept-treated patients in the ACQUIRE study achieving ACR20, -50 or -70 responses (A) over 6 months for the per protocol (PP) population (n = 693 in the s.c. abatacept-treated group, n = 678 in the i.v. abatacept-treated group; non-responder analysis) [11] and (B) over 32 months for patients who entered the LTE (all patients received s.c. abatacept + MTX, n = 1372; as-observed analysis) [26]. Fig. 1b Copyright © 2011 by the American College of Rheumatology. Fig. 1b Copyright © 2012 by the American College of Rheumatology.
The ACQUIRE study compared s.c. vs i.v. abatacept in MTX-IR patients. (A) The estimated difference between the s.c. and i.v. treatment groups for ACR20 at month 6 in the PP population (primary endpoint) was 0.3% (95% CI −4.2, 4.8). (B) Not all patients reached later time points at the time of data analysis. Data on eight patients were excluded from all efficacy analyses due to site non-compliance with study procedures. Error bars represent 95% CI.
Table 2.
Disease activity and functional outcomes
| ACQUIRE [11, 26] |
ALLOW [17, 18] |
ACCOMPANY [15] |
ATTUNE [16]c |
AMPLE [19] |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| s.c. abatacept + MTX (n = 736) | i.v. abatacept + MTX (n = 721) | s.c. abatacept + MTX (continuous) (n = 40) | s.c. abatacept + MTX (withdrawal/ reintroduction) (n = 80) | s.c. abatacept (monotherapy) (n = 49) | s.c. abatacept + MTX (combination) (n = 51) | s.c. abatacept (MTX-IR) (n = 71) | s.c. abatacept (anti-TNF IR) (n = 52) | s.c. abatacept + MTX (n = 318) | Adalimumab + MTX (n = 328) | |
| HAQ-DI, mean change from baseline (95% CI)a | Mth 6: –0.69b (s.e.: ± 0.02) | Mth 6: –0.70b (s.e.: ± 0.02) | Mth 3: –0.74 (–0.91, –0.57), Mth 6: –0.72 (–0.95, –0.50), Mth 9: –0.86 (–1.04, –0.67) | Mth 3: –0.63 (–0.76, –0.49), Mth 6: –0.50 (–0.63, –0.37), Mth 9: –0.72 (–0.85, –0.60) | Mth 4: –0.58 (–0.74, –0.42) | Mth 4: –0.31 (–0.43, –0.19) | Mth 3: –0.09 (NR), Mth 12: –0.05 (NR) | Mth 3: –0.06 (NR), Mth 12: –0.01 (NR) | Mth 12: –0.60 (s.e.: ± 0.04) | Mth 12: –0.59 (s.e.: ± 0.03) |
| LTE period: NR | LTE period: NR | LTE period: NR | LTE period: NR | LTE period: Mth 18: –0.74 (–0.95, –0.54) | LTE period: Mth 18: –0.35 (–0.53, –0.16) | No LTE period | No LTE period | |||
| DAS28 (CRP) mean change from baseline (95% CI)a | Mth 6: −2.57b (s.e.: ±0.05) | Mth 6: −2.55b (s.e.: ±0.05) | Mth 3: −1.97 (−2.18, −1.76), Mth 6: −2.03 (−2.40, −1.66), Mth 9: −2.22 (−2.50, −1.94) | Mth 3: −1.88 (−2.10, −1.66), Mth 6: −1.49 (−1.77, −1.20), Mth 9: −2.32 (−2.56, −2.09) | Mth 4: −1.94 (−2.46, −1.42) | Mth 4: −1.67 (−2.06, −1.28) | Mth 3: −0.37 (NR), Mth 12: −0.24 (NR) | Mth 3: −0.11 (NR), Mth 12: −0.07 (NR) | Mth 12: –2.30 (s.e.: ±0.08) | Mth 12: –2.27 (s.e.: ±0.08) |
| LTE period: NR | LTE period: NR | LTE period: NR | LTE period: NR | LTE period: Mth 18: −2.86 (−3.46, −2.27 | LTE period: Mth 18: −1.84 (−2.33, −1.34) | No LTE period | No LTE period | |||
| DAS28 (CRP) LDAS, % (95% CI) | Mth 6: 39.5 (35.8, 43.1) | Mth 6: 41.3 (37.6, 45.1) | Mth 9: 69.2 (54.7, 83.7) | Mth 9: 79.7 (70.6, 88.9) | Mth 4: NR | Mth 4: NR | Mth 3: 64.3, Mth 12: 58.0% | Mth 3: 40.8, Mth 12: 40.9% | Mth 12: 59.3, (53.5, 65.1) | Mth 12: 61.4, (55.6, 67.3) |
| LTE period: NR | LTE period: NR | LTE period: NR | LTE period: NR | LTE period: Mth 18: 69.4 (54.4, 84.5) | LTE period: Mth 18: 57.5 (42.2, 72.8) | No LTE period | No LTE period | |||
| DAS28 (CRP) remission, % (95% CI) | Mth 6: 24.2 (20.9, 27.4) | Mth 6: 24.8 (21.5, 28.1) | Mth 9: 51.3 (35.6, 67.0) | Mth 9: 63.5 (52.5, 74.5) | Mth 4: NR | Mth 4: NR | Mth 3: 48.6, Mth 12: 43.5% | Mth 3: 24.5%, Mth 12: 34.1% | Mth 12: 43.3 (37.4, 49.1) | Mth 12: 41.9 (36.0, 47.9) |
| LTE period: Mth 24: 24.0 (21.0, 27.0) | LTE period: Mth 24: 25.0 (22.0, 28.0) | LTE period: Mth 15: 50.0 (NR) | LTE period: Mth 15: 57.1 (NR) | LTE period: Mth 18: 58.3 (42.2, 74.4) | LTE period: Mth 18: 42.5 (27.2, 57.8) | No LTE period | No LTE period | |||
No efficacy data are available from IM101-063. During the 12-month LTE period of the ACCOMPANY study, in which patients could add or discontinue MTX, 23.3% (10/43) of patients originally randomized to the monotherapy group added MTX and one patient (1/47; 2.1%) in the combination group discontinued MTX. LDAS is defined as DAS28 (CRP) ≤3.2 and remission as DAS28 (CRP) <2.6. LDAS: low disease activity state; IR: inadequate response; Mth: month; NR: not reported. aUnless otherwise stated. bAdjusted mean ± s.e. for baseline value and weight stratification. cAll data for the ATTUNE study are from patients who had already completed ≥4 years of treatment with i.v. abatacept and were already responding to treatment.
Efficacy with or without an i.v. loading dose
An i.v. infusion of ∼10 mg/kg abatacept may be administered on day 1 of s.c. abatacept treatment initiation as an i.v. loading dose. A recent post hoc analysis of patients from the ACQUIRE (with i.v. loading) and AMPLE (without i.v. loading) studies revealed comparable onset of response, ACR20 and HAQ Disease Index (HAQ-DI) response rates, and improvements in DAS28 (CRP) with or without an i.v. loading dose over 6 months of s.c. abatacept treatment in patients who were MTX-IR [20]. PK data from the ALLOW (with i.v. loading) and ACCOMPANY (without i.v. loading) studies illustrate that target therapeutic serum concentrations were achieved by day 15 in both studies, regardless of i.v. loading [21–23]. These findings suggest that in patients not receiving an i.v. abatacept loading dose, kinetics of response is not compromised.
Efficacy of switching from i.v. to s.c. abatacept
Data from the ATTUNE study demonstrate that patients switched from long-term i.v. (≥4 years) to s.c. abatacept demonstrated continued clinical and functional efficacy, with the overall proportions of patients in DAS28-derived low disease activity state or remission at baseline being maintained through to month 12 (Table 2) [16].
Efficacy of s.c. abatacept with or without MTX
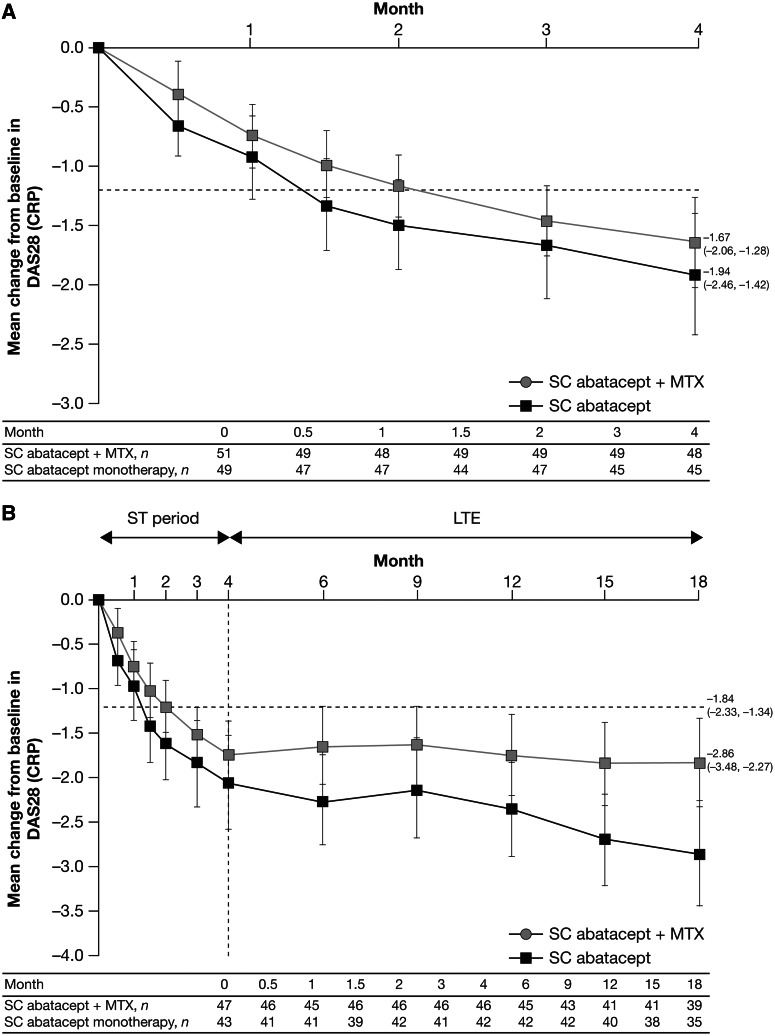
Comparable clinical and functional improvements were shown for s.c. abatacept with concomitant MTX vs s.c. abatacept monotherapy in the ACCOMPANY study (Table 2 and Fig. 2A). Overall changes in DAS28 (CRP) and HAQ-DI from baseline to month 4 were numerically greater in the monotherapy group compared with the combination group [mean changes (95% CI) from baseline to month 4: −1.94 (−2.46, −1.42) vs −1.67 (−2.06, −1.28) and −0.58 (−0.74, −0.42) vs −0.31 (−0.43, −0.19), respectively; Table 2 and Fig. 2A] [15].
Fig. 2.
Mean change from baseline DAS28 (CRP) score in patients in the ACCOMPANY study (A) over 4 months for the ITT population [n = 49 in the s.c. abatacept monotherapy group, n = 51 in the s.c. abatacept combination (plus MTX) group] and (B) over 18 months for patients who entered the LTE (n = 43 in the monotherapy group, n = 47 in the combination group) (as-observed analysis). [15]. Copyright © 2012 by the American College of Rheumatology.
The ACCOMPANY study compared s.c. abatacept with or without MTX in patients with an inadequate response to ≥1 DMARD (MTX-naïve or MTX-IR). Mean (s.d.) baseline DAS28 (CRP) was 5.4 (1.4). Error bars represent 95% CI.
Long-term efficacy
Several studies included open-label LTE periods (Table 2). During the LTE period of the ACQUIRE study, during which all patients received s.c. abatacept, ACR responses and DAS28 remission rates observed at month 6 in the s.c. and i.v. abatacept treatment groups were maintained through month 32 and remained similar between the original s.c. and i.v. groups (Table 2 and Fig. 1B) [11, 13]. Disease activity targets were maintained with continued therapy during the ACCOMPANY LTE period (Fig. 2B) [15].
Comparable efficacy to other biologic DMARDs
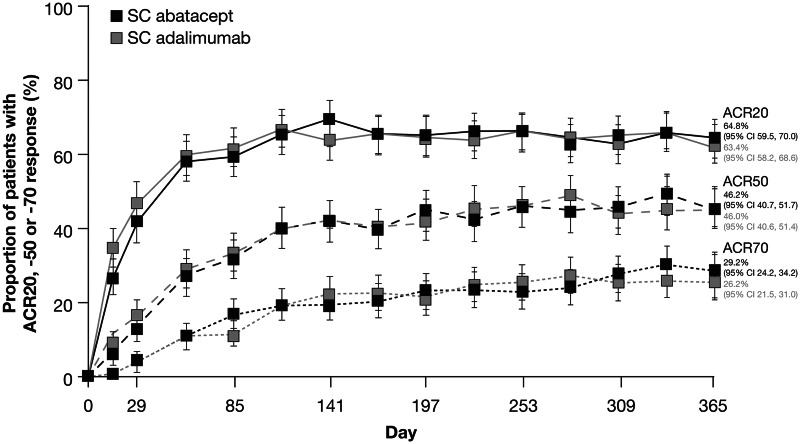
First-year data from the ongoing AMPLE trial demonstrate that s.c. abatacept in combination with MTX (no i.v. loading dose) has comparable efficacy to adalimumab plus MTX. The primary endpoint was met, demonstrating the non-inferiority of s.c. abatacept to adalimumab plus MTX by ACR20 response at month 12 [ITT population 64.8% vs 63.4%; estimated difference between the two groups 1.8 (95% CI −5.6, 9.2); Fig. 3] [19]. Year 1 ACR50 and -70 response rates were similar between the s.c. abatacept and adalimumab groups (Fig. 3). Importantly, similar kinetics of clinical response for abatacept and adalimumab were demonstrated, with similar time to onset and duration of response (Fig. 3). Similar proportions of patients achieved disease activity and physical function targets through 12 months in the s.c. abatacept and adalimumab groups [DAS28 (CRP)-defined remission 43.3% (95% CI 37.4, 49.1) vs 41.9% (95% CI 36.0, 47.9); HAQ-DI responders 60.4% (95% CI 55.0, 65.8) vs 57.0% (95% CI 51.7, 62.4)] [19].
Fig. 3.
The proportions of patients in the AMPLE study meeting ACR20, -50, and -70 responses over 1 year for the ITT population (n = 318 in the s.c. abatacept group, n = 328 in the adalimumab group). [19]. Copyright © 2013 by the American College of Rheumatology.
AMPLE is an ongoing study comparing s.c. abatacept vs adalimumab in biologic-naïve patients with background MTX. Error bars represent 95% CI.
Radiographic progression
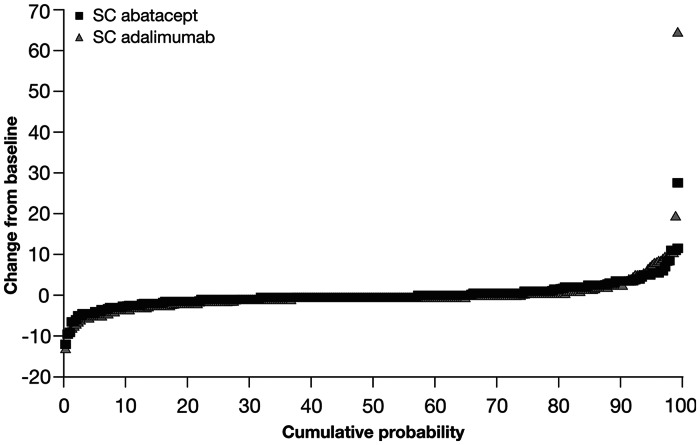
AMPLE provides the first radiographic data for the s.c. formulation of abatacept, demonstrating similar inhibition of radiographic damage in both treatment groups (Fig. 4). The proportions of patients with radiographic non-progression (defined as a change from baseline in modified total Sharp scores greater than the smallest detectable change) at month 12 were comparable [84.8% s.c. abatacept vs 88.6% adalimumab; estimated difference 4.1% (95% CI −1.5, 9.6)] [19].
Fig. 4.
Cumulative probability plot showing the distribution of change in van der Heijde modified total Sharp scores from baseline to year 1 for patients in the AMPLE study. [19]. Copyright © 2013 by the American College of Rheumatology.
AMPLE is an ongoing study comparing s.c. abatacept vs adalimumab in biologic-naïve patients with background MTX. Paired (baseline and year 1) radiographic images were available for 91.1% of patients in the abatacept group and 88.1% of patients in the adalimumab group.
Safety
Integrated safety summary for i.v. and s.c. abatacept
Safety results from five of the s.c. abatacept trials (Phase II dose-finding, ACQUIRE, ALLOW, ACCOMPANY and ATTUNE) were pooled into an integrated safety summary of 1879 patients. These data comprised 3086 patient-years (pt-yrs) of exposure to s.c. abatacept, with a mean (range) exposure of 20 (2–56) months [24, 25]. This analysis provides an opportunity to compare safety with a similar integrated analysis of i.v. abatacept, which included 4149 patients with a combined total of 12 132 pt-yrs of exposure [9]. The analysis also facilitates the assessment of changes in incidence rate (IR; expressed as events/100 pt-yrs) with increasing exposure for safety events that are of particular interest with biologic use (supplementary Table S1, available at Rheumatology Online).
Overall, 17 deaths were reported in the s.c. abatacept integrated safety summary, at an IR (95% CI) of 0.55 (0.34, 0.89), which is comparable to the IR of deaths reported for i.v. abatacept [0.60 (0.47, 0.76); supplementary Table S2, available at Rheumatology Online] [24].
Serious infections
In the integrated safety summary analysis, the IR (95% CI) of infections with s.c. abatacept was lower than reported for i.v. abatacept [53.91 (50.69, 57.33) vs 75.68 (73.00, 78.44); supplementary Table S2, available at Rheumatology Online] [24]. The IR (95% CI) of serious infections was 1.94 (1.50, 2.50) in 59 (3.1%) s.c. abatacept-treated patients and 2.87 (2.57, 3.19) in 332 (8.0%) i.v. abatacept-treated patients (supplementary Table S2, available at Rheumatology Online) [24]. The most frequent (IR > 0.10) serious infections with s.c. abatacept were pneumonia [0.36 (0.20, 0.65)], urinary tract infection [0.16 (0.07, 0.39)] and gastroenteritis [0.13 (0.05, 0.35)] [24, 25], which are consistent with those reported for the i.v. formulation [9]. Tuberculosis (TB), pulmonary TB and peritoneal TB were recorded in one s.c. abatacept-treated patient each [0.03 (0.00, 0.23) each] [24]. The IRs of serious infections did not increase with increasing exposure to s.c. abatacept up to month 24 (supplementary Table S1, available at Rheumatology Online) [25].
Autoimmune events
Autoimmune events occurred with an IR (95% CI) of 1.28 (0.93, 1.75) in 39 (2.1%) patients treated with s.c. abatacept, compared with 1.99 (1.74, 2.26) in 232 (5.6%) i.v. abatacept-treated patients (supplementary Table S2, available at Rheumatology Online) [24]. The most frequent (IR > 0.10) autoimmune events were psoriasis [0.29 (0.15, 0.56)] and SS [0.19 (0.09, 0.43)] [24, 25], which are consistent with i.v. abatacept [9]. The IRs of autoimmune events did not increase with increasing s.c. abatacept exposure (supplementary Table S1, available at Rheumatology Online) [25].
Malignancies
Malignancies, excluding non-melanoma skin cancer, occurred at comparable IRs in the s.c. and i.v. abatacept groups (supplementary Table S2, available at Rheumatology Online) [24]. The most frequent (IR > 0.10) malignancies in patients treated with s.c. abatacept were basal cell carcinoma [0.46 (0.27, 0.77)], breast cancer and squamous cell carcinoma of the skin [0.16 (0.07, 0.39) each] [25]. IRs of malignancies did not increase with increasing exposure to s.c. abatacept (supplementary Table S1, available at Rheumatology Online) [25].
Head-to-head safety comparison of s.c. and i.v. abatacept
Data from the ACQUIRE study are consistent with findings from the integrated safety analysis, demonstrating comparable safety and tolerability of s.c. and i.v. abatacept over 6 months. Similar rates of adverse events (AEs; 67.0% vs 65.2%), serious AEs (SAEs; 4.2% vs 4.9%) and discontinuation due to SAEs (1.1% vs 1.9%) were seen, along with comparable frequencies of serious infections [n = 5 (0.7%) vs n = 10 (1.4%)], malignancies [n = 3 (0.4%) vs n = 5 (0.7%)] and prespecified autoimmune events [n = 7 (1.0%) vs n = 6 (0.8%)] for s.c. and i.v. abatacept-treated patients [11].
Safety of switching from i.v. to s.c. abatacept
The primary endpoint of the ATTUNE study was met, with consistent safety observed for 3 months following the switch from i.v. to s.c. abatacept, with no serious infections, malignancies or autoimmune events during this time [16]. After month 3, one serious infection was reported (pneumonia), which did not result in discontinuation. Two malignancies occurred after month 3 (breast and uterine cancer). Two autoimmune events were reported [sarcoidosis (considered an SAE) and erythema nodosum (mild intensity)] after month 3 [16].
Injection-site reactions
The IR (95% CI) for injection-site reactions (ISRs) reported in the s.c. abatacept integrated safety summary was 2.22 (1.74, 2.82) in 66 (3.5%) patients [24, 25]. The most frequently reported ISRs were erythema, haematoma, pain and pruritus [IR 0.46 (0.27, 0.77) each]. Events were mostly (94%) mild in intensity; two patients discontinued due to ISRs. Most ISRs occurred within the first 6 months of treatment (supplementary Table S1, available at Rheumatology Online).
Comparative safety of s.c. abatacept with other s.c. biologics
The AMPLE study compares the safety of two biologic therapies head-to-head over 12 months in the same clinical trial setting [19]. The safety of s.c. abatacept and adalimumab was generally similar, with comparable rates of AEs, SAEs and malignancies (supplementary Table S3, available at Rheumatology Online) [19]. Autoimmune events were reported in more patients in the s.c. abatacept arm than the adalimumab arm (3.1% vs 1.2%; supplementary Table S3, available at Rheumatology Online); one patient in each arm discontinued treatment due to autoimmune events. Similar rates of serious infections were reported in the two arms (2.2% vs 2.7%; supplementary Table S3, available at Rheumatology Online); however, no serious infections in the abatacept arm led to discontinuation, whereas five of the nine serious infections in the adalimumab arm led to discontinuation. Fewer patients treated with s.c. abatacept discontinued treatment due to AEs and SAEs compared with adalimumab (3.5% and 1.3% vs 6.1% and 3.0%; supplementary Table S3, available at Rheumatology Online) [19]. Significantly fewer local ISRs were reported in abatacept-treated patients compared with those receiving adalimumab [3.8% vs 9.1% (95% CI −9.13, −1.62), P = 0.006; supplementary Table S3, available at Rheumatology Online]. The intensity of ISRs in both treatment arms was mostly mild, with one moderate reaction in the s.c. abatacept arm and six moderate and one severe reaction in the adalimumab arm. Three patients in the adalimumab arm discontinued due to ISRs [19].
Patient retention on s.c. abatacept treatment
High retention rates (>90%) were observed in the short-term periods of the s.c. abatacept studies (supplementary Table S4, available at Rheumatology Online). In particular, comparable retention was seen for s.c. and i.v. abatacept in the ACQUIRE study over 6 months, and 82.7% of patients remained on s.c. treatment for a maximum of 44 months in the LTE [26]. In the AMPLE study, 86.2% and 82% of patients in the s.c. abatacept and adalimumab arms completed 12 months of treatment [19].
Immunogenicity
Increased immunogenicity is a key issue with s.c. administration of biologics due to potential differences in antigen presentation [27], therefore immunogenicity was included as a primary endpoint in several studies in the s.c. abatacept clinical programme. Immunogenicity was measured using ELISA.
Comparable immunogenicity of s.c. and i.v. abatacept
During the 6-month double-blind period of ACQUIRE, immunogenicity with s.c. and i.v. abatacept was generally comparable. However, a slightly lower proportion of patients in the s.c. group than the i.v. group were found to be seropositive for anti-abatacept antibodies (0.4% vs 0.7%) and anti-CTLA-4-T antibodies (0.7% vs 1.5%). The presence of a positive antibody seroconversion did not appear to affect the efficacy or safety of abatacept [11].
Immunogenicity following a switch from i.v. to s.c. abatacept
In the ATTUNE study, eight patients were seropositive during the 3 months following the switch from i.v. to s.c. abatacept; however, six of these patients were already positive prior to enrolment and all eight patients continued treatment. These data suggest that the risk of immunogenicity is low following a switch from i.v. to s.c. abatacept and does not seem to be associated with reduced efficacy or increased safety risks [16].
Immunogenicity following withdrawal/reintroduction of s.c. abatacept
As patients may experience short-term withdrawal in clinical practice, and given the likelihood that abatacept may suppress an immunogenic response to itself by its effects upstream in the immune response [28], findings from the ALLOW study are of particular interest. This study demonstrated a non-significant increase in immunogenicity on 3-month withdrawal of abatacept (9.6% s.c. placebo vs 0% s.c. abatacept in period II; P = 0.119), which was reversed on reintroduction of s.c. abatacept (2.7% vs 2.6%, respectively, end period III) [17].
Immunogenicity in the absence of MTX
Although some biologic therapies must be administered concomitantly with MTX to avoid immunogenicity, many patients may be intolerant to MTX. Therefore the question of whether s.c. abatacept elicits an increased risk of immunogenicity when administered as monotherapy is important. Throughout the 4-month double-blind period of the ACCOMPANY study, immunogenicity rates were low in the monotherapy and combination groups (2.0% vs 3.9%), and all events were transient and associated with low titers. No positive antibody response to abatacept was observed at the end of the double-blind period, which was the primary endpoint [15].
Discussion and conclusions
RA treatment options with different routes of administration are an important consideration, given the current shift towards individualized therapy. s.c. abatacept has been developed as an alternative formulation to i.v. abatacept.
The data reviewed here demonstrate that the recommended fixed dosing for s.c. abatacept (125 mg/week) shows comparable PK parameters, clinical efficacy, low immunogenicity, safety, tolerability and patient retention to the traditional weight-tiered dosing for i.v. abatacept. Similar improvements in clinical and functional efficacy were seen for s.c. and i.v. abatacept. In addition, post hoc analyses comparing data from studies with or without i.v. loading doses suggest that an i.v. abatacept loading dose may not be required at initiation of s.c. abatacept, with patients from these studies demonstrating a similar onset of response and improvements in clinical and functional efficacy and similar PK profiles. For patients treated with s.c. abatacept who remain on treatment for more extended periods of time, improvements in clinical and functional efficacy are maintained.
With the availability of new formulations, physicians may choose to switch patients from i.v. to s.c. abatacept. Data demonstrate that efficacy is maintained in patients switched from long-term i.v. to s.c. abatacept, with high retention rates. Although comparisons of s.c. and i.v. abatacept are limited by fewer patient-years of exposure for s.c. compared with i.v., observations suggest that s.c. abatacept has a similar long-term efficacy profile to the i.v. formulation.
The safety profile of s.c. abatacept is comparable to that of i.v. abatacept, with no new safety signals detected [9, 29]. ISRs were mild and infrequent. In addition, patients switched to s.c. abatacept from long-term i.v. abatacept demonstrated no new safety events, and the incidence of serious infections, malignancies and autoimmune events did not appear to increase with increasing exposure to s.c. abatacept. An integrated safety analysis demonstrated similar mortality rates for s.c. and i.v. abatacept [IR (95% CI) 0.55 (0.34, 0.89) vs 0.60 (0.47, 0.76)]. The British Society for Rheumatology Biologics Register has reported all-cause mortality rates [events per 100 pt-yr (95% CI)] of 1.63 (1.49, 1.78) and 1.79 (1.35, 2.36) in patients receiving anti-TNF therapy (n = 12 672) and biologic-naïve patients receiving non-biologic DMARDs (n = 3522), respectively [30].
Immunogenicity has been reported with some biologics [31–34], with s.c. administration being linked to a potential increased risk [35]. The overall immunogenicity with abatacept is low and generally transient, with comparable rates reported for i.v. and s.c. administration, and with little effect on immunogenicity when switching from long-term i.v. to s.c. abatacept. Furthermore, the occurrence of immunogenicity does not seem to be associated with reduced efficacy or increased safety risks, which have been reported for some other biologic therapies [31, 32, 36]. Some biologic agents require background MTX to suppress immunogenicity. The data presented here show that s.c. abatacept was not associated with increased immunogenicity or differences in efficacy when administered as either monotherapy or in combination with MTX. In addition, in the ALLOW study, some patients were withdrawn from s.c. abatacept treatment, which is hypothesized to lead to increased risk of immunogenicity [28]. However, patients who were withdrawn experienced only a non-significant, transient increase in immunogenicity that did not persist on treatment reintroduction.
The head-to-head AMPLE study revealed that s.c. abatacept (without an i.v. loading dose) demonstrates comparable efficacy and a similar kinetics of response to adalimumab in biologic-naïve patients with RA over 12 months of treatment. The prevention of structural damage is of utmost importance in the treatment of RA, and the first radiographic data published for s.c. abatacept, from the AMPLE study, showed a similar inhibition of progression of structural damage with s.c. abatacept to adalimumab over 1 year. Safety outcomes between the two treatment groups were generally balanced, although fewer AE- and SAE-related discontinuations were observed with s.c. abatacept. Overall rates of serious infections were similar in both treatment groups; however, no abatacept-treated patients discontinued due to a serious infection, whereas five of nine patients in the adalimumab arm who experienced a serious infection discontinued. A higher rate of autoimmune events was observed with s.c. abatacept than adalimumab; however, only one case in each treatment arm resulted in discontinuation. Local ISRs were less frequent and milder in patients treated with s.c. abatacept compared with adalimumab, with no discontinuations in the s.c. abatacept arm and three patients discontinuing in the adalimumab arm. ISR-related pain was not frequently reported compared with other biologics [34, 37]. No new safety signals were observed during this study. Findings from the recent Cochrane review meta-analysis also suggest that the incidence of SAEs and serious infections with abatacept is at the lower end of the range reported for other biologics [38].
In summary, s.c. abatacept provides short-term efficacy and safety and low immunogenicity that are consistent with the established i.v. abatacept profile. In addition, data from the AMPLE study show that two agents with different mechanisms of action (T cell modulation and TNF inhibition) can demonstrate comparable clinical benefit and suggest that s.c. abatacept and adalimumab could be considered equally for the treatment of patients with RA who have responded inadequately to MTX. As a consequence of its alternative route of administration, s.c. abatacept will provide additional treatment options for patients with RA.
Supplementary Material
Acknowledgements
Professional medical writing and editorial assistance was provided by Laura McDonagh, PhD, at Caudex Medical and was funded by Bristol-Myers Squibb.
Disclosure statement: M.S. is a consultant for and received honoraria from BMS.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Yamada A, Salama A, Sayegh M. The role of novel T cell costimulatory pathways in autoimmunity and transplantation. J Am Soc Nephrol. 2002;13:559–75. doi: 10.1681/ASN.V132559. [DOI] [PubMed] [Google Scholar]
- 2.Westhovens R, Robles M, Ximenes AC, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68:1870–7. doi: 10.1136/ard.2008.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kremer JM, Genant HK, Moreland LW, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006;144:865–76. doi: 10.7326/0003-4819-144-12-200606200-00003. [DOI] [PubMed] [Google Scholar]
- 4.Kremer JM, Russell AS, Emery P, et al. Long-term safety, efficacy and inhibition of radiographic progression with abatacept treatment in patients with rheumatoid arthritis and an inadequate response to methotrexate: 3-year results from the AIM trial. Ann Rheum Dis. 2011;70:1826–30. doi: 10.1136/ard.2010.139345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smolen J, Dougados M, Gaillez C, et al. Remission according to different composite disease activity indices in biologic-naïve patients with rheumatoid arthritis treated with abatacept or infliximab plus methotrexate. Arthritis Rheum. 2011;63:1124. [Google Scholar]
- 6.Westhovens R, Kremer JM, Emery P, et al. Consistent safety and sustained improvement in disease activity and treatment response over 7 years of abatacept treatment in biologic-naïve patients with RA. Ann Rheum Dis. 2009;68:577. (Abstract SAT0108) [Google Scholar]
- 7.Genovese MC, Becker JC, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114–23. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 8.Schiff M, Pritchard C, Huffstutter JE, et al. The 6-month safety and efficacy of abatacept in patients with rheumatoid arthritis who underwent a washout after anti-tumour necrosis factor therapy or were directly switched to abatacept: the ARRIVE trial. Ann Rheum Dis. 2009;68:1708–14. doi: 10.1136/ard.2008.099218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hochberg MC, Westhovens R, Aranda R, et al. Long-term safety of abatacept: integrated analysis of clinical program data of up to 7 years of treatment. Arthritis Rheum. 2010;62:S164. [Google Scholar]
- 10.Corbo M, Valencia X, Raymond R, et al. Subcutaneous administration of abatacept in patients with rheumatoid arthritis: pharmacokinetics, safety and immunogenicity. Ann Rheum Dis. 2009;68:S574. [Google Scholar]
- 11.Genovese MC, Covarrubias A, Leon G, et al. Subcutaneous abatacept versus intravenous abatacept: a phase IIIb noninferiority study in patients with an inadequate response to methotrexate. Arthritis Rheum. 2011;63:2854–64. doi: 10.1002/art.30463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genovese MC, Covarrubias JA, Leon G, et al. The ACQUIRE (Abatacept Comparison of sub[Qu]cutaneous versus intravenous in Inadequate Responders to methotrexatE) trial: a large phase IIIb non-inferiority study. Ann Rheum Dis. 2011;70:OP0023. [Google Scholar]
- 13.Genovese MC, Cobos AC, Leon G, et al. Subcutaneous (SC) abatacept (ABA) versus intravenous (IV) ABA in patients (pts) with rheumatoid arthritis: long-term data from the ACQUIRE (Abatacept Comparison of sub[QU]cutaneous versus intravenous in Inadequate Responders to methotrexatE) trial. Arthritis Rheum. 2011;63:S150. [Google Scholar]
- 14.Murthy B, Gao L, Yin J, et al. Pharmacokinetics of subcutaneous abatacept support a fixed dosing regimen in adult patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(Suppl 3):FRI0347. [Google Scholar]
- 15.Nash P, Nayiager S, Genovese M, et al. Immunogenicity, safety and efficacy of subcutaneous abatacept with or without MTX in patients with rheumatoid arthritis: the Phase III ACCOMPANY study. Arthritis Care Res (Hoboken) 2012 Oct 24. doi: 10.1002/acr.21876. [Epub ahead of print] [Google Scholar]
- 16.Keystone EC, Kremer JM, Russell A, et al. Abatacept in subjects who switch from intravenous to subcutaneous therapy: results from the phase IIIb ATTUNE study. Ann Rheum Dis. 2012;71:857–61. doi: 10.1136/annrheumdis-2011-200355. [DOI] [PubMed] [Google Scholar]
- 17.Kaine J, Gladstein G, Strusberg I, et al. Evaluation of abatacept administered subcutaneously in adults with active rheumatoid arthritis: impact of withdrawal and reintroduction on immunogenicity, efficacy and safety (phase IIIb ALLOW study) Ann Rheum Dis. 2012;71:38–44. doi: 10.1136/annrheumdis-2011-200344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaine J, Gladstein G, Strusberg I, et al. Subcutaneous abatacept is effective and well tolerated, with low immunogenicity following temporary withdrawal and re-introduction in the ALLOW LTE. Arthritis Rheum. 2011;63:S853. [Google Scholar]
- 19.Weinblatt M, Schiff M, Valente R, et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study. Arthritis Rheum. 2012;65:28–38. doi: 10.1002/art.37711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiff M, Alten R, Weinblatt M, et al. Weekly subcutaneous abatacept confers comparable onset of treatment response and magnitude of efficacy improvement over 6 months when administered with or without an intravenous abatacept loading dose. Arthritis Rheum. 2012;64(10 Suppl):S1076. [Google Scholar]
- 21.Nash P, Ludivico C, Delaet I, et al. Improvements in disease activity and physical function in patients with RA receiving subcutaneous abatacept in the presence or absence of an initial IV loading dose. Ann Rheum Dis. 2011;70:SAT0287. [Google Scholar]
- 22.Nash P, Ludivico C, Delaet I, et al. Efficacy, safety and pharmacokinetics of subcutaneous abatacept in patients with rheumatoid arthritis, with or without an intravenous (IV) loading dose. Arthritis Rheum. 2011;63:S151. [Google Scholar]
- 23.Murthy B, Gao L, Vakkalagadda B, et al. Clinical pharmacokinetics of subcutaneous abatacept in the presence or absence of an intravenous loading dose in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(Suppl 3):SAT0284. [Google Scholar]
- 24.Alten R, Kaine J, Keystone EC, et al. Safety of subcutaneous abatacept in patients with rheumatoid arthritis (RA): integrated analysis of five clinical trials up to 4.5 years. Ann Rheum Dis. 2011;70(Suppl 3):617. [Google Scholar]
- 25.Alten R, Kaine J, Keystone EC, et al. Safety profile of subcutaneous abatacept focusing on clinically relevant events in patients with rheumatoid arthritis (RA) and up to 4.5 years of exposure. Arthritis Rheum. 2011;63:S150. [Google Scholar]
- 26.Genovese M, Pacheco-Tena C, Covarrubias A, et al. Subcutaneous abatacept: long-term data from the ACQUIRE trial. Arthritis Rheum. 2012;64(10 Suppl):S201. [Google Scholar]
- 27.Schellekens H. Factors influencing the immunogenicity of therapeutic proteins. Nephrol Dial Transplant. 2005;20(Suppl 6):vi3–9. doi: 10.1093/ndt/gfh1092. [DOI] [PubMed] [Google Scholar]
- 28.Haggerty HG, Abbott MA, Reilly TP, et al. Evaluation of immunogenicity of the T cell costimulation modulator abatacept in patients treated for rheumatoid arthritis. J Rheumatol. 2007;34:2365–73. [PubMed] [Google Scholar]
- 29.Sibilia J, Westhovens R. Safety of T-cell co-stimulation modulation with abatacept in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2007;25:S46–56. [PubMed] [Google Scholar]
- 30.Lunt M, Watson KD, Dixon WG, et al. No evidence of association between anti-tumor necrosis factor treatment and mortality in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2010;62:3145–53. doi: 10.1002/art.27660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartelds GM, Wijbrandts CA, Nurmohamed MT, et al. Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis. Ann Rheum Dis. 2007;66:921–6. doi: 10.1136/ard.2006.065615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartelds GM, Wijbrandts CA, Nurmohamed MT, et al. Anti-infliximab and anti-adalimumab antibodies in relation to response to adalimumab in infliximab switchers and anti-tumour necrosis factor naive patients: a cohort study. Ann Rheum Dis. 2010;69:817–21. doi: 10.1136/ard.2009.112847. [DOI] [PubMed] [Google Scholar]
- 33.Bendtzen K, Geborek P, Svenson M, et al. Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor alpha inhibitor infliximab. Arthritis Rheum. 2006;54:3782–9. doi: 10.1002/art.22214. [DOI] [PubMed] [Google Scholar]
- 34.Dore RK, Mathews S, Schechtman J, et al. The immunogenicity, safety, and efficacy of etanercept liquid administered once weekly in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2007;25:40–6. [PubMed] [Google Scholar]
- 35.Schellekens H. The immunogenicity of therapeutic proteins. Discov Med. 2010;9:560–4. [PubMed] [Google Scholar]
- 36.Radstake TR, Svenson M, Eijsbouts AM, et al. Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann Rheum Dis. 2009;68:1739–45. doi: 10.1136/ard.2008.092833. [DOI] [PubMed] [Google Scholar]
- 37.Weinblatt ME, Keystone EC, Furst DE, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- 38.Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011:CD008794. doi: 10.1002/14651858.CD008794.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.