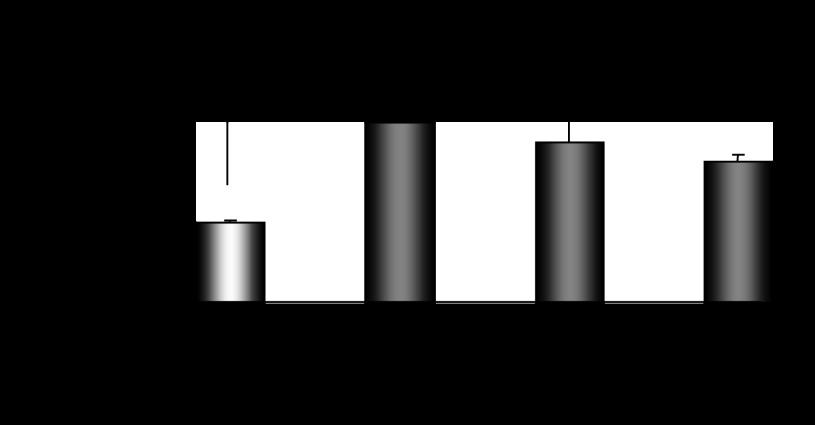Abstract
CYP2E1 is of paramount toxicological significance because it metabolically activates a large number of low-molecular-weight toxicants and carcinogens. In this context, factors that interfere with Cyp2e1 regulation may critically affect xenobiotic toxicity and carcinogenicity. The aim of this study was to investigate the role of female steroid hormones in the regulation of CYP2E1, as estrogens and progesterone are the bases of contraceptives and hormonal replacement therapy in menopausal women. Interestingly, a fluctuation in the hepatic expression pattern of Cyp2e1 was revealed in the different phases of the estrous cycle of female mice, with higher Cyp2e1 expression at estrus (E) and lower at methestrus (ME), highly correlated with that in plasma gonadal hormone levels. Depletion of sex steroids by ovariectomy repressed Cyp2e1 expression to levels similar to those detected in males and cyclic females at ME. Hormonal supplementation brought Cyp2e1 expression back to levels detected at E. The role of progesterone appeared to be more prominent than that of 17β-estradiol. Progesterone-induced Cyp2e1 upregulation could be attributed to inactivation of the insulin/PI3K/Akt/FOXO1 signaling pathway. Tamoxifen, an anti-estrogen, repressed Cyp2e1 expression potentially via activation of the PI3K/Akt/FOXO1 and GH/STAT5b-linked pathways. The sex steroid hormone-related changes in hepatic Cyp2e1 expression were highly correlated with those observed in Hnf-1α, β-catenin, and Srebp-1c. In conclusion, female steroid hormones are clearly involved in the regulation of CYP2E1, thus affecting the metabolism of a plethora of toxicants and carcinogenic agents, conditions that may trigger several pathologies or exacerbate the outcomes of various pathophysiological states.
Keywords: Cyp2e1, 17β-estradiol, progesterone, estrous cycle, mice
pituitary hormones, in particular, growth hormone (GH), are considered critical in Cyp2e1 regulation. Previous studies revealed that pituitary hormone depletion by hypophysectomy resulted in Cyp2e1 upregulation in the liver of rats and GH supplementation normalized Cyp2e1 expression to constitutive levels by suppressing Cyp2e1 transcription (11, 28, 70, 71, 80). It is of interest to note that sex steroid hormones target complex regulatory dynamics including GH secretion. On the one hand, they augment GH-secretory burst by amplifying feedforward [via both GH-releasing hormone, GH-releasing peptide(s)] and on the other hand they attenuate feedback (imposed by somatostatin and GH). The role of testosterone is less clear (49, 67). Previous studies in humans and experimental animals presented contradictory findings regarding sex differentiation in CYP2E1 constitutive expression and the role of sex steroid hormones in this regulation (5, 11, 29, 33, 34, 59). This contradiction is probably due, at least in part, to the complexity of the female hormonal state within the different phases of the estrous cycle. During the reproductive cycle, there is a fluctuation in the circulating levels of 17β-estradiol and progesterone that are produced by the ovaries and hold a determinant role in the division of the murine estrous cycle into four stages, called proestrus, estrus, methestrus, and diestrus, that generally last 4–5 days. The peak in 17β-estradiol levels comes prior to ovulation, early at estrus, whereas progesterone levels start rising late at estrus and remain high at methestrus and diestrus and then decline from proestrus until the first part of estrus (20, 69).
CYP2E1 is involved in xenobiotic-induced toxicity and carcinogenicity. It catalyzes the metabolism and bioactivation of a broad variety of low-molecular-weight (<100) and hydrophobic agents, including procarcinogens and solvents, and metabolizes drugs, such as isoniazid, chlorzoxazone, coumarin derivatives, gas anesthetics, and acetaminophen, with potential hepatotoxic and nephrotoxic properties (3, 18, 22–25, 36, 56, 79, 81). It is also worth noting that nitrosamines are metabolized by CYP2E1 to carcinogenic metabolites (81). Arachidonic acid and its metabolites that are lipid second messengers involved in cellular signaling and inflammation (4) are also substrates of CYP2E1 (15).
It should be also underscored that, in several pathophysiological states such as diabetes, obesity, and fasting, Cyp2e1 expression was detected at higher levels in both experimental animals and humans compared with normal individuals, and this increase was attributed to increased ketone body levels present in these pathologies (6, 16, 17, 22, 32, 55, 56, 63, 64, 77, 82). The determinant contribution of CYP2E1 in oxidative stress should be also added to the broad array of biological roles this cytochrome holds. Reactive oxygen species liberated during CYP2E1-catalyzed xenobiotic metabolism can trigger mitochondrial damage, DNA modification, lipid peroxidation, cytokine production, and even cell death (9, 10, 22). In addition, a novel metabolic pathway of estrogens involves CYP2E1. This CYP along with CYP1A1 and CYP2B6, is involved in estrone and estradiol conversion to quinol metabolites (50).
The multifactorial differentiation in the biological profile of males and females including drug metabolizing systems, added to the cross-talk between the steroid receptor-linked signaling pathways and those pathways regulating CYP2E1, set the necessity for further investigation of the sex-specific differences and the role of female sex steroid hormones in CYP2E1 regulation (59). Since sex steroid hormones are the basis of the widely used contraceptives and hormonal replacement therapy in menopausal women for the prevention of osteoporosis and cardiovascular events (26, 58), this study investigated the role of female sex steroid hormones in hepatic Cyp2e1 regulation, using ovariectomized mice supplemented with 17β-estradiol and/or progesterone. The role of estrogens was also evaluated in intact cyclic females treated with tamoxifen, a drug with antiestrogenic effects in the breast tissue that is used as standard endocrine treatment in women with hormone receptor-positive breast cancer. Tamoxifen, though, under certain circumstances, can also exert estrogenic agonist properties depending on the tissue (46). In addition, the hepatic Cyp2e1 expression pattern was assessed at the four distinct phases of the estrous cycle of intact cyclic female mice and compared with the male Cyp2e1 expression profile. A marked diversity in hepatic Cyp2e1 expression was observed within the different phases of the estrous cycle, with progesterone holding a critical regulatory role.
MATERIALS AND METHODS
Animals and treatment.
Wild-type and CYP2E1-humanized mice, established by insertion of the human CYP2E1 transgene into Cyp2e1-null mice on the C57BL/6J background (12), were housed in groups of three to five in plastic cages in a temperature- and light-controlled environment. Standard rodent chow and tap water were provided ad libitum. Animals were adapted to handling for an adaptation period of 1 wk prior to the experiment. All procedures were carried out in accordance with Institute of Laboratory Animal Resources guidelines and approved by the National Cancer Institute Animal Care and Use Committee.
Estrous cycle monitoring.
Intact cyclic female 6-wk-old wild-type mice were screened for their estrous cycle integrity for 2 wk. Only female mice with a normal estrous cycle were included in this study. They were divided into four groups according to the phase of the estrous cycle on the last day of the experiment. Intact male mice of the same age were also included. Estrous cycle phase was monitored by analysis of the cell types in vaginal lavages collected from all cyclic female mice. Smears were obtained daily between 1100 and 1300 for at least 15 consecutive days. The fire-polished and shortened tip of a Pasteur pipette carrying one drop of tap water was placed at the vaginal orifice, and care was taken not to insert it more than 1 mm to minimize the possibility of cervical stimulation and thus disruption of the cyclicity of the estrous cycle (48). Vaginal smears were spread gently on a microscope slide and allowed to dry. Slides were then fixed with absolute methanol (3 min), drained, and stained with Giemsa solution (2%, Merck) for at least 20 min. Staining is essential for the accurate identification of the cycle stage: cytoplasm stains blue and nuclei stain red. Identification of cell types was made microscopically according to published methods (83). Proesrus (PE), estrus (E), methestrus (ME), and diestrus (DE) are the four distinct phases of the estrous cycle. Persistent DE or E and cycles lasted longer than 5 days were considered abnormal, and animals demonstrating abnormal estrous cycles were excluded.
Ovariectomy and hormonal supplementation.
Intact cyclic female 5-wk-old wild-type mice (C57BL/6J) were bilaterally ovariectomized under gas anesthesia and implanted with pellets containing either 17β-estradiol or progesterone (3-wk release 17β-estradiol or progesterone pellets; Innovative Research of America, Sarasota, FL). Steroid hormone containing pellets released either ∼25 μg of 17β-estradiol or 425 μg of progesterone per day, levels close to those observed in intact cyclic female mice (48). The operation lasted 15–20 min, and the animals were returned to their cages after recovery, where they remained until the end of the experiment, 21 days later. Sham-operated cyclic female rats and those implanted with a placebo pellet at estrous were used as controls.
Anesthesia technique.
Anesthesia was performed using isoflurane (IsoFlo, Abbott Park, IL) in spontaneously breathing mice through a vaporizer providing standardized gas concentration to an outlet tube. Isoflurane was administered at a constant flow rate of 1.0–2.0 l/min with oxygen at a concentration of 30–35%. The animals were placed in an anesthesia induction box of 20 cm diameter and 10 cm height. A silicon tube providing the anesthetic gas mixture was connected. Anesthesia was induced by inhalation of 4% vaporized isoflurane. This led to a rapid induction of anesthesia within 15–30 s, allowing the animals to be placed in a prone position. It also allowed the type of inhalation to be changed to a tube of about 1.5 cm diameter surrounding the head and to reduce the isoflurane vaporization to 3.0–3.5%. This concentration provided deep anesthesia, allowing the surgical procedure to be performed without any clinical sign of pain or changes of macrohemodynamic parameters [mean arterial pressure (MAP) and heart rate (HR)]. This consisted of the following: no movements of the animal, no reaction to pain stimuli during surgical procedures (foot pad reaction), and stable blood pressure, HR, and respiratory rate. Under these conditions, anesthesia was maintained until the end of the operation. Buprenorphine HCl (0.25 mg/kg sc; Bedford Labs) was given after the final incision closure, and the animal was placed on a heating pad and allowed to recover before returning to its home cage. Locomotor activity, daily food and water consumption, and body weight progression showed no abnormalities 24 h after anesthesia.
Tamoxifen treatment.
Intact cyclic 8-wk-old wild-type C57BL/6J female mice were treated with tamoxifen (2 mg/kg ip) for 3 days. The mice were killed 24 h after the last dose. Controls received normal saline for 3 days and were euthanized at estrus. All mice used in this study were euthanized by carbon oxide asphyxiation.
Assessment of hepatic p-nitrophenol hydroxylase activity.
Microsomal fractions were prepared from parts of the liver dissected from individual mice by applying differential centrifugation (39). Microsomal protein content was determined by the method of Lowry et al. (42). For enzyme activity, CYP2E1-dependent p-nitrophenol hydroxylase (PNP) activity was determined spectrophotometrically by measuring the concentration of 4-nitrocatechol in 1 mg/ml microsomal protein. This compound is the metabolic product of p-nitrophenol, which was used as a substrate for CYP2E1 (53).
Quantitative real-time PCR.
For the isolation of total RNA from livers, TRIzol reagent (Invitrogen, Carlsbad, CA) was used following the manufacturer's protocol. The concentration of total RNA was determined spectrophotometrically. Quantitative real-time reverse transcriptase PCR (qPCR) was performed with cDNA generated from 1 μg of total RNA with a SuperScript III reverse transcriptase kit (Invitrogen). Gene-specific primers were designed for qPCR using the Primer Express software (Applied Biosystems, Foster City, CA). The sequences for the forward and reverse primers used are shown in Table 1. SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK) was used for the real-time reactions, which were carried out using the ABI Prism 7900 HT sequence detection system (Applied Biosystems). The PCR conditions were the following: 95°C for 10 min followed by 40 cycles of 95°C for 15 s, 60°C for 1 min, and finally one cycle of 95°C for 15 s, 60°C for 15 s, and 95°C for 15 s. Relative mRNA expression levels were normalized to β-actin, and values were quantified using the comparative threshold cycle method.
Table 1.
Forward and reverse oligonucleotide sequences used for q-PCR analysis
| Gene | Sequences |
|---|---|
| Cyp2e1 | (F) 5′-CCT GGT GGA GGA GCT CAA AA-3′ |
| (R) 5′-TGT TGA AGA GAA TAT CCG CAA TGA-3′ | |
| Hnf-1α | (F) 5′ GAC TTG ACC ATC TTC GCC AC 3′ |
| (R) 5′-CTG AAA GAG CCG GAG AAC CT-3′ | |
| β-catenin | (F) 5′-ACT GGC CTC TGA TAA AGG CAA CT-3′ |
| (R) 5′-TAG TCG TGG AAT AGC ACC CTG TT-3′ | |
| Srebp-1c | (F) 5′-AAG CAA ATC ACT GAA GGA CCT GG-3′ |
| (R) 5′-AAA GAC AAG GGG CTA CTC TGG GAG-3′ | |
| Pgc-1α | (F) 5′-TGT AGC GAC CAA TCG GAA AT-3′ |
| (R) 5′-TGA GGA CCG CTA GCA AGT TT-3′ | |
| eNos | (F) 5′-GCAGAAGAGTCCAGCGAACA-3′ |
| (R) 5′-GGCAGCCAAACACCAAAGTC-3 | |
| iNos | (F) 5′-GTG TTC CAC CAG GAG ATGTTG-3′ |
| (R) 5′-CTC CTG CCC ACT GAG TTC GTC-3′ | |
| β-actin | (F) 5′-TAT TGG CAA CGA GCG GTT CC-3′ |
| (R) 5′-GGC ATA GAG GTC TTT ACG GAT GTC-3′ |
Western blot analysis.
Immunoblot analyses of CYP2E1, total and phosphorylated-Akt, JNK, STAT5b, and FOXO1 apoprotein were carried out using microsomes, total cellular proteins, cytosol, or nuclear extracts of liver samples, respectively. RIPA buffer supplemented with protease inhibitors, PMSF (10 μM), BGP (50 μM), and NaF (50 μM) was used for the extraction of liver total cellular proteins. For the preparation of the nuclear extracts and cytosol, the NE-PER nuclear extraction kit (Pierce, Rockford, IL) was used. Protein concentration was determined in the samples using the BCA protein assay method (Pierce). Proteins were subjected to sodium dodecyl sulfate-polyacrylamide (7%) gel electrophoresis and immunoblotting using the following antibodies: mouse monoclonal CYP2E1 antibody (Cell Signaling Technology), rabbit monoclonal p-Akt (Ser473), and total-Akt antibodies (Cell Signaling Technology), mouse monoclonal total STAT5a/b IgG (Santa Cruz Biotechnology), and rabbit monoclonal p-STAT5b IgG (Tyr694; Cell Signaling Technology), rabbit polyclonal total JNK2 IgG, mouse monoclonal p-JNK IgG (Thr183 and Tyr185; Santa Cruz Biotechnology), rabbit polyclonal p-FOXO1 (Ser256) and total FOXO1 IgGs (Santa Cruz Biotechnology). Mouse monoclonal GAPDH IgG (Santa Cruz Biotechnology) was used as loading control. Secondary anti-rabbit or anti-mouse antibodies conjugated to horseradish peroxidase (Santa Cruz Biotechnology) were used, and the proteins were detected using a chemiluminescence detection kit (ECL, Amersham, GE Healthcare).
Hormonal determinations.
17β-Estradiol, the major estrogen secreted by premenauposal ovaries, and progesterone concentrations were determined in the plasma of intact and tamoxifen-treated female mice at estrus and in those mice subjected to bilateral ovariectomy and receiving hormonal replacement therapy. For determinations of estradiol and progesterone levels, an EIA corresponding kit (Cayman Chemicals) was used.
Statistical analysis.
The data are presented as means ± SE and were analyzed using two-way ANOVA followed by multiple comparisons with Bonferroni's significant difference method. The significance level for all analyses was set at a probability of ≤0.05. Moreover, correlation statistical analysis was performed using Pearson's coefficient correlations to investigate possible correlations between alterations in the relative Cyp2e1 mRNA expression and those observed in several key regulators of this CYP, such as hepatocyte nuclear factor (Hnf)-1α, β-catenin, and the sterol-regulatory element-binding protein-1c (Srebp-1c) following ovariectomy or hormonal replacement.
RESULTS
Assessment of sex differentiation in Cyp2e1 expression.
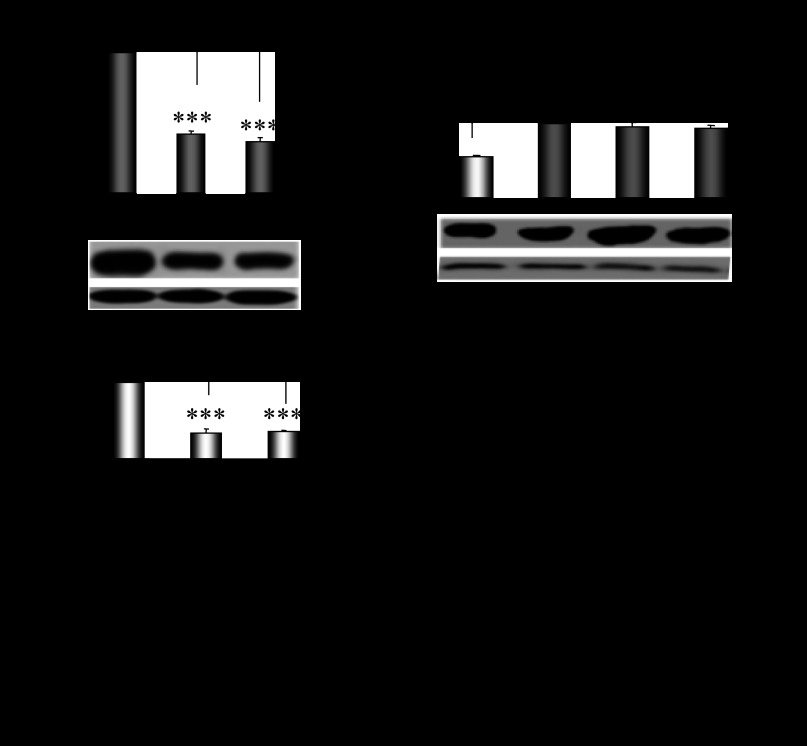
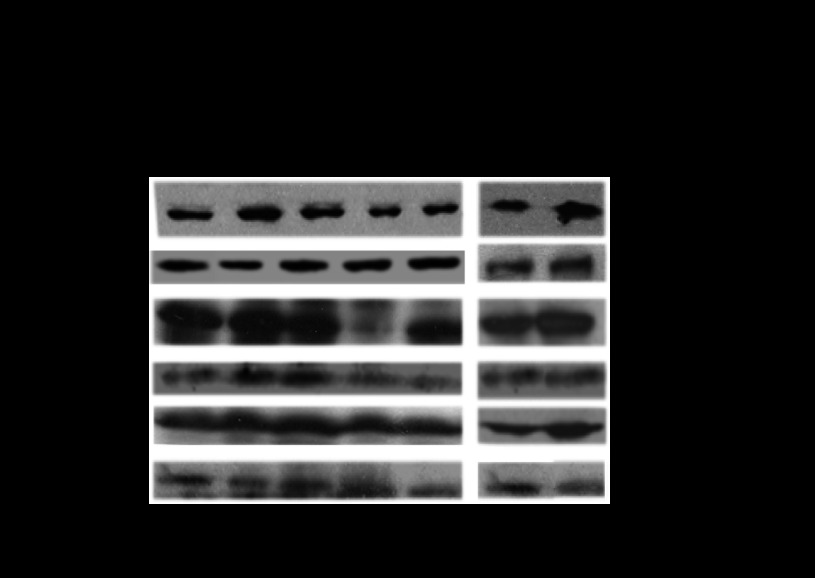
The expression profile of Cyp2e1 mRNA was evaluated in the liver of male and cyclic female mice. The hepatic expression of Cyp2e1 mRNA, protein, and activity levels were found to fluctuate within the different phases of the estrous cycle of female mice with higher expression at E and markedly lower expression at ME (Fig. 1A, P < 0.001). Pearson's coefficient correlation analysis indicated that the hepatic Cyp2e1 expression pattern in the distinct phases of the estrous cycle was highly correlated with the expression pattern of mRNAs encoding Hnf-1α (Fig. 1B, P < 0.001), β-catenin (Fig. 1C, P < 0.001) and Srebp-1c (Fig. 1D, P < 0.001), transcription factors previously found to be involved in the regulation of Cyp2e1. Hepatic Cyp2e1 mRNA, protein, and activity levels were markedly lower in males than in cyclic females at E (Fig. 2A, P < 0.001) and were at equivalent levels with those observed in cyclic females at ME and in ovariectomized mice (Figs. 1A and 2A). These data clearly indicate differentiation in the hepatic Cyp2e1 expression patterns between male and female mice. In addition, the fluctuation of Cyp2e1 expression within the different phases of the estrous cycle in females is also apparent.
Fig. 1.
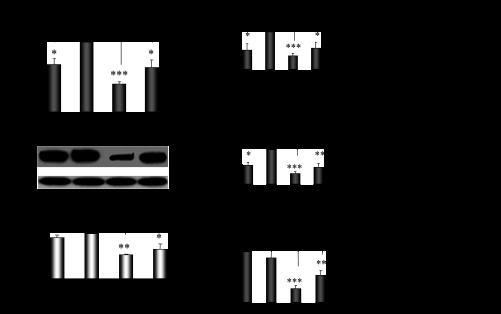
Differentiation in Cyp2e1, Hnf-1α, β-catenin, and Srebp-1c expression patterns within the different phases of the estrous cycle. A: fluctuations in Cyp2e1 expression within the distinct phases of the estrous cycle: proestrus (PE), estrus (E), methestrus (ME), diestrus (DE). All comparisons took place with basal Cyp2e1 mRNA levels at E. Values are expressed as means ± SE (n = 10). B–D: fluctuations in Hnf-1α, β-catenin, and Srebp-1c expression within the distinct phases of the estrous cycle and correlation of these changes with those in relative Cyp2e1 mRNA levels. CYP2E1-dependent p-nitrophenol hydroxylase (PNP) activity. *P < 0.05, **P < 0.01, ***P < 0.001. CYP2E1 Western blot images contain 1 sample per treatment. Each lane in the capture represents Cyp2e1 protein amount in the microsomes of 1 mouse.
Fig. 2.
Sex differentiation in hepatic Cyp2e1 expression pattern. A: Cyp2e1 mRNA, protein and activity level in cyclic females at E in ovariectomized (OV) and male mice. Comparisons took place with basal Cyp2e1 mRNA levels at E. CYP2E1-dependent PNP activity. B: effect of hormonal replacement with 17β-estradiol (E2) and/or progesterone on Cyp2e1 expression. Comparisons took place with basal Cyp2e1 mRNA levels in OV mice. Values are expressed as means ± SE (n = 10). CYP2E1 Western blot images contain 1 sample per treatment. Each lane in the capture represents CYP2E1 protein amount in the microsomes of 1 mouse. C: Correlation of changes in relative Cyp2e1 mRNA levels with plasma progesterone and E2 levels. **P < 0.01, ***P < 0.001.
Assessment of the role of female sex steroid hormones in Cyp2e1 regulation.
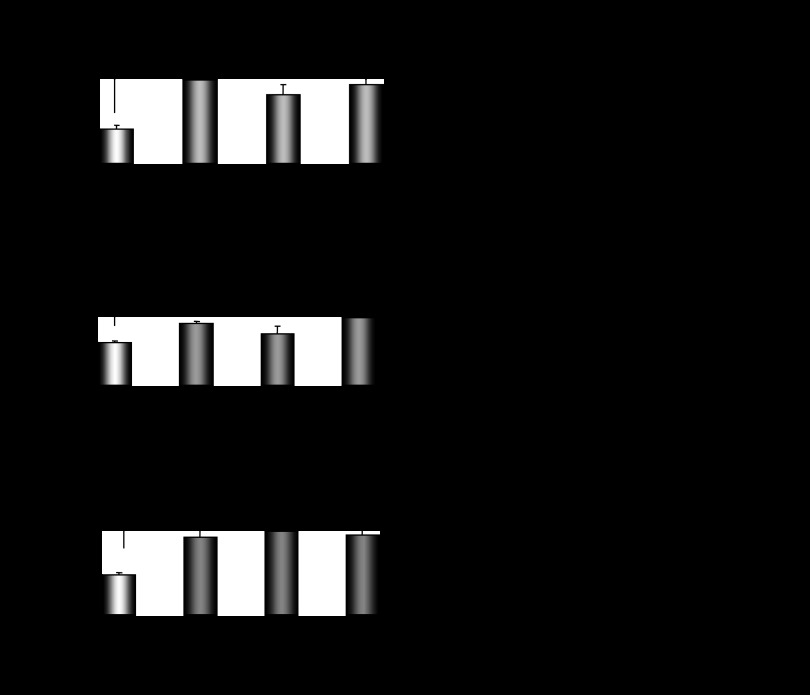
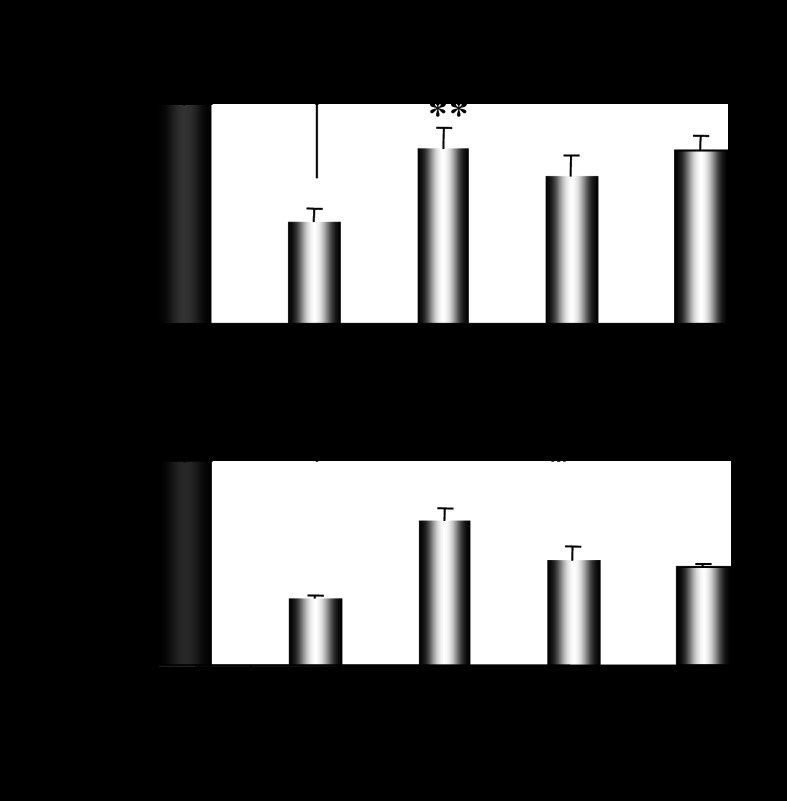
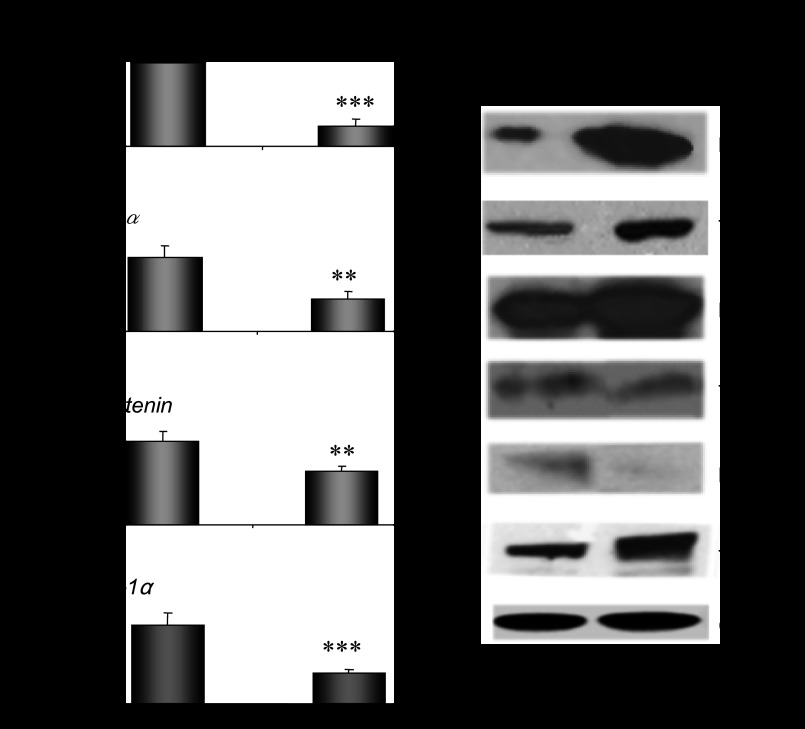
Ovariectomy-induced depletion of estrogens and progesterone was followed by a strong suppression of hepatic Cyp2e1 mRNA, protein, and activity levels compared with sham-operated females at E (Fig. 2A, P < 0.001). When ovariectomized mice were supplemented with pellets carrying 17β-estradiol, hepatic Cyp2e1 mRNA levels increased (Fig. 2B, P < 0.001), but this increase was not followed by increased CYP2E1 apoprotein and activity levels (Fig. 2B). When ovariectomized mice were implanted with progesterone pellets, hepatic Cyp2e1 mRNA, protein, and activity levels were increased (Fig. 2B, P < 0.001). Similarly, ovariectomized mice supplemented with both 17β-estradiol and progesterone had increased hepatic Cyp2e1 mRNA, protein, and activity levels (Fig. 2B, P < 0.001) compared with those receiving the placebo treatment. It is of interest to note that alterations in relative Cyp2e1 mRNA expression, which were induced by ovariectomy and/or hormonal replacement using 17β-estradiol and/or progesterone, were correlated with plasma steroid hormone levels (Fig. 2, C and D). These alterations in hepatic Cyp2e1 mRNA expression were also highly correlated with Hnf-1α (Fig. 3A, P < 0.001), β-catenin (Fig. 3B, P < 0.001), and to a lesser extent Srebp-1c (Fig. 3C, P < 0.01) mRNA levels. Pgc-1α mRNA transcripts were also increased by hormonal replacement with either 17β-estradiol and/or progesterone in ovariectomized mice compared with placebo-treated castrated mice (Fig. 4, P < 0.001).
Fig. 3.
Assessment of impact of hormonal replacement on Cyp2e1 expression. Effect of hormonal replacement with E2 and/or progesterone on Hnf-1α (A), β-catenin (B), and Srebp-1c (C) expression and correlation of changes in relative Hnf-1α, β-catenin, and Srebp-1c mRNA levels with those in relative Cyp2e1 mRNA levels. All comparisons took place with basal mRNA levels in OV mice. Values are expressed as means ± SE (n = 10). **P < 0.01, ***P < 0.001.
Fig. 4.
Effect of hormonal replacement with E2 and/or progesterone on Pgc-1α mRNA expression. Comparison took place with basal Pgc-1α mRNA levels in OV mice. ***P < 0.001.
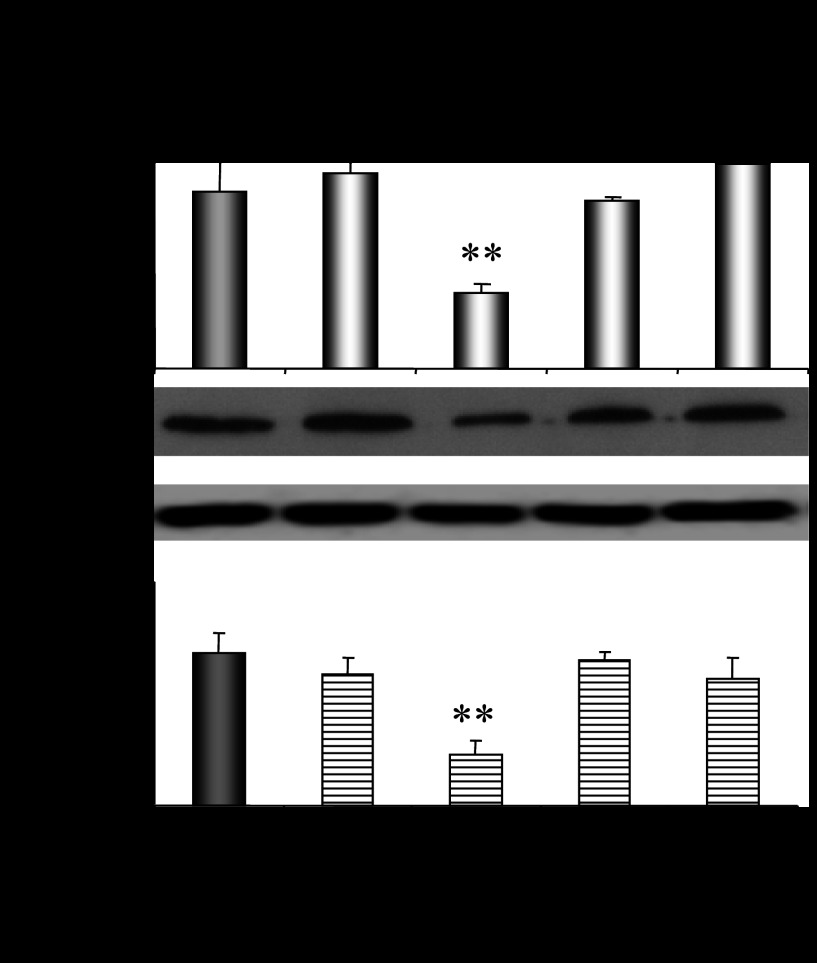
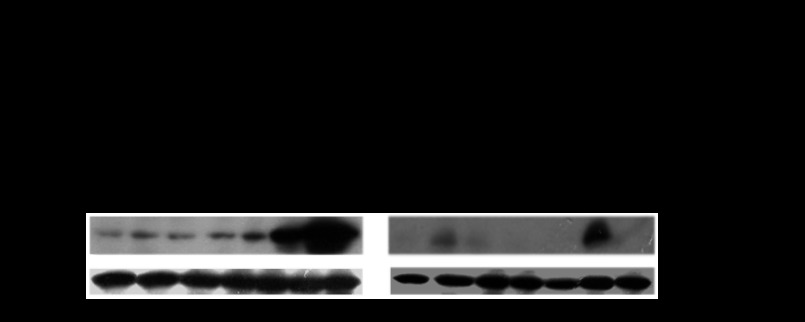
Ovariectomy had no effect on CYP2E1 mRNA levels in the livers of a CYP2E1-humanized mouse model. It should be noted that supplementation with 17β-estradiol repressed hepatic CYP2E1 expression in ovariectomized CYP2E1-humanized mice (Fig. 5, P < 0.01), whereas progesterone supplementation had no effect (Fig. 5).
Fig. 5.
Effect of hormonal replacement with E2 and/or progesterone on CYP2E1 expression in humanized CYP2E1 mice. CYP2E1 mRNA and activity levels are expressed as means ± SE (n = 10). Comparisons took place with basal CYP2E1 mRNA or PNP activity levels in OV mice, respectively. **P < 0.01. CYP2E1 Western blot images contain 1 sample per treatment. Each lane in the capture represents CYP2E1 protein amount in the microsomes of 1 transgenic mouse.
These data indicate the determinant role of sex steroid hormones in hepatic Cyp2e1 regulation, with the role of progesterone being more prominent than that of estradiol. A phenotype in hepatic CYP2E1 regulation by sex steroid hormones is also apparent between wild-type and CYP2E1-humanized mice.
Assessment of the involvement of major signal transduction pathways in steroid hormone-induced regulation of Cyp2e1.
The insulin controlled PI3K/Akt signaling pathway holds a critical role in Cyp2e1 regulation (29, 51, 62, 77). To assess the involvement of this signaling pathway in the sex steroid hormone-mediated Cyp2e1 regulation, the phosphorylated state of Akt after ovariectomy and treatment with 17β-estradiol and/or progesterone was assessed by Western blot using specific antibodies. Ovariectomy increased hepatic Akt phosphorylation and hormonal supplementation normalized its levels close to those observed in sham-operated females at E (Fig. 6). In the male liver, activation of Akt was higher than in the liver of intact females at E (Fig. 6). No significant change in FOXO1 phosphorylation was detected following ovariectomy, but it was markedly suppressed in the nucleus of ovariectomized mice supplemented with progesterone (Fig. 6). In males, FOXO1 activation in the nucleus and cytoplasm was higher than in females at E (Fig. 6).
Fig. 6.
Assessment of impact of female steroid hormones on PI3K/Akt/FOXO1 signaling pathway. Western blot analysis of Akt and FOXO1 phosphorylation (p) in hepatic nuclear, cytosolic, and total (t) cellular proteins of male mice, cyclic females at E, and OV mice that received placebo or hormonal replacement using pellets containing estradiol and/or progesterone. Western blot images contain 1 sample per treatment, which is representative of 3 samples per group tested. Each lane in the capture represents total or phosphorylated Akt or FOXO1 in total cellular, nuclear, or cytosolic proteins of 1 mouse, respectively.
It is well documented that the endothelial (eNOS)- and interfering (iNOS)-stimulated nitric oxide (NO) production is followed by a downregulation of several CYP genes including CYP2E1 (21, 31, 44). The present data indicated that eNos and iNos expression was markedly repressed in ovariectomized mice compared with intact mice at E and that hormone replacement with either 17β-estradiol and/or progesterone restored eNos and iNos expression (Fig. 7).
Fig. 7.
Effect of hormonal replacement with E2 and/or progesterone on eNos (A) and iNos (B) mRNA expression. Values are expressed as means ± SE (n = 10). All comparisons took place with basal mRNA levels in OV mice. *P < 0.05, **P < 0.01, ***P < 0.001.
GH and the GH pulse-activated transcription factor STAT5b (27) play a major role in the regulation of several CYP genes (75). It has been also reported that there is cross-talk between STAT5b and estrogen receptor (ER)α and ERβ-related pathways (7). Interestingly, STAT5b activation was detected at very low levels in the nucleus of sham and ovariectomized females compared with males (Fig. 8). Tamoxifen markedly increased STAT5b phosphorylation (Fig. 8).
Fig. 8.
Assessment of impact of female steroid hormones and tamoxifen on GH/STAT5b signaling pathway. Western blot analysis of STAT5b phosphorylation in hepatic nuclear and cytosolic proteins of male mice, cyclic females at E, treated with normal saline or tamoxifen, and OV mice that received placebo or hormonal replacement using pellets containing estradiol and/or progesterone. Western blot images contain 1 sample per treatment, which is representative of 3 samples per group tested. Each lane represents total or phosphorylated STAT5b in nuclear or cytosolic proteins of 1 mouse.
Based on these findings, it appears that the sex steroid hormone-mediated modification of Cyp2e1 regulation in female mice potentially involves the insulin/PI3K/Akt signaling pathway.
Assessment of the role of tamoxifen in Cyp2e1 regulation.
Treatment of intact females with tamoxifen reduced Cyp2e1 mRNA transcripts in the liver (Fig. 9A, P < 0.001). A similar effect of tamoxifen was observed on Hnf-1α, β-catenin, and Pgc-1α mRNAs (Fig. 9A, P < 0.01, P < 0.01, and P < 0.001, respectively). Tamoxifen also strongly increased Akt phosphorylation (Fig. 9B), which in turn stimulated FOXO1 activation (Fig. 9B). The drug also markedly decreased JNK phosphorylation (Fig. 9B). Although there is no direct evidence, the above data profoundly indicate that tamoxifen repressed Cyp2e1 expression by mechanisms involving activation of the PI3K/Akt/FOXO1 and GH/STAT5b-linked pathways.
Fig. 9.
Assessment of effect of tamoxifen on Cyp2e1 expression and PI3K/Akt/FOXO1 activation. A: effect of tamoxifen on Cyp2e1, Hnf-1α, β-catenin, and Pgc-1α mRNA expression. Values are expressed as means ± SE (n = 10). **P < 0.01, ***P < 0.001. B: assessment of tamoxifen-induced effect on Akt, FOXO1, and JNK activation by Western blot. Western blot images contain 1 sample per treatment, which is representative of 3 samples per group tested. Each lane in the capture represents total or phosphorylated Akt, FOXO1, or JNK in total cellular proteins of 1 mouse.
Assessement of hormonal replacement in ovariectomized mice.
The levels of 17β-estradiol and progesterone in sham-operated female mice at E were higher than those observed in ovariectomized placebo-treated mice (Table 2, P < 0.05 and P < 0.01, respectively). As expected, implantation of pellets carrying either 17β-estradiol and/or progesterone in bilaterally ovariectomized mice markedly supplemented the corresponding hormonal levels (Table 2, P < 0.001 and P < 0.01, respectively). It is worth noting that plasma progesterone levels in ovariectomized mice implanted with pellets carrying progesterone were equivalent to those detected in females at E (Table 2). It should be noted that plasma 17β-estradiol levels were many fold higher in ovariectomized mice supplemented with estradiol than in intact females at E (Table 2, P < 0.001). Tamoxifen treatment of cyclic females was followed by decreased serum progesterone levels compared with controls at E, whereas serum 17β-estradiol concentration was not significantly affected (Table 2, P < 0.01).
Table 2.
Plasma steroid hormone levels in C57BL/6J female mice
| Treatment | 17β-Estradiol | Progesterone |
|---|---|---|
| Sham at Estrus | 6.4 ± 0.6* | 5,151.5 ± 888.6† |
| Ovariectomized | ||
| Placebo | 3.9 ± 0.2 | 554.4 ± 79.5 |
| 17β-Estradiol | 88.7 ± 14.6 ‡ | 3,019.1 ± 866.2† |
| Progesterone | 7.7 ± 2.0 | 3,511.6 ± 361.5‡ |
| 17β-Estradiol + progesterone | 56.0 ± 8.3‡ | 5,607.3 ± 379.8‡ |
| Control at Estrus | 11.7 ± 3.5 | 4,155.0 ± 262.0 |
| Tamoxifen | 16.8 ± 5.8 | 1,697.5 ± 111.7† |
Values are expressed as means ± SE in pg/ml (n=10). Assessment of the effect of castration and hormonal replacement on plasma sex steroid levels. Plasma sex steroid hormone concentration in C57BL/6J female mice at estrus or following ovariectomy and hormonal replacement with 17β-estradiol and/or progesterone.
P < 0.05, † P < 0.01,‡P < 0.001.
DISCUSSION
The multifactorial and complex process of CYP2E1 regulation has been demonstrated by previous studies (22, 29, 37; Fig. 10) indicating that diverse factors may modify CYP2E1 expression at the levels of transcription (2, 9), mRNA stabilization (65, 80), mRNA translation (32), protein synthesis (66), and protein degradation (54, 56). Among these factors, hormones hold a critical role (8, 56). Nonetheless, despite intense investigation, the sex differentiation and the role of female sex steroid hormones in constitutive CYP2E1 regulation remain unclear (59).
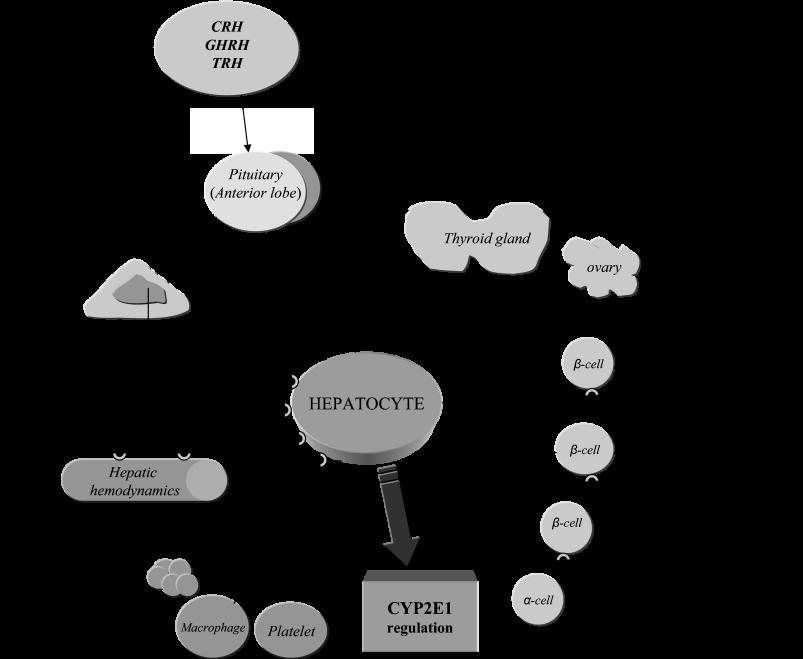
Fig. 10.
Regulation of CYP2E1 in the liver. Hormonal, cytokine-, and adrenoceptor (AR)-linked pathways involved in CYP2E1 regulation; β- and α-cells are located in pancreas. Stimulation of D2-receptors and α2-ARs, which are expressed on pancreatic β-cell membranes, restricts glucose-stimulated insulin release. The opposite is true following β2-AR stimulation (18, 53). Dashed lines depict inhibition.
Interestingly, the data of the present study revealed differential hepatic Cyp2e1 expression patterns within the distinct phases of the estrous cycle of female mice, with lower expression at methestrus and higher at estrus. Hepatic Cyp2e1 expression was markedly lower in males than in females at estrus, whereas depletion of female sex steroid hormones by ovariectomy (hormonal state similar to menopause) brought Cyp2e1 expression to levels observed in males and females at ME. When ovariectomized mice were supplemented with progesterone, Cyp2e1 expression approached that at estrus. Estrogens also increased hepatic Cyp2e1 mRNA expression in ovariectomized mice, whereas tamoxifen, an antiestrogenic agent, markedly repressed the expression of Cyp2e1 in the livers of intact female mice. This finding is in line with that of a previous study reporting that the antiestrogen toremifene alleviated ethanol induction of CYP2E1 (30). It is well established that tamoxifen competitively binds to ERs on tumors and other tissue targets, producing a nuclear complex that inhibits estrogen effects. Specifically, the ER/tamoxifen complex recruits corepressors to stop genes being switched on by estrogens (43, 46), a fact that may explain, at least in part, the suppressive effect of tamoxifen on Cyp2e1 regulation. The tamoxifen-induced repression in Cyp2e1 may also be attributed to the lower serum progesterone levels detected in drug-treated mice compared with controls. This downregulating process potentially involves several major signal transduction pathways, including PI3K/Akt/FOXO1, GH/STAT5b, and related pathways, among others. Nonetheless, further investigation is needed to directly correlate the alterations in the aforementioned pathways with those related with the sex steroids on Cyp2e1.
It is of interest to note that ovariectomy had no effect on CYP2E1 expression in the livers of mice carrying the human gene, whereas 17β-estradiol markedly downregulated CYP2E1 expression in the ovariectomized CYP2E1-humanized mice. Progesterone alone had no effect, but it blocked the downregulating effect of 17β-estradiol on CYP2E1 when castrated mice were treated with both hormones. This effect is potentially associated with the fact that progesterone acts as an antagonist of estradiol on ER gene expression (78). These differential effects on hepatic Cyp2e1 regulation, which were observed in wild-type C57BL/6J and CYP2E1-humanized mice, revealed the existence of a phenotype in the regulation of CYP2E1 expression by female sex steroid hormones. However, this experimental setting could not be used to pinpoint any differential CYP2E1 expression between rodents and humans.
It is well documented that Cyp2e1 expression is controlled by the liver-enriched homeodomain-containing transcription factor Hnf-1α (2). Nonetheless, recent reports revealed that factors other than Hnf-1α are also critical in the regulation of Cyp2e1, including β-catenin (52, 60, 68), which potentially modifies the expression of a transcriptional coactivator essential for Cyp2e1 activation by Hnf-1α or a micro-RNA destabilizing Cyp2e1 mRNA. It is also possible that β-catenin modifies Cyp2e1 expression at the translation level (22, 40). The data of the present study suggest a critical role for these two factors in the regulation of Cyp2e1, as all differences observed in the expression pattern of this CYP, those related to sex, ovariectomy, sex steroid hormones, or tamoxifen were highly correlated with Hnf-1α and β-catenin mRNA expression. It is possible that β-catenin, the main effector of the Wnt pathway, acts as a potent transcriptional coactivator of androgen (AR), estrogen (ER) and progesterone (PR) receptors (38, 47). This hypothesis is supported by a previous study using immunoprecipitation (ChIP) analysis, which revealed that Wnt and estrogen signaling pathways cross-talk in vivo through functional interaction between ERα and β-catenin (38, 47).
Insulin signaling holds a critical role in the transcriptional and posttranscriptional regulation of CYP2E1. In particular, the insulin-induced downregulation of CYP2E1 suggests involvement of phosphatidylinositol 3-kinase (PI3K) and a variety of downstream enzymes. In this regulatory pathway, a major intermediate effector is the PI3K-activated serine/threonine kinase Akt/PKB (51, 77). The present findings revealed that ovariectomy increased Akt phosphorylation, whereas supplementation with progesterone and/or estrogens brought phosphorylation levels back to those detected in females at estrus. Akt phosphorylation was also higher in males than in females at estrus and tamoxifen strongly increased it. These findings indicate that cross-talk between the sex steroid hormone receptors and insulin/PI3K/Akt signaling potentially exists (58) that may be involved in the regulation of Cyp2e1. It appears that predominantly progesterone inactivates several downstream elements in the PI3K/Akt/FOXO1 signaling pathway, an effect that was blocked by estradiol. In this pathway, SREBP-1c regulates various genes including CYP2E1 (1, 35, 84). Srebp-1c mRNA levels correlated with the fluctuations of Cyp2e1 mRNA expression in the distinct phases of the estrous cycle and the changes induced by ovariectomy, sex steroid hormones, and tamoxifen.
GH is a major endocrine factor modulating Cyp2e1 expression in the liver (11, 28, 42, 71, 76, 77, 80), with a determinant role in the sexual dimorphism observed in the expression of numerous genes (45, 74, 76). GH binds to the GH receptor on the cell membrane and activates signaling pathways including that by the GH pulse-activated transcription factor STAT5b (27), which is essential for the liver sexual dimorphism (14). The present study confirmed that, in the female liver, STAT5b activation is generally low compared with that in males (13) and is potentially connected, at least in part, with the lower Cyp2e1 expression levels detected in the male liver compared with that in intact females at E.
In the wide array of factors interfering in the regulation of various CYPs, NO, an important inflammatory mediator synthesized by both NO synthases, eNOS and iNOS, is recogniszed as an important regulator. However, in the present experimental setting, alterations in hepatic eNos and iNos mRNA expression followed ovariectomy or hormonal replacement, repression, and upregulation, respectively, appear not to be connected with those in Cyp2e1 expression, as both NOS isozymes are connected with CYP downregulation (21, 31, 44).
It is possible that transcription factors other than those mentioned above play critical roles as determinants of sexual dimorphism in Cyp2e1 regulation, and their actions are probably influenced by sex steroid hormones.
Taken together, the above data clearly indicated a role for female sex steroid hormones in the upregulation of Cyp2e1 expression in the liver of female mice. This effect is potentially mediated by inactivation of the insulin/PI3K/Akt signaling pathway. This study also confirmed a sex differentiation in the hepatic expression of Cyp2e1, with higher levels detected in intact cyclic females at E and lower in males at levels close to those detected in estrogen- and progesterone-depleted female mice (ovariectomized) and in cyclic females at ME. Notably, variation in the expression patterns of Cyp2e1 within the distinct phases of the estrous cycle is highly correlated with the fluctuations in the levels of gonadal hormones in plasma, 17β-estradiol and progesterone. On the basis of these findings and previous reports (73, 75), the present study suggests that differentiation in hepatic Cyp2e1 expression pattern between females and males is potentially mediated by the GH/STAT5b-related signaling pathway. The present data contribute to a better understanding of the selectivity that governs the CYP2E1-induced transformation of molecules in females and males. This knowledge will allow more successful predictions in pharmaco- and toxicokinetic properties of the CYP2E1 substrates, thus providing insights exploitable in novel drug development and assessment of risk associated with exposure to drugs, environmental chemicals, and procarcinogens. It also provides information about potential perturbations of endogenous regulatory circuits with associated pathophysiological consequences (72).
GRANTS
This study was supported in part by the National Cancer Institute Intramural program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.K. conception and design of research; M.K. and J.C. performed experiments; M.K. analyzed data; M.K. and F.J.G. interpreted results of experiments; M.K. prepared figures; M.K. drafted manuscript; M.K., J.C., and F.J.G. edited and revised manuscript; M.K., J.C., and F.J.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank John Buckley for valuable technical assistance.
REFERENCES
- 1.Assaf S, Hazard D, Pitel F, Morisson M, Alizadeh M, Gondret F, Diot C, Vignal A, Douaire M, Lagarrigue S. Cloning of cDNA encoding the nuclear form of chicken sterol response element binding protein-2 (SREBP-2), chromosomal localization, and tissue expression of chicken SREBP-1 and -2 genes. Poult Sci 82: 54–61, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Akiyama TE, Gonzalez FJ. Regulation of P450 genes by liver-enriched transcription factors and nuclear receptors. Biochim Biophys Acta 1619: 223–234, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Banerjee S, Shang TQ, Wilson AM, Moore AL, Strand SE, Gordon MP, Doty SL. Expression of functional mammalian P450 2E1 in hairy root cultures. Biotechnol Bioeng 77: 462–466, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Baynes JW, Dominiczak MH. Medical Biochemistry (2nd ed). Philadelphia, PA: Elsevier Mosby, p. 555, 2005 [Google Scholar]
- 5.Bebia Z, Buch SC, Wilson JW, Frye RF, Romkes M, Cecchetti A, Chaves-Gnecco D, Branch RA. Bioequivalence revisited: influence of age and sex on CYP enzymes. Clin Pharmacol Ther 76: 618–627, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Bellward GD, Chang T, Rodrigues B, McNeill JH, Maines S, Ryan DE, Levin W, Thomas PE. Hepatic cytochrome P-450j induction in the spontaneously diabetic BB rat. Mol Pharmacol 33: 140–143, 1988 [PubMed] [Google Scholar]
- 7.Bjornstrom L, Killic E, Norman M, Parker MG, Sjoberg M. Cross-talk between Stat5b and estrogen receptor-alpha and beta in mammary epithelial cells. J Mol Endocrinol 27: 93–106, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Bruckner JV, Ramanathan R, Lee KM, Muralidhara S. Mechanisms of circadian rhythmicity of carbon tetrachloride hepatotoxicity. J Pharmacol Exp Ther 300: 273–281, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Butura A, Nilsson K, Morgan K, Morgan TR, French SW, Johansson I, Schuppe-Koistinen I, Ingelman-Sundberg M. The impact of CYP2E1 on the development of alcoholic liver disease as studied in a transgenic mouse model. J Hepatol 50: 572–583, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Cederbaum AI. Cytochrome P450 2E1-dependent oxidant stress and upregulation of anti-oxidant defense in liver cells. J Gastroenterol Hepatol 21, Suppl 3: S22–S25, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Chen GF, Ronis MJ, Ingelman-Sundberg M, Badger TM. Hormonal regulation of microsomal cytochrome P4502E1 and P450 reductase in rat liver and kidney. Xenobiotica 29: 437–451, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Cheung C, Yu AM, Ward JM, Krausz KW, Akiyama TE, Feigenbaum L, Gonzalez FJ. The Cyp2e1-humanized transgenic mouse: role of Cyp2e1 in acetaminophen hepatotoxicity. Drug Metab Dispos 33: 449–457, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Choi HK, Waxman DJ. Growth hormone, but not prolactin, maintains, low-level activation of STAT5a and STAT5b in female rat liver. Endocrinology 140: 5126–5135, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol 20: 1333–1351, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Collom SL, Laddusaw RM, Burch AM, Kuzmic P, Perry Jn MD, Miller GP. CYP2E1 substrate inhibition: mechanistic interpretation through an effector site for monocyclic compounds. J Biol Chem 283: 3487–3496, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng QG, She H, Cheng JH, French SW, Koop DR, Xiong S, Tsukamoto H. Steatohepatitis induced by intragastric overfeeding in mice. Hepatology 42: 905–914, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Dong ZG, Hong JY, Ma QA, Li DC, Bullock J, Gonzalez FJ, Park SS, Gelboin HV, Yang CS. Mechanism of induction of cytochrome P-450ac (P-450j) in chemically induced and spontaneously diabetic rats. Arch Biochem Biophys 263: 29–35, 1988 [DOI] [PubMed] [Google Scholar]
- 18.Doty SL, Shang TQ, Wilson AM, Tangen J, Westergreen AD, Newman LA, Strand SE, Gordon MP. Enhanced metabolism of halogenated hydrocarbons in transgenic plants containing mammalian cytochrome P450 2E1. Proc Natl Acad Sci USA 97: 6287–6291, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Mansoury AM, Morgan NG. Activation of protein kinase C modulates alpha2-adrenergic signalling in rat pancreatic islets. Cell Signal 10: 637–643, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Fata JE, Chaudhary V, Khokha R. Cellular turnover in the mammary gland is correlated with systemic levels of progesterone and not 17β-estradiol during the estrous cycle. Biol Reprod 65: 680–688, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Gergel D, Misik V, Riesz P, Cederbaum AI. Inhibition of rat and human cytochrome P4502E1 catalytic activity and reactive oxygen radical formation by nitric oxide. Arch Biochem Biophys 337: 239–250, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez FJ. The 2006 Bernard B Brodie Award Lecture. Cyp2e1. Drug Metab Dispos 35: 1–8, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Guengerich FP, Kim DH, Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol 4: 168–4179, 1991 [DOI] [PubMed] [Google Scholar]
- 24.Guengerich FP. Catalytic selectivity of human cytochrome P450 enzymes: relevance to drug metabolism and toxicity. Toxicol Lett 70: 133–138, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Hargreaves MB, Jones BC, Smith DA, Gescher A. Inhibition of p-nitrophenol hydroxylase in rat liver microsomes by small aromatic and heterocyclic molecules. Drug Metab Dispos 22: 806–810, 1994 [PubMed] [Google Scholar]
- 26.Hel Z, Stringer E, Mestecky J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocr Rev 31: 79–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holloway MG, Laz EV, Waxman DJ. Co-dependence of growth hormone-responsive, sexually dimorphic hepatic gene expression on signal transducer and activator of transcription 5b and hepatic nuclear factor 4-α. Mol Endocrinol 20: 647–660, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Hong JY, Ning SM, Ma BL, Lee MJ, Pan JM, Yang CS. Roles of pituitary hormones in the regulation of hepatic cytochrome P450IIE1 in rats and mice. Arch Biochem Biophys 281: 132–138, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Ingelman-Sundberg M. Pharmacogenetics of cytochrome P450 and its applications in drug therapy: the past, present and future. Trends Pharmacol Sci 25: 193–200, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Järveläinen HA, Fang C, Ingelman-Sundberg M, Lukkari TA, Sippel H, Lindros KO. Kupffer cell inactivation alleviates ethanol-induced steatosis and CYP2E1 induction but not inflammatory responses in rat liver. J Hepatol 32: 900–910, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Khatsenko O, Kikkawa Y. Nitric oxide differentially affects constitutive cytochrome P450 isoforms in rat liver. J Pharmacol Exp Ther 280: 1463–1470, 1997 [PubMed] [Google Scholar]
- 32.Kim SG, Novak RF. Cytochrome P450IIE1 metabolism of pyridines: evidence for production of a reactive intermediate which exhibits redox-cycling activity and causes DNA damage. Adv Exp Med Biol 283: 753–758, 1991 [DOI] [PubMed] [Google Scholar]
- 33.Kim RB, O'Shea D. Interindividual variability of chlorzoxazone 6-hydroxylation in men and women and its relationship to CYP2E1 genetic polymorphisms. Clin Pharmacol Ther 57: 645–655, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Kim YC, Yim HK, Jung YS, Park JH, Kim SY. Hepatic injury induces contrasting response in liver and kidney to chemicals that are metabolically activated: role of male sex hormone. Toxicol Appl Pharmacol 223: 56–65, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Kohjima M, Enjoji M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, Yada M, Yada R, Harada N, Takayanagi R, Nakamuta M. Re-evaluation of fatty acid metabolism-related gene expression in non-alcoholic fatty liver disease. Int J Mol Med 20: 351–358, 2007 [PubMed] [Google Scholar]
- 36.Koop DR. Hydroxylation of p-nitrophenol by rabbit ethanol-inducible cytochrome P-450 isozyme 3a. Mol Pharmacol 29: 399–404, 1986 [PubMed] [Google Scholar]
- 37.Konstandi M, Harkitis P, Kostakis D, Marselos M, Johnson EO, Lang MA. D2-receptor-linked signaling pathways regulate the expression of hepatic CYP2E1. Life Sci 82: 1–10, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Kouzmenko AP, Takeyama K, Ito S, Furutani T, Sawatsubashi S, Maki A, Suzuki E, Kawasaki Y, Akiyama T, Tabata T, Kato S. Wnt/beta-catenin and estrogen signaling converge in vivo. J Biol Chem 279: 40255–40258, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Lang MA, Gielen JE, Nebert DW. Genetic evidence for many unique liver microsomal P-450 mediated monoxygenase activities in heterogeneic stock mice. J Biol Chem 256: 12068–12075, 1981 [PubMed] [Google Scholar]
- 40.Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest 105: 1067–1075, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li L, He D, Wilborn TW, Falany JL, Falany CN. Increased SULT1E1 activity in HepG2 hepatocytes decreases growth hormone stimulation of STAT5b phosphorylation. Steroids 74: 20–29, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 193: 265–275, 1951 [PubMed] [Google Scholar]
- 43.Massarweh S, Osborne CK, Creighton CJ, Qin L, Tsimelzon A, Huang S, Weiss H, Rimawi M, Schiff R. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res 68: 826–833, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Minamiyama Y, Takemura S, Imaoka S, Funae Y, Tanimoto Y, Inoue M. Irreversible inhibition of cytochrome P450 by nitric oxide. J Pharmacol Exp Ther 283: 1479–1485, 1997 [PubMed] [Google Scholar]
- 45.Mode A, Gustafsson JA. Sex and the liver—a journey through five decades. Drug Metab Rev 38: 197–207, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Moreira PI, Custódio JB, Nunes E, Moreno A, Seiça R, Oliveira CR, Santos MS. Estradiol affects liver mitochondrial function in ovariectomized and tamoxifen-treated ovariectomized female rats. Toxicol Appl Pharmacol 221: 102–110, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Mulholland DJ, Dedhar S, Coetzee GA, Nelson CC. Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know? Endocr Rev 26: 898–915, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Nelson JF, Felicio LS, Osterburg HH, Finch CE. Altered profiles of estradiol and progesterone associated with prolonged estrous cycles and persistent vaginal cornification in aging C57BL/6J mice. Biol Reprod 24: 784–794, 1981 [DOI] [PubMed] [Google Scholar]
- 49.Nelson LE, Sheridan MA. Regulation of somatostatins and their receptors in fish. Gen Comp Endocrinol 142: 117–133, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Ohe T, Hirobe M, Mashino T. Novel metabolic pathway of estrone and 17β-estradiol catalysed by cytochrome P-450. Drug Metab Dispos 28: 110–112, 2000 [PubMed] [Google Scholar]
- 51.Peng HM, Coon MJ. Regulation of rabbit P4502E1 expression in HepG2 cells by insulin and thyroid hormone. Mol Pharmacol 54: 740–747, 1998 [PubMed] [Google Scholar]
- 52.Persad S, Troussard AA, McPhee TR, Mulholland DJ, Dedhar S. Tumor suppressor PTEN inhibits nuclear accumulation of beta-catenin and T cell/lymphoid enhancer factor 1-mediated transcriptional activation. J Cell Biol 153: 1161–1174, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reinke LA, Moyer MJ. p-Nitrophenol hydroxylation. A microsomal oxidation which is highly inducible by ethanol. Drug Metab Dispos 13: 548–552, 1985 [PubMed] [Google Scholar]
- 54.Roberts BJ, Song BJ, Soh Y, Park SS, Shoaf SE. Ethanol induces CYP2E1 by protein stabilization. Role of ubiquitin conjugation in the rapid degradation of CYP2E1. J Biol Chem 270: 29632–29635, 1995 [DOI] [PubMed] [Google Scholar]
- 55.Robertson G, Leclercq I, Farrell GC. Nonalcoholic steatosis and steatohepatitis. II. Cytochrome P-450 enzymes and oxidative stress. Am J Physiol Gastrointest Liver Physiol 281: G1135–G1139, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Ronis M, Lindros KO, Ingelman-Sundberg M : Cytochromes P450, Metabolic and Toxicological Aspects. Boca Raton, FL: CRC, 1996, p. 211–239 [Google Scholar]
- 57.Rubí B, Ljubicic S, Pournourmohammadi S, Carobbio S, Armanet M, Bartley C, Maechler P. Dopamine D2-like receptors are expressed in pancreatic beta cells and mediate inhibition of insulin secretion. J Biol Chem 280: 36824–36832, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, Burger HG, Colditz GA, Davis SR, Gambacciani M, Gower BA, Henderson VW, Jarjour WN, Karas RH, Kleerekoper M, Lobo RA, Manson JE, Marsden J, Martin KA, Martin L, Pinkerton JV, Rubinow DR, Teede H, Thiboutot DM, Utian Endocrine Society WH. J Postmenopausal hormone therapy: an Endocrine Society scientific statement. Clin Endocrinol Metab 95, Suppl 1: S1–S66, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scandlyn MJ, Stuart EC, Somers-Edgar TJ, Menzies AR, Rosengren RJ. A new role for tamoxifen in oestrogen receptor-negative breast cancer when it is combined with epigallocatechin gallate. Br J Cancer 99: 1056–1063, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sekine S, Lan BY, Bedolli M, Feng S, Hebrok M. Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatology 43: 817–825, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Sharma M, Chuang WW, Sun Z. Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3beta inhibition and nuclear beta-catenin accumulation. J Biol Chem 277: 30935–30941, 2002 [DOI] [PubMed] [Google Scholar]
- 62.Sidhu JS, Fei L, Boyle SM, Omiecinski CJ. PI3k inhibitors reverse the Suppressive actions of insulin on CYP2E1 expression by activating stress-response pathways in primary rat hepatocytes. Mol Pharmacol 59: 1138–1146, 2001 [DOI] [PubMed] [Google Scholar]
- 63.Song BJ, Matsunaga T, Hardwick JP, Park SS, Veech RL, Yang CS, Gelboin HV, Gonzalez FJ. Stabilization of cytochrome P450j messenger ribonucleic acid in the diabetic rat. Mol Endocrinol 1: 542–547, 1987 [DOI] [PubMed] [Google Scholar]
- 64.Song BJ, Veech RL, Saenger P. Cytochrome P450IIE1 is elevated in lymphocytes from poorly controlled insulin-dependent diabetics. J Clin Endocrinol Metab 71: 1036–1040, 1990 [DOI] [PubMed] [Google Scholar]
- 65.Thummel KE, Schenkman JB. Effects of testosterone and growth hormone treatment on hepatic microsomal P450 expression in the diabetic rat. Mol Pharmacol 37: 119–129, 1990 [PubMed] [Google Scholar]
- 66.Tsutsumi M, Matsuda Y, Takada A. Role of ethanol-inducible cytochrome P-450 2E1 in the development of hepatocellular carcinoma by the chemical carcinogen, N-nitrosodimethylamine. Hepatology 18: 1483–1489, 1993 [PubMed] [Google Scholar]
- 67.Veldhuis JD, Bowers CY. Sex-steroid modulation of growth hormone (GH) secretory control: three-peptide ensemble regulation under dual feedback restraint by GH and IGF-I. Endocrine 22: 25–40, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Verras M, Sun Z. Beta-catenin is involved in insulin-like growth factor 1-mediated transactivation of the androgen receptor. Mol Endocrinol 19: 391–398, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Walmer DK, Wrona MA, Hughes CL, Nelson KG. Lactoferrin expression in the mouse reproductive tract during the natural estrous cycle: correlation with circulating estradiol and progesterone. Endocrinology 131: 1458–1466, 1992 [DOI] [PubMed] [Google Scholar]
- 70.Wauthier V, Sugathan A, Meyer RD, Dombkowski AA, Waxman DJ. Intrinsic sex differences in the early growth hormone responsiveness of sex-specific genes in mouse liver. Mol Endocrinol 24: 667–678, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Waxman DJ, Morrissey JJ, LeBlanc GA. Female-predominant rat hepatic P-450 forms j (IIE1) and 3 (IIA1) are under hormonal regulatory controls distinct from those of the sex-specific P-450 forms. Endocrinology 124: 2954–2966, 1989 [DOI] [PubMed] [Google Scholar]
- 72.Waxman DJ. P450 gene induction by structurally diverse xenochemicals: central role of nuclear receptors CAR, PXR, and PPAR. Arch Biochem Biophys 369: 11–23, 1999 [DOI] [PubMed] [Google Scholar]
- 73.Waxman DJ, Chang TKH. Hormonal regulation of liver cytochrome P450 enzymes. Cytochrome P450. Structure, Mechanism, and Biochemistry (edited by Ortiz de Montellano PR.). New York: Kluwer Academic/Plenum, 2005, p. 347–376 [Google Scholar]
- 74.Waxman DJ, O'Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol 20: 2613–2629, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol 76: 215–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Williams MT, Simonet LC. Effects of growth hormone on cytochrome P-450j. Biochem Biophys Res Commun 155: 392–397, 1988 [DOI] [PubMed] [Google Scholar]
- 77.Woodcroft KJ, Hafner MS, Novak RF. Insulin signaling in the transcriptional and posttranscriptional regulation of CYP2E1 expression. Hepatology 35: 263–273, 2002 [DOI] [PubMed] [Google Scholar]
- 78.Wu WX, Owiny J, Zhang Q, Ma XH, Nathanielsz PW. Regulation of the estrogen receptor and its messenger ribonucleic acid in the ovariectomized sheep myometrium and endometrium: the role of estradiol and progesterone. Biol Reprod 55: 762–768, 1996 [DOI] [PubMed] [Google Scholar]
- 79.Yamazaki H, Guo Z, Guengerich FP. Selectivity of cytochrome P4502E1 in chlorzoxazone 6-hydroxylation. Drug Metab Dispos 23: 438–440, 1995 [PubMed] [Google Scholar]
- 80.Yamazoe Y, Murayama N, Shimada M, Yamauchi K, Kato R. Cytochrome P450 in livers of diabetic rats: regulation by growth hormone and insulin. Arch Biochem Biophys 268: 567–575, 1989 [DOI] [PubMed] [Google Scholar]
- 81.Yang CS, Yoo JS, Ishizaki H, Hong JY. Cytochrome P450IIE1: roles in nitrosamine metabolism and mechanisms of regulation. Drug Metab Rev 22: 147–159, 1990 [DOI] [PubMed] [Google Scholar]
- 82.Yun YP, Casazza JP, Sohn DH, Veech RL, Song BJ. Pretranslational activation of cytochrome P450IIE during ketosis induced by a high fat diet. Mol Pharmacol 41: 474–479, 1992 [PubMed] [Google Scholar]
- 83.Zarrow MX : Experimental Endocrinology. A Sourcebook of Basic Techniques. New York and London: Academic, 1964, pp. 194 and 308 [Google Scholar]
- 84.Zeng T, Xie KQ. Ethanol and liver: recent advances in the mechanisms of ethanol-induced hepatosteatosis. Arch Toxicol 83: 1075–1081, 2009 [DOI] [PubMed] [Google Scholar]