Abstract
11β-Hydroxysteroid dehydrogenase type 1 (11β-HSD1) is involved in the pathogenesis of type 2 diabetes by generating active glucocorticoids (cortisol and corticosterone) that are strong inhibitors of angiogenesis. However, the mechanism of 11β-HSD1 gene expression and its relationship to adipose angiogenesis are largely unknown. To address this issue, we examined 11β-HSD1 expression in visceral and subcutaneous adipose tissue (AT) of diet-induced obese (DIO) mice during weight gain and investigated the gene regulation by hypoxia in vitro. 11β-HSD1 mRNA was reduced in the adipose tissues during weight gain in DIO mice, and the reduction was associated with an elevated expression of angiogenic factors. In vitro, 11β-HSD1 expression was induced in mRNA and protein by hypoxia. Of the two transcription factors activated by hypoxia, the nuclear factor-κB (NF-κB) enhanced but the hypoxia inducible factor-1α (HIF-1α) reduced 11β-HSD1 expression. 11β-HSD1 expression was elevated by NF-κB in epididymal fat of aP2-p65 mice. The hypoxia-induced 11β-HSD1 expression was attenuated by NF-κB inactivation in p65-deficient cells but enhanced by HIF-1 inactivation in HIF-1α-null cells. These data suggest that 11β-HSD1 expression is upregulated by NF-κB and downregulated by HIF-1α. During AT expansion in DIO mice, the reduction of 11β-HSD1 expression may reflect a dominant HIF-1α activity in the adipose tissue. This study suggests that NF-κB may mediate the inflammatory cytokine signal to upregulate 11β-HSD1 expression.
Keywords: 11β-hydroxysteroid dehydrogenase type 1, nuclear factor-κB, hypoxia-inducible factor-1α, hypoxia, hyperinsulinemia, angiogenesis, inflammation, obesity, type 2 diabetes
11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) that converts inactive glucocorticoids (GCs; cortisone and 11-dehydrocorticosterone) into active GCs (cortisol and corticosterone) plays a role in the pathogenesis of insulin resistance. Global inactivation of 11β-HSD1 by gene knockout prevents insulin resistance and reduces hepatic gluconeogenesis in obese mice (21). 11β-HSD1 overexpression in adipose tissue (AT) generates visceral obesity and systemic insulin resistance in transgenic mice (23). Liver-specific overexpression of 11β-HSD1 leads to insulin resistance in the absence of obesity in mice (31). Conversely, global inactivation of the 11β-HSD1 gene protects mice from diet-induced obesity (21, 25, 26). 11β-HSD1 has been a drug target in the study of insulin resistance, and its inhibition using a pharmacological approach improves insulin sensitivity in diabetic mice (2). 11β-HSD1 is expressed in many cell types, including adipocytes, and the expression is increased during adipocyte differentiation (7). 11β-HSD1 elevation in adipocytes is proposed to be a common molecular etiology for visceral obesity and metabolic syndrome (23). Although metabolic activities of 11β-HSD1 have been documented in the literature (16, 28), there is little information about the molecular mechanism of 11β-HSD1 regulation. In hepatocytes, the 11β-HSD1 gene promoter is activated by the transcription factor C/EBP in response to TNFα (1, 5, 18), but it is not known how 11β-HSD1 expression is regulated in adipocytes. To address this issue, we investigated the mechanism of 11β-HSD1 expression in adipose tissue during early weight gain in diet-induced obese (DIO) mice.
We hypothesize that 11β-HSD1 may inhibit AT function by suppression of angiogenesis. Active GCs mediate 11β-HSD1 activity to regulate local metabolism independently of GC level in the circulation. According to GC activity in the stimulation of adipocyte differentiation, GCs should protect AT function since new adipocytes have better metabolic activities over the old adipocytes. However, this fact is opposite of this expectation, and the mechanism remains unknown. We propose that 11β-HSD1 expression may lead to AT dysfunction through inhibition of angiogenesis, which is known to induce AT hypoxia (39). Angiogenesis that is stimulated by proangiogenic factors and inhibited by antiangiogenic factors is required for the maintenance of AT function (20, 30). Restoration of angiogenesis by VEGF overexpression in transgenic mice or by thiazolidinedione treatment improved AT function (12, 14, 15, 24, 34, 35). In contrast, elevation of the antiangiogenic factor pigment epithelium-derived factor reduced AT function (9, 37). These studies suggest that the angiogenic factor is key in the maintenance of AT function during weight gain. It is unknown why the angiogenic factors are reduced in AT in obesity (30). The active GCs from 11β-HSD1 expression provide an excellent mechanism since GCs have a strong activity in the inhibition of angiogenesis (33). This possibility was tested in AT during weight gain in the current study.
In this study, we examined the time course of 11β-HSD1 expression and angiogenic factor expression in the AT of DIO mice. Our data suggest that 11β-HSD1 reduction is associated with an increase in angiogenic factors. In addition, our data suggest that NF-κB is an activator and hypoxia-inducible factor-1α (HIF-1α) a repressor of the 11β-HSD1 gene.
MATERIALS AND METHODS
Reagents.
Antibodies to anti-11β-HSD1 (sc-20175) and p65 (sc-8008) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies to HIF-1α (H6536) and actin (ab6276) were from Sigma-Aldrich (St. Louis, MO) and Abcam (Cambridge, MA), respectively.
Animal experiments.
Male C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME) at 6 wk of age and were group-housed two to four mice per cage in the animal facility of the Pennington Biomedical Research Center with 12:12-h light-dark cycle and temperature of 22–24°C. The mice had free access to water and diet. Diet-induced obesity was induced by high-fat diet (HFD; 58% kcal in fat, D12331; Research Diets, New Brunswick, NJ) feeding for 10 wk beginning at 8 wk of age. AT (epididymal fat) was collected weekly after mice were euthanized. aP2-p65 transgenic mice were made in our laboratory and used in earlier studies (36, 43). The mice were fed HFD for 6 wk at 10–12 wk of age. All animal experiments were approved by the Institutional Animal Care and Use Committee at the Pennington Biomedical Research Center.
Cell culture.
3T3-L1 cells were purchased from American Type Culture Collection (Manassas, VA). All cells (such as HIF-1α-null and p65 null MEF) used in this study were cultured in appropriate media, as described previously (13).
Hypoxia treatment.
Cells were treated with air containing 1% O2 and 5% CO2 for 24 h in a sealed humidified chamber according to previously reported methods (42). The chamber was kept in a water bath at 37°C. Control cells for the normoxia condition were cultured at the same time in a humidified 37°C incubator.
Western blot.
Cells were collected and dissolved in lysis buffer (1% Triton X-100, 50 mM KCl, 25 mM HEPES, pH 7.8, 10 g/ml leupeptin, 20 g/ml aprotinin, 125 M dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and 1 mM sodiumorthovanadate). The whole cell lysate was sonicated in the lysis buffer, and the supernatant was collected from the homogenate after centrifugation at 12,000 rpm, 4°C, for 10 min. Supernatants were stored at −80°C until use. Western blots were conducted with equal amounts of protein, as described elsewhere (13). Between blotting with different antibodies, the same membrane was stripped in stripping buffer (GM6001) from GM Biosciences (Rockville, MD) at room temperature for 30 min. Quantitative image analysis was performed using NIH Image software (Image J) to determine the intensity of the individual protein.
Quantitative RT-PCR.
mRNA expression was determined by one-step quantitative real-time PCR using TaqMan Master Mix (Applied Biosystems, Foster City, CA). TaqMan primers for 11β-HSD1 (Mm00476182_ml), p65 (Mm00501346_ml), Hif-1α (Mm00468869_ml), heme oxygenase 1 (Hemox; Mm00516004_ml), glucose transporter (GLUT1; Mm00441473_ml), pyruvate dehydrogenase kinase 1 (PDK1; Mm00554306_ml), VEGF (Mm00437304_ml), PDGF (Mm00440678_ml), hepatocyte growth factor (HGF; Mm01135177_ml), TGFβ (Mm00441724_ml), and fibroblast growth factor 2 (FGF2; Mm00433287_ml) were used to determine mRNA with the 7900 HT Fast Real-Time PCR System (Applied Biosystems). mRNA level was normalized by 18S rRNA expression.
Statistical analysis.
The data were analyzed with Student's t-test or one-way ANOVA using SPSS software (version 11.0 for Windows; SPSS, Chicago, IL), with significance set at P < 0.05. The results are presented as means ± SE. All experiments were conducted at least three times.
RESULTS
11β-HSD1 expression is downregulated during AT expansion in DIO mice.
11β-HSD1 expression is increased in the visceral and subcutaneous fat tissues in human obesity (10, 19). In DIO mice, 11β-HSD1 expression is decreased in the fat tissue (11, 27). The mechanism underlying this discrepancy is not known (22). We propose that the difference may reflect 11β-HSD1 expression in the distinct stages of AT expansion in obesity. To test this possibility, we determined 11β-HSD1 expression in a visceral fat pad (epididymal fat) and subcutaneous fat (inguinal fat) of DIO mice during weight gain. 11β-HSD1 expression was monitored for 1–10 wk of HFD feeding. A reduction in 11β-HSD1 expression was observed in the 1st wk on HFD, and the reduction remained thereafter during the entire period (Fig. 1A). On average, 11β-HSD1 mRNA was reduced by 40% in the epididymal fat at the five observation time points (Fig. 1A). A similar reduction was observed in the subcutaneous fat (Fig. 1B).
Fig. 1.
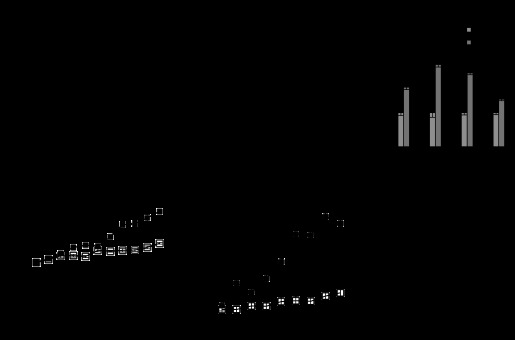
11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) expression is decreased in high-fat diet (HFD)-induced obese (DIO) mice. Beginning at 8 wk of age, mice were fed HFD for 10 wk. A: expression of 11β-HSD1 mRNA (fold change) over a 10-wk period in epididymal fat. B: expression of 11β-HSD1 mRNA (fold change) in inguinal fat. C: hypoxia-associated gene mRNA levels in epididymal fat at 1 and 5 wk of feeding is presented. D: total body weight over 10 wk. E: epididymal fat weight over 10 wk. F: p65 mRNA level at 1 and 5 wk. The data represent means ± SE (n = 3–5). P values compared with chow group. #P < 0.05; *P < 0.05 vs. chow, 1 or 5 wk.
A relationship of the 11β-HSD1 reduction and hypoxia was determined by analysis of expression of hypoxia-responsive genes, including Hif-1α, Glut1, Hemox, and Pdk1. Two of those genes (Hif-1a and Hemox) were upregulated in the 1st wk, and all of the hypoxia-responsive genes were elevated by 5 wk on HFD (Fig. 1C). The data suggest that the hypoxia-signaling pathway is activated immediately in the AT when the tissue growth is initiated in DIO mice. The weight gain and fat tissue expansion were consistently induced by the HFD in DIO mice (Fig. 1, D and E). At week 10, the body weight was increased by 90% in the mice from 24 to 46 g, and the epididymal fat mass was increased ∼10-fold from 0.25 to 2.7 g. At weeks 4 and 5, the weight and fat gain were temporarily interrupted due to an environmental change in mouse housing, which generated a transient impact in the mice. Expression of the NF-κB subunit p65 was examined, and a 70% increase was observed in the fat tissue at week 5 (Fig. 1F). These data suggest that, during early stage of fat growth, 11β-HSD1 expression is reduced, and the reduction is associated with the hypoxia in the fat pad of DIO mice.
Expression of angiogenic factors.
Angiogenesis is required for adipocyte differentiation and AT growth (8). Hypoxia is a well-known driving force in angiogenesis by inducing expression of proangiogenic factors. However, the dynamic relationship of hypoxia and angiogenic genes has not been explored previously during the AT growth in DIO mice. To address this issue, we examined five representative angiogenic factors in this study. Their expression was quantified in the epididymal fat at 1, 5, and 10 wk of HFD feeding.
The five angiogenic factors exhibited different patterns of expression during the weight gain. Vascular endothelial growth factor (VEGF) is a primary proangiogenic factor. VEGF expression was increased significantly in the 1st wk, and the increase was reduced gradually thereafter (Fig. 2A). The increase was lost by one-half at 5 wk and became insignificant by the 10th wk on HFD (Fig. 2A). Platelet-derived growth factor (PDGF) involves in maturation of capillary function. PDGF was not increased significantly during the 1st wk but exhibited a 120% increase during the 5th wk (Fig. 2B). Then, the increase disappeared during the 10th wk. Transforming growth factor-β (TGFβ) is also required for capillary maturation. Its expression was elevated during the 1st wk, and the increase was enhanced further during the 10th wk, although no increase was found during the 5th wk (Fig. 2C). HGF was increased in a pattern similar to PDGF (Fig. 2D). FGF2 was reduced modestly during the 1st wk, and then the reduction was not observed thereafter (Fig. 2E). These data suggest that the major angiogenic factors were increased during AT expansion, although their patterns of expression were different. The increase is associated 11β-HSD1 reduction in the AT. This inverse relationship suggests that 11β-HSD1 may inhibit angiogenic factor expression through active GCs in the AT.
Fig. 2.
Expression levels of angiogenic factors in DIO mice. Representative angiogenic factors were measured after 1, 5, and 10 wk on the HFD. A: VEGF mRNA. B: platelet-derived growth (PDGF) mRNA. C: transforming growth factor-β (TGFβ) mRNA. D: hepatocyte growth factor (HGF) mRNA. E: fibroblast growth factor 2 (FGF2) mRNA. The data represent means ± SE (n = 3–5). P values compared with chow group. *P < 0.05.
11β-HSD1 is inducted by NF-κB.
The association of reduced 11β-HSD1 and increased hypoxia genes suggests that 11β-HSD1 may be regulated by hypoxia in the AT during weight gain. To test this possibility, we examined 11β-HSD1 in cultured cells after treatment with hypoxia (1% oxygen). In 3T3-L1 adipocytes, the treatment induced 11β-HSD1 mRNA by 70% (Fig. 3A) at 24 h. We tested transcription factor NF-κB in the 11β-HSD1 response to hypoxia because NF-κB is activated by hypoxia in adipocytes (41). The study was conducted in wild-type (WT) mouse embryonic fibroblast (MEF) cells and NF-κB p65-null MEFs. In the basal condition, 11β-HSD1 was not detected in either WT or p65-null cells (Fig. 3B), suggesting a low expression of 11β-HSD1 in MEFs. In response to hypoxia, 11β-HSD1 expression was induced 15-fold in WT cells, and the response was not observed in p65-null cells (Fig. 3B). The data suggest that NF-κB activity is required for 11β-HSD1 induction by hypoxia.
Fig. 3.
p65 upregulates 11β-HSD1 expression. A: expression of 11β-HSD1 mRNA in 3T3-L1 cells after hypoxia treatment for 24 h. B: expression of 11β-HSD1 mRNA in p65-null mouse embryonic fibroblast (MEF) cells. Wild-type (WT) and p65-null MEFs were exposed to hypoxia for 24 h. C: 11β-HSD1 mRNA level in adipose tissue of WT and aP2-p65 mice. D: expression of 11β-HSD1 protein in 3T3-L1 cells with p65 overexpression in transient transfection. A representative blot is shown for 11β-HSD1 and p65 protein levels. The data represent means ± SE (n = 3). P value is for comparison between control and experiment group. ND, not detected.
To test this NF-κB activity in vivo, we examined 11β-HSD1 in AT of aP2-p65 mice, in which NF-κB p65 subunit is overexpressed in adipocytes and macrophages under the aP2 (FABP4) gene promoter (36). The epididymal fat pads were collected from the peritoneal cavity of aP2-p65 mice and used in mRNA assay. In the transgenic fat, 11β-HSD1 mRNA was increased remarkably, by 60%, over the WT control (Fig. 3C), suggesting that NF-κB is an activator of 11β-HSD1 gene. To test this possibility, we determined 11β-HSD1 expression in 3T3-L1 adipocytes after NF-κB activation by overexpression of p65 in a transient transfection assay. 11β-HSD1 protein was increased onefold in response to the NF-κB activation, as indicated by the immunoblot result (Fig. 3D). The increase in p65 protein was confirmed in the transfected cells (Fig. 3D). The data suggest that hypoxia induces 11β-HSD1 expression, and NF-κB represents an activator of 11β-HSD1 gene. NF-κB is likely required for 11β-HSD1 expression in response to hypoxia.
11β-HSD1 is inhibited by HIF-1α.
HIF-1α is a primary transcription factor that is activated by hypoxia to regulate gene expression, although HIF-1α is also regulated by insulin and adipogenesis in obesity (17). To test the role of HIF-1α in the regulation of 11β-HSD1 expression, we compared 11β-HSD1 expression in WT and HIF-1α-null MEFs in this study. In the absence of hypoxia, HIF-1α-null cells expressed more 11β-HSD1 mRNA than WT cells. The difference was 160-fold in the two types of cells (Fig. 4A). In response to the hypoxia treatment, the difference became 430-fold, suggesting that 11β-HSD1 expression was increased further in the null cells (Fig. 4A). The difference was observed in 11β-HSD1 protein in WT and HIF-1α-null cells (Fig. 4, B and C). The HIF-1α-null cell expressed significantly more 11β-HSD1 protein in the basal condition without hypoxia. In response to hypoxia, WT cells exhibited a threefold increase in 11β-HSD1 protein (Fig. 4C). The protein in null cells had relatively less fold increase, but it remained modestly higher than that of WT cells in response to hypoxia (Fig. 4C). These data suggest that HIF-1α is not required for induction of 11β-HSD1 expression by hypoxia. Instead, HIF-1α may inhibit 11β-HSD1 expression. To test this possibility, HIF-1α was overexpressed in 3T3-L1 adipocytes in a transient transfection. The overexpression reduced 11β-HSD1 protein expression by 50% (Fig. 4, D and E), suggesting a negative role of HIF-1α in the regulation of 11β-HSD1 expression. HIF-1α overexpression was confirmed in the transfected cells (Fig. 4D). These data suggest that HIF-1α is not required for hypoxia-induced 11β-HSD1 expression, and HIF-1α is a repressor of 11β-HSD1 gene.
Fig. 4.

Hypoxia-inducible factor-1α (HIF-1α) represses 11β-HSD1 expression during hypoxia. A: expression of 11β-HSD1 mRNA in HIF-1α-null cells. The HIF-1α-null cells were exposed to hypoxia for 24 h. The values were expressed as a fold change over WT. B: expression of 11β-HSD1 protein. C: quantification of 11β-HSD1 protein in the Western blot in B. D: expression of 11β-HSD1 protein in 3T3-L1 cells under HIF-1α overexpression. E: quantification of 11β-HSD1 protein in the Western blot in D. Data represent means ± SE (n = 3–4).
DISCUSSION
We observed that reduced 11β-HSD1 was associated with increased expression of angiogenic factors (VEGF, PDGF, TGFβ, and HGF) during adipose tissue expansion, which supports our hypothesis that 11β-HSD1 contributes to adipose tissue dysfunction in obesity by inhibition of angiogenesis. Adipose tissue dysfunction is coupled with insulin resistance in obesity, and recent studies have suggested that angiogenic failure is a risk factor for adipose dysfunction. Angiogenic failure leads to a reduction in adipose tissue blood supply, which triggers a hypoxia response in the adipose tissue (38). However, the molecular mechanism for angiogenic failure during adipose tissue expansion is largely unknown. We observed that 11β-HSD1 expression was downregulated in visceral and subcutaneous fat pads in mice from the first week on HFD. The reduction was maintained in the entire 10-wk study. Our data provide information about the time course of 11β-HSD1 change during adipose tissue expansion, which was not reported in other studies about 11β-HSD1 in adipose tissue (22, 27). More importantly, we found that the reduction was correlated to an increase in the expression of angiogenic factors in the tissues. 11β-HSD1 was reported to inhibit angiogenesis through production of active GC locally (33), and 11β-HSD1 gene inactivation was found to enhance angiogenesis in adipose tissue (24). However, 11β-HSD1 activity during adipose tissue expansion was not examined in those early studies. Our observation links 11β-HSD1 to dynamic changes of multiple angiogenic factors during adipose tissue growth.
Our data suggest that 11β-HSD1 expression is induced by hypoxia through NF-κB activation. Our gene expression data suggest that 11β-HSD1 reduction is associated with hypoxia during adipose tissue expansion in DIO mice. In vitro, 11β-HSD1 expression in response to hypoxia involves two major transcription factors, NF-κB and HIF-1α. NF-κB induced 11β-HSD1 expression in 3T3-L1 adipocytes with p65 overexpression in transient transfection and in the adipose tissue of aP2-p65 transgenic mice. Conversely, inactivation of NF-κB activity in p65-null MEFs abolished the 11β-HSD1 response to hypoxia. These data consistently suggest that NF-κB is an activator of the 11β-HSD1 gene and is required for induction of 11β-HSD1 by hypoxia. This finding provides a mechanism by which 11β-HSD1 expression is induced by TNFα (1, 18), which activates NF-κB through the TNF receptor signaling pathway. Hypoxia activates NF-κB within 2 h, but it took 24 h to induce 11β-HSD1 expression in the current study. It is possible that hypoxia acts by inducing a stronger NF-κB activator, such as TNFα, in the cultured cells for induction of 11β-HSD1. This possibility should be tested in the future since it is out of the scope of current study. It will take an extensive effort to identify the mechanism by which NF-κB mediates the hypoxia signal in the regulation of 11β-HSD1.
The study suggests that NF-κB is a target in the inhibition of 11β-HSD1 expression. 11β-HSD1 expression is induced during differentiation of adipocytes, and its maximal expression is observed in mature adipocytes (4, 6, 29). During adipocyte differentiation, NF-κB expression is elevated in the nucleus (3). This pattern of NF-κB expression correlates to 11β-HSD1 expression during adipogenesis. It was reported that active GCs inhibit 11β-HSD1 expression through the targeting of C/EBP (32). Our study suggests that NF-κB may be another target of GCs in the inhibition of 11β-HSD1 expression.
Our data suggest that HIF-1α is a negative regulator of 11β-HSD1 expression. In response to hypoxia, HIF-1α protein level is elevated in the cytoplasm as a result of protein stabilization. HIF-1α translocates to the nucleus to induce transcription of angiogenic factors, including VEGF, which stimulates endothelial cell proliferation, differentiation, and capillary formation. This angiogenic activity of VEGF promotes an increase in blood supply to the hypoxic area in tissues as being documented (12, 24, 34, 35). In obesity, HIF-1α activity is upregulated by other factors such as insulin and adipogenesis in the adipose tissue in addition to hypoxia (17). Our data from HIF-1α-null cells suggest that HIF-1α inhibits 11β-HSD1 gene expression. HIF-1α activity is dominant in the adipose tissue in the early stage of obesity, as suggested by the reduced 11β-HSD1 and increased angiogenic factors. Insulin and adipogenesis may contribute to the strong HIF-1α activity. In the DIO model, hyperinsulinemia is a feature, a result of increased β-cell sensitivity to glucose in the presence of fatty acids and islet compensatory expansion (40). The hyperinsulinemia is coupled with abundant fatty acids and glucose in the mice on a high-fat diet. In combination, the insulin and nutrients promote adipogenesis in the adipose tissue. In this condition, HIF-1α activity is enhanced in adipocytes by insulin and adipogenesis, as reported earlier (17). HIF-1α accounts for the reduced 11β-HSD1 in the adipose tissue of DIO mice in this study. In vitro, both HIF-1α and NF-κB were activated in 3T3-L1 adipocytes in the long-time (24 h) hypoxia treatment (41). However, NF-κB activity likely becomes dominant in the system to increase 11β-HSD1 expression. In vivo, a high level of NF-κB activity is associated with the chronic adipose inflammation in the late stage of obesity or in extreme obesity (ob/ob mice), which provides an explanation to the elevated 11β-HSD1 expression (28).
In summary, our study suggests that 11β-HSD1 is reduced during adipose tissue growth at the early stage of weight gain, and the reduction is associated with an increase in the angiogenic activity. In vitro, 11β-HSD1 expression was induced by hypoxia in long-time (24 h) treatment. The data from genetic models suggest that NF-κB is an activator and that HIF-1α is a repressor of the 11β-HSD1 gene. These findings provide a new mechanism by which 11β-HSD1 is regulated in the early and late stages of obesity. The 11β-HSD1 reduction in adipose tissue suggests a dominant activity of HIF-1α during adipose tissue growth in the early stage of obesity. The 11β-HSD1 elevation in the late stage of obesity suggests a dominant NF-κB activity or proinflammatory status. The study provides evidence that NF-κB and HIF-1α are molecular targets in the regulation of 11β-HSD1 expression.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-068036 and DK-085495 to J. Ye.
DISCLOSURES
No potential conflicts of interest relevant to this article are reported.
AUTHOR CONTRIBUTIONS
J.H.L. and J.Y. contributed to the conception and design of the research; J.H.L. and Z.G. performed the experiments; J.H.L., Z.G., and J.Y. analyzed the data; J.H.L., Z.G., and J.Y. interpreted the results of the experiments; J.H.L. prepared the figures; J.H.L. drafted the manuscript; J.H.L., Z.G., and J.Y. edited and revised the manuscript; J.H.L., Z.G., and J.Y. approved the final version of the manuscript.
REFERENCES
- 1. Ahasan MM, Hardy R, Jones C, Kaur K, Nanus D, Juarez M, Morgan SA, Hassan-Smith Z, Benezech C, Caamano JH, Hewison M, Lavery G, Rabbitt EH, Clark AR, Filer A, Buckley CD, Raza K, Stewart PM, Cooper MS. Inflammatory regulation of glucocorticoid metabolism in mesenchymal stromal cells. Arthritis Rheum 64: 2404–2413, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alberts P, Nilsson C, Selen G, Engblom LO, Edling NH, Norling S, Klingström G, Larsson C, Forsgren M, Ashkzari M, Nilsson CE, Fiedler M, Bergqvist E, Ohman B, Björkstrand E, Abrahmsen LB. Selective inhibition of 11 beta-hydroxysteroid dehydrogenase type 1 improves hepatic insulin sensitivity in hyperglycemic mice strains. Endocrinology 144: 4755–4762, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Berg AH, Lin Y, Lisanti MP, Scherer PE. Adipocyte differentiation induces dynamic changes in NF-κB expression and activity. Am J Physiol Endocrinol Metab 287: E1178–E1188, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Berger J, Tanen M, Elbrecht A, Hermanowski-Vosatka A, Moller DE, Wright SD, Thieringer R. Peroxisome proliferator-activated receptor-gamma ligands inhibit adipocyte 11beta -hydroxysteroid dehydrogenase type 1 expression and activity. J Biol Chem 276: 12629–12635, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Bruley C, Lyons V, Worsley AG, Wilde MD, Darlington GD, Morton NM, Seckl JR, Chapman KE. A novel promoter for the 11beta-hydroxysteroid dehydrogenase type 1 gene is active in lung and is C/EBPalpha independent. Endocrinology 147: 2879–2885, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Bujalska IJ, Kumar S, Hewison M, Stewart PM. Differentiation of adipose stromal cells: the roles of glucocorticoids and 11beta-hydroxysteroid dehydrogenase. Endocrinology 140: 3188–3196, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Bujalska IJ, Walker EA, Tomlinson JW, Hewison M, Stewart PM. 11Beta-hydroxysteroid dehydrogenase type 1 in differentiating omental human preadipocytes: from de-activation to generation of cortisol. Endocr Res 28: 449–461, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest 117: 2362–2368, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crowe S, Wu LE, Economou C, Turpin SM, Matzaris M, Hoehn KL, Hevener AL, James DE, Duh EJ, Watt MJ. Pigment epithelium-derived factor contributes to insulin resistance in obesity. Cell Metab 10: 40–47, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Desbriere R, Vuaroqueaux V, Achard V, Boullu-Ciocca S, Labuhn M, Dutour A, Grino M. 11beta-hydroxysteroid dehydrogenase type 1 mRNA is increased in both visceral and subcutaneous adipose tissue of obese patients. Obesity (Silver Spring) 14: 794–798, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Drake AJ, Livingstone DE, Andrew R, Seckl JR, Morton NM, Walker BR. Reduced adipose glucocorticoid reactivation and increased hepatic glucocorticoid clearance as an early adaptation to high-fat feeding in Wistar rats. Endocrinology 146: 913–919, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Elias I, Franckhauser S, Ferré T, Vilà L, Tafuro S, Muñoz S, Roca C, Ramos D, Pujol A, Riu E, Ruberte J, Bosch F. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes 61: 1801–1813, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gao Z, He Q, Peng B, Chiao PJ, Ye J. Regulation of nuclear translocation of HDAC3 by IkappaBalpha is required for tumor necrosis factor inhibition of peroxisome proliferator-activated receptor gamma function. J Biol Chem 281: 4540–4547, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gealekman O, Burkart A, Chouinard M, Nicoloro SM, Straubhaar J, Corvera S. Enhanced angiogenesis in obesity and in response to PPARγ activators through adipocyte VEGF and ANGPTL4 production. Am J Physiol Endocrinol Metab 295: E1056–E1064, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, Tran KV, Straubhaar J, Nicoloro S, Czech MP, Thompson M, Perugini RA, Corvera S. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation 123: 186–194, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav 59: 279–289, 2011 [DOI] [PubMed] [Google Scholar]
- 17. He Q, Gao Z, Yin J, Zhang J, Yun Z, Ye J. Regulation of HIF-1α Activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am J Physiol Endocrinol Metab 300: E877–E885, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ignatova ID, Kostadinova RM, Goldring CE, Nawrocki AR, Frey FJ, Frey BM. Tumor necrosis factor-α upregulates 11β-hydroxysteroid dehydrogenase type 1 expression by CCAAT/enhancer binding protein-β in HepG2 cells. Am J Physiol Endocrinol Metab 296: E367–E377, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Katz JR, Mohamed-Ali V, Wood PJ, Yudkin JS, Coppack SW. An in vivo study of the cortisol-cortisone shuttle in subcutaneous abdominal adipose tissue. Clin Endocrinol (Oxf) 50: 63–68, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol 29: 1575–1591, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kotelevtsev Y, Holmes MC, Burchell A, Houston PM, Schmoll D, Jamieson P, Best R, Brown R, Edwards CR, Seckl JR, Mullins JJ. 11beta-hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc Natl Acad Sci USA 94: 14924–14929, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Man TY, Michailidou Z, Gokcel A, Ramage L, Chapman KE, Kenyon CJ, Seckl JR, Morton NM. Dietary manipulation reveals an unexpected inverse relationship between fat mass and adipose 11β-hydroxysteroid dehydrogenase type 1. Am J Physiol Endocrinol Metab 300: E1076–E1084, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS. A transgenic model of visceral obesity and the metabolic syndrome. Science 294: 2166–2170, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Michailidou Z, Turban S, Miller E, Zou X, Schrader J, Ratcliffe PJ, Hadoke PW, Walker BR, Iredale JP, Morton NM, Seckl JR. Increased angiogenesis protects against adipose hypoxia and fibrosis in metabolic disease-resistant 11beta-hydroxysteroid dehydrogenase type 1 (HSD1)-deficient mice. J Biol Chem 287: 4188–4197, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morton NM, Holmes MC, Fievet C, Staels B, Tailleux A, Mullins JJ, Seckl JR. Improved lipid and lipoprotein profile, hepatic insulin sensitivity, and glucose tolerance in 11beta-hydroxysteroid dehydrogenase type 1 null mice. J Biol Chem 276: 41293–41300, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Morton NM, Paterson JM, Masuzaki H, Holmes MC, Staels B, Fievet C, Walker BR, Flier JS, Mullins JJ, Seckl JR. Novel adipose tissue-mediated resistance to diet-induced visceral obesity in 11 beta-hydroxysteroid dehydrogenase type 1-deficient mice. Diabetes 53: 931–938, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Morton NM, Ramage L, Seckl JR. Down-regulation of adipose 11beta-hydroxysteroid dehydrogenase type 1 by high-fat feeding in mice: a potential adaptive mechanism counteracting metabolic disease. Endocrinology 145: 2707–2712, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Morton NM, Seckl JR. 11beta-hydroxysteroid dehydrogenase type 1 and obesity. Front Horm Res 36: 146–164, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Napolitano A, Voice MW, Edwards CR, Seckl JR, Chapman KE. 11Beta-hydroxysteroid dehydrogenase 1 in adipocytes: expression is differentiation-dependent and hormonally regulated. J Steroid Biochem Mol Biol 64: 251–260, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Pang C, Gao Z, Yin J, Zhang J, Jia W, Ye J. Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. Am J Physiol Endocrinol Metab 295: E313–E322, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paterson JM, Morton NM, Fievet C, Kenyon CJ, Holmes MC, Staels B, Seckl JR, Mullins JJ. Metabolic syndrome without obesity: Hepatic overexpression of 11beta-hydroxysteroid dehydrogenase type 1 in transgenic mice. Proc Natl Acad Sci USA 101: 7088–7093, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sai S, Esteves CL, Kelly V, Michailidou Z, Anderson K, Coll AP, Nakagawa Y, Ohzeki T, Seckl JR, Chapman KE. Glucocorticoid regulation of the promoter of 11beta-hydroxysteroid dehydrogenase type 1 is indirect and requires CCAAT/enhancer-binding protein-beta. Mol Endocrinol 22: 2049–2060, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Small GR, Hadoke PW, Sharif I, Dover AR, Armour D, Kenyon CJ, Gray GA, Walker BR. Preventing local regeneration of glucocorticoids by 11beta-hydroxysteroid dehydrogenase type 1 enhances angiogenesis. Proc Natl Acad Sci USA 102: 12165–12170, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, Scherer PE. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci USA 109: 5874–5879, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sung HK, Doh KO, Son JE, Park JG, Bae Y, Choi S, Nelson SM, Cowling R, Nagy K, Michael IP, Koh GY, Adamson SL, Pawson T, Nagy A. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab 17: 61–72, 2013 [DOI] [PubMed] [Google Scholar]
- 36. Tang T, Zhang J, Yin J, Staszkiewicz J, Gawronska-Kozak B, Jung DY, Ko HJ, Ong H, Kim JK, Mynatt R, Martin RJ, Keenan M, Gao Z, Ye J. Uncoupling of inflammation and insulin resistance by NF-kappaB in transgenic mice through elevated energy expenditure. J Biol Chem 285: 4637–4644, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamagishi S, Adachi H, Abe A, Yashiro T, Enomoto M, Furuki K, Hino A, Jinnouchi Y, Takenaka K, Matsui T, Nakamura K, Imaizumi T. Elevated serum levels of pigment epithelium-derived factor in the metabolic syndrome. J Clin Endocrinol Metab 91: 2447–2450, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Ye J. Adipose tissue vascularization: its role in chronic inflammation. Curr Diab Rep 11: 203–210, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond) 33: 54–66, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ye J. Role of insulin in the pathogenesis of free fatty acid-induced insulin resistance in skeletal muscle. Endocr Metab Immune Disord Drug Targets 7: 65–74, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Ye J, Gao Z, Yin J, He H. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 293: E1118–E1128, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Yin J, Gao Z, He Q, Ye J. Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am J Physiol Endocrinol Metab 296: E333–E342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang J, Henagan TM, Gao Z, Ye J. Inhibition of glyceroneogenesis by histone deacetylase 3 contributes to lipodystrophy in mice with adipose tissue inflammation. Endocrinology 152: 1829–1838, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]




