Abstract
Obesity is characterized by a chronic proinflammatory state that leads to endothelial dysfunction. Saturated fatty acids (SFA) stimulate Toll-like receptors (TLR) that promote metabolic insulin resistance. However, it is not known whether TLR2 mediates impairment of vascular actions of insulin in response to high-fat diet (HFD) to cause endothelial dysfunction. siRNA knockdown of TLR2 in primary endothelial cells opposed palmitate-stimulated expression of proinflammatory cytokines and splicing of X box protein 1 (XBP-1). Inhibition of unfolding protein response (UPR) reduced SFA-stimulated expression of TNFα. Thus, SFA stimulates UPR and proinflammatory response through activation of TLR2 in endothelial cells. Knockdown of TLR2 also opposed impairment of insulin-stimulated phosphorylation of eNOS and subsequent production of NO. Importantly, insulin-stimulated vasorelaxation of mesenteric arteries from TLR2 knockout mice was preserved even on HFD (in contrast with results from arteries examined in wild-type mice on HFD). We conclude that TLR2 in vascular endothelium mediates HFD-stimulated proinflammatory responses and UPR that accompany impairment of vasodilator actions of insulin, leading to endothelial dysfunction. These results are relevant to understanding the pathophysiology of the cardiovascular complications of diabetes and obesity.
Keywords: endothelial function, vascular insulin resistance, Toll-like receptor 2, unfolding protein response, inflammation, endothelial nitric oxide synthase
insulin signaling pathways involving insulin receptor/insulin receptor substrates (IRS)/phosphatidylinositol (PI) 3-kinase/protein kinase B (Akt) contribute to glucose uptake in skeletal muscle and adipose tissue. A similar insulin signaling pathway in vascular endothelium leads to activation of endothelial nitric oxide synthase (eNOS) and production of nitric oxide (NO), which contribute to vasodilation and capillary recruitment (18). Vasodilator actions of insulin play important roles in antiatherogenesis and capillary recruitment that regulate hemodynamics as well as nutrient metabolism (23, 51). Impairment of vascular actions of insulin leads to a reduction of eNOS activity and endothelial dysfunction that contributes to both metabolic dysfunction and cardiovascular complications. This is evident from the metabolic and cardiovascular phenotype of eNOS knockout mice (9, 18, 43). High-fat diet (HFD) promotes proinflammatory responses and endoplasmic reticulum (ER) stress, major mechanisms for obesity-induced impairment of insulin actions (13, 18, 46). Unfolding protein response (UPR) is a cellular protective mechanism that copes with ER stress by reducing protein synthesis, inducing expression of chaperones, and stimulating protein degradation. UPR is stimulated by a bacterial infection that increases proinflammatory responses as a host-protective mechanism (30). Toll-like receptors (TLR) are pattern-recognizing receptors that detect pathogen-associated molecular patterns and induce innate immune responses for host defense (22, 50). TLR4 recognizes lipopolysaccharide from gram-negative bacteria, whereas TLR2 recognizes bacterial lipoproteins. Triacylated lipopeptide activates the TLR2/1 heterodimer, and diacylated lipopeptide activates the TLR2/6 heterodimer (3, 44). TLRs can be activated by various endogenous molecules, including saturated fatty acids (SFA). SFA-activated TLRs stimulate proinflammatory responses, including production of cytokines and activation of c-Jun NH2-terminal kinase (JNK) and IKKβ, leading to metabolic insulin resistance (21, 26, 33, 47). Indeed, TLR2- or TLR4-deficient mice are protected from HFD-induced insulin resistance (33, 48). Moreover, TLR4 mediates SFA-stimulated impairment of insulin signaling and NO production in vascular endothelial cells (16). However, the role of SFA-activated TLR2 in vasodilator actions of insulin with regard to UPR-mediated inflammatory responses is not known. In this study, we examine the role of TLR2 to promote SFA-induced impairment of insulin-stimulated vasodilation through a UPR-mediated mechanism. Moreover, we examine vasodilator actions of insulin ex vivo in intact vessels isolated from wild-type (WT) and TLR2-knockout mice.
MATERIALS AND METHODS
Materials.
Anti phospho (p)-eNOS, anti-p-Akt, anti-p-NF-κB, and anti-Akt were obtained from Cell Signaling Technology (Beverly, MA). Anti-eNOS and anti-p-JNK were obtained from Invitrogen (Carlsbad, CA), and anti-p65/NF-κB anti-JNK antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). siRNA for human TLR2 and scrambled siRNA were purchased from Dharmacon. dsiRNA for bovine TLR2 and nontargeted scrambled siRNA and primers for the RT-PCR were purchased from Integrated DNA technologies (Coralville, IA).
Animals.
All animal procedures were performed in accordance with the rules of and approved by the Animal Use and Care Committee at The University of Alabama at Birmingham. Both C57BL/6J (wild-type) and B6.129-Tlr2tmlkir/J (TLR2KO) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All animals were maintained in a temperature-controlled facility with a 12:12-h light-dark cycle. At 6 wk of age, mice were fed either chow (7917 Harlan Diet, 11% calories from fat) or HFD (5SPQ, 54% calories from fat; Test Diet Richmond, IN) for 10 wk. Body weight was measured every week. Mice were fasted overnight and anesthetized with pentabarbitol sodium (50 mg/kg) before euthanization.
Quantitative magnetic resonance.
These experiments were conducted by the University of Alabama at Birmingham (UAB) small animal phenotyping core facility funded by our Nutrition Obesity Research Center. In vivo body composition (total body fat and lean tissue) of mice was determined using an EchoMRI 3-in-1 quantitative magnetic resonance machine (Echo Medical Systems, Houston, TX). Quantification of results was standardized by conducting a system test using a known fat standard prior to experimental measurements being taken. Mice were weighed and then placed into a clear holding tube capped with a stopper that restricted vertical movement but allowed constant airflow. This tube was inserted into the machine, and the mouse was scanned using the normal precision mode.
Isolation of mouse primary heart endothelial cells.
Mouse heart endothelial cells (MHEC) were isolated using a modification of previously described methods (28). Briefly, after 6-wk-old mice (WT or TLR2KO mice) were euthanized by isoflurane inhalation, the heart was dissected, minced, and incubated in PBS containing 2 mg/ml collagenase II (Worthington) for 45 min at 37°C. The digested tissues were triturated using a syringe attached to a cannula, filtered through a cell strainer, and washed twice. Subsequently, the cells were incubated with microbeads (Dynal beads M-450; Invitrogen) coated with anti-CD31 (BD Biosciences) in PBS with 0.1% BSA at room temperature for 10 min. The anti-CD31 Dynabead-labeled endothelial cells were captured by a magnetic separator and seeded onto gelatin-coated 100-mm culture dishes in DMEM/high-glucose medium supplemented with 20% FBS, 45 μg/ml endothelial cell growth supplement, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10 U/ml heparin. In this study, MHECs were used between passages 3 and 6.
Cell culture and transfection.
Bovine aortic endothelial cells (BAEC) were maintained in F-12K medium containing 5% fetal bovine serum (FBS), endothelial cell growth supplement (30 mg/l; BD Biosciences), heparin sulfate (50 μg/ml), penicillin (100 U/ml), and streptomycin (100 μg/ml). Human aortic endothelial cells (HAEC; Lonza, Walkersville, MD) in primary culture were grown in F-12K medium containing EGM-2 Single Quot supplements (Lonza). All experiments were conducted on HAEC and BAEC before their sixth passage. BAEC were serum-starved (0.1% horse serum) overnight and then treated with BSA or BSA-conjugated palmitate at the indicated concentrations and for the times noted in the figure legends. HAEC were transiently transfected with 100 nM of siRNA duplex oligonucleotides [siRNA for TLR2, smartpool from Dharmacon, Lafayette, CO; dsiRNA for X-box protein-1 (XBP-1) and bovine TLR2 from Integrated DNA Technologies, Coralville, IA], using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. siRNA sequences are described in Table 1. Two days after transfection, cells were serum-starved for 2 h and then treated with BSA or palmitate, as indicated in the figure legends.
Table 1.
| Genes | Sequences |
|---|---|
| hGRP78 | |
| Forward | CATCACGCCGTCCTATGTCG |
| Reverse | CGTCAAAGACCTGTTCTG |
| hxbp-1s | |
| Forward | GGTCTGCTGAGTCCGCAGCAGG |
| Reverse | GGGCTTGGAATATGTGG |
| hCHOP | |
| Forward | GGAGAACCAGAAACGGAAAC |
| Reverse | TCTCCTTCATGCCTGCTTT |
| hIL-1β | |
| Forward | GGGCTCAAGGAAAAGAATC |
| Reverse | TTCTGCTTGAGAGGTGCTGA |
| hMCP-1 | |
| Forward | CCCCAGTCACCTGCTGTTAT |
| Reverse | TGGAATCCTGAACCCACTTC |
| hTNFα | |
| Forward | CAGAGGGCCTGTACCTCATC |
| Reverse | GGAAGACCCCTCCCAGATAG |
| hIRE-1α | |
| Forward | TCCAGTACATTGCCATCGAGCTGT |
| Reverse | TGTGAACGATGTTGAGGGAGTGGA |
| hE-selectin | |
| Forward | GGTTTGGTGAGGTGGCT |
| Reverse | TGATCTTTCCCGGAACTG |
| hIL-6 | |
| Forward | AGTGCGTCTTTGCTGCTTTCAC |
| Reverse | TGACAAACAAATTCGGTACATCCT |
| hTLR2 | |
| Forward | CCAGCAGGAACATCTGCTAT |
| Reverse | CTTGCACCACTCACTCTTCA |
| hTLR4 | |
| Forward | GTCCTCAGTGTGCTTGTAGT |
| Reverse | GG TGATGTTGGCAGCAATG |
| β-Actin | |
| Forward | CTGGCACCCAGCACAATGAAG |
| Reverse | TAGAAGCATTGCGGTGGACG |
| bTLR2 | |
| Forward | TGGAGCACTTCAACCCTCCCTTTA |
| Reverse | TACTTGCACCACTCGCTCTTCACA |
| DSi RNA | |
| hXBP1 forward | rCrUrUrGrGrArCrCrCrArGrUrCrArUrGrUrUrCrUrUrCrArAA |
| hXBP1 reverse | rUrUrUrGrArArGrArArCrArUrGrArCrUrGrGrGrUrCrCrArArGrUrU |
| hIRE1a forward | rArArGrGrArGrUrArGrArGrGrUrArGrCrArGrArArCrArArGG |
| hIRE1a reverse | rUrUrCrCrUrCrArUrCrUrCrCrArUrCrGrUrCrUrUrGrUrUrCrCrArG |
| bTLR2 forward | rGrUrArGrGrArArArUrArGrUrArArCrArGrCrUrUrCrArCrTG |
| bTLR2 reverse | rCrArGrUrGrArArGrCrUrGrUrUrArCrUrArUrUrUrCrCrUrArCrUrU |
| Dsi-scrambled forward | rCrGrUrUrArArUrCrGrCrGrUrArUrArArUrArCrGrCrGrUrAT |
| Dsi-scrambled reverse | rArUrArCrGrCrGrUrArUrUrArUrArCrGrCrGrArUrUrArArCrGrArC |
GRP78, glucose-regulated protein 78; XBP, X box protein; CHOP, C/EBP homologous protein; MCP-1, monocyte chemoattractant protein 1; IRE-1α, inositol-requiring protein-1α; TLR, Toll-like receptor; h, human; b, bovine.
Measurement of fasting glucose and insulin in serum.
Mice were fasted overnight, and the blood was collected before euthanization. Glucose was measured with a hand-held glucometer (Alphatrak; Abbott, Abbott Park, IL) right before euthanization. The serum was collected after centrifugation (2,200 g for 10 min at 4°C) of clotted blood. Radioimmunoassay was performed to determine the serum insulin levels by using the Millipore (Billerica, MA) RIA kit. These analyses were conducted by the UAB core facility. Quantitative insulin sensitivity check index (QUICKI) was calculated as 1/{log [fasting insulin (μU/ml)] + log [fasting glucose (mg/dl)]} (6, 27, 41).
Preparation of palmitate.
Preparation of palmitate was carried out as described by Mott et al. (31a). Briefly, 10.5% bovine serum albumin (Sigma A7511) was dissolved in 25 mM HEPES-DMEM and syringe-filtered (0.22 μM; Millipore). Sodium palmitate (100 mM) was heated until it was dissolved in water and added rapidly to warmed BSA solution. Then, this BSA-conjugated palmitate was added to reach the proper concentration of palmitate. We used endotoxin-free reagents and tested all of the reagents we used, including BSA, palmitate, media, and reagent diluents. We checked the endotoxin level of all of the reagents we used in this study with the Chromogenic Endotoxin Quantitation assay kit (Pierce). The levels were undetectable or <25 pg/ml.
Functional assessment for isolated mesentery arterioles.
Mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg/kg). Mesenteric arterioles were excised from the animal and placed in a cooled (4°C) chamber containing dissection buffer (145 mM NaCl, 4.7 mM KCl, 2 mM CaCl, 1.2 mM MgSO4, 1 mM NaH2PO4, 5 mM glucose, 3 mol/l MOPS buffer, 2 mM pyruvate, 0.02 mM EDTA, and 1% BSA, pH 7.4). The isolated arterioles were then cannulated with glass micropipettes with a 10-0 monofilament suture and mounted in a custom-designed tissue chamber (Living System Instrumentation, Burlington, VT). The arterioles were pressurized to 45 mmHg intraluminally with the same buffer without flow and superfused with buffer without albumin. The vessel preparations were positioned on the stage of an inverted microscope. The vessel segments were gradually warmed to 37°C during a 30-min equilibration period. After baseline diameter was established, arterioles were exposed to phenylephrine (1 μmol/l) until a maximal contraction was achieved (5 min). The vessels were subsequently stimulated with various vasodilators (10−11–10−5 mol/l, 3 min/concentration), including insulin, acetylcholine, and sodium nitroprusside. The dilator responses to insulin were observed and recorded. Measurement of vessel diameter (in μm) was performed with an electronic video caliper (Living Systems Instumentation, St. Albans, Vermont) and recorded by using a software Lab Chart (AD instruments, Colorado Springs, CO). In some experiments, vessels were incubated with the NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 100 μM) for 30 min prior to insulin stimulation.
The data are expressed as means ± SE. The vasodilator responses to insulin were calculated as percent relaxation from the maximum constriction induced by phenylephrine according to the following equation:
where IDtreat is the diameter obtained when the vessel was treated with insulin, IDPE is the diameter obtained when the vessel was constricted with penylephrine, and IDw/oCa++ is the maximal passive diameter observed when the vessel was fully dilated in buffer containing 2 mM EGTA and 100 μM adenosine without Ca++.
Preparation of cell lysates and immunoblotting.
Before lysis, cells were briefly washed with ice-cold PBS. Cells were then scraped in lysis buffer containing 50 mM Tris (pH 7.2), 125 mM NaCl, 1% Triton X-100, 0.5% NP-40, 1 mM EDTA, 1 mM Na3VO4, 20 mM NaF, 1 mM Na pyrophosphate, and complete protease inhibitor cocktail (Roche Applied Science). Cell debris was pelleted by centrifugation of samples at 17,000 g for 10 min at 4°C. Supernatants were then boiled with Laemmle sample buffer for 5 min, and proteins were resolved by 10% SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with specific antibodies, as described in the figures, using standard methods. Immunoblots were visualized and quantified by Image Analyzer (Vision Works LS) and the BioSpectrum Imaging System (UVP, Upland, CA).
Measurement of NO production.
Production of NO was assessed using the NO-specific fluorescent dye 4,5-diaminofluorescein diacetate (EMD Biosciences, Gibbstown, NJ), as described previously (17, 31). Briefly, endothelial cells were grown to 95% confluence in 24-well black plates (Denville, Metuchen, NJ) and serum-starved as indicated in the figure legends. Then, the cells were treated with BSA or palmitate. Cells were then loaded with 4,5-diaminofluorescein diacetate (final concentration, 1 μM) for 10 min at 37°C, rinsed 3 times with F-12K, and kept in the dark. Cells were then treated without or with insulin as indicated in the figure legends. After stimulation, cells were fixed in 2% paraformaldehyde for 5 min at 4°C. Fixed cells were visualized with a Zeiss inverted epifluorescence microscope (Axio Observer A1) using appropriate filters for a peak excitation wavelength of 480 nm and a peak emission wavelength of 510 nm. Images were captured and analyzed by using AV Rel 4.7 software with multichannel modules.
Immunocytochemistry.
Cells were treated as described in the figure legends. After stimulation, cells were fixed in 4% paraformaldehyde-PBS for 10 min, and the cells were washed with PBS. Cells were then permeabilized with 0.1% Triton X-100-PBS for 5 min and washed with PBS. Cells were blocked with 3% BSA-PBS for 30 min and then incubated with anti-p65/NF-κB (sc-372; Santa Cruz Biotechnology) in 3% BSA/PBS at 4°C overnight. Cells were washed with PBS three times (5 min each) and then incubated with Alexa 555-conjugated goat anti-rabbit IgG (Invitrogen, Grand Island, NY) for 1 h at room temperature. Cells were washed with PBS three times (5 min each) and then stained with Hoechst 33342 (Invitrogen, Grand Island, NY). The image was visualized with a Zeiss inverted epifluorescent microscope (Axio Observer A1). Images were captured and analyzed by using AV Rel 4.7 software with multichannel modules.
RT-PCR.
The cells were treated as described in the figure legends. Total RNA was prepared by using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. One microgram of total RNA was used for cDNA synthesis by using an Omniscript RT kit (Qiagen, Valencia, CA). Then, the cDNA was subjected to semiquantitative PCR analysis by using a Hot Star Taq Master Mix kit (Qiagen). PCR product was visualized with fluorescent dye (Envirosafe DNA/RNA Stain; Helixx Technologies), and the image was analyzed and quantified by Image Analyzer (Vision Works LS) and the BioSpectrum Imaging System (UVP). Black and white colors were inverted by using the software. The primers for each gene are described in the Table 1.
Statistical analysis.
Values are presented as means ± SE. Western blots were analyzed by one-way ANOVA. For the comparisons of vasodilatory responses, repeated measurements of two-way ANOVA combined with Bonferroni post hoc test were used.
RESULTS
TLR2 mediates palmitate-stimulated proinflammatory responses.
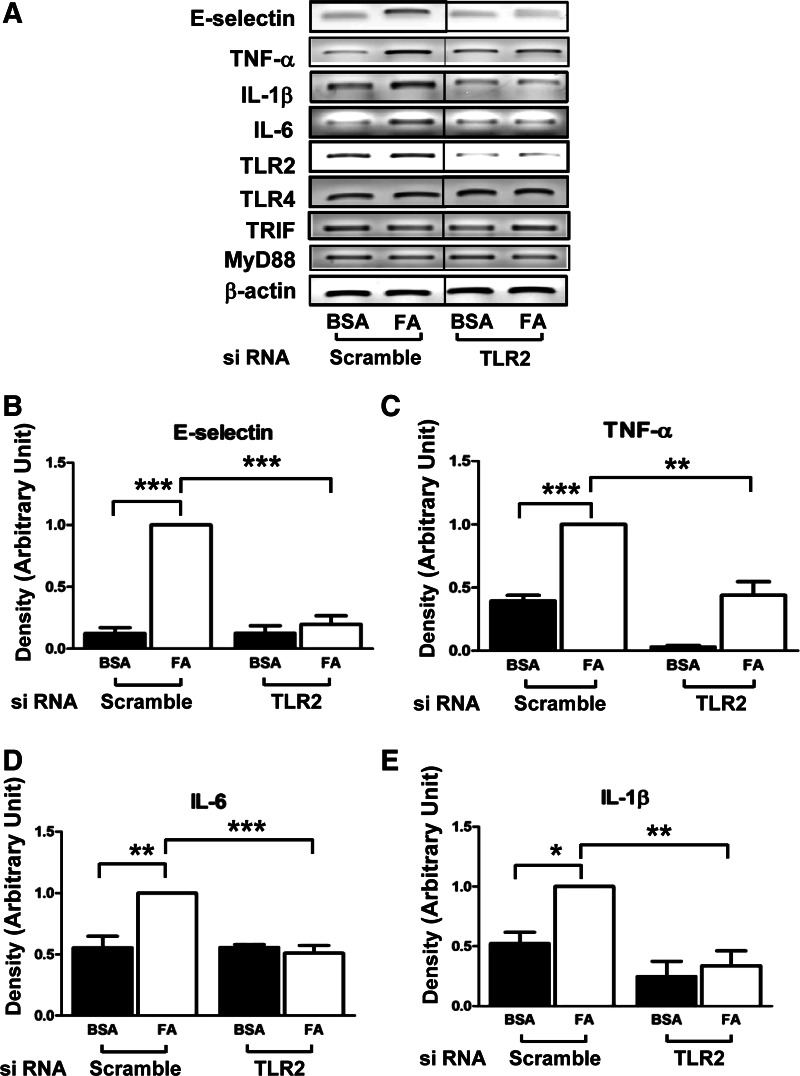
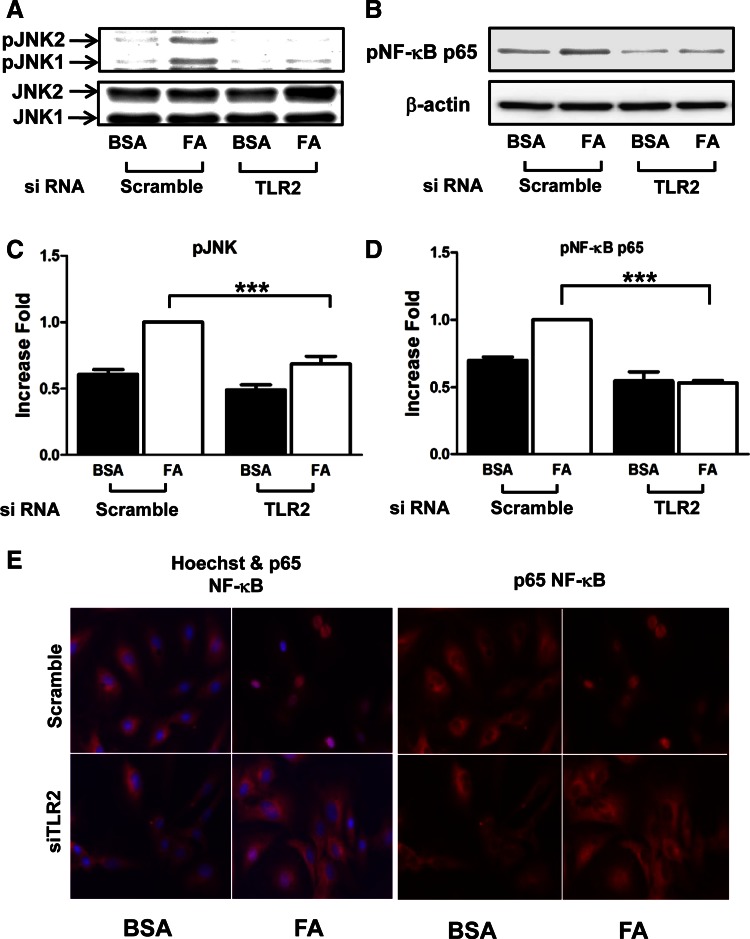
To test whether TLR2 plays a role in production of proinflammatory cytokines by SFA in vascular endothelium, we knocked down TLR2 by transiently transfecting HAEC with TLR2 siRNA and treated the cells with palmitate. mRNA expression of proinflammatory marker genes was examined by semiquantitative RT-PCR analysis. Both TLR2 and TLR4 are expressed in HAEC. siRNA knockdown of TLR2 significantly reduced expression of TLR2 (vs. scrambled control siRNA; Fig. 1A). Interestingly, knockdown of TLR2 resulted in a slight increase in the expression of TLR4 that may be compensatory (Fig. 1A). Treatment with palmitate (200 μM, 6 h) increased mRNA expression of E-selectin, TNFα, IL-6, and IL-1β (Fig. 1). Knockdown of TLR2 opposed the palmitate-stimulated increase in the expression of E-selectin, TNFα, IL-6, and IL-1β. Therefore, knockdown of TLR2 impairs the ability of palmitate to induce proinflammatory cytokines and adhesion molecules in endothelial cells. However, we were not able to detect the induction of monocyte chemotactic protein-1 by palmitate treatment (data not shown) and expression of TLR downstream molecules, including myeloid differentiation primary response 88 and Toll-receptor-associated activator of interferon-β. Next, we examined palmitate-stimulated proinflammatory signaling pathways, including phosphorylation of JNK and NF-κB. Palmitate stimulated phosphorylation of JNK and NF-κB in endothelial cells as expected. These actions of palmitate were impaired by knockdown of TLR2 (Fig. 2, A–D). Moreover, palmitate-stimulated nuclear translocation of NF-κB was inhibited by knockdown of TLR2 (Fig. 2E). These results suggest that TLR2 is required for palmitate-stimulated proinflammatory responses in vascular endothelial cells.
Fig. 1.
A: saturated fatty acid (FA)-stimulated proinflammatory responses in primary vascular endothelial cells are impaired by siRNA knockdown of Toll-like receptor (TLR)2. Human aortic endothelial cells (HAEC) were transiently transfected with scrambled control siRNA or with siRNA specifically targeting TLR2. After cells were incubated for 48 h, cells were serum-starved for 2 h and treated with either BSA or palmitate (200 μM, 6 h). Total RNA was isolated from cells and analyzed for expression of proinflammatory cytokines and adhesion molecules by semiquantitative RT-PCR analysis. The demarcated lines are due to the noncontiguous lanes, but they are from a single gel. B–E: the quantification of 3 independent experiments is shown as bar graphs (means ± SE). *P < 0.05; **P < 0.01; ***P < 0.001. TRIF, Toll-receptor-associated activator of interferon-β; MyD88, myeloid differentiation primary response 88.
Fig. 2.
Saturated FA-stimulated proinflammatory signaling in primary vascular endothelial cells is impaired by siRNA knockdown of TLR2. HAEC were transiently transfected with scrambled control siRNA or siRNA specifically targeting TLR2. Next, cells were incubated for 48 h and serum-starved for 2 h. Cells were then incubated with either BSA or palmitate (200 μM, 1 h). Cell lysates were subjected to immunoblotting with indicated antibodies. A and B: treatment with palmitate stimulated phosphorylation of JNK and NF-κB. C and D: the quantification of 3 independent experiments is shown in bar graphs (means ± SE). E: cells were plated in chamber slides and transfected with siRNA as above. Cells were then incubated with either BSA or palmitate (200 μM, 4 h) and subjected to immunofluorescence staining with anti-NF-κB p65 and anti-rabbit antibody (right). Overlapped image of anti-NF-κB p65 and nucleus staining with Hochest is shown (left). Fluorescence was visualized by Zeiss epifluorescent microscope. *** P < 0.001.
Role for TLR2 in palmitate-stimulated unfolding protein response that is associated with proinflammatory response.
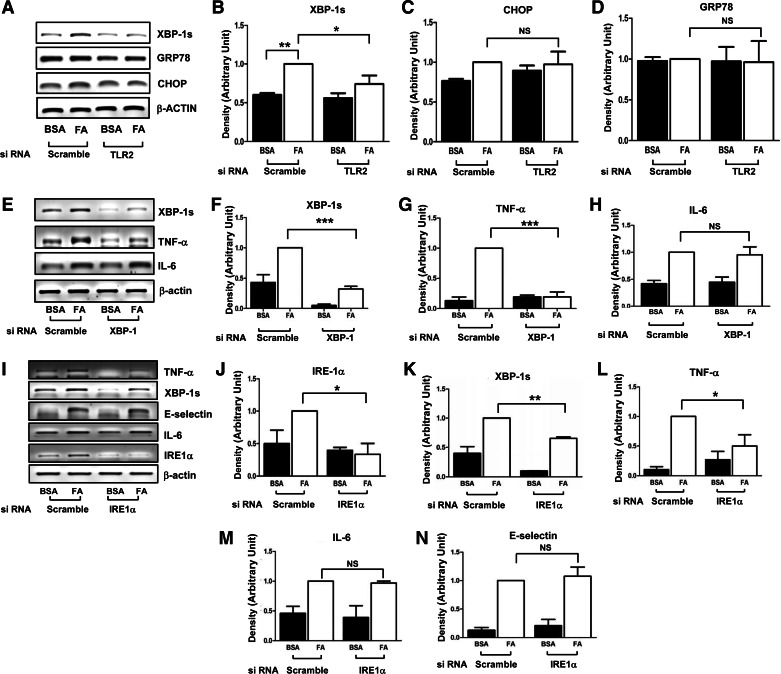
Inflammation is associated with obesity-induced ER stress (34). LPS-stimulated TLRs increase XBP-1 mRNA maturation without ER stress in macrophages (30). However, whether SFAs induce ER stress in endothelial cells through TLRs is not known. Palmitate-stimulated splicing of XBP-1 to XBP-1s was inhibited when TLR2 was knocked down. Under these same conditions, mRNA expression of CHOP and GRP78 was unaffected (Fig. 3, A–D). This suggests that treatment with palmitate stimulates UPR, similar to the response of bacteria-stimulated macrophages (30). The spliced form of XBP-1s is translated to a transcription factor that stimulates transcription of other proteins. Therefore, we next examined whether XBP-1s plays a role in palmitate-stimulated inflammatory response. siRNA knockdown of XBP-1 reduced TNFα expression (Fig. 3, E–G) but not other proinflammatory cytokines in endothelial cells (Fig. 3H). Targeted siRNA knockdown of inositol-requiring protein-1α, an endoribonuclease responsible for XBP-1 maturation, reduced the expression of TNFα but not IL-6 or E-selectin (Fig. 3, I–N). Taken together, these data suggest that TLR2 plays an important role in SFA-stimulated proinflammatory responses that are likely to involve a UPR-mediated mechanism.
Fig. 3.
Saturdated FA-mediated unfolding protein response (UPR) is impaired by siRNA knockdown of TLR2, resulting in impairment of FFA-stimulated TNFα production. HAEC were transiently transfected with siRNA targeting TLR2 (A–D), X box protein 1 (XBP-1; E–H), and inositol-requiring protein-1α (IRE-1α; I–N) or scrambled control. Forty-eight hours after transfection, cells were serum-starved for 2 h and then treated with either BSA or palmitate (200 μM, 6 h). Total RNA from cells was isolated and analyzed for the expression of genes related to the UPR. Expression of mRNA is shown in A, E, and I. The quantification of 3 independent experiments is shown in bar graphs (mean ± SE) (B, C, D, F, G, H, J, K, L, M, and N). *P < 0.05; **P < 0.01; ***P < 0.001; NSP > 0.05.
TLR2 mediates palmitate-induced impairment of insulin signaling and action in vascular endothelium.
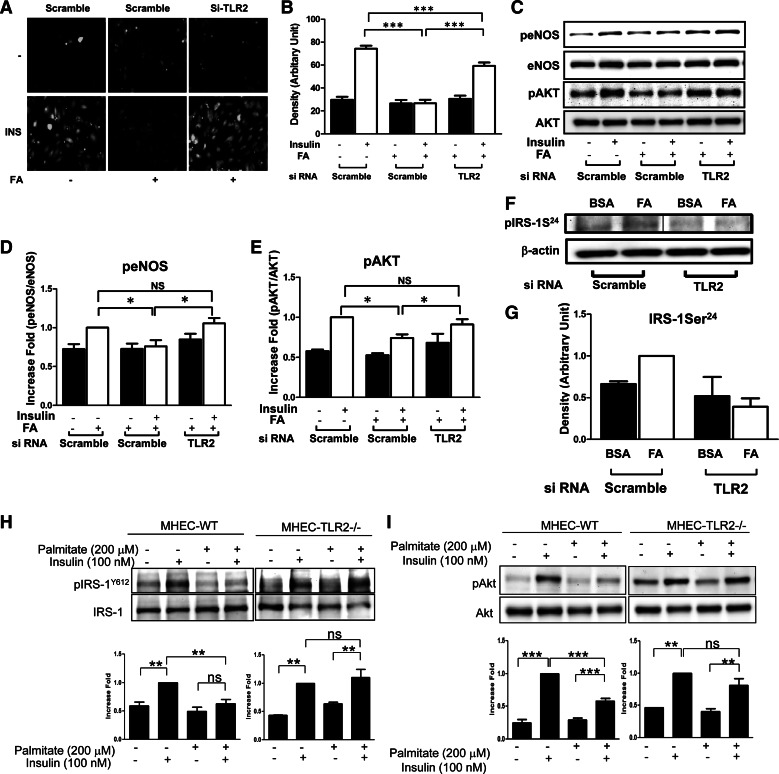
Insulin signaling through IR/IRS-1/PI 3-kinase/Akt in vascular endothelial cells stimulates phosphorylation and activation of eNOS to produce NO (57, 58). We examined whether TLR2 is involved in SFA-mediated impairment of insulin signaling and action in vascular endothelium. siRNA knockdown of TLR2 opposed the effects of palmitate in insulin-stimulated production of NO (Fig. 4, A and B). With respect to insulin signaling, pretreatment of endothelial cells with palmitate impaired insulin-stimulated phosphorylation of eNOS and Akt. Importantly, siRNA knockdown of TLR2 opposed these pathological actions of palmitate to impair insulin signaling (Fig. 4, C–E). Multiple inhibitory serine phosphorylation sites on IRS-1 have been implicated in decreased binding and activation of PI 3-kinase in response to insulin that may contribute to insulin resistance. Previously, we identified Ser24 on IRS-1 as a direct target of IL-1β receptor-activated kinase-1 and PKCδ, which are downstream components of TLR pathways (29, 32). Phosphorylation of IRS-1 at Ser24 impairs insulin-stimulated activation of PI 3-kinase and Akt (12, 19). Treatment of endothelial cells with palmitate increased phosphorylation of IRS-1 at Ser24. This was opposed by siRNA knockdown of TLR2 (Fig. 4F). Thus, SFA may impair insulin signaling pathways in part by increasing serine phsophorylation of IRS-1 at Ser24 through TLR2-mediated signaling pathways. To exclude the possibility of artifacts from treating siRNA and incomplete knockdown, we tested insulin signaling in endothelial cells isolated from WT or TLR2KO mice. Treatment with palmitate inhibited insulin-stimulated phosphorylation of IRS-1 at Tyr612 and Akt at Ser473 in MHECs isolated from WT mice. Importantly, this effect of palmitate was abolished in endothelial cells isolated from TLR2KO mice (Fig. 4, H and I). Taken together, our data suggest that TLR2 plays an important role in palmitate-induced cross-talk between proinflammatory signaling and insulin signaling in endothelial cells. This may contribute substantially to decreased NO production in response to insulin that is predicted to have adverse consequences on both metabolic and cardiovascular homeostasis.
Fig. 4.
Insulin-stimulated production of nitric oxide (NO) in primary aortic endothelial cells is impaired by pretreatment with palmitate, whereas deficiency of TLR2 opposes this action of palmitate. A: HAEC were transiently transfected with scrambled control siRNA or siRNA specifically targeting TLR2. After cells were incubated for 48 h, cells were serum-starved for 2 h, and then cells were loaded with the NO-specific fluorescent dye DAF2-DA. Cells were pretreated with BSA or palmitate (200 μM, 6 h) and then treated without or with insulin (100 nM) for 20 min. Emission of green fluorescence is indicative of NO production. Experiments shown are representative of those that were independently repeated at least 3 times. B: 3 microscopic fields/experiment were captured, and 10 randomly picked cells/field were averaged and subjected to statistical analysis by ANOVA (***P < 0.001). C–G: bovine aortic endothelial cells (BAEC) were transiently transfected with DsiRNA for scrambled or TLR2. Then, cells were incubated for 48 h in regular medium and then serum-starved overnight and pretreated with BSA or palmitate (200 μM, 6 h for C–E; 30 min for F and G) and treated without or with insulin (100 nM) for 20 min (C–E). Cell lysates were subjected to immunoblotting with indicated antibodies. The demarcated lines are due to the noncontiguous lanes, but from a single gel (F). H–I: mouse heart endothelial cells (MHEC) from C57BL/6J or TLR2KO mice were isolated. The endothelial cells were incubated in 1% FBS/F-12K media, and then cells were treated with BSA or palmitate (200 μM, 15 h), followed by insulin (100 nM, 20 min). Cell lysate were subjected to immunoblotting with indicated antibodies. The samples from wild-type (WT) and TLR2KO mice were run in parallel gels simultaneously. Values are means ± SE. Values significantly different (*P < 0.05; **P < 0.01; NSP > 0.05) from the values for the control are indicated. IRS-1, insulin receptor substrate-1; eNOS, endothelial nitric oxide synthase.
Deficiency of TLR2 protects from HFD-induced impairment of insulin signaling and action in vascular endothelium.
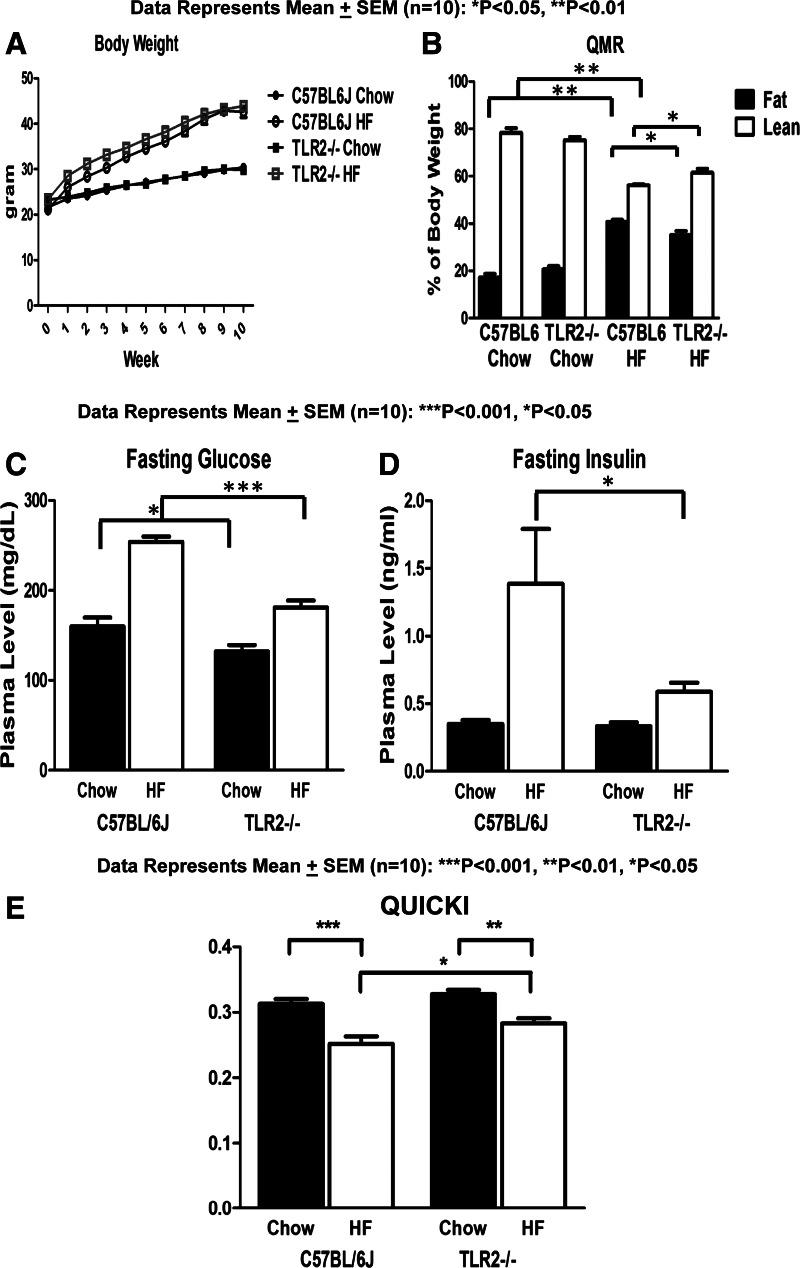
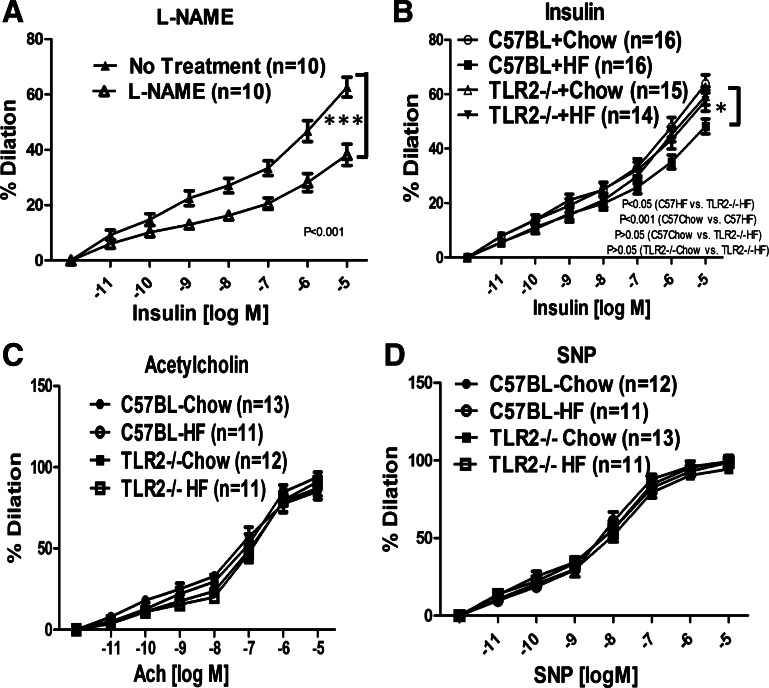
To test the role of TLR2 in obesity-induced endothelial dysfunction in intact vessels, C57BL/6J (WT) or TLR2KO mice were fed a normal chow diet (NCD; 10% calories from fat) or HFD (55% calories from fat) for 10 wk. Vasodilator actions of insulin were evaluated in mesenteric arteries isolated from these mice. As expected, both WT and TLR2KO mice gained more weight on HFD compared with NCD (Fig. 5). However, HFD TLR2KO had lower fasting glucose and insulin levels compared with HFD WT mice (Fig. 5, C and D). TLR2KO mice were partially protected from HFD-induced insulin resistance (when compared with WT mice) as assessed by QUICKI, a surrogate index for insulin sensitivity (Fig. 5E). Mesenteric arteries isolated from WT mice were dilated in response to insulin in a concentration-dependent manner (Fig. 6A). This was inhibited significantly by pretreatment with l-NAME (100 μM, competitive NOS inhibitor; Fig. 6A), indicating that insulin-stimulated vasodilator actions in these arteries are due predominantly to activity of NOS. Arteries isolated from HFD-WT mice had impaired insulin-stimulated vasodilation compared with vessels from the NCD group (P < 0.001; Fig. 6B). Of note, arteries isolated from TLR2KO mice on HFD had intact insulin-stimulated vasodilator responses comparable with those observed in arteries isolated from WT mice or TLR2KO mice on NCD (P > 0.05; Fig. 6B). Interestingly, in response to acetylcholine (ACh), we did not observe detectable differences among any of the groups with respect to vasodilation (Fig. 6C). Furthermore, the responses to sodium nitroprusside (SNP) were similar among vessels isolated from all groups of mice (Fig. 6D). This suggests strongly that vascular smooth muscle function in both WT and TLR2KO is unimpaired by HFD. Thus, consistent with our in vitro experiments in cultured endothelial cells, these ex vivo results suggest that HFD inhibits insulin-stimulated vasodilator actions through a TLR2-mediated mechanism. This may help explain in part how HFD contributes to impaired metabolic homeostasis and cardiovascular function.
Fig. 5.
Lack of TLR2 did not protect from high-fat (HF) diet-induced obesity but prevented HF diet-induced insulin resistance. A: mice fed normal chow or HF diet were weighed once/wk for 10 wk. HF diet induced obesity in WT and TLR2KO mice. B: body composition was measured by quantitative magnetic resonance (QMR). Lean body mass in TLR2KO mice fed HF diet was slightly higher compared with that in WT mice fed HF diet. C–E: mice were fasted overnight, and blood was collected before euthanization. Blood glucose levels (C) were measured by hand-held glucometer, and insulin levels (D) were measured by radioimmunoassay. Glucose and insulin levels were elevated in the WT mice fed HF diet compared with WT mice fed normal chow diet. Fasting blood glucose and insulin levels in TLR2KO mice fed HF diet were lower than in WT mice fed HFD. E: quantitative insulin sensitivity check index (QUICKI) was calculated as described in materials and methods. Values are means ± SE (n = 10). *P < 0.05, **P < 0.01, and ***P < 0.001, values significantly different from values for the control group.
Fig. 6.
Absence of TLR2 protects against HF diet induced impairment of vascular actions of insulin. C57BL/6J and TLR2−/− mice were fed with control chow or HF diet for 10 wk. Mesenteric arteries were isolated from mice. Arteries were preconstricted with phenylephrine (1 μM) and then exposed to increasing concentrations of insulin (A and B), acetylcholine (C), or sodium nitroprusside (SNP; D). The vasodilator actions in response to each agonist were observed and recorded using a data acquisition system. In some experiments (A), NG-nitro-l-arginine methyl ester (l-NAME; 100 μM, 30 min) was applied prior to insulin treatment. A: mesenteric arteries were dilated in response to insulin in a concentration-dependent manner, and this was substantially blocked by pretreatment of l-NAME. B: values that are significantly different between C57BL/6J HF vs. TLR2−/− HF are indicated. Insulin-stimulated vasodilation was impaired in WT mice but not in TLR2−/− mice fed HF diet when compared with mice fed normal chow diet. C and D: Vasodilator responses to acetylcholine or SNP were not significantly different among any of the groups (C57BL/6J vs TLR2−/− without and with HF diet). The percentage of vasorelaxation was calculated as described in materials and methods and presented as means ± SE. The data were analyzed by 2-way ANOVA for repeated measures along with a Bonferroni post hoc test (evaluating differences between entire curves). *P < 0.05; ***P < 0.001.
DISCUSSION
In the present study, we found that TLR2 plays an important role in SFA-induced inflammation and subsequent impairment of vasodilator actions of insulin. This is the first report demonstrating that TLR2 mediates HFD/SFA-induced impairment of insulin signaling and action in vascular endothelium related to endothelial dysfunction. Interestingly, SFA stimulates UPR by increasing splicing of XBP-1 to mature XBP-1s, leading to increased production of TNFα. Because TNFα causes endothelial dysfunction (37, 59), our findings suggest that SFA-mediated impairment of vascular actions of insulin may be due in part to TLR2/UPR/proinflammatory signaling and action.
Our results yield several novel insights into SFA-stimulated inflammatory responses in endothelium. First, TLR-mediated inflammatory responses are composed of both UPR-dependent and UPR-independent mechanisms (Fig. 7). Second, impaired insulin signaling and action in vascular endothelium mediated by TLR2 may contribute to endothelial dysfunction induced by HFD and/or SFA that contributes to cardiovascular complications. Third, TLR2-mediated impairment of insulin signaling and action in the vasculature may help link dysregulation of metabolic homeostasis with cardiovascular complications of diabetes, metabolic syndrome, and obesity.
Fig. 7.
Schematic diagram of proposed TLR signaling pathways that inhibit insulin-stimulated vasodilation in vascular endothelial cells. Saturated FAs stimulate TLRs that transmit signaling to activate various Ser/Thr kinases, including IL-1β receptor-associated kinase 1, PKC, JNK, and IKKβ. These Ser/Thr kinases phosphorylate IRS-1 and -2 at serine residues, including Ser24, to inhibit insulin signaling through IR/IRS-1/phosphatidylinositol (PI) 3-kinase/Akt/eNOS. TLR signaling pathways stimulate UPR to promote maturation of XBP-1 to XBP-1s that stimulates TNFα production. In addition, TLRs stimulate UPR-independent pathways, including IKKβ/NF-κB, to stimulate production of IL-6 and E-selectin.
Link between insulin resistance and impairment of vascular actions of insulin.
Obesity induces insulin resistance and endothelial dysfunction (18). The link between metabolic and vascular actions of insulin is determined in part by insulin-stimulated capillary recruitment that is responsible for ∼40% of insulin-stimulated glucose uptake in skeletal muscle (2, 23). We show that TLR2 mediates HFD-induced impairment of vascular insulin action (Fig. 6B). The insulin-stimulated capillary recruitment is NO dependent and activated through a canonical PI 3-kinase-dependent insulin signaling pathway whose components include insulin receptor/IRS-1/PI 3-kinase/PDK/Akt/eNOS (Fig. 7) (31, 57, 58). Impairment of insulin-stimulated eNOS activity contributes to reduced blood flow that helps determine metabolic insulin resistance and glucose intolerance (18, 52). Indeed, complete loss of insulin signaling in the vascular endothelium as a result of tissue-specific knockout of the insulin receptor promotes atherogenesis and increases expression of cell adhesion molecules (42). Thus, insulin signaling in vascular endothelium has specific effects to oppose inflammation and contribute to hemodynamic homeostasis. To complement our in vivo data with TLR2KO mice, our in vitro cellular studies and ex vivo data also demonstrate that TLR2 plays an important role in SFA-mediated impairment of vascular insulin action. Furthermore, we observed that there is a difference in insulin-stimulated vasodilation in WT chow vs. WT HFD (P < 0.05) but not in TLR2KO chow vs. TLR2KO HFD (P > 0.05) at 100 nM insulin (Fig. 6). These results are consistent with the results from in vitro cell culture studies. Thus, TLR2-mediated vascular insulin resistance may contribute to both metabolic and cardiovascular pathophysiology.
WT mice on HFD have impaired insulin-stimulated vasodilation compared with the same mice on a normal chow diet (Fig. 6B). By contrast, there is no detectable difference in ACh-stimulated vasodilation among these groups (Fig. 6C). Insulin and ACh stimulate production of NO by distinct mechanisms. Insulin stimulates eNOS activity using a phosphorylation-dependent mechanism. ACh stimulates eNOS activity through a Ca++-dependent G protein-coupled receptor-mediated mechanism that does not require phosphorylation of eNOS (31). Previously, we reported similar results in spontaneously hypertensive rats at 12 wk of age with intact vascular responses to ACh but impaired insulin-stimulated production of NO that contributed to hypertension, insulin resistance, and endothelial dysfunction (39, 40). In addition, mice with endothelial-specific knockout of the insulin receptor have a proatherosclerotic phenotype and impairment of insulin-stimulated vasodilation without differences in ACh-stimulated vasodilation (compared with WT mice) (42). Similarly, vessels from resistin-infused mice and a type 2 diabetic mice model showed impairment of vasodilator actions of insulin but not of ACh (11, 20). Thus, our data are consistent with studies from other groups demonstrating that impairment of insulin-stimulated vasodilation may cause endothelial dysfunction (decreased bioavailability of NO in the vasculature) without frank impairment of ACh responsiveness (15, 20, 45). It is likely that a longer period of HFD may cause more severe hyperglycemia, insulin resistance, and other abnormalities that would eventually manifest as impaired ACh actions in the vasculature.
Our data show that endothelium-independent vasodilation is not different among any of the groups tested (Fig. 6D). This suggests that vasodilator capacity of the vascular smooth muscle layer is not affected significantly by HFD or by lack of TLR2 under our experimental conditions. In addition to impaired PI 3-kinase-mediated activation of eNOS, unimpaired or augmented MAPK-dependent insulin signaling pathways may lead to increased secretion of endothelin-1 with a resultant increase in vasoconstrictor tone that augments the impaired endothelial dysfunction observed with vascular insulin resistance (39). Thus, it is possible that vasoconstrictor responses to ET-1 are augmented in HFD-induced vascular insulin resistance and that TLRs may be involved in these vascular actions of insulin. We did not address this possibility directly in the present work. However, these additional considerations merit further investigation in our future studies.
TLRs are involved in innate immunity and HFD-induced insulin resistance (4, 48). Deficiency of TLR2 does not protect from HFD-induced obesity (Fig. 5A), but it does protect from metabolic insulin resistance (Fig. 5E) and impaired insulin signaling and action in the vasculature (Figs. 4 and 6). These results suggest that SFA directly impairs insulin signaling and action in vascular endothelial cells, contributing to endothelial dysfunction. Moreover, lack of TLR2 in vascular endothelium is sufficient to uncouple obesity from both metabolic and vascular insulin resistance. Nevertheless, we cannot exclude the possibility that the inhibition of insulin actions by HFD or SFA under pathophysiological conditions may be due at least in part to other direct or indirect effects of hyperglycemia and hyperinsulinemia/insulin resistance. Cross-talk between metabolic and vascular tissues underlies reciprocal relationships between insulin resistance and endothelial dysfunction that compound deleterious effects of HFD on metabolic and cardiovascular function. Tissue-specific knockout of TLR2 in the vasculature in our study opposes this pathophysiology. Lack of TLR was able to protect against HFD-induced obesity in some (24, 38), but not all (8, 48), previous studies. From these mixed results, it remains unclear whether TLRs play a role in HFD-induced body weight gain. Differences in these results among these studies may be due to differences in the background mice strain, components of diet, the duration of diet, or gut microbiota (5).
Mechanisms for TLR-mediated insulin resistance.
TLR-mediated inflammation contributes to insulin resistance in various tissues, including liver, adipose tissue, and vascular tissue (8, 10, 16, 24, 35, 48, 54). In the present study, we show for the first time that functional consequences of TLR2 activation to impair vascular actions of insulin may contribute to overall dysregulation of metabolic homeostasis. We observed that knockdown of TLR2 increased expression of TLR4 slightly (Fig. 1A). This may be a compensatory response for reduced expression of TLR2. However, even if this increased expression is “compensatory” in some contexts, it was clearly insufficient to substitute for the loss of TLR2 with respect to the vascular and metabolic functions that we investigated and did not have a detectable effect on the fatty acid-induced inflammatory responses that we evaluated. Our results are consistent with a previous study by Nguyen et al. (33) showing that knockdown of either TLR2 or TLR4 almost completely inhibited the fatty acid-induced responses. Inflammatory cytokines inhibit insulin signaling pathways in part through activation of serine/threonine kinases that phosphorylate negative regulatory S/T residues on insulin receptor substrates (36). We observed that Ser24 on IRS-1 is phosphorylated by SFA-stimulated TLR2 in primary endothelial cells (Fig. 4F). Previously, we reported that phosphorylation of IRS-1 at Ser24 inhibits insulin-stimulated IRS-1 binding and activation of PI 3-kinase and GLUT4 translocation in adipose cells (12, 19). Ser24 is located in the pleckstrin homology domain in IRS-1 that contributes to binding of IRS-1 to phospholipids (19). Thus, phosphorylation of IRS-1 at Ser24 inhibits interaction of IRS-1 with phospholipids, including the plasma membrane. The known protein kinases responsible for phosphorylation of IRS-1 at Ser24 are IL-1β receptor-associated kinase 1 and PKCs (12, 19) that are downstream components of TLRs (1, 7, 25, 49). Production of other cytokines by TLR-mediated mechanism may potentiate this negative regulation of insulin signaling pathways. However, more detailed mechanisms for inhibition of insulin signaling by TLRs remain to be elucidated.
TLR-mediated ER stress and apoptosis have been observed in atherosclerosis (46, 53). However, mechanisms linking ER stress and inflammation are not well understood. Our study suggests, for the first time, that the UPR plays a role in SFA-induced proinflammatory response through a TLR2-mediated mechanism (Fig. 3). XBP-1s plays a role in the production of TNFα that in turn contributes to endothelial dysfunction and insulin resistance (14, 55, 59). Thus, increased production of TNFα may contribute importantly to the impairment of endothelial vasodilator properties.
We conclude that SFA-induced impairment of vasodilator actions of insulin is mediated in part through TLR2. TLR2-mediated UPR and proinflammatory responses may represent mechanisms that contribute to vascular insulin resistance. Thus, our study has uncovered an additional potential mechanism for SFA-induced impairment of vascular function. This may contribute importantly to the pathophysiology of diabetes, metabolic syndrome, obesity, and their cardiovascular complications.
GRANTS
This study was supported by the American Diabetes Association (1-09-JF-33 and 1-12-BS-99 to J. Kim) and a UAB Diabetes Research Training Center-sponsored pilot and feasibility program (P60-DK-079626) and by our Nutrition Obesity Research Center (P30-DK-056336), a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-064007 to D. H. Hwang), and program funds from the Western Human Nutrition Research Center/Agricultural Research Service/USDA and intramural research program, National Center for Complementary and Alternative Medicine, National Institutes of Health (M. J. Quon). The UAB metabolism core facility is funded by the National Institute of Diabetes and Digestive and Kidney Diseases (P30-DK-56336 and P60-DK-079626) and the National Center for Research Resources (UL-1RR-025777).
DISCLOSURES
M. J. Quon is a member of the Merck Speaker Bureau.
AUTHOR CONTRIBUTIONS
H.-J.J., H.-S.K., and J.-a.K. performed the experiments; H.-J.J., H.-S.K., M.J.Q., and J.-a.K. analyzed the data; H.-J.J., H.-S.K., and J.-a.K. prepared the figures; H.-J.J., H.-S.K., D.H.H., M.J.Q., and J.-a.K. edited and revised the manuscript; H.-J.J., H.-S.K., D.H.H., M.J.Q., and J.-a.K. approved the final version of the manuscript; H.-S.K., D.H.H., M.J.Q., and J.-a.K. interpreted the results of the experiments; J.-a.K. contributed to the conception and design of the research; J.-a.K. drafted the manuscript.
ACKNOWLEDGMENTS
We thank Simone D. Ridgeway and Pratibha Vashista for technical assistance and for reviewing manuscript. The US Department of Agriculture (USDA) is an equal opportunity provider and employer.
REFERENCES
- 1. Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2: 675–680, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Baron AD, Tarshoby M, Hook G, Lazaridis EN, Cronin J, Johnson A, Steinberg HO. Interaction between insulin sensitivity and muscle perfusion on glucose uptake in human skeletal muscle: evidence for capillary recruitment. Diabetes 49: 768–774, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, Akira S, Ulmer AJ. TLR1- and TLR6-independent recognition of bacterial lipopeptides. J Biol Chem 281: 9049–9057, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Caricilli AM, Nascimento PH, Pauli JR, Tsukumo DM, Velloso LA, Carvalheira JB, Saad MJ. Inhibition of toll-like receptor 2 expression improves insulin sensitivity and signaling in muscle and white adipose tissue of mice fed a high-fat diet. J Endocrinol 199: 399–406, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Caricilli AM, Picardi PK, de Abreu LL, Ueno M, Prada PO, Ropelle ER, Hirabara SM, Castoldi A, Vieira P, Camara NO, Curi R, Carvalheira JB, Saad MJ. Gut microbiota is a key modulator of insulin resistance in TLR 2 knockout mice. PLoS Biol 9: e1001212, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6. Chen H, Sullivan G, Quon MJ. Assessing the predictive accuracy of QUICKI as a surrogate index for insulin sensitivity using a calibration model. Diabetes 54: 1914–1925, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Dasu MR, Devaraj S, Park S, Jialal I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 33: 861–868, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davis JE, Braucher DR, Walker-Daniels J, Spurlock ME. Absence of Tlr2 protects against high-fat diet-induced inflammation and results in greater insulin-stimulated glucose transport in cultured adipocytes. J Nutr Biochem 22: 136–141, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, Vollenweider P, Pedrazzini T, Nicod P, Thorens B, Scherrer U. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation 104: 342–345, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Frisard MI, McMillan RP, Marchand J, Wahlberg KA, Wu Y, Voelker KA, Heilbronn L, Haynie K, Muoio B, Li L, Hulver MW. Toll-like receptor 4 modulates skeletal muscle substrate metabolism. Am J Physiol Endocrinol Metab 298: E988–E998, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gentile MT, Vecchione C, Marino G, Aretini A, Di Pardo A, Antenucci G, Maffei A, Cifelli G, Iorio L, Landolfi A, Frati G, Lembo G. Resistin impairs insulin-evoked vasodilation. Diabetes 57: 577–583, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Greene MW, Ruhoff MS, Roth RA, Kim JA, Quon MJ, Krause JA. PKCdelta-mediated IRS-1 Ser24 phosphorylation negatively regulates IRS-1 function. Biochem Biophys Res Commun 349: 976–986, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Hotamisligil GS. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obes (Lond) 32, Suppl 7: S52–S54, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 271: 665–668, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Katakam PV, Tulbert CD, Snipes JA, Erdos B, Miller AW, Busija DW. Impaired insulin-induced vasodilation in small coronary arteries of Zucker obese rats is mediated by reactive oxygen species. Am J Physiol Heart Circ Physiol 288: H854–H860, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res 100: 1589–1596, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Kim JA, Formoso G, Li Y, Potenza MA, Marasciulo FL, Montagnani M, Quon MJ. Epigallocatechin gallate, a green tea polyphenol, mediates NO-dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and Fyn. J Biol Chem 282: 13736–13745, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113: 1888–1904, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Kim JA, Yeh DC, Ver M, Li Y, Carranza A, Conrads TP, Veenstra TD, Harrington MA, Quon MJ. Phosphorylation of Ser24 in the pleckstrin homology domain of insulin receptor substrate-1 by Mouse Pelle-like kinase/interleukin-1 receptor-associated kinase: cross-talk between inflammatory signaling and insulin signaling that may contribute to insulin resistance. J Biol Chem 280: 23173–23183, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi T, Taguchi K, Yasuhiro T, Matsumoto T, Kamata K. Impairment of PI3-K/Akt pathway underlies attenuated endothelial function in aorta of type 2 diabetic mouse model. Hypertension 44: 956–962, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Kopp A, Buechler C, Bala M, Neumeier M, Scholmerich J, Schaffler A. Toll-like receptor ligands cause proinflammatory and prodiabetic activation of adipocytes via phosphorylation of extracellular signal-regulated kinase and c-Jun N-terminal kinase but not interferon regulatory factor-3. Endocrinology 151: 1097–1108, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Kopp A, Buechler C, Neumeier M, Weigert J, Aslanidis C, Scholmerich J, Schaffler A. Innate immunity and adipocyte function: ligand-specific activation of multiple Toll-like receptors modulates cytokine, adipokine, and chemokine secretion in adipocytes. Obesity (Silver Spring) 17: 648–656, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, Inoue M, Itoh S, Takamoto I, Sasako T, Kumagai K, Kawai T, Hashimoto S, Kobayashi T, Sato M, Tokuyama K, Nishimura S, Tsunoda M, Ide T, Murakami K, Yamazaki T, Ezaki O, Kawamura K, Masuda H, Moroi M, Sugi K, Oike Y, Shimokawa H, Yanagihara N, Tsutsui M, Terauchi Y, Tobe K, Nagai R, Kamata K, Inoue K, Kodama T, Ueki K, Kadowaki T. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab 13: 294–307, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Kuo LH, Tsai PJ, Jiang MJ, Chuang YL, Yu L, Lai KT, Tsai YS. Toll-like receptor 2 deficiency improves insulin sensitivity and hepatic insulin signalling in the mouse. Diabetologia 54: 168–179, 2011 [DOI] [PubMed] [Google Scholar]
- 25. Langlet C, Springael C, Johnson J, Thomas S, Flamand V, Leitges M, Goldman M, Aksoy E, Willems F. PKC-alpha controls MYD88-dependent TLR/IL-1R signaling and cytokine production in mouse and human dendritic cells. Eur J Immunol 40: 505–515, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem 276: 16683–16689, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Lee S, Muniyappa R, Yan X, Chen H, Yue LQ, Hong EG, Kim JK, Quon MJ. Comparison between surrogate indexes of insulin sensitivity and resistance and hyperinsulinemic euglycemic clamp estimates in mice. Am J Physiol Endocrinol Metab 294: E261–E270, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Lim YC, Garcia-Cardena G, Allport JR, Zervoglos M, Connolly AJ, Gimbrone MA, Jr, Luscinskas FW. Heterogeneity of endothelial cells from different organ sites in T-cell subset recruitment. Am J Pathol 162: 1591–1601, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loegering DJ, Lennartz MR. Protein kinase C and toll-like receptor signaling. Enzyme Res 2011: 537821, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol 11: 411–418, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179). J Biol Chem 276: 30392–30398, 2001 [DOI] [PubMed] [Google Scholar]
- 31a. Mott DM, Hoyt C, Caspari R, Stone K, Pratley R, Bogardus C. Palmitate oxidation rate and action on glycogen synthase in myoblasts from insulin-resistant subjects. Am J Physiol Endocrinol Metab 279: E561–E569, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Moynagh PN. The Pellino family: IRAK E3 ligases with emerging roles in innate immune signalling. Trends Immunol 30: 33–42, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 282: 35279–35292, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pang S, Tang H, Zhuo S, Zang YQ, Le Y. Regulation of fasting fuel metabolism by toll-like receptor 4. Diabetes 59: 3041–3048, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paz K, Hemi R, LeRoith D, Karasik A, Elhanany E, Kanety H, Zick Y. A molecular basis for insulin resistance. Elevated serine/threonine phosphorylation of IRS-1 and IRS-2 inhibits their binding to the juxtamembrane region of the insulin receptor and impairs their ability to undergo insulin-induced tyrosine phosphorylation. J Biol Chem 272: 29911–29918, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res 99: 69–77, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Poggi M, Bastelica D, Gual P, Iglesias MA, Gremeaux T, Knauf C, Peiretti F, Verdier M, Juhan-Vague I, Tanti JF, Burcelin R, Alessi MC. C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia 50: 1267–1276, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Potenza MA, Marasciulo FL, Chieppa DM, Brigiani GS, Formoso G, Quon MJ, Montagnani M. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol 289: H813–H822, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Potenza MA, Marasciulo FL, Tarquinio M, Quon MJ, Montagnani M. Treatment of spontaneously hypertensive rats with rosiglitazone and/or enalapril restores balance between vasodilator and vasoconstrictor actions of insulin with simultaneous improvement in hypertension and insulin resistance. Diabetes 55: 3594–3603, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Quon MJ. QUICKI is a useful and accurate index of insulin sensitivity. J Clin Endocrinol Metab 87: 949–951, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu IH, Chen K, Yamamoto-Hiraoka J, Goldenbogen J, Sotiropoulos KB, Clermont A, Geraldes P, Dall'Osso C, Wagers AJ, Huang PL, Rekhter M, Scalia R, Kahn CR, King GL. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab 11: 379–389, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rizzo NO, Maloney E, Pham M, Luttrell I, Wessells H, Tateya S, Daum G, Handa P, Schwartz MW, Kim F. Reduced NO-cGMP signaling contributes to vascular inflammation and insulin resistance induced by high-fat feeding. Arterioscler Thromb Vasc Biol 30: 758–765, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schenk M, Belisle JT, Modlin RL. TLR2 looks at lipoproteins. Immunity 31: 847–849, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Schulman IH, Zhou MS, Jaimes EA, Raij L. Dissociation between metabolic and vascular insulin resistance in aging. Am J Physiol Heart Circ Physiol 293: H853–H859, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Seimon TA, Nadolski MJ, Liao X, Magallon J, Nguyen M, Feric NT, Koschinsky ML, Harkewicz R, Witztum JL, Tsimikas S, Golenbock D, Moore KJ, Tabas I. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab 12: 467–482, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Senn JJ. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem 281: 26865–26875, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116: 3015–3025, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shin DM, Yang CS, Lee JY, Lee SJ, Choi HH, Lee HM, Yuk JM, Harding CV, Jo EK. Mycobacterium tuberculosis lipoprotein-induced association of TLR2 with protein kinase C zeta in lipid rafts contributes to reactive oxygen species-dependent inflammatory signalling in macrophages. Cell Microbiol 10: 1893–1905, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sorrentino R, Arditi M. Innate immunity, Toll-like receptors, and atherosclerosis: mouse models and methods. Methods Mol Biol 517: 381–399, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 94: 1172–1179, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 97: 2601–2610, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tabas I, Seimon T, Timmins J, Li G, Lim W. Macrophage apoptosis in advanced atherosclerosis. Ann NY Acad Sci 1173, Suppl 1: E40–E45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araujo EP, Vassallo J, Curi R, Velloso LA, Saad MJ. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 56: 1986–1998, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 389: 610–614, 1997 [DOI] [PubMed] [Google Scholar]
- 57. Zeng G, Nystrom FH, Ravichandran LV, Cong LN, Kirby M, Mostowski H, Quon MJ. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation 101: 1539–1545, 2000 [DOI] [PubMed] [Google Scholar]
- 58. Zeng G, Quon MJ. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest 98: 894–898, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang C, Hein TW, Wang W, Ren Y, Shipley RD, Kuo L. Activation of JNK and xanthine oxidase by TNF-alpha impairs nitric oxide-mediated dilation of coronary arterioles. J Mol Cell Cardiol 40: 247–257, 2006 [DOI] [PubMed] [Google Scholar]