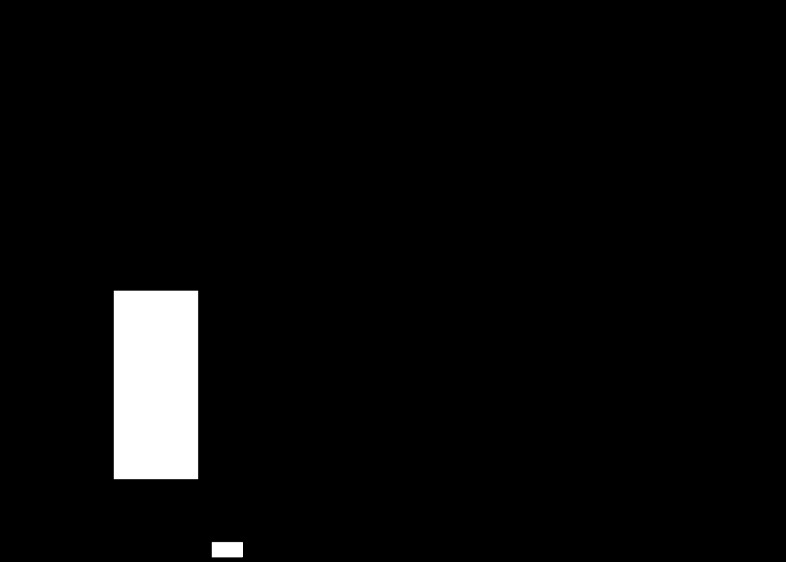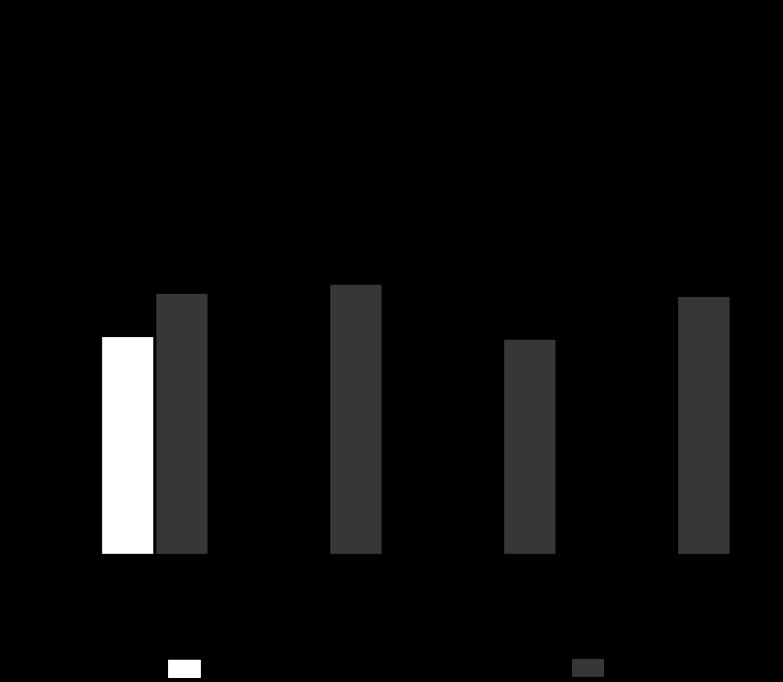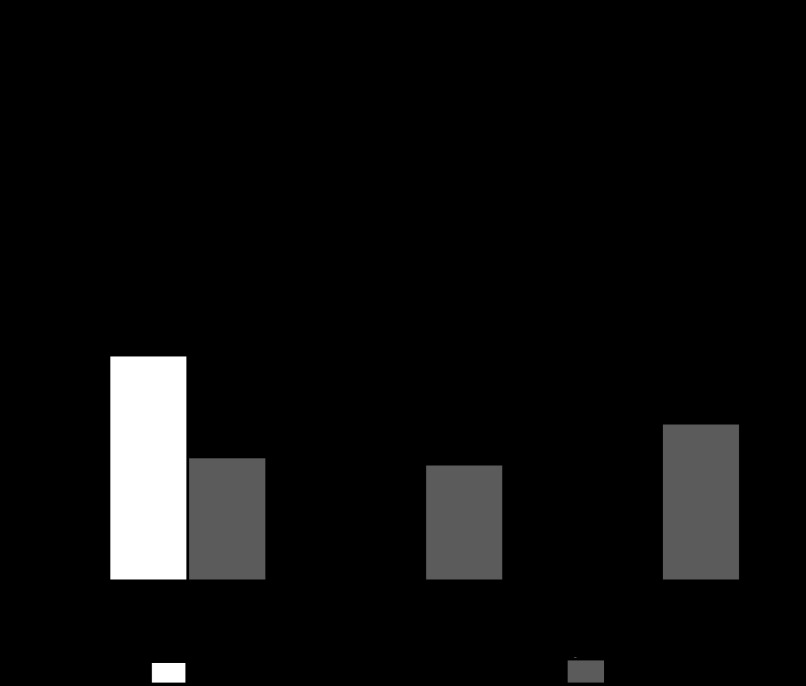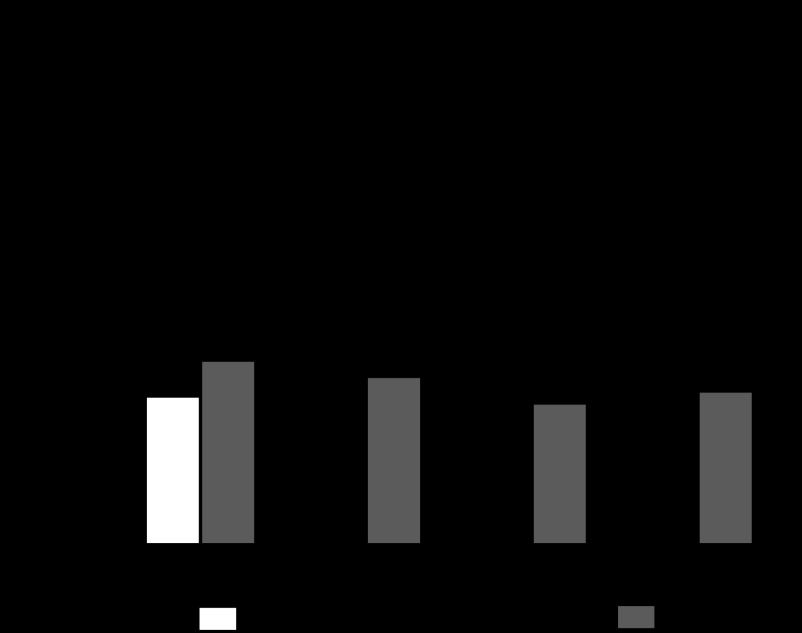Abstract
The acute direct action of angiotensin-(1–7) [ANG-(1–7)] on bicarbonate reabsorption (JHCO3−) was evaluated by stationary microperfusions on in vivo middle proximal tubules in rats using H ion-sensitive microelectrodes. The control JHCO3− is 2.82 ± 0.078 nmol·cm−2·s−1 (50). ANG-(1–7) (10−12 or 10−9 M) in luminally perfused tubules decreases JHCO3− (36 or 60%, respectively), but ANG-(1–7) (10−6 M) increases it (80%). A779 increases JHCO3− (30%) and prevents both the inhibitory and the stimulatory effects of ANG-(1–7) on it. S3226 decreases JHCO3− (45%) and changes the stimulatory effect of ANG-(1–7) to an inhibitory effect (30%) but does not affect the inhibitory effect of ANG-(1–7). Our results indicate that in the basal condition endogenous ANG-(1–7) inhibits JHCO3− and that the biphasic dose-dependent effect of ANG-(1–7) on JHCO3− is mediated by the Mas receptors via the Na+/H+ exchanger 3 (NHE3). The control value of intracellular Ca2+ concentration ([Ca2+]i), as monitored using fura-2 AM, is 101 ± 2 nM (6), and ANG-(1–7) (10−12, 10−9, or 10−6M) transiently (3 min) increases it (by 151, 102, or 52%, respectively). A779 increases the [Ca2+]i (25%) but impairs the stimulatory effect of all doses of ANG-(1–7) on it. The use of BAPTA or thapsigargin suggests a correlation between the ANG-(1–7) dose-dependent effects on [Ca2+]i and JHCO3−. Therefore, the interaction of the opposing dose-dependent effects of ANG II and ANG-(1–7) on [Ca2+]i and JHCO3− may represent an physiological regulatory mechanism of extracellular volume and/or pH changes. However, whether [Ca2+]i modification is an important direct mechanism for NHE3 activation by these peptides or is a side effect of other signaling pathways will require additional studies.
Keywords: ANG-(1–7) acute direct proximal action, Na+/H+ exchanger, cytosolic calcium
ang-(1–7) has been shown to have central nervous system effects (6) and acts to oppose the vasoconstrictive, proliferative, and profibrotic actions of ANG II in the cardiovascular system and renal tissues (8, 10). However, the renal effects of this heptapeptide are incompletely understood and the data are conflicting.
In the proximal tubule of anesthetized animals, ANG 1–7 increases urinary flow rates and sodium excretion. This effect is abolished by the Mas receptor antagonist A779 in rats (19), but in dogs, this hormonal effect is partially blocked by the AT1 receptor antagonist EXP 3174 and not by the AT2 receptor antagonist PD123319 (20). In isolated rat proximal straight tubules, similarly to ANG II, ANG-(1–7) has a biphasic effect through AT1 receptors: at physiologic levels (10−12 M), ANG-(1–7) increases fluid and bicarbonate absorption, and at high concentrations (10−8 M), ANG-(1–7) decreases fluid absorption (11). In isolated pig kidney inner cortical membranes, ANG-(1–7) inhibits Na+-ATPase activity; this effect is reversed by the AT2 receptor antagonist (5). In rabbit proximal tubular cells, ANG-(1–7) inhibits sodium reabsorption with activation of phospholipase A2 (2). It is also possible that ANG-(1–7) expresses diuresis and natriuresis via inhibition of the renal tubular Na+-K+-ATPase pump (3, 19). Taken together, these results suggest that ANG-(1–7) affects natriuresis and diuresis via activation of Mas receptors; however, AT1 and AT2 receptors may also be involved.
In contrast, in either normal or hypertensive rats (40) or in water-loaded (38) or virgin female rats (22), ANG-(1–7) has an antidiuretic effect mediated, at least in part, by the Mas receptor. In addition, in Sprague-Dawley rats, the administration of ANG-(1–7) results in antidiuresis associated with an upregulation of renal aquaporin-1 (23). In isolated rat inner medullary collecting duct cell suspensions, ANG-(1–7) (10−9 M) increases cAMP production (27); this response was attenuated by Mas receptor antagonists and by a pharmacological blockade of the vasopressin V2 receptor.
The apical Na+/H+ exchanger (isoform NHE3) mediates the majority of NaCl, NaHCO3−, and fluid reabsorption by the renal proximal tubule (18, 31). In addition, it has been shown that the NHE exchanger has calmodulin binding sites at the cytoplasmatic regulatory domain, which modulate its activity (7, 43). Confirming this finding, studies from our laboratory have shown that the dose-dependent biphasic effects of ANG II (32, 34), aldosterone (26), and arginine vasopressin (33) on the Na+/H+ exchanger are associated with either a small or large increase in intracellular Ca2+ concentration ([Ca2+]i).
Therefore, this study was designed based on the findings described above and on the fact that, under physiological conditions, plasma concentrations of ANG-(1–7) are in the picomolar range and can increase in conditions of extracellular volume expansion (24, 35, 36). In this study, we aimed to determine the effect of ANG-(1–7) (10−12, 10−9, or 10−6 M), added to an intratubular perfusion alone or in the presence of A779 [a specific ANG-(1–7) receptor Mas antagonist], S3226 (a specific inhibitor of isoform NHE3), dimethyl-BAPTA/AM (BAPTA, an intracellular calcium chelator), or thapsigargin (an intracellular calcium releaser) on JHCO3− in vivo and on [Ca2+]i in isolated superfused proximal tubules.
METHODS
Male Wistar rats (220–350 g), obtained from the Department of Physiology and Biophysics, Biomedical Sciences Institute, University of São Paulo, received a rat pellet diet and water ad libitum until the time of the experiment. They were anesthetized with tiletamine/zolazepam (Zoletil-Virbac) at 100 mg/kg via ip and were prepared for in vivo micropuncture (29). A tracheostomy was performed, and the left jugular vein and left carotid artery were cannulated for infusions and blood withdrawal, respectively. The kidney was isolated using a lumbar approach, immobilized in situ using Ringer-agar in a Lucite cup under a microscope, and adequately illuminated. During the course of the experiments, the animals received venous saline containing 3% mannitol at 0.05 ml/min.
Measurement of JHCO3−.
Net bicarbonate reabsorption was evaluated using stopped-flow microperfusion of in vivo S2 segments, with continuous measurements of the intratubular pH (29). The tubule was perfused using a double-barreled micropipette; one barrel was filled with Sudan black-colored castor oil and the other with the luminal perfusion solution colored with 0.05% FD&C green. The intratubular pH was measured as the voltage difference between the two asymmetric barrels of the H+ ion-sensitive microelectrode: the larger barrel contained an H+-sensitive ion-exchange resin (Fluka), and the smaller one contained 1 M KCl colored by FD&C green (reference barrel). The transepithelial electrical potential difference (PD; between the reference barrel and ground) was the main criterion for identifying the S2 segment (14). Intratubular pH changes were recorded continuously in the same tubule with a microcomputer equipped with an analog-to-digital conversion board (Lynx, São Paulo, Brazil) for data acquisition and processing.
JHCO3− was measured by injecting a droplet of the luminal perfusion solution between the oil columns and following the intratubular pH changes toward the steady-state level [(pH)s]. From the intratubular pH values measured along this curve and from systemic Pco2, the intratubular concentrations of HCO3− were calculated at intervals of 1 s using the Henderson-Hasselbach equation. This calculation is possible because we have previously demonstrated that renal cortical Pco2 is similar to that of arterial blood (30). The rate of tubular acidification was expressed as the half-time (t1/2) of the reduction of the injected HCO3− levels to their stationary level. JHCO3− was calculated from the following equation (29):
where k is the rate constant of the reduction of luminal bicarbonate [k = ln2/(t1/2)], r is the tubule radius, and (HCO3−)i and (HCO3−)s are the concentrations of HCO3− at the injected level and at the stationary level, respectively.
Before and after every tubular impalement on the kidney's surface, the pH microelectrodes were calibrated by superfusion with 20 mM phosphate Ringer buffer solutions containing 130 mM NaCl at 37°C. Their pH values were adjusted to 6.5, 7.0, and 7.5 with either 0.1 N NaOH or HCl.
The luminal perfusion solution contained the following (in mM): 100 NaCl, 25 NaHCO3−, 5 KCl, 1 CaCl2, and 1.2 MgSO4. The osmolality was adjusted to ∼290 mosmol/kgH2O with raffinose.
JHCO3− was evaluated in the presence of the intratubular perfusion control solution alone or plus ANG-(1–7) (10−12, 10−9, or 10−6 M), A779 [10−6 M], S3226 (10−6 M), BAPTA (5 × 10−5 M), or thapsigargin (10−5 M).
In each tubule, several measurements of JHCO3− were performed, but only one intratubular perfusion solution was used. The measurements were performed within ∼0.3 min of the start of the luminal perfusion.
Measurement of [Ca2+]i.
The kidneys were removed from anesthetized male Wistar rats (80 g), and 2-mm thick slices were prepared. Then, the proximal tubules were isolated by microdissection and transferred to glass coverslips prepared with poly-d-lysine (for tubule adhesion). The coverslips were mounted on a Leica DMI6000B (Leica Microsystem) fluorescence microscope in a thermostatically regulated perfusion chamber. Changes in [Ca2+]i were monitored fluorometrically using the calcium-sensitive probe fura-2 AM (Molecular Probes; Ref. 33). The proximal segments were loaded for 15 min with 5 μM fura-2 AM at 37°C and rinsed in a solution containing the following (in mM): 137 NaCl, 12 NaHCO3−, 2.68 KCl, 1.36 CaCl2, 0.49 MgCl2, 0.36 Na2HPO4, 5.6 glucose, and 5.0 HEPES at pH 7.4. The fura-2 intensity emitted at 510 nm was imaged using excitation at 340 and 380 nm. The images were continuously acquired (at time intervals of 600 ms) before and after substitution of experimental solutions. For each tubular Ca2+ measurement, the maximum fluorescence signals for 10 areas of the same tubular segment were averaged and then used to calculate the [Ca2+]i of this segment. The fluorescence signal was transformed to [Ca2+]i by calibration with ionomycin (5 μM; maximum Ca2+ concentration), followed by EGTA (2.5 mM; minimum Ca2+ concentration) according to the Grynkiewicz equation (15).
All applied chemicals were of analytic grade and were obtained from Sigma Chemical (St Louis, MO).
The pH and Pco2 in samples of blood were measured with a Radiometer ABL 5 blood gas system.
The data are the means ± SE; n = number of measurements/number of tubules. The differences between experimental groups were evaluated by ANOVA (one-way) with contrasts using the Bonferroni technique.
This study was approved by the Biomedical Sciences Institute, University of São Paulo-Ethical Committee for Animal Research (CEEA).
RESULTS
No significant changes in intratubular stationary (pH)s or (HCO3−)s were observed in any of the experimental groups, suggesting that the main driving force for H+ secretion (i.e., the Na+ gradient across the apical membrane) was not significantly altered.
Table 1 summarizes the main values of the parameters of tubular acidification and the transepithelial electrical PDs found in the studied groups. No significant changes in transepithelial PD were observed for any of the experimental groups, and during the intratubular perfusions, no significant changes occurred. These data indicate that the mean control value of JHCO3− is 2.82 ± 0.078 nmol·cm−2·s−1 [50/20 (number of measurements/number of tubules)].
Table 1.
Effect of intratubular microperfusion of ANG-(1–7) and/or A779, S3226, BAPTA, and/or thapsigargin on the parameters of tubular acidification in in vivo rat proximal S2 segments
| t/2, s | JHCO3−, nmol/cm−2·s−1 | n | DP, mV | |
|---|---|---|---|---|
| Control | 3.25 ± 0.097 | 2.82 ± 0.078 | 50/20 | −2 |
| ANG-(1–7) (10−12 M) | 7.51 ± 1.4* | 1.80 ± 0.21* | 52/14 | −1 |
| ANG-(1–7) (10−9 M) | 6.31 ± 0.57* | 1.11 ± 0.119* | 78/15 | −2 |
| ANG-(1–7) (10−6 M) | 1.68 ± 0.111* | 5.09 ± 0.46* | 70/18 | −3 |
| A779 (10−6 M) | 2.94 ± 0.228 | 3.68 ± 0.394* | 64/20 | −2 |
| ANG-(1–7) (10−12 M) + A779 | 2.09 ± 0.20† | 3,52 ± 0.50† | 58/21 | −1 |
| ANG-(1–7) (10−9 M) + A779 | 2.13 ± 0.159† | 2.81 ± 0.44† | 30/14 | −1 |
| ANG-(1–7) (10−6 M) + A779 | 2.73 ± 0.361† | 3.36 ± 0.421† | 64/14 | −2 |
| S3226 (10−6 M) | 5.81 ± 0.466* | 1.56 ± 0.168* | 53/11 | −2 |
| ANG-(1–7) (10−9 M) + S3226 | 5.97 ± 0.647 | 1.47 ± 0.149 | 30/11 | −2 |
| ANG-(1–7) (10−6 M) + S3226 | 4.61 ± 0.539† | 1.98 ± 0.358† | 47/15 | −3 |
| BAPTA (5 × 10−5 M) | 3.19 ± 0.27 | 2.84 ± 0.28 | 22/13 | −2 |
| BAPTA + ANG-(1–7) (10−9 M) | 3.90 ± 0.55*† | 2.14 ± 0.43*† | 18/5 | −1 |
| BAPTA + ANG-(1–7) (10−6 M) | 3.63 ± 0.50† | 2.93 ± 0.40† | 17/6 | −2 |
| Thapsigargin (10−5 M) | 5.60 ± 0.21* | 1.69 ± 0.28* | 22/8 | −2 |
| Thapsigargin + ANG-(1–7) (10−9 M) | 3.27 ± 0.24† | 2.05 ± 0.16*† | 20/8 | −1 |
| Thapsigargin + ANG-(1–7) (10−6 M) | 5.42 ± 0.96*† | 1.70 ± 0.28*† | 25/9 | −2 |
Values are means ± SE; n = number of microperfusions/number of tubules. t/2, half-time of tubular acidification; JHCO3−, net bicarbonate reabsorption; DP, transepithelial electrical potential differences; A779, a specific receptor Mas antagonist; S3226, a specific Na+/H+ exchanger (NHE3) inhibitor; BAPTA, dimethyl-BAPTA/AM, an intracellular calcium chelator; thapsigargin, an intracellular calcium releaser.
P < 0.01 vs. control;
P < 0.01 vs. angiotensin-(1–7) [ANG-(1–7)] alone in the respective dose.
Figure 1 indicates that low doses of ANG-(1–7) (10−12 or 10−9 M) in luminally perfused tubules caused a significant decrease in JHCO3− (36 and 60% of the control value, respectively) but that a high dose of ANG-(1–7) (10−6 M) increased JHCO3− (80% over the control value). Thus our results show that ANG-(1–7) has a biphasic effect on JHCO3− from in vivo middle proximal tubules.
Fig. 1.
Direct effect of angiotensin-(1–7) [ANG-(1–7)] (10−12, 10−9, or 10−6 M) tubular luminal microperfusion on net bicarbonate reabsorption (JHCO3−) in in vivo rat proximal S2 segments. Values are the means ± SE; n = number of microperfusions/number of tubules. *P < 0.01 vs. control.
Figure 2 shows that the inhibition of the Mas receptor increased JHCO3− ∼30% over the control value, indicating that A779 has an unspecific action or that, for the basal situation, endogenous intratubular ANG-(1–7) inhibits JHCO3−. Additionally, Fig. 2 indicates that the inhibition of the Mas receptor abolishes the inhibitory effect of low doses of ANG-(1–7) (10−12 or 10−9 M) and the stimulatory effect of high doses of ANG-(1–7) (10−6 M) on JHCO3−.
Fig. 2.
Direct effect of ANG-(1–7) and/or A779 (10−6 M; a specific receptor Mas antagonist) tubular luminal microperfusion on net bicarbonate reabsorption in in vivo rat proximal S2 segments. Values are the means ± SE; n = number of microperfusions/number of tubules. *P < 0.01 vs. control. #P < 0.01 vs. ANG-(1–7) alone in the respective dose. No significant difference was observed between the A779 alone and the ANG-(1–7) (10−12, 10−9, or 10−6 M) plus A779 groups.
Figure 3 shows that the inhibition of NHE3 decreased JHCO3− (45% of control value), indicating that this isoform in the basal situation is partly responsible for JHCO3−. Figure 3 also indicates that inhibition of NHE3 does not alter the inhibitory effect of a low dose of ANG-(1–7) (10−9 M) on JHCO3− but transforms the stimulatory effect of a high dose of ANG-(1–7) (10−6 M) to an inhibitory effect (30% below the control value). Thus our results indicate that the biphasic dose-dependent effect of ANG-(1–7) on JHCO3− is via the NHE3 isoform.
Fig. 3.
Direct effect of ANG-(1–7) and/or S3226 [10−6 M; a specific Na+/H+ exchanger (NHE3) inhibitor] tubular luminal microperfusion on net bicarbonate reabsorption in in vivo rat proximal S2 segments. Values are the means ± SE; n = number of microperfusions/number of tubules. *P < 0.01 vs. control. #P < 0.01 vs. ANG-(1–7) (10−6 M) alone. No significant difference was observed between the S3226 alone and the ANG-(1–7) (10−9 or 10−6 M) plus S3226 groups. No significant difference was observed between the ANG-(1–7) (10−9 M) alone and the ANG-(1–7) (10−9 M) plus S3226 groups.
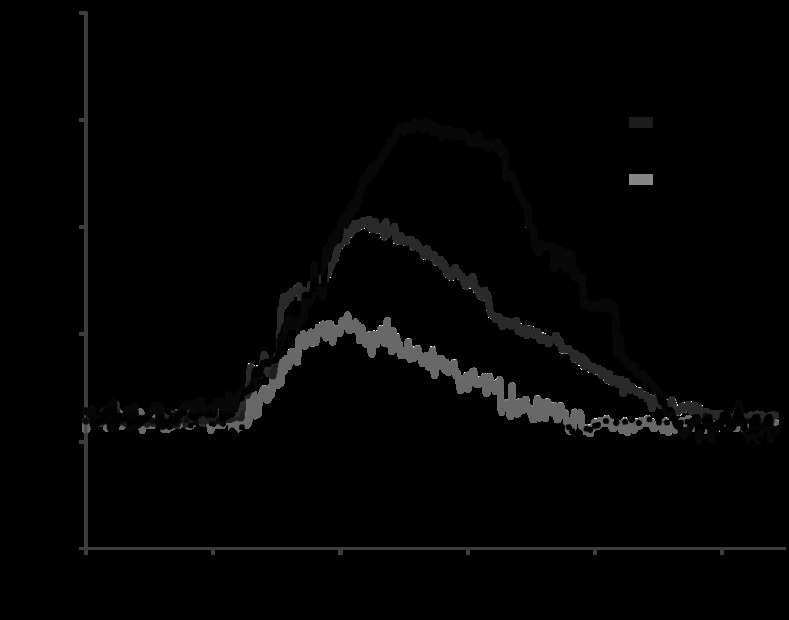
Figure 4 shows the value of fluorescence intensity of calcium continuously over time, in four individual experiments representative of the control group or in the presence of ANG-(1–7) (10−12, 10−9, or 10−6 M). We note that the fluorescence intensity in the control group was constant over time. However, after ∼20 s of the addition of ANG-(1–7) the fluorescence intensity significantly increased in a dose-dependent manner; this hormonal effect was transient and lasted ∼3 min before returning to baseline.
Fig. 4.
Cell calcium fluorescence signal tracing during 4 representative experiments. Images were continuously acquired (at time intervals of 600 ms) in the presence of the control solution or after the addition of ANG-(1–7) (10−12, 10−9, or 10−6 M), indicated by the arrow.
Table 2 shows the mean data values of the concentration of cytosolic calcium in all of the experimental groups. Our findings reveal that the [Ca2+]i in proximal tubules for the control condition is 101 ± 2 nM (6).
Table 2.
Effect of ANG-(1–7) and/or A779, BAPTA, and/or thapsigargin in maximum cytosolic calcium concentration of isolated proximal tubules from rats
| Experimental Groups | [Ca2+]i, nM | n |
|---|---|---|
| Control | 101 ± 2 | 6 |
| ANG-(1–7) (10−12 M) | 252 ± 8* | 5 |
| ANG-(1–7) (10−9 M) | 203 ± 3* | 6 |
| ANG-(1–7) (10−6 M) | 153 ± 8* | 5 |
| A779 (10−6 M) | 126 ± 2* | 5 |
| ANG-(1–7) (10−12 M) + A779 | 114 ± 7† | 5 |
| ANG-(1–7) (10−9 M) + A779 | 96 ± 4† | 5 |
| ANG-(1–7) (10−6 M) + A779 | 105 ± 5† | 5 |
| BAPTA (5 × 10−5 M) | 58 ± 3* | 5 |
| BAPTA + ANG-(1–7) (10−9 M) | 106 ± 3† | 5 |
| BAPTA + ANG-(1–7) (10−6 M) | 82 ± 2*† | 6 |
| Thapsigargin (10−5 M) | 341 ± 9* | 5 |
| Thapsigargin + ANG-(1–7) (10−9 M) | 356 ± 9*† | 5 |
| Thapsigargin + ANG-(1–7) (10−6 M) | 342 ± 12*† | 5 |
Values are the means ± SE; n = number of tubules (each tubule corresponds to the average of 10 areas). [Ca2+]i, intracellular Ca2+ concentration.
P < 0.01 vs. control;
P < 0.01 vs. ANG-(1–7) alone in the respective dose.
Figure 5 indicates that ANG-(1–7) has a dose-dependent increase in [Ca2+]i: low hormone doses (10−12 or 10−9 M) caused a large increase in [Ca2+]i (151 or 102%, respectively, over the control), whereas with the high dose of ANG-(1–7) (10−6 M), the increase in [Ca2+]i was much smaller but was significant (52% over the control). Figure 5 also indicates that inhibition of the Mas receptor causes a significant increase in [Ca2+]i with respect to control (25%) and inhibits the stimulatory effect of ANG-(1–7) all doses.
Fig. 5.
Effect of ANG-(1–7) and/or A779 (10−6 M; a specific receptor Mas antagonist) in maximum cytosolic calcium concentration of isolated proximal tubules from rats. Values are the means ± SE; n = number of tubules (each tubule corresponds to an average of 10 areas). *P < 0.01 vs. control. #P < 0.01 vs. ANG-(1–7) alone in the respective dose.
We also performed measurements to investigate the effects of an intracellular calcium chelator or releaser alone or plus ANG-(1–7) (10−9 or 10−6 M) on JHCO3− and [Ca2+]i (Tables 1 and 2, respectively). The results show that BAPTA: 1) alone does not modify the JHCO3− but decreases the [Ca2+]i to ∼58% of the control value, 2) decreases the inhibitory effect of ANG-(1–7) (10−9 M) on JHCO3− but impairs its stimulatory effect on the [Ca2+]i, and 3) impairs the stimulatory effect of ANG-(1–7) (10−6 M) on JHCO3− and on the [Ca2+]i. In addition, thapsigargin: 1) alone decreases the JHCO3−, 2) maintains the inhibitory effect of ANG-(1–7) (10−9 M) on JHCO3−, and 3) change the stimulatory effect of ANG-(1–7) (10−6 M) on JHCO3− to an inhibitory effect; however, in these three conditions, it increases the [Ca2+]i to ∼3.5 times the control value.
To detect whether the doses of ANG-(1–7) used during luminal microperfusion would affect renal function and to follow the acid-base conditions of the rats, we measured urine flow and osmolality, Na+ excretion and systemic acid-base parameters. During tubular microperfusion with all doses of ANG-(1–7), these data were similar to the basal values [urine flow = 0.0515 ± 0.006 ml·min−1·kg−1; urine osmolality = 559 ± 11.2 mosmol/kgH2O; urinary sodium excretion = 30.2 ± 1.68 eq·min−1·kg−1 (18 measurements, 6 animals); blood pH = 7.34 ± 0.14; plasma Pco2 = 35.5 ± 0.77 Torr; and plasma HCO3− = 25.2 ± 0.54 mM (12 measurements, 6 animals)]. On the basis of these findings, we conclude that in this present preparation, the luminal microperfusions of ANG-(1–7) do not have systemic effects.
DISCUSSION
The nature of the mechanism underlying ANG-(1–7) action on proximal nephron bicarbonate reabsorption is not yet clearly defined (2, 11, 19, 20). In addition, the apical Na+/H+ exchanger mediates most NaCl, NaHCO3−, and fluid reabsorption by the renal proximal tubule and is critical for the normal maintenance of extracellular fluid volume, blood pressure, and acid-base balance (18, 31). Thus the purpose of the present investigation was to clarify the acute direct effects of ANG-(1–7) (10−12, 10−9, or 10−6 M) on this exchanger in the proximal convoluted tubule.
We examined the effect of luminal perfusion of ANG-(1–7) in vivo using a stopped-flow microperfusion technique, which is not affected by glomerular filtration rates and allows us to measure the tubular acidification online. The experimental conditions are such that the measurements are performed at well-determined hormonal levels added to the tubules. Therefore, the hormonal effects are local and do not cause systemic hemodynamic changes because the amount of hormone that is introduced to the tubules is insignificant. This result is confirmed by the absence of changes in urine flow and osmolality, Na+ excretion, and systemic acid-base values during experiments where luminal hormonal microperfusions were performed. Furthermore, the luminal perfusion solution contains raffinose (a nonresorbable molecule) to reach isotonicity, which prevents fluid reabsorption induced by the hormone.
Thus it is possible that the conflicting and incompletely understood proximal effects of this heptapeptide described in the current literature can be explained because in several studies the ANG-(1–7) was injected parenterally, presenting systemic hemodynamic effects and in conditions in which the measurements were made at not determined hormonal levels into the proximal tubule. No significant changes in transepithelial PD (approximately −2 mV) were observed in any of the experimental groups. Because this value of transtubular PD is characteristic of the proximal S2 segment (14), this finding indicates that our experiments were performed in this tubular segment. In addition, during the intratubular perfusions, no significant changes of transepithelial PD were found, indicating that tubular transport capacity was maintained at a normal level.
Our results indicate that in the control situation, JHCO3− is 2.82 ± 0.078 nmol·cm−2·s−1 (50/20), a value statistically similar to that found in the proximal S2 tubular segment (13, 29). Our data also indicate that the low doses of ANG-(1–7) (10−12 or 10−9 M) cause a significant decrease in JHCO3− compared with control (36 or 60%, respectively), whereas the high dose of ANG-(1–7) (10−6 M) significantly increased JHCO3− (to 80% above the control). Therefore, our results indicate that in the S2 segment ANG-(1–7) has a biphasic effect on the Na+/H+ exchanger, that is, the low hormonal dose inhibits and the high hormonal dose stimulates the NHE3 exchanger. Consistent with this idea is the observation that ANG 1–7 enhances sodium excretion in sheep likely through a proximal tubular mechanism coherent with the current study (41). However, this biphasic action of ANG-(1–7) we are finding is inverse to the biphasic action described for ANG II, that is, it is widely accepted that ANG II stimulates the Na+/H+ at low doses and inhibits it at high doses (4, 12, 21, 32, 34).
The results obtained show that A779 alone significantly increases JHCO3− (∼30% over the control). This finding could be caused by an unspecific action of this receptor Mas specific antagonist, but it might also indicate that endogenous intratubular ANG-(1–7) in a low concentration, which inhibits the apical Na+/H+ exchanger, exists at basal conditions. As far as our knowledge reaches, the concentration of ANG-(1–7) in the proximal tubule fluid remains unknown, but a recent study (37) shows that its concentration in the cortex is 36 ± 6 pg/g protein and in medulla is 63 ± 10 pg/g. On other hand, ANG-(1–7), ACE2, and Mas protein have been immunohistochemically localized to mouse proximal tubule, suggesting the existence of a local autocrine/paracrine system for production and action of this heptapeptide (3). In addition, it is known that ANG-(1–7) is excreted in urine at concentrations higher than the ANG II (94.6 ± 11.3 and 11.4 ± 1.1 pmol/g creatinine, respectively; Ref. 42). However, the conclusion that endogenous intratubular ANG-(1–7) in a low concentration exists at basal conditions is not entirely justified. Thus Fig. 2 indicates that the effect of A779 alone on the JHCO3− is quite similar to the action of A779 plus ANG-(1–7) (10−12 or 10−6 M) on this parameter, which may indicate that this antagonist may elicit some nonspecific action instead of inhibiting the effect of ANG-(1–7).
Our data also show that the biphasic effect of ANG-(1–7) on the exchanger occurs via the Mas receptor, once A779 abolishes the inhibitory effect of ANG-(1–7) at low doses and the stimulatory effect of ANG-(1–7) at a high dose. These results are also consistent with literature findings indicating that the renal effects of ANG-(1–7) are dose dependent and mediated, at least in part, by the Mas receptor (1, 9, 19, 22, 38, 40) and with more recent studies by Silveira et al. (39) and Gwathmey et al. (17) that reveal predominant tubular localization of the Mas receptor in the kidney of both rats and sheep.
Nevertheless, in isolated rat proximal straight tubule (S3 segment), Garcia and Garvin (11) found that, like ANG II, ANG-(1–7) has a biphasic effect through AT1 receptors: at physiologic levels, it increases fluid and bicarbonate reabsorption, and at a high concentration, it decreases these parameters. This finding contrasts with our present results in in vivo rat proximal convoluted tubules (S2 segment). Whether one or more different signaling systems are involved in mediating the effect of ANG-(1–7) on NHE3 activity in the S2 and S3 proximal segments remains to be established. The renal effects of ANG-(1–7) may also involve AT1, AT2 (2, 5, 20), and V2 receptors (27), indicating a cross-talk mechanism between the Mas receptor and the ANG II and vasopressin systems. Furthermore, in the Garcia and Garvin study (11) the actions of ANG-(1–7) were blocked by AT1 antagonist, suggesting a different site than that assessed in our current study. However, our results are consistent with the idea presented in the current literature (8, 10), which suggests that both ANG-(1–7) and ANG II have opposite effects on several cardiovascular mechanisms and that at physiological doses ANG-(1–7) is a vasodilator, whereas ANG II is a vasoconstrictor.
Additionally, our data show that S3226 alone, an inhibitor of the NHE3 isoform, causes a significant decrease in JHCO3−. This result is in agreement with literature findings indicating that the luminal Na+/H+ exchanger is responsible for most secretion of H+ in the proximal tubule (18, 31). Our results also show that S3226 maintains the inhibitory effect of the low dose of ANG-(1–7) (10−9 M) on JHCO3− and changes the stimulatory effect of the high dose of ANG-(1–7) (10−6 M) to an inhibitory effect, which might be expected because in this last condition, the exchanger is inhibited. Thus our results indicate that the ANG-(1–7) affects the Na+/H+ exchanger primarily via the NHE3 isoform. Although it is known that a significant fraction of proximal apical H+ secretion is mediated by the NHE3, the H+-ATPase also contributes (18, 25, 28). If the ANG-(1–7) influences the H+-ATPase activity in in vivo proximal tubules remains to be accurately determined and is not the purpose of the present study; however, our present results indicate that in the presence of S3226, ANG (1–7) has no effect on JHCO3− (Fig. 3).
Furthermore, our results indicate that in the control situation the [Ca2+]i in the proximal tubule is 101 ± 2 nM (6), a value statistically similar to that found in this tubular segment (26). Our data also indicate that ANG-(1–7) (10−12, 10−9, or 10−6 M) increases the [Ca2+]i (151, 102, or 52% relative to controls, respectively). In addition, the inhibition of the Mas receptor increases the [Ca2+]i in the control condition but inhibits the stimulatory effect of all doses of ANG-(1–7) on this parameter. The present data are compatible with the demonstration that in astrocytes from the rostral ventrolateral medulla of rats, ANG-(1–7) causes a dose-dependent increase in [Ca2+]i that is abolished by the Mas receptor antagonist A779 (16). Our findings also support the data indicating that ANG-(1–7) at relatively low doses binds to the Mas receptor, promoting the release of arachidonic acid (2). Thus the activation of phospholipase A2 (with the consequent release of arachidonic acid and via metabolites dependent on cytochrome P450 epoxygenase) raises a lot the [Ca2 +]i (for activation of voltage-dependent calcium channel in the membrane), thereby inhibiting the exchanger, which also occurs via complex Ca-calmodulin binding sites (26, 32, 33, 34).
According to Wakabayashi et al. (43) and Eguti et al. (7), the NHE exchanger has two calmodulin binding sites at the cytoplasmatic regulatory domain, which modulate its activity. A high-affinity site (region A, containing the residues 636–657), which is tonically inhibitory, binds to low levels of Ca2+/calmodulin, thus suppressing the inhibition, i.e., stimulating the exchanger at low Ca2+/calmodulin levels. Our current data reveal that this occurs in presence of ANG-(1–7) (10−6 M). A low-affinity site (region B, comprised of residues 664–684), however, binds with calcium and calmodulin only at high concentrations and, under these conditions, inhibits the exchanger activity. Our present results show that this occurs in presence of ANG-(1–7) (10−12 or 10−9 M). Confirming this finding, the biphasic action of ANG-(1–7) on the NHE exchanger we are finding is inverse to the biphasic action previously described for ANG II (32, 34), aldosterone (26), and arginine vasopressin (33), that is, low doses of these hormones cause a small increase in [Ca2+]i, stimulating the exchanger, whereas high hormonal doses cause a large increase in [Ca2+]i, inhibiting the exchanger. However, it is necessary to be cautious in the interpretation of the present data since the comparison of Figs. 1 vs. 5 indicates that the differences in the [Ca2+]i responses at ANG-(1–7) (10−12, 10−9, or 10−6 M) are not entirely comparables to the changes in JHCO3− responses at the same doses of ANG-(1–7), with the possibility that other signaling pathways are also involved in the hormonal effects.
In addition, our results with the A779 also concur with the data from the literature presented above since the inhibition of the Mas receptor during basal conditions causes a small increase in [Ca2+]i (25%) and stimulates the Na+/H+ exchanger (30%) compared with controls; however, the inhibition of the Mas receptor abolishes the stimulatory effect of all doses of ANG-(1–7) on [Ca2+]i, preventing the inhibitory effect of low doses of ANG-(1–7) (10−12 and 10−9 M) and the stimulatory effect of the high dose of ANG-(1–7) (10−6 M) on the Na+/H+ exchanger.
Our experiments with an intracellular calcium chelator or releaser too support these findings. BAPTA alone does not modify the JHCO3− once it decreases the [Ca2+]i to ∼58% of the control value, which by itself does not affect the NHE exchanger. As BAPTA alone decreases the basal [Ca2+]i but does not change the basal JHCO3−, it seems difficult to compare the responses with ANG-(1–7) plus BAPTA. However, BAPTA decreases the inhibitory effect of ANG-(1–7) (10−9 M) on JHCO3− and impairs the stimulatory effect of ANG-(1–7) (10−6 M) on JHCO3− because it impairs the stimulatory effect of ANG-(1–7) (10−9 or 10−6 M) on the [Ca2+]i (7, 43). On the other hand, thapsigargin alone decreases the JHCO3−, maintains the inhibitory effect of ANG-(1–7) (10−9 M) on JHCO3−, and changes the stimulatory effect of ANG-(1–7) (10−6 M) on JHCO3− to an inhibitory effect because, in these three conditions, thapsigargin increases the [Ca2+]i to about 3,5 times the control value, which by itself inhibits the NHE exchanger.
The physiological relevance of the opposite dose-dependent effects of ANG-(1–7) and ANG II on the Na+/H+ exchanger and [Ca2+]i demonstrated in the present study remains to be established. However, since 1) the apical NHE3 isoform mediates the majority of NaCl, NaHCO3− and fluid reabsorption by the renal proximal tubule and is critical for the normal maintenance of extracellular fluid volume, blood pressure, and acid-base balance (18, 31); and 2) the NHE exchanger calmodulin binding sites at the cytoplasmatic regulatory domain (which modulate its activity) are associated with either a small or large increase in [Ca2+]i (7, 43), it is reasonable to assume that, in intact animals, the interaction of these renal proximal opposite dose-dependent hormonal effects may represent an important physiological regulatory mechanism in terms of intra- and extracellular volume and/or pH changes. However, further studies are needed to clarify if the [Ca2+]i change is a important direct mechanism for the exchanger activation by these hormones or is a side effect of other signaling pathways.
In conclusion, we have undertaken in vivo stationary microperfusion studies in the cortical proximal tubule of rats to evaluate the acute direct effects of ANG-(1–7) (10−12, 10−9, or 10−6 M) on HCO3− reabsorption. Our results indicate that ANG-(1–7) has a biphasic dose-dependent effect on JHCO3−, mediated by the Mas receptor and the NHE3 isoform of the Na+/H+ exchanger. The results are compatible with stimulation of this exchanger by a moderate increase in [Ca2+]i in the presence of the high dose of ANG-(1–7) (10−6 M) and the inhibition of this exchanger by large increases in [Ca2+]i induced by low doses of ANG-(1–7) (10−12 or 10−9 M); these findings are the opposite of those found for ANG II (32, 34). Additionaly, our experiments with BAPTA or thapsigargin confirm the role for cytosolic calcium in the regulation of NHE3. Thus the interaction of the opposite dose-dependent effects of ANG-(1–7) and ANG II on the renal proximal Na+/H+ exchanger and [Ca2+]i levels may represent an important physiological regulatory mechanism of extracellular volume and/or pH changes. Nevertheless, it is a complex mechanism and additional factors need to be investigated.
GRANTS
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Pesquisas (CNPq).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.C.C.-B., D.C.A.L.-D., and M.d.M.-A. conception and design of research; R.C.C.-B., D.C.A.L.-D., and M.d.M.-A. performed experiments; R.C.C.-B., D.C.A.L.-D., and M.d.M.-A. analyzed data; R.C.C.-B., D.C.A.L.-D., and M.d.M.-A. interpreted results of experiments; R.C.C.-B., D.C.A.L.-D., and M.d.M.-A. prepared figures; R.C.C.-B. and M.d.M.-A. drafted manuscript; R.C.C.-B., D.C.A.L.-D., and M.d.M.-A. edited and revised manuscript; R.C.C.-B., D.C.A.L.-D., and M.d.M.-A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Gerhard Malnic for English assistance in the responses to the reviewers.
REFERENCES
- 1. Alenina N, Xu P, Rentzsch B, Patkin EL, Bader M. Genetically altered animal models for Mas and angiotensin-(1–7). Exp Physiol 93: 528–537, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Andreatta-van Leyen S, Romero MF, Khosla MC, Douglas JG. Modulation of phospholipase A2 activity and sodium transport by angiotensin-(1–7). Kidney Int 44: 932–936, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Chappell MC, Modrall JG, Diz DI, Ferrario CM. Novel aspects of the renal renin-angiotensin system: angiotensin-(1–7), ACE2 and blood pressure regulation. Contrib Nephrol 143: 77–89, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Coppola S, Frömter E. An electrophysiological study of angiotensin II regulation of Na-HCO3 cotransport and K+ conductance in renal proximal tubules. II. Effect of micromolar concentrations. Pflügers Arch 427: 151–156, 1994 [DOI] [PubMed] [Google Scholar]
- 5. De Souza AM, Lopes AG, Pizzino CP, Fossari RN, Miguel NC, Cardozo FP, Abi-Abib R, Fernandes MS, Santos DP, Caruso-Neves C. Angiotensin II and angiotensin-(1–7) inhibit the inner cortex Na+-ATPase activity through AT2 receptor. Regul Pept 120: 167–175, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Diz DI, Pirro NT. Differential actions of angiotensin II and angiotensin (1–7) on transmitter release. Hypertension 19: 1141–1148, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Eguti DM, Thieme K, Leung G, Mello-Aires M, Oliveira-Souza M. Regulation of Na+/H+ exchanger isoform 1 (NHE1) by calmodulin-binding sites: role of angiotensin II. Cell Physiol Biochem 26: 541–552, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Ferrario CM. New physiological concepts of the renin-angiotensin system from the investigation of precursors and products of angiotensin I metabolism. Hypertension 55: 445–452, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferrario CM, Varagic J. The ANG-(1–7)/ACE2/Mas axis in the regulation of nephron function. Am J Physiol Renal Physiol 298: F1297–F1305, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1–7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol 289: H2281–H2290, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garcia NH, Garvin JL. Angiotensin-(1–7) has a biphasic effect in the proximal straight tubule. J Am Soc Nephrol 5: 1133–1138, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Geibel J, Giebisch G, Boron WF. Angiotensin II stimulates both Na+/JHCO3− cotransport in the rabbit proximal tubule. Proc Natl Acad Sci USA 87: 7917–7920, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giebisch G, Malnic G, De Mello GB, Mello-Aires M. Kinetics of luminal acidification in cortical tubules of the rat kidney. J Physiol 267: 571–600, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gil FZ, Malnic G. Effects of amphotericin B on renal tubular acidification in the rat. Pflügers Arch 413: 280–286, 1989 [DOI] [PubMed] [Google Scholar]
- 15. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985 [PubMed] [Google Scholar]
- 16. Guo F, Liu B, Tang F, Lane S, Souslova EA, Chudakov DM, Paton JF, Kasparov S. Astroglia are a possible cellular substrate of angiotensin (1–7) effects in the rostral ventrolateral medulla. Cardiovasc Res 87: 578–584, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gwathmey TM, Westwood BM, Pirro NT, Tang L, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin-(1–7) receptor is functionally coupled to the formation of nitric oxide. Am J Physiol Renal Physiol 299: F983–F990, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamm LL. Renal acidification mechanisms. In: The Kidney, edited by Brenner BM. Philadelphia, PA: Saunders, 497–534, 2004 [Google Scholar]
- 19. Handa RK, Ferrario CM, Strandhoy JW. Renal actions of angiotensin-(1–7): in vivo and in vitro studies. Am J Physiol Renal Fluid Electrolyte Physiol 270: F141–F147, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Heller J, Kramer HJ, Maly J, Cervenka L, Horacek V. Effect of intrarenal infusion of angiotensin-(1–7) in the dog. Kidney Blood Press Res 23: 89–94, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Houillier P, Chambrey R, Achard JM, Froissart M, Poggioli J, Paillard M. Signaling pathways in the biphasic effect of angiotensin II on apical Na+/H+ antiport activity in proximal tubule. Kidney Int 50: 1496–1505, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Joyner J, Neves L, Ferrario C, Brosnihan K. Administration of d-alanine-[ANG-(1–7)] (A-779) prior to pregnancy in Sprague Dawley rats produces antidiuresis in late gestation. J Am Soc Hypertens 2: 425–430, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joyner J, Neves LA, Stovall K, Ferrario CM, Brosnihan KB. Angiotensin-(1–7) serves as an aquaretic by increasing water intake and diuresis in association with downregulation of aquaporin-1 during pregnancy in rats. Am J Physiol Regul Integr Comp Physiol 294: R1073–R1080, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Joyner J, Neves LA, Granger JP, Alexander BT, Merrill DC, Chappell MC, Ferrario CM, Davis WP, Brosnihan KB. Temporal-spatial expression of ANG-(1–7) and angiotensin-converting enzyme 2 in the kidney of normal and hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol 293: R169–R177, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Leite-Dellova DC, Malnic G, Mello-Aires M. Genomic and nongenomic stimulatory effect of Aldosterone on H+-ATPase in proximal S3 segment. Am J Physiol Renal Physiol 300: F662–F691, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Leite-Dellova DCA, Oliveira-Souza M, Malnic G, Mello-Aires M. Genomic and nongenomic dose-dependent biphasic effect of aldosterone on Na+/H+ exchanger in proximal S3 segment: role of cytosolic calcium. Am J Physiol Renal Physiol 295: F1342–F1352, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Magaldi AJ, Cesar KR, de Araujo M, Simões e Silva AC, Santos RA. Angiotensin-(1–7) stimulates water transport in rat inner medullary collecting duct: evidence for involvement of vasopressin V2 receptors. Pflügers Arch 447: 223–230, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Malnic G, Geibel JP. Cell pH and H+ secretion by S3 segment of mammalian kidney: role of H+-ATPase and Cl−. J Membr Biol 178: 115–125, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Malnic G, Mello-Aires M. Kinetic study of bicarbonate reabsorption in proximaltubule of the rat. Am J Physiol 220: 1759–1767, 1971 [DOI] [PubMed] [Google Scholar]
- 30. Mello-Aires M, Lopes MJ, Malnic G. Pco2 in renal cortex. Am J Physiol Renal Fluid Electrolyte Physiol 259: F357–F365, 1990 [DOI] [PubMed] [Google Scholar]
- 31. Moe OW. Acute regulation of proximal tubule apical membrane Na+/H+ exchanger NHE3: role of phosphorylation, protein trafficking and, regulatory factors. J Am Soc Nephrol 10: 2412–2425, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Musa-Aziz R, Oliveira-Souza M, Mello-Aires M. Signaling pathways in the biphasic effect of ANGII on Na+/H+ exchanger in epithelial colon cells. J Membr Biol 205: 49–60, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Oliveira-Souza M, Mello-Aires M. Effect of arginine vasopressin and ANP on pHi and [Ca2+]i regulation in MDCK cells. Kidney Int 60: 1800–1808, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Oliveira-Souza M, Mello-Aires M. Interaction of angiotensin II and atrial natriuretic peptide on pHi regulation in MDCK cells. Am J Physiol Renal Physiol 279: F944–F953, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Pendergrass KD, Pirro NT, Westwood BM, Ferrario CM, Brosnihan KB, Chappell MC. Sex differences in circulating and renal angiotensins of hypertensive mRen(2). Lewis but not normotensive Lewis rats. Am J Physiol Heart Circ Physiol 295: H10–H20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2 Lewis rat. Am J Physiol Renal Physiol 290: F1497–F1506, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Prieto MC, González-Villalobos RA, Botros FT, Martin VL, Pagán J, Satou R, Lara LS, Feng Y, Fernandes FB, Kobori H, Casarini DE, Navar LG. Reciprocal changes in renal ACE/ANG II and ACE2/ANG 1–7 are associated with enhanced collecting duct renin in Goldblatt hypertensive rats. Am J Physiol Renal Physiol 300: F749–F755, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Santos RA, Simões e Silva AC, Magaldi AJ, Khosla MC, Cesar KR, Passaglio KT, Baracho NC. Evidence for a physiological role of angiotensin-(1–7) in the control of hydroelectrolyte balance. Hypertension 27: 875–884, 1996 [DOI] [PubMed] [Google Scholar]
- 39. Silveira KD, Bosco KSP, Diniz LR, Carmona AK, Cassali GD, Bruna-Romero O, Sousa LP, Teixeira MM, Santos RA, Silva AC, Vieira MA. ACE2-angiotensin-(1–7)-Mas axis in renal ischaemia/reperfusion injury in rats. Clin Sci 119: 385–394, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Simões e Silva AC, Bello AP, Baracho NC, Khosla MC, Santos RA. Diuresis and natriuresis produced by long term administration of a selective angiotensin-(1–7) antagonist in normotensive and hypertensive rats. Regul Pept 74: 177–184, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Tang L, Carey LC, Bi J, Valego N, Sun X, Deibel P, Perrott J, Figueroa JP, Chappell MC, Rose JC. Gender differences in the effects of antenatal betamethasone exposure on renal function in adult sheep. Am J Physiol Regul Integr Comp Physiol 296: R309–R317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Valdés G, Germain AM, Corthorn J, Berrios C, Foradori AC, Ferrario CM, Brosnihan KB. Urinary vasodilator and vasoconstrictor angiotensins during menstrual cycle, pregnancy, and lactation. Endocrine 16: 117–122, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Wakabayashi S, Bertrand B, Ikeda T, Poyssegur J, Shiekawa M. Mutation of calmodulin-binding site renders the Na+/H+ exchanger (NHE1) highly H+-sensitive and Ca2+ regulation defective. J Biol Chem 269: 13710–13715, 1994 [PubMed] [Google Scholar]