Abstract
Techniques to measure morphological parameters, such as glomerular (and thereby nephron) number, glomerular size, and kidney volume, have been vital to understanding factors contributing to chronic kidney disease (CKD). These techniques have also been important to understanding the associations between CKD and other systemic and cardiovascular diseases and have led to the identification of developmental risk factors for these pathologies. However, existing techniques in quantitative kidney morphology are resource- and time-consuming and are destructive to the organ. This review discusses the emerging generation of techniques to study kidney morphology quantitatively using magnetic resonance imaging (MRI) using the intravenous injection of the superparamagnetic nanoparticle cationic ferritin, which binds to the glomerular basement membrane. A primary advantage of MRI over previously established techniques is the ability to quantify morphology in the intact organ with minimal sample preparation. We highlight areas of research where MRI-based morphological measurements will be helpful in animal models and possibly diagnostic clinical nephrology, discuss technical challenges in light of the progress in MRI techniques to date, and identify novel measurements that may be possible using MRI, both ex vivo and in vivo.
Keywords: MRI, magnetic resonance imaging, kidney, glomerular counting, glomerular sizing, glomerular filtration rate, stereology
noninvasive imaging is a valuable tool for in vivo diagnostics and research. Several new imaging markers correlate well with traditional markers of kidney function such as glomerular filtration, and noninvasive techniques are used to detect the presence of gross structural abnormalities. There is a significant, emerging interest in noninvasive imaging tools to assess kidney morphology near the resolution and specificity of histological diagnosis, with the aim of obtaining molecular, structural, and functional information without biopsy. Indeed, measuring renal morphology in situ may yield more information than conventional histology because the tissue remains intact. Magnetic resonance imaging (MRI) techniques are particularly suited for a wide range of studies of kidney morphology and may have strong potential to facilitate a link between kidney structure and function in vivo. This article discusses emerging MRI techniques to study renal morphology in animal models and humans and identifies key technical and regulatory challenges to bringing imaging technology to the clinic.
For many years the human kidney was believed to contain ∼1 million nephrons. However, recent studies have indicated that while the mean value may well be close to 1 million, glomerular (and thereby nephron) number in adult human kidneys varies widely. In the largest study to date of more than 400 human kidneys obtained at autopsy (the Monash Series), nephron number ranged from ∼200,000 to more than 2.7 million, a 12.8-fold range (7, 27). Much research attention has focused on glomerular (and thereby nephron) number in recent years. The catalyst for this interest was the landmark article by Brenner et al. (10), who proposed that low nephron number (and thereby low glomerular filtration surface area), either congenital or acquired, was associated with increased risk of hypertension. Since this report, low nephron number has also been associated with greater susceptibility to kidney disease.
Knowledge of glomerular endowment, the number of glomeruli (nephrons) present in the kidney at the conclusion of nephrogenesis, is of interest to developmental biologists, pediatric nephrologists, and those interested in the developmental origins of adult health and disease. This is because nephron endowment is an index of the success or efficiency of nephrogenesis. Nephrogenesis is a complex process involving numerous events (including ureteric budding and branching, mesenchymal condensation, mesenchyme to epithelial conversion, glomerulogenesis, and tubule segmentation) regulated by hundreds of genes and feto-maternal environmental factors (24, 26). Whether a specific gene or environmental factor plays a significant role in nephrogenesis can be easily determined by assessing nephron endowment.
Glomerular volume has emerged in recent years as an important microanatomical marker of glomerular health. Glomerular volume is usually reported as a mean value for an entire kidney. Like total glomerular number, mean glomerular volume varies widely in normal human kidneys. In the Monash Series referred to above, mean glomerular volume varied 6.7-fold (7, 27). In humans, mean glomerular volume is generally directly correlated with kidney weight and body size and is often inversely correlated with total glomerular number and birth weight. Given that mean glomerular volume provides a single estimate of glomerular size for a kidney that may contain more than 1 million or perhaps 2 million glomeruli, there have been several recent reports of individual glomerular volumes within single kidneys (27). In these studies, typically the volumes of 30 glomeruli were determined per kidney using a disector/Cavalieri-based method. Increased heterogeneity of glomerular volume has been shown to be associated with several risk factors for kidney disease, including birth weight, age, race, body size, and hypertension (21).
Accurate quantitative data of total glomerular number, mean glomerular volume, total renal glomerular volume, individual glomerular volumes, total renal glomerular filtration surface area and so forth can be correlated with physiological measures of renal blood flow, glomerular filtration rate, and blood pressure. Patients with chronic kidney disease (CKD) often suffer from hypertension, and efforts to treat hypertension can lead to compensatory renal hyperperfusion that can damage the nephron. There is a strong need to detect patients at risk for CKD to apply therapy early. There is thus an interest in detecting kidney morphology for basic science and clinical diagnostics. A multidisciplinary approach to measuring clinically relevant parameters can provide powerful new insights into renal biology in health and disease.
Histological Approaches
Glomerular number.
Researchers have been attempting to estimate glomerular, and thereby nephron number, for more than a century. Currently, four main approaches are utilized: 1) acid maceration; 2) counts of glomerular profiles; 3) model-based stereology; and 4) design-based stereology. All four of these approaches have limitations. Acid maceration involves digestion of kidney tissue in an acid solution, followed by mechanical dissociation and counting of glomeruli in aliquots of known volume. Counts of glomerular profiles (2-dimensional samples of glomeruli as observed in histological sections) have significant problems in terms of data interpretation and biological relevance. In short, counts of glomerular profiles are usually expressed as number per area of section (NA). The problems with this approach are multiple, with estimates influenced by glomerular size and size variation, glomerular shape, section thickness, section compression, and tissue shrinkage associated with fixation and processing. The final estimate of NA is a density and tells us nothing about the total number of glomeruli in the kidney. Counts of glomerular profiles should be avoided.
Model-based stereological approaches do provide estimates of the total number of glomeruli in the kidney. With these approaches, glomerular numerical density (NV, number per volume of cortex for example) is estimated using an approach that requires knowledge of glomerular geometry (mean caliper diameter, size distribution, and shape; see Refs. 1, 16, 17, 33). Unfortunately, these key geometric parameters are typically assumed rather than measured, and to the extent that the assumptions are biased, so too are the resulting estimates. Numerical density estimates can be multiplied by the reference volume (cortex and kidney) to provide total glomerular number, and this overcomes the problems of interpreting densities. However, the reliance on geometric assumptions remains.
Design-based stereological methods require no knowledge of glomerular geometry and are currently considered the gold-standard approach for estimating total glomerular number. These methods are based on the disector principle of Sterio (31) and include the disector/Cavalieri combination [when disector estimates of glomerular number per volume are multiplied by a Cavalieri estimate of kidney volume; see Bertram (9)] and the disector/fractionator combination [when disector estimates of glomerular number in a known fraction of kidney tissue are multiplied by the reciprocal of the fraction; see Bertram et al. (8) and Bertram (9)]. Despite the unquestionable theoretical breakthrough represented by the disector technique and its many contributions to nephrology (25), this approach has only been adopted by a handful of laboratories across the world for glomerular counting. There are several reasons for this. First, the method requires alignment of fields in two histological sections. While possible in paraffin sections, this is much easier in glycolmethacrylate sections, but most histology laboratories are not equipped for glycolmethacrylate embedding and sectioning. Second, the technique requires exhaustive sectioning of tissue blocks. Third, counting glomeruli with disectors is slow and tedious. In our experience, even after tissue sampling, processing, embedding, sectioning, staining, and coverslipping, 4–5 h of counting are required to estimate total glomerular number in a rat kidney. For human kidneys, an additional 6 h at least are required to count glomeruli.
Given the limitations of the four methods currently used to estimate glomerular number, a new method is required. Ideally, such a method would be far less labor intensive than stereology, would provide accurate (no bias) and precise (low variance) estimates, would count and size every glomerulus, and would be noninvasive, allowing longitudinal studies in both animal models of kidney disease and in patients.
Glomerular volume.
Despite the important insights on glomerular volume, the current method for estimating individual glomerular volumes is slow and tedious, requiring serial sectioning of individual glomeruli and measurement of all, or most, profiles from that glomerulus. A noninvasive method that could provide the volume of each and every glomerulus in a kidney, and thereby the glomerular size distribution, would represent a significant technological breakthrough and almost certainly generate many new insights into glomerular growth and shrinkage during development, adolescence, adult life, and disease.
MRI
MRI is critical to many biological, preclinical, and clinical applications. One of the major advantages of MRI is a high level of flexibility in contrast. MRI has been useful for both qualitative and quantitative measurements in small animal models of kidney disease (34). The basis of MRI is the interaction between a nuclear moment of an atom and an applied magnetic field. The polarization of the nucleus leads to a net alignment of magnetization, which has a corresponding resonance radiofrequency (RF) that depends on the external magnetic field strength. One or several RF antennae, tuned to the nuclear resonance frequency, are used to detect this magnetization to form the MR signal. An MR image is created by spatially varying the resonance frequency using another electromagnetic gradient coil, with windings and current applied to slightly change the magnetic field in space. These gradient coils can be combined to dynamically adjust the magnetic field in three dimensions, so that the locations of water protons can be identified in three dimensions. Current MRI acquisition strategies can incorporate either retrospective or dynamic motion correction, compensation for local changes in the RF field, and rapid whole body coverage.
Measuring total kidney volume with MRI.
There have been several successful imaging approaches to measuring kidney volume. Both cortical volume and segmental intrarenal volumes have strong associations with renal diseases such as fibrosis, nephritis, and stenosis or urinary obstruction. Recently, Pedersen et al. (26) recommended a combined approach of histology with MRI-based volumetry to determine kidney and cortical volume without the use of an imaging contrast agent. However, biopsy carries significant discomfort and risk, so there is still a need for a technique to make direct measurements in vivo.
Identifying, counting, and measuring the volume of individual glomeruli with MRI.
More recently, an approach has emerged to directly measure whole kidney glomerular numbers (Nglom) and individual glomerular volumes with MRI (5, 20). This work was based on the observation that ferritin, a protein-based nanoparticle, can be modified to pass through the glomerular endothelium, label the glomerular basement membrane, and allow for whole kidney detection of each labeled glomerulus with MRI (Fig. 1) (6).
Fig. 1.
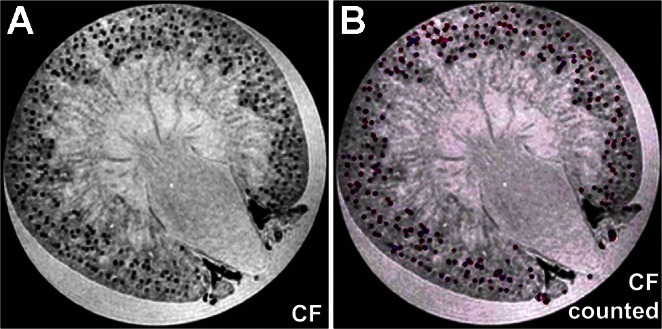
Whole kidney identification of individual glomeruli. A: representative axial kidney image from a three-dimensional (3D) MRI dataset from a cationized ferritin (CF)-injected rat. Data were analyzed with a 3D counting algorithm to identify individual glomeruli. Regions defined as glomeruli by the computational 3D counting algorithm were assigned an arbitrary color exclusively for visualization purposes (B). UNx, unilateral nephrectomy; Nglom, whole kidney glomerular numbers. [From Beeman SC and colleagues (5).]
In the 2008 study (6) that first introduced this approach, cationized ferritin (CF) was injected intravenously into healthy rats, and individual glomeruli were identified in gradient-echo MR images using a magnetic field strength of 11.7 T. It was suggested that every glomerulus in the kidney could be identified in the images. Subsequent work (5, 20) showed that postprocessing of three-dimensional (3D) MR images after intravenous injection of CF could be used to count every glomerulus in the healthy rat kidney and also to estimate the intrarenal distribution of glomerular volumes. The MRI counts in these studies were validated with acid maceration and fractionator/disector stereology. Total MRI-based counts were lower than stereological counts but higher than those obtained by acid maceration of the kidneys. However, all techniques reported numbers for glomerular counts within 10%, and the MRI-based technique also yielded an estimate of the distribution of glomerular volumes in each kidney.
GLOMERULUS-SPECIFIC PROBE.
The ferritin protein forms a spherical, 13-nm 24-mer with an inner cavity of approximately 5–7 nm. Ferritins contain a ferroxidase at the particle surface, allowing Fe2+ and other metals to form a crystal in the core. This iron oxide core also makes ferritin weakly superparamagnetic in native form (19). Native and modified ferritins were previously developed as MRI contrast agents (11, 14, 18, 24). CF had been developed originally by Danon et al. in the 1970s (15) to study cellular and tissue charge distribution with electron microscopy. CF was formed by a simple modification of horse spleen ferritin through the chemical addition of multiple amine groups to the surface of the ferritin particle.
Apoferritin from various species has been used and expressed recombinantly and can be synthetically filled with iron (32). Alternatively, apoferritin can be created to contain a wide range of products by opening the protein and reassembling it.
IMAGE ANALYSIS.
The advantages of 3D MRI in measuring renal morphology (like those described above) are accompanied by a need for quantitative, accurate image processing techniques. Future image processing will ideally be automated, so as to eliminate observer bias in image segmentation. One of the challenges to automated image processing is the high level of flexibility in MRI contrast though the choice of MRI pulse sequence and local variability in tissue contrast. Furthermore, the signal-to-noise ratio (SNR) in MRI can vary widely from systems of different field strength, RF coil configurations, and hardware and temporal limitations to the image resolution.
A first step in image processing is to segment the images to identify specific structures and to segment the kidney from the surrounding tissue or background. This can be accomplished manually, although adaptive automatic segmentation is possible based on image intensity and geometric features of the image. The image must then be analyzed to extract the morphologic features. Individual, CF-labeled glomeruli have been segmented from the surrounding cortex based on the local decrease in T2*-weighted image intensity. Because the effect of CF labeling can depend on the amount of CF injection and accumulation, the field strength, and the detailed parameters used in MRI acquisition, the performance of the image processing method must be validated. One of the major limiting factors in identifying CF-labeled glomeruli is the size of the artifact created by the CF relative to the imaging voxel size. For example, the recent images have been acquired in the rat kidney with an image spatial resolution of approximately 100- to 200-μm resolution (4, 19). In three dimensions, the artifact from a single glomerulus creates a perturbation, the size of which depends on the image acquisition parameters, magnetic field strength, amount of CF in the glomerulus, and ferritin relaxivity. Distinguishing between interglomerular variability in CF uptake and glomerular size is an important direction for future work. The glomeruli can be identified automatically by applying a local threshold, coupled with a geometric criterion on the number of connected voxels meeting this threshold.
Future Directions
Morphology in disease.
There are numerous current scientific and future clinical applications of MRI-based renal morphology. There are many important animal models of renal and cardiovascular disease. Among them are rat and mouse models of CKD and developmental diseases that affect kidney morphology. The most recent application of cationized ferritin to measure glomeruli has been attempted in a rat model of focal and segmental glomerulosclerosis (6).
The size of glomeruli is thought to be essential for the assessment of hyperfiltration. This has, however, never been proven. The new technology allows for the first time to size glomeruli. This is neatly depicted in Fig. 2 (unpublished data), in which the size of glomeruli of sham-operated and unilaterally nephrectomized (UNx) Sprague-Dawley rats are compared. The body weight of the animals is similar, while kidney weight is 1.4-fold greater in UNx rats. Glomerular numbers are similar with the exception of animal UNx3, which has a higher count. As a consequence, average glomerular volume is 1.5 times higher in the UNx animals (with the exception of UNx3, which is lower, as expected from the higher glomerular count). Thus the changes in kidney weight seem to be reflected by the glomerular size. These findings are also replicated by the distribution patterns of the glomerular sizes of the different animals. In the future these data need to be correlated with measured glomerular filtration data to come to a new definition of hyperfiltration.
Fig. 2.
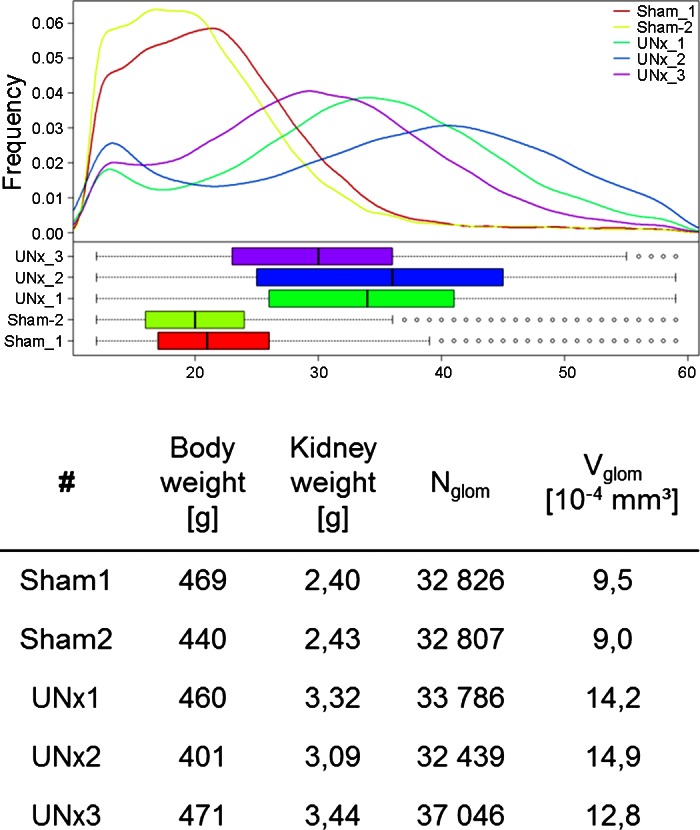
Quantification of glomerular volume distribution in rat kidneys 6 wk after UNx or sham operation. Top: distribution of the volume under the different conditions. Glomerular basement membranes of these animals were labeled using CF in vivo. In total 6.6 mg/kg body wt was administered over three intravenous injections over a period of 3 h. After UNx compensatory renal growth is induced. Glomerular volume distribution is shifted towards larger volumes (Nglom, number of glomeruli; Vglom, mean glomerular volume). eNOS, endothelial nitric oxide synthase. Changes can easily be detected by MRI. [From the laboratory of Gretz and colleagues, unpublished observations.]
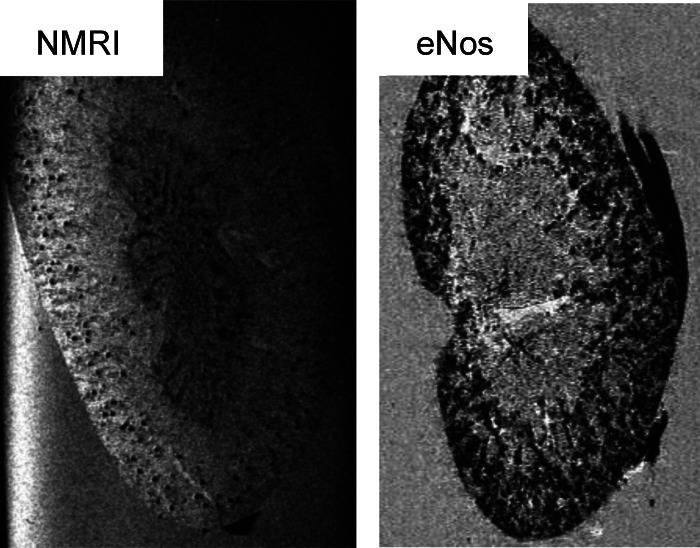
In cases where the negative charge of the basement membrane is reduced and proteinuria occurs, the cationic ferritin will also be filtered and appear in tubules from where it cannot be removed while perfusing the kidney. This is depicted with MRI in Fig. 3 from Enos−/− mice. In such situations, automated glomerular counting cannot be performed. On the other hand, one could make use of that finding to assess charge changes in the basement membrane while evaluating new treatment modalities.
Fig. 3.
Increased glomerular permeability causes increased nonspecific diffusion of intravenously injected CF in the Enos knockout (Enos−/−) mouse. [From the laboratory of Gretz N and colleagues, unpublished observations.]
Fig. 4.
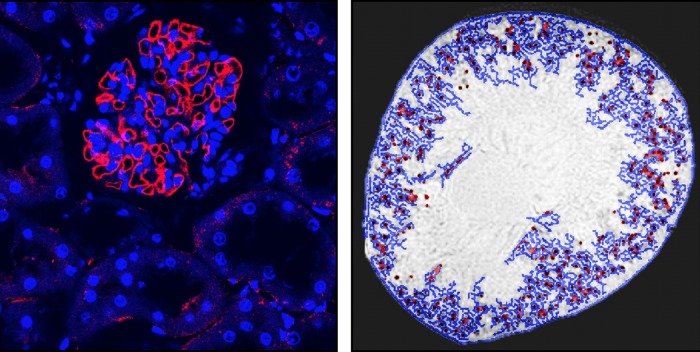
Intravenously injected CF is detected with fluorescence immunohistochemistry and MRI in low levels in the proximal tubule of a perfused kidney. A: red immunofluorescence of ferritin is seen in a glomerulus and surrounding tubules. B: image processing is used to detect CF labeling of the glomeruli (red dots) and apparent tubules in the surrounding cortex (blue lines) based on the intensity of the MRI voxels. (From Bennett KM and colleagues, unpublished observations).
Technical challenges.
The principal technical challenges to in vivo MRI include image sensitivity, resolution, acquisition techniques, and motion correction. The prescribed resolution in clinical MRI is dictated primarily by the strength of the applied gradients and the RF line width. However, the SNR for a given resolution is dictated by the amount of signal available within the MRI receiver bandwidth. All other factors being similar, this is dictated by the quality of the RF coil and its proximity to the organ being imaged and also by the field strength. There have been rapid advances in the development of RF technology, with specialized RF coils for every organ and each application. For example, Qian et al. (28–30) have recently demonstrated the use of implantable resonators to dynamically detect individual glomeruli in vivo before and after labeling with CF. The spatial resolution and spatial range also limit the total acquisition time. For in vivo MRI, the dynamic image acquisition time has been shortened through the development of parallel imaging technology, with the tradeoff of a moderate decrease in SNR.
Anatomical motion poses another major hurdle for translation of imaging techniques to detect kidney morphology. Previous attempts to detect glomeruli in vivo in rats have involved both respiratory gating and supine positioning to reduce motion during the scan (6). These images were acquired in 3D with an ∼50-μm resolution and over several tens of hours. Compared with preclinical studies, a current typical resolution for clinical imaging is approximately 1–2 mm3, with a requirement for shorter (<1–2 h) scan times. At these resolutions, partial volume effects will impact glomerular number and volume estimates and may reduce sensitivity to labeling with CF inside the voxel. Motion during the scan may further decrease this local sensitivity, although respiratory gating should be useful to reduce motion-related noise. The reduced sensitivity to CF labeling may eventually be overcome by employing CF with increased relaxivity (13).
One of the principal advantages of noninvasive imaging is the ease of translation of novel imaging techniques from preclinical studies to clinical trials. There are many examples of diagnostic imaging techniques that spent a relatively short time in testing before receiving regulatory approval. Many of these advantages do not exist for MRI contrast agents, however, because they are held to the standards of new pharmaceuticals in both efficacy and toxicity. Thus obtaining regulatory approval is one of the primary hurdles to developing molecular probes.
Toxicity and regulatory challenges.
A major hurdle for developing novel diagnostic agents is obtaining government and regulatory approval. In the United States, regulatory approval of investigational new drugs is obtained through a careful progression through preclinical and clinical trials, with input from the Food and Drug Administration. Phase 1 clinical trials can be commenced after complete preclinical studies demonstrating both efficacy of the agent and identifying any potential acute toxicity. We have recently demonstrated that MRI-detectable CF causes no acute or chronic change in kidney or liver metabolic markers for toxicity and does not significantly increase total blood lymphocyte counts (4). Further work will be required to establish the minimum detectable, nontoxic dose. However, an extensive review of the literature reveals that CF from horse spleen has been used as an antigen to induce a rodent model of nephropathy. Most of these studies exposed animals to CF followed by a systemic injection of anti-ferritin antibody, causing a focal immune complex in the glomerulus. However, there is one report in the literature of temporary proteinuria and reversible morphological changes in the glomerulus of a rabbit using high doses of CF similar to what our groups have used to image (∼50 mg/kg; Ref. 3). This pathology only presented several days after the initial injections, suggesting an autoimmune reaction to the horse spleen CF rather than a destruction of the glomerular basement membrane anionic barrier. Another group reported that a much larger direct injection of CF into the renal artery caused acute proteinuria, possibly through clogging of the glomerular basement membrane and tubules (22). To our knowledge these studies have not been independently repeated. If these early studies are confirmed, it may be critical to optimize CF dose, bolus timing, and possibly to use a recombinant version of CF specific to the rodent model for use in rodent models of CKD or in eventual use in humans. Alternatively, CF created from modified apoferritin with a higher relaxivity, such as “magnetoferritin,” may be detectable in ∼10 × lower doses. Further work will be needed to prove efficacy and safety, and long-term carcinogenesis, but the latter preclinical studies can be performed during the initial clinical trials.
Associating tubules and glomeruli.
The association between the glomerulus and the tubule is critical to the function of the nephron. There is evidence for a correlation between reduced nephron function and injury at the site of the glomerulotubular junction, suggesting a potential relationship between the number of atubular glomeruli and severity of a number of chronic renal diseases (12, 23). A noninvasive way to measure the number of atubular glomeruli may be important to discover novel mechanisms and therapies for these diseases and may be useful for early kidney diagnostics.
Conclusions
Noninvasive techniques to measure kidney morphology are critical to advancing both research and clinical diagnostic nephrology. MRI techniques have been developed to provide a high-resolution, nondestructive view of the kidney at near-microscopic resolution. These MRI-based tools may eventually be complimentary or be combined with techniques such as contrast-enhanced microcomputed tomography and also have the potential to enable kidney glomerular measurements both in vivo and in excised organs in animals (2). There are a number of technical and regulatory challenges to developing contrast agents for use in the clinic, which may make these agents more immediately suited for preclinical studies. Other techniques such as diffusion-weighted MRI, magnetization transfer, and endogenous contrast may eventually provide insight into glomerular structure and function without the need for injected contrast agents. Because these techniques are noninvasive, they may be more readily translated to clinical use. With collaborative efforts between nephrologists, physiologists, and imaging scientists, noninvasive MRI may be important to understanding structure-function relationships in the kidney and in improving early diagnosis of kidney disease.
GRANTS
The work by N. Gretz was supported by the EC FP7 project, PLACE-it and the Marie-Curie project NephroTools, as well as by the Erasmus Project. The work by K. Bennett was supported by American Heart Association Grant 12BGIA9840020, National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-091722, and National Institutes of Health Diabetic Complications Consortium.
DISCLOSURES
K. Bennett owns Nanodiagnostics LLC.
AUTHOR CONTRIBUTIONS
Author contributions: K.M.B., J.F.B., S.C.B., and N.G. conception and design of research; K.M.B., J.F.B., S.C.B., and N.G. performed experiments; K.M.B., J.F.B., S.C.B., and N.G. analyzed data; K.M.B., J.F.B., S.C.B., and N.G. interpreted results of experiments; K.M.B., S.C.B., and N.G. prepared figures; K.M.B., J.F.B., S.C.B., and N.G. drafted manuscript; K.M.B., J.F.B., S.C.B., and N.G. edited and revised manuscript; K.M.B., J.F.B., S.C.B., and N.G. approved final version of manuscript.
REFERENCES
- 1. Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec 94: 239–247, 1946 [DOI] [PubMed] [Google Scholar]
- 2. Badea CT, Drangova M, Holdsworth DW, Johnson GA. In vivo small-animal imaging using micro-CT and digital subtraction angiography. Phys Med Biol 53: R319–50, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Batsford SR, Sasaki M, Takamiya H, Vogt A. Cationic macromolecule-induced nephrotic syndrome in rabbits. Lack of immune complex involvement. Lab Invest 49: 260–269, 1983 [PubMed] [Google Scholar]
- 4. Beeman SC, Georges JF, Bennett KM. Toxicity, biodistribution, and ex vivo MRI detection of intravenously injected cationized ferritin. Magn Reson Med 69: 853–861, 2013 [DOI] [PubMed] [Google Scholar]
- 5. Beeman SC, Zhang M, Gubhaju L, Wu T, Bertram JF, Frakes DH, Cherry BR, Bennett KM. Measuring glomerular number and size in perfused kidneys using MRI. Am J Physiol Renal Physiol 300: F1454–F1457, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Bennett KM, Zhou H, Sumner JP, Dodd SJ, Bouraoud N, Doi K, Star RA, Koretsky AP. MRI of the basement membrane using charged nanoparticles as contrast agents. Magn Reson Med 60: 564–574, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: implications for health and disease. Pediatr Nephrol 26: 1529–1533, 2011 [DOI] [PubMed] [Google Scholar]
- 8. Bertram JF, Soosaipillai MC, Ricardo SD, Ryan GB. Total numbers of glomeruli and individual glomerular cell types in the normal rat kidney. Cell Tissue Res 270: 37–45, 1992 [DOI] [PubMed] [Google Scholar]
- 9. Bertram JF. Analyzing renal glomeruli with the new stereology. Int Rev Cytol 161: 111–172, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Brenner B, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more of the other? Am J Hypertens 1: 335–347, 1988 [DOI] [PubMed] [Google Scholar]
- 11. Bulte JW, Douglas T, Mann S, Frankel RB, Moskowitz BM, Brooks RA, Baumgarner CD, Vymazal J, Strub MP, Frank JA. Magnetoferritin: characterization of a novel superparamagnetic MR contrast agent. J Magn Reson Imaging 4: 497–505, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Chevalier RL, Forbes MS. Generation and evolution of atubular glomeruli in the progression of renal disorders. J Am Soc Nephrol 19: 197–206, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Clavijo-Jordan V, Caplan MR, Bennett KM. Simplified synthesis and relaxometry of magnetoferrin for magnetic resonance imaging. Magn Reson Med 64: 1260–1266, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Cohen B, Dafni H, Meir G, Harmelin A, Neeman M. Ferritin as an endogenous MRI reporter for noninvasive imaging of gene expression in C6 glioma tumors. Neoplasia 7: 109–117, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Danon D, Goldstein L, Marikovsky Y, Skutelsky E. Use of cationized ferritin as a label of negative charges on cell surfaces. J Ultrastruct Res 38: 500–510, 1972 [DOI] [PubMed] [Google Scholar]
- 16. DeHoff R, Rhines F. Determination of number of particles per unit volume from measurements made on random plane sections: the general cylinder and the ellipsoid. Trans Metall Soc AIME, 1961 [Google Scholar]
- 17. Floderus S. Untersuchungen uber den Bau der menschlichen hypophse mit besondener Berucksichtigung der quantitativen mikromorphologischen Verhaltnisse. Acta Pathol Microbiol Scand 53: 1–276, 1944 [Google Scholar]
- 18. Genove G, DeMarco U, Xu H, Goins WF, Ahrens ET. A new transgene reporter for in vivo magnetic resonance imaging. Nat Med 11: 450–454, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Gossuin Y, Muller R, Gillis P, Bartel L. Relaxivities of human liver and spleen ferritin. Magn Reson Imaging 23: 1001–1004, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Heilmann M, Neudecker S, Wolf I, Gubhaju L, Sticht C, Schock-Kusch D, Kriz W, Bertram JF, Schad LR, Gretz N. Quantification of glomerular number and size distribution in normal rat kidneys using magnetic resonance imaging. Nephrol Dial Transplant 27: 100–107, 2012 [DOI] [PubMed] [Google Scholar]
- 21. Hoy WE, Hughson MD, Zimanyi M, Samuel T, Douglas-Denton R, Holden L, Mott S, Bertram JF. Distribution of volumes of individual glomeruli in kidneys at autopsy: association with age, nephron number, birth weight and body mass index. Clin Nephrol 74, Suppl 1: S105–12, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Kubosawa H, Nakayama H, Sano T, Kondo Y. Morphological alterations of glomerulus induced by infusion of cationized ferritin. Acta Pathol Jpn 43: 445–455, 1993 [DOI] [PubMed] [Google Scholar]
- 23. Marcussen N. Atubular glomeruli and the structural basis for chronic renal failure. Lab Invest 66: 265–284, 1992 [PubMed] [Google Scholar]
- 24. Heywood BR, Mann S, Meldrum FC. Magnetoferritin: in vitro synthesis of a novel magnetic protein. Science 257: 522–523, 1992 [DOI] [PubMed] [Google Scholar]
- 25. Moritz KM, Wintour EM, Black MJ, Bertram JF, Caruana G. Factors influencing mammalian kidney development: implications for health in adult life. Adv Anat Embryol Cell Biol 196: 1–78, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Pedersen M, Karstoft K, Lødrup A, Jespersen B, Nyengaard JR. Advantages and controversies in the era of intrarenal volumetry. Am J Nephrol 33, Suppl 1: 40–45, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Puelles VG, Zimanyi MA, Samuel T, Hughson MD, Douglas-Denton RN, Bertram JF, Armitage JA. Estimating individual glomerular volume in the human kidney: clinical perspectives. Nephrol Dial Transplant 27: 1880–1888, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qian C, Murphy-Boesch J, Dodd S, Koretsky A. Sensitivity enhancement of remotely coupled NMR detectors using wirelessly powered parametric amplification. Magn Reson Med 68: 989–996, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qian C, Yu X, Chen DY, Dodd S, Bouraoud N, Pothayee N, Chen Y, Beeman S, Bennett K, Murphy-Boesch J, Koretsky A. Wireless Amplified Nuclear MR Detector (WAND) for high-spatial-resolution MR imaging of internal organs: preclinical demonstration in a rodent model. Radiology 2013. February 7 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qian C, Zabow G, Koretsky A. Engineering novel detectors and sensors for MRI. J Magn Reson 229: 67–74, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc 134: 127–136, 1984 [DOI] [PubMed] [Google Scholar]
- 32. Uchida M, Terashima M, Cunningham CH, Suzuki Y, Willits DA, Willis AF, Yang PC, Tsao PS, McConnell MV, Young MJ, Douglas T. A human ferritin iron oxide nano-composite magnetic resonance contrast agent. Magn Reson Med 60: 1073–1081, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Weibel ER, Gomez DM. A principle for counting tissue structures on random sections. J Appl Physiol 17: 343–348, 1962 [DOI] [PubMed] [Google Scholar]
- 34. Xie L, Cianciolo RE, Hulette B, Lee HW, Qi Y, Cofer G, Johnson GA. Magnetic resonance histology of age-related nephropathy in the Sprague Dawley rat. Toxicol Pathol 40: 764–778, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]