Abstract
The importance of innate immunity for survival is underscored by its presence at almost every level of the evolutionary tree of life. The task of “danger” recognition by the innate immune system is carried out by a broad class of pattern recognition receptors. These receptors are expressed in both hematopoietic and nonhematopoietic cells such as renal epithelial cells. Upon activation, pattern recognition receptors induce essentially two types of defensive responses: inflammation and phagocytosis. In this review, we highlight evidence that renal epithelial cells are endowed with such defensive capabilities and as such fully participate in renal innate immune responses.
Keywords: epithelial cells, innate immunity, pattern recognition receptors, Toll-like receptor, endotoxin
the fundamental role of the innate immune system is to initiate a quick response immediately after detecting “danger signals” in the setting of infection (nonself) or tissue injury (self). Swiftness is key, because, for example, the doubling time of a single bacterium would allow it to produce millions of progeny within a day if not kept in check. Innate immune cells carry several families of receptors, collectively called pattern recognition receptors (PRRs), which recognize conserved features of pathogens. Some PRRs, such as the mannose receptor and complement system, bind microbes and facilitate phagocytosis. Other PRRs, such as Toll- and NOD-like receptors, induce a wide array of proinflammatory and reactive cytokines in response to danger signals (65).
Most mammalian species have 10–13 types of Toll-like receptors (TLRs) (9). TLRs are responsible for triggering innate immune responses to many forms of pathogens. TLRs are heavily expressed in myeloid cells where they have been extensively investigated. However, some TLRs, such as TLR4, are also expressed and functional in other cell types, including renal epithelial cells (124, 125, 140). Renal tubular TLRs participate in the inflammatory response characteristic of many forms of acute kidney injury. In addition, during injury, tubules can also exhibit phagocytic function (55). Finally, renal epithelial cells express major histocompatibility complex (MHC) class II protein, costimulatory molecules, and produce a plethora of inflammatory and chemotactic cytokines (10). Accordingly, it is increasingly appreciated that epithelial cells and traditional innate immune cells can exhibit remarkable similarities in behavior and function. In this review, we will primarily focus on the less explored “innate immune cells”, i.e., renal epithelial cells.
Epithelial Cells Are Not Alone
The kidney is a complex organ, consisting of at least 12 functionally different epithelial cell types (1). Epithelial cells are surrounded by a dense network of immune cells. In particular, macrophages and dendritic cells, collectively called mononuclear phagocytes, are the most predominant immune cells in the kidney. The visually stunning mononuclear phagocytic network was beautifully described by Soos et al. (129) in 2006. This remarkable network may come as a surprise given the fact that, unlike the gut, the kidney is a nonlymphoid organ and lacks microbial exposure under normal conditions.
It is now increasingly recognized that mononuclear phagocytes have markedly diverse functions: from traditional phagocytic function to versatile, trophic roles (23, 42, 75, 79, 91, 94, 128). Under normal conditions, the mononuclear phagocytic system is believed to play an important role in maintaining the integrity of the tissue microenvironment. In fact, mononuclear phagocytes (CSF1R+) are abundantly present even in early embryonic kidneys. Interestingly, when the embryonic kidney was cultured with CSF1 and endotoxin, significant growth in the branch tips and nephrons was observed, presumably because CSF1 and endotoxin induced mononuclear phagocytes to stimulate nephron growth (105).
Like for many other organs, the conventional classification of dendritic cells and macrophages in the kidney remains controversial because of overlap in function and surface markers (37, 54, 96). Multidimensional data such as multicolor flow cytometry and microarray studies continue to reveal the complexity of immune cells in various organs. As detailed in the Immunological Genome Project, it is now evident that each organ has unique sets of immune cell makeup (36, 89). The modern classification of immune cell subpopulations now considers subset-specific transcription factors and ontogenies (72, 116). The interested reader is referred to Refs. 15, 38, and 48 (general) and 23, 78, 96, 108, and 132 (kidney) for further details.
Epithelial Cells and PRRs
Much progress has been made in understanding the origins of microbe sensing and innate immune responses. Among diverse PRR families, TLRs are the most extensively studied receptors over the past decade. TLR4 is a functional homomer and requires coreceptors CD14 and MD2 for tighter binding with its ligand, lipopolysaccharide (LPS). TLR2 is heteromeric and complexes with TLRs 1 or 6. Crystallographic analysis showed detailed interactions between the TLR4:MD2 and LPS complex. Similarly, TLR2/TLR1 and its ligand lipopeptide, as well as TLR3 and its ligand poly (I:C), have been crystallized (60, 80, 100). TLRs are strategically located in different cellular compartments, allowing them to sense distinctive pathogen-associated molecular patterns and assemble downstream signaling cascades (62). Some TLRs are exclusively expressed in myeloid cells whereas others are relatively ubiquitous and can be expressed by renal epithelial cells.
Renal expression of TLRs has been studied and confirmed by many investigators. Nevertheless, some uncertainty remains regarding the precise distribution of TLRs in the kidney. This is partly because TLRs are such potent receptors that the expression levels are naturally low at the levels of mRNA and protein. In monocytes, it is estimated that TLR4 is present at 1,300 molecules, whereas CD14 is expressed at 115,000 molecules/cell (135). In nonmyeloid cells, TLR4 expression is likely much lower. Furthermore, due to the inherently complex kidney architecture, one needs to combine technically intricate microdissection, in situ hybridization, and immunostaining to adequately characterize TLRs expression and distribution among various renal cell populations. In that regard, immunostaining remains very challenging because of lack of firm antibodies in this class (41, 71, 123, 140). Nevertheless, collective evidence strongly supports that a number of TLRs are indeed expressed in renal epithelial cells. We and others have previously reviewed the expression of TLRs in the kidney (13, 25, 39, 126).
TLRs in Kidney Injury
Many investigators have reported that tubular expression of TLR2 and TLR4 is increased by experimental ischemia-reperfusion injury in rats and mice (66, 113, 140). For example, TLRs are believed to be activated by various damage-associated molecular patterns, such as the high-morbidity group box 1 (3, 4, 58, 82, 112). Importantly, Wu et al. (141) examined bone marrow chimeric mice between TLR4 knockout and wild-type mice. Chimeric mice lacking renal TLR4 had significantly less tubular damage and azotemia compared with mice lacking hematopoietic TLR4, indicating that intrinsic TLR4 in the kidney is instrumental in mediating tubular damage (141). Pulskens et al. (104) also demonstrated the importance of intrinsic renal TLR4 after ischemic injury. Similarly, Leemans et al. (76) examined bone marrow chimeric mice between TLR2 knockout and wild-type mice and found that intrinsic renal TLR2 has a central role in the unfolding of the injury process.
In models of urinary tract infections, TLR4, TLR5, and TLR11 have been shown to play protective roles (12). When these receptors are defective or absent, clearance of the infection is hindered. It has also been shown that urinary tract epithelial TLR4 and hematopoietic TLR4 are both crucial in mounting a proper inflammatory response to infected bladder mucosa or even pyelonephritis (101, 117). A role for renal TLR4 was also proposed in more chronic models of injury such as obstructive uropathy (103).
In human kidney transplantation, Kruger et al. (69) found that TLR4 expression in proximal and distal tubules is increased in deceased donor kidneys compared with living donor kidneys. Furthermore, the authors determined donor TLR4 genotypes in a cohort of 276 subjects and found 30 subjects with loss-of-function single nucelotide polymorphisms (SNPs), Asp299Gly and Thr399Ile. These two loss-of-function SNPs diminish receptor binding of endotoxin but do not affect TLR4 gene or protein expression (5, 106). Compared with kidneys with wild-type alleles, kidneys with a TLR4 loss-of-function allele had fewer proinflammatory cytokines, and the rate of immediate graft function was higher. It remains to be determined whether this acute protection in TLR4-mutant receivers translates into long-term protection. In summary, compelling evidence indicates that renal epithelial TLRs are central to the regulation of tissue immunity and inflammation.
The Danger Model in the Kidney: S1 Proximal Tubules as the First Line of Defense
To best illustrate the role of renal TLR4 in innate immunity, we next discuss in some detail an animal model of endotoxemia. As opposed to cecal ligation and puncture, ischemia, or transplant models, endotoxin injury models can circumvent concerns such as simultaneous activation of multiple TLRs induced by often uncharacterized damage-associated molecular patterns or polymicrobial infections (20). As such, they are more useful models to characterize specific cellular and molecular pathways of injury.
Endotoxin, released from bacteria in various molecular sizes, can be filtered by nephrons and interact with TLR4 expressed on the proximal tubules. We have recently shown in vivo that systemically administered endotoxin is indeed filtered and taken up by proximal tubules, resulting in tubular oxidative stress (63). Importantly, endotoxin-induced tubular toxicity has an absolute requirement for tubular TLR4. Conversely, TLR4-expressing hematopoietic cells are not essential or sufficient for endotoxin-induced tubular oxidative stress. Note that circulating hematopoietic cells are the primary source of systemic cytokines (11). Taken together, the direct endotoxin-tubular interaction is an important pathway leading to acute kidney injury in endotoxemia.
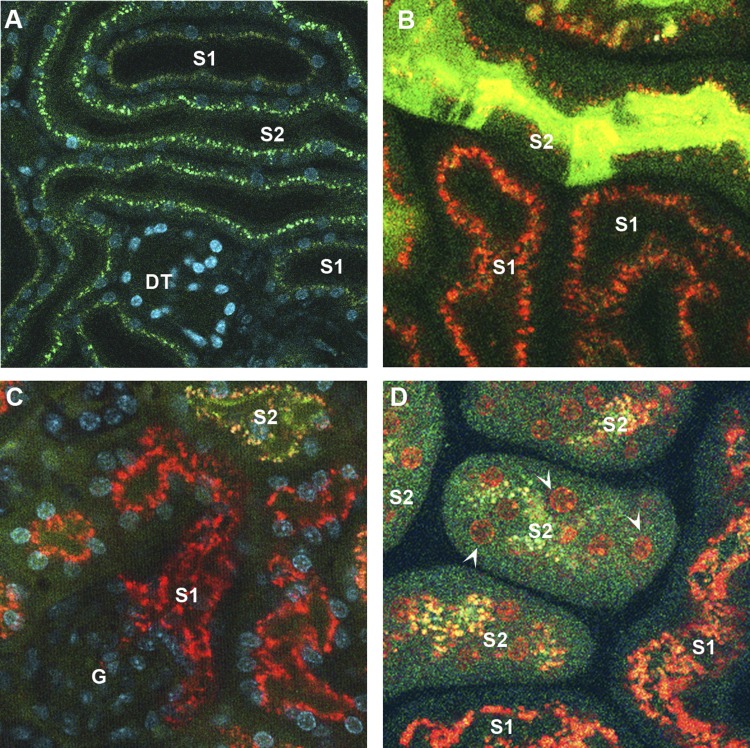
We also found that filtered endotoxin is internalized predominantly by S1 proximal tubules where TLR4 appears to be expressed the most (63). Two more observations support a role for S1 as the first line of defense in the kidney against endotoxemia. First, S1 endotoxin uptake can be upregulated by endotoxin preexposure, indicating that it is a receptor-mediated process rather than nonspecific endocytosis. Second, and interestingly, this S1-endotoxin interaction does not result in any apparent immediate injury to S1 (Fig. 1). This is due in part to the upregulation of cytoprotective molecules such as heme oxygenase-1 and sirtuin-1 in S1 tubules (43, 47, 49, 95). The lack of injury (e.g., oxidative stress) to S1 segments, despite their direct interaction with endotoxin, underscores their high potential for autoprotection. Such a phenomenon has been reported in mononuclear phagocytes after TLR4-mediated exposure to endotoxin (114). Like mononuclear phagocytes, the S1 autoprotection mechanism seems to be dependent, in part, on upregulation of cytoprotective molecules with antioxidant properties. In this model of endotoxemia, the S1 segment acts as the “sensor” of endotoxin in the filtrate and as such autoprotects itself while simultaneously signaling to neighboring segments such as S2 and S3. We note that while S1 autoprotects itself, there is widespread oxidative stress in S2 and S3 (Fig. 1). Whether this represents unavoidable collateral damage or is actually part of a broad signaling cascade is unknown. This function of S1 segments is remarkably similar to that of Kupffer cells in the liver, which also signal the presence of endotoxin to neighboring hepatocytes (110). In summary, S1 segments may play a sentinel role similar to innate immune cells by sensing danger signals and signaling to neighboring cells.
Fig. 1.
Endotoxin-induced tubular oxidative stress. A: live 2-photon microscopy of the mouse kidney. S1 and S2 proximal tubules can be discerned by their autofluorescence signatures. S1 exhibits brown autofluorescence, whereas S2 exhibits bright green punctate autofluorescence. Nuclei were stained blue with Hoechst. Distal tubules emit minimal autofluorescence (DT). B: endotoxin-induced oxidative stress was measured with carboxy-2′,7′-dichlorodihydrofluorescein diacetate (green) using 2-photon intravital imaging. Systemically administered endotoxin (red) was filtered and internalized predominantly by S1 proximal tubules and yet prominent oxidative stress was observed in S2 proximal tubules. Endotoxin uptake observed in S2 is secondary to fluid-phase endocytosis. C: because glomeruli are located at depths beyond the reach of 2-photon microscopy, kidney tissues were freshly dissected to image the deeper segments. This further confirms the internalization of endotoxin by S1 proximal tubules and tubular oxidative stress in downstream S2 and S3 segments. G, glomerulus. D: endotoxin-induced oxidative stress was measured live with dihydroethidium, which emits nuclear fluorescence in the presence of cytosolic superoxide (orange; arrowheads). Similar to B, S2 proximal tubules exhibited oxidative stress whereas S1 tubules exhibited no oxidative stress despite their greater endotoxin uptake.
Teleologically, the role of S1 tubules as sentinels for immunity is appealing. The kidney is a highly vascular organ which filters hundreds of liters of blood per day, and a significant part of the filtrate is reabsorbed by proximal tubules. S1 cells, with their upstream location, are strategically poised to screen the filtrate and “watch” for alarm signals coming from the circulation. In particular, it is increasingly appreciated that endotoxemia is a rather common event, occurring daily at subclinical levels during routine breaches to mucosal integrity in various locations (84, 102, 119). An attractive possibility is that, through this pathway, S1 could act as a “sink” for the uptake and degradation of filtered endotoxin. This function is very similar to that proposed for the liver, a major detoxifying center for endotoxemia, particularly that originating from the gut (57, 83). The mechanisms of hepatic endotoxin removal have been reviewed elsewhere (16, 85, 110). These detoxifying functions of the liver, and possibly the kidney, could represent one aspect of the widely recognized ability of many organisms to develop endotoxin tolerance (6).
We also note that TLR4 signaling pathways in the tubules, both in their molecular details and ultimate functions, may not be identical to those present in hematopoietic cells. For example, SIGIRR, a TLR4-inhibitory molecule, while expressed in tubular cells, does not seem to inhibit TLR signaling (73). Feulner et al. (32) reported that murine proximal tubules produce and secrete acyloxyacyl hydrolase into the urinary lumen. Acyloxyacyl hydrolase is an endotoxin-detoxifying enzyme, which could act to minimize the downstream effects of endotoxin that evaded proximal reabsorption. Watts et al. (138) demonstrated that endotoxin inhibits HCO3− absorption in medullary thick ascending limbs of the loop of Henle (TAL) through a TLR4-dependent pathway. Such downstream effects of endotoxin are likely very varied and will require better characterization in in vivo models.
In this section, we highlighted the roles of renal tubular TLRs in tissue inflammation and immunity. This epithelial cell-centric view could well apply to other organs (33, 74, 87, 127). Polly Matzinger (86), who proposed the danger model, states:
The danger model says that it is a tissue that controls whether you turn on an immune response, by sending alarm signals. It is also a tissue that induces tolerance by allowing its antigens to be presented without alarm signals. Perhaps, therefore, it could also be the tissue that determines the class of immunity.
The Danger Model in the Kidney: Thick Ascending Limbs Regulate Innate Immunity
If S1 proximal tubules are the first line of defense against endotoxemia, TAL, on the other hand, may be essential regulators of the innate immune response within the kidney. TAL tubules span across all areas of the kidney except the inner medulla (28). Consequently, TAL cells are contiguous to most cell types (epithelial and hematopoietic) within the kidney. With this distribution, they are strategically positioned to sense and react to changes (physiological and pathological) in these microenvironments, and possibly mediate various forms of horizontal cross talk (27, 29, 40). Furthermore, TAL in the highly susceptible outer stripe are resistant to acute kidney injury compared with neighboring proximal tubules (18, 27). Therefore, it is plausible to consider that tubules such as S1 and TAL, with an essential modulatory role during activation of innate immunity, must be more resilient to injury.
A unique feature of TAL is the production of Tamm-Horsfall protein (THP; also known as uromodulin). THP is a heavily glycosylated protein that is uniquely produced in the kidney by TAL (29, 107, 120). While predominantly targeted to the apex of the TAL by a GPI anchor signal (107), THP is also released basolaterally by an unknown mechanism (26). Although the functions of THP were elusive for many decades, there has been a recent surge in our understanding of the important role of this glycoprotein in various kidney diseases (29). Interestingly, THP appears to function as an essential effector produced by TAL during kidney injury to modulate innate immunity. In fact, the immunomodulatory functions of THP were a subject of controversy (29). Initially, THP was shown to have anti-inflammatory properties, by suppressing T cells in vitro (93) and binding renal cytokines and lymphokines (IL-1 and TNF) (50). However, a number of subsequent studies also performed in vitro suggested a proinflammatory role of THP, specifically in activating neutrophils (53, 67, 139) and monocytes (130, 144). In addition, Saemann and colleagues (115) demonstrated that THP activates myeloid dendritic cells via TLR4 to acquire a fully mature phenotype. With the availability of THP knockout mice, we provided strong in vivo evidence confirming that the role of THP is indeed anti-inflammatory and protective during kidney injury (26, 27, 30). In fact, the presence of THP, produced in TAL, inhibits the production of cytokines and chemokines such as CXCL2 (27) and CCL2 (26) in injured neighboring proximal tubules. Therefore, THP mediates a regulatory cross talk between TAL and proximal tubules, aimed at suppressing tubular activation of innate immunity and promoting recovery (29). This is thought to occur, in part, through basolateral THP released in the interstitium and interacting with the basolateral domain of proximal tubules, where its putative receptor was localized (26, 27). In addition, systemic levels of THP increase during recovery from acute kidney injury (26), suggesting a broader role for THP such as mediating cross talk between the kidney and other organs. Interestingly, recent data also showed that THP regulates the levels of circulating cytokines by acting as a urinary cytokine trap (81). Therefore, through the production of THP, TAL tubules directly modulate innate immunity by regulating tubular epithelial production of cytokines/chemokines and their systemic levels. However, the extent of the interaction of THP with the renal phagocytic system remains uncertain. Dong et al. (21) suggested that THP may be part of the renal antigens presented by dendritic cells after injury caused by LPS injection. Whatever the extent of the interaction between THP and the renal phagocytic system, the outcome, based on the in vivo data from THP knockout mice (27, 30), must be to limit injury and promote repair.
Finally, the role of TAL in renal defense comes full circle through the functions of urinary THP in defense against bacterial colonization of the bladder mucosa. In fact, THP knockout mice are more susceptible to bladder colonization by uropathogenic Escherichia coli (7, 90). This occurs because of the binding of THP to E. coli, which prevents the interaction of these pathogens with uroplakins on the surface of urothelial cells (99). Therefore, THP serves as a decoy for pathogenic bacteria in the bladder and limits their interaction with cell surface receptors.
In summary, TAL tubules regulate innate immunity by shaping the evolving response to danger signals during kidney injury and by defending the urinary tract from pathogens. This complex task is accomplished through the production of THP, a unique kidney-specific glycoprotein.
Epithelial Cell-Immune Cell Interactions in the Kidney
Many mononuclear phagocyte markers are elevated in the kidney after acute tubular injury and even in chronic diseases such as polycystic kidney disease (44, 92, 147). It was also shown that many of these proteins are upregulated not only in mononuclear phagocytes but also in epithelial cells. Renal epithelial cells secrete chemokines in response to direct stimulation with TLR ligands (133). MHC I and II are highly expressed on proximal tubules after transplant and other stimuli (8, 34, 142). Tubular injury also increases tubular expression of costimulatory molecules (77, 97, 136). There are even some data to suggest that proximal tubules could present antigen to T cells (17, 45, 59, 70, 142).
The generation and activation of mononuclear phagocytes is dependent on CSF1R and its ligand CSF1 (46). Interestingly, CSF1R and its ligand CSF1 are upregulated in the tubules after ischemia-reperfusion injury and transplant (88, 146). Tubular recovery is CSF1R and CSF1 dependent and requires the presence of mononuclear phagocytes (2). Proximal tubules also express GM-CSF (19), a molecule which induces differentiation of monocytes into phagocytes (46). Finally, kidney injury molecule-1 (KIM-1) was shown to be highly expressed in injured proximal tubules. Ichimura et al. (55) demonstrated that KIM-1 is in fact a phosphatidylserine receptor and as such can function as a scavenger receptor. Therefore, during tubular injury, proximal tubules are transformed into “semiprofessional phagocytes.” Of note, KIM-1 can be upregulated anywhere from S1 to S3 proximal tubules, depending on the type of injury (145).
We have reviewed similarities between epithelial cells and innate immune cells. However, one important difference remains between the two cell types: mobility. Renal epithelial cells do not typically translocate. Therefore, epithelial cells alone will not be able to accomplish higher levels of immune activities (such as remote information transfer) unless they are supported by immune cells (56, 122). Ultimately, epithelial cells and immune cells are both essential in shaping renal immunity.
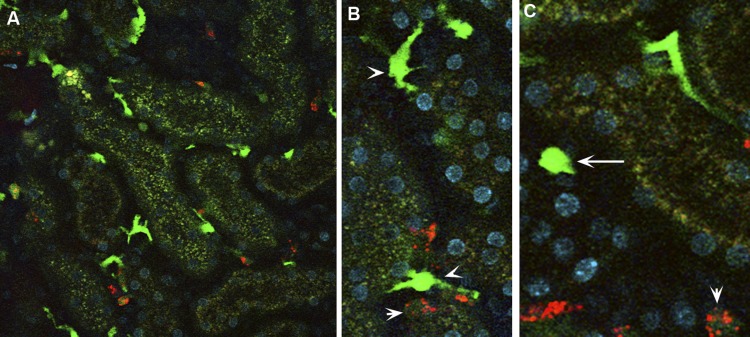
Figure 2 and a Supplemental Movie show CX3CR1+ myeloid cells in the live kidney (all supplementary material for this article are accessible on the journal website). The chemokine receptor CX3CR1 is widely expressed in mononuclear phagocytes, and CX3CR1 has been central to define its lineage and subsets (121, 143). The CX3CR1+ renal mononuclear phagocytes are remarkably heterogeneous in shape, signal intensity, and motion, likely reflecting their functional diversity.
Fig. 2.
Mononuclear phagocytic network in the kidney. A–C: CX3CR1-enhanced green fluorescence protein (EGFP) mouse was injected with rhodamine-labeled (poly I:C) (red), a TLR3 ligand, and the kidney was imaged live. The CX3CR1-EGFP kidney shows stationary dendritic cells with high EGFP fluorescence (CX3CR1high dendritic; arrowhead). Mobile spheroidal cells with either high or intermediate EGFP fluorescence are denoted as CX3CR1high spheroidal (long arrow) and CX3CR1int spheroidal (short arrow). Poly (I:C) localized to CX3CR1int spherical cells. CX3CR1high dendritic cells and CX3CR1high spheroidal cells did not take up poly (I:C). See also Supplemental Movie (supplementary material for this article is accessible on the journal website).
Traditionally, immune cells are thought to exacerbate tubular injury through inflammatory cytokines. However, it is increasingly recognized that certain subsets of immune cells play protective roles via immune cell-epithelial cell interactions. Many groups have reported intriguing epithelial cell-immune cell cross talk. Lee et al. (75) demonstrated that M1 macrophages switch to a M2 phenotype when cocultured with proximal tubular cells. Wang et al. (137) showed that proximal tubules stimulated by endotoxin inhibit macrophage activation. Others also showed that proximal tubules modulate mononuclear phagocyte function, maturation, and differentiation (64, 68). The interactions between renal epithelial cells and mononuclear phagocytic cells are not restricted to proximal tubules. Collecting duct epithelial cells also influence macrophage phenotypes (35). To investigate the role of myeloid cells in vivo, clodronate and CD11b- or CD11c-diphtheria toxin transgenic mice are often used. Although the outcomes may vary depending on the timing of depletion and models used (22, 24, 51, 52, 61, 75, 118), beneficial roles of mononuclear phagocytes have been demonstrated by multiple groups (98). In a model of cisplatin nephrotoxicity, CD11c depletion of diphtheria toxin transgenic mice resulted in more severe injury, suggesting that renal CD11c+ mononuclear phagocytes mediate protection in this model (131). Recently, Ferenbach et al. (31) showed that clodronate does not deplete alternative M2 macrophages and gives rise to less severe renal ischemia-reperfusion injury. Taken together, compelling evidence indicates that reciprocal interactions between mononuclear phagocytes and renal epithelial cells are instrumental in maintaining the integrity of the tissue environment. In other organs, even stronger evidence exists that epithelial-immune cell interactions shape overall organ immunity (14, 109, 127, 134).
Concluding Remarks
The kidney is a nonlymphoid, sterile organ, and yet renal epithelial cells are surrounded by an extensive mononuclear phagocytic network. These mononuclear phagocytes are pivotal in maintaining the tissue environment in health and disease. There exists considerable cross talk between mononuclear phagocytes and epithelial cells. In fact, renal epithelial cells share many phenotypic and functional characteristics with mononuclear phagocytes. Because renal epithelial cells are positioned at the interface between the internal milieu and external environment, it comes as no surprise that they can serve as primary guardians of the kidney and the body as a whole. As an example, we featured renal epithelial TLR4, which is strategically located on the tubules so it can respond to both systemic infection and local injury. Although innate immune cells activated by injured renal epithelial cells are commonly viewed as amplifiers of injury, protective roles of innate immune cells are increasingly appreciated. Investigations of immunity at the whole organ level will likely reveal more facets to the functions of renal epithelial and myeloid cells. Some of these functions will be unique to one cell type but others are likely shared by these sisters in arms.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant R01 DK080067, an O'Brien Center Grant P30DK079312 through NIH, Dialysis Clinics, Inc. to P. C. Dagher, and a Veteran Affairs Merit Award to T. M. El-Achkar.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: T.H. and P.C.D. provided conception and design of research; T.H. performed experiments; T.H. and P.C.D. analyzed data; T.H. and P.C.D. interpreted results of experiments; T.H. prepared figures; T.H. drafted manuscript; T.H., T.M.E.-A., and P.C.D. edited and revised manuscript; T.H. and P.C.D. approved final version of manuscript.
Supplementary Material
REFERENCES
- 1. Al-Awqati Q, Schwartz GJ. A fork in the road of cell differentiation in the kidney tubule. J Clin Invest 113: 1528–1530, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alikhan MA, Jones CV, Williams TM, Beckhouse AG, Fletcher AL, Kett MM, Sakkal S, Samuel CS, Ramsay RG, Deane JA, Wells CA, Little MH, Hume DA, Ricardo SD. Colony-stimulating factor-1 promotes kidney growth and repair via alteration of macrophage responses. Am J Pathol 179: 1243–1256, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anders HJ. Toll-like receptors and danger signaling in kidney injury. J Am Soc Nephrol 21: 1270–1274, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Anders HJ, Muruve DA. The inflammasomes in kidney disease. J Am Soc Nephrol 22: 1007–1018, 2011. [DOI] [PubMed] [Google Scholar]
- 5. Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, Frees K, Watt JL, Schwartz DA. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet 25: 187–191, 2000. [DOI] [PubMed] [Google Scholar]
- 6. Ayres JS, Schneider DS. Tolerance of infections. Annu Rev Immunol 30: 271–294, 2012. [DOI] [PubMed] [Google Scholar]
- 7. Bates JM, Raffi HM, Prasadan K, Mascarenhas R, Laszik Z, Maeda N, Hultgren SJ, Kumar S. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int 65: 791–797, 2004. [DOI] [PubMed] [Google Scholar]
- 8. Benson EM, Colvin RB, Russell PS. Induction of IA antigens in murine renal transplants. J Immunol 134: 7–9, 1985. [PubMed] [Google Scholar]
- 9. Beutler BA. TLRs and innate immunity. Blood 113: 1399–1407, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci 25: 1788–1796, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chassin C, Tourneur E, Bens M, Vandewalle A. A role for collecting duct epithelial cells in renal antibacterial defences. Cell Microbiol 13: 1107–1113, 2011. [DOI] [PubMed] [Google Scholar]
- 13. Cheung KP, Kasimsetty SG, McKay DB. Innate immunity in donor procurement. Curr Opin Organ Transplant 18: 154–160, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med 203: 2841–2852, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chow A, Brown BD, Merad M. Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol 11: 788–798, 2011. [DOI] [PubMed] [Google Scholar]
- 16. Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol 27: 147–163, 2009. [DOI] [PubMed] [Google Scholar]
- 17. Deckers JG, De Haij S, van der Woude FJ, van der Kooij SW, Daha MR, van Kooten C. IL-4 and IL-13 augment cytokine- and CD40-induced RANTES production by human renal tubular epithelial cells in vitro. J Am Soc Nephrol 9: 1187–1193, 1998. [DOI] [PubMed] [Google Scholar]
- 18. di Mari JF, Davis R, Safirstein RL. MAPK activation determines renal epithelial cell survival during oxidative injury. Am J Physiol Renal Physiol 277: F195–F203, 1999. [DOI] [PubMed] [Google Scholar]
- 19. Disteldorf EM, Panzer U. Is there a role for proximal tubular cells in regulating dendritic cell maturation and function in renal disease? Nephrol Dial Transplant 28: 239–241, 2013. [DOI] [PubMed] [Google Scholar]
- 20. Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest 119: 2868–2878, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD. Antigen presentation by dendritic cells in renal lymph nodes is linked to systemic and local injury to the kidney. Kidney Int 68: 1096–1108, 2005. [DOI] [PubMed] [Google Scholar]
- 22. Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD. Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int 71: 619–628, 2007. [DOI] [PubMed] [Google Scholar]
- 23. Duffield JS. Macrophages and immunologic inflammation of the kidney. Semin Nephrol 30: 234–254, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duffield JS, Tipping PG, Kipari T, Cailhier JF, Clay S, Lang R, Bonventre JV, Hughes J. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am J Pathol 167: 1207–1219, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. El-Achkar TM, Dagher PC. Renal Toll-like receptors: recent advances and implications for disease. Nat Clin Pract Nephrol 2: 568–581, 2006. [DOI] [PubMed] [Google Scholar]
- 26. El-Achkar TM, McCracken R, Liu Y, Heitmeier MR, Bourgeois S, Ryerse J, Wu XR. Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium and circulation during recovery from acute kidney injury. Am J Physiol Renal Physiol. (First published February 6, 2013). doi:10.1152/ajprenal.00543.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. El-Achkar TM, McCracken R, Rauchman M, Heitmeier MR, Al-Aly Z, Dagher PC, Wu XR. Tamm-Horsfall protein-deficient thick ascending limbs promote injury to neighboring S3 segments in an MIP-2-dependent mechanism. Am J Physiol Renal Physiol 300: F999–F1007, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. El-Achkar TM, Plotkin Z, Marcic B, Dagher PC. Sepsis induces an increase in thick ascending limb Cox-2 that is TLR4 dependent. Am J Physiol Renal Physiol 293: F1187–F1196, 2007. [DOI] [PubMed] [Google Scholar]
- 29. El-Achkar TM, Wu XR. Uromodulin in kidney injury: an instigator, bystander, or protector? Am J Kidney Dis 59: 452–461, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. El-Achkar TM, Wu XR, Rauchman M, McCracken R, Kiefer S, Dagher PC. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am J Physiol Renal Physiol 295: F534–F544, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferenbach DA, Sheldrake TA, Dhaliwal K, Kipari TM, Marson LP, Kluth DC, Hughes J. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int 82: 928–933, 2012. [DOI] [PubMed] [Google Scholar]
- 32. Feulner JA, Lu M, Shelton JM, Zhang M, Richardson JA, Munford RS. Identification of acyloxyacyl hydrolase, a lipopolysaccharide-detoxifying enzyme, in the murine urinary tract. Infect Immun 72: 3171–3178, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fritz JH, Le Bourhis L, Magalhaes JG, Philpott DJ. Innate immune recognition at the epithelial barrier drives adaptive immunity: APCs take the back seat. Trends Immunol 29: 41–49, 2008. [DOI] [PubMed] [Google Scholar]
- 34. Fuggle SV, McWhinnie DL, Chapman JR, Taylor HM, Morris PJ. Sequential analysis of HLA-class II antigen expression in human renal allografts. Induction of tubular class II antigens and correlation with clinical parameters. Transplantation 42: 144–150, 1986. [DOI] [PubMed] [Google Scholar]
- 35. Fujiu K, Manabe I, Nagai R. Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J Clin Invest 121: 3425–3441, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma'ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ, Immunological Genome Consortium. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 13: 1118–1128, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol 10: 453–460, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science 327: 656–661, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gluba A, Banach M, Hannam S, Mikhailidis DP, Sakowicz A, Rysz J. The role of Toll-like receptors in renal diseases. Nat Rev Nephrol 6: 224–235, 2010. [DOI] [PubMed] [Google Scholar]
- 40. Gobe GC, Johnson DW. Distal tubular epithelial cells of the kidney: potential support for proximal tubular cell survival after renal injury. Int J Biochem Cell Biol 39: 1551–1561, 2007. [DOI] [PubMed] [Google Scholar]
- 41. Good DW, George T, Watts BA., 3rd Lipopolysaccharide directly alters renal tubule transport through distinct TLR4-dependent pathways in basolateral and apical membranes. Am J Physiol Renal Physiol 297: F866–F874, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 32: 593–604, 2010. [DOI] [PubMed] [Google Scholar]
- 43. Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol 50: 323–354, 2010. [DOI] [PubMed] [Google Scholar]
- 44. Grigoryev DN, Liu M, Hassoun HT, Cheadle C, Barnes KC, Rabb H. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol 19: 547–558, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hagerty DT, Allen PM. Processing and presentation of self and foreign antigens by the renal proximal tubule. J Immunol 148: 2324–2330, 1992. [PubMed] [Google Scholar]
- 46. Hamilton JA, Achuthan A. Colony stimulating factors and myeloid cell biology in health and disease. Trends Immunol 34: 81–89, 2013. [DOI] [PubMed] [Google Scholar]
- 47. Hasegawa K, Wakino S, Yoshioka K, Tatematsu S, Hara Y, Minakuchi H, Sueyasu K, Washida N, Tokuyama H, Tzukerman M, Skorecki K, Hayashi K, Itoh H. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J Biol Chem 285: 13045–13056, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity 35: 323–335, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. He W, Wang Y, Zhang MZ, You L, Davis LS, Fan H, Yang HC, Fogo AB, Zent R, Harris RC, Breyer MD, Hao CM. Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest 120: 1056–1068, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hession C, Decker JM, Sherblom AP, Kumar S, Yue CC, Mattaliano RJ, Tizard R, Kawashima E, Schmeissner U, Heletky S, Chow EP, Burne CA, Shaw A, Muchmore AV. Uromodulin (Tamm-Horsfall glycoprotein): a renal ligand for lymphokines. Science 237: 1479–1484, 1987. [DOI] [PubMed] [Google Scholar]
- 51. Heymann F, Meyer-Schwesinger C, Hamilton-Williams EE, Hammerich L, Panzer U, Kaden S, Quaggin SE, Floege J, Grone HJ, Kurts C. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J Clin Invest 119: 1286–1297, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hochheiser K, Engel DR, Hammerich L, Heymann F, Knolle PA, Panzer U, Kurts C. Kidney dendritic cells become pathogenic during crescentic glomerulonephritis with proteinuria. J Am Soc Nephrol 22: 306–316, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Horton JK, Davies M, Topley N, Thomas D, Williams JD. Activation of the inflammatory response of neutrophils by Tamm-Horsfall glycoprotein. Kidney Int 37: 717–726, 1990. [DOI] [PubMed] [Google Scholar]
- 54. Hume DA. Macrophages as APC and the dendritic cell myth. J Immunol 181: 5829–5835, 2008. [DOI] [PubMed] [Google Scholar]
- 55. Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest 118: 1657–1668, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ingersoll MA, Platt AM, Potteaux S, Randolph GJ. Monocyte trafficking in acute and chronic inflammation. Trends Immunol 32: 470–477, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jacob AI, Goldberg PK, Bloom N, Degenshein GA, Kozinn PJ. Endotoxin and bacteria in portal blood. Gastroenterology 72: 1268–1270, 1977. [PubMed] [Google Scholar]
- 58. Jang HR, Rabb H. The innate immune response in ischemic acute kidney injury. Clin Immunol 130: 41–50, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jevnikar AM, Wuthrich RP, Takei F, Xu HW, Brennan DC, Glimcher LH, Rubin-Kelley VE. Differing regulation and function of ICAM-1 and class II antigens on renal tubular cells. Kidney Int 38: 417–425, 1990. [DOI] [PubMed] [Google Scholar]
- 60. Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, Lee JO. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130: 1071–1082, 2007. [DOI] [PubMed] [Google Scholar]
- 61. Jo SK, Sung SA, Cho WY, Go KJ, Kim HK. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant 21: 1231–1239, 2006. [DOI] [PubMed] [Google Scholar]
- 62. Kagan JC. Signaling organelles of the innate immune system. Cell 151: 1168–1178, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kalakeche R, Hato T, Rhodes G, Dunn KW, El-Achkar TM, Plotkin Z, Sandoval RM, Dagher PC. Endotoxin uptake by S1 proximal tubular segment causes oxidative stress in the downstream S2 segment. J Am Soc Nephrol 22: 1505–1516, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kassianos AJ, Sampangi S, Wang X, Roper KE, Beagley K, Healy H, Wilkinson R. Human proximal tubule epithelial cells modulate autologous dendritic cell function. Nephrol Dial Transplant 28: 303–312, 2013. [DOI] [PubMed] [Google Scholar]
- 65. Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34: 637–650, 2011. [DOI] [PubMed] [Google Scholar]
- 66. Kim BS, Lim SW, Li C, Kim JS, Sun BK, Ahn KO, Han SW, Kim J, Yang CW. Ischemia-reperfusion injury activates innate immunity in rat kidneys. Transplantation 79: 1370–1377, 2005. [DOI] [PubMed] [Google Scholar]
- 67. Kreft B, Jabs WJ, Laskay T, Klinger M, Solbach W, Kumar S, van Zandbergen G. Polarized expression of Tamm-Horsfall protein by renal tubular epithelial cells activates human granulocytes. Infect Immun 70: 2650–2656, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kronsteiner B, Peterbauer-Scherb A, Grillari-Voglauer R, Redl H, Gabriel C, van Griensven M, Wolbank S. Human mesenchymal stem cells and renal tubular epithelial cells differentially influence monocyte-derived dendritic cell differentiation and maturation. Cell Immunol 267: 30–38, 2011. [DOI] [PubMed] [Google Scholar]
- 69. Kruger B, Krick S, Dhillon N, Lerner SM, Ames S, Bromberg JS, Lin M, Walsh L, Vella J, Fischereder M, Kramer BK, Colvin RB, Heeger PS, Murphy BT, Schroppel B. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci USA 106: 3390–3395, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kuroiwa T, Schlimgen R, Illei GG, McInnes IB, Boumpas DT. Distinct T cell/renal tubular epithelial cell interactions define differential chemokine production: implications for tubulointerstitial injury in chronic glomerulonephritides. J Immunol 164: 3323–3329, 2000. [DOI] [PubMed] [Google Scholar]
- 71. Laestadius A, Soderblom T, Aperia A, Richter-Dahlfors A. Developmental aspects of Escherichia coli-induced innate responses in rat renal epithelial cells. Pediatr Res 54: 536–541, 2003. [DOI] [PubMed] [Google Scholar]
- 72. Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol 11: 750–761, 2011. [DOI] [PubMed] [Google Scholar]
- 73. Lech M, Garlanda C, Mantovani A, Kirschning CJ, Schlondorff D, Anders HJ. Different roles of TiR8/Sigirr on toll-like receptor signaling in intrarenal antigen-presenting cells and tubular epithelial cells. Kidney Int 72: 182–192, 2007. [DOI] [PubMed] [Google Scholar]
- 74. Lech M, Grobmayr R, Weidenbusch M, Anders HJ. Tissues use resident dendritic cells and macrophages to maintain homeostasis and to regain homeostasis upon tissue injury: the immunoregulatory role of changing tissue environments. Mediators Inflamm 2012: 951390, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest 115: 2894–2903, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li H, Nord EP. CD40 ligation stimulates MCP-1 and IL-8 production, TRAF6 recruitment, and MAPK activation in proximal tubule cells. Am J Physiol Renal Physiol 282: F1020–F1033, 2002. [DOI] [PubMed] [Google Scholar]
- 78. Li L, Okusa MD. Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Semin Nephrol 30: 268–277, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci USA 107: 4194–4199, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liu L, Botos I, Wang Y, Leonard JN, Shiloach J, Segal DM, Davies DR. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science 320: 379–381, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Liu Y, El-Achkar TM, Wu XR. Tamm-Horsfall protein regulates circulating and renal cytokines by affecting glomerular filtration rate and acting as a urinary cytokine trap. J Biol Chem 287: 16365–16378, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lu CY, Winterberg PD, Chen J, Hartono JR. Acute kidney injury: a conspiracy of Toll-like receptor 4 on endothelia, leukocytes, and tubules. Pediatr Nephrol 27: 1847–1854, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lumsden AB, Henderson JM, Kutner MH. Endotoxin levels measured by a chromogenic assay in portal, hepatic and peripheral venous blood in patients with cirrhosis. Hepatology 8: 232–236, 1988. [DOI] [PubMed] [Google Scholar]
- 84. Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev 31: 817–844, 2010. [DOI] [PubMed] [Google Scholar]
- 85. Mathison JC, Ulevitch RJ. The clearance, tissue distribution, and cellular localization of intravenously injected lipopolysaccharide in rabbits. J Immunol 123: 2133–2143, 1979. [PubMed] [Google Scholar]
- 86. Matzinger P. The evolution of the danger theory. Interview by Lauren Constable, Commissioning Editor. Expert Rev Clin Immunol 8: 311–317, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nat Rev Immunol 11: 221–230, 2011. [DOI] [PubMed] [Google Scholar]
- 88. Menke J, Iwata Y, Rabacal WA, Basu R, Yeung YG, Humphreys BD, Wada T, Schwarting A, Stanley ER, Kelley VR. CSF-1 signals directly to renal tubular epithelial cells to mediate repair in mice. J Clin Invest 119: 2330–2342, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, Pandey G, Leboeuf M, Elpek KG, Helft J, Hashimoto D, Chow A, Price J, Greter M, Bogunovic M, Bellemare-Pelletier A, Frenette PS, Randolph GJ, Turley SJ, Merad M, Immunological Genome Consortium. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol 13: 888–899, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mo L, Zhu XH, Huang HY, Shapiro E, Hasty DL, Wu XR. Ablation of the Tamm-Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. Am J Physiol Renal Physiol 286: F795–F802, 2004. [DOI] [PubMed] [Google Scholar]
- 91. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8: 958–969, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mrug M, Zhou J, Woo Y, Cui X, Szalai AJ, Novak J, Churchill GA, Guay-Woodford LM. Overexpression of innate immune response genes in a model of recessive polycystic kidney disease. Kidney Int 73: 63–76, 2008. [DOI] [PubMed] [Google Scholar]
- 93. Muchmore AV, Decker JM. Uromodulin: a unique 85-kilodalton immunosuppressive glycoprotein isolated from urine of pregnant women. Science 229: 479–481, 1985. [DOI] [PubMed] [Google Scholar]
- 94. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11: 723–737, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int 70: 432–443, 2006. [DOI] [PubMed] [Google Scholar]
- 96. Nelson PJ, Rees AJ, Griffin MD, Hughes J, Kurts C, Duffield J. The renal mononuclear phagocytic system. J Am Soc Nephrol 23: 194–203, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Niemann-Masanek U, Mueller A, Yard BA, Waldherr R, van der Woude FJ. B7–1 (CD80) and B7–2 (CD 86) expression in human tubular epithelial cells in vivo and in vitro. Nephron 92: 542–556, 2002. [DOI] [PubMed] [Google Scholar]
- 98. Noessner E, Lindenmeyer M, Nelson PJ, Segerer S. Dendritic cells in human renal inflammation—Part II. Nephron Exp Nephrol 119: e91–e98, 2011. [DOI] [PubMed] [Google Scholar]
- 99. Pak J, Pu Y, Zhang ZT, Hasty DL, Wu XR. Tamm-Horsfall protein binds to type 1 fimbriated Escherichia coli and prevents E. coli from binding to uroplakin Ia and Ib receptors. J Biol Chem 276: 9924–9930, 2001. [DOI] [PubMed] [Google Scholar]
- 100. Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458: 1191–1195, 2009. [DOI] [PubMed] [Google Scholar]
- 101. Patole PS, Schubert S, Hildinger K, Khandoga S, Khandoga A, Segerer S, Henger A, Kretzler M, Werner M, Krombach F, Schlondorff D, Anders HJ. Toll-like receptor-4: renal cells and bone marrow cells signal for neutrophil recruitment during pyelonephritis. Kidney Int 68: 2582–2587, 2005. [DOI] [PubMed] [Google Scholar]
- 102. Piya MK, Harte AL, McTernan PG. Metabolic endotoxaemia: is it more than just a gut feeling? Curr Opin Lipidol 24: 78–85, 2013. [DOI] [PubMed] [Google Scholar]
- 103. Pulskens WP, Rampanelli E, Teske GJ, Butter LM, Claessen N, Luirink IK, van der Poll T, Florquin S, Leemans JC. TLR4 promotes fibrosis but attenuates tubular damage in progressive renal injury. J Am Soc Nephrol 21: 1299–1308, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pulskens WP, Teske GJ, Butter LM, Roelofs JJ, van der Poll T, Florquin S, Leemans JC. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PloS one 3: e3596, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rae F, Woods K, Sasmono T, Campanale N, Taylor D, Ovchinnikov DA, Grimmond SM, Hume DA, Ricardo SD, Little MH. Characterisation and trophic functions of murine embryonic macrophages based upon the use of a Csf1r-EGFP transgene reporter. Dev Biol 308: 232–246, 2007. [DOI] [PubMed] [Google Scholar]
- 106. Rallabhandi P, Bell J, Boukhvalova MS, Medvedev A, Lorenz E, Arditi M, Hemming VG, Blanco JC, Segal DM, Vogel SN. Analysis of TLR4 polymorphic variants: new insights into TLR4/MD-2/CD14 stoichiometry, structure, and signaling. J Immunol 177: 322–332, 2006. [DOI] [PubMed] [Google Scholar]
- 107. Rampoldi L, Scolari F, Amoroso A, Ghiggeri G, Devuyst O. The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int 80: 338–347, 2011. [DOI] [PubMed] [Google Scholar]
- 108. Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest 118: 3522–3530, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, Nespoli A, Viale G, Allavena P, Rescigno M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol 6: 507–514, 2005. [DOI] [PubMed] [Google Scholar]
- 110. Roberts RA, Ganey PE, Ju C, Kamendulis LM, Rusyn I, Klaunig JE. Role of the Kupffer cell in mediating hepatic toxicity and carcinogenesis. Toxicol Sci 96: 2–15, 2007. [DOI] [PubMed] [Google Scholar]
- 112. Rosin DL, Okusa MD. Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol 22: 416–425, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rusai K, Sollinger D, Baumann M, Wagner B, Strobl M, Schmaderer C, Roos M, Kirschning C, Heemann U, Lutz J. Toll-like receptors 2 and 4 in renal ischemia/reperfusion injury. Pediatr Nephrol 25: 853–860, 2010. [DOI] [PubMed] [Google Scholar]
- 114. Rushworth SA, Chen XL, Mackman N, Ogborne RM, O'Connell MA. Lipopolysaccharide-induced heme oxygenase-1 expression in human monocytic cells is mediated via Nrf2 and protein kinase C. J Immunol 175: 4408–4415, 2005. [DOI] [PubMed] [Google Scholar]
- 115. Saemann MD, Weichhart T, Zeyda M, Staffler G, Schunn M, Stuhlmeier KM, Sobanov Y, Stulnig TM, Akira S, von Gabain A, von Ahsen U, Horl WH, Zlabinger GJ. Tamm-Horsfall glycoprotein links innate immune cell activation with adaptive immunity via a Toll-like receptor-4-dependent mechanism. J Clin Invest 115: 468–475, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nat Immunol 13: 1145–1154, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Schilling JD, Martin SM, Hung CS, Lorenz RG, Hultgren SJ. Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc Natl Acad Sci USA 100: 4203–4208, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Scholz J, Lukacs-Kornek V, Engel DR, Specht S, Kiss E, Eitner F, Floege J, Groene HJ, Kurts C. Renal dendritic cells stimulate IL-10 production and attenuate nephrotoxic nephritis. J Am Soc Nephrol 19: 527–537, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev 90: 859–904, 2010. [DOI] [PubMed] [Google Scholar]
- 120. Serafini-Cessi F, Malagolini N, Cavallone D. Tamm-Horsfall glycoprotein: biology and clinical relevance. Am J Kidney Dis 42: 658–676, 2003. [DOI] [PubMed] [Google Scholar]
- 121. Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol 26: 421–452, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 11: 762–774, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Shigeoka AA, Holscher TD, King AJ, Hall FW, Kiosses WB, Tobias PS, Mackman N, McKay DB. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J Immunol 178: 6252–6258, 2007. [DOI] [PubMed] [Google Scholar]
- 124. Shigeoka AA, Kambo A, Mathison JC, King AJ, Hall WF, da Silva Correia J, Ulevitch RJ, McKay DB. Nod1 and nod2 are expressed in human and murine renal tubular epithelial cells and participate in renal ischemia reperfusion injury. J Immunol 184: 2297–2304, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Shigeoka AA, Mueller JL, Kambo A, Mathison JC, King AJ, Hall WF, Correia Jda S, Ulevitch RJ, Hoffman HM, McKay DB. An inflammasome-independent role for epithelial-expressed Nlrp3 in renal ischemia-reperfusion injury. J Immunol 185: 6277–6285, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Shirali AC, Goldstein DR. Tracking the toll of kidney disease. J Am Soc Nephrol 19: 1444–1450, 2008. [DOI] [PubMed] [Google Scholar]
- 127. Shulzhenko N, Morgun A, Hsiao W, Battle M, Yao M, Gavrilova O, Orandle M, Mayer L, Macpherson AJ, McCoy KD, Fraser-Liggett C, Matzinger P. Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat Med 17: 1585–1593, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122: 787–795, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Soos TJ, Sims TN, Barisoni L, Lin K, Littman DR, Dustin ML, Nelson PJ. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int 70: 591–596, 2006. [DOI] [PubMed] [Google Scholar]
- 130. Su SJ, Chang KL, Lin TM, Huang YH, Yeh TM. Uromodulin and Tamm-Horsfall protein induce human monocytes to secrete TNF and express tissue factor. J Immunol 158: 3449–3456, 1997. [PubMed] [Google Scholar]
- 131. Tadagavadi RK, Reeves WB. Renal dendritic cells ameliorate nephrotoxic acute kidney injury. J Am Soc Nephrol 21: 53–63, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Teteris SA, Engel DR, Kurts C. Homeostatic and pathogenic role of renal dendritic cells. Kidney Int 80: 139–145, 2011. [DOI] [PubMed] [Google Scholar]
- 133. Tsuboi N, Yoshikai Y, Matsuo S, Kikuchi T, Iwami K, Nagai Y, Takeuchi O, Akira S, Matsuguchi T. Roles of toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J Immunol 169: 2026–2033, 2002. [DOI] [PubMed] [Google Scholar]
- 134. Unkel B, Hoegner K, Clausen BE, Lewe-Schlosser P, Bodner J, Gattenloehner S, Janssen H, Seeger W, Lohmeyer J, Herold S. Alveolar epithelial cells orchestrate DC function in murine viral pneumonia. J Clin Invest 122: 3652–3664, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Van Amersfoort ES, Van Berkel TJ, Kuiper J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin Microbiol Rev 16: 379–414, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Wahl P, Schoop R, Bilic G, Neuweiler J, Le Hir M, Yoshinaga SK, Wuthrich RP. Renal tubular epithelial expression of the costimulatory molecule B7RP-1 (inducible costimulator ligand). J Am Soc Nephrol 13: 1517–1526, 2002. [DOI] [PubMed] [Google Scholar]
- 137. Wang Y, Tay YC, Harris DC. Proximal tubule cells stimulated by lipopolysaccharide inhibit macrophage activation. Kidney Int 66: 655–662, 2004. [DOI] [PubMed] [Google Scholar]
- 138. Watts BA, George T, 3rd, Sherwood ER, Good DW. Basolateral LPS inhibits NHE3 and HCOFormula absorption through TLR4/MyD88-dependent ERK activation in medullary thick ascending limb. Am J Physiol Cell Physiol 301: C1296–C1306, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wimmer T, Cohen G, Saemann MD, Horl WH. Effects of Tamm-Horsfall protein on polymorphonuclear leukocyte function. Nephrol Dial Transplant 19: 2192–2197, 2004. [DOI] [PubMed] [Google Scholar]
- 140. Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemen MA, Hiemstra PS, van 't Veer C. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J Immunol 168: 1286–1293, 2002. [DOI] [PubMed] [Google Scholar]
- 141. Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest 117: 2847–2859, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Wuthrich RP, Glimcher LH, Yui MA, Jevnikar AM, Dumas SE, Kelley VE. MHC class II, antigen presentation and tumor necrosis factor in renal tubular epithelial cells. Kidney Int 37: 783–792, 1990. [DOI] [PubMed] [Google Scholar]
- 143. Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38: 79–91, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Yu CL, Tsai CY, Lin WM, Liao TS, Chen HL, Sun KH, Chen KH. Tamm-Horsfall urinary glycoprotein enhances monokine release and augments lymphocyte proliferation. Immunopharmacology 26: 249–258, 1993. [DOI] [PubMed] [Google Scholar]
- 145. Zhang J, Brown RP, Shaw M, Vaidya VS, Zhou Y, Espandiari P, Sadrieh N, Stratmeyer M, Keenan J, Kilty CG, Bonventre JV, Goering PL. Immunolocalization of Kim-1, RPA-1, and RPA-2 in kidney of gentamicin-, mercury-, or chromium-treated rats: relationship to renal distributions of iNOS and nitrotyrosine. Toxicol Pathol 36: 397–409, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, Yin H, Wong K, Miyazawa T, Chen J, Chang I, Singh A, Harris RC. CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest 122: 4519–4532, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zhou J, Ouyang X, Cui X, Schoeb TR, Smythies LE, Johnson MR, Guay-Woodford LM, Chapman AB, Mrug M. Renal CD14 expression correlates with the progression of cystic kidney disease. Kidney Int 78: 550–560, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.