Abstract
The rate of urine secretion by insect Malpighian tubules (MTs) is regulated by multiple diuretic and antidiuretic hormones, often working either synergistically or antagonistically. In the Drosophila melanogaster MT, only diuretic factors have been reported. Two such agents are the biogenic amine tyramine (TA) and the peptide drosokinin (DK), both of which act on the stellate cells of the tubule to increase transepithelial chloride conductance. In the current study, TA and DK signaling was quantified by microelectrode recording of the transepithelial potential in isolated Drosophila MTs. Treatment of tubules with cGMP caused a significant reduction in the depolarizing responses to both TA and DK, while cAMP had no effect on these responses. To determine whether a specific cGMP-dependent protein kinase (PKG) was mediating this inhibition, PKG expression was knocked down by RNAi in either the principal cells or the stellate cells. Knockdown of Pkg21D in the stellate cells eliminated the modulation of TA and DK signaling. Knockdown of Pkg21D with a second RNAi construct also reduced the modulation of TA signaling. In contrast, knockdown of the expression of foraging or CG4839, which encodes a known and a putative PKG, respectively, had no effect. These data indicate that cGMP, acting through the Pkg21D gene product in the stellate cells, can inhibit signaling by the diuretic agents TA and DK. This represents a novel function for cGMP and PKG in the Drosophila MT and suggests the existence of an antidiuretic hormone in Drosophila.
Keywords: Malpighian tubule, tyramine, Drosophila, kinin, insect, excretory system
precise control of water and osmotic balance is essential to the survival of all organisms. In insects, these functions are performed, in part, by the excretory system, including the Malpighian tubules (MTs)—blind-ended epithelial tubes that secrete the primary urine and empty into the digestive tract at the junction between the midgut and hindgut (1, 7, 25, 63)—and the rectum—the main site of water reabsorption and the adjustment of fecal water content (7). To respond to the changing physiological state of the animal, the rate of urine secretion by the MTs is controlled by many diuretic and antidiuretic agents (2, 44, 47).
Much is known about different families of diuretic factors, the second messenger pathways through which they signal, and their effects on transepithelial ion transport; however, mechanisms of antidiuresis are less well-understood. Three classes of antidiuretic peptides have been identified that act directly on the MTs, and the second messenger cGMP is central to the signaling of all three. The first two classes are represented by the unrelated peptides Tenmo-ADFa and Tenmo-ADFb. These peptides were isolated from the beetle Tenebrio molitor, stimulate an increase in cGMP levels in the MTs of both Tenebrio and the mosquito Aedes aegypti, and their antidiuretic actions are mimicked by treatment of MTs with cGMP (26, 27, 37, 64). The third class contains the CAPA peptides, which cause antidiuresis in MTs from a number of heteropteran species and Aedes aegypti (12, 13, 31, 49, 55); they are most well-characterized in the blood-sucking insect Rhodnius prolixus (47). Like other antidiuretic peptides, CAPA peptides cause an increase in cGMP levels and their actions are mimicked by cGMP (12, 13, 31, 49, 55). However, the precise molecular events linking the peptides to a rise in cGMP levels are unclear; the Rhodnius CAPA receptor is a GPCR, as opposed to a receptor guanylate cyclase (50), and CAPA only causes a rise in cGMP levels in tubules that have been stimulated previously with a diuretic (51).
The molecular mechanisms of ion transport and its regulation are well-characterized in the Drosophila melanogaster MT (22, 25). The main segment of the Drosophila MT, where urine secretion takes place, is composed of two cell types: the principal cells and the smaller, less abundant stellate cells (24, 60). Transport of cations from the hemolymph to the lumen occurs through the principal cells, energized by a V-type proton ATPase in the apical membrane (19, 42). Anions, primarily chloride, then flow passively into the lumen, driven by the lumen-positive transepithelial potential (TEP) (42). The precise route of chloride transport across the epithelium—either paracellular or transcellular through the stellate cells—remains unclear, but chloride transport is stimulated by increased intracellular calcium levels in the stellate cells. The diuretic peptide drosokinin (DK) or related kinins from other species increase urine production and chloride conductance by stimulating the release of calcium from intracellular stores in the stellate cells (42, 43, 54, 56, 58, 62), and the biogenic amine tyramine (TA) has identical effects on chloride conductance and appears to act through the same signaling pathway (3, 4). In addition to DK and TA, another diuretic agent identified in Drosophila is the CAPA peptide, which stimulates cation transport by the principal cells through a pathway that includes a rise in intracellular calcium, activation of nitric oxide synthase, and stimulation of a soluble guanylate cyclase (16, 17, 33, 58). Finally, both the calcitonin-like peptide DH31 and the corticotropin releasing factor-like peptide DH44 stimulate principal cell cation transport through the production of cAMP (9, 14). To date, there have been no reports of an antidiuretic agent in the Drosophila MT.
As mentioned above, cGMP production in the Drosophila MT is stimulated by CAPA and triggers an increase in urine production. This diuresis can also be triggered by treatment of MTs with cGMP itself (16, 23), as the tubules are able to take up and transport cyclic nucleotides (28, 57). Transport of cGMP by the Drosophila MT is mediated largely by the product of the white (w) gene; however, cGMP-dependent diuresis is still observed in MTs isolated from w mutant flies (28), suggesting the existence of an additional transporter. The cGMP-mediated diuresis is accompanied by a hyperpolarization of the TEP and thus is thought to occur through stimulation of cation transport by the principal cells (42). Consistent with this model, the effects of cGMP are additive to those of the kinins, which act on the stellate cells (16, 23). Within the principal cells, cGMP has also recently been linked to the activation of innate immune responses by salt stress; in this case, the first messenger is the peptide NPLP1-VQQ acting through the receptor guanylate cyclase Gyc76C (46). There is also some evidence for a role of cGMP within the stellate cells. Production of cGMP specifically in the stellate cells by ectopic expression of a mammalian receptor guanylate cyclase causes diuresis (34).
A major effector of cGMP signaling is PKG (30). The Drosophila genome encodes two characterized PKGs and one putative PKG (38). The first, DG1, is encoded by the gene Pkg21D and is highly expressed in the MT, with an expression level at least 10-fold higher than that of any other tissue examined (11, 29). Overexpression of DG1 in the principal cells results in an enhanced diuresis in response to exogenous cGMP (36). The second PKG, DG2, is encoded by the foraging (for) gene and is also expressed in the MT (11). Overexpression of DG2 in the principal cells causes a specific hypersensitivity to CAPA-induced diuresis (36). In addition, the natural forR and fors polymorphisms result in differential sensitivity to CAPA-induced diuresis (35), suggesting an endogenous role of DG2 in this signaling pathway in the principal cells. More broadly, DG2 has been linked to a number of physiological responses in Drosophila, including foraging behavior, olfactory learning, thermotolerance, and response to anoxia (10, 18, 20, 32, 39, 45). The Drosophila genome contains a third gene, CG4839, which is predicted to encode a PKG and is expressed in the MT (11, 38), but there have been no reports on its biochemical or physiological function.
In this paper, we present a novel role for cGMP in the Drosophila MT. We find that activation of DG1 in the stellate cells inhibits the activation of transepithelial chloride conductance mediated by the diuretic agents TA and DK. Signaling through DG1 thus would be predicted to mediate context-dependent antidiuresis and would represent the first example of antidiuretic signaling in Drosophila.
METHODS
Fly stocks and genetics.
Stocks of Drosophila melanogaster were maintained on cornmeal/yeast/molasses/agar food at 24°C on a 12:12-h light-dark cycle. Fly stocks used in these experiments include Canton S, y1 v1; P{TRiP.JF02766}attP2 (FBst0027686, called Pkg21D-RNAi1), y1 v1; P{TRiP.JF02372}attP2 (FBst0027046, called CG4839-RNAi), w * ; P{KK101298}VIE-260B [FBst0480105, from the Vienna Drosophila RNAi Center (VDRC), called for-RNAi], w * ; P{KK112484}VIE-260B (FBst0475371, from VDRC, called Pkg21D-RNAi2), P{UAS-Dcr-2.D}1, w1118; P{Act5C-GAL4}25FO1/CyO (FBst0025708), w1118; P{UAS-Dcr-2.D}10 (FBst0024651), w * ; P{GawB}c42 (FBst0030835, gift of Prof. Julian Dow), and w * ; P{GawB}c710 (FBti0009567, gift of Prof. Julian Dow). Except where noted, fly stocks were obtained from the Bloomington Drosophila Stock Center. Genes studied in this work include Pkg21D (FBgn0000442), foraging (FBgn0000721), and CG4839 (FBgn0032187).
To minimize the effect of genetic background on TA sensitivity, the for-RNAi and Pkg21D-RNAi2 lines were cantonized by back-crossing for at least six generations against a cantonized w * stock; the resulting stocks were balanced with CyO. For these balanced stocks, only straight-winged progeny of crosses with Gal4 lines was used for physiological assays. The c42-gal4 and c710-gal4 stocks had previously been cantonized (6). To increase the efficiency of RNAi, a UAS-Dcr-2 transgene was recombined onto the c42-gal4 and c710-gal4 chromosomes by conventional genetic crosses. In each case, recombinants were identified by eye color for the presence of the gal4 driver and by PCR for the presence of the UAS-Dcr-2 transgene (as indicated by a 638-bp amplimer), using the primers L: GGA CAC GGT AAT GAT GTT TC and R: GAA GAG AAC TCT GAA TAG GG. All RNAi experiments in this study were performed using the resulting recombinant stocks.
Electrophysiology.
MTs were dissected from 6- to 8-day-old adult Drosophila females and placed in dishes in which 100 μl of 31 μg/ml poly-l-lysine were allowed to dry and that were rinsed with deionized water briefly before dissection, and the TEP was recorded with a conventional microelectrode as described (6). Data acquisition and analysis were performed with pClamp 9 software (Molecular Devices, Sunnyvale, CA). The dissecting and recording saline consisted of the following (in mM): 85 NaCl, 20 KCl, 3 CaCl2, 12 MgCl2, 7.5 NaHCO3, 10 HEPES, 15 glucose, pH 6.8 (260–265 mosmol/kgH2O). For recordings in standard bathing medium (SBM), MTs were dissected in saline and after the tubule was placed in the recording dish, the saline was replaced with SBM consisting of a 1:1 mixture of Schneider's Drosophila Medium (Life Technologies, Grand Island, NY) and a “diluting saline” containing (in mM) 36 NaCl, 21 KCl, 15 MgCl2, 5 CaCl2, 4.8 NaHCO3, 2 NaH2PO4, 11.1 glucose, 15 HEPES, pH 6.75. Agents added to the recording solution included sucrose (for hyperosmotic saline), cAMP sodium salt (Sigma, St. Louis, MO), cGMP sodium salt (Sigma), TA (Sigma), and DK (AnaSpec, Fremont, CA). For the experiments described in Figs. 2 and 3 (see Figs. 2 and 3), two 20-s applications of agonist were separated by 4–6 min of saline or cGMP. For the experiments described in Figs. 4–6 (see Figs. 4–6), two 20-s applications of agonist were separated by 4 min of saline, hyperosmotic saline, or cGMP, as described in results. Recording saline osmolality was measured with a vapor-pressure osmometer (Wescor, Logan, UT).
Fig. 2.
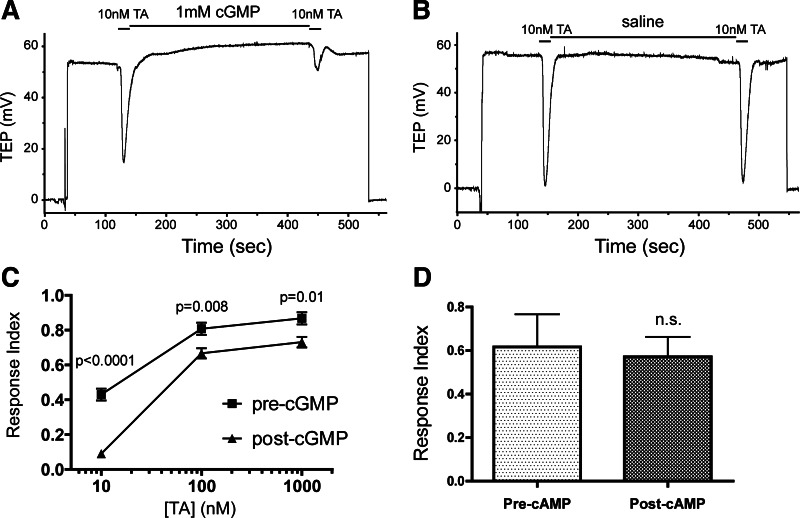
Modulation of tyramine (TA) responses by cGMP. A and B: TEP recordings of MTs bathed in saline and treated with 2 20-s applications of 10 nM TA separated by treatment with either 1 mM cGMP (A) or saline (B). C: mean amplitude of responses to different doses of TA before and after treatment with 1 mM cGMP. P values indicate significant differences of the second response from the first by paired t-test; n = 6–10 tubules per condition. D: mean responses to 10 nM TA before and after treatment with 1 mM cAMP, showing no significant change in the response by paired t-test; n = 8 tubules. Error bars indicate SD.
Fig. 3.
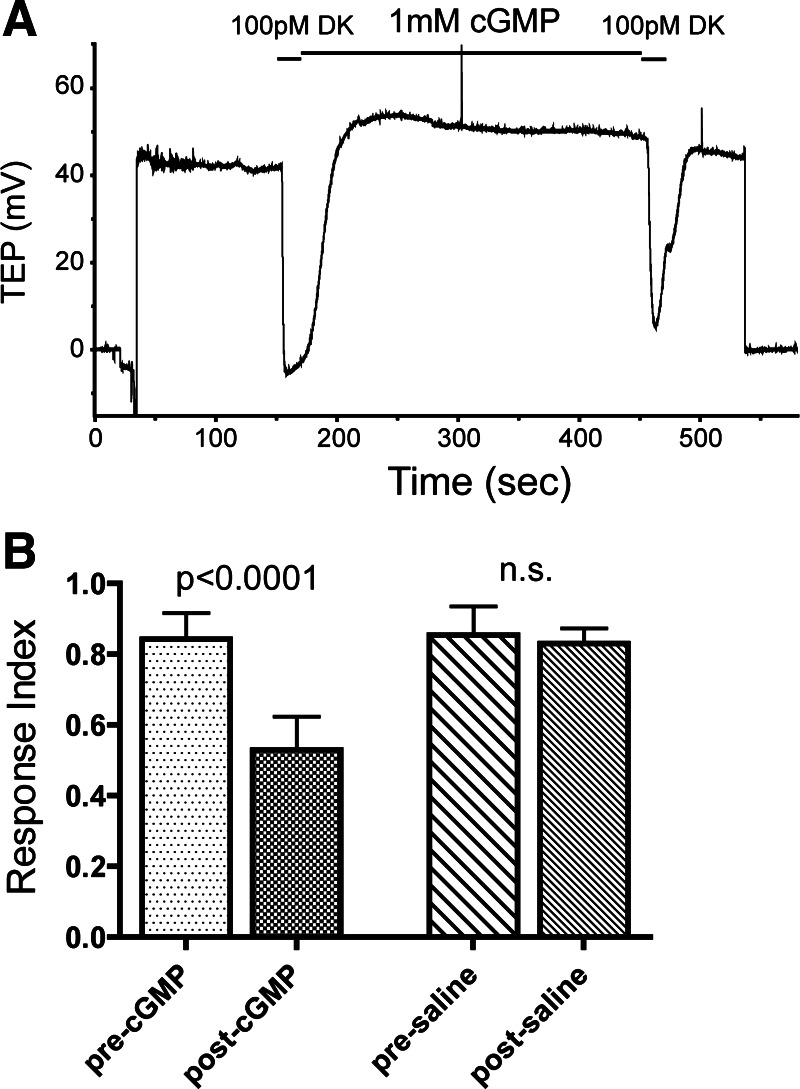
Modulation of drosokinin (DK) responses by cGMP. A: TEP recording of a MT bathed in saline and treated with 2 20-s applications of 100 pM DK separated by treatment with 1 mM cGMP. B: mean responses to 100 pM DK before and after treatment with either 1 mM cGMP (left bars) or saline (right bars). P values indicate comparisons between the 2 responses by paired t-test; n = 8 tubules per condition. Error bars indicate SD.
Fig. 4.
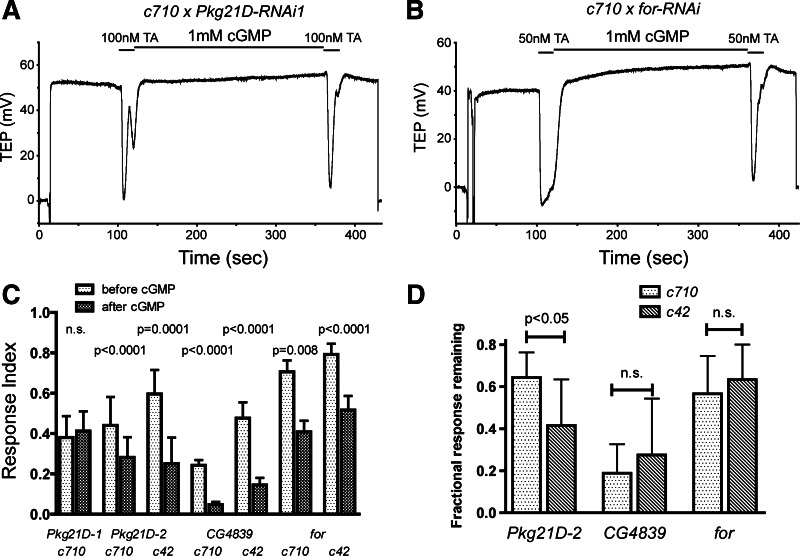
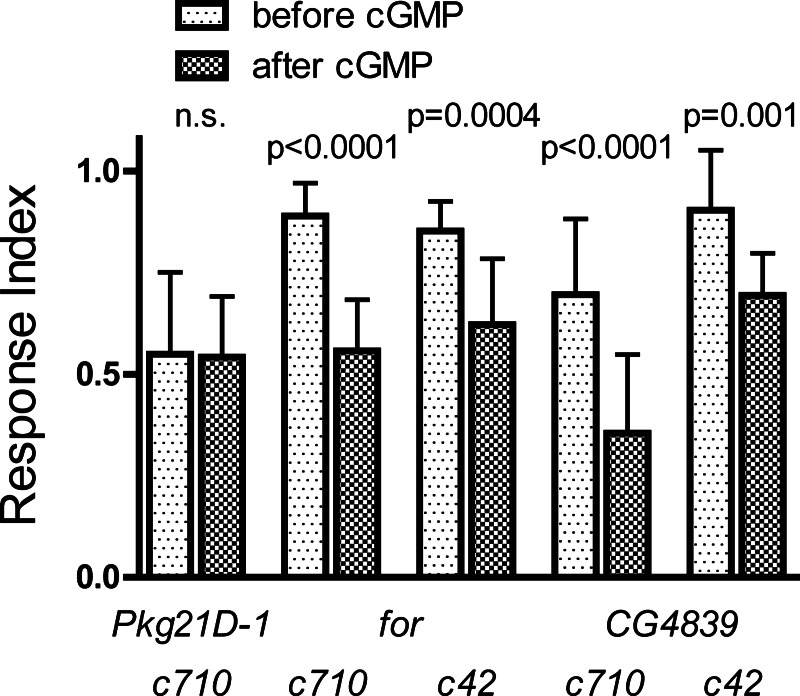
Role of specific PKG isoforms in the modulation of the TA response. A and B: TEP recordings of MTs dissected from progeny of w; UAS-Dcr-2 c710-gal4 × w; Pkg21D-RNAi1 (A) or w; for-RNAi/CyO (B). MTs were bathed in saline and treated with 2 20-s applications of 100 nM (A) or 50 nM (B) TA separated by 1 mM cGMP. C: mean TA responses before and after treatment with 1 mM cGMP for progeny of crosses between either w; UAS-Dcr-2 c42-gal4 or w; UAS-Dcr-2 c710-gal4 and the 4 RNAi lines. TA was applied at 50 nM except for progeny of w; UAS-Dcr-2 c710-gal4 × w; Pkg21D-RNAi1, which were treated with 100 nM TA to obtain responses of similar amplitude to the other genotypes; n = 8–10 MTs per condition. P values result from comparisons of the first and second responses by paired t-test or Wilcoxon signed rank test. D: data from C are replotted to show the fraction of the initial TA response remaining after treatment with cGMP for the progeny of the 6 crosses that responded to cGMP. For each RNAi line, the responses of c42 and c710 progeny were compared by 1-way ANOVA and Bonferroni posttest. Error bars indicate SD.
Fig. 6.
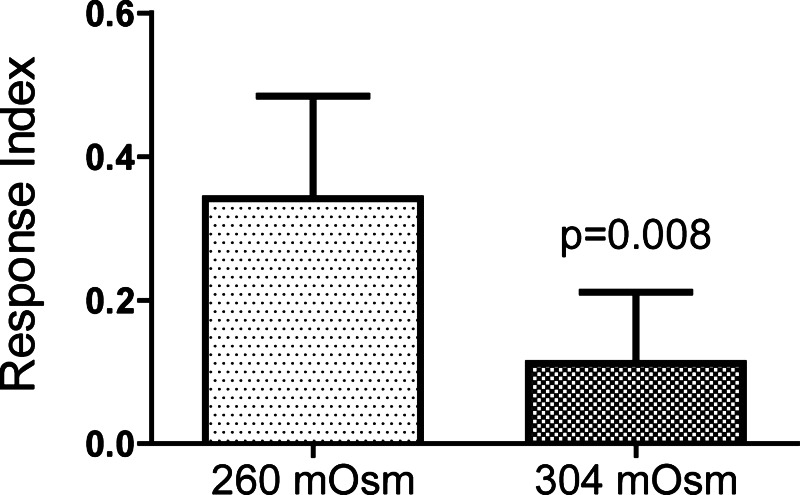
Modulation of TA response by hyperosmolality after PKG knockdown. Mean responses of MTs dissected from progeny of w; UAS-Dcr-2 c710-gal4 × w; Pkg21D-RNAi1 treated with 2 applications of 100 nM TA separated by a 4-min treatment with high-osmolality saline (304 instead of 260 mosmol/kgH2O). P value shown is from a Wilcoxon matched pairs signed rank test comparing the first and second responses; n = 8 tubules. Error bars indicate SD.
Response index values for TA and DK responses were calculated as previously described (6) for the 20-s period beginning 5 s after the start of agonist application. For two responses to 1 mM TA and four responses to 100 pM DK, the response index was calculated to be slightly above 1; in these cases, the value was set to 1.0 for the data analysis.
Statistics.
For statistical analysis, response index values were transformed by taking the arcsine of the square root of each value. Negative values were transformed by taking the negative arcsine of the square root of the absolute value of the response index. Statistical tests were performed with Graphpad Prism v5.02 for Windows (GraphPad Software, San Diego, CA, www.graphpad.com). Datasets were tested for normality using a D'Agostino-Pearson normality test (this test is only valid for n ≥ 8). Two datasets failed this test (c710-gal4 × for-RNAi first response, see Fig. 4C and w; UAS-Dcr-2 c710-gal4 × w; Pkg21D-RNAi1 second response, see Fig. 6) and were analyzed using a nonparametric Wilcoxon signed rank test instead of a paired t-test.
RESULTS
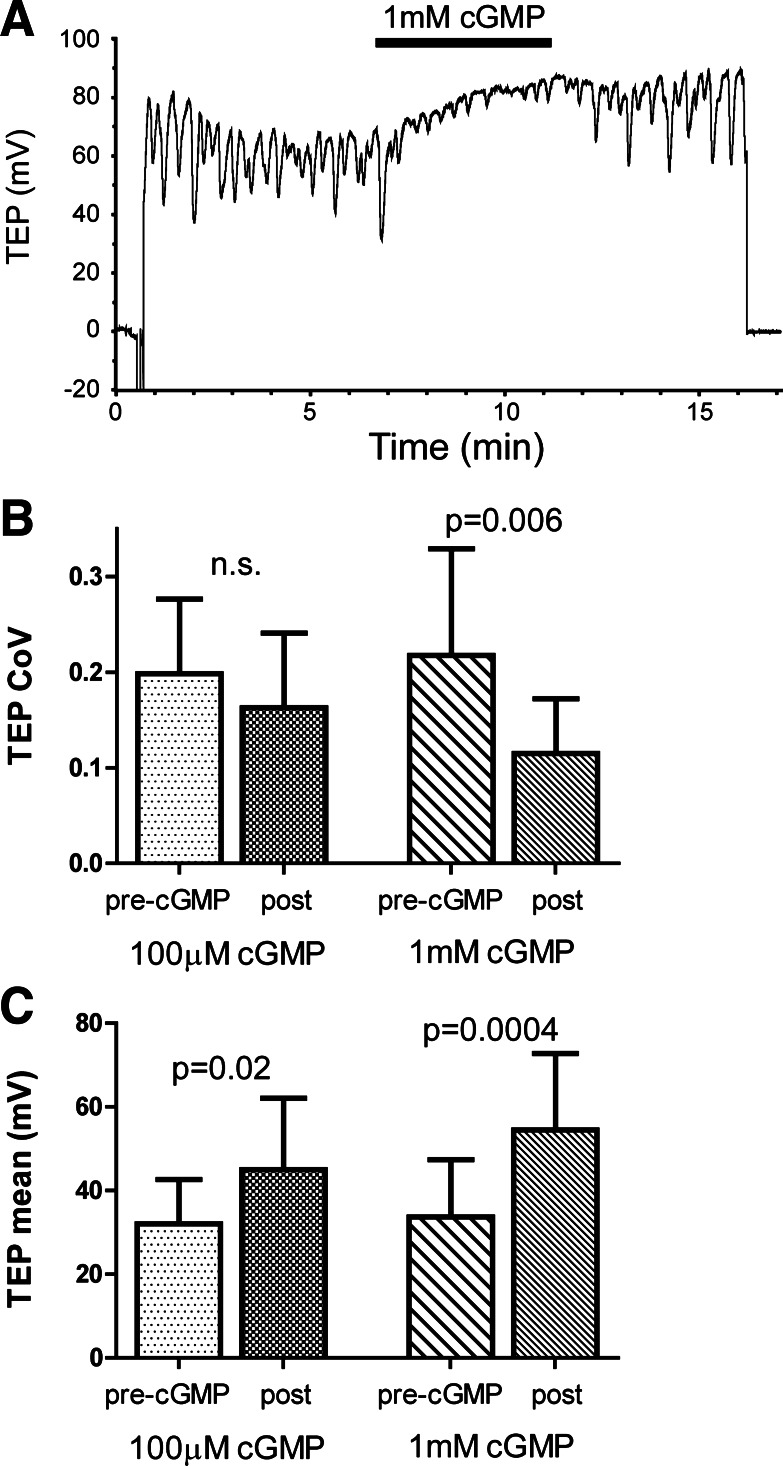
Conventional microelectrode recording of isolated MTs bathed in SBM, which contains tissue culture medium, revealed oscillations in the TEP. We previously reported that these oscillations are due to TA signaling, as the MT is able to convert tyrosine in the SBM to TA (4). The addition of 1 mM cGMP to the peritubular bath caused two changes to the TEP: an increase in TEP amplitude and a decrease in the magnitude of the oscillations, as quantified by the TEP coefficient of variation (CoV; Fig. 1). Both of these effects were observed within 2 min of the addition of cGMP. While the first response has been demonstrated to result from the stimulation of active cation transport through the principal cells (42), the second effect, on oscillations, has not previously been reported. The suppression of the TEP oscillations required a high concentration of cGMP, as treatment of tubules with 100 μM cGMP still increased the TEP amplitude but had no effect on the CoV (Fig. 1, B and C).
Fig. 1.
Suppression of transepithelial potential (TEP) oscillations by cGMP. A: TEP recording of a Malpighian tubule (MT) bathed in standard bathing medium (SBM) and treated for 4.5 min with 1 mM cGMP as indicated by the bar. B and C: effect of 100 μM and 1 mM cGMP on the TEP coefficient of variation (CoV; B) and amplitude (C) in MTs bathed in SBM. TEP parameters were measured over 2-min windows ending with the addition of cGMP (pre-cGMP) and beginning 2 min after the addition of cGMP (post-cGMP); n = 7–8 MTs/condition. P values are from comparisons of pre-cGMP and post-cGMP values by paired t-test. Error bars indicate SD. n.s., Not significant.
To determine more directly whether cGMP inhibited the MT response to TA, tubules were bathed in saline and treated with two 20-s applications of TA separated by 4–6 min of either saline or 1 mM cGMP (see methods). As shown in Fig. 2, the cGMP treatment almost completely eliminated the second response to 10 nM TA, which would otherwise have been identical to the first response (P = 0.63, paired t-test comparison of first and second responses in saline-treated tubules, n = 8). The responses to 100 nM and 1 μM TA were also inhibited by cGMP, although to a lesser degree. With these higher concentrations of TA, as with the lower concentration, treatment of tubules with saline in the absence of cGMP resulted in a second TA response that was identical to the first (100 nM, P = 0.22, n = 7; 1 μM, P = 0.34, n = 4, paired t-tests). At all three concentrations of TA tested, there was no difference in the amplitude of the initial response between cGMP-treated and saline-treated MTs (unpaired t-test, P = 0.10, 0.41, 0.48 for 10 nM, 100 nM, 1 μM TA, respectively). The modulation of TA sensitivity was specific to cGMP, as treatment of tubules with 1 mM cAMP had no effect on the response to 10 nM TA (Fig. 2D).
Since TA and the peptide hormone DK share a common signaling pathway, we tested whether DK responses were also inhibited by cGMP. As shown in Fig. 3, sensitivity to 100 pM DK was significantly suppressed following treatment with 1 mM cGMP. As with the TA responses, treatment of tubules with saline between the two applications resulted in an unchanged DK response (P = 0.19 by paired t-test, n = 8), and the initial responses of the two groups were identical (P = 0.74, unpaired t-test).
The action of cGMP is often mediated by the activation of a cGMP-dependent protein kinase (PKG), and so we sought to test the involvement of PKGs in the modulation of the TA response. We obtained fly stocks carrying inducible UAS-RNAi transgenes against the three Drosophila PKG genes; two stocks were directed against Pkg21D (Pkg21D-RNAi1, Pkg21D-RNAi2) and one stock each was directed against the other two genes (for-RNAi, CG4839-RNAi). None of these transgenes is predicted to induce any off-target effects on the expression of other genes (21, 40, 41). These UAS-RNAi stocks were crossed with three different Gal4 driver lines to induce RNAi ubiquitously (Act5C-gal4), in the principal cells of the MT (c42-gal4), and in the stellate cells of the MT (c710-gal4). In each case, a UAS-Dcr-2 transgene was included in the background to increase the RNAi efficiency. Ubiquitous expression of each of the four UAS-RNAi transgenes with Act5C-gal4 was lethal, indicating that each of the PKG genes is required for viability. Targeted knockdown of the genes with c42-gal4 or c710-gal4 resulted in viable, apparently healthy flies, with one exception. Adult progeny from crossing Pkg21D-RNAi1 with c42-gal4 were rarely obtained, and those few viable adults appeared unhealthy and never survived long enough to be used in electrophysiological experiments. In contrast, crossing Pkg21D-RNAi2 with c42-gal4 did yield viable progeny. This difference is consistent with our phenotypic data described below and suggests that we were able to drive more complete knockdown of Pkg21D expression with Pkg21D-RNAi1 compared with Pkg21D-RNAi2. However, this interpretation could not be tested directly by quantitative PCR due to the expression of Pkg21D in multiple cell types within the MT.
As shown in Fig. 4, knockdown of Pkg21D expression in the stellate cells by crossing c710-gal4 with Pkg21D-RNAi1 completely eliminated the modulation of TA sensitivity by cGMP. In tubules challenged with either 50 nM (data not shown) or 100 nM TA, the response to a second application of TA following a 4-min incubation with 1 mM cGMP was identical to the first response. Inhibition of the TA response by cGMP was still observed after knockdown of Pkg21D by Pkg21D-RNAi2 in either the principal or the stellate cells (Fig. 4C). However, the degree of inhibition was significantly reduced after stellate cell knockdown with Pkg21D-RNAi2, compared with principal cell knockdown, indicating a partial elimination of the cGMP-dependent modulation (Fig. 4D). In contrast, knockdown of either for or CG4839 in either cell type did not eliminate the cGMP-dependent modulation, and we did not observe any difference in the degree of modulation between stellate cell and principal cell knockdown for either of these PKG genes (Fig. 4, B, C, D).
The modulation of the DK response showed the same dependence on Pkg21D expression as that of the TA response. As shown in Fig. 5, knockdown of Pkg21D in the stellate cells with Pkg21D-RNAi1 eliminated the cGMP-dependent inhibition of the response to 100 pM DK. Modulation of the DK response was still present after knockdown of either for or CG4839 in either cell type.
Fig. 5.
Role of specific PKG isoforms in the modulation of the DK response. Mean responses to 100 pM DK before and after treatment with 1 mM cGMP for progeny of crosses between either w; UAS-Dcr-2 c42-gal4 or w; UAS-Dcr-2 c710-gal4 and the RNAi lines Pkg21D-RNAi1, for-RNAi/CyO, and CG4839-RNAi; n = 8 MTs per condition. P values result from comparisons of the first and second responses by paired t-tests. Error bars indicate SD.
We previously reported that increasing the osmolality of the peritubular bath causes an inhibition of the MT TA and DK responses very similar to that seen with cGMP treatment (5). To examine a possible relationship between these two modulatory responses, we examined the response to hyperosmotic saline in tubules isolated from progeny of a cross between c710-gal4 and Pkg21D-RNAi1. As described above, such tubules fail to show a cGMP-dependent modulation of the TA response. In eight tubules challenged with 100 nM TA, a 4-min increase in the peritubular osmolality from 260 to 304 mosmol/kgH2O caused a significant reduction in the TA response (Fig. 6). All eight tubules showed a decreased response following the increase in osmolality.
DISCUSSION
In this study, we report a new role for cGMP in the Drosophila MT, that of inhibiting the ability of the diuretics TA and DK to increase transpithelial chloride conductance. This modulation requires the expression of DG1 in the stellate cells; thus, we conclude that the exogenously applied cGMP is entering the stellate cells and activating DG1. The stellate cell-specific requirement for DG1 expression is consistent with our knowledge of TA and kinin signaling; both agents bind to receptors that are expressed in the stellate cells (56) (Zhang and Blumenthal, manuscript in preparation), and kinins cause a specific elevation of intracellular calcium levels in the stellate cells (43). The point in the diuretic signaling pathway that is affected by DG1 is still unknown, but it is likely to be downstream of the receptor as TA and kinins bind to separate receptors but are both similarly inhibited by cGMP (56) (Zhang and Blumenthal, manuscript in preparation).
The modulation observed in the current work appears similar to that we previously reported in response to an increase in peritubular osmolality (5). However, these two phenomena are independent, as osmotic inhibition of the TA response is still observed following knockdown of Pkg21D expression that eliminates the cGMP-dependent modulation. Thus, there appear to be multiple inhibitory pathways that impinge on TA and kinin signaling. The dual levels of control exerted on TA- and kinin-mediated diuresis could be due to the potency of these agents, which not only can elevate the rate of urine secretion by several-fold but also can depolarize the TEP, affecting all electrogenic transport processes ongoing in the MT.
Our observation of cGMP-dependent modulation of diuretic signaling in the stellate cells (c710-gal4) suggests the existence of a hormone that activates this modulatory pathway, possibly by binding to a receptor guanylate cyclase on the stellate cells. Consistent with this possibility, it is reported that stellate cells in the trichopteran insect Rhyacophila dorsalis acutidens, which has MTs that are morphologically similar to those of Drosophila, contain high levels of particulate guanylate cyclase activity (59). The hypothesized extracellular factor would act as a context-specific antidiuretic hormone, only reducing urine secretion in tubules that were stimulated by TA or kinin. Diuresis by agents that act on the principal cells, such as CAPA, DH31, or DH44, would not be affected by this hormone. Such antagonistic relationships among hormones are a common theme in insect excretory control (44); a similar situation occurs in Rhodnius, where CAPA specifically antagonizes the diuresis caused by serotonin and not that caused by a corticotropin releasing factor-related peptide (48). Although the physiological roles of TA- and DK-mediated diuresis in the intact fly are not yet known, DK signaling has recently been shown to be required for normal fluid balance (15).
We showed that cGMP inhibits the depolarization induced by TA and kinin and conclude that this inhibition will have an antidiuretic effect, but we have not directly demonstrated an antidiuresis caused by treating tubules with cGMP. Such an experiment is complicated by the fact that in Drosophila and other dipterans, cGMP also causes a diuresis by acting on the principal cells (16, 23, 53). The stimulatory effects of cGMP are seen at much lower concentrations, as we show in Fig. 1. Indeed, previous studies showed that kinin-mediated diuresis and depolarization still occur in cGMP-treated tubules (16, 23, 42). Such results are not inconsistent with our data, as at the high doses of kinin used in the prior work, we find that cGMP inhibits but does not abolish the depolarizing effects of TA. A direct demonstration of an antidiuretic action of cGMP in the stellate cells will only be feasible when it becomes possible to increase cGMP levels specifically in the stellate cells and thus must await the identification of the peptide or other agent linked to stellate cell cGMP production.
As mentioned in the introduction, Dow and co-workers (34) previously reported a diuretic effect of cGMP in the stellate cells. In that study, the rat atrial natriuretic peptide (ANP) receptor guanylate cyclase was expressed in the stellate cells; treatment of such tubules with ANP triggered a diuresis. We offer two possible explanations to explain this apparent conflict between this result and our data. First, there could be compartmentalization of cGMP within the stellate cells, as has been hypothesized in the principal cells (8) and demonstrated in mammalian cells (52, 61), such that elevation in one compartment causes diuresis and in the other antidiuresis. Another possibility is that the diuresis seen by Dow and colleagues resulted from the stimulation of an electroneutral transport pathway, an effect that we would not have detected in our TEP recordings. The possibility of two opposing roles of cGMP in the stellate cells, in addition to the many functions of this second messenger in the principal cells, indicates the astonishing regulatory complexity of the insect MT.
GRANTS
Support was provided by Marquette University and National Science Foundation (NSF) Grant IOS-0744619 to E. M. Blumenthal. K. A. Ruka was supported by a Research Experience for Undergraduates supplement to the NSF grant.
Present address of K. A. Ruka: Dept. of Molecular and Integrative Physiology, University of Michigan, 7725 Medical Science II, 1137 E Catherine St., Ann Arbor, MI 48109-5622.
Present address of A. P. Miller: Graduate School of Biomedical Sciences, Medical College of Wisconsin, 8701 Watertown Plank Rd., Milwaukee, WI 53226.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.A.R. and E.M.B. conception and design of research; K.A.R., A.P.M., and E.M.B. performed experiments; K.A.R., A.P.M., and E.M.B. analyzed data; K.A.R., A.P.M., and E.M.B. interpreted results of experiments; K.A.R., A.P.M., and E.M.B. edited and revised manuscript; K.A.R., A.P.M., and E.M.B. approved final version of manuscript; E.M.B. prepared figures; E.M.B. drafted manuscript.
ACKNOWLEDGMENTS
We thank Prof. Julian Dow, the Vienna Drosophila RNAi Center (VDRC), and the Bloomington Drosophila Stock Center at Indiana University for providing fly stocks and the TRiP at Harvard Medical School (National Institutes of Health/National Institute of General Medical Sciences R01-GM084947) and for providing transgenic RNAi fly stocks used in this study.
REFERENCES
- 1.Beyenbach KW. Mechanism and regulation of electrolyte transport in Malpighian tubules. J Insect Physiol 41: 197–207, 1995 [Google Scholar]
- 2.Beyenbach KW. Transport mechanisms of diuresis in Malpighian tubules of insects. J Exp Biol 206: 3845–3856, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Blumenthal EM. Characterization of transepithelial potential oscillations in the Drosophila Malpighian tubule. J Exp Biol 204: 3075–3084, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Blumenthal EM. Regulation of chloride permeability by endogenously produced tyramine in the Drosophila Malpighian tubule. Am J Physiol Cell Physiol 284: C718–C728, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Blumenthal EM. Modulation of tyramine signaling by osmolality in an insect secretory epithelium. Am J Physiol Cell Physiol 289: C1261–C1267, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal EM. Isoform- and cell-specific function of tyrosine decarboxylase in the Drosophila Malpighian tubule. J Exp Biol 212: 3802–3809, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Bradley TJ. The excretory system: structure and physiology. In: Comprehensive Insect Physiology Biochemistry and Pharmacology: Regulation: Digestion, Nutrition, Excretion, edited by Kerkut GA, Gilbert LI. Oxford: Pergamon, 1985 [Google Scholar]
- 8.Broderick KE, MacPherson MR, Regulski M, Tully T, Dow JA, Davies SA. Interactions between epithelial nitric oxide signaling and phosphodiesterase activity in Drosophila. Am J Physiol Cell Physiol 285: C1207–C1218, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Cabrero P, Radford JC, Broderick KE, Costes L, Veenstra JA, Spana EP, Davies SA, Dow JA. The Dh gene of Drosophila melanogaster encodes a diuretic peptide that acts through cyclic AMP. J Exp Biol 205: 3799–3807, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Chen A, Kramer EF, Purpura L, Krill JL, Zars T, Dawson-Scully K. The influence of natural variation at the foraging gene on thermotolerance in adult Drosophila in a narrow temperature range. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 197: 1113–1118, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet 39: 715–720, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Coast GM, Nachman RJ, Lopez J. The control of Malpighian tubule secretion in a predacious hemipteran insect, the spined soldier bug Podisus maculiventris (Heteroptera, Pentatomidae). Peptides 32: 493–499, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Coast GM, Te Brugge VA, Nachman RJ, Lopez J, Aldrich JR, Lange A, Orchard I. Neurohormones implicated in the control of Malpighian tubule secretion in plant sucking heteropterans: the stink bugs Acrosternum hilare and Nezara viridula. Peptides 31: 468–473, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Coast GM, Webster SG, Schegg KM, Tobe SS, Schooley DA. The Drosophila melanogaster homologue of an insect calcitonin-like diuretic peptide stimulates V-ATPase activity in fruit fly Malpighian tubules. J Exp Biol 204: 1795–1804, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Cognigni P, Bailey AP, Miguel-Aliaga I. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab 13: 92–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies SA, Huesmann GR, Maddrell SH, O'Donnell MJ, Skaer NJ, Dow JA, Tublitz NJ. CAP2b, a cardioacceleratory peptide, is present in Drosophila and stimulates tubule fluid secretion via cGMP. Am J Physiol Regul Integr Comp Physiol 269: R1321–R1326, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Davies SA, Stewart EJ, Huesmann GR, Skaer NJ, Maddrell SH, Tublitz NJ, Dow JA. Neuropeptide stimulation of the nitric oxide signaling pathway in Drosophila melanogaster Malpighian tubules. Am J Physiol Regul Integr Comp Physiol 273: R823–R827, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Dawson-Scully K, Bukvic D, Chakaborty-Chatterjee M, Ferreira R, Milton SL, Sokolowski MB. Controlling anoxic tolerance in adult Drosophila via the cGMP-PKG pathway. J Exp Biol 213: 2410–2416, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Day JP, Wan S, Allan AK, Kean L, Davies SA, Gray JV, Dow JA. Identification of two partners from the bacterial Kef exchanger family for the apical plasma membrane V-ATPase of Metazoa. J Cell Sci 121: 2612–2619, 2008 [DOI] [PubMed] [Google Scholar]
- 20.de Belle JS, Hilliker AJ, Sokolowski MB. Genetic localization of foraging (for): a major gene for larval behavior in Drosophila melanogaster. Genetics 123: 157–163, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Dow JA, Davies SA. The Malpighian tubule: rapid insights from post-genomic biology. J Insect Physiol 52: 365–378, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Dow JA, Maddrell SH, Davies SA, Skaer NJ, Kaiser K. A novel role for the nitric oxide-cGMP signaling pathway: the control of epithelial function in Drosophila. Am J Physiol Regul Integr Comp Physiol 266: R1716–R1719, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Dow JA, Maddrell SH, Gortz A, Skaer NJ, Brogan S, Kaiser K. The malpighian tubules of Drosophila melanogaster: a novel phenotype for studies of fluid secretion and its control. J Exp Biol 197: 421–428, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Dow JAT, Davies SA. The Drosophila melanogaster Malpighian tubule. Adv Insect Physiol 28: 1–83, 2001 [Google Scholar]
- 26.Eigenheer RA, Nicolson SW, Schegg KM, Hull JJ, Schooley DA. Identification of a potent antidiuretic factor acting on beetle Malpighian tubules. Proc Natl Acad Sci USA 99: 84–89, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eigenheer RA, Wiehart UM, Nicolson SW, Schoofs L, Schegg KM, Hull JJ, Schooley DA. Isolation, identification and localization of a second beetle antidiuretic peptide. Peptides 24: 27–34, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Evans JM, Day JP, Cabrero P, Dow JA, Davies SA. A new role for a classical gene: white transports cyclic GMP. J Exp Biol 211: 890–899, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Foster JL, Higgins GC, Jackson FR. Biochemical properties and cellular localization of the Drosophila DG1 cGMP-dependent protein kinase. J Biol Chem 271: 23322–23328, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Hofmann F, Bernhard D, Lukowski R, Weinmeister P. cGMP regulated protein kinases (cGK). Hand Exp Pharmacol 191: 137–162, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Ionescu A, Donini A. AedesCAPA-PVK-1 displays diuretic and dose dependent antidiuretic potential in the larval mosquito Aedes aegypti (Liverpool). J Insect Physiol 58: 1299–1306, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Kaun KR, Riedl CA, Chakaborty-Chatterjee M, Belay AT, Douglas SJ, Gibbs AG, Sokolowski MB. Natural variation in food acquisition mediated via a Drosophila cGMP-dependent protein kinase. J Exp Biol 210: 3547–3558, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Kean L, Cazenave W, Costes L, Broderick KE, Graham S, Pollock VP, Davies SA, Veenstra JA, Dow JA. Two nitridergic peptides are encoded by the gene capability in Drosophila melanogaster. Am J Physiol Regul Integr Comp Physiol 282: R1297–R1307, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Kerr M, Davies SA, Dow JA. Cell-specific manipulation of second messengers; a toolbox for integrative physiology in Drosophila. Curr Biol 14: 1468–1474, 2004 [DOI] [PubMed] [Google Scholar]
- 35.MacPherson MR, Broderick KE, Graham S, Day JP, Houslay MD, Dow JA, Davies SA. The dg2 (for) gene confers a renal phenotype in Drosophila by modulation of cGMP-specific phosphodiesterase. J Exp Biol 207: 2769–2776, 2004 [DOI] [PubMed] [Google Scholar]
- 36.MacPherson MR, Lohmann SM, Davies SA. Analysis of Drosophila cGMP-dependent protein kinases and assessment of their in vivo roles by targeted expression in a renal transporting epithelium. J Biol Chem 279: 40026–40034, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Massaro RC, Lee LW, Patel AB, Wu DS, Yu MJ, Scott BN, Schooley DA, Schegg KM, Beyenbach KW. The mechanism of action of the antidiuretic peptide Tenmo ADFa in Malpighian tubules of Aedes aegypti. J Exp Biol 207: 2877–2888, 2004 [DOI] [PubMed] [Google Scholar]
- 38.McQuilton P, St Pierre SE, Thurmond J. FlyBase 101–the basics of navigating FlyBase. Nucleic Acids Res 40: D706–D714, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mery F, Belay AT, So AK, Sokolowski MB, Kawecki TJ. Natural polymorphism affecting learning and memory in Drosophila. Proc Natl Acad Sci USA 104: 13051–13055, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ni JQ, Liu LP, Binari R, Hardy R, Shim HS, Cavallaro A, Booker M, Pfeiffer BD, Markstein M, Wang H, Villalta C, Laverty TR, Perkins LA, Perrimon N. A Drosophila resource of transgenic RNAi lines for neurogenetics. Genetics 182: 1089–1100, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ni JQ, Markstein M, Binari R, Pfeiffer B, Liu LP, Villalta C, Booker M, Perkins L, Perrimon N. Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat Methods 5: 49–51, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Donnell MJ, Dow JA, Huesmann GR, Tublitz NJ, Maddrell SH. Separate control of anion and cation transport in malpighian tubules of Drosophila melanogaster. J Exp Biol 199: 1163–1175, 1996 [DOI] [PubMed] [Google Scholar]
- 43.O'Donnell MJ, Rheault MR, Davies SA, Rosay P, Harvey BJ, Maddrell SHP, Kaiser K, Dow JAT. Hormonally controlled chloride movement across Drosophila tubules is via ion channels in stellate cells. Am J Physiol Regul Integr Comp Physiol 274: R1039–R1049, 1998 [DOI] [PubMed] [Google Scholar]
- 44.O'Donnell MJ, Spring JH. Modes of control of insect Malpighian tubules: synergism, antagonism, cooperation and autonomous regulation. J Insect Physiol 46: 107–117, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Osborne KA, Robichon A, Burgess E, Butland S, Shaw RA, Coulthard A, Pereira HS, Greenspan RJ, Sokolowski MB. Natural behavior polymorphism due to a cGMP-dependent protein kinase of Drosophila. Science 277: 834–836, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Overend G, Cabrero P, Guo AX, Sebastian S, Cundall M, Armstrong H, Mertens I, Schoofs L, Dow JA, Davies SA. The receptor guanylate cyclase Gyc76C and a peptide ligand, NPLP1-VQQ, modulate the innate immune IMD pathway in response to salt stress. Peptides 34: 209–218, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Paluzzi JP. Anti-diuretic factors in insects: the role of CAPA peptides. Gen Comp Endocrinol 176: 300–308, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Paluzzi JP, Naikkhwah W, O'Donnell MJ. Natriuresis and diuretic hormone synergism in R. prolixus upper Malpighian tubules is inhibited by the anti-diuretic hormone, RhoprCAPA-alpha2. J Insect Physiol 58: 534–542, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Paluzzi JP, Orchard I. Distribution, activity and evidence for the release of an anti-diuretic peptide in the kissing bug Rhodnius prolixus. J Exp Biol 209: 907–915, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Paluzzi JP, Park Y, Nachman RJ, Orchard I. Isolation, expression analysis, and functional characterization of the first antidiuretic hormone receptor in insects. Proc Natl Acad Sci USA 107: 10290–10295, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paluzzi JP, Russell WK, Nachman RJ, Orchard I. Isolation, cloning, and expression mapping of a gene encoding an antidiuretic hormone and other CAPA-related peptides in the disease vector, Rhodnius prolixus. Endocrinology 149: 4638–4646, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Piggott LA, Hassell KA, Berkova Z, Morris AP, Silberbach M, Rich TC. Natriuretic peptides and nitric oxide stimulate cGMP synthesis in different cellular compartments. J Gen Physiol 128: 3–14, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pollock VP, McGettigan J, Cabrero P, Maudlin IM, Dow JA, Davies SA. Conservation of capa peptide-induced nitric oxide signaling in Diptera. J Exp Biol 207: 4135–4145, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Pollock VP, Radford JC, Pyne S, Hasan G, Dow JA, Davies SA. NorpA and itpr mutants reveal roles for phospholipase C and inositol (1,4,5)-trisphosphate receptor in Drosophila melanogaster renal function. J Exp Biol 206: 901–911, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Quinlan MC, Tublitz NJ, O'Donnell MJ. Anti-diuresis in the blood-feeding insect Rhodnius prolixus Stal: the peptide CAP2b and cyclic GMP inhibit Malpighian tubule fluid secretion. J Exp Biol 200: 2363–2367, 1997 [DOI] [PubMed] [Google Scholar]
- 56.Radford JC, Davies SA, Dow JA. Systematic G protein-coupled receptor analysis in Drosophila melanogaster identifies a leucokinin receptor with novel roles. J Biol Chem 277: 38810–38817, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Riegel JA, Maddrell SHP, Farndale RW, Caldwell FM. Stimulation of fluid secretion of Malpighian tubules of Drosophila melanogaster Meig by cyclic nucleotides of inosine, cytidine, thymidine and uridine. J Exp Biol 201: 3411–3418, 1998 [DOI] [PubMed] [Google Scholar]
- 58.Rosay P, Davies SA, Yu Y, Sözen MA, Kaiser K, Dow JA. Cell-type specific calcium signaling in a Drosophila epithelium. J Cell Sci 110: 1683–1692, 1997 [DOI] [PubMed] [Google Scholar]
- 59.Secca T, Sciaccaluga M, Marra A, Barberini L, Bicchierai MC. Biochemical activity and multiple locations of particulate guanylate cyclase in Rhyacophila dorsalis acutidens (Insecta: Trichoptera) provide insights into the cGMP signaling pathway in Malpighian tubules. J Insect Physiol 57: 521–528, 2011 [DOI] [PubMed] [Google Scholar]
- 60.Sözen MA, Armstrong JD, Yang M, Kaiser K, Dow JA. Functional domains are specified to single-cell resolution in a Drosophila epithelium. Proc Natl Acad Sci USA 94: 5207–5212, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Su J, Scholz PM, Weiss HR. Differential effects of cGMP produced by soluble and particulate guanylyl cyclase on mouse ventricular myocytes. Exp Biol Med (Maywood) 230: 242–250, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Terhzaz S, O'Connell FC, Pollock VP, Kean L, Davies SA, Veenstra JA, Dow JAT. Isolation and characterization of a leucokinin-like peptide of Drosophila melanogaster. J Exp Biol 202: 3667–3676, 1999 [DOI] [PubMed] [Google Scholar]
- 63.Wessing A, Eichelberg D. Malpighian tubules, rectal papillae and excretion. In: The Genetics and Biology of Drosophila, edited by Ashburner M, Wright TRF. London: Academic, 1978 [Google Scholar]
- 64.Wiehart UI, Nicolson SW, Eigenheer RA, Schooley DA. Antagonistic control of fluid secretion by the Malpighian tubules of Tenebrio molitor: effects of diuretic and antidiuretic peptides and their second messengers. J Exp Biol 205: 493–501, 2002 [DOI] [PubMed] [Google Scholar]