Abstract
Nucleotides within the airway surface liquid promote fluid secretion via activation of airway epithelial purinergic receptors. ATP is stored within and released from mucin granules as co-cargo with mucins, but the mechanism by which ATP, and potentially other nucleotides, enter the lumen of mucin granules is not known. We assessed the contribution of the recently identified SLC17A9 vesicle nucleotide transporter (VNUT) to the nucleotide availability within isolated mucin granules and further examined the involvement of VNUT in mucin granule secretion-associated nucleotide release. RT-PCR and Western blot analyses indicated that VNUT is abundantly expressed in airway epithelial goblet-like Calu-3 cells, migrating as a duplex with apparent mobility of 55 and 60 kDa. Subcellular fractionation studies indicated that VNUT55 was associated with high-density mucin granules, whereas VNUT60 was associated with low-density organelles. Immunofluorescence studies showed that recombinant VNUT localized to mucin granules and other organelles. Mucin granules isolated from VNUT short hairpin RNA-expressing cells exhibited a marked reduction of ATP, ADP, AMP, and UTP levels within granules. Ca2+-regulated vesicular ATP release was markedly reduced in these cells, but mucin secretion was not affected. These results suggest that VNUT is the relevant nucleotide transporter responsible for the uptake of cytosolic nucleotides into mucin granules. By controlling the entry of nucleotides into mucin granules, VNUT contributes to the release of purinergic signaling molecules necessary for the proper hydration of co-released mucins.
Keywords: VNUT, SLC17A9, mucin granules, ATP release, mucin secretion
extracellular nucleotides are key components of the mucociliary clearance process that traps and removes inhaled particles and microorganisms from the lung. Nucleotides/nucleosides within the airway surface liquid (ASL) promote mucin secretion via activation of P2Y2 receptors in mucous (goblet) cells and stimulate fluid secretion/mucin hydration via A2B and P2Y2 receptor activation in ciliated cells (11). Deficient fluid secretion results in abnormal mucous hydration/clearance, leading to lung failure in cystic fibrosis, the most prevalent potentially lethal genetic disease in the United States and a major factor contributing to the progressive airway obstruction associated with chronic obstructive lung disease (4).
Despite the importance of ASL nucleotides in airway physiology, the mechanisms by which airway epithelial cells release nucleotides have just begun to be addressed. For example, we recently demonstrated that plasma membrane pannexin 1 largely contributes to conductive ATP release from normal airways dominated by ciliated cells (22). Deletion of the pannexin 1 gene resulted in impaired ATP release in mouse tracheas ex vivo (22).
We also demonstrated that mucin granules are an important additional source of nucleotide release (8, 9, 18). That is, primary cultures of human airway epithelial cells that were induced to develop goblet cell hyperplasia via respiratory syncytial virus infection or IL-13 exposure acquired enhanced ATP release from the secretory pathway that correlated with increased mucin secretion and was not affected by pannexin inhibitors (18). Purified mucin granules, i.e., free of other organelle markers, store ATP and, to a greater extent, ADP and AMP (9), which are released as co-cargo with mucins via Ca2+-regulated mucin granule exocytosis (8, 9). Thus, nucleotide release from mucin granules signals in a paracrine fashion to P2Y2 and A2B receptors on ciliated cells, promoting ion/water secretion and mucin hydration. While these observations provide insights into how mucin secretion and nucleotide release are coordinated, the mechanisms responsible for the uptake/storage of nucleotides in mucin granules remain uncertain.
Recently, Sawada et al. (20) identified SLC17A9 as a vesicular nucleotide transporter (VNUT) that displays features of the ATP transporter endogenously expressed in chromaffin granules: 1) strong VNUT immunoreactivity was observed in mouse adrenal glands, 2) reconstitution of purified recombinant SLC17A9 into liposomes resulted in Δψ-driven Cl−-dependent ATP (and ADP) transport activity that was inhibited by DIDS and by Evans blue, and 3) knockdown of SLC17A9 by small interfering RNA decreased ATP release in chromaffin-like PC12 cells (20). VNUT immunoreactivity also was observed in zymogen granules isolated from pancreatic acini. Zymogen granules exhibited nucleotide uptake, with kinetics, ion dependence, and susceptibility to inhibitors similar to SLC17A9 (6). Thus, VNUT-mediated ATP uptake into zymogen granules contributes to ATP release with digestive enzymes (24).
Since VNUT was reported to be expressed in various tissues, including the lung (25), we hypothesized that VNUT mediates nucleotide uptake in airway epithelial cell mucin granules, thereby contributing to the release of nucleotides from mucin-secreting goblet cells. In this study, we used Calu-3 cells as a model of airway epithelial goblet cells (8, 9) to investigate the expression of VNUT in mucin granules and further examined VNUT's contribution to mucin granule nucleotide content and release.
MATERIALS AND METHODS
Cell culture.
Human airway epithelial Calu-3 cells were grown in MEM containing 10% FBS, as previously reported (8).
Cell fractionation and mucin granule isolation.
Mucin granules were isolated following a protocol developed in our laboratory (9). Briefly, Calu-3 cells were detached, resuspended in ice-cold lysis buffer (20 mM PIPES, pH 6.8, 130 mM potassium glutamate, 3 mM MgCl2, 0.1 mM CaCl2, 3 mM EGTA, and 300 mM sucrose), and disrupted by cavitation (800–1,000 psi for 30 min on ice). Lysates were centrifuged for 1 min at 200 g, and the pellet (P1) and supernatant (S1) were separated. S1 was pelleted at 3,000 g for 3 min, and the resulting supernatant (S2) and pellet (P2) were separately processed. P2 was subjected to two consecutive Percoll gradients, and 100-μl fractions were collected and processed immediately or stored at −80°C for further analyses. Typically, a 75-cm2 confluent culture yields 5–10 mg of protein in the cavitation homogenate and 10–30 μg of protein in the isolated mucin granule fraction (9).
For isolation of particulate components other than mucin granules, S2 was centrifuged at 10,000 g for 30 min, yielding a mitochondria-rich pellet (P3). The resulting supernatant (S3) was diluted 10-fold in lysis buffer (containing no sucrose) and centrifuged at 150,000 g for 3 h, yielding a microsome-rich pellet (P4) and supernatant (S4).
Plasma membranes from Calu-3 cells were isolated using the Plasma Membrane Protein Extraction kit (BioVision Research Products, Mountain View, CA) following the manufacturer's instructions. The plasma membrane was separated from cytosolic fractions by differential centrifugation and detergent/aqueous partitioning, as described elsewhere (28).
Subcellular fractions were identified using organelle markers by immuno-slot blotting using antibodies against MUC5AC (mucin granules), GM130 (Golgi), protein disulfide isomerase [endoplasmic reticulum (ER)], lysosomal-associated membrane protein (LAMP)-1 (lysosome), and mitochondrial cytochrome oxidase subunits III/IV, as we previously described (9). Antibodies against MUC1 and, when indicated, CFTR were used for plasma membrane identification, as previously described (8). Immunoblots were developed using a horseradish peroxidase chemiluminescence substrate (Millipore, Billerica, MA), and immunoreactive bands were quantified as previously described (9, 22).
Purification of recombinant human VNUT.
Human VNUT (hVNUT; GenBank accession no. BC025312) was expressed in Escherichia coli C43 (DE3) and subsequently purified, as previously described (15). Briefly, hVNUT-transformed cells grown in TB medium were induced with isopropyl-β-d-thiogalactopyranoside, harvested, and disrupted by sonication. E. coli lysates were centrifuged at 5,856 g for 10 min, and the resultant supernatant was further centrifuged at 150,000 g for 1 h. The pellet (suspended at 10 mg/ml) was treated with 1.5% Fos-choline 14 (Affymetrix) and centrifuged at 150,000 g for 1 h, and the supernatant (diluted twice with 20 mM Tris·HCl, pH 7.5, 100 mM NaCl, 10 mM KCl, and 2 mM phenylmethylsulfonyl fluoride) was applied to a column containing 1 ml of Ni-NTA Superflow resin (Qiagen, Valencia, CA). After 1 h, the column was washed, and hVNUT was eluted with washing buffer containing 300 mM imidazole. All procedures were carried out at 4°C, as described elsewhere (15).
VNUT antibodies.
Rabbit polyclonal antibodies against hVNUT were prepared by repeated injection of purified hVNUT with Freund's complete adjuvant and successive injection of Freund's incomplete adjuvant (Santa Cruz Biotechnology). The antiserum was further purified by affinity chromatography, as described elsewhere (20).
Western blotting.
Purified hVNUT was resolved on 10% SDS-PAGE. Calu-3 cell proteins were resolved on 4–20% SDS-PAGE. Proteins were analyzed by Western blotting using the anti-VNUT antiserum (1:5,000 dilution) generated as described above.
Quantification of nucleotides in isolated mucin granules and cell lysates.
Freshly isolated mucin granules and the postnuclear supernatant (S1) were disrupted/denatured with 5% trichloroacetic acid (TCA), the TCA was extracted with ethyl ether, and ATP, ADP, and AMP were quantified using the etheno-derivatization technique, as previously described (12). UTP was measured using the UDP-glucose pyrophosphorylase assay, which quantifies the UTP-dependent formation of [14C]glucose 1-phosphate to UDP-[14C]glucose, via HPLC, as previously described (13).
ATP release.
ATP release to thin ASL was assessed in real time using the luciferin-luciferase assay, as described elsewhere (17). Briefly, Calu-3 cells were infected with the indicated short hairpin RNA (shRNA) lentivirus and selected with puromycin (see shRNA) for 1 wk. Cells were transferred to collagen-coated 12-mm Transwell supports (Costar) and grown to confluence in an air-liquid interface (14) in the continued presence of puromycin. Cultures were bilaterally rinsed and transferred to a luminometer (model TD-20/20, Turner Biosystems, Sunnyvale, CA), and 50 μl of HBSS-HEPES containing luciferase and luciferin were added to the mucosal compartment. The basolateral compartment was incubated in the presence of 1 ml HEPES-buffered (pH 7.4) HBSS supplemented with 1.6 mM CaCl2 and 0.8 mM MgCl2. Luminescence was recorded as arbitrary light units at 15-s intervals. Calibration curves using known concentrations of ATP were performed at the end of each assay. Parallel cultures were lysed with 5% TCA, and the intracellular ATP content was assessed offline, as previously described (22).
Mucin secretion.
MUC5AC secreted into the mucosal medium was measured by slot-blot analysis, as previously described (8). Slot blots were scanned and quantified on a LI-COR Odyssey system (Lincoln, NE).
shRNA.
Lentiviruses bearing vector-expression clones (pLKO1/puromycin) containing scrambled and VNUT shRNAs [VNUT shRNA#2 (GCACACTGTAGGATGCTTAAA) and VNUT shRNA#6 (CCACAGTGGCATTTCTGTTAA)] were obtained from the University of North Carolina Lenti-shRNA core facility. Calu-3 cells were infected with the desired lentivirus (0.5 × 106 colony-forming units/35-mm dish for 2 h) and subsequently selected with puromycin, as described elsewhere (22). Puromycin-selected cultures were used for an initial screening of VNUT expression and for ATP release studies. To assess the effect of VNUT shRNA on the composition of isolated mucin granules, we infected 1-mo-old confluent cultures with viruses bearing scramble or VNUT shRNAs, rinsed, and maintained for 2 days with medium without puromycin. On the 2nd day after infection, cells were rinsed and incubated for 20 min with 100 μM protease-activated receptor 2-activating peptide to induce secretion of preformed mucin granules (9). Cultures were rinsed and maintained with medium for an additional day. This protocol maximizes the relative expression of mucin granules formed de novo after shRNA infection. Cells were detached and fractionated, as described above.
RT-PCR analysis.
Total RNA was isolated using the RNeasy kit (Qiagen) and reverse-transcribed into cDNA using SuperScript (Invitrogen). Primers used for VNUT amplification are described in Table 1. Amplified PCR products were identified by sequence analysis at the University of North Carolina DNA sequencing facility. Quantitative PCR (qPCR) was performed using the LightCycler 480 Real-Time PCR system, and relative expression levels of the genes of interest was normalized to expression of the reference gene 18S, as previously described (21).
Table 1.
Forward and reverse primers used for RT-PCR amplification
| Set | Target | Oligonucleotide Composition |
|---|---|---|
| 1F | VNUT isoform 1 | CGCCGTCTGAGCACCCCAAG |
| 1R | VNUT isoform 1 | AGCTGCTGAGCACGATGCCG |
| 2F | VNUT isoform 2 | ACCACCCTGTGTATGCATGACCCT |
| 2R | VNUT isoform 2 | AGCTGCTGAGCACGATGCCG |
| 3F | VNUT isoforms 1 and 2 | AGACCTTCCCCGACGCCA |
| 3R | VNUT isoforms 1 and 2 | GGACGGGGCCAAGTCCTGGA |
| 5F | MUC5AC | TCTATGAGGGCTGCGTCTTT |
| 5R | MUC5AC | CGTAGCAGTAGGAGGGGTTG |
Sequences are in 5′ to 3′ order. F, forward; R, reverse; VNUT, vesicular nucleotide transporter.
Overexpression of VNUT and immunofluorescence studies.
VNUT cDNA containing a COOH-terminal Myc/His tag was constructed by subcloning SLC17A9 cDNA [GenBank accession no. BC025312 (20)] into a pcDNA3.1 Myc/His vector (Invitrogen). Calu-3 cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. VNUT and mucin granules where visualized by immunofluorescence with anti-Myc and anti-MUC5AC antibodies, respectively, 4 days after transfection, as previously described (7, 23). Cells were examined using a Zeiss 510 Meta confocal microscopy system, and images were assembled using Adobe Photoshop.
Reagents.
Luciferase from Photinus pyralis, Percoll, and anti-α-tubulin monoclonal antibody were obtained from Sigma (St. Louis, MO); the antibody against cytochrome oxidase subunit III/IV from Molecular Probes (Eugene, OR); and monoclonal anti-MUC5AC (45M1) and MUC1 antibodies from LabVision, Thermo Scientific (Fremont, CA). Anti-human CFTR monoclonal antibodies 596 and 769 (8, 16) were a kind gift from Dr. John Riordan (University of North Carolina Chapel Hill). Luciferin and antibodies against GM130, p230, protein disulfide isomerase, and LAMP-1 were obtained from BD Biosciences Pharmingen (San Jose, CA); anti-Myc polyclonal antibody from Upstate (Lake Placid, NY); and secondary antibodies from Santa Cruz Biotechnology. The protease-activated recepptor 2-activating peptide SLIGKV (PAR2-AP) was synthesized at Tufts University Peptide Synthesis Core Facility. Other chemicals were of the highest purity available and were obtained from sources previously reported.
Data analysis.
Statistical analysis was performed using Student's paired t-test (Microsoft Excel 2003) or ANOVA with post hoc Tukey's honestly significant difference (JMP Genomics, version 4.1, SAS, Cary, NC). P < 0.05 was considered statistically significant.
RESULTS
Expression of VNUT in Calu-3 cells.
Human lung adenocarcinoma-derived Calu-3 cells retain several features of goblet cells, including the abundant expression of dense-core mucin granules and susceptibility to Ca2+-regulated mucin secretion (8). In addition, they represent a valuable source for mucin granule isolation (9). Therefore, we used Calu-3 cells to test the hypothesis that VNUT regulates ATP levels in airway epithelial mucin granules.
VNUT expression in Calu-3 cells was first investigated by RT-PCR analysis. Primers designed to amplify the 296-bp segment between positions 986 and 1282 of the VNUT coding sequence (GenBank accession no. BC025312) were used to demonstrate expression of VNUT transcripts (Fig. 1A). BC025312 encodes a 430-amino acid protein (UniProt identifier Q9BYT1-2) that exhibits nucleotide transport activity (20). An additional VNUT isoform (GenBank accession no. NM_022082.3) encodes a 436-amino acid putative protein (UniProt identifier Q9BYT1-1), but this isoform has not been isolated/characterized. The two VNUT isoforms result from an alternative splicing at the NH2 terminus (19, 20). The 19 and 13 residues comprising the NH2-terminal tail of Q9BYT1-1 and Q9BYT1-2 (VNUT-1 and VNUT-2, respectively) are unique for each variant, but both isoforms share 100% homology along the remaining 417 COOH-terminal amino acids. Both isoforms were robustly amplified when forward primers specific for each variant were used (Fig. 1A, right); because of limitations in designing isoform-specific primers with similar amplification coefficients, the relative levels of VNUT-1 and VNUT-2 mRNAs could not be assessed by qPCR.
Fig. 1.
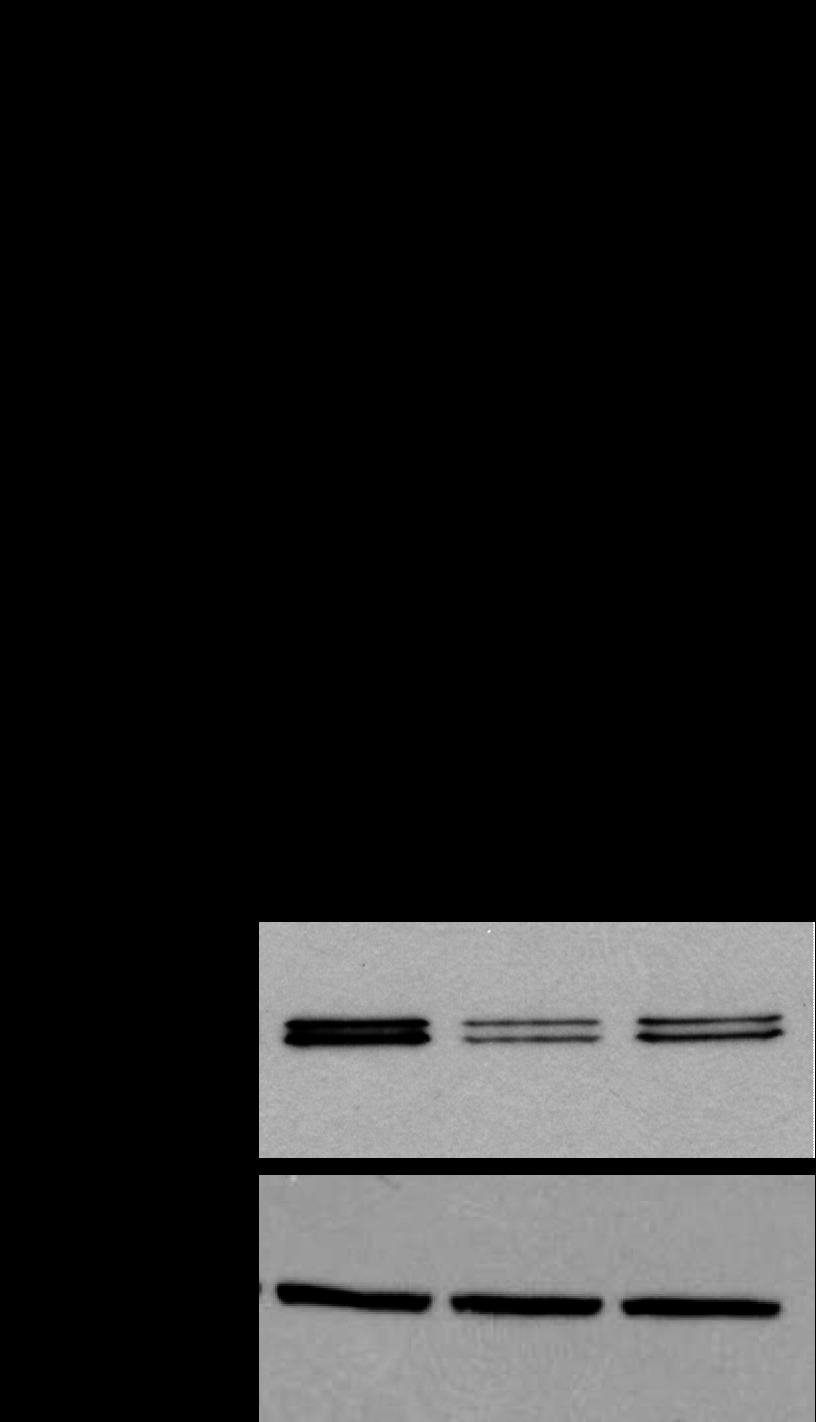
Vesicular nucleotide transporter (VNUT) is expressed in Calu-3 cells. A: RT-PCR analysis of VNUT expression using primers common to VNUT-1 and VNUT-2 mRNAs (predicted amplification size 296 bp; left) or specific for VNUT-1 (V-1, 209 bp) or VNUT-2 (V-2, 181 bp) (right); see Table 1 for primer sequences. B: Calu-3 cell lysate proteins were electrophoresed on 4–10% SDS-PAGE and transferred and analyzed by Western blotting with anti-VNUT antibody (V) or its antigen-preabsorbed antiserum (PA). C: Coomassie blue (left) and Western (right) blots of extracts from uninduced (a) and induced Escherichia coli (b) and partially purified human VNUT (hVNUT; c); samples were electrophoresed on 10% SDS-PAGE. Numbers at left indicate size in bp (A) or kDa (B and C).
An antibody generated against purified VNUT-2 (see materials and methods) was used to confirm VNUT protein expression in Calu-3 cells by Western blotting. As shown in Fig. 1B, two strong immunoreactive species with apparent motility of 55 and 60 kDa (VNUT55 and VNUT60, respectively) were revealed with the anti-VNUT antibody, but not with its antigen-preabsorbed antiserum. Complementary data verified that this antibody recognizes recombinant hVNUT-2 (Q9BYT1-2) purified from E. coli (Fig. 1Cc) or in crude extracts of E. coli overexpressing hVNUT-2 (Fig. 1Cb), but not in noninduced cells (Fig. 1Ca). Further validation of this antibody was obtained after knockdown of VNUT expression in Calu-3 cells via shRNA (see Fig. 6). Since this antibody was raised against full-length VNUT-2, which exhibits >90% homology with VNUT-1, whether VNUT-1, VNUT-2, or both contributed to the immunoreactive species described in Fig. 1B could not be determined.
Fig. 6.
VNUT short hairpin RNA2 [shRNA2 (sh2)] and shRNA6 (sh6) reduced VNUT expression in Calu-3 cells. Cells were infected with lentivirus carrying scramble shRNA (scr), VNUT shRNA2, or VNUT shRNA6 and selected for 10 days with puromycin before RNA and protein extraction. A: quantification of VNUT mRNA levels using primers for the common region for both VNUT isoforms (see Table 1). B: representative Western blot. TUB, tubulin. Values are means ± SD from 3 independent experiments. *P < 0.05.
VNUT55 is expressed in nucleotide-storing mucin granules.
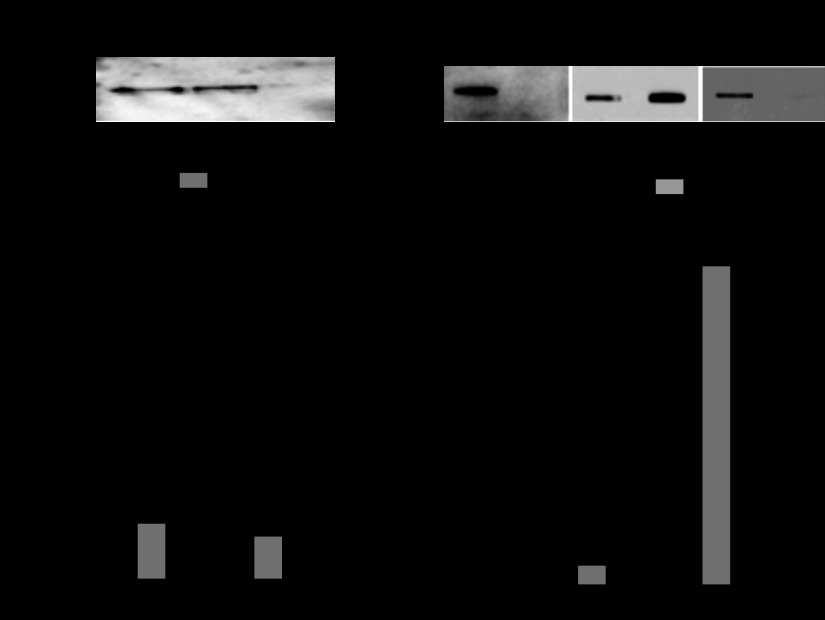
To examine the expression of VNUT in mucin granules, our recently described protocol for mucin granule isolation was implemented (9). Calu-3 cells were harvested, lysed by N2 cavitation, and subjected to mild centrifugation (200 g) to sediment nuclei and cell debris. A subsequent 2,000-g centrifugation of the postnuclear supernatant (S1) yielded a pellet (P2) and a supernatant (S2) enriched with VNUT55 and VNUT60, respectively (Fig. 2A). The gel-forming mucin MUC5AC distributed ∼58% and ∼42% in P2 and S2, respectively, while mitochondria, Golgi, ER, and plasma membrane markers were largely recovered in S2 (Fig. 2B). Centrifugation of S2 at 10,000 g yielded a mitochondria-rich pellet (P3) nearly devoid of VNUT immunoreactivity; i.e., >90% VNUT60 was recovered in the postmitochondrial supernatant (S3) (Fig. 2A), together with markers of lysosomes, ER, Golgi, and plasma membrane (Fig. 2B). S2-associated MUC5AC also remained mostly in the postmitochondrial supernatant (S3) (Fig. 2B).
Fig. 2.
Subcellular distribution of VNUT in Calu-3 cells. A: Western blot illustrating distribution of VNUT immunoreactivity in supernatant (S1, S2, and S3) and particulate (P2 and P3) cell fractions. B: quantification of VNUT immunoreactivity and cell markers in fractions depicted in A. Values [percentage of recovery relative to postnuclear supernatant (S1)] are means ± SD from ≥3 independent experiments. For simplicity, markers for lysosomes (Lys), Golgi (Gol), endoplasmic reticulum (ER), and plasma membrane (PM) are indicated with a single bar. MIT, mitochondria.
Using Percoll density gradients, we previously showed that highly purified intact mucin granules storing ATP can be isolated from P2 (9). Since ∼95% VNUT55 sedimented with P2 (Fig. 2), the presence of VNUT in mucin granules isolated over Percoll gradients was examined. As shown in Fig. 3, VNUT55 coeluted with the mucin granule-rich fraction (fraction 4), identified by its MUC5AC content (Fig. 3, A and B). Markers for mitochondria (cytochrome oxidase), lysosomes (LAMP-1), Golgi (GM130), ER (protein disulfide isomerase), and plasma membrane (MUC1 and CFTR) were negligible in the mucin granule-rich fraction and other fractions from the Percoll gradient, consistent with the >80% recovery of these organelles with S2, as shown in Fig. 2 and elsewhere (9).
Fig. 3.
VNUT55 is expressed in mucin granules. A: Western blot illustrating VNUT55 immunoreactivity in fractions isolated after centrifugation of P2 on Percoll density gradients. B: VNUT immunoreactivity coeluted with the mucin granule-rich fraction, assessed by its MUC5AC content (fraction 4). C: ATP and UTP levels are concentrated in the VNUT55- and mucin granule-rich fraction 4.
In agreement with our previous report (9), the mucin granule-rich fraction was enriched with ATP (∼1 nmol/mg protein) and, to greater extent, ATP metabolites, i.e., ADP and AMP (not shown) (9). The total adenylyl nucleotide content (ATP + ADP + AMP) inside the mucin granule (∼10 nmol/mg) represented ∼5–10% of the cellular adenine nucleotide pool. The fact that ATP (or its metabolites) levels in fractions 1–3 and 5–8 of the Percoll gradient were negligible (Fig. 3C and data not shown) (9) suggests that fraction 4 contained nonleaking granules. In addition, the mucin granule-rich fraction contained UTP (∼0.3 nmol/mg protein). Since the ratio of ATP to UTP within the mucin granule (∼3.0) was similar to the ATP-to-UTP ratio measured in S3 (Fig. 3C), the data suggest that ATP and UTP enter the mucin granule by a common mechanism that reflects their cytosolic concentrations.
VNUT60 is associated with low-density vesicles.
The postmitochondrial supernatant (S3) was centrifuged for 3 h at 150,000 g, and VNUT60 was nearly completely recovered with the pellet (P4) (Fig. 4A). Markers of lysosomes, ER, Golgi, and plasma membrane also were largely recovered with P4. Relative to their initial input (S1), 85% VNUT60 was recovered in P4, together with >80% of ER/Golgi, lysosomes, and plasma membrane markers. In contrast, only a minor fraction of MUC5AC was recovered with P4 (18 ± 7%) and S4 (14 ± 7%) (Fig. 4A), suggesting that the MUC5AC immunoreactivity not associated with the mucin granule-rich fraction isolated in Fig. 3 reflected mucins released from damaged granules during the cell cavitation/fractionation.
Fig. 4.
Distribution of VNUT60 in Calu-3 cells. A: postmitochondrial supernatant (S3, see Fig. 2) was centrifuged at 150,000 g for 3 h, and the resulting pellet (P4) and supernatant (S4) were analyzed by Western blot. Values [percentage of recovery relative to postnuclear supernatant (S1)] are means ± SD from ≥3 independent experiments. B: plasma membrane-rich fraction was isolated using the Plasma Membrane Protein Extraction kit. Equivalent aliquots of the resulting plasma membrane and cellular organelle membrane (Org) fractions were analyzed by Western blotting (VNUT) and slot blotting (MUC1 and GM130) and quantified as described in Fig. 2 legend. Values are means ± SD from ≥3 independent experiments.
Although VNUT has been described as a vesicular transporter (20), its expression as a plasma membrane protein has not been formally examined. Our above-described protocol for cell homogenization and fractionation was optimized for mucin granule isolation, but not for plasma membrane purification. Therefore, to isolate the plasma membrane, Calu-3 cells were homogenized and subjected to differential centrifugation and detergent/aqueous partitioning, as described elsewhere (28). As shown in Fig. 4B, the plasma membrane-rich fraction (identified by its MUC1 content) displayed negligible VNUT immunoreactivity; VNUT60 was entirely recovered within cellular organelle membranes (identified with the Golgi marker GM130).
Collectively, the data in Figs. 2–4 indicate that VNUT55 is associated with nucleotide-storing mucin granules and that VNUT60 is associated with low-density vesicles.
Recombinant VNUT localizes to mucin granules and vesicles.
Since the anti-VNUT antibody (see above) is not suitable for immunohistochemistry, Calu-3 cells were transfected with Myc-tagged VNUT-2, and the cellular distribution of Myc immunoreactivity was examined by confocal microscopy. Strong vesicular Myc immunoreactivity was observed in transfected Calu-3 cells (Fig. 5A). Myc immunoreactivity was observed co-localizing with the mucin granule marker MUC5AC and also in vesicular compartments that stained negative for MUC5AC (Fig. 5A). Calu-3 cells transfected with empty vector showed no Myc immunoreactivity (Fig. 5B).
Fig. 5.
Recombinant VNUT-2 localizes to mucin granules and vesicles. Calu-3 cells were transfected with Myc-VNUT (A) or empty vector (B). Confocal microscopy images of the differential interference contrast (DIC) and anti-Myc (green) and anti-MUC5AC (red) immunostaining confocal planes are shown. Arrows, mucin granules stained for MUC5AC; arrowheads, non-mucin-containing vesicles. VNUT-transfected cells exhibit Myc immunoreactivity localizing to mucin granules (A, arrow, right) and non-mucin-containing vesicles (A, arrowhead, right). No Myc immunoreactivity is observed in empty vector-transfected cells (B). Scale bars, 5 μm.
VNUT regulates the nucleotide content in mucin granules.
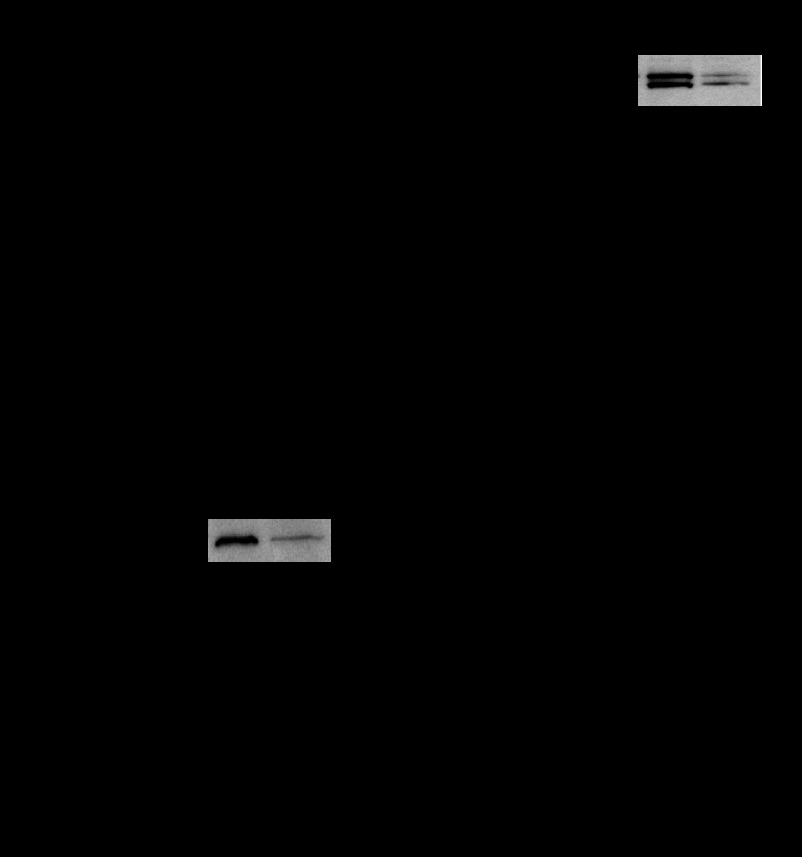
Having shown that nucleotide-storing mucin granules contain one form of VNUT (i.e., VNUT55), we investigated the extent to which VNUT knockdown affects the nucleotide content within granules. By screening a shRNA lentiviral library, two shRNA constructs that were stably expressed in Calu-3 cells caused robust VNUT expression reduction. Calu-3 cells expressing VNUT shRNA2 and VNUT shRNA6 displayed 75% and 67% reduction of VNUT mRNA levels, respectively, relative to scramble shRNA-expressing cells (Fig. 6A). Consistent with these results, VNUT immunoreactivity (VNUT55 and VNUT60), but not the housekeeping protein tubulin, was markedly reduced in Western blots of Calu-3 cells stably expressing VNUT shRNA2 and VNUT shRNA6 (Fig. 6B).
Next, mucin granules were isolated (as shown in Figs. 2 and 3) from Calu-3 cells that were freshly infected with the above-described shRNA lentiviruses (experiments using VNUT shRNA6 are shown in Figs. 7 and 8, and VNUT shRNA2-infected cells rendered nearly identical results). Acute VNUT shRNA infection decreased VNUT expression (Fig. 7, A and B) to levels similar to those observed above with stably infected cells. That is, Calu-3 cells acutely expressing VNUT shRNA exhibited a marked reduction of VNUT mRNA levels (Fig. 7A), which was accompanied by ∼70% reduction of VNUT55- and VNUT60-immunoreactive bands, relative to scramble shRNA (Fig. 7B). No effect was observed on MUC5AC mRNA expression (Fig. 7, A and B). As predicted, mucin granules isolated from VNUT shRNA-infected cells exhibited a marked reduction of VNUT55 immunoreactivity; MUC5AC levels in mucin granules were not affected by VNUT shRNA (Fig. 7C).
Fig. 7.
VNUT knockdown reduces nucleotide content in mucin granules. Calu-3 cells were infected with lentiviruses bearing scramble or VNUT shRNA6 and studied at 3 days postinfection. A and B: mRNA and proteins were quantified in the whole cell extract. C and D: mucin granules (MG) were isolated, and their content was measured as described in Fig. 3 legend. Values are means ± SD from 3 independent experiments. *P < 0.05. Insets: representative Western blots for VNUT in whole cells (B) and mucin granules (C) from scramble- and VNUT shRNA-infected cells.
Fig. 8.

VNUT knockdown reduces mucin secretion-associated nucleotide release. A and B: polarized cultures of Calu-3 cells were exposed to 5 μM ionomycin, and ATP was assessed in real time. A: representative trace of ATP release (recorded in real time every 15 s; ionomycin was added at time 0). B: peak ATP release from 2 independent shRNA6 lentiviral infections, each assessed in quadruplicate. Values (ratio of extracellular ATP to intracellular ATP) are means ± SD. Significantly different from vehicle: **P < 0.0001 and *P < 0.001 (by ANOVA). Significantly different from scramble/ionomycin: ‡P < 0.005 (by ANOVA). C: mucin secretion was assessed 15 min following addition of 5 μM ionomycin. Values are means ± SD from 3 independent measurements performed in quadruplicate. Significantly different from vehicle: *P < 0.05 (by ANOVA).
VNUT shRNA have no apparent effect on the total cell content of ATP and UTP. That is, ATP levels in the total cell lysate from scramble shRNA- and VNUT shRNA-infected cells were 108 ± 9 and 94.5 ± 5 nmol/mg protein, respectively; the total cellular UTP content was 33 ± 5 and 31 ± 3 nmol/mg protein in VNUT shRNA-expressing and control cells, respectively. In contrast, nucleotide levels in the mucin granule fraction were markedly reduced in VNUT shRNA-expressing cells. As shown in Fig. 7D, ATP levels in the mucin granule fraction isolated from VNUT shRNA-infected cells were reduced to one-third relative to levels measured in mucin granules from control cells (0.4 ± 0.1 and 1.1 ± 0.2 nmol/mg protein in VNUT shRNA- and scramble shRNA-infected cells, respectively). Levels of ADP and AMP in mucin granules also were reduced in VNUT shRNA-infected cells (45% and 55%, respectively, compared with scramble shRNA-infected cells). Similar to ATP, VNUT knockdown markedly reduced the UTP content in the mucin granule fraction compared with scramble shRNA-infected cells (0.12 ± 0.03 and 0.36 ± 0.04 nmol/mg protein, respectively; Fig. 7D).
VNUT knockdown reduces Ca2+-promoted ATP release from Calu-3 cells.
Previously, we showed that Ca2+-regulated mucin secretion is accompanied by nucleotide release from mucin granules (8, 9). To assess the extent to which VNUT knockdown affects the magnitude of ATP release from mucin-secreting cells, ATP release was measured in response to the Ca2+ ionophore ionomycin, a potent mucin secretagogue (8). As shown in Fig. 8, A and B, ionomycin-promoted ATP release was markedly reduced in cells expressing VNUT shRNA compared with scramble shRNA. Ionomycin-promoted mucin secretion was not affected by the VNUT knockdown (Fig. 8C).
DISCUSSION
Mucin granules represent an important source of nucleotide release from mucin-secreting airway epithelia (9). Therefore, identifying the mechanisms of nucleotide storage within mucin granules is highly relevant to airway pathophysiology. Utilizing knockdown approaches that efficiently reduced VNUT expression in mucin granules, we provide evidence that VNUT is the relevant mucin granule nucleotide transporter. By controlling the nucleotide content within mucin granules, VNUT contributes to the release of nucleotides from mucin-secreting goblet cells.
VNUT has been initially identified as the vesicular nucleotide transporter responsible for the uptake of cytosolic ATP into secretory granules of chromaffin cells (20). VNUT is predicted to encode 12 transmembrane domains with putative NH2- and COOH-terminal tails facing the cytosol. Two splice variants of human VNUT, Q9BYT1-1 and Q9BYT1-2, have been described. Q9BYT1-2 (VNUT-2) encodes a functional protein that, when reconstituted in liposomes, exhibits nucleotide transport activities consistent with the ATP transporter endogenously expressed in chromaffin (1, 20) and zymogen (6) granules. VNUT-1 has not been characterized. VNUT-1 and VNUT-2 differ from each other at the NH2-terminal cytoplasmic tail, but whether differences in the NH2-terminal tail confer tissue specificity and/or subcellular localization selectivity is not known. Since mRNA for both splice variants was amplified from Calu-3 cells, it could be speculated that the two major VNUT-immunoreactive bands detected in these cells, VNUT55 and VNUT60, represent Q9BYT1-2 and Q9BYT1-1, respectively. However, the migratory pattern of VNUT may be affected by posttranslational modifications, as previously proposed for Q9BYT1-2 (20). The anti-VNUT antibody used in the current study was raised against full-length Q9BYT1-2, which shares >90% homology with Q9BYT1-1. Thus we could not conclusively identify the VNUT isoform(s) present in Calu-3 cells. Furthermore, because of the lack of isoform-specific primers with similar amplification coefficients for qPCR analysis, the relative abundance of VNUT mRNA variants could not be determined. Similarly, attempts to selectively knock down VNUT variants were hampered by the unavailability of small interference oligonucleotides specific for either VNUT species.
However, differential centrifugation protocols were used to resolve VNUT-immunoreactive bands in two subcellular fractions. VNUT55 was recovered with the mucin granule-rich fraction (Figs. 2 and 3), while VNUT60 sedimented with low-density vesicles associated with the lysosome/ER/Golgi-rich fraction (Fig. 4). Importantly, overexpression of recombinant Myc-tagged Q9BYT1-2 (VNUT-2) resulted in strong Myc immunoreactivity that localized to mucin granules and other vesicular compartments (Fig. 5). One interpretation of this observation is that posttranslational modifications of a single VNUT isoform could have accounted for the multiple subcellular localization of VNUT in Calu-3 cells. A more conclusive assessment of VNUT-1 and VNUT-2 localization/function in goblet cells awaits the generation of VNUT isoform-specific probes (e.g., isoform-specific antibodies).
VNUT knockdown markedly reduced mucin granule ATP, ADP, and AMP content. Our data showing that VNUT shRNA decreased UTP concentrations within mucin granules suggest that VNUT transports UTP in addition to ATP. This notion is in agreement with published studies indicating that UTP reduced the uptake of ATP in VNUT-reconstituted proteoliposomes (20) and that ATP and UTP were equally transported into chromaffin granules (now known to express VNUT) (1). While ATP and UTP content in the mucin granule represented a small (∼1%) fraction of the total cellular ATP and UTP content, the ratio of ATP to UTP in the mucin granule fraction coincided with the cytosolic ATP-to-UTP ratio, suggesting that these nucleotides are transported with kinetics that reflect their cytosolic concentrations. Together, these results reinforce the hypothesis that VNUT transports UTP in addition to ATP. While ATP represented ∼80% of the total adenine nucleotide pool in the cell lysate, ADP and AMP accounted for 90% of the purine pool in the mucin granule fraction, suggesting that ATP is largely dephosphorylated upon entry into the mucin granule, as previously proposed (9). However, we cannot rule out the possibility that VNUT transported cytosolic ADP, as previously suggested (20).
Whether luminal ATP is necessary for mucin maturation/organization in the mucin granule is not known. Our data indicate that a 70% reduction of ATP levels inside the mucin granule has no apparent effect on mucin content and secretion. Lack of complete reduction of intragranular ATP prevented us from speculating on the role of intragranular ATP in the content and quality of packed mucins.
Regardless of the role of ATP inside the mucin granule, VNUT knockdown reduced the amount of ATP released with secreted mucins, strongly suggesting that VNUT contributes to the cellular release of nucleotide signaling molecules from mucin granules.
A scenario in which ATP released from secretory granules contributes in an autocrine fashion to the physiological secretion of co-cargo molecules has been recently described with insulin-secreting β-cells. That is, in pancreatic β-cells, extracellular glucose promotes Ca2+-dependent insulin granule exocytosis. ATP released from insulin granules further stimulates P2X receptors on β-cells, which in turn enhances insulin granule exocytosis (5). Reduction of VNUT expression in β-cells via shRNA markedly reduced ATP (but not insulin) levels in insulin granules and reduced glucose-evoked ATP release and insulin secretion (5).
Like β-cells, ATP released with mucins is predicted to act as an autocrine stimulus, further enhancing mucin secretion via activation of P2Y2 receptors on goblet cells (2). Our results in Fig. 8 indicate that VNUT knockdown reduces ATP release without affecting mucin secretion. However, unlike native airway epithelial goblet cells, the Calu-3 cells used in our studies do not express P2Y2 receptors; therefore, assessment of the impact of mucin granule ATP depletion on mucin secretion could not be appreciated in this study.
However, it is worth noting that ATP represents a minor (<10%) fraction of the adenine nucleotide pool stored in mucin granules, with ADP (60%) and AMP (30%) representing the predominant species (9; present study). Thus, mucin granules release ADP/AMP in preference to ATP (9). Such a pattern of nucleotide release from goblet cells offers a potential physiological advantage of selectively activating ion/water transport activities on neighboring ciliated cells. Specifically, released ADP/AMP > ATP provides an abundant source for the production of extracellular adenosine, which promotes liquid secretion via an A2b receptor/CFTR-mediated mechanism on ciliated cells (3, 11). Such a mechanism maximizes the capacity to hydrate mucins in normal airways while minimizing autocrine feedback on mucin secretion via goblet cell P2Y2 receptors. Although we cannot rule out the possibility that vesicular pathways, in addition to mucin granules (23), represent an additional source of nucleotide release in mucin-secreting cells, our results suggest that mucin granule-associated VNUT is an important contributor to the cellular release of nucleotides as extracellular warning signals at the initial steps of mucin secretion.
Calu-3 cells, which constitute a mixed population of nonmucous and goblet cells (8), exhibit pannexin 1-dependent and Ca2+-regulated exocytotic pathways for ATP release. We have shown that pannexin 1 shRNA reduced ATP release in Calu-3 cells exposed to hypotonicity (22), and thrombin-stimulated ATP release from Calu-3 cells was reduced by the pannexin inhibitor carbenoxolone (9). However, residual ATP release that was associated with Ca2+-regulated exocytosis of mucin granules persisted in thrombin-stimulated Calu-3 cells after complete inhibition of pannexin (9). We have also demonstrated that treatment of Calu-3 cells with ionomycin resulted in robust mucin granule exocytosis that was accompanied by ATP release; mucin secretion and ATP release in response to ionomycin were blunted after treatment of Calu-3 cells with inhibitors of the secretory pathway, and bafilomycin A1 [an inhibitor of the H+-ATPase that facilitates vesicular ATP transport via VNUT (20)] markedly reduced ionomycin-promoted ATP release from Calu-3 cells (8). Lastly, as shown in the current study, ionomycin induced Ca2+-regulated mucin secretion and VNUT-dependent ATP release in Calu-3 cells. Collectively, our results are consistent with the notion that pannexin 1- and VNUT-dependent pathways contribute separately to the release of ATP from airway epithelial cells.
In summary, our finding that VNUT55 is functionally expressed in mucin granules supports the concept that VNUT is the relevant nucleotide transporter of canonical secretory granules, as reported with synaptic vesicles (10) and chromaffin (20) and zymogen (6) granules. The finding that VNUT immunoreactivity is also associated with mucin-free vesicles in Calu-3 cells is consistent with recent studies illustrating VNUT expression in vesicles and/or VNUT-dependent ATP release in cells lacking dense-core, bona fide secretory granules, e.g., biliary epithelial cells, T lymphocytes, and alveolar epithelial-like A549 cells (19, 26, 27). Future studies are needed to address whether VNUT60-rich vesicles are additional contributors to the ATP release observed in mucin-secreting goblet cells.
GRANTS
Research from the authors' laboratories was supported by National Heart, Lung, and Blood Institute Grant P01-HL-0343223.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.I.S., S.M.K., W.K.O., M.H., Y.M., and E.R.L. are responsible for conception and design of the research; J.I.S., S.F.O., C.v.H., L.M., L.C.J., N.T., and E.R.L. performed the experiments; J.I.S., S.M.K., C.v.H., M.H., Y.M., and E.R.L. analyzed the data; J.I.S., S.M.K., S.F.O., Y.M., and E.R.L. interpreted the results of the experiments; J.I.S., S.F.O., M.H., Y.M., and E.R.L. prepared the figures; J.I.S., S.M.K., and E.R.L. edited and revised the manuscript; J.I.S., S.M.K., and E.R.L. approved the final version of the manuscript; E.R.L. drafted the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Tal Kafri (University of North Carolina Lenti-shRNA core facility) for providing shRNA lentiviruses and for useful suggestions. We also thank Dr. Neal Kramarcy for assistance and use of the Zeiss confocal microscope system at the Michael Hooker Microscopy Facility and Dr. Hong Dang for assistance with ANOVA tests.
REFERENCES
- 1. Bankston LA, Guidotti G. Characterization of ATP transport into chromaffin granule ghosts: synergy of ATP and serotonin accumulation in chromaffin granule ghosts. J Biol Chem 271: 17132–17138, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol 70: 487–512, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Davis CW, Lazarowski E. Coupling of airway ciliary activity and mucin secretion to mechanical stresses by purinergic signaling. Respir Physiol Neurobiol 163: 208–213, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Evans CM, Koo JS. Airway mucus: the good, the bad, the sticky. Pharmacol Ther 121: 332–348, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Geisler JC, Corbin KL, Li Q, Feranchak AP, Nunemaker CS, Li C. Vesicular nucleotide transporter-mediated ATP release regulates insulin secretion. Endocrinology 154: 675–684, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haanes KA, Novak I. ATP storage and uptake by isolated pancreatic zymogen granules. Biochem J 429: 303–311, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Kreda SM, Gentzsch M. Imaging CFTR protein localization in cultured cells and tissues. Methods Mol Biol 742: 15–33, 2011 [DOI] [PubMed] [Google Scholar]
- 8. Kreda SM, Okada SF, van Heusden CA, O'Neal W, Gabriel S, Abdullah L, Davis CW, Boucher RC, Lazarowski ER. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol 584: 245–259, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kreda SM, Seminario-Vidal L, van Heusden CA, O'Neal W, Jones L, Boucher RC, Lazarowski ER. Receptor-promoted exocytosis of airway epithelial mucin granules containing a spectrum of adenine nucleotides. J Physiol 588: 2255–2267, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Larsson M, Sawada K, Morland C, Hiasa M, Ormel L, Moriyama Y, Gundersen V. Functional and anatomical identification of a vesicular transporter mediating neuronal ATP release. Cereb Cortex 22: 1203–1214, 2012 [DOI] [PubMed] [Google Scholar]
- 11. Lazarowski ER, Boucher RC. Purinergic receptors in airway epithelia. Curr Opin Pharmacol 9: 262–267, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lazarowski ER, Boucher RC, Harden TK. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem 275: 31061–31068, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Lazarowski ER, Harden TK. Quantitation of extracellular UTP using a sensitive enzymatic assay. Br J Pharmacol 127: 1272–1278, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem 279: 36855–36864, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leviatan S, Sawada K, Moriyama Y, Nelson N. Combinatorial method for overexpression of membrane proteins in Escherichia coli. J Biol Chem 285: 23548–23556, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mall M, Kreda SM, Mengos A, Jensen TJ, Hirtz S, Seydewitz HH, Yankaskas J, Kunzelmann K, Riordan JR, Boucher RC. The ΔF508 mutation results in loss of CFTR function and mature protein in native human colon. Gastroenterology 126: 32–41, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem 281: 22992–23002, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okada SF, Zhang L, Kreda SM, Abdullah LH, Davis CW, Pickles RJ, Lazarowski ER, Boucher RC. Coupled nucleotide and mucin hypersecretion from goblet cell metaplastic human airway epithelium. Am J Respir Cell Mol Biol 45: 253–260, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sathe MN, Woo K, Kresge C, Bugde A, Luby-Phelps K, Lewis MA, Feranchak AP. Regulation of purinergic signaling in biliary epithelial cells by exocytosis of SLC17A9-dependent ATP-enriched vesicles. J Biol Chem 286: 25363–25376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci USA 105: 5683–5686, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seminario-Vidal L, Kreda S, Jones L, O'Neal W, Trejo J, Boucher RC, Lazarowski ER. Thrombin promotes release of ATP from lung epithelial cells through coordinated activation of Rho- and Ca2+-dependent signaling pathways. J Biol Chem 284: 20638–20648, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seminario-Vidal L, Okada SF, Sesma JI, Kreda SM, van Heusden CA, Zhu Y, Jones LC, O'Neal WK, Penuela S, Laird DW, Boucher RC, Lazarowski ER. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem 286: 26277–26286, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sesma JI, Esther CR, Jr, Kreda SM, Jones L, O'Neal W, Nishihara S, Nicholas RA, Lazarowski ER. ER/Golgi nucleotide sugar transporters contribute to the cellular release of UDP-sugar signaling molecules. J Biol Chem 284: 12572–12583, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sorensen CE, Novak I. Visualization of ATP release in pancreatic acini in response to cholinergic stimulus. Use of fluorescent probes and confocal microscopy. J Biol Chem 276: 32925–32932, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Sreedharan S, Shaik JH, Olszewski PK, Levine AS, Schioth HB, Fredriksson R. Glutamate, aspartate and nucleotide transporters in the SLC17 family form four main phylogenetic clusters: evolution and tissue expression. BMC Genomics 11: 17, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takai E, Tsukimoto M, Harada H, Sawada K, Moriyama Y, Kojima S. Autocrine regulation of TGF-β1-induced cell migration by exocytosis of ATP and activation of P2 receptors in human lung cancer cells. J Cell Sci 125: 5051–5060, 2012 [DOI] [PubMed] [Google Scholar]
- 27. Tokunaga A, Tsukimoto M, Harada H, Moriyama Y, Kojima S. Involvement of SLC17A9-dependent vesicular exocytosis in the mechanism of ATP release during T cell activation. J Biol Chem 285: 17406–17416, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zatyka M, Ricketts C, da S, X , Minton J, Fenton S, Hofmann-Thiel S, Rutter GA, Barrett TG. Sodium-potassium ATPase 1 subunit is a molecular partner of wolframin, an endoplasmic reticulum protein involved in ER stress. Hum Mol Genet 17: 190–200, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]