Abstract
The Xin repeat-containing proteins were originally found in the intercalated discs of cardiac muscle with implicated roles in cardiac development and function. A pair of paralogous genes, Xinα (Xirp1) and Xinβ (Xirp2), is present in mammals. Ablation of the mouse Xinα (mXinα) did not affect heart development but caused late-onset adulthood cardiac hypertrophy and cardiomyopathy with conductive defects. Both mXinα and mXinβ are also found in the myotendinous junction (MTJ) of skeletal muscle. Here we investigated the structural and functional significance of mXinα in skeletal muscle. In addition to MTJ and the contact sites between muscle and perimysium, mXinα but not mXinβ was found in the blood vessel walls, whereas both proteins were absent in neuromuscular junctions and nerve fascicles. Coimmunoprecipitation suggested association of mXinα with talin, vinculin, and filamin, but not β-catenin, in adult skeletal muscle, consistent with our previous report of colocalization of mXinα with vinculin. Loss of mXinα in mXinα-null mice had subtle effects on the MTJ structure and the levels of several MTJ components. Diaphragm muscle of mXinα-null mice showed hypertrophy. Compared with wild-type controls, mouse extensor digitorum longus (EDL) muscle lacking mXinα exhibited no overt change in contractile and relaxation velocities or maximum force development but better tolerance to fatigue. Loaded fatigue contractions generated stretch injury in wild-type EDL muscle as indicated by a fragmentation of troponin T. This effect was blunted in mXinα-null EDL muscle. The results suggest that mXinα play a role in MTJ conductance of contractile and stretching forces.
Keywords: Xinα, myotendinous junction, muscle fatigue, fast troponin T fragmentation
the family of xin repeat-containing proteins is a relatively new class of actin-binding protein primarily found in striated muscle cells (10, 19, 33, 44, 57, 59, 62). The majority of Xin proteins in muscle cells is present in adherence junctions: the intercalated disc of cardiac muscle and the myotendinous junction (MTJ) of skeletal muscle (20, 44, 49, 57, 59). In mammals, a pair of paralogous genes encodes two protein isoforms, Xinα [also called cardiomyopathy-associated 1 (Cmya1) or Xin actin-binding repeat-containing 1 (Xirp1)] and Xinβ (also called Cmya3, myomaxin, or Xirp2; Ref. 19). Through alternative splicing, each gene is capable of producing several protein variants (20, 43, 57, 61).
In the heart, mXinα directly interacts with β-catenin at the intercalated discs, providing a link between N-cadherin/catenin complex and the underlying actin cytoskeleton. Mouse hearts deficient in mXinα exhibited progressive structural defects in the intercalated discs and late-onset cardiac hypertrophy and cardiomyopathy with conductive abnormalities (6, 20, 30, 43). Therefore, mXinα may play an important role in the N-cadherin-mediated adhesion and signaling between cardiomyocytes (10, 20, 43). Mouse hearts without mXinβ had mislocalization of mXinα and N-cadherin and severe growth retardation, diastolic dysfunction, and postnatal lethality (61).
In skeletal muscle, which has no intercalated discs, the majority of mXinα is localized to the MTJs. MTJ is the interface between skeletal muscle cells and tendon and as such bears the contractile and stretching forces. The molecular mechanisms for the MTJ components to regulate and/or transmit these forces remain largely unknown. The mouse Xinα (mXinα) is a modular protein located at MTJ, capable of binding to actin filaments and interacting with many actin-binding proteins, including filamin, Mena/VASP, α-actinin, tropomyosin, gelsolin, and vinculin (10, 19, 24, 44, 57).
During development, skeletal muscles differentially express three types of cadherin (M-, N-, and R-cadherin), and each type of cadherin-mediated adhesion is specifically required for the cellular events such as commitment, recognition, alignment, fusion, and terminal differentiation in myogenesis (8, 17, 40, 46, 64). However, none of them was detected at the MTJs of adult skeletal muscle (27), suggesting minimal contribution to MTJ adhesion by the cadherin/catenin-mediated system. Therefore, the role of mXinα in the function of MTJ requires further investigation.
Several components of the dystrophin-glycoprotein complex-mediated and integrin-mediated adhesion systems have been shown to concentrate at the MTJs (1, 7, 9, 11, 29, 48, 55). The integrin specifically enriched in the MTJ is α7β1 (4, 22, 45). The β1-subunit of integrin has been further shown to bind talin (23, 53) and filamin (18, 37, 56). We (10) and others (57) previously showed that mXinα was capable of interacting with filamin and vinculin but not talin. It has been demonstrated that filamin can bind γ/δ-sarcoglycans of dystrophin-glycoprotein complex (21, 54), whereas vinculin is a known talin-binding protein (5). Therefore, mXinα may potentially play a role at the MTJ in linking these two adhesion systems for the transmission of forces.
We previously reported that in pressure overload-induced hypertrophic hearts both mXinα and mXinβ are significantly upregulated and integrated in the intercalated discs (62), suggesting a load-related function. In skeletal muscle, eccentric contractions involving stretched/loaded shortenings cause injuries in myofibril and cytoskeleton components (15, 31, 32, 42). The morphological and molecular changes detected in the stretch-induced muscle injury include Z-band dissolution, sarcolemmal damage, misorganized desmin intermediate filaments, misaligned myofibrils, and upregulation of talin and vinculin. In contrast, electron microscopy detected only very moderate morphological alteration at MTJ (15). Despite the minimal alteration at MTJ, studies of human and rabbit muscle strain injury have demonstrated that the region of muscle near MTJ is most vulnerable to eccentric contractions (41, 52).
In the present study, we investigated the localization and function of mXinα in skeletal muscle. Immunofluorescence microscopy using Xin isoform-specific antibodies determined the detailed localization of mXinα. Coimmunoprecipitation revealed the association of mXinα with known MTJ-associated proteins. Contractility and fatigability were investigated in muscles from wild-type and mXinα-null mice. Compared with wild-type control, mXinα-null muscle was more tolerant to fatigue and more resistant to stretch injury. The results suggest a role of mXinα in skeletal muscle force transductions.
MATERIALS AND METHODS
Animals.
All animal procedures were performed using protocols approved by the Wayne State University and University of Iowa Institutional Animal Care and Use Committees. The mXinα-null mouse line was generated as described previously (20) and has been backcrossed to and maintained in C57BL/6J strain. Age-matched adult wild-type and mXinα-deficient mice were used in all experiments for comparisons.
Histology and immunofluorescence assay.
Immediately after euthanasia, gastrocnemius (Ga), tibialis anterior (TA), extensor digitorum longus (EDL), soleus (Sol), tongue (Ton), and diaphragm (Di) muscles were rapidly dissected and frozen in liquid nitrogen-cooled isopentane as described previously (26). Seven-micrometer cryosections were examined with hematoxylin and eosin, Masson's trichrome, and immunofluorescence staining as previously described (20).
Single- and double-label indirect immunofluorescence microscopy was carried out as previously described (20, 49). Several rabbit polyclonal antibodies against mXin isoforms were used: U1013 recognizing both mXinα and mXinβ (49), U1697 recognizing only mXinα, U1741 recognizing only mXinβ, and U1040 recognizing only mXinβ (20, 61). The specificities of the anti-Xin antibodies have been characterized in details in the cited publications. The endogenous mouse IgG in mouse muscle samples studied is not recognized by the goat anti-rabbit IgG second antibody used in our study.
Other antibodies used were mouse monoclonal antibody (mAb) CGβ6 recognizing smooth muscle and nonmuscle tropomyosins (16, 34) and anti-neurofilament 200 mAb N52 (Sigma, St Louis, MO). Alexa Fluor 488-conjugated α-bungarotoxin (B-13422) was purchased from Molecular Probes (Eugene, OR).
Coimmunoprecipitation and Western blot analysis.
Gastrocnemius muscle of adult mice was homogenized in an immunoprecipitation buffer (150 mM NaCl, 50 mM Tris·HCl, pH7.5, 1% Nonidet P-40, and 0.1% SDS plus protease inhibitor mixture; Roche Applied Science, Indianapolis, IN). After preincubation with protein G-Sepharose beads (GE Healthcare, Piscataway, NJ) for 30 min at 4°C, the homogenate (150 μg of total protein/immunoprecipitation) was cleared by centrifugation at 12,000 g for 15 min. The precleaned homogenate was incubated with anti-tropomyosin mAb LC24 (16, 35, 47, 63), anti-filamin mAb FLMN01 (Abcam, Cambridge, MA), anti-vinculin mAb hVIN-1 (Sigma), anti-talin mAb 8D4 (Sigma), anti-β-catenin mAb 15B8 (Sigma), or normal mouse serum control at 4°C overnight. Incubated again with protein G-Sepharose beads at 4°C for 1 h, the beads were collected by centrifugation and washed three times with immunoprecipitation buffer and one time with PBS. The bound proteins were eluted with SDS-PAGE sample buffer, resolved on 7.5% SDS-PAGE (49), and transferred to nitrocellulose membrane for Western blot analysis using anti-mXin U1013 as described previously (20).
In situ measurement of muscle contraction.
Littermates of adult wild-type and mXinα-KO mice of body weight ∼22 g were used to study muscle function employing an in situ force measurement protocol (3). The mouse was anesthetized with intraperitoneal injection of pentobarbital (100 mg/kg body wt). A small incision was made at the ankle to expose the distal tendon of EDL muscle. Carefully to avoid stretch injury, the tendon was dissected from surrounding connective tissues and cut near the bone attachment. The animal was then placed on a platform maintained at 37°C with circulating warm water, and the knee was immobilized by mounting between a pair of screws instrumented on the platform (Aurora Scientific, Aurora, Ontario, Canada). The end segment of tendon was folded back and tied with 3–0 silk suture to securely attach to the lever arm of a servomotor (model 300B; Aurora Scientific) that controls the length of the muscle and measures the force development. The small incision site and the exposed muscle and tendon tissue were kept under moisture with dripping Krebs solution (118 mM NaCl, 4.7 mM KCl, 2.25 mM MgSO4, 1.2 mM KH2PO4, 2.25 mM CaCl2, 11 mM glucose, and 21 mM NaHCO3) equilibrated in 5% CO2-95% O2, pH 7.4 at 37°C.
Contraction of EDL muscle was induced with electrical stimulation of the peroneal or sciatic nerve through a pair of wire electrodes. The stimulation voltage and, subsequently, muscle length were adjusted to optimum for the development of maximum isometric twitch force. The muscle was stimulated to contract at increasing frequencies until the force development reached plateau (Po), typically at 300 Hz. The optimal in situ muscle length (Lo) was measured with a caliper, based on defined anatomical landmarks. Fiber length (Lf) of mouse EDL muscle was estimated by multiplying Lo by the Lf/Lo ratio of 0.45 (2). The mean Lf values of EDL were 5.58 ± 0.03 and 5.61 ± 0.02 mm (means ± SE) for muscles of adult wild-type and mXinα-KO groups, respectively.
Fatigue contraction protocol.
After baseline contractions were measured, the EDL muscle was treated with a fatigue protocol consisting of 150 repeats of 200 ms/s stimulation at 300 Hz. After the fatigue protocol, the muscle was allowed to recover under 200-ms 300 Hz stimulation/min for 20 min when a plateau of recovery in tetanic force development occurred.
Stretch-contraction protocol.
A stretch-contraction protocol mimicking eccentric contractions was carried out after the baseline functional measurement or fatigue protocol. During each of the 200 ms/s 300-Hz stimulations, stretch was applied from the 100th ms to 200th ms in a ramp to increase the muscle fiber length (Lf) by 20%. This contraction-stretch cycle was repeated for 150 times. The length changes corresponded to a 4–20% of the EDL muscle fiber length and are within the physiological range for most skeletal muscles (12). The same protocol as that used for recovery after fatigue contractions was applied to allow the muscle to recover from the stretch contraction protocol and to evaluate the tolerance of muscles to such loaded contractions.
After in situ contractile measurements, the mice were euthanized under anesthesia and the EDL muscles were rapidly dissected, trimmed to remove tendons, weighed after blotting dry, and frozen at −80°C for protein analysis. Total muscle fiber cross-sectional area was calculated by dividing the muscle wet mass by the product of the Lf and the specific density of skeletal muscle tissue, 1.06 mg/mm3. The EDL muscles subjected to contractile measurements, fatigue and/or stretch-contractions were examined compared with the control muscle from the resting leg.
Examination of the integrity of myofilament proteins.
Immediately after being taken out from deep freezer, the frozen muscle tissue was homogenized in 40 vol (wt/vol) of SDS-gel sample buffer containing 2% SDS, 10% glycerol, 50 mM Tris-base, and 2% 2-mercaptoethanol, pH 8.8, using a high speed mechanical homogenizer (PRO Scientific, Oxford, CT). The homogenized muscle samples were immediately heated at 80°C for 5 min, clarified by high speed centrifugation, and stored at −80°C for SDS-PAGE and Western blot analysis. The total muscle protein extracts were resolved on 14% Laemmli gel with an acrylamide:bisacrylamide ratio of 180:1. The protein bands were visualized by staining the gel with Coomassie Brilliant Blue R250.
Duplicate gels were electrically blotted on nitrocellulose membranes. TBS containing 1% BSA was used to block the nitrocellulose membranes at room temperature for 30 min. The membranes were then incubated with mAb T12 recognizing fast skeletal muscle troponin T (TnT; Ref. 36) and mAb TnI-1 recognizing all three muscle type TnI isoforms (25), both diluted in TBS containing 0.1% BSA, at 4°C overnight. After high stringency washes with TBS plus 0.5% Triton X-100 and 0.05% SDS, the membranes were incubated with alkaline phosphatase-conjugated goat anti-mouse IgG second antibody (Santa Cruz Biotechnology), washed again, and developed in 5-bromo-4-chloro-3-indolylphosphate/nitro blue tetrazolium substrate solution.
Data analysis.
Two-dimensional densitometry was carried out to quantify SDS-PAGE gel and Western blots on images scanned at 600 dots/in. Statistical significance for all quantitative data was determined using Student's t-test or two-way ANOVA test.
RESULTS
Similar but distinguishable localizations for mXinα and mXinβ in mouse skeletal muscles.
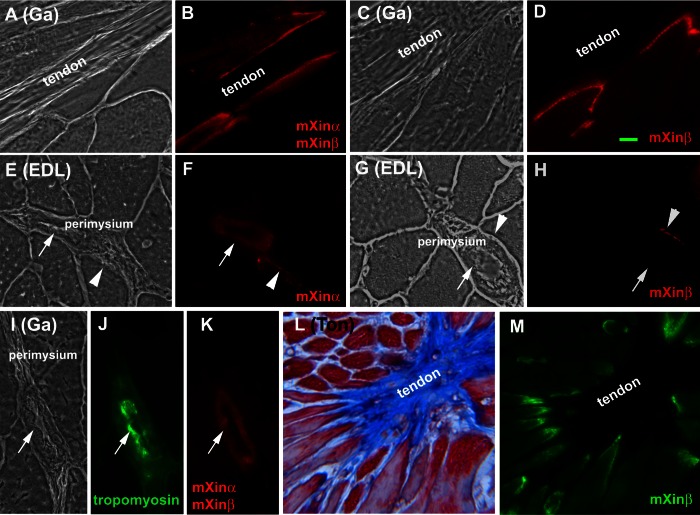
Immunofluorescence microscopy using anti-mXin polyclonal antibodies showed major staining in the MTJ of gastrocnemius (Ga; Fig. 1, B and D), TA (data not shown), EDL (data not shown), and Ton (Fig. 1M). An additional contact site between the muscle fibers and perimysium of EDL was stained with anti-mXinα U1697 and with anti-mXinβ U1040 (arrowheads in Fig. 1, F and H, respectively). Therefore, mXinα and mXinβ were similarly localized to the MTJs and the contact sites between muscle and perimysium. It should be noted that the presence of these two proteins was uneven at these contact sites, particularly at the perimysium sites. Not all of the cells in contact with the perimysium were labeled (Fig. 1, F and H). This variation suggests that cells surrounding the perimysium make focal contacts at different planes of the section throughout the perimysium.
Fig. 1.
Both mXinα and mXinβ are localized to the myotendinous junctions and the contact sites between muscle fibers and perimysium whereas only mXinα is found in the blood vessel walls of perimysium. A–H and L–M: single-label indirect immunofluorescence microscopic images of cryosections of gastrocnemius (Ga), extensor digitorum longus (EDL), and tongue (Ton) muscles were obtained with antibody U1013 against both mXinα and mXinβ (B), anti-mXinβ antibody U1741 (D), anti-mXinα antibody U1697 (F), or anti-mXinβ antibody U1040 (H and M). All muscles were from adult wild-type C57BL/6J mice, except for the gastrocnemius muscle shown in C and D, which was from adult mXinα-null mouse. A, C, E, and G are phase-contrast images and B, D, F, and H are the corresponding fluorescent (red) images, respectively. I–K: double-label immunofluorescence images of gastrocnemius muscle sections were obtained with anti-smooth muscle tropomyosin mAb CGβ6 (green) and anti-mXin antibody U1013 (red). Arrowheads in E–H indicate that mXin is also present at the cell adhesion to the perimysium of muscle fiber. The arrows in E–K indicate the blood vessels within the perimysium recognized by U1013 (K) and anti-mXinα U1697 (F) but not anti-mXinβ U1040 (H) antibodies. L: Massion's trichrome-stained images of tongue; M: their corresponding fluorescent (green) images obtained using anti-mXinβ U1040 antibody. Bar = 10 μm.
In addition to MTJ and the contact sites, U1013 (recognizing mXinα and mXinβ) and U1697 (specific to mXinα) both stained blood vessels within the perimysium of wild-type EDL, and Ga muscles (Fig. 1, F and K, arrows). These blood vessels were confirmed by double-staining of mAb CGβ6 against smooth muscle and nonmuscle tropomyosins (Fig. 1J). In contrast, U1040 (mXinβ-specific) antibody did not stain the blood vessel within the perimysium (Fig. 1H, arrow). Moreover, blood vessels in mXinα-null muscles were not stained by any of these anti-mXin antibodies (data not shown). The results suggest that mXinα but not mXinβ is present in the blood vessel walls.
Absence of mXin proteins in neuromuscular junctions and nerve fascicles.
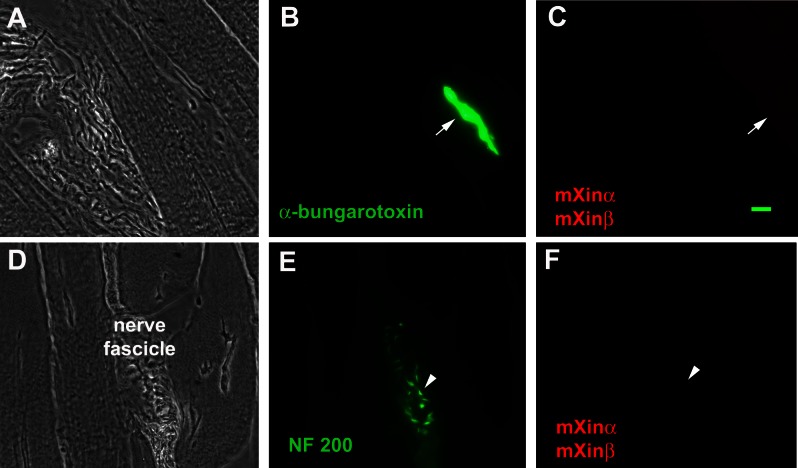
In adult skeletal muscle, neuromuscular junctions are specialized contact sites for motor neuron to control muscle activity, and nerve fascicles are formed by a bundle of nerves running through the perimysium. Double-label immunofluorescence microscopy on wild-type gastrocnemius muscle cryosections using anti-mXin antibody U1013 together with either fluorescence-tagged α-bungarotoxin for neuromuscular junction or anti-neurofilament 200 for nerve fascicle showed that neither of these structures (Fig. 2, B, C, E, and F) contained a detectable amount of mXin protein.
Fig. 2.
Neither mXinα nor mXinβ is found in the neuromuscular junctions or perimysium nerve fascicles. Double-label immunofluorescence images of gastrocnemius muscle sections were obtained with either a combination of Alex Fluor 488-conjugated anti-α-bungarotoxin and antibody U1013 (red) (A–C) or a mixture of anti-neurofilament 200 (NF 200) (green) and U1013 (red) (D–F) antibodies. Neither neuromuscular junction (indicated by arrow) nor nerve fascicle (indicated by arrowhead) contains mXin protein. Bar = 10 μm.
Coimmunoprecipitation of mXin with known MTJ components.
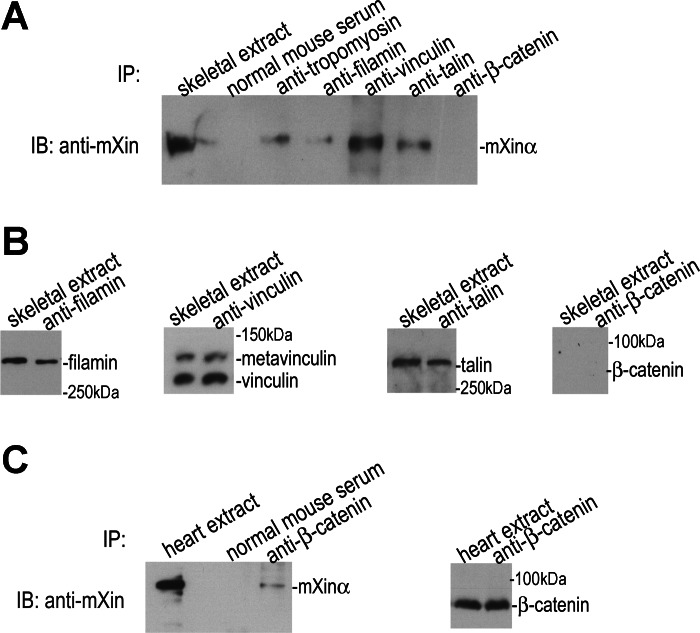
Several known adhesion components, such as filamin, talin, vinculin, β1-integrin, and dystrophin also concentrate in MTJs of skeletal muscle (49, 55, 57). Our previous immunofluorescence microscopy on muscle sections showed the colocalization of mXinα with vinculin (49). Yeast two-hybrid assay using a heart cDNA library identified filamin, vinculin, and tropomyosin as mXinα-interacting partners (10). As shown in Fig. 3A, mXinα was coimmunoprecipitated from gastrocnemius muscle extracts using anti-tropomyosin (LC24), anti-filamin, anti-vinculin, or anti-talin mAb but not anti-β-catenin mAb or normal mouse serum control. mAb LC24 recognizes tropomyosin isoform 4 that is known to locate together with γ-actin external to the sarcomere but adjacent to the Z-line and in the subsarcolemmal region including MTJ in skeletal muscle cells (28, 58). The antibodies used in our experiments specifically precipitated their respective antigens from skeletal muscle extract except for anti-β-catenin (Fig. 3B and data not shown for LC24), while the same anti-β-catenin mAb was able to coimmunoprecipitate mXinα from heart extract (Fig. 3C) (10). These results suggested that mXinα formed complexes with talin, vinculin, filamin, and nonmuscle tropomyosin at the contact sites with potential roles in adhesion and/or cell signaling. The fact that skeletal muscle extract contained very little β-catenin suggests that the N-cadherin/β-catenin-mediated adhesion may play only minor roles at those contact sites in adult skeletal muscle.
Fig. 3.
mXinα is associated with tropomyosin, filamin, vinculin and talin in skeletal muscle extract. A: coimmunoprecipitates (IP) from total proteins extracted from gastrocnemius muscle using various antibodies were analyzed by immunoblot (IB) using anti-mXin antibody. mXinα was present in the immunoprecipitates of anti-tropomyosin, anti-filamin, anti-vinculin, and anti-talin but not anti-β-catenin antibodies or control normal mouse serum. B: various IP products were examined with Western blot using respective antibodies. Results showed that the gastrocnemius muscle extract contained very little β-catenin. C: same anti-β-catenin antibody was able to coimmunoprecipitate mXinα from total protein extract of adult mouse heart (10).
Structure and molecular changes in mXinα-null muscles.
To study the role of mXinα in skeletal muscle, we histologically and molecularly characterized muscle tissues from wild-type and mXinα knockout mice. Figure 4 shows the comparisons of hematoxylin and eosin-stained muscle sections from Ton, Di, and Sol of wild-type (mXinα+/+) and mXinα−/− mice. No overt difference in histology of these muscles was observed. At a similar plane, the roundness values (as a measure of fiber shape complexity) for wild-type and mXinα-null diaphragm fibers were the same (Table 1). Using dynamic image analysis system (DIAS) software (50, 51) to analyze muscle size, we found that the cross-sectional area of myofibers was significantly larger for the mXinα-null diaphragm muscle (Fig. 4, C and D, and Table 1). However, hypertrophy was not observed in any other muscles examined in mXinα-null mice.
Fig. 4.
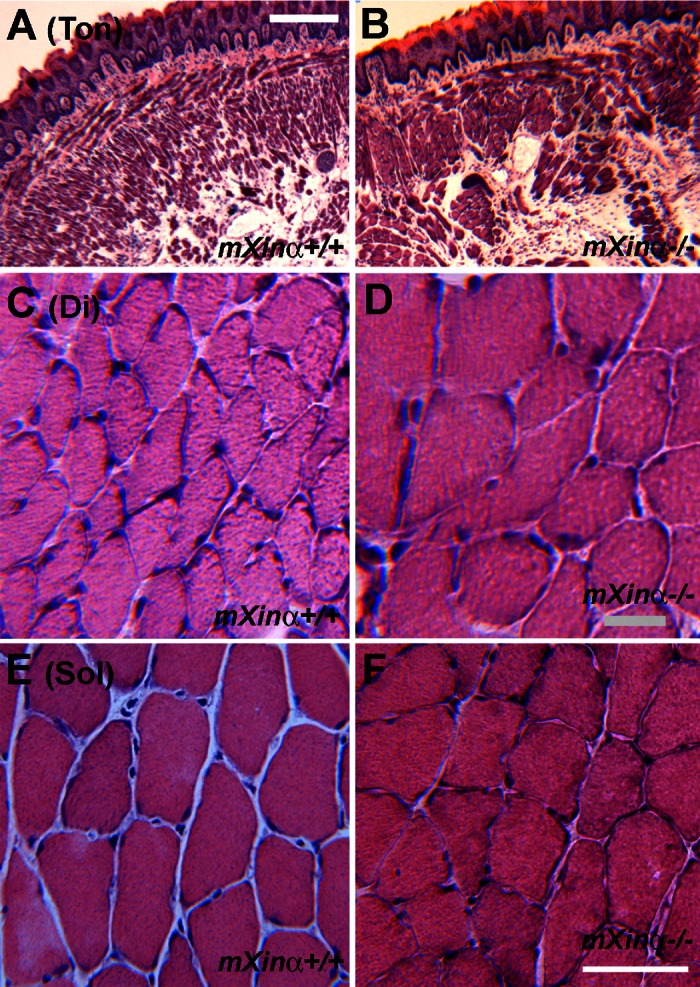
mXinα-null diaphragm muscle fibers showed hypertrophy. Hematoxylin and eosin-stained cryosections were prepared from Ton (A and B), diaphragm (Di; C and D), and soleus (Sol; E and F) muscles of 7-mo-old wild-type (mXinα+/+) and mXinα−/− mice. No centrally localized nuclei or obvious alterations in gross morphology was detected in mXinα-null muscles. However, the cross-sectional area of mXinα−/− diaphragm muscle fibers appeared larger than the wild-type counterparts, indicative of hypertrophy. Bar in A for A and B = 250 μm; bar in D for C and D = 20 μm; bar in F for E and F = 50 μm.
Table 1.
Comparisons of cross-sectional area and roundness of soleus and diaphragm muscle fibers of wild-type and mXinα-null mice
| Soleus |
Diaphragm |
|||||
|---|---|---|---|---|---|---|
| n | CSA, μm2 | Roundness, % | n | CSA, μm2 | Roundness, % | |
| mXinα+/+ | 60 | 1,506.14 ± 71.16 | 73.78 ± 1.23 | 38 | 809.1 ± 59.75 | 70.28 ± 1.72 |
| mXinα−/− | 72 | 1,433.79 ± 39.26 | 75.43 ± 1.27 | 32 | 1,113.61 ± 82.6 | 70.29 ± 1.81 |
| P value | NS | NS | 0.003 | NS | ||
Data are expressed as means ± SE. Cross sections of muscle fibers were stained with hematoxylin and eosin and examined under Leitz Laborlux 12 microscope equipped with Leica DFC320 digital camera. Images were taken and the fiber perimeters were traced manually and analyzed using the dynamic image analysis system (DIAS) software (50, 51). Fiber cross-sectional area (CSA) was computed from the perimeters, whereas the roundness was computed using the formula 100 × 4π (area/perimeter2), with a perfect circle having 100% roundness and a straight line having 0% of roundness value. NS, not significant (P > 0.5 in Student's t-test).
Western blots showed that wild-type mouse diaphragm muscle does not contain particularly high level of Xinα. Actually, the relative expression levels of mXinα and mXinβ in diaphragm were 2.6∼4.4 folds lower than the highest expression detected in soleus muscle (data not shown). Therefore, the expression level could not account for the hypertrophic phenotype in mXinα-null diaphragm but not soleus muscle. An alternative hypothesis is that diaphragm is a muscle that continuously works rhythmically to sustain respiration, which could be a reason for its sensitivity to the loss of mXinα. The hypertrophic phenotype of Xinα-null diaphragm muscle may be analogous to the progressive myocardial hypertrophy seen in mXinα-deficient hearts (20).
The SDS gels and Western blots further showed no difference in the overall protein profile or the expression of representative thick and thin filament proteins in the diaphragm muscle of mXinα-null mice compared with wild-type controls (data not shown).
The effect of mXinα loss on the structure and protein components of MTJ was examined in 5.5 to 13.5-mo-old mXinα-null mice using double-label immunofluorescence microscopy. There was no obvious alteration in the location of talin in MTJ of mXinα-deficient muscles (data not shown). In addition, no overt changes in the other MTJ protein components were found in the mXinα-null muscle (data not shown). The results that talin, vinculin, and integrin were still coassociated in mXinα-null muscle indicate that mXinα was not required for the association or the stabilization of the protein complex.
The lack of changes in expression levels of mXinβ, dystrophin, talin, metavinculin, vinculin, filamin, α-tubulin, and β-tubulin was further confirmed by Western blot analyses of Ga, EDL, and Di muscle samples from 5.5 and 8.5-mo-old wild-type and mXinα−/− and mXinα+/− mice (data not shown).
Effects of mXinα-null on muscle function.
Isometric and stretched/eccentric type contractile protocols (Fig. 5) were used to compare the function and fatigability of mXinα-null and wild-type muscles. mXinα-null and wild-type EDL muscles showed no significant difference in contractility (Table 2), indicating that deletion of mXinα did not affect the baseline contractility.
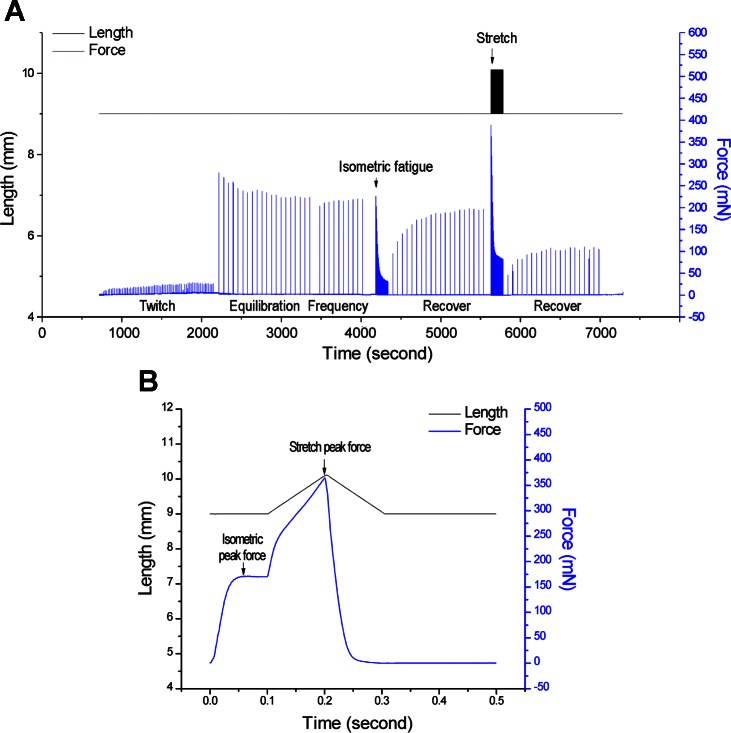
Fig. 5.
Protocol of contractile studies. A: experimental protocol used on EDL muscle for in situ functional measurement is outlined with representative muscle length and force traces. During initial twitch contractions, the muscle was stimulated with single pulse of 0.1-ms duration at constant voltage of 25 V. Muscle length was increased to reach the maximum activation of twitch force. Tetanic contractions were then induced with 200 ms of such pulse stimulations at 300 Hz every minute for 20 min. A fatigue protocol was followed consisting of 150 repeats of 200 ms/s tetanic contractions. One minute after the fatigue protocol, the muscle was allowed to recover under 200 ms/min tetanic contractions for 20 min. Eccentric contractions were induced at last with the same fatigue stimulations together with 20% stretching of Fiber length (Lf) during the last 100 ms. The above recovery protocol was applied 1 min after the eccentric contractions. B: eccentric contraction cycle is illustrated to show the ramp increase of muscle length during each cycle of tetanic contractions. Isometric peak force was measured during the first 100 ms under the optimal muscle length whereas peak force was shown at the end of stretch. Muscle length was returned to the optimal length by a ramp decrease in 100 ms.
Table 2.
Baseline contractile parameters
| Wild Type | mXinα Null | |
|---|---|---|
| Body weight, g | 21.44 ± 0.32 | 21.57 ± 1.28 |
| Muscle mass, mg | 9.20 ± 0.31 | 8.73 ± 0.47 |
| Length, mm | 12.40 ± 0.06 | 12.49 ± 0.05 |
| CSA, mass/length | 0.74 ± 0.03 | 0.70 ± 0.04 |
| Twitch force, mN | 22.23 ± 1.27 | 19.97 ± 7.46 |
| Twitch force/CSA | 30.07 ± 2.21 | 29.49 ± 12.27 |
| Twitch +dF/dt, mN/s | 4,281.13 ± 365.71 | 3,892.86 ± 984.91 |
| Twitch −dF/dt, mN/s | −2,756.17 ± 181.36 | −2,204.42 ± 565.63 |
| Twitch TPT, ms | 10.13 ± 1.38 | 10.00 ± 0.69 |
| Twitch TP50, ms | 4.30 ± 0.26 | 4.20 ± 0.38 |
| Twitch TR75, ms | 13.33 ± 0.57 | 14.50 ± 1.77 |
| Tetanic force, mN | 238.55 ± 6.34 | 225.12 ± 22.06 |
| Tetanic/CSA, mN/mm2 | 322.86 ± 20.08 | 326.80 ± 47.17 |
| Tetanic +dF/dt, mN/s | 8,965.34 ± 747.94 | 9,904.12 ± 995.34 |
| Tetanic −dF/dt, mN/s | −16,426.45 ± 955.81 | −15,824.13 ± 1,190.50 |
| Stretch Force, mN | 412.79 ± 15.90 | 396.61 ± 9.90 |
| Stretch/CSA, mN/mm2 | 557.23 ± 23.94 | 569.73 ± 28.07 |
| Stretch +dF/dt, mN/s | 5,156.26 ± 152.62 | 5,250.16 ± 489.19 |
| Stretch −dF/dt, mN/s | −16,349.41 ± 725.86 | −14,847.85 ± 450.22 |
Values are presented as means ± SE; n =3 mice each in mXinα-null and wild-type groups. Contractile function of extensor digitorum longus muscle was examined in situ under central anesthesia. No difference was found in the twitch contraction of mXinα-null muscle as compared with wild-type control. Tetanic contractions with stretch or without stretch also did not show significant difference between mXinα-null and wild-type muscles. TPT, contractile time to peak twitch tension; TP50, contractile time to 50% of peak twitch tension; TR75, relaxation time to 25% peak tension. Statistical analysis was done using Student's t-test.
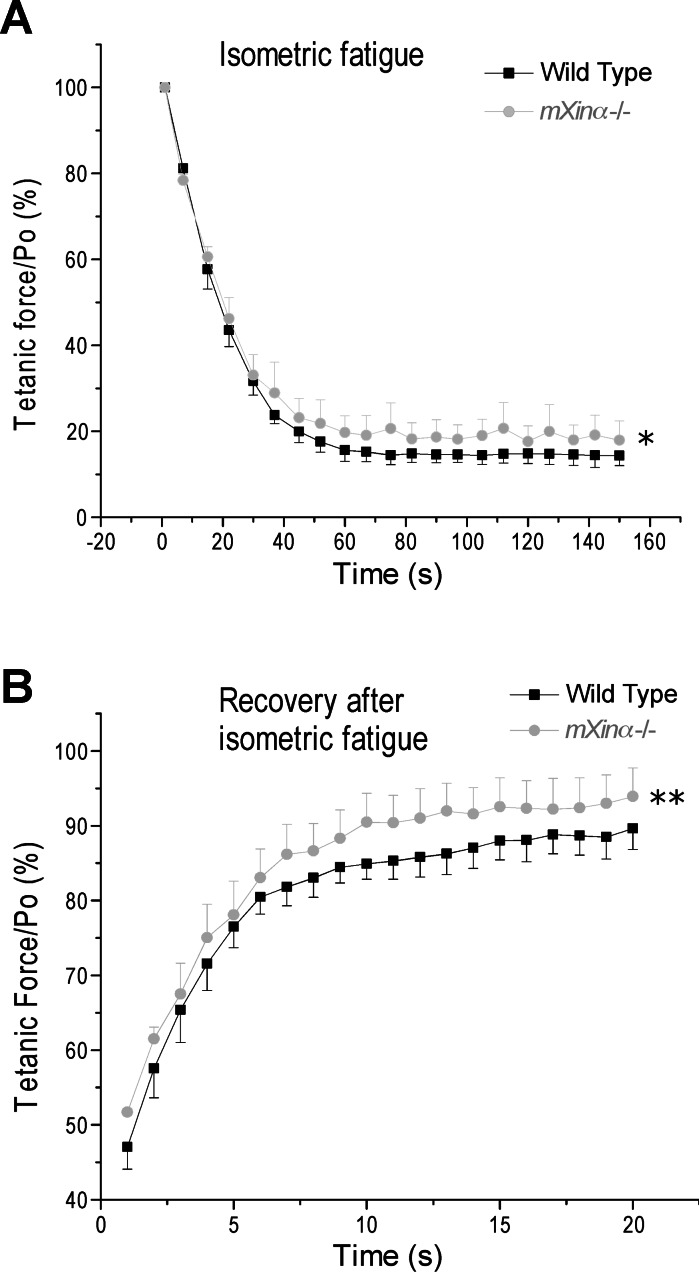
In isometric fatigability test, a higher resistance to fatigue with better recovery was found for mXinα-null EDL muscle compared with that of wild-type controls (Fig. 6). In the stretch-loaded fatigue contraction test (Fig. 5B), EDL muscle of mXinα-null mice showed the same fatigability, as well as recovery, as that of wild-type control muscle (data not shown).
Fig. 6.
Fatigability and recovery of mXinα-null and wild-type mouse EDL muscles. During isometric tetanic contractions in situ, force data were sampled every 15 s to plot the curve of fatigability. Shown as the percentage of the maximum force before fatigue, tetanic force decreased rapidly during the 150-s fatigue protocol (A) with an ∼85–90% recovery within 20 min (B). EDL muscle of mXinα-null (mXinα−/−) mice showed less fatigability and better recovery than that of wild-type muscle (*P < 0.05; **P < 0.01 as tested using two-way ANOVA). Values are presented as means ± SE; n = 3 mice each in wild-type and mXinα−/− groups.
mXinα-null EDL muscle exhibited minimized fast TnT fragmentation.
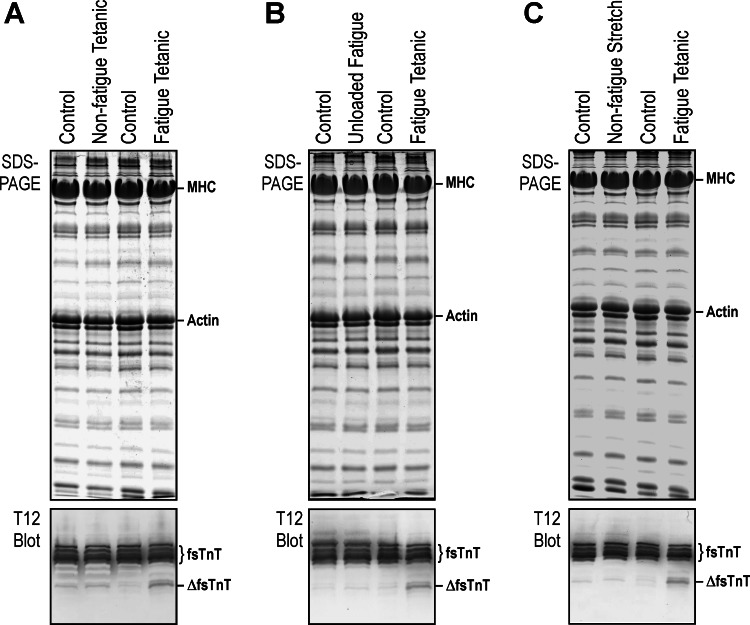
Western blot analysis detected a fragment of fast skeletal muscle TnT (fsTnT) in wild-type mouse EDL muscles, which increased after loaded fatigue contractions (Fig. 7). In contrast, no significant production of the fast TnT fragment after loaded nonfatigue contractions (Fig. 7A) or unloaded fatigue contractions (Fig. 7B). Stretch-loaded nonfatigue contractions did not increase the level of fast TnT fragmentation either (Fig. 7C).
Fig. 7.
Upregulated fragmentation of fast skeletal muscle troponin T (TnT) in EDL muscle after loaded fatigue contractions. Contraction of left EDL muscle was induced in situ with electrical stimulation while the right EDL muscle received no treatment as the resting control. The lower tendon was attached to the force transducer for the measuring of loaded contractions or cut free as an unloaded contraction control. A: EDL muscle treated with loaded tetanic isometric contractions (200 ms/min for 40 min) or loaded fatigue contractions (200 ms/s for 150 s). mAb T12 Western blot detected a fragmentation of fast TnT (ΔfsTnT) after loaded fatigue contractions but not after loaded nonfatigue tetanic contractions. B: fatigue protocol was performed on loaded and unloaded EDL muscles. Western blot showed no change in EDL muscle after unloaded fatigue contractions. C: stretch-loaded protocol was tested with 200 ms/min nonfatigue tetanic contractions for 80 min. Western blot showed no increase of fast TnT fragmentation in EDL after stretch-loaded nonfatigue contractions compared with the resting control. MHC, myosin heavy chain.
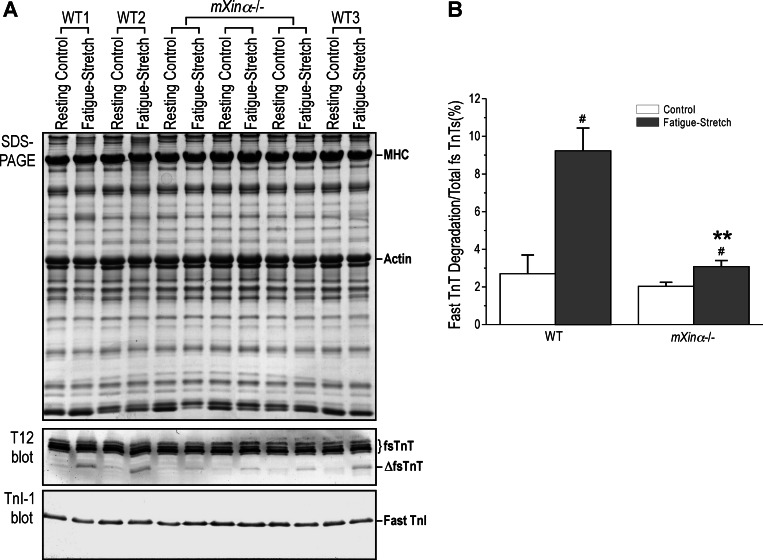
SDS-PAGE showed no apparent change of protein profile in mXinα-null EDL muscle compared with the wild-type control (Fig. 8A). The fast TnT fragmentation in wild-type EDL muscles reflecting an injury after loaded fatigue contractions was not detected in EDL muscle of the resting leg. No fragmentation was found for TnI, another subunit of the troponin complex (Fig. 8A).
Fig. 8.
EDL muscle of mXinα-null mice had minimized fast TnT fragmentation during loaded fatigue contractions. A: SDS-PAGE and Western blot of EDL muscles from the testing and control legs of mXinα-null (mXinα−/−) and wild-type (WT) mice showed that the fragmentation of fast TnT detected by mAb T12 in muscles undergone loaded fatigue contractions compared with that in the control muscle. The level of fast TnT fragmentation was significantly lower in mXinα-null EDL muscle than that in wild-type muscle. No degradation of TnI was detected in the Western blot using mAb TnI-1. B: densitometry analysis quantified the difference and statistical significance of the reduced level of fast TnT fragmentation in mXinα-null EDL muscle during loaded fatigue contraction was examined using Student's t-test. Values are presented as means ± SE; n = 3 mice each in wild-type and mXinα-null groups. **P < 0.01 vs. wild-type; #P < 0.05 vs. resting control.
In comparison with the wild-type control, mXinα-null mouse EDL muscle had a significantly lower level of fast TnT fragmentation (Fig. 8A). The results were quantified in Fig. 8B, and the data suggest that the lack of mXinα in MTJ altered force transduction and minimized the stretch injury. In contrast to the fast fiber muscle EDL, the fast TnT fragmentation was minimum in wild-type mouse soleus muscle (slow/oxidative fiber) after the loaded fatigue protocol (data not shown), possibly due to the lower number of fast muscle fibers present in slow type muscle or the different tissue environment.
DISCUSSION
The vast majority of both mXinα and mXinβ was localized to the MTJ and the contact sites between muscle and perimysium. mXinα but not mXinβ was found in the blood vessel walls in the muscle tissues. Similar blood vessel localization of mXinα was observed in mouse heart (data not shown). It was previously reported by Otten et al. (43) that a transiently increased perivascular fibrosis occurred in mXinα knockout mouse hearts. Our mXinα knockout mouse hearts and skeletal muscles did not show any detectable fibrosis phenotype. This discrepancy may be due to the different knockout alleles used. Nonetheless, the function of mXinα in the blood vessel walls merits further investigation.
In mXinα-null mice at 5.5 to 13.5 mo of age, there were subtle histological and molecular changes in most muscles examined (data not shown). Hypertrophy was only detected in mXinα-null diaphragm fibers. Neither the relative expression level of mXinα in diaphragm muscle (data not shown) nor the subtle changes in myofibrillar proteins in mXinα-null muscle (data not shown) could account for the hypertrophy of diaphragm muscle. Previously, we reported that mXinα-null hearts exhibited defects in intercalated discs between 1 and 3 mo of age. During aging, they developed progressive cardiac hypertrophy and cardiomyopathy (20). Therefore, the continuous rhythmic contractions of diaphragm muscle analogous to that of the cardiac muscle may correlate to the development of hypertrophy in these muscles of mXinα-null mice.
MTJ is an essential structure for the function of skeletal muscle. It transduces forces during muscle contraction and stretch. The presence of the vast majority of both isoforms of mXin in MTJ may suggest a role in the structural integrity as that seen for mXin proteins in the formation of intercalated discs and heart chambers. The evolutionarily more conserved mXinβ is essential for initiating the formation and maturation of intercalated discs (19, 60–62). mXinβ-null mice die postnatally with cardiac chamber defects (61). During the second week postnatal, upregulated mXinβ colocalized with N-cadherin puncta to form bigger aggregates along the developing intercalated discs between cardiomyocytes (60). In this regard, we also observed similar mXinβ aggregates along the MTJ of mXinα-null muscles (data not shown), suggesting an analogous mechanism for the MTJ formation. The presence of mXinβ in mXinα-null muscle may account for its ability to form MTJs and to maintain functionality.
The potential role of mXinα in the mechanical property of MTJ may also be of functional significance. When twitch and tetanic contractions were compared between wild-type and mXinα-null EDL muscles, there was no significant difference in baseline contractile parameters (Table 2). However, during isometric tetanic contraction, mXinα-null EDL muscles fatigued slower and recovered from fatigue faster (Fig. 6). The results suggest that the deletion of mXinα at the MTJ may have caused a functional change in transducing contractile forces.
Our coimmunoprecipitation results suggest that mXinα can form a complex with filamin, vinculin, metavinculin, and talin. Both filamin and talin are known to bind the β1-subunit of integrin (18, 23, 37, 53, 56). α7β1-Integrin is a transmembrane structural protein concentrated at the MTJ, linking to actin cytoskeleton through talin/vinculin. Alterations in compliance have been observed in α7-integrin (Itga7) knockout mouse muscle (38), consistent with the possibility of the α7β1-integrin being a load-bearing protein. In our previous yeast two-hybrid study, we showed that mXinα interacted with vinculin and filamin (10). Therefore, it is likely that mXinα associates with filamin, vinculin, and talin at MTJ and plays structural roles and mXinα-null MTJ may have an altered mechanical compliance.
We previously demonstrated that in cardiac muscle pressure overload induced a restrictive proteolytic truncation of cardiac TnT (13). Since pressure overload increases the resistance to the ejection force of the ventricle and thus applies resistance to the shortening of activated cardiac muscle, this stress condition may be considered analogous to that in the loaded fatigue contraction of skeletal muscle. Therefore, the fragmentation of fast skeletal muscle TnT in EDL muscle during loaded fatigue contractions may be a physiological adaptation to this stress condition rather than a simple sign of muscle injury.
The ubiquitin-proteasome system is not anticipated to have a major change here since the outcome of reduced fast TnT degradation in Xinα−/− mouse EDL muscle is a highly selective response, rather different from the universal housekeeping function of the ubiquitin-proteasome activity (39).
μ-Calpain associated with the myofilaments contributes to the production of cardiac TnT truncation in the heart (65). Since the level of free Ca2+ naturally fluctuates during contraction and relaxation in striated muscle cells, the peak concentration of cytosolic Ca2+ would be sufficient to activate μ-calpain. Therefore, we proposed that induction of the restrictive fragmentation of cardiac TnT in pressure overload of cardiac muscle is most likely regulated by the substrate sensitivity to the protease rather than by increasing μ-calpain activity. This mechanism may guide future investigations on the production of fast TnT fragmentation in skeletal muscle cells during loaded fatigue contraction, in which high loads on myofibrils during activated contraction may alter the molecular conformation of TnT and increase the sensitivity to proteases.
It is known that the truncated cardiac TnT remains in the myofilaments (65) to have a functional effect on decreasing the contractile velocity, which is energetically compensatory (14). A decrease in contractility due to such truncation of fast skeletal muscle TnT could also be protective in the cases of loaded fatigue contractions in skeletal muscle, whereas future structural and functional characterizations of the fast TnT fragmentation will verify this hypothesis.
In summary, our study documented detailed information for the location of mXinα in MTJs and its association with other cell junction proteins. The implication of the role of Xinα in the structure and compliance of the MTJ is supported by the finding that the mXinα-null mouse EDL muscles had higher tolerance to fatigue and a protective phenotype during loaded fatigue contractions as shown by the significantly reduced fragmentation of fast TnT. The location and function of mXinα in skeletal muscle MTJ suggest that it is an interesting target to understand muscle function and injury. The physiological and pathophysiological significance of mXinα in skeletal muscle function and adaptation merits further investigation.
GRANTS
This study was supported in part by National Institutes of Health Grants AR-048816, HL-086720, and HL-098945 (to J.-P. Jin) and HL-107383 (to J. J. Lin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.-Z.F., Q.W., R.S.R., and J.L.L. performed experiments; H.-Z.F. analyzed data; H.-Z.F., J.J.L., and J.-P.J. interpreted results of experiments; H.-Z.F., J.J.L., and J.-P.J. prepared figures; H.-Z.F., J.J.L., and J.-P.J. drafted manuscript; H.-Z.F., J.J.L., and J.-P.J. edited and revised manuscript; H.-Z.F., Q.W., R.S.R., J.L.L., J.J.L., and J.-P.J. approved final version of manuscript; J.J.L. and J.-P.J. conception and design of research.
ACKNOWLEDGMENTS
We thank Geoff Cady and Hui Wang for technical assistance.
REFERENCES
- 1. Bozyczko D, Decker C, Muschler J, Horwitz AF. Integrin on developing and adult skeletal muscle. Exp Cell Res 183: 72–91, 1989 [DOI] [PubMed] [Google Scholar]
- 2. Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol 404: 71–82, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brooks SV, Faulkner JA. The magnitude of the initial injury induced by stretches of maximally activated muscle fibres of mice and rats increases in old age. J Physiol 497: 573–580, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burkin DJ, Kaufman SJ. The alpha7beta1 integrin in muscle development and disease. Cell Tissue Res 296: 183–190, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Burridge K, Mangeat P. An interaction between vinculin and talin. Nature 308: 744–746, 1984 [DOI] [PubMed] [Google Scholar]
- 6. Chan FC, Cheng CP, Wu KH, Chen YC, Hsu CH, Gustafson-Wagner EA, Lin JL, Wang Q, Lin JJ, Lin CI. Intercalated disc-associated protein, mXin-alpha, influences surface expression of ITO currents in ventricular myocytes. Front Biosci 3: 1425–1442, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang WJ, Iannaccone ST, Lau KS, Masters BS, McCabe TJ, McMillan K, Padre RC, Spencer MJ, Tidball JG, Stull JT. Neuronal nitric oxide synthase and dystrophin-deficient muscular dystrophy. Proc Natl Acad Sci USA 93: 9142–9147, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Charrasse S, Meriane M, Comunale F, Blangy A, Gauthier-Rouviere C. N-cadherin-dependent cell-cell contact regulates Rho GTPases and beta-catenin localization in mouse C2C12 myoblasts. J Cell Biol 158: 953–965, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Q, Sealock R, Peng HB. A protein homologous to the Torpedo postsynaptic 58K protein is present at the myotendinous junction. J Cell Biol 110: 2061–2071, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi S, Gustafson-Wagner EA, Wang Q, Harlan SM, Sinn HW, Lin JL, Lin JJ. The intercalated disk protein, mXinalpha, is capable of interacting with beta-catenin and bundling actin filaments [corrected]. J Biol Chem 282: 36024–36036, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crosbie RH, Lebakken CS, Holt KH, Venzke DP, Straub V, Lee JC, Grady RM, Chamberlain JS, Sanes JR, Campbell KP. Membrane targeting and stabilization of sarcospan is mediated by the sarcoglycan subcomplex. J Cell Biol 145: 153–165, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cutts A. The range of sarcomere lengths in the muscles of the human lower limb. J Anat 160: 79–88, 1988 [PMC free article] [PubMed] [Google Scholar]
- 13. Feng HZ, Biesiadecki BJ, Yu ZB, Hossain MM, Jin JP. Restricted N-terminal truncation of cardiac troponin T: a novel mechanism for functional adaptation to energetic crisis. J Physiol 586: 3537–3550, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feng HZ, Chen M, Weinstein LS, Jin JP. Removal of the N-terminal extension of cardiac troponin I as a functional compensation for impaired myocardial beta-adrenergic signaling. J Biol Chem 283: 33384–33393, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frenette J, Cote CH. Modulation of structural protein content of the myotendinous junction following eccentric contractions. Int J Sports Med 21: 313–320, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Gallant C, Appel S, Graceffa P, Leavis P, Lin JJ, Gunning PW, Schevzov G, Chaponnier C, DeGnore J, Lehman W, Morgan KG. Tropomyosin variants describe distinct functional subcellular domains in differentiated vascular smooth muscle cells. Am J Physiol Cell Physiol 300: C1356–C1365, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. George-Weinstein M, Gerhart J, Blitz J, Simak E, Knudsen KA. N-cadherin promotes the commitment and differentiation of skeletal muscle precursor cells. Dev Biol 185: 14–24, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Gontier Y, Taivainen A, Fontao L, Sonnenberg A, van der Flier A, Carpen O, Faulkner G, Borradori L. The Z-disc proteins myotilin and FATZ-1 interact with each other and are connected to the sarcolemma via muscle-specific filamins. J Cell Sci 118: 3739–3749, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Grosskurth SE, Bhattacharya D, Wang Q, Lin JJ. Emergence of Xin demarcates a key innovation in heart evolution. PLoS One 3: e2857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gustafson-Wagner EA, Sinn HW, Chen YL, Wang DZ, Reiter RS, Lin JL, Yang B, Williamson RA, Chen J, Lin CI, Lin JJ. Loss of mXinalpha, an intercalated disk protein, results in cardiac hypertrophy and cardiomyopathy with conduction defects. Am J Physiol Heart Circ Physiol 293: H2680–H2692, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guyon JR, Kudryashova E, Potts A, Dalkilic I, Brosius MA, Thompson TG, Beckmann JS, Kunkel LM, Spencer MJ. Calpain 3 cleaves filamin C and regulates its ability to interact with gamma- and delta-sarcoglycans. Muscle Nerve 28: 472–483, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Hodges BL, Hayashi YK, Nonaka I, Wang W, Arahata K, Kaufman SJ. Altered expression of the alpha7beta1 integrin in human and murine muscular dystrophies. J Cell Sci 110: 2873–2881, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Horwitz A, Duggan K, Buck C, Beckerle MC, Burridge K. Interaction of plasma membrane fibronectin receptor with talin–a transmembrane linkage. Nature 320: 531–533, 1986 [DOI] [PubMed] [Google Scholar]
- 24. Huang HT, Brand OM, Mathew M, Ignatiou C, Ewen EP, McCalmon SA, Naya FJ. Myomaxin is a novel transcriptional target of MEF2A that encodes a Xin-related alpha-actinin-interacting protein. J Biol Chem 281: 39370–39379, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Jin JP, Yang FW, Yu ZB, Ruse CI, Bond M, Chen A. The highly conserved COOH terminus of troponin I forms a Ca2+-modulated allosteric domain in the troponin complex. Biochemistry 40: 2623–2631, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Jorgensen AO, Arnold W, Pepper DR, Kahl SD, Mandel F, Campbell KP. A monoclonal antibody to the Ca2+-ATPase of cardiac sarcoplasmic reticulum cross-reacts with slow type I but not with fast type II canine skeletal muscle fibers: an immunocytochemical and immunochemical study. Cell Motil Cytoskeleton 9: 164–174, 1988 [DOI] [PubMed] [Google Scholar]
- 27. Kaufmann U, Martin B, Link D, Witt K, Zeitler R, Reinhard S, Starzinski-Powitz A. M-cadherin and its sisters in development of striated muscle. Cell Tissue Res 296: 191–198, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Kee AJ, Schevzov G, Nair-Shalliker V, Robinson CS, Vrhovski B, Ghoddusi M, Qiu MR, Lin JJ, Weinberger R, Gunning PW, Hardeman EC. Sorting of a nonmuscle tropomyosin to a novel cytoskeletal compartment in skeletal muscle results in muscular dystrophy. J Cell Biol 166: 685–696, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khurana TS, Watkins SC, Chafey P, Chelly J, Tome FM, Fardeau M, Kaplan JC, Kunkel LM. Immunolocalization and developmental expression of dystrophin related protein in skeletal muscle. Neuromuscul Disord 1: 185–194, 1991 [DOI] [PubMed] [Google Scholar]
- 30. Lai YJ, Huang EY, Yeh HI, Chen YL, Lin JJ, Lin CI. On the mechanisms of arrhythmias in the myocardium of mXinalpha-deficient murine left atrial-pulmonary veins. Life Sci 83: 272–283, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lieber RL, Schmitz MC, Mishra DK, Friden J. Contractile and cellular remodeling in rabbit skeletal muscle after cyclic eccentric contractions. J Appl Physiol 77: 1926–1934, 1994 [DOI] [PubMed] [Google Scholar]
- 32. Lieber RL, Woodburn TM, Friden J. Muscle damage induced by eccentric contractions of 25% strain. J Appl Physiol 70: 2498–2507, 1991 [DOI] [PubMed] [Google Scholar]
- 33. Lin JJ, Gustafson-Wagner EA, Sinn HW, Choi S, Jaacks SM, Wang DZ, Evans S, Li-Chun LJ. Structure, expression, and function of a novel intercalated disc protein, Xin. J Med Sci 25: 215–222, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin JJ, Chou CS, Lin JL. Monoclonal antibodies against chicken tropomyosin isoforms: production, characterization, and application. Hybridoma 4: 223–242, 1985 [DOI] [PubMed] [Google Scholar]
- 35. Lin JJ, Warren KS, Wamboldt DD, Wang T, Lin JL. Tropomyosin isoforms in nonmuscle cells. Int Rev Cytol 170: 1–38, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Lin JJC, Feramisco JR, Blose SH, Matsumura F. Monoclonal antibodies to cytoskeletal proteins. In: Monoclonal Antibodies and Functional Cell Lines: Progress and Applications, edited by Kennett RH, Bechtol KB, McKearn TJ. New York: Plenum, 1984, p. 119–151 [Google Scholar]
- 37. Loo DT, Kanner SB, Aruffo A. Filamin binds to the cytoplasmic domain of the beta1-integrin. Identification of amino acids responsible for this interaction. J Biol Chem 273: 23304–23312, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Lopez MA, Mayer U, Hwang W, Taylor T, Hashmi MA, Jannapureddy SR, Boriek AM. Force transmission, compliance, and viscoelasticity are altered in the α7-integrin-null mouse diaphragm. Am J Physiol Cell Physiol 288: C282–C289, 2005 [DOI] [PubMed] [Google Scholar]
- 39. MacGurn JA, Hsu PC, Emr SD. Ubiquitin and membrane protein turnover: from cradle to grave. Annu Rev Biochem 81: 231–259, 2012 [DOI] [PubMed] [Google Scholar]
- 40. Meriane M, Roux P, Primig M, Fort P, Gauthier-Rouviere C. Critical activities of Rac1 and Cdc42Hs in skeletal myogenesis: antagonistic effects of JNK and p38 pathways. Mol Biol Cell 11: 2513–2528, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nikolaou PK, Macdonald BL, Glisson RR, Seaber AV, Garrett WE., Jr Biomechanical and histological evaluation of muscle after controlled strain injury. Am J Sports Med 15: 9–14, 1987 [DOI] [PubMed] [Google Scholar]
- 42. Ogilvie RW, Armstrong RB, Baird KE, Bottoms CL. Lesions in the rat soleus muscle following eccentrically biased exercise. Am J Anat 182: 335–346, 1988 [DOI] [PubMed] [Google Scholar]
- 43. Otten J, van der Ven PF, Vakeel P, Eulitz S, Kirfel G, Brandau O, Boesl M, Schrickel JW, Linhart M, Hayess K, Naya FJ, Milting H, Meyer R, Furst DO. Complete loss of murine Xin results in a mild cardiac phenotype with altered distribution of intercalated discs. Cardiovasc Res 85: 739–750, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Pacholsky D, Vakeel P, Himmel M, Lowe T, Stradal T, Rottner K, Furst DO, van der Ven PF. Xin repeats define a novel actin-binding motif. J Cell Sci 117: 5257–5268, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Paul AC, Sheard PW, Kaufman SJ, Duxson MJ. Localization of alpha 7 integrins and dystrophin suggests potential for both lateral and longitudinal transmission of tension in large mammalian muscles. Cell Tissue Res 308: 255–265, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Rosenberg P, Esni F, Sjodin A, Larue L, Carlsson L, Gullberg D, Takeichi M, Kemler R, Semb H. A potential role of R-cadherin in striated muscle formation. Dev Biol 187: 55–70, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Schevzov G, Whittaker SP, Fath T, Lin JJ, Gunning PW. Tropomyosin isoforms and reagents. Bioarchitecture 1: 135–164, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shear CR, Bloch RJ. Vinculin in subsarcolemmal densities in chicken skeletal muscle: localization and relationship to intracellular and extracellular structures. J Cell Biol 101: 240–256, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sinn HW, Balsamo J, Lilien J, Lin JJ. Localization of the novel Xin protein to the adherens junction complex in cardiac and skeletal muscle during development. Dev Dyn 225: 1–13, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Soll DR. The use of computers in understanding how animal cells crawl. Int Rev Cytol 8: 439–454, 1995 [PubMed] [Google Scholar]
- 51. Soll DR, Voss E. Two and three-dimensional computer systems for analyzing how cells crawl. In: Motion Analysis of Living Cells, edited by Soll D, Wessels D. New York: Wiley-Liss, 1998, p. 25–52 [Google Scholar]
- 52. Speer KP, Lohnes J, Garrett WE., Jr Radiographic imaging of muscle strain injury. Am J Sports Med 21: 89–95; discussion 96, 1993 [DOI] [PubMed] [Google Scholar]
- 53. Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. Talin binding to integrin beta tails: a final common step in integrin activation. Science 302: 103–106, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Thompson TG, Chan YM, Hack AA, Brosius M, Rajala M, Lidov HG, McNally EM, Watkins S, Kunkel LM. Filamin 2 (FLN2): a muscle-specific sarcoglycan interacting protein. J Cell Biol 148: 115–126, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tidball JG, O'Halloran T, Burridge K. Talin at myotendinous junctions. J Cell Biol 103: 1465–1472, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. van der Flier A, Kuikman I, Kramer D, Geerts D, Kreft M, Takafuta T, Shapiro SS, Sonnenberg A. Different splice variants of filamin-B affect myogenesis, subcellular distribution, and determine binding to integrin β subunits. J Cell Biol 156: 361–376, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van der Ven PF, Ehler E, Vakeel P, Eulitz S, Schenk JA, Milting H, Micheel B, Furst DO. Unusual splicing events result in distinct Xin isoforms that associate differentially with filamin c and Mena/VASP. Exp Cell Res 312: 2154–2167, 2006 [DOI] [PubMed] [Google Scholar]
- 58. Vlahovich N, Schevzov G, Nair-Shaliker V, Ilkovski B, Artap ST, Joya JE, Kee AJ, North KN, Gunning PW, Hardeman EC. Tropomyosin 4 defines novel filaments in skeletal muscle associated with muscle remodelling/regeneration in normal and diseased muscle. Cell Motil Cytoskeleton 65: 73–85, 2008 [DOI] [PubMed] [Google Scholar]
- 59. Wang DZ, Reiter RS, Lin JL, Wang Q, Williams HS, Krob SL, Schultheiss TM, Evans S, Lin JJ. Requirement of a novel gene, Xin, in cardiac morphogenesis. Development 126: 1281–1294, 1999 [DOI] [PubMed] [Google Scholar]
- 60. Wang Q, Lin JL, Chan SY, Lin JJ. The Xin repeat-containing protein, mXinbeta, initiates the maturation of the intercalated discs during postnatal heart development. Dev Biol 374: 264–280, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang Q, Lin JL, Reinking BE, Feng HZ, Chan FC, Lin CI, Jin JP, Gustafson-Wagner EA, Scholz TD, Yang B, Lin JJ. Essential roles of an intercalated disc protein, mXinbeta, in postnatal heart growth and survival. Circ Res 106: 1468–1478, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang Q, Lin JL, Wu KH, Wang DZ, Reiter RS, Sinn HW, Lin CI, Lin JJC. Xin proteins and intercalated disc maturation, signaling and diseases. Front Biosci 17: 2566–2593, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Warren KS, Lin JL, McDermott JP, Lin JJ. Forced expression of chimeric human fibroblast tropomyosin mutants affects cytokinesis. J Cell Biol 129: 697–708, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zeschnigk M, Kozian D, Kuch C, Schmoll M, Starzinski-Powitz A. Involvement of M-cadherin in terminal differentiation of skeletal muscle cells. J Cell Sci 108: 2973–2981, 1995 [DOI] [PubMed] [Google Scholar]
- 65. Zhang Z, Biesiadecki BJ, Jin JP. Selective deletion of the NH2-terminal variable region of cardiac troponin T in ischemia reperfusion by myofibril-associated mu-calpain cleavage. Biochemistry 45: 11681–11694, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]