Abstract
Minocycline is a second-generation, semi-synthetic tetracycline that has been in therapeutic use for over 30 years because of its antibiotic properties against both gram-positive and gram-negative bacteria. It is mainly used in the treatment of acne vulgaris and some sexually transmitted diseases. Recently, it has been reported that tetracyclines can exert a variety of biological actions that are independent of their anti-microbial activity, including anti-inflammatory and anti-apoptotic activities, and inhibition of proteolysis, angiogenesis and tumour metastasis. These findings specifically concern to minocycline as it has recently been found to have multiple non-antibiotic biological effects that are beneficial in experimental models of various diseases with an inflammatory basis, including dermatitis, periodontitis, atherosclerosis and autoimmune disorders such as rheumatoid arthritis and inflammatory bowel disease. Of note, minocycline has also emerged as the most effective tetracycline derivative at providing neuroprotection. This effect has been confirmed in experimental models of ischaemia, traumatic brain injury and neuropathic pain, and of several neurodegenerative conditions including Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis, Alzheimer's disease, multiple sclerosis and spinal cord injury. Moreover, other pre-clinical studies have shown its ability to inhibit malignant cell growth and activation and replication of human immunodeficiency virus, and to prevent bone resorption. Considering the above-mentioned findings, this review will cover the most important topics in the pharmacology of minocycline to date, supporting its evaluation as a new therapeutic approach for many of the diseases described herein.
Keywords: minocycline, tetracyclines, antibiotic, anti-inflammatory, immunomodulatory, neuroprotection
Introduction
Tetracyclines are bacteriostatic antibiotics that are considered broad-spectrum antibiotics because they are active against a wide range of aerobic and anaerobic gram-positive and gram-negative bacteria, and against other microorganisms, including Rickettsia, Chlamydia and Plasmodium spp., and Mycoplasma pneumoniae (Figure 1). The mechanism of action behind the antibiotic properties of tetracyclines is mainly related to their ability to bind to the bacterial 30S ribosomal subunit and inhibit protein synthesis. In an attempt to improve their efficacy, various structural changes have been employed, for example, ring D modification through carbons 7–9, which is the basis for the higher efficacy obtained with the semi-synthetic compounds minocycline and doxycycline (Nelson, 1998). Minocycline (7-dimethylamino-6-dimethyl-6-deoxytetracycline) is a second-generation, semi-synthetic tetracycline analogue that has been used for over 30 years (Yong et al., 2004). It retains the efficacy against both gram-positive and gram-negative bacteria and has been approved by the Medicines and Healthcare Products Regulatory Agency for the treatment of acne vulgaris, and by the US Food and Drug Administration (FDA) for the treatment of some sexually transmitted diseases and rheumatoid arthritis (Good and Hussey, 2003; Blum et al., 2004). Minocycline shows a better pharmacokinetic profile than the first-generation tetracyclines when used orally, being rapidly and completely absorbed, even in elderly populations, with a longer half-life and excellent tissue penetration, and an almost complete bioavailability (Barza et al., 1975; Kramer et al., 1978; Klein and Cunha, 1995). In addition, it is a highly lipophilic molecule that can easily pass through the blood–brain barrier (Brogden et al., 1975), thus promoting its accumulation in cells of the CSF and CNS (Aronson, 1980; Yrjänheikki et al., 1999; Kielian et al., 2007) and enabling its use in the treatment of many CNS diseases (Saivin and Houin, 1988; Wang et al., 2003; Yong et al., 2004). Moreover, minocycline has a good safety record when used chronically. Long-term treatment with minocycline at dosages of up to 200 mg·day−1, the highest dosage recommended by the US FDA, is generally safe and well-tolerated in humans. Minocycline's known and most common side effects, including nausea, vertigo and mild dizziness, occur mainly early after its administration and disappear shortly following therapy discontinuation. However, according to the British National Formulary, for treatments continued for more than 6 months, it is recommended to monitor every 3 months for hepatotoxicity, pigmentation and systemic lupus erythematosus, and it has been advised that treatment should be discontinued if these develop or if pre-existing systemic lupus erythematosus worsens. The higher risk of lupus-erythematosus-like syndrome and irreversible pigmentation associated with minocycline in comparison with other tetracyclines has limited its extensive use in human infections, being currently indicated just for the treatment of acne vulgaris (Williams et al., 1974; Klein and Cunha, 1995).
Figure 1.
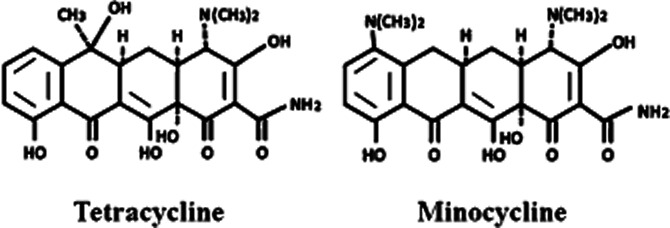
Chemical structure of minocycline and its parent, tetracycline.
The antibiotic properties of tetracyclines were initially described in the late 1940s; but more recently, numerous studies have focused on their non-antibiotic properties. In fact, it has been reported that tetracyclines can exert a variety of biological actions that are independent of their anti-microbial activity, including anti-inflammatory and anti-apoptotic activities, and inhibitory effects on proteolysis, angiogenesis and tumour metastasis (Golub et al., 1991; 1992; Greenwald and Golub, 1993; Sapadin and Fleischmajer, 2006). This is the case for minocycline (Zemke and Majid, 2007; Kielian et al., 2007), as it has recently been considered beneficial for diseases with an inflammatory basis, including rosacea, bullous dermatoses, neutrophilic diseases, pyoderma gangrenosum, sarcoidosis, aortic aneurysms, cancer metastasis, periodontitis and autoimmune disorders such as rheumatoid arthritis and scleroderma (reviewed in Sapadin and Fleischmajer, 2006; Soory, 2008; Griffin et al., 2010). Minocycline has also emerged as the most effective tetracycline derivative regarding neuroprotection, an effect that has been confirmed in experimental models of ischaemia (Yrjänheikki et al., 1998; 1999; Koistinaho et al., 2005), traumatic brain injury (Sanchez Mejia et al., 2001) and neuropathic pain (Raghavendra et al., 2003; Mei et al., 2011), and of several neurodegenerative conditions such as Parkinson's (Du et al., 2001; Thomas and Le, 2004; Abdel-Salam, 2008) and Huntington's (Chen et al., 2000; Thomas et al., 2004) diseases, amyotrophic lateral sclerosis (Zhu et al., 2002), Alzheimer's disease (Choi et al., 2007), multiple sclerosis (Brundula et al., 2002; Nessler et al., 2002) and spinal cord injury (SCI) (Golub et al., 1991; Yong et al., 2004; Festoff et al., 2006). These pre-clinical studies have prompted the evaluation of minocycline in clinical trials in patients with neuronal disease, where it has shown promising neuroprotective properties (Lampl et al., 2007). Moreover, the growing interest in minocycline has led to evaluations of its therapeutic efficacy in many other experimental disease models, such as inflammatory bowel disease (Huang et al., 2009b; Garrido-Mesa et al., 2011a,b), diabetes (Bain et al., 1997; Wang et al., 2003; Cai et al., 2011), fragile X syndrome (FXS) (Paribello et al., 2010), cardiac ischaemia (Scarabelli et al., 2004) and human immunodeficiency virus (HIV) infection (Szeto et al., 2010; Campbell et al., 2011) (Figure 2).
Figure 2.
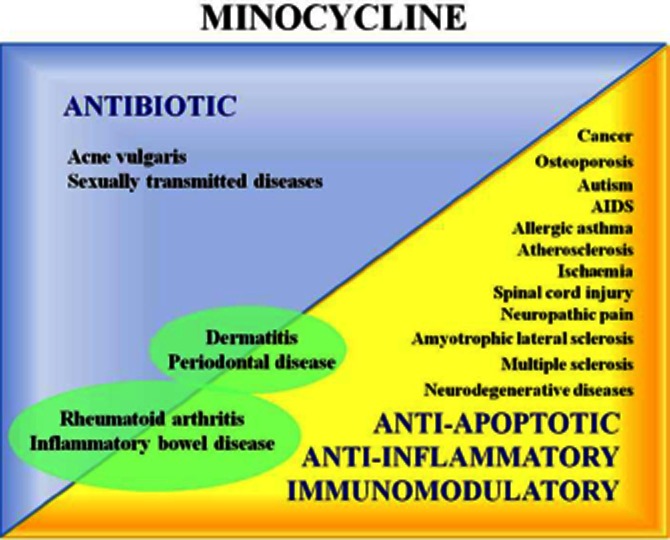
Clinical potential of minocycline due to its antibiotic activity; its anti-apoptotic, anti-inflammatory and immunomodulatory properties; and the association of both its non-antibiotic and anti-microbial effects.
Many of these studies have proposed the mechanisms that may be involved in minocycline's anti-inflammatory, immunomodulatory and neuroprotective effects. These include (i) inhibitory effects on the activities of key enzymes, like iNOS (Amin et al., 1997), MMPs (Golub et al., 1991) and PLA2 (Pruzanski et al., 1992); (ii) reduction of protein tyrosine nitration because of its peroxynitrite-scavenging properties (Whiteman and Halliwell, 1997); (iii) inhibition of caspase-1 and caspase-3 activation (Chen et al., 2000); (iv) enhancement of Bcl-2-derived effects, thus protecting the cells against apoptosis (Wang et al., 2003; Domercq and Matute, 2004; Jordan et al., 2007); (v) reduction of p38 MAPK phosphorylation (Corbacella et al., 2004); and (vi) inhibition of PARP-1 activity (Alano et al., 2006). Tetracyclines' well-known ability of binding to Ca2+ and Mg2+ may account for some of these biological activities via the chelation of these cations and their transport into intracellular compartments (White and Pearce, 1982) (Figure 3).
Figure 3.
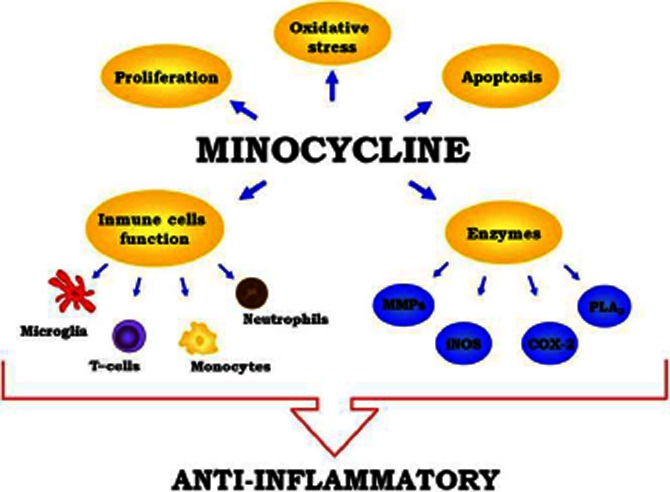
Mechanisms involved in the anti-inflammatory activity of minocycline: inhibitory effects on enzyme activities, like iNOS, MMPs, COX-2 or PLA2; inhibition of apoptosis, through the inhibition of caspase-1 and caspase-3 activation and the enhancement of Bcl-2-derived effects; antioxidant properties and inhibition of immune cell activation and proliferation.
In this review, we aim to summarize the effects reported for minocycline regarding its non-antibiotic properties in different experimental models, thereby supporting its evaluation as a new therapeutic approach for diseases associated with a deregulated immune response. This compound may have additional value in conditions in which an altered immune response and a microbial aetiology are involved, considering its ability to combine both immunomodulatory and anti-microbial properties.
Effects of minocycline on dermatitis
As tetracyclines, including minocycline, have been reported to reduce neutrophil chemotaxis (Esterly et al., 1978; 1984), different studies have evaluated their effectiveness in cutaneous inflammation. Ishikawa et al. (2009) reported that minocycline, at concentrations similar to those obtained therapeutically in serum (5 or 10 μM) (Agwuh and MacGowan, 2006), reduced the protease-activated receptor 2-mediated production of IL-8, and thus attenuated the pro-inflammatory process in epidermal keratinocytes. They proposed that the ability of tetracyclines to chelate Ca2+ may contribute to these effects, as protease-activated receptor 2 activation transiently increases intracellular Ca2+ levels in keratinocytes, triggering the downstream binding of NF-κB to DNA (Macfarlane et al., 2005; Stefansson et al., 2008). Open clinical studies have confirmed minocycline's efficacy in non-infectious forms of dermatitis (Humbert et al., 1991), supporting its clinical use in various skin disorders, such as inflammatory acne, rosacea, bullous dermatoses and neutrophilic dermatoses (Sapadin and Fleischmajer, 2006). Maintenance of remission in some of these conditions frequently required concomitant administration of corticosteroids; however, most of these reports were generally non-placebo-controlled or uncontrolled studies, and included small number of patients. Therefore, further evaluation of larger numbers of patients in randomized, well-controlled studies is still necessary. Finally, although minocycline is recommended for the aforementioned skin disorders, current therapy has moved towards the use of doxycycline, which has a similar efficacy for the treatment of these conditions with a reduced incidence of adverse effects, such as drug hypersensitivity syndrome, hyperpigmentation and dizziness, frequently associated with minocycline therapy (Bachelez et al., 2001; Sapadin and Fleischmajer, 2006; Korting and Schöllmann, 2009).
Effects of minocycline on periodontal disease
The pharmacological profile of tetracyclines, which combines anti-microbial with anti-inflammatory and anti-apoptotic properties, makes them suitable for periodontal disease treatment, which is characterized by an inflammatory process in addition to its well-known microbial aetiology (Soory, 2008). At the levels conventionally detected in the plasma and gingival crevicular fluid, minocycline causes a significant stimulation of osteoblastic cells, whereas long-term exposure of these cells to tetracyclines results in a proportional increase in the mineralized bone matrix (Gomes and Fernandes, 2007). These effects are achieved without affecting the survival and protein expression of human gingival fibroblasts, epithelial cells and periodontal ligament fibroblasts (Suzuki et al., 2006). Taken together with their anti-microbial activity, these effects may explain the efficacy, in particular of minocycline, in reducing disease progression and promoting periodontal healing when administered at doses of 100–200 mg·day−1 for 7–14 days (Kirkwood et al., 2007; Basegmez et al., 2011).
Effects on minocycline on rheumatoid arthritis
Because of the anti-collagenase activity of minocycline initially described by Golub et al. (1983), and later confirmed by Greenwald et al. (1987), in human rheumatoid tissue and synovial cultures, many studies over the last four decades have focused on its effects in rheumatoid arthritis. In the adjuvant arthritis model of rheumatoid arthritis, tetracyclines reduced collagenase activity in the inflamed tissue, although they did not show anti-inflammatory effects in rats (Greenwald et al., 1992). When combined with non-steroidal anti-inflammatory drugs in the same study, tetracyclines had a synergistic effect, as evidenced by a total inhibition of degradative enzyme activity and a normalization of radiological bone damage. Similarly, they were shown to inhibit the synthesis and/or activity of cartilage proteinases both in vitro and in vivo (Arsenis et al., 1992). In another study, Zernicke et al. (1997) reported that tetracycline derivatives could reverse the deleterious effects of adjuvant disease on the mechanical strength of the femur in rats. These studies led to clinical trials with different tetracyclines, including minocycline. Initially, two double-blind, placebo-controlled trials were conducted: one by Kloppenburg et al. (1994) and the minocycline in rheumatoid arthritis trial (Tilley et al., 1995). The former demonstrated that minocycline had clinically useful anti-inflammatory properties in patients with rheumatoid arthritis and was superior to the placebo, whereas no clear conclusions could be drawn from the latter. A few years later, Langevitz et al. (2000) summarized the results of two previous open trials and three double-blind controlled studies. They concluded that minocycline might be beneficial in patients with rheumatoid arthritis, especially when administered early in the course of the disease or in patients with only mild disease, showing beneficial effects with respect to joint swelling and/or tenderness, laboratory parameters and patient assessments. A meta-analysis from 2003 that summarized the results from the clinical trials conducted until 2002 confirmed these beneficial effects (Stone et al., 2003). Despite these promising results and the FDA approval of semi-synthetic tetracyclines for rheumatoid arthritis, in a recent review, Greenwald (2011) acknowledged that the weak anti-inflammatory properties of tetracyclines are easily surpassed by many other agents. Nevertheless, the potential of tetracyclines in osteoarthritis treatment still seems attractive and the in vitro inhibition of cartilage degradation by minocycline presents a solid rationale for forward progress.
Effects of minocycline on CNS pathologies
As previously mentioned, minocycline, because of its high lipid solubility (Good and Hussey, 2003), easily crosses the blood–brain barrier (Brogden et al., 1975; Saivin and Houin, 1988; Yong et al., 2004), and in recent years, has been shown to be beneficial in animal models of CNS diseases. Many of these studies were initially based on minocycline's ability to inhibit microglia activation, a process that has deleterious effects on neurogenesis and neuronal survival, which would justify its potential effectiveness in the treatment of neuroinflammatory and/or neurodegenerative disorders (Yrjänheikki et al., 1998; Chen et al., 2000; Du et al., 2001; Zhu et al., 2002; Metz et al., 2004; Tomás-Camardiel et al., 2004). In fact, different in vitro studies have described minocycline's ability to block LPS-stimulated inflammatory cytokine secretion and Toll-like-receptor (TLR)-2 surface expression in the BV-2 microglia-derived cell line and on microglia isolated from the brains of adult mice. Minocycline also attenuated the mRNA expression of inflammatory genes, including IL-6, IL-1β, major histocompatibility complex (MHC) II and TLR-2 (Nikodemova et al., 2006; Henry et al., 2008).
Minocycline's ability to mitigate cytokine expression in the brain during systemic inflammatory events may be useful in preventing cognitive and behavioural deficits. Indeed, minocycline attenuates sickness behaviour and anhedonia associated with LPS-induced neuroinflammation, in parallel with a decrease in neuroinflammatory markers in the hippocampus and cortex and in indolamine 2,3-dioxygenase (IDO) mRNA expression (Henry et al., 2008). These data are consistent with those from another report showing a causal relationship between IDO activity and acute depressive effects in adult CD-1 mice. In that report, minocycline's ability to block IDO induction prevented depressive-like immobility (O'Connor et al., 2009). In addition, the authors observed that, while minocycline pre-treatment attenuated LPS-induced brain IL-1β production, it had no effect on plasma IL-1β levels, suggesting that minocycline has anti-inflammatory effects within the brain, which accounts for the recovery from sickness and the reduction in the frequency of neurobehavioural complications.
Consistent with its anti-inflammatory properties, minocycline has been reported to act as a neuroprotective agent in models of both global and focal ischaemia, processes driven by the infiltration of the ischaemic brain area by inflammatory cells (Feuerstein et al., 1997; Koistinaho and Hökfelt, 1997). In a gerbil model of forebrain ischaemia, minocycline prevented microglial activation, reducing the infarct size and increasing the survival of hippocampal neurons, even when treatment began after the ischaemic insult. These effects were accompanied by a reduction of IL-1β-converting enzyme, COX-2 and iNOS mRNA levels in the affected brain regions (Yrjänheikki et al., 1998; 1999). Koistinaho et al. (2005) showed that this effect of minocycline seemed to be MMP-dependent, as this compound protected against permanent cerebral ischaemia in wild-type mice, but not in MMP-9-deficient mice. Moreover, Park et al. (2011) reported that minocycline, similar to other MMP inhibitors, was effective in treating neuroinflammation following experimental photothrombotic cortical ischaemia. In those studies, both pre- and post-ischaemic minocycline treatment significantly reduced the infarct size and the expression of neuroinflammatory mediators in the ischaemic cortex, confirming previous reports (Romanic et al., 1998; Koistinaho et al., 2005) and clearly attributing this effect to MMP inhibition.
Reduction in the expression of inflammatory mediators within the brain after minocycline treatment has been repeatedly reported in pre-clinical studies, accounting for its inhibitory effects on infarct size in ischaemia models and positive effects on behavioural complications associated with neuroinflammatory processes. Given these promising experimental results, translational research is required to provide new insights into the usefulness of minocycline in these CNS pathologies.
The potential efficacy of minocycline in the treatment of Parkinson's, Alzheimer's and Huntington's diseases has been proposed. Chen et al. (2000) evaluated minocycline's effects in the transgenic R6/2 mouse model of Huntington's disease and reported that minocycline delayed disease progression and mortality. The mechanism was determined to be inhibition of caspase-1 and caspase-3 expression, and reduction of iNOS activation, preventing the detrimental effect that these enzymes exert in Huntington's disease (Ona et al., 1999). Moreover, minocycline-treated mice showed significantly inhibited generation of the endogenous Huntington cleavage fragment and lower mature IL-1β levels in the brain. The anti-inflammatory effects of minocycline may also account for the beneficial effects observed in both in vitro and in animal models of Parkinson's disease. Minocycline was found to prevent nigrostriatal dopaminergic neurodegeneration in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease, an effect related to the prevention of dopamine depletion in the striatum and nucleus accumbens, and is associated with marked reductions in iNOS and caspase-1 expression (Du et al., 2001). In a different study, minocycline's ability to protect nigrostriatal dopaminergic neurons was related to reduced MPTP-induced activation of microglia, inhibition of mature IL-1β formation, and NADPH oxidase and iNOS activation (Wu et al., 2002). In addition, in vitro studies using primary cultures of mesencephalic and cerebellar granule neurons (CGN) and glia confirmed that minocycline inhibited 1-methyl-4-phenylpyridinium (MPP+)-mediated iNOS expression and NO-induced neurotoxicity. This effect was related to the inhibition of p38 MAPK activation in CGN (Du et al., 2001). Together, these results suggest that minocycline blocks MPTP neurotoxicity in vivo by indirectly inhibiting MPTP/MPP+-induced glial iNOS expression and neurotoxicity, most likely by inhibiting the phosphorylation of p38 MAPK. In contrast to these promising results, minocycline's ineffectiveness and/or deleterious effects have also been reported in studies on animal models of Huntington's (Diguet et al., 2003; 2004a; Smith et al., 2003) and Parkinson's (Yang et al., 2003; Diguet et al., 2004a) diseases. Therefore, minocycline seems to have variable and even contradictory effects in different species and models of these neurological disorders. Could these discrepancies be due to differences in the mode of administration and dose used? Additional experimental work should be undertaken to determine whether minocycline has a neuroprotective effect in Huntington's and Parkinson's diseases before conducting further clinical trials (Diguet et al., 2004b).
In view of the pathogenesis of amyotrophic lateral sclerosis, which has been related to up-regulation of the expression and the increased activity of different pro-inflammatory signals, including caspase-1, caspase-3, iNOS and p38 MAPK (Friedlander et al., 1997; Li et al., 2000), there is also a rationale for minocycline to be beneficial in this disease. Indeed, minocycline delayed disease onset and extended survival in an experimental model of amyotrophic lateral sclerosis-transgenic mice expressing the mutant human SOD1-G93A transgene. In this model, minocycline inhibited mitochondrial permeability transition-mediated cytochrome c release, a mechanism of action that was confirmed in vitro both in cells and in isolated mitochondria (Zhu et al., 2002). Similar findings were reported by Kriz et al. (2002) and Van Den Bosch et al. (2002), who showed that minocycline delayed the onset of motor neuron degeneration and muscle strength decline and increased the longevity of amyotrophic lateral sclerosis mice. Additional in vitro studies revealed that minocycline reduced the apoptosis of cultured neurons from patients with motor neuron diseases, including amyotrophic lateral sclerosis (Tikka et al., 2002). As is the case for Huntington's and Parkinson's diseases, the usefulness of minocycline in this neurological condition remains controversial, however. Despite the promising results from these animal models, a multi-centre, randomized placebo-controlled phase III trial revealed minocycline to have a detrimental effect. Amyotrophic lateral sclerosis patients treated with the drug deteriorated significantly faster than patients in the control group (Gordon et al., 2007).
Pre-clinical studies have confirmed that minocycline's pharmacological profile could be of interest in the treatment of Alzheimer's disease. The first report describing the effects of minocycline in a model of Alzheimer's disease appeared in 2004, describing the drug's beneficial effects in an experimental Alzheimer's disease model induced by i.c.v. injection of μ-p75-saporin in mice. Minocycline ameliorated cholinergic cell loss and reduced the simultaneous activation of microglia and astrocytes that occur after the administration of the immunotoxin, and led to the down-regulation of transcription of pro-inflammatory mediators and mitigation of cognitive impairment (Hunter et al., 2004). Minocycline treatment has also been reported to suppress microglial production of IL-1β, IL-6, TNF and nerve growth factor in amyloid precursor protein (APP) transgenic mice, but did not affect amyloid β peptide (Aβ) deposition in this model (Seabrook et al., 2006). Moreover, Choi et al. (2007) reported that minocycline could attenuate the phosphorylation of eukaryotic initiation translation factor 2 alpha (eIF-2α), which may ultimately impair cognitive functions by decreasing the efficacy of the de novo protein synthesis required for synaptic plasticity (Harding et al., 2000; Chen et al., 2003; Taylor et al., 2005) and caspase-12 activation, in Aβ1–42-treated or C-terminal fragments of APP (APP-CTs)-transfected differentiated PC 12 neuronal cells. The increases in p-eIF2a were also attenuated by minocycline administration in two animal models: Aβ1–42 infused rats and Tg2576 mice, in which minocycline reduced neuronal cell death, improved cognitive impairment and attenuated the deficits in learning and memory (Choi et al., 2007). Similarly, minocycline treatment was able to correct behavioural impairments and lower levels of inflammatory markers and Aβ trimers in an early, pre-plaque inflammatory process in an Alzheimer's disease-like transgenic rat model (Cuello et al., 2010). Aβ accumulation was sufficient to induce cognitive impairment and biochemical alterations in the cerebral cortex and hippocampus in the absence of amyloid plaques, together with up-regulation of pro-inflammatory markers, such as MHC II, iNOS and COX-2, and these responses were successfully arrested by minocycline (Cuello et al., 2010). Thus, minocycline treatment ameliorates the cognitive impairment and deficits in learning and memory that characterize Alzheimer's disease by a variety of actions, and future work must aim to determine its usefulness in patients.
Finally, and more importantly, increasing experimental evidence suggests that minocycline, alone or combined with other drugs, could ameliorate multiple sclerosis severity and progression. This finding has been derived mainly on the basis of the promising results obtained in an established animal model of multiple sclerosis, the experimental autoimmune encephalomyelitis (EAE). In 2002, two different groups described minocycline's ability to attenuate the clinical and histological severity of EAE, an effect associated with decreased inflammation and the inhibition of microglial activation (Brundula et al., 2002; Popovic et al., 2002). Popovic et al. (2002) reported that minocycline administration, following a curative protocol, suppressed ongoing disease activity and limited disease progression in EAE induced in dark agouti rats. Similarly, Brundula et al. (2002) showed that minocycline, most probably because of its ability to inhibit MMP activity, delayed the onset of clinical symptoms and attenuated the severity of the neuroinflammation that occurred in EAE, even when it was administered after the onset of the clinical signs. Later, Nikodemova et al. (2007) reported that minocycline treatment, when started at the onset of the symptoms, considerably decreased the severity of the clinical course of the disease by reducing both lesion number and size. This effect was associated with a reduced macrophage migration from the periphery into the CNS, thus resulting in decreased leukocyte infiltration into the parenchyma of the spinal cord and decreased microglia MHC II expression and proliferation. Moreover, these neuroprotective effects were improved when high minocycline concentrations were locally delivered into the CNS (Xue et al., 2010).
Minocycline was also effective in combination with atorvastatin, a statin previously reported to be effective in attenuating clinical EAE in mice (Youssef et al., 2002). Combination therapy caused greater reduction in disease severity than individual drugs used alone, in both acute and chronic disease phases, along with attenuation of inflammation, demyelination and axonal loss (Luccarini et al., 2008).
The mechanisms responsible for the pharmacological actions of minocycline in EAE are its influence on T-cell activity, its ability to inhibit microglial activation and its neuroprotective effects. In vitro studies have revealed that minocycline inhibited antigen processing for presentation to human T-cells (Kalish and Koujak, 2004), T-cell proliferation and production of inflammatory cytokines (Kloppenburg et al., 1995; 1996), and T-cell transmigration across a fibronectin matrix barrier, most likely via the inhibition of MMPs. In vivo assays have found that this antibiotic promotes immune differentiation from a type 1 helper T-cell (Th1) to a type 2 helper T-cell (Th2) phenotype, thus modulating the susceptibility to EAE (Popovic et al., 2002). Regarding microglial activation, Popovic et al. (2002) found in relapsing-remitting EAE that activated microglia were absent in rats treated with minocycline. Moreover, minocycline inhibited microglia MHC II expression and the subsequent reactivation of T-cells, which resulted in attenuation of the clinical severity of EAE (Nikodemova et al., 2007) and reduced infiltration of T lymphocytes into the CNS parenchyma (Brundula et al., 2002; Popovic et al., 2002). In addition, the direct antioxidant potential of minocycline attenuated reactive oxygen species (ROS)-mediated neuronal and axonal destruction in vitro (Wilkins et al., 2004) and its ability to chelate Ca2+ may prevent the activation of calpains and preserve axonal integrity, as observed in minocycline-treated EAE rats (Stirling, 2004; Yong et al., 2004; Maier et al., 2007). The success of minocycline in multiple sclerosis treatment in experimental models prompted its evaluation in phase I/II clinical trials in humans. These confirmed its beneficial effects and found it to be safe and well-tolerated (Metz et al., 2004; 2009; Zabad et al., 2007). The results revealed that minocycline significantly reduced relapse rates, MRI – active lesions, and local brain atrophy. The trials also showed that the clinical response to minocycline was accompanied by beneficial immune changes that may be desirable in the control of multiple sclerosis. Therefore, of all the neurological conditions considered here, it appears that minocycline has the most potential in the treatment of multiple sclerosis. Strong experimental evidence supports minocycline's ability to reduce the severity and progression of the disease, which has been confirmed by the clinical trials performed to date. Phase III clinical trials should be conducted to determine the drug's potential for multiple sclerosis treatment.
Effects of minocycline on neuropathic pain
The contribution of glial cells to the initiation of neuropathic pain sensitization and peripheral nerve injury-induced neuropathic pain has been well-characterized (Inoue and Tsuda, 2009; Zhuo et al., 2011). As mentioned earlier, minocycline inhibits microglial activation in various pathological conditions, without affecting astroglia and neurons (Yrjänheikki et al., 1998; Tikka et al., 2001), and this may justify its reported ability to reverse neuronal sensitization in neuropathic animal models when applied to the spinal cord (Tikka et al., 2001; Raghavendra et al., 2003). In fact, several studies have revealed that minocycline can exert anti-nociceptive effects on experimental neuropathic pain induced by peripheral nerve injury, inflammation or SCI, after both systemic (i.p.) and local (intrathecal) administration (Raghavendra et al., 2003; Hains and Waxman, 2006; Owolabi and Saab, 2006; Mika et al., 2007). Minocycline's beneficial effects in these cases are clearly enhanced when it is administered as early as possible, especially during the initiation stage (Ledeboer et al., 2005; Owolabi and Saab, 2006; Mei et al., 2011). Similarly, minocycline has been recently found to reverse microglial reactivity and thermal hyperalgesia secondary to sciatic neuropathy when injected into the ventral posterolateral thalamus (LeBlanc et al., 2011). Special attention has been given to minocycline's effects in the development of diabetic neuropathy. Pabreja et al. (2011) recently reported that the chronic administration of minocycline significantly prevented cold allodynia and thermal hyperalgesia in diabetic rats. This beneficial effect was associated with decreased levels of pro-inflammatory cytokines and an attenuated oxidative stress balance in the spinal cord of these diabetic animals. Moreover, the beneficial effects of minocycline in diabetes were associated with the prevention of retinal complications, most probably because of the inhibition of diabetes-induced cytokine and cytotoxin production (Krady et al., 2005). Consistent with this finding, Wang et al. (2005) showed that minocycline inhibited the up-regulation and increased release of IL-1β, TNFα and NO caused by bacterial LPS in retinal microglia. Cai et al. (2011) investigated the neuroprotective mechanisms of minocycline against diabetic brain injury and reported its ability to improve the behavioural deficits caused by altered glucose metabolism in diabetic rats. In addition, they demonstrated that the drug down-regulates the increased Aβ in the hippocampus by inhibiting signal transduction molecules upstream of the NF-κB pathway and by attenuating oxidative stress. The anti-nociceptive effects of minocycline have subsequently been confirmed in different models of neuropathic pain (spinal nerve transection, peripheral nerve injury, inflammation, sciatic neuropathy and SCI), particularly when it was administered during the initiation stage, and have been attributed to the inhibition of microglia activation. In diabetes, in addition to the amelioration of diabetic neuropathy, minocycline prevented retinal complications and improved behavioural deficits. Human trials have not yet been conducted, but based on the available experimental data, minocycline therapy seems like a rational approach for these conditions.
Effects of minocycline on SCI
Because the neuropathic pain and motor weakness that result from microglia activation are believed to trigger nociceptive hypersensitivity in SCI, minocycline could be a rational approach for treating the complications of this condition (Colburn et al., 1999). Support for this proposition comes from the results of different studies that show that minocycline may reduce neuropathic pain after SCI. However, few data exist regarding minocycline's ability to promote motor recovery after SCI, rendering this possibility controversial. In rodent models of SCI, minocycline administration significantly improved both hindlimb function and strength, reduced the gross lesion size in the spinal cord and induced axonal sparing. Minocycline-treated mice demonstrated superior behavioural recovery than that shown by methylprednisolone-treated mice, the approved treatment for acute SCI in humans (Wells et al., 2003). In rats with SCI, minocycline inhibited the release of cytochrome c from the mitochondria, markedly enhancing long-term hindlimb locomotion (Teng et al., 2004). More recently, Saganová et al. (2008) showed that both short- and long-term treatment with minocycline had a neuroprotective effect on the spinal cord rostral to the injury epicentre. Previous data had indicated that minocycline exerts a protective effect on white matter and motor neuron number at sites both rostral, but also caudal, to the lesion epicentre (Teng et al., 2004).
Minocycline has also been shown to improve functional recovery after SCI through the inhibition of pro-nerve growth factor production by microglia, thereby reducing oligodendrocyte death and apoptosis after traumatic SCI. It also inhibited the expression of p75 neurotrophin receptors and the activation of the Ras homolog gene family member A (RhoA) after SCI (Yune et al., 2007). Furthermore, Festoff et al. (2006) reported that minocycline might also exert a neuroprotective effect in SCI by reducing microgliosis and inhibiting caspase expression. A recent study reported minocycline's effects on motor neuron recovery and neuropathic pain in a rat model of thoracic SCI (Cho et al., 2011). The study revealed that, at post-operative day 2, the locomotor score was higher and mechanical hyperalgesia was reduced in minocycline-treated animals than in the corresponding controls. The attenuation of neuropathic pain behaviour and motor recovery correlated with reductions in microglia and astrocyte activation respectively. Experimental data have subsequently clarified that minocycline could be of benefit in SCI in different ways: relieving pain and promoting motor recovery after lesion development. However, clinical studies evaluating its potential application in human SCI are still missing.
Effects of minocycline on ischaemia
Minocycline's ability to limit tissue damage in the kidney, heart, lung and neural cells in the setting of ischaemia both in vitro and in vivo has been documented (Yong et al., 2004; Stirling et al., 2005; Sutton et al., 2005; Jordan et al., 2007). When considering stroke, blood–brain barrier disruption after stroke can worsen ischaemic injury by increasing oedema and causing haemorrhage. Minocycline, probably because of its ability to inhibit microglia activation, was able to attenuate infarct volume and neurological deficits in mice after experimental stroke, as a result of a marked reduction in blood–brain barrier disruption and haemorrhage (Yenari et al., 2006). Based on this preliminary evidence, clinical studies in patients with stroke have been performed. Lampl et al. (2007) reported that the oral administration of minocycline (200 mg) for 5 days, with a therapeutic window of 6–24 h after stroke onset, promoted a better outcome compared with the placebo. This beneficial effect could be associated with significant blunting of ischaemic tissue oxidative stress, consistent with previous reports (Kraus et al., 2005). In another ischaemic condition, the myocardial ischaemia-reperfusion (I/R) injury, in which the damage has been associated with the activation of MMPs and serine proteases, minocycline exerted a protective effect, as confirmed by various pre-clinical studies. The evaluation in ex vivo heart systems and cultured cardiac myocytes revealed minocycline's ability to affect apoptotic cell pathways (Scarabelli et al., 2004). Various in vivo studies have also described the cardioprotective effects of minocycline, as antibiotic pre- and post-treatment of rats subjected to I/R resulted in a significant reduction in infarct size, an effect that was accompanied by a reduction in MMP-9 activity and oxidative stress (Romero-Perez et al., 2008). These assays confirmed previous results that the accumulation of tetracyclines in infarcted myocardium was directly related to the degree of tissue damage (Holman et al., 1973; Holman and Zweiman, 1975). In fact, the levels of minocycline that accumulated in the myocardium were several fold higher than those in the plasma, with increased accumulation in ischaemic compared with normal myocardium. Thus, it is possible that some of the cardioprotective effects of minocycline may be attributable to its high tissue levels, which allow for notable effects on its targets: MMP-9 inhibition and ROS-scavenging, together with anti-apoptotic effects. In a more recent study, the protective effect of minocycline against myocardial ischaemia and I/R injury was also attributed to inhibition of the expression of high mobility group box 1 (HMGB1), a protein that has been also found to act as an early mediator of inflammation and cell damage during I/R injury (Andrassy et al., 2008; Hu et al., 2010). PARP-1 inhibition has also been proposed as a possible mechanism underlying minocycline's cardioprotective activity (Tao et al., 2010). During myocardial I/R injury, there is an increase in reactive oxygen and nitrogen species that leads to oxidative DNA damage and the activation of nuclear repair enzymes, such as PARP-1, which promote DNA repair under normal conditions, but which could lead to cell death if excessive (Zhang and Niu, 1994; Yu et al., 2002; Alano et al., 2006). In cultured adult rat cardiac myocytes in which I/R injury was simulated using oxygen-glucose deprivation, minocycline, at micromolar concentrations, significantly reduced cell death and biochemical markers of PARP-1 activation and prevented mitochondrial permeability transition (Tao et al., 2010). Moreover, minocycline has also been reported to be effective in preventing ischaemia-induced ventricular arrhythmias in rats. The incidence of ventricular fibrillation, the duration and the number of episodes of ventricular tachycardia plus unidentifiable and low-voltage QRS complexes, and the severity of arrhythmias were significantly reduced by minocycline treatment (Hu et al., 2011). In that study, the authors postulated that the anti-arrhythmic effect of minocycline may have been associated with activation of the PI3K/Akt signalling pathway and mitochondrial KATP channels, which are known to participate in the anti-arrhythmic effect of ischaemic or pharmacological preconditioning during myocardial ischaemia (Végh and Parratt, 2002; Gourine et al., 2005). Similar clinical benefits of minocycline as those shown in stroke patients could be expected in myocardial ischaemia patients, especially considering its cardiotropisms, antioxidant properties and cardioprotective activity shown in experimental models, in vitro and in vivo.
Effects of minocycline on atherosclerosis
Various studies have proposed minocycline to prevent atherosclerosis, in relation to its cytoprotective effects in vascular cells. Accordingly, minocycline has been shown to protect against diabetic microvascular complications (Krady et al., 2005) and to reduce neointima formation in macrovascular disease following acute vascular injury of the rat carotid artery (Pinney et al., 2003). In these studies, a reduction in the number of vascular smooth muscle cells (VSMC) was seen after minocycline treatment, which was attributed to an inhibition of MMP activity and cytokine-induced VSMC migration (Pinney et al., 2003; Yao et al., 2004). Moreover, minocycline has been shown to inhibit VEGF-induced MMP-9 mRNA transcription and protein activation in human aortic VSMCs in vitro (Pinney et al., 2003; Yao et al., 2004). More recently, Shahzad et al. (2011) showed minocycline to reduce plaque size and vascular stenosis in diet-induced atherosclerosis through a PARP-1 and p27Kip1-dependent mechanism. In vitro assays revealed that minocycline reduced the proliferative process in different cell types, including human aortic smooth muscle cells (HASMC) and murine primary aortic VSMCs. These data may explain the lower number of VSMC observed within atherosclerotic plaques of ApoE-/- high-fat diet (HFD) mice treated with minocycline (Shahzad et al., 2011). These authors also established that minocycline's anti-proliferative effect in VSMC depended on p27Kip1, as it induced in vitro expression in both VSMCs and HASMCs, and in atherosclerotic plaques analysed ex vivo. Of note, the knockdown of p27Kip1 in primary mouse aortic cells abolished the anti-proliferative effect of minocycline. In addition, minocycline reduced poly(ADP-ribose) formation, a marker of PARP-1 activity, in plaques of the truncus brachiocephalicus of ApoE-/- HFD mice and markedly reduced PARP-1 expression, particularly in low-density lipoprotein-treated HASMCs. The reduction of atherosclerotic plaque size after minocycline treatment, mainly due to its anti-proliferative effects on VSMCs, suggests a potential application of minocycline on vascular complications. However, no clinical trials have been conducted yet.
Effects of minocycline on inflammatory bowel disease
The effects of minocycline on experimental colitis were first described in 2009 by Huang et al. (2009b), who reported its ability to prevent and treat dextran sodium sulphate-induced colitis, significantly diminishing the mortality rate and attenuating the severity of the disease. These beneficial effects were associated with reduced iNOS and MMP expression in the intestinal tissue. Supporting these observations, Garrido-Mesa et al. (2011a) confirmed the intestinal anti-inflammatory effect of minocycline in experimental models of colitis in both mice and rats, showing it to have a higher efficacy than other antibiotics like metronidazole, traditionally used to treat human inflammatory bowel disease, although it was devoid of immunomodulatory properties (Perencevich and Burakoff, 2006). That study proposed that minocycline's ability to modulate both the immune and the microbiological parameters of the disease contribute to its beneficial effects. Regarding the latter, these studies revealed that the imbalance in the composition of the intestinal microbiota that characterizes experimental colitis was restored after minocycline treatment, while the other antibiotics tested did not show this effect. Furthermore, in a mouse model of reactivated colitis, the association of minocycline with the probiotic Escherichia coli Nissle 1917, which has been reported to show beneficial effects in these intestinal conditions (Schultz, 2008), exerted a greater intestinal anti-inflammatory effect than the individual treatments and, in addition, was able to attenuate the reactivation of the colitis (Garrido-Mesa et al., 2011b). Therefore, combined immunomodulatory and anti-microbial properties of minocycline could be very useful in the treatment of multifactorial conditions, like inflammatory bowel disease.
Effects of minocycline on allergic asthma
Recently, Joks et al. (2010) demonstrated that minocycline can suppress ongoing human and murine IgE responses, without affecting those of IgM, IgG and IgA. In addition, they also reported that minocycline strongly suppressed the in vitro induction of the memory IgE antibody-forming responses of spleen and mesenteric lymph node cells from BPO-KLH sensitized mice. The suppression was dose-dependent and IgE isotype-specific (Joks et al., 2010). More recently, it has been described that minocycline suppressed in vitro IgE production by peripheral blood mononuclear cells (PBMCs) from asthmatic subjects, whereas there was no change in IgE levels in PBMC cultures from non-asthmatic subjects. The presence of both CD4+ and CD8+ T lymphocytes was found to be required for the minocycline-mediated suppression of IgE responses by PBMCs of allergic asthmatic humans. Finally, these minocycline-mediated decreases in IgE responses were associated with the suppression of p38 MAPK in the T lymphocytes of these patients (Joks and Durkin, ,b). These effects may account for the improvement observed in allergic asthmatic patients after minocycline treatment in a randomized, double-blind, placebo-controlled, crossover study (Daoud et al., 2008). In that trial, the patients' asthma symptoms and spirometric outcomes were significantly improved, and their oral steroid requirements reduced, findings that indicate the potential usefulness of minocycline for treating asthma.
Effects of minocycline on HIV infection
Several in vitro studies have shown minocycline's ability to inhibit HIV activation, proliferation and viral replication in microglia, macrophages and lymphocytes (Si et al., 2004; Zink et al., 2005; Nikodemova et al., 2007). Similarly, it has also been shown to reduce virus infection and immune responses in several experimental models (Si et al., 2004; Zink et al., 2005; Follstaedt et al., 2008; Ratai et al., 2010; Szeto et al., 2010). One of the first studies describing this potential use of minocycline in HIV infection was performed in a simian immunodeficiency virus (SIV) macaque model of HIV-associated neurological damage (Zink et al., 2005). Minocycline-treated SIV-infected macaques were noted to have less severe encephalitis, reduced expression of CNS inflammatory markers and reduced axonal degeneration. In addition, the authors found that treatment with minocycline significantly decreased the viral load in the CSF and plasma and cytotoxic lymphocyte infiltration into the brain. In vitro assays revealed that minocycline also decreased p38 activation and HIV replication in primary human lymphocytes, in association with a reduction in MCP-1/CCL2 production (Zink et al., 2005). These findings led to a study focusing on whether minocycline might improve performance in cognitively impaired HIV-infected subjects by the Acquired Immunodeficiency Syndrome (AIDS) Clinical Trials Group (http://clinicaltrials.gov/ct2/show/NCT00361257). In this setting, Szeto et al. (2010) demonstrated minocycline to have significant anti-HIV effects in primary human CD4+ T-cells. Antibiotic treatment reduced single-cycle replication, reactivation from a primary CD4+ T-cell-derived model of HIV latency and viral RNA expression after de novo infection with the reference strain HIV NL4-3. These ex vivo experiments described, for the first time, the ability of minocycline to decrease virus expression from the resting CD4+ reservoirs of HIV-infected patients during highly active anti-retroviral treatment (HAART) and that the anti-HIV effects of minocycline applied to both laboratory and clinical strains of HIV (Zink et al., 2005). Minocycline has been shown to have many effects on CD4+ T-cells that impair HIV by reducing its permissiveness and reactivation from latency. It has been shown to alter T-cell activation, blunting changes in the expression of activation/proliferation markers and cytokine secretion, which are critical for the activation pathways that regulate HIV replication. However, minocycline has also been shown to affect HIV replication, as it dose-dependently decreased the level of productive virus by inhibiting DNA integration or transcription. All these data support the proposition that minocycline would be effective as a novel maintenance therapy in combination with HAART (Szeto et al., 2010).
In addition to the effects of minocycline on CD4+ T-cells and viral replication, the decreased monocyte/macrophage activation caused by this antibiotic can also play a neuroprotective role in SIV-AIDS. Ratai et al. (2010) reported, in a non-human primate model of accelerated neuro-AIDS, that none of the minocycline-treated animals developed SIV encephalitis. More recently, Campbell et al. (2011) also concluded that, not only its effects on T-cells, but also its inhibitory effect on monocyte activation, correlate with neuronal protection in SIV neuro-AIDS. The authors observed that the reduction of viral replication in CD14+ monocytes in vitro after minocycline treatment was directly related to impaired traffic of these cells into the brain. Therefore, there was a correlation between the expansion of activated monocytes and neuronal protection with minocycline. This may result in decreased replication or abundance of CD14+CD16+ target cells for HIV and SIV in vivo, as shown in a rapid model of SIV-neuropathogenesis in rhesus macaques. In this model, minocycline treatment resulted in neuronal protection: it reduced the activation of monocytes and their accumulation in the lymph nodes of treated animals, and inhibited the expression of several markers critical for monocyte traffic and function (CCR2, CD163, CD11b and CD64). These results indicate that the anti-viral effects of minocycline are linked to its ability to reduce the activation of monocytes and their permissiveness to viral infection.
Unfortunately, despite the evidence mentioned earlier, a small pilot study reported that minocycline failed to modulate CSF HIV infection or immune activation in chronic untreated HIV-1 infection (Ho et al., 2011). The chronic infection of the patients enrolled in the study could have presented a level of immune activation and viral replication that minocycline was too weak to modify, as it showed little effect on CNS or systemic macrophage activation and CSF infection. In addition, the absence of encephalitis meant that there might have been little CNS disease to target. Differences in the species (human compared with macaques) and in the disease targets (the duration of the disease and the presence of encephalitis) should be considered. Furthermore, the authors acknowledged limitations in the design of their study (e.g. its small size, short duration and the absence of an untreated control group) that could have led to a type II error and a reduced power to detect effects of minocycline. Nevertheless, the usefulness of minocycline's neuroprotective properties in the treatment of HIV-infection associated cognitive impairment was also ruled out by a clinical trial recently conducted by Sacktor et al. (2011). Considering these outcomes and the limitations of the aforementioned trials, larger studies are needed to fully test the effectiveness of minocycline on these measures in AIDS patients. Moreover, given the effects of minocycline reported in vitro and in experimental models (Zink et al., 2005; Szeto et al., 2010), there may still be a basis for further study, for example, in well-treated patients in which the level of immunoactivation is partially attenuated or in combination with anti-retroviral drugs.
Effects of minocycline on autism
FXS is the most common genetic determinant of cognitive impairment and autism spectrum disorders (Koukoui and Chaudhuri, 2007; Penagarikano et al., 2007). Minocycline has been revealed recently as a new possible FXS drug treatment, based on the evidence from various studies involving either experimental animals or humans. In a mouse model of FXS, minocycline promoted the maturation of hippocampal dendritic spines to normal morphology and repressed anxiety and memory defects, effects that may be related to the specific inhibition of MMP-9 (Bilousova et al., 2009). In addition, Siller and Broadie (2011) showed, in a Drosophila model of FXS, that neural circuit architecture defects were alleviated by minocycline treatment, which effectively restored the normal synaptic structure. Their results support minocycline as a promising potential FXS treatment and confirmed that it might act via MMP inhibition, as previously reported (Bilousova et al., 2009). In fact, minocycline has been effective in FXS patients, as reported in an open-label add-on trial on FXS patients, which revealed that a wide variety of symptoms were improved by minocycline treatment, including irritability, stereotypy, hyperactivity and inappropriate speech subscales (Paribello et al., 2010; Utari et al., 2010).
Effects of minocycline on osteoporosis
Minocycline benefits bone physiology in several ways. In ovariectomized aged rats, a model for post-menopausal osteoporosis, minocycline was able to both increase bone formation and decrease bone loss in trabecular bone, with a similar efficacy as that observed with oestrogen therapy (Williams et al., 1996). In a subsequent study, minocycline treatment prevented the decrease in bone mineral density induced after ovariectomy and abolished the detrimental effects induced in the femoral trabecular bone area (Williams et al., 1998). In that study, minocycline showed dual effects, modestly reducing bone resorption while substantially stimulating bone formation. In addition, minocycline was found to stimulate the colony-forming efficiency of marrow stromal cells derived from ovariectomized rats, possibly explaining its stimulatory effect on bone formation. Of note, in a rat model of synchronized osseous remodelling, minocycline significantly impaired the disorganization of both the osteoid seam and the layer of osteoblasts, preserved the synthetic activity of osteoblasts and inhibited interstitial collagenase activity and thus bone resorption (Klapisz-Wolikow and Saffar, 1996). Finally, the natural osteotropism that characterize tetracyclines increases their effectiveness in inhibiting MMPs produced by osteoclasts or bone tumour cells (Saikali and Singh, 2003).
Effects of minocycline on cancer
Degradation of the extracellular matrix by MMPs is a critical phenomenon in cancer invasion and metastasis (Hua et al., 2011). Considering the potent inhibitory effects of tetracyclines against MMPs, their anti-cancer potential has been studied in a variety of cancers, including melanoma, lung, breast and prostate cancers (Lokeshwar, 2011). Minocycline has been shown to inhibit in vitro invasion and experimental pulmonary metastasis in mouse renal adenocarcinoma (MRAC-PM2) cells (Masumori et al., 1994). In addition, the i.p. administration of minocycline reduced the number of metastatic nodules in the lung when MRAC-PM2 cells were i.v. injected to mice. Minocycline also suppressed the type IV collagenolytic activity of these cells, which can contribute to the suppression of their metastatic potential (Masumori et al., 1994). Considering its osteotropism, minocycline was highly effective in inhibiting MMPs produced by tumour cells in the bone (Saikali and Singh, 2003). Moreover, when combined with celecoxib, minocycline inhibited the osseous metastasis of breast cancer in nude mice, by increasing tumour cell death and decreasing tumour expression of MMP-9 and VEGF (Niu et al., 2008). In addition, minocycline has recently been shown to be a promising new candidate for adjuvant therapy against malignant gliomas, as it reduced glioma growth both in vitro and in an experimental mouse model, an effect that was associated with a strongly attenuated expression of membrane type 1 MMP (MT1-MMP) in glioma-associated microglia (Markovic et al., 2011). Furthermore, minocycline has been described to inhibit tumour growth in the xenograft tumour model of C6 glioma cells. This effect was associated with an induction of autophagic cell death, although minocycline still induced cell death through the activation of caspase-3 when autophagy was inhibited (Liu et al., 2011). Therefore, these experimental data confirm that the anti-proliferative, anti-angiogenic and matrix-stimulatory activities displayed by minocycline increase its anti-metastasic and anti-tumour potential. Moreover, minocycline may benefit patients undergoing standard cancer chemotherapy by alleviating drug-induced gut damage. In a model of 5-fluorouracil-induced small intestinal mucositis, minocycline protected mice from gut injury. Body weight loss, diarrhoea and villi measurements were improved by minocycline treatment, which also repressed the expression of TNFα, IL-1β and iNOS, decreased the apoptotic index, and inhibited PARP-1 activity in the mouse small intestine. In addition, minocycline treatment appeared to enhance the anti-tumour effects of 5-fluorouracil in tumour CT-26 xenograft mice (Huang et al., 2009a).
Conclusions
In summary, minocycline has long been used as an antibiotic for acne vulgaris, perioral disease and cutaneous sarcoidosis, and it is currently used for the treatment of inflammatory diseases such as rheumatoid arthritis. However, minocycline has now been demonstrated to have anti-inflammatory, immunomodulatory and neuroprotective effects. Various studies in animal models and some clinical trials have confirmed the beneficial effects and safety of minocycline, alone or combined with other drugs, as a promising therapeutic for diseases with an inflammatory background. Multiple mechanisms of action have been proposed for minocycline, which stands out as a drug affecting multiple targets. Its antioxidant properties, calcium chelation and ability to inhibit pro-inflammatory enzymes such as iNOS or MMPs may have prominent roles in its beneficial effects in most of the diseases considered in this review. However, other tetracyclines, especially doxycycline, have been reported to share some of these properties, yet have not exhibited a similar efficacy in the same models. Therefore, it seems that it is minocycline's multiple targets that makes it such an outstanding drug among the tetracyclines. In addition, minocycline has been shown to display several attractive advantages that deserve our attention: it is generally considered a safe drug in humans, with a known side-effect profile; it is relatively inexpensive, which makes it suitable for long-term use in these diseases; and, lastly, it is established as an oral therapy, being well absorbed (95–100%) and reaching most of the compartments of the body, including the CNS. Although a better understanding of the mechanisms involved in the action of minocycline in vivo is required before its therapeutic potential can be accurately assessed, the encouraging results and attractive merits of this antibiotic indicate that it will have potential as a therapeutic approach for treating many of the diseases described in this review.
Acknowledgments
This work was supported by the Spanish Ministry of Science and Innovation (SAF2011-29648), with funds from the European Union and Junta de Andalucia (CTS 164). CIBER-EHD is founded by Instituto de Salud Carlos III. N. Garrido-Mesa is a predoctoral fellow of Spanish Ministry of Education and Science.
Glossary
- AIDS
acquired immunodeficiency syndrome
- Aβ
amyloid β peptide
- EAE
experimental autoimmune encephalomyelitis
- eIF-2α
eukaryotic initiation translation factor-2 alpha
- FDA
Food and Drug Administration
- FXS
fragile X syndrome
- HASMC
human aortic smooth muscle cell
- HIV
human immunodeficiency virus
- I/R
ischaemia-reperfusion
- MHC
major histocompatibility complex
- SCI
spinal cord injury
- SIV
simian immunodeficiency virus
- Th
helper T-cell
- TLR
toll-like-receptor
- VSMC
vascular smooth muscle cell
Conflicts of interest
The authors declare no conflicts of interest.
References
- Abdel-Salam OME. Drugs used to treat Parkinson's disease, present status and future directions. CNS Neurol Disord Drug Targets. 2008;7:321–342. doi: 10.2174/187152708786441867. [DOI] [PubMed] [Google Scholar]
- Agwuh KN, MacGowan A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother. 2006;58:256–265. doi: 10.1093/jac/dkl224. [DOI] [PubMed] [Google Scholar]
- Alano CC, Kauppinen TM, Valls AV, Swanson RA. Minocycline inhibits poly(ADP-ribose) polymerase-1 at nanomolar concentrations. Proc Natl Acad Sci U S A. 2006;103:9685–9690. doi: 10.1073/pnas.0600554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin AR, Patel RN, Thakker GD, Lowenstein CJ, Attur MG, Abramson SB. Post-transcriptional regulation of inducible nitric oxide synthase mRNA in murine macrophages by doxycycline and chemically modified tetracyclines. FEBS Lett. 1997;410:259–264. doi: 10.1016/s0014-5793(97)00605-4. [DOI] [PubMed] [Google Scholar]
- Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, et al. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117:3216–3226. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- Aronson AL. Pharmacotherapeutics of the newer tetracyclines. J Am Vet Med Assoc. 1980;176:1061–1068. [PubMed] [Google Scholar]
- Arsenis C, Moak SA, Greenwald RA. Tetracyclines (TETs) inhibit the synthesis and/or activity of cartilage proteinases in vivo and in vitro. Matrix Suppl. 1992;1:314. [PubMed] [Google Scholar]
- Bachelez H, Senet P, Cadranel J, Kaoukhov A, Dubertret L. The use of tetracyclines for the treatment of sarcoidosis. Arch Dermatol. 2001;137:69–73. doi: 10.1001/archderm.137.1.69. [DOI] [PubMed] [Google Scholar]
- Bain S, Ramamurthy NS, Impeduglia T, Scolman S, Golub LM, Rubin C. Tetracycline prevents cancellous bone loss and maintains near-normal rates of bone formation in streptozotocin diabetic rats. Bone. 1997;21:147–153. doi: 10.1016/s8756-3282(97)00104-x. [DOI] [PubMed] [Google Scholar]
- Barza M, Brown RB, Shanks C, Gamble C, Weinstein L. Relation between lipophilicity and pharmacological behavior of minocycline, doxycycline, tetracycline, and oxytetracycline in dogs. Antimicrob Agents Chemother. 1975;8:713–720. doi: 10.1128/aac.8.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basegmez C, Berber L, Yalcin F. Clinical and biochemical efficacy of minocycline in nonsurgical periodontal therapy: a randomized controlled pilot study. J Clin Pharmacol. 2011;51:915–922. doi: 10.1177/0091270010373929. [DOI] [PubMed] [Google Scholar]
- Bilousova TV, Dansie L, Ngo M, Aye J, Charles JR, Ethell DW, et al. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet. 2009;46:94–102. doi: 10.1136/jmg.2008.061796. [DOI] [PubMed] [Google Scholar]
- Blum D, Chtarto A, Tenenbaum L, Brotchi J, Levivier M. Clinical potential of minocycline for neurodegenerative disorders. Neurobiol Dis. 2004;17:359–366. doi: 10.1016/j.nbd.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Brogden RN, Speight TM, Avery GS. Minocycline: a review of its antibacterial and pharmacokinetic properties and therapeutic use. Drugs. 1975;9:251–291. doi: 10.2165/00003495-197509040-00005. [DOI] [PubMed] [Google Scholar]
- Brundula V, Rewcastle NB, Metz LM, Bernard CC, Yong VW. Targeting leukocyte MMPs and transmigration: minocycline as a potential therapy for multiple sclerosis. Brain. 2002;125:1297–1308. doi: 10.1093/brain/awf133. [DOI] [PubMed] [Google Scholar]
- Cai Z, Zhao Y, Yao S, Bin Zhao B. Increases in β-amyloid protein in the hippocampus caused by diabetic metabolic disorder are blocked by minocycline through inhibition of NF-κB pathway activation. Pharmacol Rep. 2011;63:381–391. doi: 10.1016/s1734-1140(11)70504-7. [DOI] [PubMed] [Google Scholar]
- Campbell JH, Burdo TH, Autissier P, Bombardier JP, Westmoreland SV, Soulas C, et al. Minocycline inhibition of monocyte activation correlates with neuronal protection in SIV neuroAIDS. Plos ONE. 2011;6:e18688. doi: 10.1371/journal.pone.0018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Muzzio IA, Malleret G, Bartsch D, Verbitsky M, Pavlidis P, et al. Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins. Neuron. 2003;39:655–669. doi: 10.1016/s0896-6273(03)00501-4. [DOI] [PubMed] [Google Scholar]
- Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- Cho DC, Cheong JH, Yang MS, Hwang SJ, Kim JM, Kim CH. The effect of minocycline on motor neuron recovery and neuropathic pain in a rat model of spinal cord injury. J Korean Neurosurg Soc. 2011;49:83–91. doi: 10.3340/jkns.2011.49.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Kim H-S, Shin KY, Kim E-M, Kim M, Kim H-S, et al. Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer's disease models. Neuropsychopharmacology. 2007;32:2393–2404. doi: 10.1038/sj.npp.1301377. [DOI] [PubMed] [Google Scholar]
- Colburn RW, Rickman AJ, DeLeo JA. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp Neurol. 1999;157:289–304. doi: 10.1006/exnr.1999.7065. [DOI] [PubMed] [Google Scholar]
- Corbacella E, Lanzoni I, Ding D, Previati M, Salvi R. Minocycline attenuates gentamicin induced hair cell loss in neonatal cochlear cultures. Hear Res. 2004;197:11–18. doi: 10.1016/j.heares.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Cuello AC, Ferretti MT, Leon WC, Iulita MF, Melis T, Ducatenzeiler A, et al. Early-stage inflammation and experimental therapy in transgenic models of the Alzheimer-like amyloid pathology. Neurodegener Dis. 2010;7:96–98. doi: 10.1159/000285514. [DOI] [PubMed] [Google Scholar]
- Daoud A, Gloria CJ, Taningco G, Hammerschlag MR, Weiss S, Gelling M, et al. Minocycline treatment results in reduced oral steroid requirements in adult asthma. Allergy Asthma Proc. 2008;29:286–294. doi: 10.2500/aap.2008.29.3121. [DOI] [PubMed] [Google Scholar]
- Diguet E, Rouland R, Tison F. Minocycline is not beneficial in a phenotypic mouse model of Huntington's disease. Ann Neurol. 2003;54:841–842. doi: 10.1002/ana.10818. [DOI] [PubMed] [Google Scholar]
- Diguet E, Fernagut P-O, Wei X, Du Y, Rouland R, Gross C, et al. Deleterious effects of minocycline in animal models of Parkinson's disease and Huntington's disease. Eur J Neurosci. 2004a;19:3266–3276. doi: 10.1111/j.0953-816X.2004.03372.x. [DOI] [PubMed] [Google Scholar]
- Diguet E, Gross CE, Tison F, Bezard E. Rise and fall of minocycline in neuroprotection: need to promote publication of negative results. Exp Neurol. 2004b;189:1–4. doi: 10.1016/j.expneurol.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Domercq M, Matute C. Neuroprotection by tetracyclines. Trends Pharmacol Sci. 2004;25:609–612. doi: 10.1016/j.tips.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterly NB, Furey NL, Flanagan LE. The effect of antimicrobial agents on leukocyte chemotaxis. J Invest Dermatol. 1978;70:51–55. doi: 10.1111/1523-1747.ep12543487. [DOI] [PubMed] [Google Scholar]
- Esterly NB, Koransky JS, Furey NL, Trevisan M. Neutrophil chemotaxis in patients with acne receiving oral tetracycline therapy. Arch Dermatol. 1984;120:1308–1313. [PubMed] [Google Scholar]
- Festoff BW, Ameenuddin S, Arnold PM, Wong A, Santacruz KS, Citron BA. Minocycline neuroprotects, reduces microgliosis, and inhibits caspase protease expression early after spinal cord injury. J Neurochem. 2006;97:1314–1326. doi: 10.1111/j.1471-4159.2006.03799.x. [DOI] [PubMed] [Google Scholar]
- Feuerstein GZ, Wang X, Barone FC. Inflammatory gene expression in cerebral ischemia and trauma. Potential new therapeutic targets. Ann N Y Acad Sci. 1997;825:179–193. doi: 10.1111/j.1749-6632.1997.tb48428.x. [DOI] [PubMed] [Google Scholar]
- Follstaedt SC, Barber SA, Zink MC. Mechanisms of minocycline-induced suppression of simian immunodeficiency virus encephalitis: inhibition of apoptosis signal-regulating kinase 1. J Neurovirol. 2008;14:376–388. doi: 10.1080/13550280802199898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander RM, Brown RH, Gagliardini V, Wang J, Yuan J. Inhibition of ICE slows ALS in mice. Nature. 1997;388:31. doi: 10.1038/40299. [DOI] [PubMed] [Google Scholar]
- Garrido-Mesa N, Camuesco D, Arribas B, Comalada M, Bailón E, Cueto-Sola M, et al. The intestinal anti-inflammatory effect of minocycline in experimental colitis involves both its immunomodulatory and antimicrobial properties. Pharmacol Res. 2011a;63:308–319. doi: 10.1016/j.phrs.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Garrido-Mesa N, Utrilla P, Comalada M, Zorrilla P, Garrido-Mesa J, Zarzuelo A, et al. The association of minocycline and the probiotic Escherichia coli Nissle 1917 results in an additive beneficial effect in a DSS model of reactivated colitis in mice. Biochem Pharmacol. 2011b;82:1891–1900. doi: 10.1016/j.bcp.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Golub L, Greenwald R, Ramamurthy N, Zucker S, Ramsammy L, McNamara T. Tetracyclines (TCs) inhibit matrix metalloproteinases (MMPs): in vivo effects in arthritic and diabetic rats and new in vitro studies. Matrix Suppl. 1992;1:315–316. [PubMed] [Google Scholar]
- Golub LM, Lee HM, Lehrer G, Nemiroff A, McNamara TF, Kaplan R, et al. Minocycline reduces gingival collagenolytic activity during diabetes. Preliminary observations and a proposed new mechanism of action. J Periodont Res. 1983;18:516–526. doi: 10.1111/j.1600-0765.1983.tb00388.x. [DOI] [PubMed] [Google Scholar]
- Golub LM, Ramamurthy NS, McNamara TF, Greenwald RA, Rifkin BR. Tetracyclines inhibit connective tissue breakdown: new therapeutic implications for an old family of drugs. Crit Rev Oral Biol Med. 1991;2:297–321. doi: 10.1177/10454411910020030201. [DOI] [PubMed] [Google Scholar]
- Gomes PS, Fernandes MH. Effect of therapeutic levels of doxycycline and minocycline in the proliferation and differentiation of human bone marrow osteoblastic cells. Arch Oral Biol. 2007;52:251–259. doi: 10.1016/j.archoralbio.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Good ML, Hussey DL. Minocycline: stain devil? Br J Dermatol. 2003;149:237–239. doi: 10.1046/j.1365-2133.2003.05497.x. [DOI] [PubMed] [Google Scholar]
- Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6:1045–1053. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Molosh AI, Poputnikov D, Bulhak A, Sjöquist P-O, Pernow J. Endothelin-1 exerts a preconditioning-like cardioprotective effect against ischaemia/reperfusion injury via the ET(A) receptor and the mitochondrial K(ATP) channel in the rat in vivo. Br J Pharmacol. 2005;144:331–337. doi: 10.1038/sj.bjp.0706050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald R, Golub LM. Tetracyclines in arthritis. J Rheumatol. 1993;20:1990. [PubMed] [Google Scholar]
- Greenwald RA. The road forward: the scientific basis for tetracycline treatment of arthritic disorders. Pharmacol Res. 2011;64:610–613. doi: 10.1016/j.phrs.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Greenwald RA, Golub LM, Lavietes B, Ramamurthy NS, Gruber B, Laskin RS, et al. Tetracyclines inhibit human synovial collagenase in vivo and in vitro. J Rheumatol. 1987;14:28–32. [PubMed] [Google Scholar]
- Greenwald RA, Moak SA, Ramamurthy NS, Golub LM. Tetracyclines suppress matrix metalloproteinase activity in adjuvant arthritis and in combination with flurbiprofen, ameliorate bone damage. J Rheumatol. 1992;19:927–938. [PubMed] [Google Scholar]
- Griffin MO, Fricovsky E, Ceballos G, Villarreal F. Tetracyclines: a pleitropic family of compounds with promising therapeutic properties. Review of the literature. Am J Physiol Cell Physiol. 2010;299:C539–C548. doi: 10.1152/ajpcell.00047.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, et al. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho EL, Spudich SS, Lee E, Fuchs D, Sinclair E, Price RW. Minocycline fails to modulate cerebrospinal fluid HIV infection or immune activation in chronic untreated HIV-1 infection: results of a pilot study. AIDS Res Ther. 2011;8:17. doi: 10.1186/1742-6405-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman BL, Zweiman FG. Time course of 99mTc(Sn)-tetracycline uptake in experimental acute myocardial infarction. J Nucl Med. 1975;16:1144–1146. [PubMed] [Google Scholar]
- Holman BL, Idoine J, Fliegel CP, Davis MA, Treves S, Eldh P, et al. Detection and localization of experimental myocardial infarction with 99m Tc-tetracycline. J Nucl Med. 1973;14:595–599. [PubMed] [Google Scholar]
- Hu X, Zhou X, He B, Xu C, Wu L, Cui B, et al. Minocycline protects against myocardial ischemia and reperfusion injury by inhibiting high mobility group box 1 protein in rats. Eur J Pharmacol. 2010;638:84–89. doi: 10.1016/j.ejphar.2010.03.059. [DOI] [PubMed] [Google Scholar]
- Hu X, Wu B, Wang X, Xu C, He B, Cui B, et al. Minocycline attenuates ischemia-induced ventricular arrhythmias in rats. Eur J Pharmacol. 2011;654:274–279. doi: 10.1016/j.ejphar.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Hua H, Li M, Luo T, Yin Y, Jiang Y. Matrix metalloproteinases in tumorigenesis: an evolving paradigm. Cell Mol Life Sci. 2011;68:3853–3868. doi: 10.1007/s00018-011-0763-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T-Y, Chu H-C, Lin Y-L, Ho W-H, Hou H-S, Chao Y-C, et al. Minocycline attenuates 5-fluorouracil-induced small intestinal mucositis in mouse model. Biochem Biophys Res Commun. 2009a;389:634–639. doi: 10.1016/j.bbrc.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Huang T-Y, Chu H-C, Lin Y-L, Lin C-K, Hsieh T-Y, Chang W-K, et al. Minocycline attenuates experimental colitis in mice by blocking expression of inducible nitric oxide synthase and matrix metalloproteinases. Toxicol Appl Pharmacol. 2009b;237:69–82. doi: 10.1016/j.taap.2009.02.026. [DOI] [PubMed] [Google Scholar]
- Humbert P, Treffel P, Chapuis JF, Buchet S, Derancourt C, Agache P. The tetracyclines in dermatology. J Am Acad Dermatol. 1991;25:691–697. doi: 10.1016/0190-9622(91)70255-z. [DOI] [PubMed] [Google Scholar]
- Hunter CL, Bachman D, Granholm A-C. Minocycline prevents cholinergic loss in a mouse model of Down's syndrome. Ann Neurol. 2004;56:675–688. doi: 10.1002/ana.20250. [DOI] [PubMed] [Google Scholar]
- Inoue K, Tsuda M. Microglia and neuropathic pain. Glia. 2009;57:1469–1479. doi: 10.1002/glia.20871. [DOI] [PubMed] [Google Scholar]
- Ishikawa C, Tsuda T, Konishi H, Nakagawa N, Yamanishi K. Tetracyclines modulate protease-activated receptor 2-mediated proinflammatory reactions in epidermal keratinocytes. Antimicrob Agents Chemother. 2009;53:1760–1765. doi: 10.1128/AAC.01540-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joks R, Durkin HG. Effect of tetracyclines on IgE allergic responses and asthma. Recent Pat Inflamm Allergy Drug Discov. 2011a;5:221–228. doi: 10.2174/187221311797264919. [DOI] [PubMed] [Google Scholar]
- Joks R, Durkin HG. Non-antibiotic properties of tetracyclines as anti-allergy and asthma drugs. Pharmacol Res. 2011b;64:602–609. doi: 10.1016/j.phrs.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Joks R, Smith-Norowitz T, Nowakowski M, Bluth MH, Durkin HG. Tetracycline-mediated IgE isotype-specific suppression of ongoing human and murine IgE responses in vivo and murine memory IgE responses induced in vitro. Int Immunol. 2010;22:281–288. doi: 10.1093/intimm/dxq004. [DOI] [PubMed] [Google Scholar]
- Jordan J, Fernandez-Gomez FJ, Ramos M, Ikuta I, Aguirre N, Galindo MF. Minocycline and cytoprotection: shedding new light on a shadowy controversy. Curr Drug Deliv. 2007;4:225–231. doi: 10.2174/156720107781023938. [DOI] [PubMed] [Google Scholar]
- Kalish RS, Koujak S. Minocycline inhibits antigen processing for presentation to human T cells: additive inhibition with chloroquine at therapeutic concentrations. Clin Immunol. 2004;113:270–277. doi: 10.1016/j.clim.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Kielian T, Esen N, Liu S, Phulwani NK, Syed MM, Phillips N, et al. Minocycline modulates neuroinflammation independently of its antimicrobial activity in Staphylococcus aureus-induced brain abscess. Am J Pathol. 2007;171:1199–1214. doi: 10.2353/ajpath.2007.070231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood KL, Cirelli JA, Rogers JE, Giannobile WV. Novel host response therapeutic approaches to treat periodontal diseases. Periodontol 2000. 2007;43:294–315. doi: 10.1111/j.1600-0757.2006.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapisz-Wolikow M, Saffar JL. Minocycline impairment of both osteoid tissue removal and osteoclastic resorption in a synchronized model of remodeling in the rat. J Cell Physiol. 1996;167:359–368. doi: 10.1002/(SICI)1097-4652(199605)167:2<359::AID-JCP22>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Klein NC, Cunha BA. Tetracyclines. Med Clin North Am. 1995;79:789–801. doi: 10.1016/s0025-7125(16)30039-6. [DOI] [PubMed] [Google Scholar]
- Kloppenburg M, Terwiel JP, Mallée C, Breedveld FC, Dijkmans BA. Minocycline in active rheumatoid arthritis. A placebo-controlled trial. Ann N Y Acad Sci. 1994;732:422–423. doi: 10.1111/j.1749-6632.1994.tb24773.x. [DOI] [PubMed] [Google Scholar]
- Kloppenburg M, Verweij CL, Miltenburg AM, Verhoeven AJ, Daha MR, Dijkmans BA, et al. The influence of tetracyclines on T cell activation. Clin Exp Immunol. 1995;102:635–641. doi: 10.1111/j.1365-2249.1995.tb03864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppenburg M, Brinkman BM, de Rooij-Dijk HH, Miltenburg AM, Daha MR, Breedveld FC, et al. The tetracycline derivative minocycline differentially affects cytokine production by monocytes and T lymphocytes. Antimicrob Agents Chemother. 1996;40:934–940. doi: 10.1128/aac.40.4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinaho J, Hökfelt T. Altered gene expression in brain ischemia. Neuroreport. 1997;8:i–viii. [PubMed] [Google Scholar]
- Koistinaho M, Malm TM, Kettunen MI, Goldsteins G, Starckx S, Kauppinen RA, et al. Minocycline protects against permanent cerebral ischemia in wild type but not in matrix metalloprotease-9-deficient mice. J Cereb Blood Flow Metab. 2005;25:460–467. doi: 10.1038/sj.jcbfm.9600040. [DOI] [PubMed] [Google Scholar]
- Korting HC, Schöllmann C. Current topical and systemic approaches to treatment of rosacea. J Eur Acad Dermatol Venereol. 2009;23:876–882. doi: 10.1111/j.1468-3083.2009.03167.x. [DOI] [PubMed] [Google Scholar]
- Koukoui SD, Chaudhuri A. Neuroanatomical, molecular genetic, and behavioral correlates of fragile X syndrome. Brain Res Rev. 2007;53:27–38. doi: 10.1016/j.brainresrev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Krady JK, Basu A, Allen CM, Xu Y, LaNoue KF, Gardner TW, et al. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005;54:1559–1565. doi: 10.2337/diabetes.54.5.1559. [DOI] [PubMed] [Google Scholar]
- Kramer PA, Chapron DJ, Benson J, Mercik SA. Tetracycline absorption in elderly patients with achlorhydria. Clin Pharmacol Ther. 1978;23:467–472. doi: 10.1002/cpt1978234467. [DOI] [PubMed] [Google Scholar]
- Kraus RL, Pasieczny R, Lariosa-Willingham K, Turner MS, Jiang A, Trauger JW. Antioxidant properties of minocycline: neuroprotection in an oxidative stress assay and direct radical-scavenging activity. J Neurochem. 2005;94:819–827. doi: 10.1111/j.1471-4159.2005.03219.x. [DOI] [PubMed] [Google Scholar]
- Kriz J, Nguyen MD, Julien J-P. Minocycline slows disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2002;10:268–278. doi: 10.1006/nbdi.2002.0487. [DOI] [PubMed] [Google Scholar]
- Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, et al. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology. 2007;69:1404–1410. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- Langevitz P, Livneh A, Bank I, Pras M. Benefits and risks of minocycline in rheumatoid arthritis. Drug Saf. 2000;22:405–414. doi: 10.2165/00002018-200022050-00007. [DOI] [PubMed] [Google Scholar]
- LeBlanc BW, Zerah ML, Kadasi LM, Chai N, Saab CY. Minocycline injection in the ventral posterolateral thalamus reverses microglial reactivity and thermal hyperalgesia secondary to sciatic neuropathy. Neurosci Lett. 2011;498:138–142. doi: 10.1016/j.neulet.2011.04.077. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, et al. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Li M, Ona VO, Guégan C, Chen M, Jackson-Lewis V, Andrews LJ, et al. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science. 2000;288:335–339. doi: 10.1126/science.288.5464.335. [DOI] [PubMed] [Google Scholar]
- Liu W-T, Lin C-H, Hsiao M, Gean P-W. Minocycline inhibits the growth of glioma by inducing autophagy. Autophagy. 2011;7:166–175. doi: 10.4161/auto.7.2.14043. [DOI] [PubMed] [Google Scholar]
- Lokeshwar BL. Chemically modified non-antimicrobial tetracyclines are multifunctional drugs against advanced cancers. Pharmacol Res. 2011;63:146–150. doi: 10.1016/j.phrs.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luccarini I, Ballerini C, Biagioli T, Biamonte F, Bellucci A, Rosi MC, et al. Combined treatment with atorvastatin and minocycline suppresses severity of EAE. Exp Neurol. 2008;211:214–226. doi: 10.1016/j.expneurol.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Macfarlane SR, Sloss CM, Cameron P, Kanke T, McKenzie RC, Plevin R. The role of intracellular Ca2+ in the regulation of proteinase-activated receptor-2 mediated nuclear factor kappa B signalling in keratinocytes. Br J Pharmacol. 2005;145:535–544. doi: 10.1038/sj.bjp.0706204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier K, Merkler D, Gerber J, Taheri N, Kuhnert AV, Williams SK, et al. Multiple neuroprotective mechanisms of minocycline in autoimmune CNS inflammation. Neurobiol Dis. 2007;25:514–525. doi: 10.1016/j.nbd.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Markovic DS, Vinnakota K, van Rooijen N, Kiwit J, Synowitz M, Glass R, et al. Minocycline reduces glioma expansion and invasion by attenuating microglial MT1-MMP expression. Brain Behav Immun. 2011;25:624–628. doi: 10.1016/j.bbi.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Masumori N, Tsukamoto T, Miyao N, Kumamoto Y, Saiki I, Yoneda J. Inhibitory effect of minocycline on in vitro invasion and experimental metastasis of mouse renal adenocarcinoma. J Urol. 1994;151:1400–1404. doi: 10.1016/s0022-5347(17)35268-0. [DOI] [PubMed] [Google Scholar]
- Mei X-P, Xu H, Xie C, Ren J, Zhou Y, Zhang H, et al. Post-injury administration of minocycline: an effective treatment for nerve-injury induced neuropathic pain. Neurosci Res. 2011;70:305–312. doi: 10.1016/j.neures.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Metz LM, Zhang Y, Yeung M, Patry DG, Bell RB, Stoian CA, et al. Minocycline reduces gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol. 2004;55:756. doi: 10.1002/ana.20111. [DOI] [PubMed] [Google Scholar]
- Metz LM, Li D, Traboulsee A, Myles ML, Duquette P, Godin J, et al. Glatiramer acetate in combination with minocycline in patients with relapsing–remitting multiple sclerosis: results of a Canadian, multicenter, double-blind, placebo-controlled trial. Mult Scler. 2009;15:1183–1194. doi: 10.1177/1352458509106779. [DOI] [PubMed] [Google Scholar]
- Mika J, Osikowicz M, Makuch W, Przewlocka B. Minocycline and pentoxifylline attenuate allodynia and hyperalgesia and potentiate the effects of morphine in rat and mouse models of neuropathic pain. Eur J Pharmacol. 2007;560:142–149. doi: 10.1016/j.ejphar.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Nelson ML. Chemical and biological dynamics of tetracyclines. Adv Dent Res. 1998;12:5–11. doi: 10.1177/08959374980120011901. [DOI] [PubMed] [Google Scholar]
- Nessler S, Dodel R, Bittner A, Reuss S, Du Y, Hemmer B, et al. Effect of minocycline in experimental autoimmune encephalomyelitis. Ann Neurol. 2002;52:689–690. doi: 10.1002/ana.10353. author reply 690. [DOI] [PubMed] [Google Scholar]
- Nikodemova M, Duncan ID, Watters JJ. Minocycline exerts inhibitory effects on multiple mitogen-activated protein kinases and IκBα degradation in a stimulus-specific manner in microglia. J Neurochem. 2006;96:314–323. doi: 10.1111/j.1471-4159.2005.03520.x. [DOI] [PubMed] [Google Scholar]
- Nikodemova M, Watters JJ, Jackson SJ, Yang SK, Duncan ID. Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC)/betaII. J Biol Chem. 2007;282:15208–15216. doi: 10.1074/jbc.M611907200. [DOI] [PubMed] [Google Scholar]
- Niu G, Liao Z, Cai L, Wei R, Sun L. The combined effects of celecoxib and minocycline hydrochloride on inhibiting the osseous metastasis of breast cancer in nude mice. Cancer Biother Radiopharm. 2008;23:469–476. doi: 10.1089/cbr.2008.0475. [DOI] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ona VO, Li M, Vonsattel JP, Andrews LJ, Khan SQ, Chung WM, et al. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington's disease. Nature. 1999;399:263–267. doi: 10.1038/20446. [DOI] [PubMed] [Google Scholar]
- Owolabi SA, Saab CY. Fractalkine and minocycline alter neuronal activity in the spinal cord dorsal horn. FEBS Lett. 2006;580:4306–4310. doi: 10.1016/j.febslet.2006.06.087. [DOI] [PubMed] [Google Scholar]
- Pabreja K, Dua K, Sharma S, Padi SSV, Kulkarni SK. Minocycline attenuates the development of diabetic neuropathic pain: possible anti-inflammatory and anti-oxidant mechanisms. Eur J Pharmacol. 2011;661:15–21. doi: 10.1016/j.ejphar.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Paribello C, Tao L, Folino A, Berry-Kravis E, Tranfaglia M, Ethell IM, et al. Open-label add-on treatment trial of minocycline in fragile X syndrome. BMC Neurol. 2010;10:91. doi: 10.1186/1471-2377-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Shin TK, Lee HY, Kim SJ, Lee WS. Matrix metalloproteinase inhibitors attenuate neuroinflammation following focal cerebral ischemia in mice. Korean J Physiol Pharmacol. 2011;15:115. doi: 10.4196/kjpp.2011.15.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagarikano O, Mulle JG, Warren ST. The pathophysiology of fragile x syndrome. Annu Rev Genomics Hum Genet. 2007;8:109–129. doi: 10.1146/annurev.genom.8.080706.092249. [DOI] [PubMed] [Google Scholar]
- Perencevich M, Burakoff R. Use of antibiotics in the treatment of inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:651–664. doi: 10.1097/01.MIB.0000225330.38119.c7. [DOI] [PubMed] [Google Scholar]
- Pinney SP, Chen HJ, Liang D, Wang X, Schwartz A, Rabbani LE. Minocycline inhibits smooth muscle cell proliferation, migration and neointima formation after arterial injury. J Cardiovasc Pharmacol. 2003;42:469–476. doi: 10.1097/00005344-200310000-00003. [DOI] [PubMed] [Google Scholar]
- Popovic N, Schubart A, Goetz BD, Zhang S-C, Linington C, Duncan Ian D. Inhibition of autoimmune encephalomyelitis by a tetracycline. Ann Neurol. 2002;51:215–223. doi: 10.1002/ana.10092. [DOI] [PubMed] [Google Scholar]
- Pruzanski W, Greenwald RA, Street IP, Laliberte F, Stefanski E, Vadas P. Inhibition of enzymatic activity of phospholipases A2 by minocycline and doxycycline. Biochem Pharmacol. 1992;44:1165–1170. doi: 10.1016/0006-2952(92)90381-r. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, DeLeo Joyce A. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- Ratai E-M, Bombardier JP, Joo C-G, Annamalai L, Burdo TH, Campbell J, et al. Proton magnetic resonance spectroscopy reveals neuroprotection by oral minocycline in a nonhuman primate model of accelerated NeuroAIDS. Plos ONE. 2010;5:e10523. doi: 10.1371/journal.pone.0010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke. 1998;29:1020–1030. doi: 10.1161/01.str.29.5.1020. [DOI] [PubMed] [Google Scholar]
- Romero-Perez D, Fricovsky E, Yamasaki KG, Griffin M, Barraza-Hidalgo M, Dillmann W, et al. Cardiac uptake of minocycline and mechanisms for in vivo cardioprotection. J Am Coll Cardiol. 2008;52:1086–1094. doi: 10.1016/j.jacc.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, Miyahara S, Deng L, Evans S, Schifitto G, Cohen BA, et al. Minocycline treatment for HIV-associated cognitive impairment: results from a randomized trial. Neurology. 2011;77:1135–1142. doi: 10.1212/WNL.0b013e31822f0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saganová K, Orendácová J, Cízková D, Vanický I. Limited minocycline neuroprotection after balloon-compression spinal cord injury in the rat. Neurosci Lett. 2008;433:246–249. doi: 10.1016/j.neulet.2008.01.041. [DOI] [PubMed] [Google Scholar]
- Saikali Z, Singh G. Doxycycline and other tetracyclines in the treatment of bone metastasis. Anticancer Drugs. 2003;14:773–778. doi: 10.1097/00001813-200311000-00001. [DOI] [PubMed] [Google Scholar]
- Saivin S, Houin G. Clinical pharmacokinetics of doxycycline and minocycline. Clin Pharmacokinet. 1988;15:355–366. doi: 10.2165/00003088-198815060-00001. [DOI] [PubMed] [Google Scholar]
- Sanchez Mejia RO, Ona VO, Li M, Friedlander RM. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery. 2001;48:1393–1399. doi: 10.1097/00006123-200106000-00051. discussion 1399–1401. [DOI] [PubMed] [Google Scholar]
- Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258–265. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Scarabelli TM, Stephanou A, Pasini E, Gitti G, Townsend P, Lawrence K, et al. Minocycline inhibits caspase activation and reactivation, increases the ratio of XIAP to smac/DIABLO, and reduces the mitochondrial leakage of cytochrome C and smac/DIABLO. J Am Coll Cardiol. 2004;43:865–874. doi: 10.1016/j.jacc.2003.09.050. [DOI] [PubMed] [Google Scholar]
- Schultz M. Clinical use of E. coli Nissle 1917 in inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1012–1018. doi: 10.1002/ibd.20377. [DOI] [PubMed] [Google Scholar]
- Seabrook TJ, Jiang L, Maier M, Lemere CA. Minocycline affects microglia activation, Abeta deposition, and behavior in APP-tg mice. Glia. 2006;53:776–782. doi: 10.1002/glia.20338. [DOI] [PubMed] [Google Scholar]
- Shahzad K, Thati M, Wang H, Kashif M, Wolter J, Ranjan S, et al. Minocycline reduces plaque size in diet induced atherosclerosis via p27(Kip1) Atherosclerosis. 2011;219:74–83. doi: 10.1016/j.atherosclerosis.2011.05.041. [DOI] [PubMed] [Google Scholar]
- Si Q, Cosenza M, Kim M-O, Zhao M-L, Brownlee M, Goldstein H, et al. A novel action of minocycline: inhibition of human immunodeficiency virus type 1 infection in microglia. J Neurovirol. 2004;10:284–292. doi: 10.1080/13550280490499533. [DOI] [PubMed] [Google Scholar]
- Siller SS, Broadie K. Neural circuit architecture defects in a Drosophila model of fragile X syndrome are alleviated by minocycline treatment and genetic removal of matrix metalloproteinase. Dis Models Mech. 2011;4:673–685. doi: 10.1242/dmm.008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, Woodman B, Mahal A, Sathasivam K, Ghazi-Noori S, Lowden PAS, et al. Minocycline and doxycycline are not beneficial in a model of Huntington's disease. Ann Neurol. 2003;54:186–196. doi: 10.1002/ana.10614. [DOI] [PubMed] [Google Scholar]
- Soory M. A role for non-antimicrobial actions of tetracyclines in combating oxidative stress in periodontal and metabolic diseases: a literature review. Open Dent J. 2008;2:5–12. doi: 10.2174/1874210600802010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson K, Brattsand M, Roosterman D, Kempkes C, Bocheva G, Steinhoff M, et al. Activation of proteinase-activated receptor-2 by human kallikrein-related peptidases. J Invest Dermatol. 2008;128:18–25. doi: 10.1038/sj.jid.5700965. [DOI] [PubMed] [Google Scholar]
- Stirling DP. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci. 2004;24:2182–2190. doi: 10.1523/JNEUROSCI.5275-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling DP, Koochesfahani KM, Steeves JD, Tetzlaff W. Minocycline as a neuroprotective agent. Neuroscientist. 2005;11:308–322. doi: 10.1177/1073858405275175. [DOI] [PubMed] [Google Scholar]
- Stone M, Fortin PR, Pacheco-Tena C, Inman RD. Should tetracycline treatment be used more extensively for rheumatoid arthritis? Metaanalysis demonstrates clinical benefit with reduction in disease activity. J Rheumatol. 2003;30:2112–2122. [PubMed] [Google Scholar]
- Sutton TA, Kelly KJ, Mang HE, Plotkin Z, Sandoval RM, Dagher PC. Minocycline reduces renal microvascular leakage in a rat model of ischemic renal injury. Am J Physiol Renal Physiol. 2005;288:F91–F97. doi: 10.1152/ajprenal.00051.2004. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Yagisawa J, Kumakura S, Tsutsui T. Effects of minocycline and doxycycline on cell survival and gene expression in human gingival and periodontal ligament cells. J Periodont Res. 2006;41:124–131. doi: 10.1111/j.1600-0765.2005.00843.x. [DOI] [PubMed] [Google Scholar]
- Szeto GL, Brice AK, Yang H, Barber SA, Siliciano RF, Clements JE. Minocycline attenuates HIV infection and reactivation by suppressing cellular activation in human CD4+ T cells. J Infect Dis. 2010;201:1132–1140. doi: 10.1086/651277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Kim SH, Honbo N, Karliner JS, Alano CC. Minocycline protects cardiac myocytes against simulated ischemia–reperfusion injury by inhibiting poly(ADP-ribose) polymerase-1. J Cardiovasc Pharmacol. 2010;56:659–668. doi: 10.1097/FJC.0b013e3181faeaf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SS, Haste NM, Ghosh G. PKR and eIF2alpha: integration of kinase dimerization, activation, and substrate docking. Cell. 2005;122:823–825. doi: 10.1016/j.cell.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Teng YD, Choi H, Onario RC, Zhu S, Desilets FC, Lan S, et al. Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci U S A. 2004;101:3071–3076. doi: 10.1073/pnas.0306239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Le WD. Minocycline: neuroprotective mechanisms in Parkinson's disease. Curr Pharm Des. 2004;10:679–686. doi: 10.2174/1381612043453162. [DOI] [PubMed] [Google Scholar]
- Thomas M, Ashizawa T, Jankovic J. Minocycline in Huntington's disease: a pilot study. Mov Disord. 2004;19:692–695. doi: 10.1002/mds.20018. [DOI] [PubMed] [Google Scholar]
- Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikka TM, Vartiainen NE, Goldsteins G, Oja SS, Andersen PM, Marklund SL, et al. Minocycline prevents neurotoxicity induced by cerebrospinal fluid from patients with motor neurone disease. Brain. 2002;125:722–731. doi: 10.1093/brain/awf068. [DOI] [PubMed] [Google Scholar]
- Tilley BC, Alarcón GS, Heyse SP, Trentham DE, Neuner R, Kaplan DA, et al. Minocycline in rheumatoid arthritis. A 48-week, double-blind, placebo-controlled trial. MIRA Trial Group. Ann Intern Med. 1995;122:81–89. doi: 10.7326/0003-4819-122-2-199501150-00001. [DOI] [PubMed] [Google Scholar]
- Tomás-Camardiel M, Rite I, Herrera AJ, de Pablos RM, Cano J, Machado A, et al. Minocycline reduces the lipopolysaccharide-induced inflammatory reaction, peroxynitrite-mediated nitration of proteins, disruption of the blood-brain barrier, and damage in the nigral dopaminergic system. Neurobiol Dis. 2004;16:190–201. doi: 10.1016/j.nbd.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Utari A, Chonchaiya W, Rivera SM, Schneider A, Hagerman RJ, Faradz SMH, et al. Side effects of minocycline treatment in patients with fragile X syndrome and exploration of outcome measures. Am J Intellect Dev Disabil. 2010;115:433–443. doi: 10.1352/1944-7558-115.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Bosch L, Tilkin P, Lemmens G, Robberecht W. Minocycline delays disease onset and mortality in a transgenic model of ALS. Neuroreport. 2002;13:1067–1070. doi: 10.1097/00001756-200206120-00018. [DOI] [PubMed] [Google Scholar]
- Végh A, Parratt JR. The role of mitochondrial K(ATP) channels in antiarrhythmic effects of ischaemic preconditioning in dogs. Br J Pharmacol. 2002;137:1107–1115. doi: 10.1038/sj.bjp.0704966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AL, Yu ACH, Lau LT, Lee C, Wu LM, Zhu X, et al. Minocycline inhibits LPS-induced retinal microglia activation. Neurochem Int. 2005;47:152–158. doi: 10.1016/j.neuint.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, et al. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington's disease. Proc Natl Acad Sci U S A. 2003;100:10483–10487. doi: 10.1073/pnas.1832501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JEA, Hurlbert RJ, Fehlings MG, Yong VW. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003;126:1628–1637. doi: 10.1093/brain/awg178. [DOI] [PubMed] [Google Scholar]
- White JR, Pearce FL. Characterization of chlortetracycline (aureomycin) as a calcium ionophore. Biochemistry. 1982;21:6309–6312. doi: 10.1021/bi00267a041. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Halliwell B. Prevention of peroxynitrite-dependent tyrosine nitration and inactivation of alpha1-antiproteinase by antibiotics. Free Radic Res. 1997;26:49–56. doi: 10.3109/10715769709097783. [DOI] [PubMed] [Google Scholar]
- Wilkins A, Nikodemova M, Compston A, Duncan I. Minocycline attenuates nitric oxide-mediated neuronal and axonal destruction in vitro. Neuron Glia Biol. 2004;1:297–305. doi: 10.1017/S1740925X05000104. [DOI] [PubMed] [Google Scholar]
- Williams D, Laughlin L, Lee Y-H. Minocycline: possible vestibular side-effects. Lancet. 1974;304:744–746. doi: 10.1016/s0140-6736(74)90941-6. [DOI] [PubMed] [Google Scholar]
- Williams S, Wakisaka A, Zeng QQ, Barnes J, Martin G, Wechter WJ, et al. Minocycline prevents the decrease in bone mineral density and trabecular bone in ovariectomized aged rats. Bone. 1996;19:637–644. doi: 10.1016/s8756-3282(96)00302-x. [DOI] [PubMed] [Google Scholar]
- Williams S, Wakisaka A, Zeng QQ, Barnes J, Seyedin S, Martin G, et al. Effect of minocycline on osteoporosis. Adv Dent Res. 1998;12:71–75. doi: 10.1177/08959374980120012401. [DOI] [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Mikliaeva EI, Casha S, Zygun D, Demchuk A, Yong VW. Improving outcomes of neuroprotection by minocycline: guides from cell culture and intracerebral hemorrhage in mice. Am J Pathol. 2010;176:1193–1202. doi: 10.2353/ajpath.2010.090361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Sugama S, Chirichigno JW, Gregorio J, Lorenzl S, Shin DH, et al. Minocycline enhances MPTP toxicity to dopaminergic neurons. J Neurosci Res. 2003;74:278–285. doi: 10.1002/jnr.10709. [DOI] [PubMed] [Google Scholar]
- Yao JS, Chen Y, Zhai W, Xu K, Young WL, Yang G-Y. Minocycline exerts multiple inhibitory effects on vascular endothelial growth factor-induced smooth muscle cell migration: the role of ERK1/2, PI3K, and matrix metalloproteinases. Circ Res. 2004;95:364–371. doi: 10.1161/01.RES.0000138581.04174.2f. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke. 2006;37:1087–1093. doi: 10.1161/01.STR.0000206281.77178.ac. [DOI] [PubMed] [Google Scholar]
- Yong VW, Wells J, Giuliani F, Casha S, Power C, Metz LM. The promise of minocycline in neurology. Lancet Neurol. 2004;3:744–751. doi: 10.1016/S1474-4422(04)00937-8. [DOI] [PubMed] [Google Scholar]
- Youssef S, Stüve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- Yrjänheikki J, Keinänen R, Pellikka M, Hökfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrjänheikki J, Tikka T, Keinänen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S-W, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- Yune TY, Lee JY, Jung GY, Kim SJ, Jiang MH, Kim YC, et al. Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J Neurosci. 2007;27:7751–7761. doi: 10.1523/JNEUROSCI.1661-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabad RK, Metz LM, Todoruk TR, Zhang Y, Mitchell JR, Yeung M, et al. The clinical response to minocycline in multiple sclerosis is accompanied by beneficial immune changes: a pilot study. Mult Scler. 2007;13:517–526. doi: 10.1177/1352458506070319. [DOI] [PubMed] [Google Scholar]
- Zemke D, Majid A. The potential of minocycline for neuroprotection in human neurologic disease. Clin Neuropharmacol. 2004;27:293–298. doi: 10.1097/01.wnf.0000150867.98887.3e. [DOI] [PubMed] [Google Scholar]
- Zernicke RF, Wohl GR, Greenwald RA, Moak SA, Leng W, Golub LM. Administration of systemic matrix metalloproteinase inhibitors maintains bone mechanical integrity in adjuvant arthritis. J Rheumatol. 1997;24:1324–1331. [PubMed] [Google Scholar]
- Zhang J, Niu X. Changes of monoamines, purines and amino acids in rat striatum as measured by intercerebral microdialysis during ischemia/reperfusion. Chin Med Sci J. 1994;9:225–229. [PubMed] [Google Scholar]
- Zhu S, Stavrovskaya IG, Drozda M, Kim BYS, Ona V, Li M, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Wu G, Wu L-J. Neuronal and microglial mechanisms of neuropathic pain. Mol Brain. 2011;4:31. doi: 10.1186/1756-6606-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink MC, Uhrlaub J, DeWitt J, Voelker T, Bullock B, Mankowski J, et al. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. JAMA. 2005;293:2003–2011. doi: 10.1001/jama.293.16.2003. [DOI] [PubMed] [Google Scholar]


