Abstract
Background and Purpose
Bone resorption induced by interleukin-1β (IL-1β) and tumour necrosis factor (TNF-α) is synergistically potentiated by kinins, partially due to enhanced kinin receptor expression. Inflammation-induced bone resorption can be impaired by IL-4 and IL-13. The aim was to investigate if expression of B1 and B2 kinin receptors can be affected by IL-4 and IL-13.
Experimental Approach
We examined effects in a human osteoblastic cell line (MG-63), primary human gingival fibroblasts and mouse bones by IL-4 and IL-13 on mRNA and protein expression of the B1 and B2 kinin receptors. We also examined the role of STAT6 by RNA interference and using Stat6-/- mice.
Key Results
IL-4 and IL-13 decreased the mRNA expression of B1 and B2 kinin receptors induced by either IL-1β or TNF-α in MG-63 cells, intact mouse calvarial bones or primary human gingival fibroblasts. The burst of intracellular calcium induced by either bradykinin (B2 agonist) or des-Arg10-Lys-bradykinin (B1 agonist) in gingival fibroblasts pretreated with IL-1β was impaired by IL-4. Similarly, the increased binding of B1 and B2 ligands induced by IL-1β was decreased by IL-4. In calvarial bones from Stat6-deficient mice, and in fibroblasts in which STAT6 was knocked down by siRNA, the effect of IL-4 was decreased.
Conclusions and Implications
These data show, for the first time, that IL-4 and IL-13 decrease kinin receptors in a STAT6-dependent mechanism, which can be one important mechanism by which these cytokines exert their anti-inflammatory effects and impair bone resorption.
Keywords: interleukin-4, interleukin-13, interleukin-1β, tumour necrosis factor-α, kinin receptors, STAT6
Introduction
In inflammatory conditions such as periodontitis and rheumatoid arthritis, increased osteoclast activity can be stimulated by different bone resorbing proinflammatory cytokines such as interleukin-1 (IL-1) and tumour necrosis factors-α and -β (TNFs) (Lerner, 2006). The proinflammatory peptide bradykinin (BK) (Couture et al., 2001; Moreau et al., 2005), as well as several kinin analogues, have also been shown to stimulate bone resorption (Lerner et al., 1987; Ljunggren and Lerner, 1990; Worthy et al., 1990; Lerner, 1997). Interestingly, kinins not only stimulate bone resorption, but also synergistically potentiate the bone resorptive effect of IL-1 (Lerner, 1991; Lerner and Modeer, 1991), by a mechanism dependent on prostaglandin production (Lerner et al., 1989). In contrast to these observations, it has been reported that mice lacking both subtypes of kinin receptors have decreased bone mass as assessed by dual emission x-ray absorptiometry (Kakoki et al., 2010). Since histomorphometric analysis was not performed, it is not possible to know if the decreased bone mass was a consequence of increased resorption or decreased formation of bone. Nor is it possible to know if the phenotype was a result by direct or indirect effect on bone cells. The data suggest the interesting possibility that kinins may be involved in physiological remodelling of bone. It remains to be shown the role of kinin receptors in vivo in inflammation-induced bone resorption.
IL-4 (19-kDa) and IL-13 (10-kDa) are immunoregulatory cytokines secreted mainly by activated T helper type 2 (TH2) cells and mast cells. IL-4 inhibits the ability of macrophages to produce a number of inflammatory cytokines (e.g. IL-1, TNF-α and IL-6), and has therefore been considered as an anti-inflammatory cytokine (Hart et al., 1989; 1991; te Velde et al., 1990). During recent years, it has been clear that macrophages have remarkable heterogeneity, including M1 and M2 macrophages, microglial cells in the brain, tumour-associated macrophages, dendritic cells in skin and mucosa, Kupffer cells in liver and osteoclasts in bone, and that the polarization is controlled by a complex transcriptional response to molecules in the microenvironment (Lawrence and Natoli, 2011). In this context, IL-4 has been shown to be an activator of alternatively activated M2 macrophages, rather than an anti-inflammatory cytokine (Gordon and Martinez, 2010). IL-4 and IL-13 have also been shown to affect bone remodelling and both cytokines inhibit osteoclast formation and bone resorption in vitro and in vivo (Riancho et al., 1993; Miossec et al., 1994; Palmqvist et al., 2006). IL-4 and IL-13 exhibit their effects on osteoclasts as well as on osteoblasts since both cytokines have been shown not only to cause a decrease in receptor activator of nuclear factor κB (RANK) expression in osteoclast progenitor cells (Moreno et al., 2003; Palmqvist et al., 2006), but also to down-regulate RANK ligand (RANKL) and up-regulate osteoprotegerin (OPG) in osteoblasts isolated from mouse calvarial bones (Palmqvist et al., 2006).
Although IL-4 and IL-13 exhibit only 25% homology in their amino acid sequences, they seem to share many properties including a receptor subunit [the α subunit of the IL-4 receptor (IL-4Rα)]. There are at least two types of IL-4 receptors, called Type 1 and 2. IL-4R Type 1 is formed by heterodimerization of IL-4Rα chain and the common IL-2γ chain (γC), a receptor component that is also found in many other receptors (i.e. IL-2, IL-7, IL-15 and IL-21). The IL-4 receptor Type 2, with affinity to both IL-4 and IL-13, consists of IL-4Rα and the IL-13 receptor α1 (IL-13Rα1) protein (Zurawski et al., 1995; Kelly-Welch et al., 2003). IL-13 also binds to the other IL-13 receptor subtype α2 (IL-13Rα2) with an even higher affinity. This receptor has been thought to be a decoy receptor due to its short cytoplasmic tail, but it has been shown that IL-13Rα2 may have some signalling capabilities, regulating IL-13 signalling pathways (Tabata and Khurana Hershey, 2007; Andrews et al., 2009). In the canonical pathway triggered by IL-4 and IL-13, binding of either IL-4 to IL-4Rα in IL-4R Type 1 or 2, or IL-13 to IL-13Rα1 in IL-13 receptors results in an activation of janus tyrosine kinase 1 (JAK1), which leads to phosphorylation of tyrosine residues of IL-4Rα, which subsequently liaisons with the transcription factor signal transducers and activators of transcription 6 (STAT6). Upon activation, STAT6 homodimerizes and translocates into the nucleus (Hebenstreit et al., 2006).
The effects of kinins are linked to two molecularly and pharmacologically distinct subtypes of kinin receptors, called B1 and B2 (Leeb-Lundberg et al., 2005). The B2 receptor is constitutively expressed in many cell types, whereas the B1 receptor is believed to be induced following injury and by various proinflammatory cytokines (Marceau et al., 1998). We have shown that both B2 and B1 receptors are constitutively expressed on the osteoblastic cell line MG-63, on human gingival fibroblasts and in intact mouse calvariae (Brechter and Lerner, 2002; Brechter et al., 2008), and that both receptors are up-regulated by IL-1β and TNF-α, but not by IL-6, IL-11, IL-17, leukaemia inhibitory factor or oncostatin M (Brechter et al., 2008). The activation of both B1 and B2 receptors results in increased prostaglandin formation (Brechter et al., 2008), and kinins synergistically potentiate prostaglandin formation stimulated by IL-β and TNF-α (Brechter and Lerner, 2007; Brechter et al., 2008). The synergistic interactions between signalling through kinin receptors, on one hand, and IL-1β and TNF-α receptors, on the other hand, leading to synergistic stimulation of prostaglandin formation and bone resorption, involve stimulation of mRNA and protein expression of cyclooxygenase-2, as well as IL-1β and TNF-α induced enhanced expression of B1 and B2 receptors (Brechter and Lerner, 2007).
We have, in the present study, investigated the possibility that inhibition of inflammation-induced bone resorption by IL-4 (Joosten et al., 1999; Lubberts et al., 2000; Woods et al., 2001) may not only be due to effects by IL-4 on osteoclasts and osteoblasts, but also on effects by more upstream events. We here demonstrate that IL-4/IL-13 regulates the expression of kinin receptors on osteoblasts, human gingival fibroblasts and in neonatal mouse calvarial bones, which may be an important mechanism by which these cytokines decrease inflammatory induced bone resorption.
Methods
Bone cell culture
MG-63 cells (obtained at passage 87 from the American Type Culture Collection) are a human osteoblastic osteosarcoma cell line which expresses several osteoblastic phenotypes including biosynthesis of type I collagen and osteocalcin (Clover and Gowen, 1994). The cells used in the present study responded to 1,25(OH)2-vitamin D3 (1 nmol·L−1) with a four- to fivefold stimulation of osteocalcin biosynthesis and to recombinant human insulin-like growth factor-I (25 ng·mL−1) (R&D Systems, Abdington, UK) with a two- to fourfold stimulation of type I collagen formation (data not shown). The cells were cultured in plastic flasks (Nunc, Roskilde, Denmark) in α-modification of minimal essential medium (α-MEM) (Gibco, Grand Island, NE, USA) with 10% fetal calf serum (FCS) at 37°C in humidified atmosphere containing 5% CO2 in air. For experiments, cells were seeded at an initial density of 4–5 × 104 cells·cm−2 in 9.5 cm2 culture dishes (Nunc, Roskilde, Denmark). To the dishes, α-MEM/10% FCS (Gibco) was added and cells were cultured for 1–2 days until 80–90% confluent monolayers were obtained. Then, the cells were washed two times in phosphate buffered saline (PBS) and once in serum free α-MEM and incubated in α-MEM/1% FCS, with or without IL-1β, TNF-α, IL-4 and IL-13 (R&D Systems).
Isolation of gingival fibroblasts
Human gingival fibroblasts were isolated from explants of human papillary gingiva obtained by surgery of clinically healthy gingiva as previously described (Lerner and Hänstrom, 1987). Cells growing out from the explants were detached and subcultured in α-MEM/10% FCS in 75 cm2 flasks. When used in experiments, the cells were seeded at a density 2 × 104 cells·cm−2 and cultured to 80% confluency in 6-wells plates (9.5 cm2). The cells were then washed two times in PBS (Gibco) and once in serum free α-MEM and subsequently incubated in α-MEM/1%FCS with and without test substances. After 24 h of incubation, RNA was extracted for subsequent analysis of gene expression. The study was approved by the Human Studies Ethical Committee of Umeå University and informed consent was obtained by all patients.
Animals
Mice homozygous for the STAT6tm1Gru mutation in a Balb/c background (C.129S2-Stat6tm1Gru/J; stock no. 002828) and their corresponding wild type mice Balb/cJ (stock no. 000651) were purchased from JAX®MICE (The Jackson Laboratory, Sacramento, CA, USA) and were bred in our animal facility unit. Animal care and experiments were approved and made in accordance with accepted standards of humane animal care and use, as considered appropriate by the Animal Care and Use Committee of Umeå University, Umeå, Sweden. All studies involving animals are reported in accordance with ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Culture of calvarial bones
Calvarial bones from 5 to 7 days old mice (Stat6-/-, Balb/cJ) were dissected and divided into two halves along the sagittal suture. The bones were pre-incubated overnight in α-MEM containing 0.1% BSA and 1 μM indomethacin (Merck, Sharp and Dohme, Haarlem, The Netherlands). Following pre-incubation, the bones were extensively washed and subsequently incubated for 24 h, in 24-well culture dishes, containing 1.0 mL indomethacin-free α-MEM /BSA medium, with or without test substances.
RNA extraction
After incubation with or without test substances, the MG-63 cells, gingival fibroblasts or calvarial bones were washed two times in PBS and total RNA was isolated using RNAqueous™-4PCR kit (Ambion Ltd. Huntingdon, Cambridgeshire, UK) by following the manufacturer's protocol. The calvarial bones were homogenized (Ultra-Turrax®, Jenke & Kunkel KG, Staufen, Germany) before RNA extraction. Extracted RNA was quantified spectrophotometrically and analysed by agarose gel electrophoresis. The RNA isolated was DNase-treated with a commercially available DNA-free™-kit (Ambion Ltd.), by following the instructions from the manufacturer. Only RNA preparations showing intact species were used for subsequent analyses.
Semi-quantitative reverse transcription polymerase chain reaction (PCR)
Total RNA (1 μg) from MG-63 cells or from human gingival fibroblasts was reverse transcribed into single stranded cDNA with a first strand cDNA synthesis kit using random p(dN)6 primers. The cDNA was amplified in PCR utilizing a PCR Core Kit and PC-960 G Gradient Thermal Cycler (Corbett Research Pty Ltd., Mortlake, Australia). The PCR reactions for B1 and B2 receptors, and for the IL-4 and IL-13 receptor subunits, were performed as described previously (Brechter et al., 2008). No amplification was detected in samples where the reverse transcription reaction had been excluded (data not shown). The primers sequences for BDKRB1, BDKRB2 and GAPDH were previously described (Brechter and Lerner, 2002) and the sequences, position of the 3′and 5′ends and GenBank accession numbers and predicted size of the amplicon for the IL-4 and IL-13 receptors subunits are described in the Supporting Information Table S1. The PCR products were separated by 1.5% agarose gel electrophoresis and visualized using ethidium bromide staining. The identity of the PCR products was confirmed using QIAquick purification kit and a Thermo Sequenase™ II DYEnamic ET® terminator cycle sequencing premix kit with sequences analysed on an ABI 377 XL DNA Sequencer (Amersham Biosciences, Uppsala, Sweden).
Quantitative real-time polymerase chain reaction (qPCR)
A 0.25–0.5 μg of total RNA, following DNase treatment, was reverse transcribed into cDNA with a first strand cDNA Synthesis Kit using random p(dN)6 primers, following the manufacturer's instructions (Roche, Mannheim, Germany). qPCR analysis was performed to assess the relative quantification of mRNA levels of BDKRB1, BDKRB2, Bdkrb1, Bdkrb2 and Tnfsf11 (the gene encoding for the ligand of the receptor activator of NF-κB; RANKL), using TaqMan Universal PCR Master Mix kit (Applied Biosystems, Foster City, CA, USA), specific primers and a fluorescent probe for each target mRNA. The primers and probes were previously described (Brechter and Lerner, 2002; Brechter et al., 2008). IL4R and IL2RG mRNAs were also analysed by qPCR using SYBR Green Universal PCR master mix (Applied Biosystems, Foster City, CA, USA) and primers amplifying specific products as confirmed by melting curve analysis. IL13RA1 and IL13RA2 mRNA analysis was performed using pre-made primer-probe mix from Applied Biosystems (assay numbers Hs00609817_m1 and Hs00152924_m1). Amplifications were performed using the ABI PRISM 7900 HT Sequence Detection System and Software (Applied Biosystems). To rule out DNA contamination, samples in which the reverse transcription reaction had been omitted were also submitted to the PCR reaction, yielding no amplification. To control cDNA input, RPL13A (human) or Actb (mouse) were used as internal standard. The relative expression of target mRNA was computed from the target Ct values and RPL13A/Actb Ct values using the standard curve method (User Bullentin #2, Applied Biosystems).
Radioligand binding assays
The assays were performed as described previously (Brechter and Lerner, 2002). In brief, human gingival fibroblasts were incubated in MEM/HEPES/0.1% BSA with [3H]-BK 4 nmol·L−1 (Perkin-Elmer, Waltham, MA, USA) or [3H]-des-Arg10-Lys-BK 14 nmol·L−1 (Perkin-Elmer) for 90 min, with or without different kinin receptor agonists or antagonists. Following extensive washings, the cells were detached and the radioactivity analysed using liquid scintillation counter. The binding of [3H]-BK was strongly competed for by BK (Sigma-Aldrich, St. Louis, MO, USA) and Hoe140 (Neosystem, Strasbourg, France), whereas des-Arg10-Lys-BK (Bachem, Bubendorf, Switzerland) or des-Arg10-Hoe140 (Neosystem) were without effects (data not shown). The binding of [3H]-des-Arg10-Lys-BK to the human gingival cells was strongly inhibited for by des-Arg10-Lys-BK and des-Arg10-Hoe140, with much less effect by BK or Hoe140 (data not shown). Identical experiments were performed with MG-63 cells which exhibited similar characteristics (data not shown).
Calcium mobilization
Human gingival fibroblasts were seeded in 42 mm diameter glass coverslips at 1 × 105 cells per coverslip in 3 mL of α-MEM supplemented with 10% FCS. After overnight attachment, the media were replaced by α-MEM supplemented with 10% FCS with or without IL-1β (100 pg·mL−1) or IL-1β in combination with IL-4 (30 ng·mL−1). To assess intracellular Ca2+ ([Ca2+]i) mobilization, the media were removed 24 h after treatment with the cytokines and then the cells were washed three times with PBS 1X and loaded with Fluo-3/AM (5 μM) in humidified 37°C incubator gassed with 5% of CO2 for 30 min.
Cells were imaged in buffer (135 mM NaCl, 5 mM KCl, 10 mM HEPES, 1 mM MgCl2, 2 mM glucose, 2 mM CaCl2, pH 7.2) and BK or des-Arg10-Lys-bradykinin (DALBK) (1 μM) were added to the buffer. The change in fluorescence in 5–10 different cells was independently recorded over time. Fluorescence imaging experiments were performed in a scanning laser confocal microscope (Leica SP5, Leica, Bensheim, Germany) with a 63 X water immersion objective. Fluo-3 fluorescence dye was excited with the wavelength 488 nm using an argon ion laser, and the emitted fluorescence was measured at 510 nm. Digital image analysis was made with Image J (W.S. Rasband/National Institutes of Health, Bethesda, MD, USA, http://rsb.info.nih.gov/ij). Time-course software was used to capture images of the cells, in the Live Data Mode acquisition. ΔF/F0 (ΔF = (F(t) − F0), where F(t) is the observed fluorescence at time t, and F0 the fluorescence at t = 0. F(t) and F0 were computed by adding the intensity of the pixels in a circular area enclosing a single cell. All experiments were made at room temperature (23–25°C). The maximum ΔF/F0 for each group was calculated and the values were used for statistical analysis.
STAT6 knock down
Human gingival fibroblasts were seeded in 6-well plates at 1 × 105 cells/well in 2 mL of α-MEM supplemented with 10% FCS. After overnight attachment, the cells were washed twice with PBS and transfected with a scrambled siRNA (30 nM) or a mix of three different siRNAs targeting STAT6 (30 nM; IDs: s13540, s13541, s13542, Ambion, Austin, TX, USA) with lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), in α-MEM supplemented with 10% FBS without antibiotics. Twenty-four hours after transfection, the media were changed, and α-MEM supplemented with 10% FBS and antibiotics was added with or without test substances. The silencing was confirmed at protein level by Western blot and at the mRNA level by qPCR using Taqman Assay (Hs0059618); at least 70% of silencing was reached (Souza et al. 2012).
Statistics
Statistical analysis of multiple treatment groups was performed using one-way anova, with Levene's homogenecity test, and subsequently Bonferroni's or Dunnett's T3 post hoc test. Data shown in figures are expressed as means ± SEM for 4–6 observations. SEM is shown when the height of the error bar is larger than the radius of the symbol. The figures are representative for 2–3 independent experiments.
Results
IL-4 and IL-13 inhibit the mRNA expression of kinin receptors in unstimulated and in IL-1β and TNF-α stimulated MG-63 cells
Semi-quantitative RT-PCR demonstrated that IL-4 (30 ng·mL−1) and IL-13 (30 ng·mL−1) inhibited the basal mRNA expression of both BDKRB1 and BDKRB2 in MG-63 cells (Figure 1A). In contrast, IL-1β (100 pg·mL−1) up-regulated the mRNA expression of BDKRB1 and BDKRB2, in agreement with our previous observations (Brechter et al., 2008). qPCR confirmed that IL-4 and IL-13 significantly decreased the levels of basal, as well as IL-1β (Figure 1B,C) and TNF-α (Figure 1D,E) stimulated, mRNA expression of BDKRB1 and BDKRB2. The inhibitory effect in MG-63 cells on IL-1β induced enhancement of the mRNA expressions of both kinin receptors was dependent on the concentration (0.05–50 ng·mL−1) of IL-4 (Figure 1F, G).
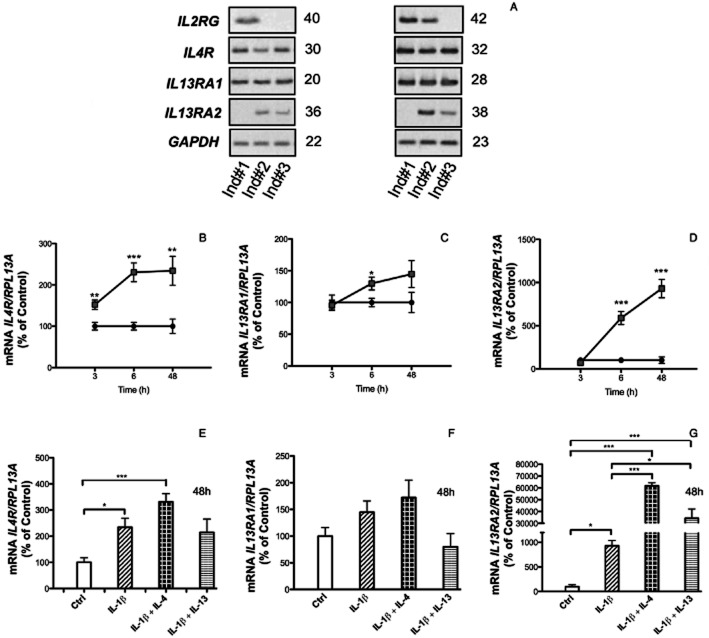
Figure 1.
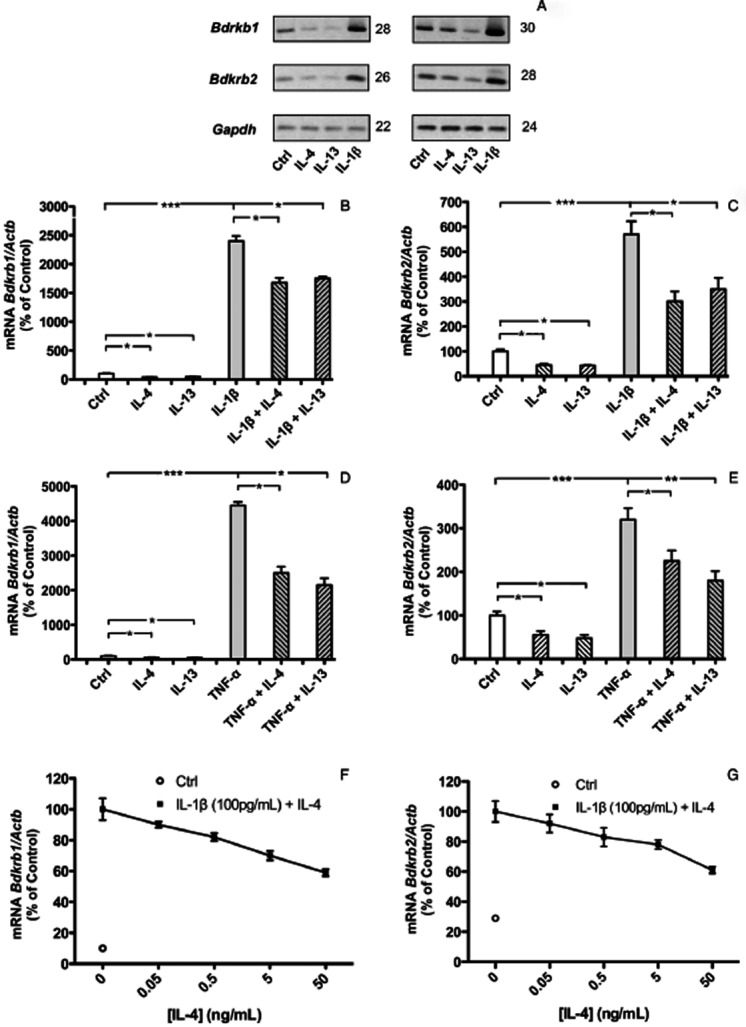
Semi-quantitative (A) and quantitative RT-PCR analyses (B–G) of the mRNA expressions of BDKRB1 (A, B, D, F) and BDKRB2 (A, C, E, G), in MG-63 cells. Effects of IL-4 (30 ng·mL−1), IL-13 (30 ng·mL−1) and IL-1β (100 pg·mL−1), on the mRNA expressions of BDKRB1 and BDKRB2 (A). Quantitative RT-PCR analyses of the effects of IL-4 (50 ng·mL−1), IL-13 (50 ng·mL−1), IL-1β (100 pg·mL−1), TNF-α (10 ng·mL−1) and the combination of IL-1β with either IL-4 or IL-13 (B, C) or TNF-α with either IL-4 or IL-13 (D,E) on the mRNA expressions of BDKRB1 and BDKRB2. IL-1β stimulated mRNA expressions of both BDKRB1 and BDKRB2 were inhibited by IL-4 in a concentration-dependent manner (F, G). The values represent means ± SEM for four wells per group. SEM is shown as vertical bars when larger than the radius of the symbol. In quantitative RT-PCR reactions, samples were analysed in duplicates and normalized with RPL13A. In B, C, D and E, the statistically significant effects are shown by asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001). In F and G, statistically significant (P < 0.05) effects were obtained at 0.5–50 ng·mL−1 IL-4. Figures to the right of the gels in (A) denotes the number of cycles used in the PCR reactions.
IL-4 and IL-13 inhibit kinin receptor mRNA expression in human gingival fibroblasts stimulated by IL-1β and TNF-α
We next studied if the inhibitory effects by IL-4 and IL-13 on kinin receptors could be observed in primary human cells and, therefore, used human gingival fibroblasts which express functional B1 and B2 receptors (Brechter et al., 2008). The basal mRNA expression of both kinin receptors was significantly reduced by IL-4 and IL-13, respectively (Figure 2A,B). IL-1β and TNF-α up-regulated the mRNA expression of BDKRB1 as well asBDKRB2, and these up-regulations were significantly inhibited by both IL-4 and IL-13 (Figure 2A,B).
Figure 2.
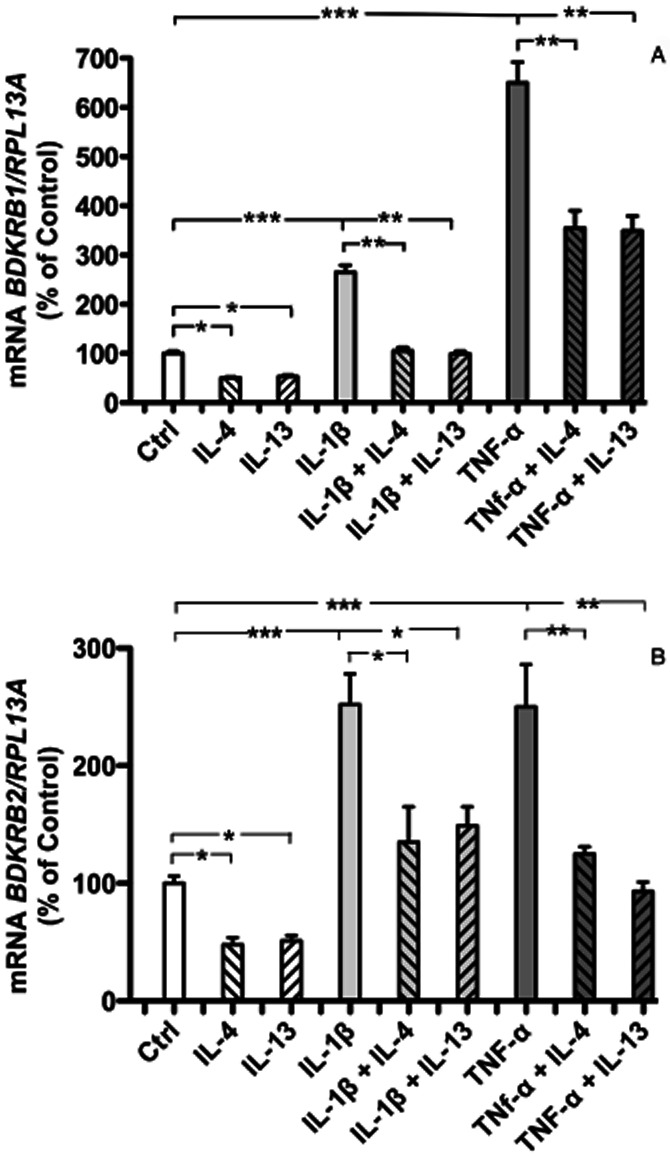
Quantitative RT-PCR analyses of the mRNA expressions of BDKRB1 (A), and BDKRB2 (B), in human gingival fibroblasts. Effects of IL-4 (50 ng·mL−1), IL-13 (50 ng·mL−1), IL-1β (100 pg·mL−1), the combination of IL-1β with either IL-4 or IL-13, TNF-α (10 ng·mL−1) and the combination of TNF-α with either IL-4 or IL-13, on the mRNA expressions of BDKRB1 (A) and BDKRB2 (B). The values represent means ± SEM for four wells per group. Samples were analysed in duplicates and normalized with RPL13A. The statistically significant effects are shown by asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
IL-4 inhibits IL-1β and TNF-α induced up-regulation of kinin binding to B1 and B2 receptors and their function
To assess if IL-4 induced down-regulation of kinin receptor mRNA was associated with decreased amount of kinin receptors, we performed radioligand binding studies. In agreement with previous observations (Brechter et al., 2008), we found that pretreament of human gingival fibroblasts for 24 h with IL-1β or TNF-α increased the binding of both [3H]-BK and [3H]-des-Arg10-Lys-BK, effects that were partially impaired by IL-4 (Figure 3A,B). Similarly, IL-1β and TNF-α increased binding of [3H]-BK and [3H]-des-Arg10-Lys-BK to MG-63 cells were partially impaired by IL-4 (data not shown).
Figure 3.
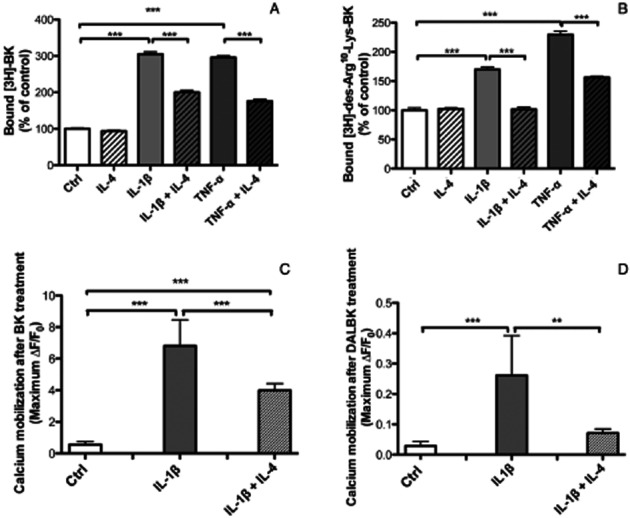
Radioligand binding showing the specific binding of [H3]-BK (A) and [H3]-DALBK (B) to human gingival fibroblasts. Cells were pre-incubated for 24 h with either control media or media containing IL-4 (30 ng·mL−1), IL-1β (100 pg·mL−1) without or with IL-4 or TNF-α (25 ng·mL−1) without or with IL-4. Then, the cells were challenged with radiolabelled ligands for 90 min. Values represent means ± SEM of cell bound radioactivity for four wells per group. In figures (C) and (D) is shown the increase in transient [Ca2+]i concentration in response to BK (C) or DALBK (D). Human gingival fibroblasts were pre-incubated without (Ctrl) or with IL-1β (100 pg·mL−1) in the absence of presence of IL-4 (30 ng·mL−1) for 24 h. Then the cells were challenged with the B1 and B2 receptor agonists (1 μM) and intracellular calcium levels were followed by recording the fluorescence intensity. Values represent means ± SEM for 5–10 recordings. The statistically significant effects are shown by asterisks (**, P < 0.01; ***, P < 0.001).
To further examine if the IL-4 induced decrease of the mRNA expression of the two kinin receptors was associated with decreased levels of functional kinin receptor proteins, we then investigated whether IL-4 affected BK and DALBK induced increase in transient [Ca2+]i in human gingival fibroblasts which had been pretreated by IL-1β with and without IL-4. Gingival fibroblasts pretreated with 100 pg·mL−1 of IL-1β for 24 h evoked a more profound increase in [Ca2+]i when stimulated by BK or DALBK, as compared with the kinin response in cells not pretreated with IL-1β. Pretreatment with IL-1β influenced only the [Ca2+]i transient amplitude, suggesting that IL-1β up-regulates the number of kinin receptors (data not shown). When the cells were pretreated with IL-1β in combination with IL-4 (30 ng·mL−1), the [Ca2+]i mobilization response to BK and DALBK was decreased, suggesting that IL-4 could prevent up-regulation of both B1 and B2 functional receptors induced by IL-1β (Figure 3C,D).
The effects of IL-4 in gingival fibroblasts is mediated by IL-4Rα/IL-13Rα1 and STAT6
IL-4 can bind to two different dimeric receptor subtypes consisting of either IL-4Rα/IL-2RγC or IL-4Rα/IL-13Rα1 (Callard et al., 1996; Murata et al., 1998). Heterodimerization of IL-4 receptors induced by ligand binding results in activation of JAKs and subsequent phosphorylation of STAT6 which then dimerizes, translocates to the nucleus and acts as a transcription factor regulating different genes. Using semi-quantitative RT-PCR, we found that human gingival fibroblasts from three different individuals constitutively expressed IL4R and IL13RA1 mRNA (Figure 4A). IL2RG and IL13RA2 mRNA could be detected in fibroblasts from 2/3 individuals, but only at much lower expression levels (Figure 4A). Using qPCR, we could detect IL4R, IL13RA1 and IL13RA2 mRNA, but not IL2RG mRNA; IL4R was most abundantly expressed (average Ct for the control at 3 h was 24.5) followed by IL13RA1 (average Ct for the control at 3 h was 28.3) and IL13RA2 (average Ct for the control at 3 h was 34.3). Over a time period of 3–48 h, IL-1β robustly increased IL13RA2 mRNA, modestly enhanced IL4RmRNA, whereas IL13RA1 mRNA was not affected (Figure 4B–D). Co-stimulation of the human gingival fibroblasts with IL-1β or either IL-4 or IL-13 did not affect the mRNA expression of IL4R and IL13RA1, but the mRNA expression of IL13RA2 induced by IL-1β was synergistically enhanced by both IL-14 and IL-13 (Figure 4E–G). These data indicate that IL-4 and IL-13 act on human gingival fibroblast mainly by Type 2 IL-4 receptors.
Figure 4.
Semi-quantitative RT-PCR (A) and qPCR (B–G) analyses of the expression of the IL-4 receptor subunits in human gingival fibroblasts. The RT-PCR analysis was performed in cells isolated from three different individuals (A). The numbers on the right of the images represent the number of cycles in the PCR reaction. In B–D is shown the time-dependent effect of IL-1β (100 pg·mL−1) on the mRNA expression of IL4R, IL13RA1 and IL13RA2. In E–G is shown the effect by IL-4 (30 ng·mL−1) on IL-1β induced regulation of IL4R, IL13RA1 and IL13RA2. The values represent means ± SEM for four wells per group. Samples were analysed in duplicates and normalized with RPL13A. The statistically significant effects are shown by asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
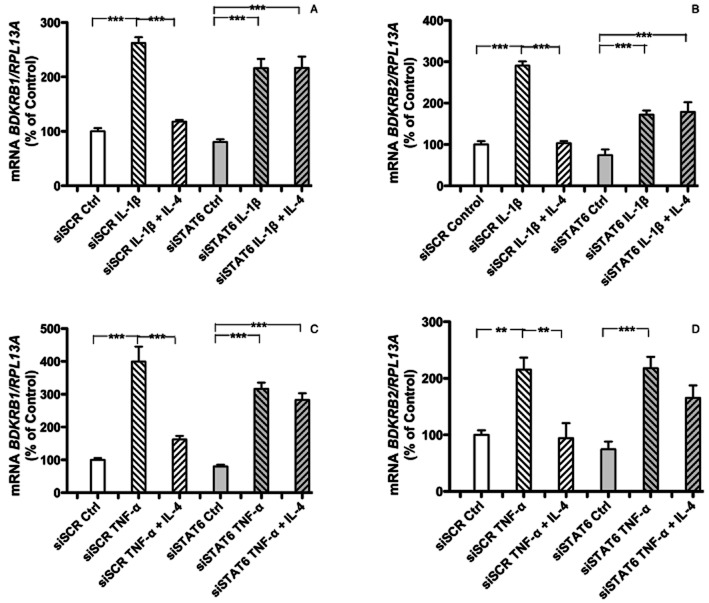
We have observed that treatment of human gingival fibroblasts with IL-4 stimulates phosphorylation of STAT6, both in the presence and absence of IL-1β (Souza et al., 2012). Therefore, the relative importance of STAT6 was assessed using silencing interference strategy. In human gingival fibroblasts rendered STAT6 deficient using an siRNA approach, it was observed that the inhibition of BDKRB1 and BDKRB2 mRNA by IL-4 in IL-1β (Figure 5A, B) or TNF-α (Figure 5C, D) stimulated cells was substantially decreased. In contrast, transfection with scrambled siRNA did not affect IL-4 induced inhibition of BDKRB1 and BDKRB2 mRNA expression in IL-1β and TNF-α stimulated cells (Figure 5A–D).
Figure 5.
Quantitative RT-PCR analyses of the mRNA expression of BDKRB1 (A, C), the BDKRB2 (B, D) in human gingival fibroblasts transfected with scrambled siRNA (siSCR) or STAT6 siRNA (siSTAT6). In A, B is shown the effects of IL-1β (100 pg·mL−1) without or with IL-4 (30 ng·mL−1) on BDKRB1 (A) and BDKRB2 (B) mRNA. In C, D is shown the effects of TNF-α (50 ng·mL−1) without or with IL-4 (30 ng·mL−1) on BDKRB1 (C) and BDKRB2 (D) mRNA. The values represent means ± SEM for four wells per group. The samples were analysed in duplicates and normalized with RPL13A. The statistically significant effects are shown by asterisks (*, P < 0.05; **P < 0.01).
We next evaluated the importance of STAT6 for the inhibitory effect of IL-4 on kinin receptor expression by using calvarial bones from mice with deletion of the Stat6 gene. IL-4 inhibited IL-1β-stimulated up-regulation of Bdkrb1 mRNA expressions in calvarial bones from wild type mice (Figure 6A), but had no effect in bones from Stat6-/- mice (Figure 6B). Similarly, the inhibition of Bdkrb2 mRNA by IL-4 in IL-1β stimulated calvarial bones from wild type mice (Figure 6C) was not observed in bones from Stat6-/- mice (Figure 6D).
Figure 6.
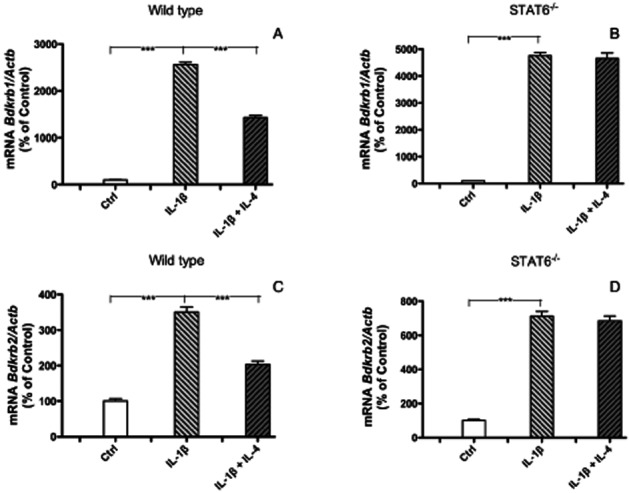
Quantitative RT-PCR analyses of the mRNA expressions of Bdkrb1 (A, B), Bdkrb2 (C, D), in calvarial bones from wild type mice (A, C) or Stat6-deficient mice (B, D). Bones were incubated for 24 h without (control) or with IL-1β (100 pg·mL−1) in the absence or presence of IL-4 (50 ng·mL−1). The values represent means ± SEM for 5–6 bones per group. The statistically significant effects are shown by asterisks (***P < 0.001).
IL-4 decreased Tnfsf11 in calvarial bones stimulated by kinins in the presence of either IL-1β or TNF-α
In order to examine if decreased expression and number of IL-4 receptors may affect osteoclast formation, we assessed if IL-4 regulated the mRNA expression of Tnfs11 (encoding for RANKL) in mouse calvarial bones. Previously, we reported that BK and DALBK synergistically potentiated IL-1β induced Tnfsf11 mRNA expression and RANKL protein in the calvarial bones, without affecting Tnfrsf11a (encoding for RANK) or Tnfrsf11b (encoding for OPG) mRNA. The robust enhancement of Tnfsf11 mRNA expression by co-treatment with kinins and IL-1β was significantly decreased by IL-4 (Figure 7), indicating that IL-4 not only decreased kinin receptors but also decreased the expression of a key molecule promoting osteoclast differentiation.
Figure 7.
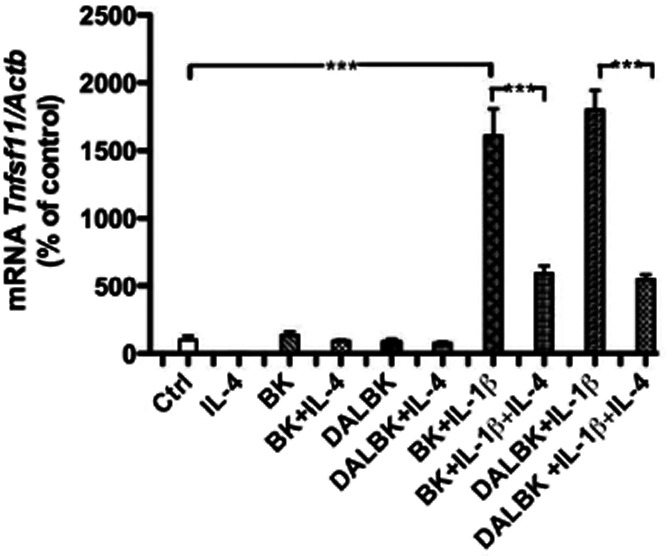
Quantitative RT-PCR analysis of the mRNA expression of Tnfsf11 in calvarial bones incubated for 24 h without or with BK (1 μM), DALBK (1 μM), IL-4 (30 ng·mL−1 and IL-1β (100 pg·mL−1) in different combinations. The values represent means ± SEM for 5–6 bones per group. The statistically significant effects are shown by asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Discussion
In the present study, we demonstrate that IL-4 and IL-13 inhibit the mRNA expression of BDKRB1 and BDKRB2 in human MG-63 cells, as well as in intact mouse calvarial bones. IL-4 and IL-13 inhibit both constitutive expression of BDKRB1 and BDKRB2 mRNA, as well as the IL-1β and TNF-α-induced enhanced expression of the two kinin receptors in the MG-63 cells. IL-4 and IL-13 also inhibited the up-regulation of both receptor subtypes in calvarial bones stimulated by IL-1β. These findings may explain the observations showing that local administration of IL-4 inhibited, in a dose-dependent manner, hyperalgesic responses caused by BK and TNF-α in rats (Cunha et al., 1999), and that IL-4 depressed the B1 receptor sensitization process, in human umbilical veins (Sardi et al., 2002). IL-4 has been shown to inhibit bone resorption in inflammation-induced apical periodontitis (Stashenko et al., 2007), an effect which could be explained by its inhibitory effect on RANK expression in osteoclasts and/or RANKL expression in osteoblasts (Palmqvist et al., 2006). Similarly, IL-4 has also been shown to inhibit bone resorption in juxtaarticular bone in collagen type II-induced arthritis in mice (Joosten et al., 1999; Lubberts et al., 2000) and in adjuvant-induced arthritis in rats (Woods et al., 2001). Our data in the present study indicate that IL-4 may inhibit inflammation-induced osteoclast formation not only by its effect on RANKL-RANK-OPG pathway, but also by decreasing the expression of kinin receptors in osteoblasts. We here show that IL-4 inhibits the robust up-regulation of Tnfsf11 mRNA induced by co-treatment with IL-1β and either BK or DALBK. Although these data do not show that IL-4 inhibits Tnfsf11 through decreased kinin receptor expression, the data demonstrate the effect by IL-4 on inflammation-induced RANKL. Final proof for that the effect is mediated, totally or partially, by decreased kinin receptor expression must await in vivo data in mice lacking IL-4 receptors and kinin receptors in osteoblasts.
Based upon the observations that kinins can stimulate prostaglandin E2 (PGE2) formation and synergistically potentiate the stimulatory effects by IL-1β and TNF-α on PGE2 in human gingival fibroblasts (Lerner, 1991), together with the fact that PGE2 is a potent stimulator of RANKL in osteoblasts (Nagai and Sato, 1999; Okada et al., 2000), we have suggested that kinin receptors in gingival fibroblasts may be important in the pathogenesis of periodontal disease (Brechter and Lerner, 2002; 2007). We here show that IL-4 and IL-13 inhibit basal and cytokine-induced mRNA expression of kinin receptors also in primary human gingival fibroblasts. That not only mRNA expression was decreased by IL-4, but also kinin receptor protein, is demonstrated by our observations that IL-4 strongly decreased IL-1β and TNF-α induced increased specific binding of radiolabelled BK and DALBK to surface receptors on the human gingival fibroblasts.
In order to further the evidence that not only the mRNA levels of kinin receptors and receptor binding, but also the expression of functional receptors was affected by IL-4 treatment, we took advantage of the fact that B1 and B2 receptor activation leads to intracellular calcium mobilization (Bertram et al., 2007; Zubakova et al., 2008). Corroborating with previous data showing a potentiation of [Ca2+]i by BK in human gingival fibroblasts primed with IL-1β, (Nakao et al., 2001), we observed a higher calcium transient amplitude in response to BK when the human gingival fibroblasts were pretreated with IL-1β for 24 h. This response was markedly inhibited when the cells were co-treated with IL-4 together with IL-1β. We have previously shown that BK, but not DALBK, induces a transient increase in [Ca2+]i in human gingival fibroblasts (Lerner et al., 1992), which is most likely due to the fact that the B1 receptor is not constitutively expressed at the same levels as the B2 receptor. Here we show using DALBK as a B1 agonist, that pretreatment with IL-1β induces a transient rise of calcium in cells subsequently exposed to DALBK. This observation is in agreement with our previous finding showing that IL-1β up-regulates B1 receptor mRNA and binding in human gingival fibroblasts (Brechter et al., 2008). Also, the DALBK induced burst of intracellular calcium was substantially decreased when cells were treated with IL-4 in addition to IL-1β (Figure 3D), indicating down-regulation of functional receptor protein. Taken together, the radioligand data and the intracellular calcium assays show that B1 and B2 receptor proteins are up-regulated by IL-1β and that this effect is impaired by IL-4.
To further investigate the mechanisms involved in the inhibitory effects of IL-4, we analysed the expression of the receptor subunits that recognize IL-4 and IL-13 in human gingival fibroblasts. These cells were found to mainly express the IL-4Rα and IL-13Rα1 receptor subunits, which is typical for non-hematopoietic cells and which indicate that IL-4 and IL-13 exert their effect on kinin receptor expression through Type 2 IL-4 receptors, known to be activated by both cytokines (Callard et al., 1996). The IL13-Rα2 subunit, known to be involved in IL-13 signalling (Fichtner-Feigl et al., 2006), was also detected, but at much lower levels compared to the expression of the Type 2 IL-4 receptors subunits. Surprisingly, its expression was strongly enhanced by IL-1β, an effect that was synergistically potentiated by IL-4 and IL-13. Similarly, enhanced expression of IL-13-Rα2 mRNA and protein induced by the combination of TNF-α and IL-4 or IL-13 has previously been reported in human keratinocytes and macrophages (David et al., 2003; Fichtner-Feigl et al., 2006). However, regulation of the expression of this receptor subunit does not interfere with IL-4 signalling, but interferes with IL-13 signalling, probably because of the affinity of IL-13 for the IL-13Rα2 subunit (Fichtner-Feigl et al., 2006). Therefore, Type 2 receptors are the strongest candidates mediating the inhibitory effects by IL-4 and IL-13 on kinin receptors in human gingival fibroblasts.
In order to investigate the molecules that act downstream to the IL-4 receptors, we assessed the importance of phosphorylation of STAT6 in human gingival fibroblasts exposed to IL-4. Activation of STAT-6 is a well-known downstream mechanism in IL-4 receptor signalling (Callard et al., 1996; Kelly-Welch et al., 2003; Hebenstreit et al., 2006). We have recently reported that STAT6 is phosphorylated when human gingival fibroblasts are exposed to IL-4, without IL-4 affecting total STAT6 protein (Souza et al., 2012). Enhanced IL-4 induced STAT6 phosphorylation was observed both in the presence and absence of IL-1β. To evaluate the importance of this transcription factor for the inhibition of B1 and B2 receptors expression by IL-4, we silenced STAT6 using transfection with specific oligonucleotides in these cells. In the human gingival fibroblasts in which STAT6 had been knocked down, the impairment on IL-1β induced B2 and B1 receptor up-regulation by IL-4 was abolished, showing that STAT6 is crucial for the effects by IL-4 on kinin receptor expression in these cells. In order to confirm if this is true also for bone cells, we used calvarial bones from Stat6-deficient mice and compared these results with those from the corresponding Balb wild type mice. The inhibitory effect, caused by IL-4 on IL-1β induced B1 or B2 receptor mRNA expression in calvarial bones from wild type mice could not be observed in calvarial bones from Stat6-/- mice, indicating that STAT6 is critically important in this regulation.
In conclusion, we here demonstrate, for the first time, that B1 and B2 receptors are down-regulated by IL-4 and IL-13 via a STAT6-dependent pathway. These inhibitory effects might be important in IL-4 induced inhibition of inflammation-induced bone resorption, in diseases such as periodontitis and rheumatoid arthritis.
Acknowledgments
We would like to thank Mrs. Inger Lundgren and Mrs. Ingrid Boström for skilful technical assistance. This work was supported by the Swedish Research Council for Medicine, the Swedish Rheumatism Association, the Royal 80 Year Found of King Gustav V, the County Council of Västerbotten, the Swedish Dental Society, Combine, Salus Ansvar, the Lundberg Foundation, ALF/LUA grants from the Sahlgrenska University Hospital, Fundação de Amparo à Pesquisa do Estado de São Paulo (Grants 2008/58958-7, 2008/07221-4) and CNPq (Grant 301291/2010 PQ).
Glossary
- α-MEM
α-modification of minimal essential medium
- BK
bradykinin
- DALBK
des-Arg10-Lys-bradykinin
- FCS
fetal calf serum
- IL
interleukin
- IL-13R
interleukin-13 receptor
- IL-2γC
common IL-2γ chain
- IL-4R
interleukin-4 receptor
- JAK
janus tyrosine kinase
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- PGE2
prostaglandin E2
- qPCR
quantitative real-time polymerase chain reaction
- RANK
receptor activator of nuclear factor κB
- RANKL
receptor activator of nuclear factor κB ligand
- STAT6
signal transducers and activators of transcription 6
- TH2
T helper type 2
- TNF
tumour necrosis factor
Conflicts of interests
The authors declare that they have no conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Table S1 Table showing the sequences, Gene Bank accession numbers of the primers used for the semi-quantitative analyses.
References
- Andrews AL, Nordgren IK, Kirby I, Holloway JW, Holgate ST, Davies DE, et al. Cytoplasmic tail of IL-13Ralpha2 regulates IL-4 signal transduction. Biochem Soc Trans. 2009;37(Pt 4):873–876. doi: 10.1042/BST0370873. [DOI] [PubMed] [Google Scholar]
- Bertram CM, Baltic S, Misso NL, Bhoola KD, Foster PS, Thompson PJ, et al. Expression of kinin B1 and B2 receptors in immature, monocyte-derived dendritic cells and bradykinin-mediated increase in intracellular Ca2+ and cell migration. J Leukoc Biol. 2007;81:1445–1454. doi: 10.1189/jlb.0106055. [DOI] [PubMed] [Google Scholar]
- Brechter AB, Lerner UH. Characterization of bradykinin receptors in a human osteoblastic cell line. Regul Pept. 2002;103:39–51. doi: 10.1016/s0167-0115(01)00325-1. [DOI] [PubMed] [Google Scholar]
- Brechter AB, Lerner UH. Bradykinin potentiates cytokine-induced prostaglandin biosynthesis in osteoblasts by enhanced expression of cyclooxygenase 2, resulting in increased RANKL expression. Arthritis Rheum. 2007;56:910–923. doi: 10.1002/art.22445. [DOI] [PubMed] [Google Scholar]
- Brechter AB, Persson E, Lundgren I, Lerner UH. Kinin B1 and B2 receptor expression in osteoblasts and fibroblasts is enhanced by interleukin-1 and tumour necrosis factor-alpha. Effects dependent on activation of NF-kappaB and MAP kinases. Bone. 2008;43:72–83. doi: 10.1016/j.bone.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Callard RE, Matthews DJ, Hibbert L. IL-4 and IL-13 receptors: are they one and the same? Immunol Today. 1996;17:108–110. doi: 10.1016/0167-5699(96)80600-1. [DOI] [PubMed] [Google Scholar]
- Clover J, Gowen M. Are MG-63 and HOS TE85 human osteosarcoma cell lines representative models of the osteoblastic phenotype? Bone. 1994;15:585–591. doi: 10.1016/8756-3282(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Couture R, Harrisson M, Vianna RM, Cloutier F. Kinin receptors in pain and inflammation. Eur J Pharmacol. 2001;429:161–176. doi: 10.1016/s0014-2999(01)01318-8. [DOI] [PubMed] [Google Scholar]
- Cunha FQ, Poole S, Lorenzetti BB, Veiga FH, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-4. Br J Pharmacol. 1999;126:45–50. doi: 10.1038/sj.bjp.0702266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David MD, Bertoglio J, Pierre J. Functional characterization of IL-13 receptor alpha2 gene promoter: a critical role of the transcription factor STAT6 for regulated expression. Oncogene. 2003;22:3386–3394. doi: 10.1038/sj.onc.1206352. [DOI] [PubMed] [Google Scholar]
- Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Hart PH, Vitti GF, Burgess DR, Whitty GA, Piccoli DS, Hamilton JA. Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci U S A. 1989;86:3803–3807. doi: 10.1073/pnas.86.10.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart PH, Cooper RL, Finlay-Jones JJ. IL-4 suppresses IL-1 beta, TNF-alpha and PGE2 production by human peritoneal macrophages. Immunology. 1991;72:344–349. [PMC free article] [PubMed] [Google Scholar]
- Hebenstreit D, Wirnsberger G, Horejs-Hoeck J, Duschl A. Signaling mechanisms, interaction partners, and target genes of STAT6. Cytokine Growth Factor Rev. 2006;17:173–188. doi: 10.1016/j.cytogfr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Joosten LA, Lubberts E, Helsen MM, Saxne T, Coenen-de Roo CJ, Heinegard D, et al. Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis. Arthritis Res. 1999;1:81–91. doi: 10.1186/ar14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakoki M, Sullivan KA, Backus C, Hayes JM, Oh SS, Hua K, et al. Lack of both bradykinin B1 and B2 receptors enhances nephropathy, neuropathy, and bone mineral loss in Akita diabetic mice. Proc Natl Acad Sci U S A. 2010;107:10190–10195. doi: 10.1073/pnas.1005144107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003;300:1527–1528. doi: 10.1126/science.1085458. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg LM, Marceau F, Muller-Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- Lerner U, Hänstrom L. Human gingival fibroblasts secrete non-dialyzable, prostanoid-independent products which stimulate bone resorption in vitro. J Periodontal Res. 1987;22:284–289. doi: 10.1111/j.1600-0765.1987.tb01586.x. [DOI] [PubMed] [Google Scholar]
- Lerner UH. Bradykinin synergistically potentiates interleukin-1 induced bone resorption and prostanoid biosynthesis in neonatal mouse calvarial bones. Biochem Biophys Res Commun. 1991;175:775–783. doi: 10.1016/0006-291x(91)91633-n. [DOI] [PubMed] [Google Scholar]
- Lerner UH. The role of kallikrein-kinin system in inflammation-induced bone metabolism. In: Farmer SG, editor. The Kinin System. New York: Academic Press; 1997. pp. 219–234. [Google Scholar]
- Lerner UH. Inflammation-induced bone remodeling in periodontal disease and the influence of post-menopausal osteoporosis. J Dent Res. 2006;85:596–607. doi: 10.1177/154405910608500704. [DOI] [PubMed] [Google Scholar]
- Lerner UH, Modeer T. Bradykinin B1 and B2 receptor agonists synergistically potentiate interleukin-1-induced prostaglandin biosynthesis in human gingival fibroblasts. Inflammation. 1991;15:427–436. doi: 10.1007/BF00923340. [DOI] [PubMed] [Google Scholar]
- Lerner UH, Jones IL, Gustafson GT. Bradykinin, a new potential mediator of inflammation-induced bone resorption. Studies of the effects on mouse calvarial bones and articular cartilage in vitro. Arthritis Rheum. 1987;30:530–540. doi: 10.1002/art.1780300507. [DOI] [PubMed] [Google Scholar]
- Lerner UH, Ransjo M, Ljunggren O. Bradykinin stimulates production of prostaglandin E2 and prostacyclin in murine osteoblasts. Bone Miner. 1989;5:139–154. doi: 10.1016/0169-6009(89)90092-5. [DOI] [PubMed] [Google Scholar]
- Lerner UH, Brunius G, Anduren I, Berggren PO, Juntti-Berggren L, Modeer T. Bradykinin induces a B2 receptor-mediated calcium signal linked to prostanoid formation in human gingival fibroblasts in vitro. Agents Actions. 1992;37:44–52. doi: 10.1007/BF01987889. [DOI] [PubMed] [Google Scholar]
- Ljunggren O, Lerner UH. Evidence for BK1 bradykinin-receptor-mediated prostaglandin formation in osteoblasts and subsequent enhancement of bone resorption. Br J Pharmacol. 1990;101:382–386. doi: 10.1111/j.1476-5381.1990.tb12718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubberts E, Joosten LA, Chabaud M, van Den Bersselaar L, Oppers B, Coenen-De Roo CJ, et al. IL-4 gene therapy for collagen arthritis suppresses synovial IL-17 and osteoprotegerin ligand and prevents bone erosion. J Clin Invest. 2000;105:1697–1710. doi: 10.1172/JCI7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau F, Hess JF, Bachvarov DR. The B1 receptors for kinins. Pharmacol Rev. 1998;50:357–386. [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P, Chomarat P, Dechanet J, Moreau JF, Roux JP, Delmas P, et al. Interleukin-4 inhibits bone resorption through an effect on osteoclasts and proinflammatory cytokines in an ex vivo model of bone resorption in rheumatoid arthritis. Arthritis Rheum. 1994;37:1715–1722. doi: 10.1002/art.1780371202. [DOI] [PubMed] [Google Scholar]
- Moreau ME, Garbacki N, Molinaro G, Brown NJ, Marceau F, Adam A. The kallikrein-kinin system: current and future pharmacological targets. J Pharmacol Sci. 2005;99:6–38. doi: 10.1254/jphs.srj05001x. [DOI] [PubMed] [Google Scholar]
- Moreno JL, Kaczmarek M, Keegan AD, Tondravi M. IL-4 suppresses osteoclast development and mature osteoclast function by a STAT6-dependent mechanism: irreversible inhibition of the differentiation program activated by RANKL. Blood. 2003;102:1078–1086. doi: 10.1182/blood-2002-11-3437. [DOI] [PubMed] [Google Scholar]
- Murata T, Obiri NI, Puri RK. Structure of and signal transduction through interleukin-4 and interleukin-13 receptors (review) Int J Mol Med. 1998;1:551–557. doi: 10.3892/ijmm.1.3.551. [DOI] [PubMed] [Google Scholar]
- Nagai M, Sato N. Reciprocal gene expression of osteoclastogenesis inhibitory factor and osteoclast differentiation factor regulates osteoclast formation. Biochem Biophys Res Commun. 1999;257:719–723. doi: 10.1006/bbrc.1999.0524. [DOI] [PubMed] [Google Scholar]
- Nakao S, Ogata Y, Modeer T, Segawa M, Furuyama S, Sugiya H. Bradykinin induces a rapid cyclooxygenase-2 mRNA expression via Ca2+ mobilization in human gingival fibroblasts primed with interleukin-1 beta. Cell Calcium. 2001;29:446–452. doi: 10.1054/ceca.2001.0206. [DOI] [PubMed] [Google Scholar]
- Okada Y, Lorenzo JA, Freeman AM, Tomita M, Morham SG, Raisz LG, et al. Prostaglandin G/H synthase-2 is required for maximal formation of osteoclast-like cells in culture. J Clin Invest. 2000;105:823–832. doi: 10.1172/JCI8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist P, Lundberg P, Persson E, Johansson A, Lundgren I, Lie A, et al. Inhibition of hormone and cytokine-stimulated osteoclastogenesis and bone resorption by interleukin-4 and interleukin-13 is associated with increased osteoprotegerin and decreased RANKL and RANK in a STAT6-dependent pathway. J Biol Chem. 2006;281:2414–2429. doi: 10.1074/jbc.M510160200. [DOI] [PubMed] [Google Scholar]
- Riancho JA, Zarrabeitia MT, Olmos JM, Amado JA, Gonzalez-Macias J. Effects of interleukin-4 on human osteoblast-like cells. Bone Miner. 1993;21:53–61. doi: 10.1016/s0169-6009(08)80120-1. [DOI] [PubMed] [Google Scholar]
- Sardi SP, Rey-Ares V, Pujol-Lereis VA, Serrano SA, Rothlin RP. Further pharmacological evidence of nuclear factor-kappa B pathway involvement in bradykinin B1 receptor-sensitized responses in human umbilical vein. J Pharmacol Exp Ther. 2002;301:975–980. doi: 10.1124/jpet.301.3.975. [DOI] [PubMed] [Google Scholar]
- Souza PP, Palmqvist P, Lundberg P, Lundgren I, Hanstrom L, Souza JA, et al. Interleukin-4 and interleukin-13 inhibit the expression of leukemia inhibitory factor and interleukin-11 in fibroblasts. Mol Immunol. 2012;49:601–610. doi: 10.1016/j.molimm.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Stashenko P, Goncalves RB, Lipkin B, Ficarelli A, Sasaki H, Campos-Neto A. Th1 immune response promotes severe bone resorption caused by Porphyromonas gingivalis. Am J Pathol. 2007;170:203–213. doi: 10.2353/ajpath.2007.060597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata Y, Khurana Hershey GK. IL-13 receptor isoforms: breaking through the complexity. Curr Allergy Asthma Rep. 2007;7:338–345. doi: 10.1007/s11882-007-0051-x. [DOI] [PubMed] [Google Scholar]
- te Velde AA, Huijbens RJ, Heije K, de Vries JE, Figdor CG. Interleukin-4 (IL-4) inhibits secretion of IL-1 beta, tumor necrosis factor alpha, and IL-6 by human monocytes. Blood. 1990;76:1392–1397. [PubMed] [Google Scholar]
- Woods JM, Katschke KJ, Volin MV, Ruth JH, Woodruff DC, Amin MA, et al. IL-4 adenoviral gene therapy reduces inflammation, proinflammatory cytokines, vascularization, and bony destruction in rat adjuvant-induced arthritis. J Immunol. 2001;166:1214–1222. doi: 10.4049/jimmunol.166.2.1214. [DOI] [PubMed] [Google Scholar]
- Worthy K, Figueroa CD, Dieppe PA, Bhoola KD. Kallikreins and kinins: mediators in inflammatory joint disease? Int J Exp Pathol. 1990;71:587–601. [PMC free article] [PubMed] [Google Scholar]
- Zubakova R, Gille A, Faussner A, Hilgenfeldt U. Ca2+ signalling of kinins in cells expressing rat, mouse and human B1/B2-receptor. Int Immunopharmacol. 2008;8:276–281. doi: 10.1016/j.intimp.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Zurawski SM, Chomarat P, Djossou O, Bidaud C, McKenzie AN, Miossec P, et al. The primary binding subunit of the human interleukin-4 receptor is also a component of the interleukin-13 receptor. J Biol Chem. 1995;270:13869–13878. doi: 10.1074/jbc.270.23.13869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.