Abstract
Purpose
Glutathione depletion has been documented in several disease states, and exogenous administration has been hypothesized to have therapeutic potential for some conditions. In an effort to reach target tissues of the sinuses and central nervous system (CNS), glutathione is being prescribed as an intranasal spray, although no literature exists to support this mode of administration. The objective of this study was to describe patient-reported outcomes in a population of individuals who have been prescribed intranasal reduced glutathione, (in)GSH.
Methods
A survey was designed to assess individuals' perception of tolerability, adverse events, and health benefits associated with (in)GSH use. Using a pharmacy database, 300 individuals were randomly selected to receive a survey; any individual who had received one or more prescriptions for (in)GSH between March 2009 and March 2011 was eligible for participation.
Results
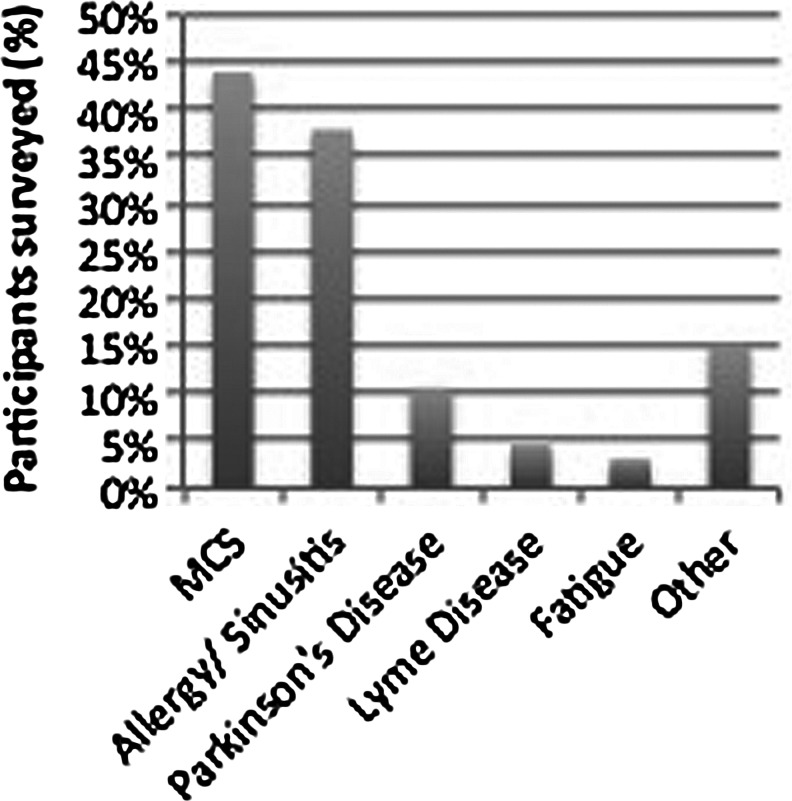
Seventy (70) individuals returned the survey (23.3% response rate) from 20 different states. Reported indications for (in)GSH prescriptions were multiple chemical sensitivity (MCS) (n=29), allergies/sinusitis (n=25), Parkinson disease (PD) (n=7), Lyme disease (n=3), fatigue (n=2), and other (n=10). Of the respondents, 78.8% (n=52) reported an overall positive experience with (in)GSH, 12.1% (n=8) reported having experienced adverse effects, and 62.1% (n=41) reported having experienced health benefits attributable to (in)GSH use. Over 86% of respondents considered the nasal spray to be comfortable and easy to administer.
Conclusions
This is the first study to evaluate patient-reported outcomes among individuals across the country who have been prescribed (in)GSH. The majority of survey respondents considered (in)GSH to be effective and without significant adverse effects. (in)GSH should be further evaluated as a method of treating respiratory and CNS diseases where free-radical burden is a suspected contributor to disease progression.
Introduction
Glutathione is an endogenously synthesized tripeptide consisting of cysteine, glutamate, and glycine. Glutathione is an essential antioxidant, and an imbalance of glutathione homeostasis has been implicated in the pathogenesis of many diseases of the respiratory, immune, and central nervous systems (CNS). While diminished glutathione levels have been described in numerous diseases,1–4 little attention has been given to exogenous repletion as a therapeutic strategy. This unpatentable molecule is being compounded by pharmacists and used nationwide, and yet no published information has been available regarding safety or efficacy.
Glutathione plays an important role in the detoxification of reactive oxygen species (ROS) in the central nervous system, where it directly quenches radicals in nonenzymatic reactions and reduces peroxides generated by glutathione peroxidase. Antioxidant activity occurs in the cytosol, membranes, and within the mitochondria.5 In addition, glutathione plays a role in detoxification, the transport of cysteine, cell proliferation, and the regulation of apoptosis.6
Intranasal administration of glutathione has been hypothesized to be advantageous over other methods of administration, when the target tissue is the brain or upper respiratory tract.7 While the rich vasculature of the nasal mucosa might facilitate systemic absorption, intranasal administration provides direct contact with the mucous membranes of the nasal passages and sinuses, which may be advantageous in conditions of the upper respiratory tract. For diseases of the CNS, intranasal administration may be a unique means of bypassing the blood–brain barrier. Absorption into the CNS after intranasal administration has been postulated to occur via the olfactory and trigeminal neuronal pathways (intraneuronal) and diffusion across mucosal barrier and olfactory plate (extraneuronal), depending on the size, polarity, and odorant nature. Intraneuronal absorption requires axonal transport and thus occurs over hours to days to reach target tissue throughout the brain. Diffusion across the olfactory plate results in immediate delivery and has been shown to occur for small, water-soluble particles less than 1000 daltons (Da) in size.8 Glutathione is a small, odorant, polar molecule of only 307.33 Da, suggesting that the molecule may be well absorbed without enhancers, but pharmacokinetic studies of intranasally administered glutathione have not been conducted in either healthy or diseased populations (Table 1).
Table 1.
Advantages and Limitations of Intranasal Administration of Medications
| Advantages | Limitations |
|---|---|
| No modification of therapy is required | Delivery is expected to decrease with increasing molecular size |
| Reduces systemic exposure | Mucosal irritation or damage may occur |
| Noninvasive, easy to administer | Nasal congestion may interfere with delivery |
| Drug degradation and metabolism are minimized | Unknown delivery to various brain tissues |
| May bypass the blood–brain barrier | Limited data on central nervous system absorption, utilization, and metabolism |
The history of intranasal reduced glutathione [(in)GSH] dates back to 2003, when environmental medicine practitioners began using it to treat MCS. In 2004, one of us (LKM) began using it for PD, Huntington disease, Down syndrome, and autism. Over 2000 individuals have received prescriptions for intranasal glutathione through Key Pharmacy, a compounding pharmacy in Kent, WA, since 2003.9 Because it is a sterile product, it is regulated in all states by the respective state board of pharmacy according to USP sterile guidelines 797. Only one peer-reviewed publication exists in the literature that mentions (in)GSH, and it is not indexed on PubMed.7
Surveys are an inexpensive and efficient instrument for collecting patient-reported outcomes. In response to increasing prescribing and a paucity of data, the authors decided to survey patients who have used (in)GSH about their experiences.
Methods
Key Pharmacy, a pharmacy with 14 years of experience compounding glutathione products, offered to make their database available for this study. This particular pharmacy was chosen because they compound the glutathione using a stabilization formula that results in >97.4% stability of reduced glutathione after 30 days, and 94% stability at 60 days.10 Institutional Review Board approval was obtained from Bastyr University to send surveys to 300 randomly selected individuals from the Key Pharmacy database. Thousands of prescriptions have been written by hundreds of practitioners11 across the country. In an effort to maximize the number of patients who returned the survey, only individuals who had received a prescription between March 31, 2009 and March 31, 2011, the 24 months prior to mailing (n=558), were eligible. Pharmacy staff used their database to identify all individuals who received a prescription for (in)GSH, which was exported into Microsoft Excel. The list was sorted by last name, and then a blank column A was added, in which the formula “=RAND()” was added to every cell. This function allocates a random rational number between 0 and 1 in each cell where the function is used. The entire database was then sorted by column A (the random number) in ascending order, and the top 300 patients on the list were selected as our sample group. Key Pharmacy conducted the mailing to protect individuals' privacy, and anonymous surveys were returned to the principal investigator at Bastyr University. Key Pharmacy did not play a role in data collection, analysis, or manuscript preparation.
Data were compiled 2 months after the surveys were mailed. Patients were asked when they began using (in)GSH. When an exact date was provided, that start date was used. So as not to over- or underestimate treatment duration, the midpoint of the date provided was entered (e.g., when only a month and year were provided, the 15th of that month was entered; when only a year was provided, July 15 was entered).
Results
Of the 70 respondents, 66 (94.3%) reported having filled a prescription for (in)GSH. Of those 66 respondents, 51 (77.3%) were female. The age of (in)GSH-treated respondents ranged from 20 to 78 years, with a mean age of 56.8 years (standard deviation [SD], 12.9). The mean age of women treated with (in)GSH was 56.2 (SD 13.1), compared with 58.6 (SD 12.5) for men. Of the 66 respondents, 36 (54.6%) resided in Washington State; the remaining respondents resided in 19 other states. The duration of prescription use ranged from 2 to 178 months, with a mean of 37.4 months (SD 37.3) (Fig. 1) (Tables 2 and 3).
FIG. 1.
Reported indications for use of intranasal reduced glutathione [(in)GSH], presented as percent (%) of individuals surveyed. MCS, multiple chemical sensitivity.
Table 2.
Participant-Reported Experiences Attributable to Intranasal Glutathione
| |
MCS |
Allergy/sinusitis |
Parkinson's |
Other |
Total |
|---|---|---|---|---|---|
| Diagnosis | 42.0% (N=29) | 36.2% (N=25) | 10.1% (N=7) | 18.2% (N=12) | 100% (N=66) |
| Single diagnosis selected | 75.9% (n=22) | 84.0% (n=21) | 100.0% (n=7) | 41.7% (n=5) | 83.3% (n=55) |
| Multiple diagnoses selected | 24.1% (n=7) | 16.0% (n=4) | 0.0% (n=0) | 58.3% (n=7) | 16.7% (n=11) |
| Demographics | |||||
| Female | 93.1% (n=27) | 76.0% (n=19) | 14.3% (n=1) | 75.0% (n=9) | 77.3% (n=51) |
| Mean/median age | 54.1/54.0 | 54.2/53.0 | 69.6/68.0 | 55.3.54.0 | 56.8/58.0 |
| Overall experience | |||||
| Positive | 72.4% (n=21) | 88.0% (n=22) | 57.1% (n=4) | 66.7% (n=8) | 78.8% (n=52) |
| Neutral | 13.8% (n=4) | 12.0% (n=3) | 42.9% (n=3) | 16.7% (n=2) | 15.2% (n=10) |
| Negative | 6.9% (n=2) | – | – | – | 3.0% (n=2) |
| Median duration of use: months (p25/p75) | 32.5 (16.0/65.0) | 22.0 (10.0/52.0) | 9.4 (9.0/11.1) | 34.0 (22.0/44.5) | 24.0 (10.0/56.0) |
| Frequency of use | |||||
| Consistent | 55.2% (n=16) | 44.0% (n=11) | 42.9% (n=3) | 50.0% (n=6) | 50.0% (n=33) |
| Intermittent | 31.0% (n=9) | 44.0% (n=11) | 42.9% (n=3) | 25.0% (n=3) | 36.4% (n=24) |
| Discontinued | 10.3% (n=3) | 12.0% (n=3) | 14.3% (n=1) | 16.7% (n=2) | 12.1% (n=8) |
| Negative effects | 20.7% (n=6) | – | – | 25.0% (n=3) | 12.1% (n=8) |
| Health benefits | 62.1% (n=18) | 60.0% (n=15) | 42.9% (n=3) | 58.3% (n=8) | 62.1% (n=41) |
| Perceived consequences of (in)GSH use | |||||
| Irritation of sinuses or nasal passages | 31.0% (n=9) | 4% (n=1) | 14.3% (n=1) | 16.7% (n=2) | 18.2% (n=12) |
| Headaches | 20.7% (n=6) | – | – | 8.3% (n=1) | 9.1% |
| Loss of smell | – | – | – | – | – |
| Worsening of disease symptoms | 3.4% (n=1) | – | – | – | 1.5% |
| Bloody nose | 13.8% (n=4) | 4% (n=1) | – | – | 7.6% |
| More frequent sinus infections | – | – | – | – | – |
| More frequent ear infections | – | – | – | – | – |
| Improved energy | 17.2% (n=5) | 20% (n=5) | 28.6% (n=2) | 25.0% (n=3) | 24.2% (n=16) |
| Improved sense of well-being | 31.0% (n=9) | 16% (n=4) | 14.3% (n=1) | 33.3% (n=4) | 28.8% (n=19) |
| Improvement in sense of smell | 10.3% (n=3) | 12% (n=3) | 14.3% (n=1) | 8.3% (n=1) | 12.1% (n=8) |
| Improvement in disease symptoms | 44.8% (n=13) | 52% (n=13) | 28.6% (n=2) | 33.3% (n=4) | 45.5% (n=30) |
| Reduced frequency of headaches | 13.8% (n=4) | 16% (n=4) | – | 16.7% (n=2) | 15.2% (n=10) |
| Less frequent sinus infections | 13.8% (n=4) | 48% (n=12) | – | 16.7% (n=2) | 27.3% (n=18) |
| Less frequent ear infections | 3.4% (n=1) | 4% (n=1) | – | – | 3.0% (n=2) |
MCS, multiple chemical sensitivities; (in)GSH, intranasal reduced glutathione.
Table 3.
Experience with Administration of Intranasal Glutathione
| Yes | No | Total | |
|---|---|---|---|
| Do you use the spray bottle provided by Key Pharmacy? | 93.9% (n=62) | 4.6% (n=3) | 98.5% (n=65; 1 missing) |
| Comfortable | Uncomfortable | ||
|---|---|---|---|
| Do you consider the administration to be comfortable or uncomfortable? | 86.4% (n=57) | 7.6% (n=5) | 94.0% (n=62; 4 missing) |
| Easy | Difficult | Total | |
|---|---|---|---|
| Do you consider the nasal spray to be physically easy or difficult to administer? | 87.9% (n=58) | 9.1% (n=6) | 97.0% (n=64; 2 missing) |
Discussion
This study is the first to utilize a survey to evaluate patient-reported outcomes among users of (in)GSH. From the results of the survey, it appears the therapy is well tolerated with few reported side-effects. That the therapy has been in use for years and that 78% of total survey respondents consider their overall experience with (in)GSH to be positive are among the most notable findings of this survey. For all conditions combined, the most frequently reported benefits include a reported improvement in disease symptoms (45.5%), improved sense of well-being (28.8%), decreased frequency of sinus infections (27.3%), and improved energy (24.2%). The adverse events most commonly reported were irritation to the nasal passages (18.2%), headaches (9.1%), and bloody nose (7.6%). These data suggest that individuals who claim to have a diagnosis of multiple chemical sensitivity (MCS) are approximately twice as likely to have these adverse events than those who identify with other diseases, a finding that warrants greater attention.
The diversity of indications for which (in)GSH is being prescribed warrants separate discussions of clinical significance, so the three primary indications will be discussed separately below.
Multiple chemical sensitivity
MCS is a chronic condition where a diverse array of symptoms are attributed to heightened sensitivity to low-level exposure to chemicals, including solvents, volatile organic compounds, perfumes, etc. Symptoms are nonspecific and include odor intolerance, fatigue, headaches, dizziness, anorexia, and shortness of breath; comorbidities include chronic fatigue syndrome and fibromyalgia.12 MCS is a poorly understood condition; theories regarding its pathogenesis include genetically determined impairments of detoxification enzymes and CNS sensitization,13 and an elevation of nitric oxide/peroxynitrite.14 In all of these proposed pathogenetic mechanisms, excessive ROS are involved. Recently, decreased levels of reduced and oxidized glutathione, as well as of glutathione-metabolizing enzyme activities, were reported in erythrocytes of individuals with MCS.15 At least one published report exists of an individual being prescribed glutathione as a therapy for MCS,16 numerous websites encourage the therapy, and this survey suggests improvement in patient-reported outcomes with (in)GSH therapy in people with self-reported MCS.
Chronic sinusitis/allergies
Excessive free-radical formation and depleted antioxidant defenses have been associated with the pathogenesis of several chronic inflammatory disorders of the respiratory tract. While extensive reviews have been published on the therapeutic potential of glutathione in the lower respiratory tract,2 few have addressed the upper respiratory tract. Decreased levels of reduced glutathione have been observed in patients with chronic sinusitis,17 providing scientific rationale for repletion as a therapeutic strategy. One study administered 600 mg GSH per day or placebo by nasal aerosol to children with chronic otitis media. GSH levels were dramatically increased in the nasal mucosa in the first hour after treatment and resulted in a statistically significant improvement in nasal obstruction, rhinorrhea, and ear fullness.18,19
Parkinson's disease
Decreases in glutathione concentrations are the earliest reported biochemical event to occur in the parkinsonian substantia nigra.20,21 This is supported by the finding that GSH is decreased to almost the same degree in patients with incidental Lewy body disease, considered to be a preclinical form of PD.3 Depletion in levels of GSH in the substantia nigra precede the loss of complex I activity and subsequent dopaminergic cell death.20,22 The loss of this primary antioxidant so early in the course of the disease suggests that GSH deficiency may be involved with disease initiation.23 In 2008, Zeevalk et al. published an extensive review on the role of glutathione deficiency and redox perturbation in the pathophysiology of PD.24 Two (2) studies have attempted intravenous augmentation of glutathione,25,26 and both concluded that further research into glutathione supplementation in PD was warranted. A phase I safety and tolerability study of (in)GSH is under way in a population of individuals with PD.27
The major limitations of this study are the lack of verifiable diagnoses reported by respondents, lack of objective symptom improvement, and low survey response rate, all of which were anticipated weaknesses given the study design. A limitation unique to this study is the degree to which this population is reflective of the rest of the population with the same diagnosis. It is possible that providers utilizing unconventional, non-U.S. Food and Drug Administration-approved therapies may attract a unique population of patients, and these results may not be generalized to the rest of the population. Future studies should be condition-specific, randomized controlled trials focusing on both objective clinical improvements as well as patient-reported outcomes. Future studies should also seek to determine whether therapeutic efficacy is dependent on endogenous glutathione status.
Conclusions
The three self-reported conditions for which most individuals are using (in)GSH are MCS, chronic sinusitis/allergies, and PD. In these conditions, diminished glutathione has been implicated in the disease pathogenesis, thus providing scientific rationale for glutathione augmentation as a therapeutic strategy. (in)GSH is inexpensive (∼$50/month), can be self-administered, and may be a novel method of directly reaching target tissues of the respiratory tract and CNS. This survey of patient-reported experiences suggests (in)GSH is easy and comfortable to administer, with few reported adverse events and results in perceived improvement in health among those returning the survey. Future intervention studies should be conducted in each of these conditions to determine whether the individual's perception of improvement can be objectively verified and whether such benefits are generalizable to a larger population.
Acknowledgments
The authors would like to thank Key Compounding Pharmacy for database access and providing a historical context for the use of intranasal glutathione. We would also like to thank NIH National Center for Complementary and Alternative Medicine, the Bernard Osher Foundation, and Bastyr University Research Institute for providing funding for the study authors to conduct this study. Laurie K. Mischley was supported by an NIH NCCAM/Bernard Osher Career Development Award (K01); John S. Finnell was supported by an NIH NCCAM F32 Career Training Award.
Disclosure Statement
The authors have no partnerships or financial interests to disclose.
References
- 1.Mueller SG. Trabesinger AH. Boesiger P. Wieser HG. Brain glutathione levels in patients with epilepsy measured by in vivo (1)H-MRS. Neurology. 2001;57:1422–1427. doi: 10.1212/wnl.57.8.1422. [DOI] [PubMed] [Google Scholar]
- 2.Prousky J. The treatment of pulmonary diseases and respiratory-related conditions with inhaled (nebulized or aerosolized) glutathione. Evid Based Complement Alternat Med. 2008;5:27–35. doi: 10.1093/ecam/nem040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulz JB. Lindenau J. Seyfried J. Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem FEBS. 2000;267:4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- 4.Wu G. Fang YZ. Yang S, et al. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 5.Sims NR. Nilsson M. Muyderman H. Mitochondrial glutathione: A modulator of brain cell death. J Bioenerg Biomembr. 2004;36:329–333. doi: 10.1023/B:JOBB.0000041763.63958.e7. [DOI] [PubMed] [Google Scholar]
- 6.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 7.Mischley LK. Glutathione deficiency in Parkinson's disease: Intranasal administration as a method of augmentation. J Orthomol Med. 2011;26:1–5. [Google Scholar]
- 8.McMartin C. Hutchinson LE. Hyde R. Peters GE. Analysis of structural requirements for the absorption of drugs and macromolecules from the nasal cavity. J Pharm Sci. 1987;76:535–540. doi: 10.1002/jps.2600760709. [DOI] [PubMed] [Google Scholar]
- 9.Collier C. Count so far. In: Mischley L, editor. Email communication regarding update on the number of individuals identified thus far who have received a prescription for intransally-administered glutathione. Seattle, WA: 2011. [Google Scholar]
- 10.Eagle Analytical Services. Laboratory Report: Glutathione 200 mg/mL. Houston: 2011. Contract No.: Sample ID #: 248260. [Google Scholar]
- 11.Seymour J. In: Use of compounded glutathione by CAM practitioners in the Pacific Northwest. Mischley LK, editor. personal communication; Las Vegas, NV: 2007. [Google Scholar]
- 12.Brown MM. Jason LA. Functioning in individuals with chronic fatigue syndrome: Increased impairment with co-occurring multiple chemical sensitivity and fibromyalgia. Dyn Med. 2007;6:6. doi: 10.1186/1476-5918-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorg BA. Multiple chemical sensitivity: Potential role for neural sensitization. Crit Rev Neurobiol. 1999;13:283–316. doi: 10.1615/critrevneurobiol.v13.i3.30. [DOI] [PubMed] [Google Scholar]
- 14.Pall ML. Elevated nitric oxide/peroxynitrite theory of multiple chemical sensitivity: Central role of N-methyl-D-aspartate receptors in the sensitivity mechanism. Environ Health Perspect. 2003;111:1461–1464. doi: 10.1289/ehp.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Luca C. Scordo MG. Cesareo E, et al. Biological definition of multiple chemical sensitivity from redox state and cytokine profiling and not from polymorphisms of xenobiotic-metabolizing enzymes. Toxicol Appl Pharmacol. 2010;248:285–292. doi: 10.1016/j.taap.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Inomata N. Osuna H. Fujita H, et al. Multiple chemical sensitivities following intolerance to azo dye in sweets in a 5-year-old girl. Allergol Int. 2006;55:203–205. doi: 10.2332/allergolint.55.203. [DOI] [PubMed] [Google Scholar]
- 17.Westerveld GJ. Dekker I. Voss HP, et al. Antioxidant levels in the nasal mucosa of patients with chronic sinusitis and healthy controls. Arch Otolaryngol Head Neck Surg. 1997;123:201–204. doi: 10.1001/archotol.1997.01900020089013. [DOI] [PubMed] [Google Scholar]
- 18.Testa B. Mesolella M. Testa D, et al. Glutathione in the upper respiratory tract. Ann Otol Rhinol Laryngol. 1995;104:117–119. doi: 10.1177/000348949510400205. [DOI] [PubMed] [Google Scholar]
- 19.Testa B. Testa D. Mesolella M, et al. Management of chronic otitis media with effusion: The role of glutathione. Laryngoscope. 2001;111:1486–1489. doi: 10.1097/00005537-200108000-00028. [DOI] [PubMed] [Google Scholar]
- 20.Chinta SJ. Andersen JK. Reversible inhibition of mitochondrial complex I activity following chronic dopaminergic glutathione depletion in vitro: Implications for Parkinson's disease. Free Radical Biol Med. 2006;41:1442–1448. doi: 10.1016/j.freeradbiomed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Perry TL. Godin DV. Hansen S. Parkinson's disease: A disorder due to nigral glutathione deficiency? Neurosci Lett. 1982;33:305–310. doi: 10.1016/0304-3940(82)90390-1. [DOI] [PubMed] [Google Scholar]
- 22.Dexter DT. Sian J. Rose S, et al. Indices of oxidative stress and mitochondrial function in individuals with incidental Lewy body disease. Ann Neurol. 1994;35:38–44. doi: 10.1002/ana.410350107. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura K. Wang W. Kang UJ. The role of glutathione in dopaminergic neuronal survival. J Neurochem. 1997;69:1850–1858. doi: 10.1046/j.1471-4159.1997.69051850.x. [DOI] [PubMed] [Google Scholar]
- 24.Zeevalk GD. Razmpour R. Bernard LP. Glutathione and Parkinson's disease: Is this the elephant in the room? Biomed Pharmacother. 2008;62:236–249. doi: 10.1016/j.biopha.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Sechi G. Deledda MG. Bua G, et al. Reduced intravenous glutathione in the treatment of early Parkinson's disease. Prog Neuro-psychopharmacol Biol Psychiatry. 1996;20:1159–1170. doi: 10.1016/s0278-5846(96)00103-0. [DOI] [PubMed] [Google Scholar]
- 26.Hauser RA. Lyons KE. McClain T, et al. Randomized, double-blind, pilot evaluation of intravenous glutathione in Parkinson's disease. Mov Disord. 2009;24:979–983. doi: 10.1002/mds.22401. [DOI] [PubMed] [Google Scholar]
- 27.National Institutes of Health. Intranasal Glutathione in Parkinson's Disease. 2011. http://clinicaltrials.gov/ct2/show/NCT01398748?term=Intranasal+Glutathione+in+Parkinson%E2%80%99s+Disease&rank=1. [Jul 26;2012 ]. http://clinicaltrials.gov/ct2/show/NCT01398748?term=Intranasal+Glutathione+in+Parkinson%E2%80%99s+Disease&rank=1 <. >.