Abstract
Improvement of blood flow and promotion of angiogenesis are important therapeutic measures for the treatment of ischemic peripheral vascular diseases. Since apolipoprotein (a) (apo (a)) is a glycoprotein with repetitive kringle domains exhibiting 75% to 98% structural homology with plasminogen (Plg), apo (a) may also have a negative effect on endothelial progenitor cell (EPC)-induced angiogenesis through Plg-like inhibitory effects on EPC proliferation, adhesion, migration, and angiogenesis. To evaluate the effect of apo (a) on EPCs-induced angiogenesis, EPCs were isolated from the bone marrow of apo (a) transgenic mice, wild-type litter mates, and normal mice. These cells were cultured without or with apo (a) before transplantation. Hindlimb ischemia models were surgically induced in mice, which then received an intravenous injection of 3×105 EPCs. At 3, 7, and 14 days post EPC transplantation, the adhesion, migration abilities, and capillary density in calf muscles were assessed. Results indicate that apo (a) significantly reduced the adhesion and migration abilities of EPCs. Furthermore, the tubule-like formation of EPCs on Matrigel gels was damaged. In vivo experiments showed the homing of EPCs to ischemic peripheral vascular, and the number of capillary vessels decreased significantly in apo(a) transgenic mice. This study demonstrated that apo (a) could attenuate the adhesion, migration, and homing abilities of EPCs and could impair the angiogenesis ability of EPCs.
Homing of endothelial progenitor cells to ischemic peripheral vascular sites and the number of capillaries were decreased in mice transgenic for Apo (a). This gene attenuated adhesion, migration, and homing abilities of EPCs and impaired angiogenesis.
Introduction
Cardiovascular diseases and peripheral vascular disease are major health problems in western countries. Peripheral vascular disease generally leads to an increased risk of arterial occlusions in the diseased region of the body, and can severely hinder blood flow in the region. In the later stages, over one-third of the patients suffering from one or more arterial occlusions will eventually require amputation because of atherosclerosis and diabetes which result in the formation of ulcers and gangrene (Foubert et al., 2007). Current standards of treatment in these situations, including interventional therapy or surgical bypass grafts, may eventually result in vascular endothelial injury, late restenosis, and other complications which may have a significant impact on a patient's health and quality of life. The discovery of endothelial progenitor cells (EPCs) opens up the possibility of developing angiogenesis therapies and is a major breakthrough in the treatment of ischemic diseases. EPCs are now known to be members of the hematopoietic lineage. Increasing evidence suggests that varieties of haematopoietic cells play critical roles in angiogenesis, although do not directly participate in vasculogenesis. Only rare circulating endothelial colony forming cells (ECFCs) and resident ECFC of human blood vessels display true vasculogenic activity in vivo when implanted with several types of matrix molecules(Critser et al., 2011). So, EPCs have been recently identified as a cell population that is circulating cells displayed the ability to display cell surface antigens similar to endothelial cells in vitro, to circulate and lodge in areas of ischemia or vascular injury, and to facilitate the repair of damaged blood vessels or augment development of new vessels as needed by a tissue (Critser et al., 2010; Richardson and Yoder, 2011). However, circulating blood contains from 70 to 210 EPCs per milliliter of blood which account for only 0.002% of total peripheral blood mononuclear cells. Patients with ischemic vascular disease have low numbers of EPCs and those EPCs have a limited ability to move to other sites (Peichev et al., 2000) so the number of circulating EPCs can serve as a biological marker of vascular function. In addition, the loss or reduction of EPCs is an important predictive index of cardiovascular diseases (Schmidt-Lucke et al., 2005; Głowińska-Olszewska et al., 2011). Unfortunately, identifying the factors affecting the biological activity of EPCs has thus far, been a challenge.
Apo (a) is a glycoprotein with repetitive kringle domains exhibiting 75%–98% structural homology with plasminogen (Plg). In particular, apo (a) consists of ten types of kringle-IV-like domains, one copy of a kringle-V-like domain and the Plg counterpart of a protease domain (McLean et al., 1987). Some studies show Plg plays a crucial role in angiogenesis and K-V can inhibit endothelial cell growth (Cao et al., 1997). Evidence also shows N-terminal fragments of Plg are secreted into urine and consist of kringle structures (K-I to K-IV) which inhibit angiogenesis (Schulter et al., 2001). With regard to the high homology with Plg, one might expect that apo (a) affects angiogenesis in a similar way that Plg does. Furthermore, Lp (a) plasma concentrations are mainly controlled by the rate of apo (a) de novo biosynthesis, whereas Lp (a) catabolism might have only small effects (Lawn et al. 1996; Ikenaga et al., 2011). Iwabayashi et al. (2012) documented the effect of Lp (a) on EPCs, showing the presence of LP (a) could significantly diminish the angiogenic function of EPCs via acceleration of senescence, reactive oxygen species production, and functional impairment of the endothelial cell lineage. However, little data is available related to whether apo (a) has a negative effect on the therapeutic potential of EPCs in ischemic settings. Therefore, we studied the usefulness of EPCs in treating severe hindlimb ischemia to demonstrate the impact of apo (a) on EPC angiogenesis both in vivo and in vitro.
Materials and Methods
Animals
Female BALB/c mice (inbred 6–7 weeks old mice weighing 15–19 g used as a negative control, i.e., normal mice group) were purchased from the Institute of Experimental Animals, Hengyang, Hunan, China, and both male and female C57BL/6×SJL wild-type litter mates and apo (a) transgenic mice were purchased from Stanford University School of Medicine; body weights and ages of these mice were similar to those of BALB/c mice. Transgenic mice have a murine plasma apo (a) concentration of ∼10 mg/dL, which is comparable to the median level in humans. Animal use experimental protocols were approved and carried out in accordance with the principles and guidelines of both the West Virginia University Institutional Animal Care and Use Committee and the Institute of Laboratory Animal Resources Guide for the Care and Use of Laboratory Animals; our local ethics committee also approved the study.
Plasma apo (a) measurements
The apo (a) transgenic mice were bred onto C57BL/6×SJL strains background for the study. They express a natural human apo (a) with 17 kringle (K) IV repeats, followed by KV and a protease-like domain from a cDNA transgene controlled by the mouse transferrin promoter. All mice were kept on a 12 h light/dark cycle in a temperature-controlled room at 21°C–23°C, fed a standard rodent chow diet and water ad libitum to 12 weeks of age. The plasma levels of apo (a) in the mice were determined with an apo (a)-specific double sandwich ELISA assay using the monoclonal antibody MAb a-6 (Diasorin, Inc.) as the capture antibody and the monoclonal antibody MAb a-1-1 (Diasorin, Inc.) labeled with horse radish peroxidase as the detection antibody. Statistical analyses were performed using SPSS 13.0 (IBM Corporation) and CurveExpert 1.3 software.
Isolation and culture of mouse marrow-derived EPCs
Mice were anesthetized with 60 mg/kg sodium pentobarbital intraperitoneally before surgery. After immersing in 75% ethanol for 30 min, their thighbone and shinbone marrow cavities were washed with 8 mL EGM-2 (Lonza Corporation) containing hEGF, VEGF, hFGF-B, IGF-1, ascorbic acid, heparin, and 5% fetal bovine serum (Hyclone Corporation). The wash fluid collected was slowly added into a 15 mL centrifugal tube, prefilled with 6 mL of mouse Ficoll-Paque™ (GE Amersham Biosciences Co.) taking care not to disturb the boundary between these two layers. These samples were subjected to horizontal high-speed centrifugation at 1800 rpm at 20°C for 20 min, after which, four distinct layers became visible. The bottom was predominately red blood cells, above which, was a clear Ficoll layer, followed by a slightly pink and hazy layer containing the majority of the mononuclear cells, and finally an upper layer of phosphate-buffered saline (PBS). The upper layer was carefully aspirated and the mononuclear layer along with the two-thirds of the Ficoll layer was collected into 15 mL tubes and centrifuged at 1100 rpm for 10 min to pellet cells, washed once with PBS, resuspended in EGM-2 media and counted; these cells were finally transferred to culture bottles coated with 0.1% fibronectin (Sigma Chemicals Co.) (Tie et al., 2010). After 3 days in culture, nonadherent cells were removed and new medium was added, the culture was maintained for 2–3 weeks; then cells were identified and treated without or with 0.2, 1, 5, 10, 15 μg/mL apo (a) (ARP, Inc.™) for 24 h before the following experiments (Schulter et al., 2001). Recombinant human apo (a) containing 17 kringle IV-like domains followed by KV and a protease-like domain was isolated from COS-7 cells culture media and purified by lysine–Sepharose chromatography. The engineered protein was 487 kDa.
EPCs characterization
To confirm EPCs, adherent cells were assessed for the uptake of Dil Ac-LDL (Sigma Chemicals Co.), binding of FITC-UEA1 (Sigma Chemicals Co.) and immunostaining with CD133, CD34 (Abcam Corporation), and VEGFR-2 (Cell Signaling Technology). EPCs were fixed, washed and centrifuged, then blocked with 5% goat serum for 30 min, followed by overnight incubation (4°C) with mono-clonal primary antibodies specific for CD133 (1:100), CD34 (1:100), and VEGFR-2 (1:100) diluted with 1% goat serum. Control groups were prepared in a similar manner with the exception that primary antibodies were replaced with PBS. After incubation, cells were washed with PBS, followed by incubation with secondary antibody (1:100) diluted with 5% goat serum in PBS, and incubated at room temperature for 30 min. Subsequently, cells were quantitatively analyzed for biomarker expression using flow cytometry. In this study, attached cells that showed uptake of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine-labeled acetylated low density lipoprotein (Dil-AcLDL) and binding of FITC-Ulex europaeus agglutinin (UEA) were considered EPCs. Briefly, cells were plated on the sterile glass slides coated with mouse fibronectin in six-well culture plates to culture. After two washes with PBS, 10 μg/mL of Dil-AcLDL was added to culture for 4 h at 5% CO2 at 37°C. Cells were washed twice and then fixed in 4% paraformaldehyde for 30 min, incubated with 80 μg/mL of UEA-1 for 1 h under the same culture conditions. The functional characteristics of EPCs were detected by fluorescent microscopy. Red or green fluorescent cells suggested either uptake of Dil-AcLDL or binding of UEA-1, respectively; double positive fluorescent cells indicated the presence of EPCs.
EPCs transplantation into ischemic hindlimbs animal model
Mice were anaesthetized with 60 mg/kg pentobarbital. An ischemic model of mouse hindlimb was established through ligation of the proximal end of the femoral artery and the distal portion of the saphenous artery; then excised the femoral artery and attached side-branches. One day after surgery, each mouse (n=8 for each group) received an intravenous injection with approximately 3×105 normal EPCs. Both injected EPCs were prelabeled with a 2 μg/mL CM-Dil (molecular probe). Thirty minutes before euthanasia, mice received a final intravenous injection of 50 mg Bandeiraea simplicifolia lectin 1 (BS lectin-1) (Cell Signaling Technology) to label the ischemic tissue endothelial cells (Couffinhal et al., 1998). At 3, 7, and 14 days after the EPCs were transplanted, calf muscles from the mice injected with Dil-labeled EPCs and BS-lectin-I were embedded frozen section samples. A total of 20 different fields (five fields in each section and four cross-sections from each animal) were examined to assess cell homing and capillary-like angiogenesis. Tissues from ischemic and normal healthy limbs muscles were also harvested to examine the expression of adhesive molecules.
Migration analysis
The chemotactic effect of VEGF on EPCs migration was assessed by an in vitro chemotaxis assay in a transwell chamber (8 mm pores; Coastar). Approximately 1×105 (1.5 mL) of EPCs treated with 0.2, 1, 5, 10, 15 μg/mL apo (a) for 24 h were suspended in the upper compartment. VEGF (PeproTech Corp.) diluted to 1, 10, or 50 ng/mL in 2.6 mL of EBM2 containing 0.5% BSA was placed in the lower compartment and allowed to incubate for 4 h at 37°C. Nonmigratory cells were removed and the number of cells found in the lower compartment was counted. The bottom cell was fixed with 95% alcohol for 10 min and stained with hematoxylin for 5 min. To analyze whether the migration ability is also inhibited in response to the stromal cell-derived factor-1 (SDF-1) in addition to VEGF, EPCs were treated by SDF-1 (PeproTech Corp.) in the same manner. Five fields were randomly selected to determine the migratory rate under a microscope for averaging.
Adhesion analysis
After apo (a) treatment, EPCs were incubated with 10 μg/mL of Dil-AcLDL for 4 h under 37°C, washed twice carefully with PBS to remove nonadherent cells, the adherent cells were stained with the nuclear stain 4,6-diamino-phenylindole (DAPI) (Sigma Chemicals Co.) and washed twice, then EPCs were counted by fluorescence microscopy. Five fields were randomly selected for the average number of adherent cells labeled with Dil-AcLDL.
Apoptosis assay
EPCs apoptosis was assessed by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay according to the manufacturer's instructions (Roche Molecular Biochemicals). Nuclei were stained with DAPI, and the percentage of TUNEL-positive cells was evaluated in a blinded manner. We also used annexin V/propidium iodide binding assay according to the manufacturer's instructions to detect apoptosis. To exclude dead cells, only annexin V-positive and propidium iodide-negative cells were counted.
Tubule-like formation on matrigel gels
To investigate the effect of apo (a) on the integration of EPCs into vascular structures, cells were cultured on Matrigel gels. Briefly, Matrigel gels (B&D Biosciences) were thawed at 4°C overnight and 100 μL/well gels was placed on a 24-well culture plate for 1 h at 37°C to allow solidification. EPCs (2×104) treated without or with apo (a) were plated on the Matrigel gels and incubated in EGM-2 for 3–7 days. Five random fields were selected and tubule formation was defined as observed structures exhibiting a length four times of its width.
Western blot and reverse transcriptase–polymerase chain reaction
Calf muscles from ischemic and normal healthy limbs were harvested 7 days after surgery for VEGF assays and EPCs were also harvested for PGSL-1 and CXCR4 assays. Tissues and EPCs were lysed to collect protein, then proteins were separated on SDS-PAGE gels and transferred to polyvinylidene difluoride membranes (Merck Millipore) blocked by incubation in Tris-buffered saline, followed by a 3h incubation at room temperature with antibodies to VEGF, PGSL-1, CXCR4 (1:200; Santa Cruz Biotechnology), and β-actin (1:400; ZhongShan Biotech). The membranes were washed in Tris-buffered Saline and incubated with a secondary antibody for 1 h. After being washed extensively, relative quantification of proteins was determined with BanScan software. In reverse transcriptase–polymerase chain reaction (RT-PCR) test, total RNA was extracted from ischemic and normal hindlimb muscles, PCR was performed with primers: GAPDH forward: 5′-CCATGGAGAAGGCTGGGG-3′, reverse: 5′-CAAAGTTGTCATGGATGACC-3′; E-selectin forward: 5′-GCTGTCCAGTGTGAAGCCTTATC-3′, reverse: 5′-GCAATGAGGATGTCAGGA-3′; P-selectin forward: 5′-GCTTCAGGACAATGGACAGC-3′, reverse: 5′-CTTTCTTAGCAGAGCCAGGAGTG-3′.
Statistical analysis
Data were presented as mean±SEM. The statistical significance of differences between groups was analyzed by ANOVA and multiple comparison t-test using the SPSS13.0 software (IBM Corporation). Differences were considered significant when p<0.05.
Results
Apo (a) attenuated EPCs adhesion and migration
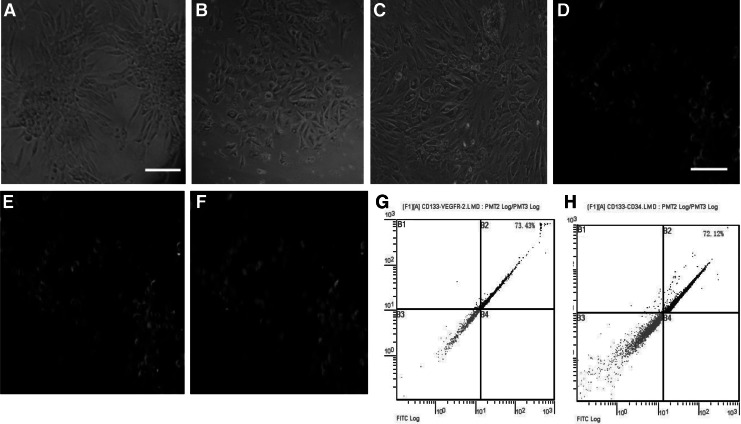
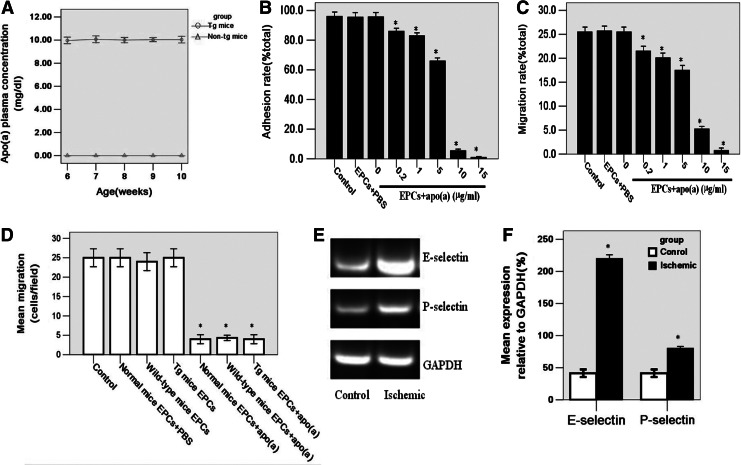
When EPCs were isolated and cultured for 5–7 days; typically colonies were observed to grow radially (Fig. 1A), and became confluent after 2–3 weeks (Fig. 1B, C). EPCs which could take up Dil-AcLDL (Fig. 1D) and bind to FITC UEA-1 (Fig. 1E) could be identified by their orange color (Fig. 1F). The number of EPCs expressing CD133+/VEGFR-2+ and CD133+/CD34+ was 73.43% and 72.12%, respectively (Fig. 1G, H). Adhesion and migration were very important steps in EPC homing to impaired tissues; the expression of P-selectin glycoprotein ligand-1 (PGSL-1), a ligand of P/E selectin, on EPCs has been previously reported (Urao et al., 2008; Raemer et al., 2009). To investigate the effect of apo (a) on EPC adhesion, we treated EPCs with 0.2, 1, 5, 10, 15 μg/mL apo (a) and adopted an RT-PCR method to detect P/E-selectin expressed in the ECs of ischemic tissues, which could promote adhesion of circular EPCs to the endothelium of injured tissues. The results demonstrated EPCs adhesion ability was attenuated after apo (a) treatment for 24 h. When EPCs were subjected to an adhesion assay, they responded to stimulation by apo (a) in a dose dependent manner. Addition of 10 μg/mL apo (a), which is comparable to the median level in the Tg mice and humans (Fig. 2A) (Lawn et al., 1992), reduced the number of adhesive EPCs significantly. At the lowest concentration tested [0.2 μg/mL apo (a)], inhibition of 94.2±6.9% compared with control group was observed, at a concentration of 15 μg/mL apo (a); EPCs almost completely expressed apoptosis (n=3, p<0.01) (Fig. 2B). An in vitro migration experiment also produced the same results (normal mice EPCs+PBS, 25±3; wild-type mice EPCs, 24±3; Tg mice EPCs, 25±2; normal mice EPCs+apo (a), 3±1; wild-type mice EPCs+apo (a), 4±1; Tg mice EPCs+apo (a), 3±1 vs. control 25±3 cells/field; p<0.01) (Fig. 2C, D). RT-PCR examination disclosed a high expression of P/E-selectin in the endothelium of ischemic tissues compared with normal hindlimb tissues (n=3, p<0.05) (Fig. 2E, F) and a down regulated expression of PGSL-1 on apo (a)-treated EPCs detected by western blot (n=3, p<0.05) (Fig. 5A, D).
FIG. 1.
Phenotypic and functional characterization of mouse marrow-derived endothelial progenitor cells (EPCs). (A, B, C) Cellular morphologies after 5 days (A), 15 days (B), and 20 days (C) in culture. Scale Bars: 10 μm. (D, E) EPCs could take up 1,1′-dioctadecyl-3,3,3′,3′- tetramethylindocarbocyanine-labeled acetylated low density lipoprotein (Dil-AcLDL) (D) and bind to FITC-Ulex europaeus agglutinin (UEA) (E). Scale Bars: 10 μm. (F) Double positive Cells showed uptake of Dil-AcLDL and binding of UEA appeared (orange fluorescence; grey). Scale Bars: 10 μm. (G) The cellular surface markers, CD133+/VEGFR-2+ EPCs, were 73.43%, quantitatively analyzed by flow cytometry. (H) CD133+/CD34+ EPCs were 72.12%.
FIG. 2.
Apo (a) attenuated EPCs adhesion and migration abilities. (A) The serum apo (a) concentration in transgenic mice was approximately 10 μg/mL, detected by ELISA, and was comparable to the median human level. (B, C) Apo (a) attenuated EPCs adhesion (B) and migration abilities (C) in a dose-dependent manner (n=3; *p<0.01). (D) The migration ability of EPCs treated with 10 μg/mL apo (a) was attenuated severely, but there were no significant differences between these groups and the control group which was not treated with apo (a) (n=3; *p<0.01). (E) mRNA expression of P/E-selectin in the endothelium of ischemic tissues, detected by reverse transcriptase–polymerase chain reaction analysis. (F) The quantitative analysis showed that the expression of P/E-selectin in the endothelium of ischemic tissues was increased compared with those in healthy tissues from hindlimbs (n=3; *p<0.05). The data are expressed as means±SEM.
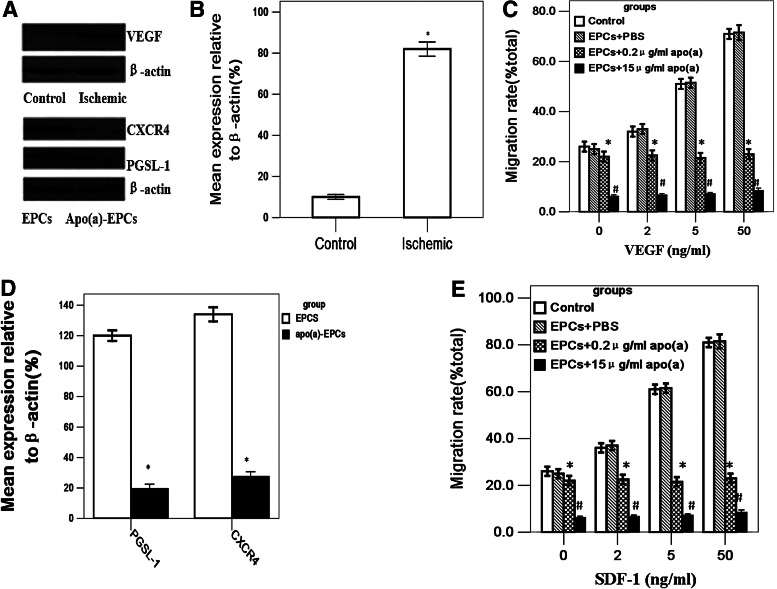
FIG. 5.
Detection of relevant molecular expression in EPCs and muscle tissues. (A) VEGF, PGSL-1and CXCR4 detected by Western blot. (B) The expression of VEGF increased in ischemic tissues, compared with healthy nonischemic control limbs in the same mouse (n=3, *p<0.05). (C) EPC migration was induced by VEGF in a dose-dependent manner (n=8; *p<0.05). (D) The expression of PGSL-1 and CXCR4 decreased in apo (a)-treated EPCs (n=3; *p<0.05). (E) Although the expression of CXCR4 in apo (a)-treated EPCs decreased; there was still a chemotactic effect to SDF-1, but the number of EPCs which had migrated was limited (n=8; *p<0.05 vs. control group, #p<0.05 vs. control, EPCs+PBS and EPCs+0.2 μg/ml apo(a) groups). The data are expressed as means±SEM.
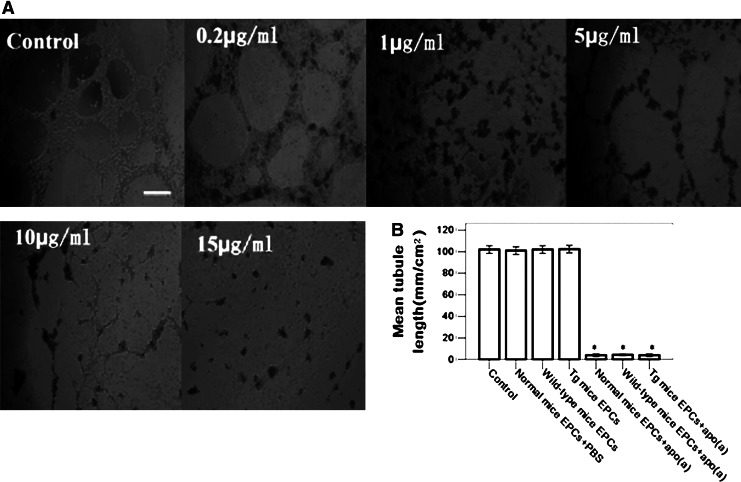
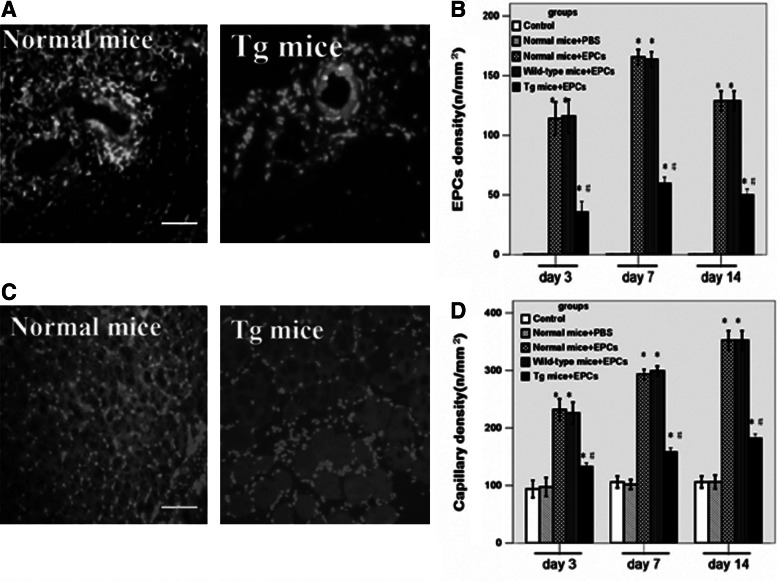
Apo (a) impaired EPCs tubule-like formation, homing and angiogenesis abilities
EPCs could form a tubule-like formation on Matrigel gels, but this formation could be damaged by apo (a). In this experiment, EPCs were cultured on Matrigel gels in the absence or presence of 0.2, 1, 5, 10, 15 μg/mL apo (a); after 7 days cultured, tubule-like formation was impaired 92.1%±3.8% treated with 10 μg/mL apo (a) and almost completely eliminated at the concentration of 15 μg/mL in normal mice EPCs, wild-type mice EPCs, and Tg mice EPCs groups (Fig. 3A), but no significant difference between these groups was observed when they were not exposed to apo (a) treatment compared with the control group (Fig. 3B). These results indicated that the adhesion of EPCs and their migration and in vitro tubule-like formation abilities were impaired potentially because of the influence of apo (a). Previous studies had shown that EPCs could repair injured vessels in ischemic tissues and promote the proliferation of new vessels (Kwon et al., 2011). To elucidate the mechanisms by which apo (a) impaired the angiogenesis of EPCs in vivo, each mouse received an intravenous injection with 3×105 (100 μL) normal EPCs labeled with molecular probe CM-Dil. The host vascular endothelial cells (ECs) were labeled with BS lectin-1. EPCs were identified in tissue sections stained red (greyish white spots), whereas host ECs stained with BS lectin-1 appear green (grey lumens). Histological examination revealed reduced local accumulation of EPCs in apo (a) transgenic mice compared with control group (day 3, normal mice+EPCs, 118±9, wild-type mice+EPCs, 121±10, Tg mice+EPCs, 42±6 vs. control, 0 cells/mm2; day 7, normal mice+EPCs, 163±11, wild-type mice+EPCs, 159±11, Tg mice+EPCs, 56±6 vs. control, 0 cells/mm2; day 14, normal mice+EPCs, 131±10, wild-type mice+EPCs, 130±10, Tg mice+EPCs, 51±5 vs. control, 0 cells/mm2; p<0.05) (Fig. 4A, B). Likewise, a decreased number of capillary ECs was observed in apo (a) transgenic mice compared with control groups (day 3, normal mice+PBS, 95±16, normal mice+EPCs, 226±21, wild-type mice+EPCs, 219±20, Tg mice+EPCs, 142±28 vs. control, 92±13 cells/mm2; day 7, normal mice+PBS, 103±15, normal mice+EPCs, 285±26, wild-type mice+EPCs, 298±31, Tg mice+EPCs, 156±29 vs. control, 108±22 cells/mm2; day 14, normal mice+PBS, 108±20, normal mice+EPCs, 342±19, wild-type mice+EPCs, 344±24, Tg mice+EPCs, 178±28 vs. control, 109±24 cells/mm2; p<0.05) (Fig. 4C, D). We deployed an immunoblotting method to analyze VEGF, which is an effective chemotactic agent of EPCs. Several studies have reported that the expression of VEGF increased in ischemic tissues; we also found there were bright protein bands for mouse VEGF in ischemic tissues at 7 days after surgery, but no visible bands for normal hindlimb tissues (n=3, p<0.05) (Fig. 5A, B). When subjected to chemotactic assays, EPCs responded to VEGF stimulation in a dose-dependent manner (n=8, p<0.05) (Fig. 5C). The expression of CXCR4 on apo (a)-EPCs was lower (n=3, p<0.05) (Fig. 5A, D); apo (a)-EPCs still exhibited a chemotactic effect on SDF-1, but the number which had migrated was limited (n=8, p<0.05) (Fig. 5E).
FIG. 3.
Apo (a) impaired tubule-like formation dose-dependently. (A) The tubule-like structure of EPCs formed on Matrigel gels, cultured with 0, 0.2, 1, 5, 10, 15 μg/mL apo (a) for 7 days. Scale Bars: 1 mm. (B) The quantitative analysis showed that the tubule-like structures was impaired by 92.1%±3.8% after being treated with 10 μg/mL apo (a) in normal mice EPCs, wild-type mice EPCs, and Tg mice EPCs groups (n=3; *p<0.01). The data are expressed as means±SEM.
FIG. 4.
Apo (a) impaired EPCs homing and angiogenesis abilities. (A) Micrographs of Dil-labeled EPCs (red fluorescence; grayish-white spots) homing to ischemic perivascular area of normal mice and transgenic mice; the host vascular ECs labeled by BS lectin-1 were green (grey lumens), and the nuclei dyed with 4,6-diamino-phenylindole (DAPI) were blue (white spots). Scale Bars: 50 μm. (B) The statistical analysis showed that the number of homing EPCs was significantly decreased in transgenic mice group after EPCs transplantation 3, 7, and 14 days (n=8; *p<0.05 vs. normal control mice; *,#p<0.05 vs. EPCs-injected normal mice and EPCs-injected wild-type mice). (C) The capillary density in ischemic tissues from EPCs-injected normal mice and EPCs-injected Tg mice, and the BS lectin-1-labeled capillary endothelium was green (grey spots). Scale bars: 100 μm. (D) Transgenic mice had fewer capillaries (n=8, *p<0.05 vs. normal control mice, *,#p<0.05 vs. EPCs-injected normal mice and EPCs-injected wild-type mice). The data are expressed as means±SEM.
Discussion
Angiogenesis, the formation of new microvessels, is a complex process involving the proliferation and migration of endothelial cells, formation of vessel lumen, and synthesis of basal lamina. Several studies have demonstrated homing EPCs can be further differentiated into endothelial cells and form a vessel network (Grisar et al., 2011; Bikfalvi, 2012; Segal et al., 2012). To investigate the effect of apo (a) on EPC homing, we prestained mouse marrow-derived EPCs with CM-Dil and injected them into normal mice, wild-type mice, and apo (a) transgenic mice. BS lectin-1 was used to stain mouse vascular endothelium. The EPCs labeled with CM-Dil were found to localize in the ischemic hindlimbs 3 days after the mouse received an intravenous injection. Compared with the normal control and PBS control groups, the number of homing EPCs and capillaries increased in EPCs-injected normal mice and EPCs-injected wild-type mice ischemic tissues, but decreased in apo (a) transgenic mice ischemic tissues significantly. This indicated that apo (a) impaired the homing ability of EPCs and reduced angiogenesis. Migration and adhesion are two important steps in EPC homing. Several reports showed that EPCs expressed the P-selectin glycoprotein ligand-1 (PGSL-1), the ligand of P/E selectin, and adhesion molecules on ischemic ECs would have an important role in recruiting circulating EPCs to the murine vasculature (Raemer et al., 2009). In the present study, we observed that endothelial cells in ischemic tissue vessels expressed high-levels of P-selectin and E-selectin, but the expression of PGSL-1 and CXCR4 in apo (a)-treated EPCs decreased by western blot assay, suggesting that apo (a) inhibited the expression of PGSL-1 and CXCR4 in EPCs. The migration ability of apo (a)-treated EPCs was also inhibited significantly. The expression of CXCR4 on apo (a)-EPCs was lower than in the controls; apo (a)-EPCs still exhibited a chemotactic effect on SDF-1, but the number which had migrated was limited. In an experiment of in vitro angiogenesis, EPCs showed superior ability in forming tubule-like formations on Matrigel gels, but the formations were severely damaged after apo (a) treatment with 10 μg/mL apo (a); approximately 92.1%±3.8% of tubule-like formation was impaired and almost completely eliminated at the concentration of 15 μg/mL. These results indicated that EPCs adhesion, migration, and in vitro tubule formation abilities were impaired potentially due to the influence of apo (a). Some studies also showed that VEGF could recruit hemopoietic stem cells to new vessels and further induced proliferation and differentiation of EPCs to vascular endothelial cells (Song et al., 2011). Furthermore, recruited EPCs could also secrete cytokines, such as VEGF (Asahara et al., 1999; Aicher et al., 2005). In our study, we found the expression of VEGF in ischemic tissues was higher compared with that of normal control tissues. For apo (a) transgenic mice, the expression of VEGF in ischemic tissues was also increased, but the number of EPCs found to have homing action was limited. It is likely that the expression of VEGF increased in ischemic tissues; apo (a) may also inhibit the migration and homing of EPCs. Moreover, apo (a) inhibited the expression of PGSL-1 and CXCR4 on EPCs which severely impaired the adhesion ability of EPCs. As a result, the number of EPCs which were able to home in on ischemic tissues decreased, which further inhibited angiogenesis.
Apo (a) could have negative effects on the proliferation, adhesion, migration, and angiogenesis of EPCs, but patients with peripheral vascular disease are often diagnosed with other diseases, including hypertension, diabetes, and atherosclerosis. These other comorbidities may also affect the biological activity of EPCs. Also, since the Lp (a) complex is produced from apo (a) and apo B100 by the formation of a disulfide bond, and Lpa (a) is present only in primates; therefore, this model cannot be used to represent the effects of in-vivo high Lp (a) levels on angiogenesis, and a complete simulation of the effect of apo (a) on EPCs angiogenesis in vivo is currently impossible; this is one limitation of this study. Despite this limitation, the research also provided an experimental basis for studying the effect of apo (a) on EPC angiogenesis: apo (a) downregulates the expression of PGSL-1 and CXCR4 which impairs EPC migration and adhesion abilities, and the number of homing EPCs decreased in ischemic tissues accordingly, which resulted in the inhibition of angiogenesis. Even though the level of VEGF increased, capillaries had a limited number in apo (a) transgenic mice ischemic tissues.
Acknowledgments
This work was supported by Grant 81070221 from the National Natural Science Foundation of China. The authors would like to thank Prof. Zhisheng Jiang and Prof. Zuo Wang for their overall guidance in the scientific research, design, and experimental techniques.
Disclosure Statement
No competing financial interests exist.
References
- Aicher A. Zeiher A.M. Dimmeler S. Mobilizing endothelial progenitor cells. Hypertension. 2005;45:321–325. doi: 10.1161/01.HYP.0000154789.28695.ea. [DOI] [PubMed] [Google Scholar]
- Asahara T. Takahashi T. Masuda H. Kalka C. Chen D. Iwaguro H. Inai Y. Silver M. Isner J.M. VEGF contribute to postnatal neovascularization by mobilizing bone marrow-derived Endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikfalvi A. Angiogenesis and invasion in cancer. Handb Clin Neurol. 2012;104:35–43. doi: 10.1016/B978-0-444-52138-5.00003-7. [DOI] [PubMed] [Google Scholar]
- Cao Y. Chen A. An S.S.A. Ji R.W. Davidson D. Llinas M. Kringle 5 of plasminogen is a novel inhibitor of endothelial cell growth. J Biol Chem. 1997;272:22924–22928. doi: 10.1074/jbc.272.36.22924. [DOI] [PubMed] [Google Scholar]
- Couffinhal T. Silver M. Zheng L.P. Kearney M. Witzenbichler B. Isner J.M. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- Critser P.J. Voytik-Harbin S.L. Yoder M.C. Isolating and defining cells to engineer human blood vessels. Cell Prolif. 2011;44:15–21. doi: 10.1111/j.1365-2184.2010.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critser P.J. Kreger S.T. Voytik-Harbin S.L. Yoder M.C. Collagen matrix physical properties modulate endothelial colony forming cell derived vessels in vivo. Microvasc Res. 2010;80:23–30. doi: 10.1016/j.mvr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foubert P. Silvestre J.S. Souttou B. Bamouceeau V. Martin C. Ebrahimian T.G. Leré-Déan C. Contreres J.O. Sulpece E. Levy B.I. Plouët J. Tobelem G. Le Ricousse-Roussanne S. PSGL-1–mediated activation of EphB4 increases the proangiogenic potential of endothelial progenitor cells. J Clin Invest. 2007;117:1527–1537. doi: 10.1172/JCI28338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Głowińska-Olszewska B. Luczyński W. Bossowski A. Endothelial progenitor cells as a new marker of endothelial function with respect to risk of cardiovascular disorders. Postepy Hig Med Dosw. 2011;65:8–15. doi: 10.5604/17322693.931086. [DOI] [PubMed] [Google Scholar]
- Grisar J.C. Haddad F. Gomari F.A. Wu J.C. Endothelial progenitor cells in cardiovascular disease and chronic inflammation: from biomarker to therapeutic agent. Biomark Med. 2011;5:731–744. doi: 10.2217/bmm.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenaga H. Ishihara M. Inoue I. Kawagoe T. Shimatani Y. Miura F. Makama Y. Dai K. Otani T. Ejiri K. Oda N. Nakamura M. Miki T. Usefulness of lipoprotein (a) for predicting progression of non-culprit coronary lesions after acute myocardial infarction. Circ J. 2011;75:2847–2852. doi: 10.1253/circj.cj-11-0365. [DOI] [PubMed] [Google Scholar]
- Iwabayashi M. Taniyama Y. Sanada F. Azuma J. Iekushi K. Okayama K. Chatterjee A. Rakugi H. Morishita R. Inhibition of Lp(a)-induced functional impairment of endothelial cells and endothelial progenitor cells by hepatocyte growth factor. Biochem Biophys Res Commun. 2012;423:79–84. doi: 10.1016/j.bbrc.2012.05.086. [DOI] [PubMed] [Google Scholar]
- Kwon S.M. Lee Y.K. Yokoyama A. Jung S.Y. Masuda H. Kawamoto A. Lee Y.M. Asahara T. Differential activity of bone marrow hematopoietic stem cell subpopulations for EPC development and ischemic neovascularization. J Mol Cell Cardiol. 2011;51:308–317. doi: 10.1016/j.yjmcc.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Lawn R.M. Pearle A.D. Kunz L.L. Rubini E.M. Reckless J. Metcalfe J.C. Grainger D.J. Feedback mechanism of focal vascular lesion formation in transgenic apolipoprotein(a) mice. J Biol Chem. 1996;271:31367–31371. doi: 10.1074/jbc.271.49.31367. [DOI] [PubMed] [Google Scholar]
- Lawn R.M. Wade D.P. Hammer R.E. Chiesa G. Verstuyft J.G. Rubin E.M. Atherogenesis in transgenic mice expressing human apolipoprotein(a) Nature. 1992;360:670–672. doi: 10.1038/360670a0. [DOI] [PubMed] [Google Scholar]
- McLean J.W. Tomlinson J.E. Kuang W.J. Eaton D.L. Chen E.Y. Fless G.M. Scanu A.M. Lawn R.M. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 1987;330:132–137. doi: 10.1038/330132a0. [DOI] [PubMed] [Google Scholar]
- Peichev M. Naiyer A.J. Pereira D. Zhu Z. Lane W.J. Williams M. Oz M.C. Hicklin D.J. Witte L. Moore M.A. Rafili S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- Richardson M.R. Yoder M.C. Endothelial progenitor cells: quo vadis? J Mol Cell Cardiol. 2011;50:266–272. doi: 10.1016/j.yjmcc.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raemer P.C. Haemmerling S. Giese T. Canaday D.H. Katus H.A. Dengler T.J. Sivangandam V.G. Endothelial progenitor cells possess monocyte-like antigen-presenting and T-cell-co-stimulatory capacity. Transplantation. 2009;87:340–349. doi: 10.1097/TP.0b013e3181957308. [DOI] [PubMed] [Google Scholar]
- Schmidt-Lucke C. Rössig L. Fichtlscherer S. Vasa M. Britten M. Kämper U. Dimmeler S. Zeiher A.M. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- Schulter V. Koolwijk P. Peters E. Frank S. Hrzenjak A. Graier W.F. Van Hinsbergh V.W. Kostner G.M. Impact of apolipoprotein(a) on in vitro angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21:433–438. doi: 10.1161/01.atv.21.3.433. [DOI] [PubMed] [Google Scholar]
- Segal M.S. Sautina L. Li S. Diao Y. Agoulnik A.I. Kielczewski J. Mcguane J.T. Grant M.B. Conrad K.P. Relaxin increases human endothelial progenitor cell NO and migmouceion and vasculogenesis in mice. Blood. 2012;119:629–636. doi: 10.1182/blood-2011-04-346007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X. Liu S. Qu X. Hu Y. Zhang X. Wang T. Wei F. BMP2 and VEGF promote angiogenesis but retard terminal differentiation of osteoblasts in bone regeneration by up-regulating Id1. Acta Biochim Biophys Sin(Shanghai) 2011;43:796–804. doi: 10.1093/abbs/gmr074. [DOI] [PubMed] [Google Scholar]
- Tie G. Yan J. Yang Y. Park B.D. Messina J.A. Raffai R.L. Nowicki P.T. Messina L.M. Oxidized low-density lipoprotein induces apoptosis in endothelial progenitor cells by inactivating the phosphoinositide 3-kinase/Akt pathway. J Vasc Res. 2010;47:519–530. doi: 10.1159/000313879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urao N. Inomata H. Razvi M. Kim H.V.V. Wary K. Mckinney R. Fukai T. Ushio-Fukai M. Role of Nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res. 2008;103:212–220. doi: 10.1161/CIRCRESAHA.108.176230. [DOI] [PMC free article] [PubMed] [Google Scholar]