Abstract
Salvinorin A, the active ingredient of the hallucinogenic plant Salvia divinorum is the most potent known naturally occurring hallucinogen and is a selective κ-opioid receptor agonist. To better understand the ligand-receptor interactions, a series of dicarboxylic ester-type of salvinorin A derivatives were synthesized and evaluated for their binding affinity at κ, δ, and μ-opioid receptors. Most of the analogues show high affinity to the κ-opioid receptor. Methyl malonyl derivative 4 shows the highest binding affinity (Ki = 2 nM), analogues 5, 7, and 14 exhibit significant affinity for the κ-receptor (Ki = 21, 36 and 39 nM).
Keywords: Salvia divinorum, Salvinorin A and B, Dicarboxylic ester-type ligands, Kappa opioid receptor agonists
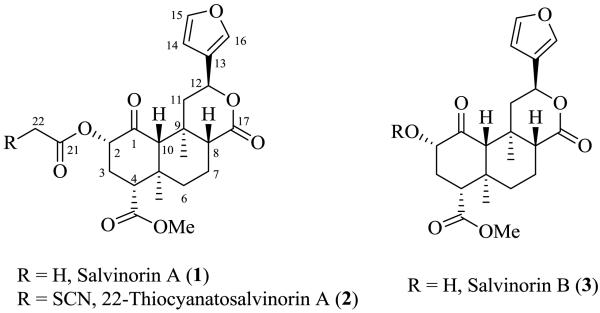
The neoclerodane diterpenoid salvinorin A (1) isolated from the leaves of hallucinogenic sage Salvia divinorum, is a potent and selective κ-opioid receptor (KOR) agonist.1, 2 Since its discovery, a large number of analogues have been prepared by semi-synthesis to probe the pharmacophore and mode of binding.3 Some of these analogues present interesting pharmacological profiles from full KOR agonist to partial δ-opioid receptor (DOR) or μ-opioid receptor (MOR) agonist and antagonists. The current objective is to utilize the knowledge about salvinorin A-KOR interactions to rationally design salvinorin A derivatives with different pharmacological profiles and therapeutic potential. In the course of our work on the molecular mechanism of interaction of salvinorin A with KOR, we reported irreversible binding of 22-thiocyanatosalvinorin A (2) (Fig. 1) with the sulfhydryl group of Cys-315 at the κ-opioid receptor.4, 5 Our previous work on the KOR model and analysis of the mode of binding of 2 suggest that Cys-315 may be a good anchoring amino acid at the binding site in KOR.4, 6 The presence of ester substituents at C-22 may enhance electrophilicity of this center and thereby leads to stronger binding with the thiol group of Cys-315 of KOR.
Figure 1.
The structures of salvinorin A, 22-thiocyanatosalvinorin A, and salvinorin B.
Herein, we report the synthesis of series of new dicarboxylic ester salvinorin A derivatives and their binding affinity to κ, δ and μ-opioid receptors.
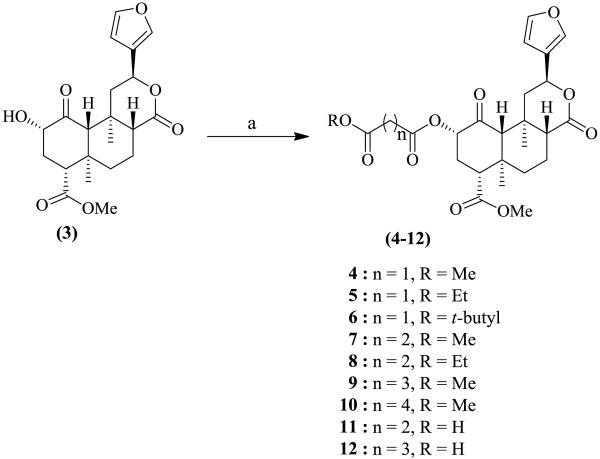
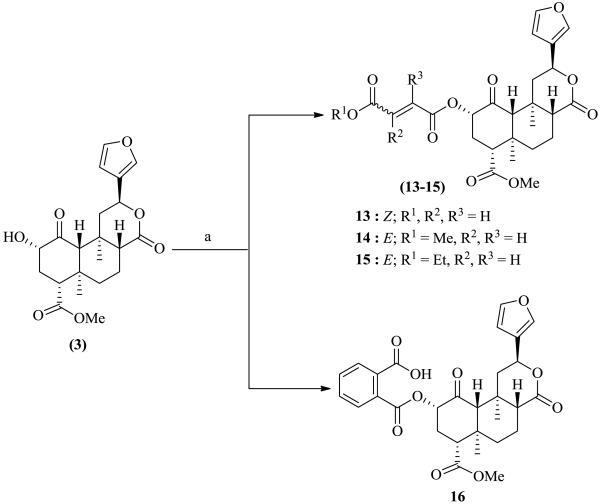
Salvinorin A (1) was isolated from dried leaves of Salvia divinorum and purified as previously reported.7 It was hydrolyzed to salvinorin B (3), which served as the starting material for the preparation of the C(2)-modified dicarboxylic ester analogue library 4-16 (Schemes 1 and 2). Compounds 4-10, 14, and 15 were prepared8 in yields of 57-69% by reacting 3 with the corresponding acid halides in the presence of Et3N in DCM (Schemes 1 and 2). Analogues 11-13 and 16 were synthesized9 in yields of 40-51% via the reaction of 3 with appropriate anhydrides using DBU (1, 8-diazabicyclo [5.4.0] undec-7-ene) as base (Schemes 1 and 2). The physical data (1H NMR, 13C NMR and HR-ESIMS) were consistent with the proposed structures.
Scheme 1.
Synthesis of dicarboxylic ester-type salvinorin A analogues 4-12 Reagents and conditions: (a) appropriate acid chloride, Et3N, dry DCM, N2 r.t, 2-3 h, or appropriate acid anhydride, DBU, dry DCM, N2, r.t, 6-8 h.
Scheme 2.
Synthetic route for the designed ligands 13-16. Reagents and conditions: (a) appropriate acid chloride, Et3N, dry DCM, N2, r.t, 3 h, or appropriate acid anhydride, DBU, dry DCM, N2, r.t, 6-8 h.
The κ, δ and μ-opioid receptor binding affinities of synthesized compounds 4-16 were evaluated at the NIMH-sponsored Psychoactive Drug Screening Program (PDSP), University of North Carolina at Chapel Hill using radioligand binding assays conducted as previously described, 1, 5 and the data are collated in Tables 1 and 2. For comparison purposes, opioid binding affinity data for compounds 1-3 and positive controls for MOR and DOR are included in Table 1. Most of the dicarboxylic ester derivatives show appreciable binding affinity to KOR, and no affinity to MOR and DOR. The methyl malonyl derivative 4 had higher affinity than 1 at KOR (Ki = 2.0 vs 6.2 nM) (Table 1). The ethyl malonyl and methyl succinyl ligands 5 and 7, also have significant affinities to KOR, these being respectively 3- and 6-fold reduced compared to 1 (Ki = 21.0 and 36, respectively, vs 6.2 nM). The KOR affinities of analogues 6, 8, and 9 were decreased 24-, 70-, and 49-fold as compared to 1 (Ki = 148, 437 and 302, respectively, vs 6.2 nM). Similarly, compounds 10, 12, and 15 show reduced affinity (Ki = 575, 263, and 427 nM respectively) at KOR. Interestingly, the methyl fumaryl ligand 14 had a 6-fold reduced but still considerable affinity for the κ-opioid receptor compared to 1 (Ki = 39 vs 6.2 nM). Analogues 11 and 16 exhibited less affinity for the κ-receptor (Ki = 4070 and 2291 nM, respectively), and derivative 13 has no affinity for any of the opioid receptors.
Table 1.
Binding affinities of C(2)-dicarboxylic ester-derived salvinorin A analogues (4-16) at MOR, DOR, and KOR
| Affinitiesb, | Ki± SD [nM] | Selectivity | |||
|---|---|---|---|---|---|
|
| |||||
| Compound | MORc | DORd | KORe | MOR/KOR | DOR/KOR |
| 1 | >10000 | >10000 | 6.2±2.2 | ||
| Naltrindole | ND | 0.9±0.3 | ND | ||
| DAMGO | 0.9±0.2 | ND | ND | ||
| 2 a | >10000 | >10000 | 0.6±0.2 | ||
| 3 | >10000 | >10000 | >10000 | ||
| 4 | >10000 | 4320±680 | 2.0±0.3 | >5000 | 2160 |
| 5 | >10000 | >10000 | 21.0±5.6 | >476 | >476 |
| 6 | >10000 | >10000 | 148±32 | >67 | >67 |
| 7 | >10000 | >10000 | 36±8 | >277 | >277 |
| 8 | >10000 | >10000 | 437±129 | >23 | >23 |
| 9 | 5012±1400 | >10000 | 302±94 | 16 | >33 |
| 10 | >10000 | >10000 | 575±183 | >17 | >17 |
| 11 | >10000 | >10000 | 4070±1300 | >2 | >2 |
| 12 | 4570±1300 | >10000 | 263±86 | 17 | >38 |
| 13 | >10000 | >10000 | >10000 | 1 | 1 |
| 14 | 711±134 | >10000 | 39±11 | 18 | >256 |
| 15 | 724±137 | >10000 | 427±134 | 2 | >23 |
| 16 | >10000 | >10000 | 2291±492 | >4 | >4 |
see ref. 4, Ki of 2 is 0.6 vs 1.9 nM of 1 for the KOR.
Data are mean of three experiments performed in duplicate.
The binding affinity constant (Ki) determined against [3H]DAMGO ligand.
Ki determined against [3H]DADLE ligand.
Ki determined against [3H]U69,553 ligand.
N.D - not determined.
Table 2.
Kappa opioid receptor functional assay: Efficacies of Gαi activation in live HEK293 cells using a cAMP biosensor Glosensor-22F (Promega)
| Compound | EC50±SD [nM]a |
|---|---|
| 1 | 5±3 |
| 4 | 137±15 |
| 5 | 52±23 |
| 7 | 855±130 |
| 14 | 70±25 |
Data are mean of four experiments
To characterize the relative efficacy of these dicarboxylic ester ligands, compounds 4, 5, 7, and 14 together with salvinorin A (1) were selected for a functional assay. Table 2 shows the EC50 values of these ligands in stimulating Gαi signaling mediated by the κ-opioid receptor.
In summary, a series of new dicarboxylic ester-type analogues of salvinorin A were synthesized in an effort to explore the effects of C-2 substitution towards selectivity at κ, δ, and μ-opioid receptors. Most of the analogues showed high binding affinity to the κ-opioid receptor with little affinity for δ- and μ-opioid receptors. Compound 4 possessing a C-2 methyl malonyl group exhibited increased selectivity for KOR compared to the parent salvinorin A (1).
Supplementary Material
Acknowledgements
This work was supported by the NIH Grant R01 DA017204 and the NIMH Psychoactive Drug Screening Program (PDSP), University of North Carolina at Chapel Hill, NC 27599. We thank Mr. Daniel J. Siebert from the Salvia divinorum Research and Information Center for supplying the plant material, and Dr. Bin Wang for the assistance with HRESIMS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Supplementary data associated with this article can be found, in the online version.
References and notes
- 1.Ortega A, Blount JF, Manchand PS. J. Chem. Soc., Perkin Trans.1. 1982:2505–2508. [Google Scholar]
- 2.Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Beguin C, Potuzak J, Xu W, Liu - Chen LY, Streicher JM, Groer CE, Bohn LM, Carlezon WA, Jr., Cohen BM. Bioorg. Med. Chem. Lett. 2012;22:1023–1026. doi: 10.1016/j.bmcl.2011.11.128. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lovell KM, Vasiljevik T, Araya JJ, Lozama A, Prevatt-Smith KM, Day VW, Dersch CM, Rothman RB, Butelman ER, Kreek MJ, Prisinzano TE. Bioorg. Med. Chem. 2012;20:3100–3110. doi: 10.1016/j.bmc.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Cunningham CW, Rothman RB, Prisinzano TE. Pharmacol. Rev. 2011;63:316–347. doi: 10.1124/pr.110.003244. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Lozama A, Cunningham CW, Caspers MJ, Douglas JT, Dersch CM, Rothman RB, Prisinzano TE. J. Nat. Prod. 2011;74:718–726. doi: 10.1021/np1007872. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Tidgewell K, Harding WW, Lozama A, Cobb H, Shah K, Kannan P, Dersch CM, Parrish D, Deschamps JR, Rothman RB, Prisinzano TE. J. Nat. Prod. 2006;69:914–918. doi: 10.1021/np060094b. [DOI] [PubMed] [Google Scholar]; (f) Bikbulatov RV, Yan F, Roth BL, Zjawiony JK. Bioorg. Med. Chem. Lett. 2007;17:2229–2232. doi: 10.1016/j.bmcl.2007.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Lee DY, He M, Liu-Chen L-Y, Wang Y, Li J-G, Xu W, Ma Z, Carlezon WA, Jr., Cohen B. Bioorg. Med. Chem. Lett. 2006;16:5498–5502. doi: 10.1016/j.bmcl.2006.08.051. [DOI] [PubMed] [Google Scholar]; (h) Stewart DJ, Fahmy H, Roth BL, Yan F, Zjawiony JK. Arzneimittelforschung. 2006;56:269–275. doi: 10.1055/s-0031-1296720. [DOI] [PubMed] [Google Scholar]; (i) Beguin C, Richards MR, Li JG, Wang Y, Xu W, Liu - Chen LY, Carlezon WA, Jr., Cohen BM. Bioorg. Med. Chem. Lett. 2006;16:4679–4685. doi: 10.1016/j.bmcl.2006.05.093. [DOI] [PubMed] [Google Scholar]; (j) Harding WW, Tidgewell K, Byrd N, Cobb H, Dersch CM, Butelman ER, Rothman RB, Prisinzano TE. J. Med. Chem. 2005;48:4765–4771. doi: 10.1021/jm048963m. [DOI] [PubMed] [Google Scholar]; (k) Lee DY, Yang L, Xu W, Deng G, Guo L, Liu-Chen LY. Bioorg. Med. Chem. Lett. 2010;20:5749–5752. doi: 10.1016/j.bmcl.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan F, Bikbulatov RV, Mocanu V, Dicheva N, Parker CE, Westel WC, Mosier PD, Westkaemper RB, Allen JA, Zjawiony JK, Roth BL. Biochem. 2009;48:6898–6908. doi: 10.1021/bi900605n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, Liu W, Thompson AA, Huang XP, Carroll FI, Mascarella SW, Westkaemper RB, Mosier PD, Roth BL, Cherezov V, Stevens RC. Nature. 2012;485:327–332. doi: 10.1038/nature10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan F, Mosier PD, Westkaemper RB, Stewart J, Zjawiony JK, Vortherms TA, Sheffler DJ, Roth BL. Biochem. 2005;44:8643–8651. doi: 10.1021/bi050490d. [DOI] [PubMed] [Google Scholar]
- 7.Kutrzeba LM, Karamayan VT, Speth RC, Williamson JS, Zjawiony JK. Pharm. Biol. 2009;47:1078–1084. [Google Scholar]
- 8.Procedure for the synthesis of analogues 4-10, 14 and 15: Compound 3 (15 mg, 0.05 mmol) and a catalytic amount of triethylamine were dissolved in DCM (3 mL). An appropriate acid chloride (0.125 mmol) was added, and the reaction mixture was stirred for 2-3 h at rt. After TLC indicated completion of the reaction, the mixture was quenched with water and the organic layer separated. The organic phase was washed with dilute aqueous HCl (0.01 mol/L, 2 mL) followed by saturated NaHCO3 (2 mL). The organic layer was dried over anhydrous Na2SO4, evaporated, and the residue was purified by column chromatography (SiO2; eluent: n-hexane / EtOAc) to obtain the target product. In some cases purification was done by preparative HPLC (C18 column, MeCN-water).
- 9.Method for the synthesis of derivatives 11-13 and 16: To a solution of compound 3 (15 mg, 0.05 mmol) in DCM (3 mL), a catalytic amount of DBU and an appropriate acid anhydride (0.15 mmol) were added. The mixture was stirred at room temperature for 6-8 h. After TLC indicated completion of the reaction, the mixture was concentrated under reduced pressure and the residue was purified by column chromatography (SiO2; eluent: n-hexane / EtOAc) and HPLC (C18 column, MeCN-water) to yield the target product.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.