Abstract
Fibrocytes are unique cells possessing the proinflammatory properties of macrophages and the tissue remodeling properties of fibroblasts. Because these cells display a strong association with many human diseases characterized by chronic and dysregulated inflammatory responses the study of fibrocytes is important and timely. This review presents recent data regarding fibrocyte origin, identification, differentiation, and appearance in diseased tissue. The available data regarding the association of fibrocytes with several forms of chronic tissue inflammation seen in the setting of lung disease, autoimmunity, liver disease, and normal aging will be presented. This review concludes by putting these data in perspective and by suggesting future areas of investigation. It is hoped that this information will lead to additional investigations in this burgeoning field and improve our understanding of the novel role fibrocytes may play in human disease.
Introduction
Chronic inflammatory responses are characterized by replacement of normal organ structure with inflammatory cells and excess connective tissue. This pathology is seen in the setting of persistent injury that causes ongoing immune activation and impeded repair. It was until recently believed that normal and dysregulated repair responses result from the recruitment, proliferation, and activation of local connective tissue cells such as fibroblasts [1]. However, an expanding body of research now supports the involvement of bone marrow derived progenitor cells called fibrocytes in these processes. These cells are believed to originate from a monocyte derived precursor and are implicated in the pathogenesis of many chronic inflammatory states including those involving the lung [2], autoimmunity [3–5][6], liver [7], skin [8], and even normal aging [5]. In the last year alone there has been an explosion of data regarding the pathways regulating fibrocyte differentiation and phenotype. This review will discuss the current criteria for fibrocyte identification in the circulation and tissue, present the most recent data regarding their differentiation pathways and known functions, highlight recent literature regarding the role of fibrocytes in chronic inflammatory diseases affecting the lung, liver, autoimmunity, and aging, and will conclude by suggesting areas for further study.
Identification of Fibrocytes
Identification of fibrocytes in the circulation or diseased organs involves co-detection of characteristic cell surface proteins and collagens or extracellular matrix components (ECM) [9]. The hematopoietic origin of human fibrocytes is reflected by their expression of CD45 and Leukocyte specific protein-1 (LSP-1) [10]. Their presumed monocyte origin is reflected by expression of CD11b, CD11c, and CD11d [10]. Their role in immunologic responses is reflected by their expression of chemokine receptors such as CCR2 [11] and CXCR4 [12] and by their expression of proteins important for host defense (CD16/32, CD163) [10] and antigen presentation (Major Histocompatibility Complex [MHC] I and II, CD80, and CD86) [13]. Perhaps relating to their multilineage potential, circulating and cultured fibrocytes express CD34 [5], a motility protein that identifies certain stem cell populations When present, CD34 can be used to distinguish fibrocytes from related cell populations such as fibroblasts and macrophages [14]. For tissue analysis, morphologic identification of the fibrocyte’s typical spindle shape is an additional method of detection [9]. In addition to these cell surface markers, fibrocytes also produce a wide array of ECM components including a Collagens 1, IV, and V, structural proteins, and glycosaminoglycans (GAGs) [15,16]. The markers displayed by fibrocytes are compiled in Table 1.
Table 1.
Fibrocyte Marker Expression
| Marker | Expression |
|---|---|
| Adhesion and Motility | |
| CD9, CD11a, CD11b, CD11c, CD43, CD164, Mac2, LSP-1 |
+/++ |
| CD34 | + |
| CD29, CD44, CD81, ICAM-1, CD49 complex, CD81 |
+ |
| Cell Surface Enzymes | |
| CD10, CD172a | + |
| CD13, Prolyl-4-hydroxylase | + |
| FAP | + |
| Scavenging receptors and host defense | |
| CD14, CD68, CD163, CD206, CD209, CD35, CD36 |
+/− |
| Fcγ receptors | |
| CD16, CD32a, CD32b, CD32c | +/++ |
| Chemokine receptors | |
| CCR2, CCR5, CCR4, CCR7, CCR9, CXCR1, CXCR4, CXC3R1 |
+/++ |
| Antigen Presentation | |
| CD80, CD86, MHCI, MCHII | + |
| Extracellular matrix | |
| Collagen-I/III/IV, vimentin, tenascin | + |
| Fibronectin, α-SMA | +/− |
| Collagen V | ++ |
| Glycosaminoglycans | |
| Perlecan, Veriscan, Hyaluronan | ++/+ |
| Decorin | + |
| Miscellaneous | |
| Semaphorin 7a | + |
| CD115 | − |
| Thy1.1 | + |
| CD105 | + |
++ high level, + moderate, +/− conflicting reports or equivocal, - no expression
Fibrocyte Origin and Differentiation
Human fibrocyte precursors co-purify with CD14+ monocytes [4]. Murine modeling shows that fibrocyte outgrowth from monocytes is enhanced by enrichment for CD11b, CD115, and Gr1 [17]. These effects are require direct contact with activated CD4+ lymphocytes and occur via an mTOR-PI3 kinase dependent pathway [17]. Fibrocyte differentiation is also regulated by the Fcγ receptors CD64 and CD32 [18]. Inhibition of these receptors with Serum Amyloid P attenuates fibrocyte accumulation in human [19] rat [20] and murine samples [21] via an ITIM-dependent mechanism [22]. The effects on human cells are promoted by high molecular weight hyaluronan (HA) and opposed by low molecular weight HA, suggesting that the immunologic effects of SAP are at least partially mediated via CD44 [23]. Fibrocyte differentiation is also reduced by TH1 cytokines (IFNγ, TNF, and IL-12) and is augmented by TH2 cytokines (IL-4 and IL-13) [24], TGF-β1, and engagement of the β1 integrin subunit [15,25]. These latter effects require Erk phoshorylation [26]. Treatment with TLR2 agonists inhibit human fibrocyte differentiation; however, the mechanism of these effects is not clear [27]. The immunomodulatory and neuronal guidance protein Semaphorin 7a controls human and murine fibrocyte differentiation via a β1-integrin-dependent pathway that is opposed by Plexin C1 [25]. Primary human monocytes obtained from patients with scleroderma related lung disease display enhanced differentiation into fibrocytes; this phenotype is reversed by the restoration of normal caveolin-1 scaffolding domains, indicating that Caveolins also control fibrocyte fate [28]. Further studies show that the monocyte to fibrocyte transition is controlled by caspase activation, as inhibition of caspase phosphorylation reduces fibrocytes in cultures of primary human cell and in a mouse model of pulmonary fibrosis [4]. While these data support the contention that fibrocytes derive from monocytes under a variety of stimuli, careful lineage tracing studies are required to confirm this to be true in all circumstances.
Fibrocyte Homing
Murine fibrocytes express a several chemokine receptors including CCR1, CCR2 [7,11], CCR7, and CXCR4 which in several studies have been shown to control migration and recruitment of fibrocytes to injured tissue [12,29]. Human fibrocytes also express the chemokine receptors CCR2 [11], CCR3, and CCR5 [30] CXCR4 [12], as well as the β1 integrin subunit and Semaphorin 7a [25]. One study found an association between concentrations of soluble factors such as TNF, IL-10, MCP-1 and IL-1 receptor antagonist (IL-1Ra) in the blood of patients with Scleroderma related interstitial lung disease (SSc-ILD) [5] suggesting that fibrocytes migrate in response to one or more of these factors. Peripheral blood mononuclear cells from these patients express high levels of Plexin C1, an inhibitory receptor for Sema 7a that in studies of primary human cells reduces fibrocyte outgrowth, suggesting that the increase in Plexin C1 detected in these patients is a counterregulatory response [25]. A separate set of studies found high levels of CXCL12 (the cognate ligand for CXCR4) in the lungs and blood of patients with idiopathic pulmonary fibrosis (IPF) and these levels correlate with circulating fibrocyte concentrations [31]. The study of fibrocyte trafficking is an area of investigation with the potential to lead to new therapies for fibrosing diseases.
Fibrocyte Function
While a definite contribution to disease pathogenesis is lacking, fibrocytes display many functions that could influence chronic inflammatory responses. Early studies of fibrocyte biology centered on their role as a circulating source of contractile myofibroblasts. However, because lineage tracing studies show only minimal contribution of fibrocytes to α-SMA production in several models [32,33] it is likely that fibrocytes possess other properties that promote tissue remodeling. Thus it is particularly relevant that human fibrocytes respond to Interleukin-1 beta (IL-1β) by increasing secretion of Interleukin-6 (IL-6), Interleukin-8 (IL-8), Chemokine (C-C motif) ligand 2 (CCL2), Chemokine (C-C motif) ligand 3(CCL3), and by increasing expression of Intercellular adhesion molecule-1 (ICAM-1) which would be expected to recruit and activate leukocytes [34]. Notably, it has been found that fibrocytes from asthmatic patients respond to IL17A by secreting proinflammatory mediators, and respond to TH2 cytokines by producing collagen [35]. Human fibrocytes possess antigen-presenting properties [36] and under certain conditions can induce a mixed TH1/TH2 response in naïve human CD4 cells [8]. Consistent with these proinflammatory properties, a proinflammatory CD45+Col-Iα+ population is found in the spleens of mice that have been exposed to a variety of stimuli including TGF-β1, LPS and viral infection. These fibrocyte-like cells express MHCII at high levels and present antigen to CD8 cells [13], suggesting an important role for fibrocytes in the early events mediating inflammation.
In addition to these proinflammatory functions, fibrocytes also respond to IL-1β increasing Interleukin-10 (IL-10) production which would be expected to reduce inflammation [34]. Their distinctive pattern of ECM production includes Collagen V, hyaluronan, and versican, and perlecan [15] and would be expected to recruit inflammatory cells and promote tissue repair. Fibrocytes also produce soluble mediators that induce myofibroblast transformation in culture such as PDGF and TGF-β1, and have been shown to control angiogenesis via secretion of soluble mediators including matrix metalloproteinases (MMPs) vascular endothelial growth factor (VEGF), PDGF-A, hepatocyte growth factor (HGF), granulocyte–macrophage colony stimulating factor (GM-CSF), basic fibroblast growth factor (b-FGF), IL-8 and IL-1β [37] Through their surface expression of Semaphorin 7a [3] fibrocytes could activate macrophages and dendritic cells and negatively regulate T cell responses. Fibrocyte-like cells are also identified in human malignancies and permit tumor metastasis in rodent models of malignancy by suppression of IFNγ and TNF [38], as well as through overexpression of MMP9 [39]. Taken together, these data suggest a paradigm in which fibrocytes possess a remarkable array of functional characteristics characterized by both inflammation and ECM production that is dictated by the local milieu. This paradigm is illustrated in Figure 1.
Figure 1. Potential contributions of fibrocytes to autoimmune pathogenesis.
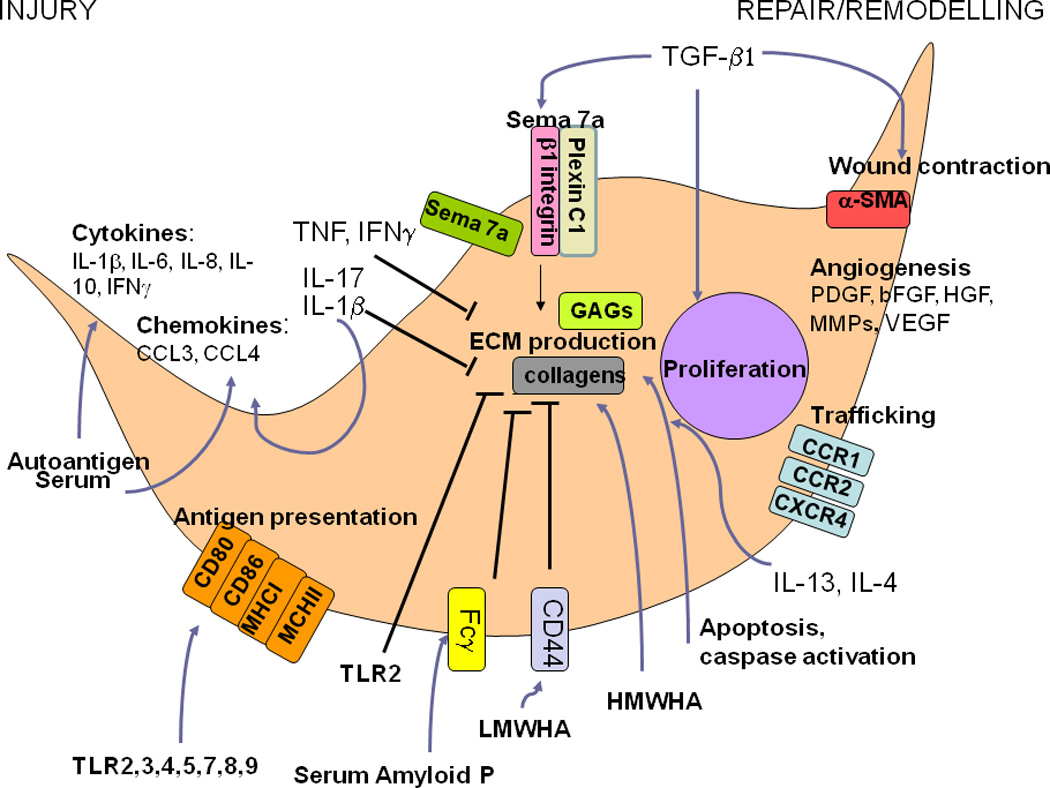
In response to the local milieu, fibrocytes demonstrate marked phenotypic plasticity. For example, exposure to autoantigen, acute injury, IL-1β, IL-17, serum factors, and innate immune stimuli with Toll-like receptors (TLRs) and viral infection, fibrocytes adopt a proinflammatory phenotype characterized by secretion of IFNγ, IL-6, IL-8, CCL3, CCL and the increased expression of MHC I and MCHII and the costimulatory molecules CD80 and CD86. In the setting of stimulation with TH2 cytokines such as IL4 and IL13, a more reparative phenotype ensues that is characterized by secretion of ECM components such as collagens and glycosaminoglycans (GAGs). Collagen production is promoted by exposure to high molecular weight hyaluronan (HMWHA) and inhibited via low molecular weight hyaluronan (LMWHA). These latter effects may occur via LMWHA-induced increased expression of CD44, which impedes fibrocyte differentiation. TLR2 activation and Serum amyloid P (SAP) also attenuate fibrocyte outgrowth, with SAP’s effects occurring via components of the Fcγ receptor. Collagen production is also stimulated by TGF-β1 in a Semaphorin 7a (Sema 7a) dependent manner. The effects occur via the β1 integrin subunit and are opposed by Plexin C1. TGF-β1 also stimulates fibrocyte proliferation and wound contraction via α-SMA. Angiogenesis is promoted via the secretion of PDGFα, IL-10, VEGF, HGF, and b-HGF and trafficking is promoted via the chemokine receptors CCR1, CCR2, and CXCR4.
Fibrocyte Associations with Chronic Inflammatory Disease
Given the combination of ongoing inflammation and fibroblast-driven end-organ remodeling seen in chronic inflammatory disease, fibrocytes have emerged as a novel area of investigation in this area. Because the most recent studies of fibrocytes have focused on lung, automimmunity, liver, and normal aging, information regarding fibrocytes in these disorders is summarized below.
Lung
Fibrocytes are associated with many forms of chronic inflammatory lung diseases. Elevations in both circulating [31] and intrapulmonary [4] fibrocytes have been reported in patients with Idiopathic Pulmonary Fibrosis (IPF), where their presence portends short term clinical decline [2]. Similarly, compared to mechanically ventilated patients without Acute Respiratory Distress Syndrome (ARDS) high numbers of fibrocytes are seen in the bronchoalveolar lavage fluid of patients with ARDS who went on to show increased mortality [40]. Elevations in circulating and intrapulmonary fibrocytes are seen in patients with several forms of airway remodeling including chronic persistent asthma, where they have been shown to secrete increased levels of several matrix metalloproteinases including MMP2,3,7,8, [41] and correlate with basement membrane thickness and disease severity [42], and bronchiolitis obliterans following both lung [43] and bone marrow [44] transplantation. Because most of the work in this area has focused on describing these disease associations, there exist ample opportunities for future studies aimed at understanding fibrocyte biology in the setting of chronic lung disease.
Autoimmune
Fibrocytes are increasingly recognized as potential mediators of several autoimmune diseases. Perhaps the strongest association is seen in scleroderma related lung fibrosis, in which both the blood [4,5,25,28] and lungs [4,28] of patients with this disease are enriched for fibrocytes that show increased migration in Boyden chambers compared to fibrocytes obtained from normal controls [28]. Similar findings are seen in the setting of lung disease caused by amyopathic antisynthetase syndrome [4], where ex vivo fibrocyte outgrowth is inhibited by caspase inhibition. Fibrocyte abnormalities are seen in autoimmune thyroiditis [45], where the peripheral blood and retro-ortibal fad pat are enriched for fibrocytes displaying high expression of several pro-inflammatory mediators [45], and in the synovial fluid of patients with rheumatoid arthritis, where fibrocytes possess both the proinflammatory properties generally attributed to synovial macrophages and the tissue remodeling properties generally attributed to synovial fibroblasts [6]. When viewed in combination, these data suggest that ongoing cell death responses characterizing autoimmune disease causes recruitment of fibrocytes which then modulate their phenotype according to the local milieu. Further studies employing animal modeling will be necessary to confirm this hypothesis.
Liver
As early as 2006, fibrocytes were identified in the bile-duct ligation model of murine liver fibrosis [32]. However, because these bone-marrow derived cells comprised a small fraction of αSMA+, desmin+ hepatic stellate cells (which mediate collagen deposition in this model) further study was required to determine the relevance of fibrocytes. An elegant murine imaging study using in vivo imaging of a Col-1 driven luciferase reporter gene found that fibrocytes traffic to diseased liver in response to engagement of the chemokine receptors CCR1 and CCR2 [7]. A similar population has been identified in the spleens of mice treated with TGF-β1, LPS, viral infection or carbon tetrachloride. Because these cells express MHCII and stimulate naïve T cells [13], it is possible that in addition to their modest production of ECM and αSMA, fibrocytes participate in the early phases of fibrosis via initiation and perpetuation of host immune responses. While a similar population has yet to be identified in humans, further characterization of these cells and their participation in chronic liver inflammation remains an important area of study.
Aging
Dysregulated immune responses are seen in otherwise healthy, aged individuals. While the factors regulating this phenotype remain unclear, recent studies suggest a role for fibrocytes. Murine modeling using the senescence-accelerated mice shows increased circulating and intrapulmonary fibrocytes, which may at least partially explain the increased sensitivity to bleomycin seen in these mice [46]. Similarly, imaging studies using the Col-1-luciferase mouse described above show increased egress of fibrocytes into the circulation of aged mice compared to young mice, suggesting that abnormalities in fibrocyte trafficking may be related to CCR2 and CCR3 [7]. Interestingly, similar abnormalities were seen in the circulation of aged but otherwise healthy humans, where increased fibrocyte precursors were seen compared to younger subjects [5]. Further work is required to determine whether fibrocytes play a role in age-related tissue dysfunction.
Conclusions
Fibrocytes may serve as novel targets for intervention in chronic inflammatory disease. However, development of fibrocyte based-therapies requires better understanding of their function. Thus, development of modeling systems allowing better characterization of antigen presentation, production of soluble mediators, and tissue remodeling are sorely needed. In addition, due to the considerable overlap in cell surface markers shared by fibrocytes, monocytes, and fibroblasts, development of fibrocyte-directed therapies will be difficult in the absence of known fibrocyte-specific markers. Pursuit of these areas may yield new insight into chronic inflammation and lead to improved therapies for these difficult to treat disorders.
Highlights.
Fibrocytes are bone marrow derived cells that are implicated in tissue remodeling
Fibrocytes demonstrate marked phenotypic plasticity in response to local cues
Fibrocytes secrete cytokines and present antigen in response to inflammatory stimuli
Fibrocytes secrete extracellular matrix components in response to TH2 cytokines
Fibrocytes may serve as biomarkers and/or therapeutic targets in chronic inflammatory disease
Acknowledgments
Sources of Support: NIH (R01 HL109033), Scleroderma Foundation, and a joint research award from the American Thoracic Society and Pulmonary Fibrosis Foundation (all to ELH)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69(2):213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 2.Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, Margetts PJ, Farkas L, Dobranowski J, Boylan C, O'Byrne PM, et al. Circulating Fibrocytes Are an Indicator for Poor Prognosis in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2009;179(7):588–594. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 3. Gan Y, Reilkoff RA, Peng x, Russell TR, Chen QC, Mathai SK, Gulati M, Homer RJ, Elias JA, Bucala RJ, Herzog EL. Role of Semaphorin 7a in TGF b1 induced lung fibrosis, and scleroderma-related interstitial lung disease. Arth Rheum. 2011;63(8):2484–2494. doi: 10.1002/art.30386. This manuscript shows that the immunomodulatory protein Semaphorin 7a controls fibrocyte differentiation in a β1 integrin dependent manner that is opposed by Plexin C1. This is the first paper to demonstrate a connection between Semaphorin 7a, Plexin C1, and fibrocytes.
- 4.Peng X, Mathai SK, Murray LA, Russell T, Reilkoff R, Chen Q, Gulati M, Elias JA, Bucala R, Gan Y, Herzog EL. Local apoptosis promotes collagen production by monocyte-derived cells in transforming growth factor beta1-induced lung fibrosis. Fibrogenesis Tissue Repair. 2011;4(1):12. doi: 10.1186/1755-1536-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathai SK, Gulati M, Peng X, Russell TR, Shaw AC, Rubinowitz AN, Murray LA, Siner JM, Antin-Ozerkis DE, Montgomery RR, Reilkoff RA, et al. Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab Invest. 2010;90(6):812–823. doi: 10.1038/labinvest.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galligan CL, Siminovitch KA, Keystone EC, Bykerk V, Perez OD, Fish EN. Fibrocyte activation in rheumatoid arthritis. Rheumatology (Oxford) 49(4):640–651. doi: 10.1093/rheumatology/kep265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scholten D, Reichart D, Paik YH, Lindert J, Bhattacharya J, Glass CK, Brenner DA, Kisseleva T. Migration of fibrocytes in fibrogenic liver injury. Am J Pathol. 2011;179(1):189–198. doi: 10.1016/j.ajpath.2011.03.049. This manuscript uses state of the art imaging techniques to track the migration of fibrocytes in an in vivo murine model of hepatic fibrosis. The Col-1 GFP and Col-1 Luciferase mice used in this paper can be used for isolation of live fibrocytes, a technique which to date has been impossible to achieve.
- 8.Medina A, Ghahary A. Reprogrammed fibrocytes induce a mixed Th1/Th2 cytokine response of naive CD4(+) T cells. Mol Cell Biochem. 2011;346(1–2):89–94. doi: 10.1007/s11010-010-0595-2. [DOI] [PubMed] [Google Scholar]
- 9.Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol. 2011;11(6):427–435. doi: 10.1038/nri2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4(10):e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekert JE, Murray LA, Das AM, Sheng H, Giles-Komar J, Rycyzyn MA. Chemokine (CC motif) ligand 2 mediates direct and indirect fibrotic responses in human and murine cultured fibrocytes. Fibrogenesis Tissue Repair. 2012;4(1):23. doi: 10.1186/1755-1536-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehrad B, Burdick MD, Strieter RM. Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis. Int J Biochem Cell Biol. 2009;41(8–9):1708–1718. doi: 10.1016/j.biocel.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kisseleva T, von Kockritz-Blickwede M, Reichart D, McGillvray SM, Wingender G, Kronenberg M, Glass CK, Nizet V, Brenner DA. Fibrocyte-like cells recruited to the spleen support innate and adaptive immune responses to acute injury or infection. J Mol Med (Berl) 2012;89(10):997–1013. doi: 10.1007/s00109-011-0756-0. This paper suggests a central role for fibrocytes, or fibrocyte-like cells, in the initiation of proinflammatory responses caused by a variety of stimuli.
- 14.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171(1):380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 15. Bianchetti L, Barczyk M, Cardoso J, Schmidt M, Bellini A, Mattoli S. Extracellular matrix remodelling properties of human fibrocytes. J Cell Mol Med. 2011 doi: 10.1111/j.1582-4934.2011.01344.x. in press. This paper shows that in addition to type I collagen, fibrocytes express an array of ECM proteins including other types of Collagen and glycosaminoglycans.
- 16.Curnow SJ, Fairclough M, Schmutz C, Kissane S, Denniston AK, Nash K, Buckley CD, Lord JM, Salmon M. Distinct types of fibrocyte can differentiate from mononuclear cells in the presence and absence of serum. PLoS One. 2010;5(3):e9730. doi: 10.1371/journal.pone.0009730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niedermeier M, Reich B, Gomez MR, Denzel A, Schmidbauer K, Gobel N, Talke Y, Schweda F, Mack M. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc Natl Acad Sci U S A. 2009;106(42):17892–17897. doi: 10.1073/pnas.0906070106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilling D, Tucker NM, Gomer RH. Aggregated IgG inhibits the differentiation of human fibrocytes. J Leukoc Biol. 2006;79(6):1242–1251. doi: 10.1189/jlb.0805456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;171(10):5537–5546. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilling D, Roife D, Wang M, Ronkainen SD, Crawford JR, Travis EL, Gomer RH. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol. 2007;179(6):4035–4044. doi: 10.4049/jimmunol.179.6.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray LA, Chen Q, Kramer MS, Hesson DP, Argentieri RL, Peng X, Gulati M, Homer RJ, Russell T, van Rooijen N, Elias JA, et al. TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum Amyloid P. Int J Biochem Cell Biol. 2011;43(1):154–162. doi: 10.1016/j.biocel.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Castano AP, Lin SL, Surowy T, Nowlin BT, Turlapati SA, Patel T, Singh A, Li S, Lupher ML, Jr, Duffield JS. Serum amyloid P inhibits fibrosis through Fc gamma Rdependent monocyte-macrophage regulation in vivo. Sci Transl Med. 2009;1(5):5ra13. doi: 10.1126/scitranslmed.3000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maharjan AS, Pilling D, Gomer RH. High and low molecular weight hyaluronic acid differentially regulate human fibrocyte differentiation. PLoS ONE. 2011;6(10):e26078. doi: 10.1371/journal.pone.0026078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83(6):1323–1333. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gan y, Reilkoff RA, Peng x, Russell TR, Chen QC, Mathai SK, Gulati M, Homer RJ, Elias JA, Bucala RJ, Herzog EL. Role of Semaphorin 7a in TGF b1 induced lung fibrosis, and scleroderma-related interstitial lung disease. Arth Rheum. 2011;63(8):2484–2494. doi: 10.1002/art.30386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikam VS, Wecker G, Schermuly R, Rapp U, Szelepusa K, Seeger W, Voswinckel R. Treprostinil Inhibits Adhesion and Differentiation of Fibrocytes via cAMP and Rap Dependent ERK Inactivation. Am J Respir Cell Mol Biol. 2011;45(4):692–703. doi: 10.1165/rcmb.2010-0240OC. [DOI] [PubMed] [Google Scholar]
- 27.Maharjan AS, Pilling D, Gomer RH. Toll-like receptor 2 agonists inhibit human fibrocyte differentiation. Fibrogenesis Tissue Repair. 2011;3:23. doi: 10.1186/1755-1536-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tourkina E, Bonner M, Oates J, Hofbauer A, Richard M, Znoyko S, Visconti RP, Zhang J, Hatfield CM, Silver RM, Hoffman S. Altered monocyte and fibrocyte phenotype and function in scleroderma interstitial lung disease: reversal by caveolin-1 scaffolding domain peptide. Fibrogenesis Tissue Repair. 2011;4(1):15. doi: 10.1186/1755-1536-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, Kaneko S. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci U S A. 2006;103(38):14098–14103. doi: 10.1073/pnas.0511200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166(12):7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 31.Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353(1):104–108. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 32.Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, Brenner DA. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45(3):429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114(3):438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160(1):419–425. [PubMed] [Google Scholar]
- 35. Bellini A, Marini MA, Bianchetti L, Barczyk M, Schmidt M, Mattoli S. Interleukin (IL)-4, IL-13, and IL-17A differentially affect the profibrotic and proinflammatory functions of fibrocytes from asthmatic patients. Mucosal Immunol. 2012 doi: 10.1038/mi.2011.60. in press. This paper illustrates that TH17 cytokines promote a proinflammatory phenotype in human fibrocytes and that TH2 cytokines promote a profibrotic phenotype in these cells.
- 36.Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A. 1997;94(12):6307–6312. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartlapp I, Abe R, Saeed RW, Peng T, Voelter W, Bucala R, Metz CN. Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. Faseb J. 2001;15(12):2215–2224. doi: 10.1096/fj.01-0049com. [DOI] [PubMed] [Google Scholar]
- 38.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330(6005):827–830. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 39.van Deventer HW, Wu QP, Bergstralh DT, Davis BK, O'Connor BP, Ting JP, Serody JS. C-C chemokine receptor 5 on pulmonary fibrocytes facilitates migration and promotes metastasis via matrix metalloproteinase 9. Am J Pathol. 2008;173(1):253–264. doi: 10.2353/ajpath.2008.070732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quesnel C, Piednoir P, Gelly J, Nardelli L, Garnier M, Lecon V, Lasocki S, Bouadma L, Philip I, Elbim C, Mentre F, et al. Alveolar fibrocyte percentage is an independent predictor of poor outcome in patients with acute lung injury. Crit Care Med. 2012;40(1):21–28. doi: 10.1097/CCM.0b013e31822d718b. [DOI] [PubMed] [Google Scholar]
- 41. Garcia-de-Alba C, Becerril C, Ruiz V, Gonzalez Y, Reyes S, Garcia-Alvarez J, Selman M, Pardo A. Expression of matrix metalloproteases by fibrocytes: possible role in migration and homing. Am J Respir Crit Care Med. 2010;182(9):1144–1152. doi: 10.1164/rccm.201001-0028OC. This paper performs functional analysis of fibrocytes and finds them to express detectable levels of various MMPs, thus further supporting a role for these cells in tissue remodeling.
- 42.Nihlberg K, Larsen K, Hultgardh-Nilsson A, Malmstrom A, Bjermer L, Westergren-Thorsson G. Tissue fibrocytes in patients with mild asthma: a possible link to thickness of reticular basement membrane? Respir Res. 2006;7:50. doi: 10.1186/1465-9921-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LaPar DJ, Burdick MD, Emaminia A, Harris DA, Strieter BA, Liu L, Robbins M, Kron IL, Strieter RM, Lau CL. Circulating fibrocytes correlate with bronchiolitis obliterans syndrome development after lung transplantation: a novel clinical biomarker. Ann Thorac Surg. 2011;92(2):470–477. doi: 10.1016/j.athoracsur.2011.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersson-Sjoland A, Erjefalt JS, Bjermer L, Eriksson L, Westergren-Thorsson G. Fibrocytes are associated with vascular and parenchymal remodelling in patients with obliterative bronchiolitis. Respir Res. 2009;10:103. doi: 10.1186/1465-9921-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Douglas RS, Afifiyan NF, Hwang CJ, Chong K, Haider U, Richards P, Gianoukakis AG, Smith TJ. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2011;95(1):430–438. doi: 10.1210/jc.2009-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu J, Gonzalez ET, Iyer SS, Mac V, Mora AL, Sutliff RL, Reed A, Brigham KL, Kelly P, Rojas M. Use of senescence-accelerated mouse model in bleomycin-induced lung injury suggests that bone marrow-derived cells can alter the outcome of lung injury in aged mice. J Gerontol A Biol Sci Med Sci. 2009;64(7):731–739. doi: 10.1093/gerona/glp040. [DOI] [PMC free article] [PubMed] [Google Scholar]


