Abstract
Cyclooxygenase (COX) inhibiting drugs augment muscle mass and strength improvements during resistance exercise based treatment of sarcopenia in older individuals. Initial evidence suggests a potential mechanism of COX inhibitor blunted prostaglandin (PG) E2 stimulation of interleukin (IL)-6 and the ubiquitin ligase MuRF-1, reducing their inhibition on muscle growth. The purpose of this investigation was to determine if PGE2 stimulates IL-6 and MuRF-1 transcription in skeletal muscle. Muscle biopsies were obtained from 10 young individuals and incubated ex vivo with PGE2 or control and analyzed for IL-6 and MuRF-1 mRNA levels. PGE2 upregulated (P<0.05) expression of both IL-6 (195%) and MuRF-1 (51%). A significant relationship was found between IL-6 and MuRF-1 expression after incubation with PGE2 (r=0.77, P<0.05), suggesting regulation through a common pathway. PGE2 induces IL-6 and MuRF-1 transcription in human skeletal muscle, providing a mechanistic link between COX inhibiting drugs, PGE2, and the regulation of muscle mass.
Keywords: PGE2, Skeletal muscle, IL-6, MuRF-1
1. INTRODUCTION
The prostaglandin (PG) producing cyclooxygenase (COX) pathway has been shown to be a key regulator of skeletal muscle protein turnover through the actions of PGF2α and PGE2 [1–5]. Recent evidence shows that blocking this pathway with common COX inhibitors (i.e., acetaminophen and ibuprofen) in older humans during resistance exercise training over several months promotes substantial additional skeletal muscle hypertrophy [6]. The mechanism underlying this effect is not completely clear, but COX inhibitor suppression of intramuscular levels of the myokine interleukin-6 (IL-6) and the ubiquitin ligase muscle RING finger-1 (MuRF-1) appears to play a central role [7]. This purported mechanism is based on findings that low level elevation of IL-6 reduces human muscle protein synthesis and increases net muscle protein degradation [8], is associated with a reduction in muscle mass and functional independence in older individuals [9–12], and retards growth and promotes muscle atrophy in animals [13, 14]. In addition, MuRF-1 is a central mediator of skeletal muscle proteolysis [15–17]. Thus, a COX inhibitor mediated reduction in the intramuscular levels of these two factors would reduce their inhibitory effects on muscle growth.
A connection between the COX pathway and IL-6 and MuRF-1 production in skeletal muscle has not been established. However, studies in non-skeletal muscle cells suggest PGE2 can stimulate IL-6 transcription [18–21]. No such evidence exists connecting PGs to MuRF-1 transcription. Therefore, the goal of the current investigation was to address the hypothesis, using ex vivo incubation studies, that PGE2 stimulates the transcription of IL-6 and MuRF-1 in human skeletal muscle. If true, these findings would have implications for understanding how COX inhibiting drugs promote muscle hypertrophy in older individuals and provide insight into PG regulation of inflammation in the development and treatment of sarcopenia, the age related loss of skeletal muscle mass and function [22–24].
2. MATERIALS AND METHODS
Participants
Ten male subjects (Age: 24±1y; Height: 181±2cm; Weight: 80.3±3.3kg; BMI: 24.2±1.1kg/m2) were recruited to participate in this investigation and before enrollment each subject completed a detailed health and exercise history questionnaire. Subjects were excluded if they had any known acute or chronic illness, cardiac, pulmonary, liver, or kidney abnormalities, uncontrolled hypertension, insulin- or non-insulin dependent diabetes or other metabolic disorders, arthritis, a history of neuromuscular problems, if they used tobacco or regularly consumed analgesics/anti-inflammatory drug(s), prescription or non-prescription. All subjects were considered moderately physically active. This study was approved by the Ball State University Institutional Review Board. All procedures, risks, and benefits associated with the experimental testing were explained to the subjects before providing written consent to participate.
Muscle Biopsy
Subjects underwent a muscle biopsy of the vastus lateralis [25, 26] in the early morning (~0700) after at least 30 minutes of supine rest. Prior to the muscle biopsy, subjects were supplied their evening meals in liquid form (Ensure Plus; 57% carbohydrate, 15% protein, 28% fat) that provided 50% of the estimated daily caloric need to standardize the composition, amount, and timing (i.e., ~12h fast) of the final meal consumed prior to the biopsy. In addition, subjects were instructed to refrain from physical activity beyond their normal daily activity for three days prior to the biopsy. Following the biopsy, the muscle was cleansed of excess blood, visible fat, connective tissue and divided into ~10mg samples and immediately processed for the ex vivo incubation experiments.
Ex vivo PGE2 Stimulation Experiments
Four ~10mg muscle samples were immediately placed in individual pre-weighed incubation vials containing 1ml of pre-gassed (95% O2/5% CO2) Krebs-Henseleit Buffer (KHB) (118.5mM NaCl, 1.2mM MgSO4, 4.7mM KCl, 1.2mM KH2PO4, 25mM NaHCO3, 2.5mM CaCl2; pH 7.4) supplemented with 5mM glucose, re-weighed to determine muscle weight (11.60±0.41mg), and then completed a pre-incubation of 30min. The muscle samples were then transferred to new vials containing 1ml of fresh pre-gassed KHB, with two vials receiving PGE2 (20μM) (PGE2 powder dissolved in 100% ethanol; experimental samples) (BML-PG007, Enzo Life Sciences, Farmingdale, NY) and two vials receiving the same amount of ethanol (7μL; control samples). The amount of PGE2 was chosen based on preliminary experiments on human skeletal muscle completed using various PGE2 concentrations (data not shown) and on studies in human nerve and bone cells [19, 21, 27, 28]. The four vials were then incubated in a shaking water bath (110 cycles/min) under constant temperature (37°C) and received continuous gassing (95% O2/5% CO2) for 1 or 2 hours. At the end of each 1h and 2h incubation period, an experimental and control muscle sample were removed from their incubation vials, blotted on KHB soaked gauze and frozen in liquid nitrogen (−190°C). After freezing, the muscle samples were placed in RNAlater-Ice (Ambion, Austin, TX) at −20°C until mRNA analysis.
Muscle mRNA Measurements
qPCR was completed on the incubated samples to determine the mRNA levels of IL-6 and MuRF-1 as we have previously described [7, 29]. Total RNA was extracted in TRI Reagent (Molecular Research Center, Cincinnati, OH). The quality and integrity (RIN of 7.1±0.1) of extracted RNA (88.3±5.8 ng/μl) was evaluated using a RNA 6000 Nano LabChip kit on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA) [6, 30, 31]. Oligo (dT) primed first-strand cDNA was synthesized (150ng of total RNA) using SuperScript II RT (Invitrogen, Carlsbad, CA). Quantification of mRNA levels (in duplicate) was performed in a 72-well Rotor-Gene 3000 Centrifugal Real-Time Cycler (Corbett Research, Mortlake, NSW, Australia). Housekeeping gene GAPDH was used as a reference gene [30, 32, 33]. All primers used in this investigation were mRNA-specific (on different exons and/or crossing over an intron) and designed for qPCR (Vector NTI Advance 9 software, Invitrogen) using SYBR Green chemistry. Details about primer characteristics and sequences have been described previously [7, 29]. A melting curve analysis was generated for all qPCR runs to validate that only one product was present. A serial dilution curve (cDNA made from 500ng of total RNA of human skeletal muscle; Ambion, Austin, TX) was generated for each qPCR run to evaluate reaction efficiencies. The amplification calculated by the Rotor-Gene software was specific and highly efficient (1.03±0.02; R2=0.99±0.00; slope=3.25±0.05). Gene expression of IL-6 and MuRF-1 was determined after 1h and 2h of incubation by using the 2−ΔCT (arbitrary units) relative quantification method [31, 33, 34].
Statistical Analysis
Data were analyzed with a one-tailed paired t-test to compare gene expression between control and PGE2 stimulated samples after 1h and 2h of incubation. Muscle sample weight between the control and PGE2 stimulated samples was also compared with a paired t-test. The association between the IL-6 and MuRF-1 gene expression was evaluated using a Pearson r correlation. For all variables, significance was accepted at P<0.05. Data are presented as means ± SE.
3. RESULTS
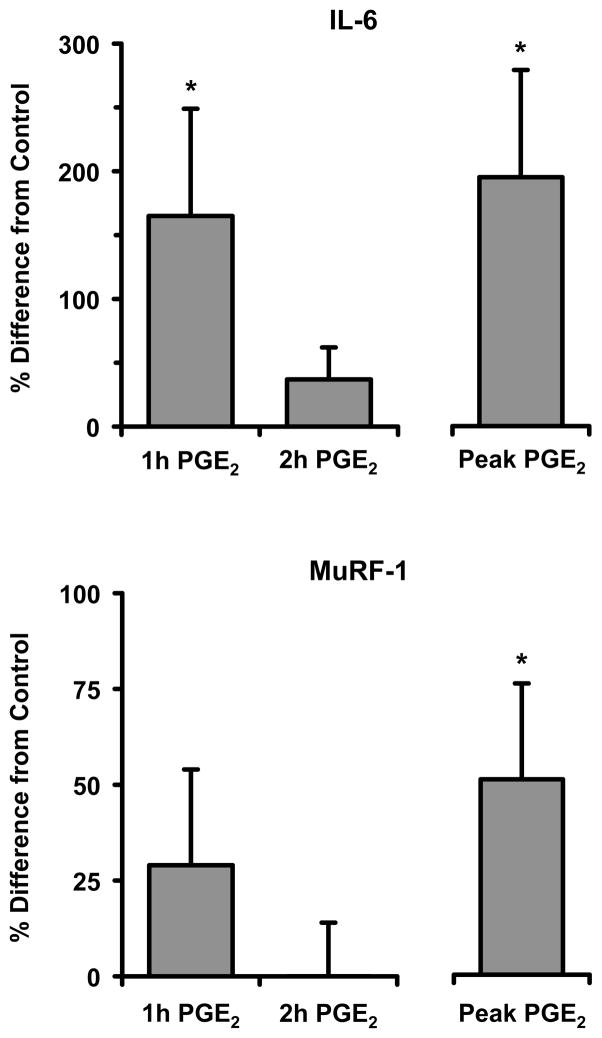
On average, IL-6 was upregulated by PGE2 (165%) at 1h (P<0.05) but was similar to control after 2h (P>0.05) and MuRF-1 expression in response to PGE2 was similar to control at 1h and 2h (P>0.05) (Figure 1). However, not all subjects had their largest induction of IL-6 or MuRF-1 at the same time points; therefore, the largest induction from each subject was grouped (Peak), resulting in an upregulation (P<0.05) of IL-6 (195%) and MuRF-1 (51%) (Figure 1). A significant relationship was found between IL-6 and MuRF-1 gene expression after 1h and 2h of incubation with PGE2 (r=0.77, P<0.05). There were no differences in muscle weight between the control and PGE2 samples (control: 11.77±0.62; PGE2: 11.42±0.56mg) (P>0.05).
Figure 1.
Percent difference in IL-6 and MuRF-1 gene expression from control after human skeletal muscle samples (~10mg) were incubated (Krebs-Henseleit Buffer, shaking 37°C water bath, continuous gassing (95% O2/5% CO2)) with and without PGE2 (20μM) for 1h or 2h. Not all subjects had their largest induction at the same time points; therefore, the largest induction of gene expression that occurred for both genes from either the 1h or 2h experiments by each subject were grouped together to determine peak expression independent of time (Peak). IL-6 and MuRF-1 mRNA expression was determined by qPCR. Data are means ± SE from 10 subjects. * P<0.05 from control.
4. DISCUSSION AND CONCLUSIONS
The primary findings from this investigation were prostaglandin E2 induces intramuscular transcription of IL-6 and MuRF-1, both regulators of human skeletal muscle mass, and this transcriptional activation may be regulated through a common pathway.
The PGE2 stimulated IL-6 gene transcription response in the current study (Figure 1) is similar to studies that have examined this response in cultured human nerve and bone cells [19, 21]. The fact that some subjects had their highest induction of IL-6 with PGE2 at 1h while others responded more at 2h suggests a variation in sensitivity to PGE2 simulation among the subjects. To a greater extent this variation in response was also seen in the MuRF-1 expression increase with PGE2 stimulation, as 40% of the subjects had a peak response at 1h while 60% had their peak expression at 2hrs. The specific basis for the variability of MuRF-1 (and to some degree IL-6) expression induced by PGE2 ex vivo is unclear. Differences in the number of PGE2 receptors on the incubated muscle and components associated with the stimulated receptor pathway would likely alter the responsiveness of the muscle to PGE2. Exercise training does impact human skeletal muscle PGE2 receptor levels [7], although the training status of the current subjects was relatively similar (~30min/day of exercise). Considering the known fiber type influence on metabolic and molecular processes [35–38], including IL-6 and MuRF-1 levels [39, 40], it is possible that differences in fiber type among the subjects or between the different incubated muscle samples contributed to the variable transcription response. This variability raises interesting questions regarding the PG/COX pathway in human skeletal muscle and further investigation into these issues is clearly warranted.
The significant relationship between IL-6 and MuRF-1 expression in response to PGE2 suggests that activation of the PGE2 receptor may stimulate both IL-6 and MuRF-1 gene transcription through a similar mechanism in human skeletal muscle. In support of this notion, PGE2 stimulates IL-6 transcription in non-skeletal muscle cells through a NF-κB-mediated mechanism [18, 20, 21, 41], and MuRF-1 has been shown to be regulated via NF-κB [17, 42]. Based on these studies and the current data it is reasonable to speculate that PGE2 stimulates IL-6 and MuRF-1 transcription through a common PGE2 receptor - NF-κB linked mechanism.
The findings from the current ex vivo studies of adult human skeletal muscle provide strong support for the proposed mechanism of COX inhibitors augmenting muscle growth by up to 50% in resistance training older individuals through a reduction in PGE2 stimulation of IL-6 and MuRF-1 [6, 7]. This mechanism is based on the findings that: 1) Intramuscular levels of IL-6 and MuRF-1 are lower after several months of resistance training in individuals consuming COX inhibitors (e.g., acetaminophen and ibuprofen) daily compared to a placebo group [7], 2) IL-6 and MuRF-1 are elevated after exercise [29] and generally exhibit the aforementioned inhibitory effects on muscle growth, and 3) The elevation in intramuscular PGE2 in response to exercise can be eliminated with consumption of a COX inhibitor [2]. Further support comes from studies that show NF-κB activation and binding to the IL-6 promoter is elevated with resistance exercise in humans [43], and NF-κB regulators (i.e., IKKβ) appear to be reduced in skeletal muscle from individuals consuming a COX inhibitor [7].
It is unclear if the PGE2 and COX inhibitor regulated effect on muscle mass is age-specific, as might be expected if differences existed between young and old in their production of intramuscular PGE2 or their PGE2 stimulation of IL-6 and MuRF-1 in response to repeated resistance exercise sessions. While the studies in the current investigation were limited to young males to establish whether or not the proposed pathway was present in skeletal muscle, there is no evidence to suggest this pathway would not be intact in both young and old men and women. Further studies examining potential gender and aging differences are needed. In addition, the current methodology opens the possibility to test further hypotheses in this area and should greatly expand our insight into PG regulation of metabolic and molecular processes in human skeletal muscle.
In summary, we have established a connection between the COX pathway product PGE2 and the stimulation of IL-6 and MuRF-1 transcription in human skeletal muscle. These findings establish a novel mechanism regulating skeletal muscle adaptation in humans, add to our understanding of the molecular and metabolic effects of the most commonly consumed drugs, COX inhibitors, and identify a new pathway and potential targets for the treatment of sarcopenia.
Acknowledgments
Grant Support: NIH grant R01 AG020532.
Thank you to Jeff Ryder PhD, who provided expert guidance in the setup and performance of the incubation experiments.
Footnotes
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rodemann HP, Goldberg AL. Arachidonic acid, prostaglandin E2 and F2α influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem. 1982;257:1632–1638. [PubMed] [Google Scholar]
- 2.Trappe TA, Fluckey JD, White F, Lambert CP, Evans WJ. Skeletal muscle PGF2α and PGE2 in response to eccentric resistance exercise: influence of ibuprofen and acetaminophen. J Clin Endocrinol Metab. 2001;86:5067–5070. doi: 10.1210/jcem.86.10.7928. [DOI] [PubMed] [Google Scholar]
- 3.Trappe TA, White F, Lambert CP, Cesar D, Hellerstein M, Evans WJ. Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. Am J Physiol Endo Metab. 2002;282:E551–556. doi: 10.1152/ajpendo.00352.2001. [DOI] [PubMed] [Google Scholar]
- 4.Palmer RM. Prostaglandins and the control of muscle protein synthesis and degradation. Prostaglandins Leukot Essent Fatty Acids. 1990;39:95–104. doi: 10.1016/0952-3278(90)90017-f. [DOI] [PubMed] [Google Scholar]
- 5.Markworth JF, Cameron-Smith D. Prostaglandin F2α stimulates PI3K/ERK/mTOR signaling and skeletal myotube hypertrophy. Am J Physiol Cell Physiol. 2011;300:C671–682. doi: 10.1152/ajpcell.00549.2009. [DOI] [PubMed] [Google Scholar]
- 6.Trappe TA, Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Sullivan BE, Lee JD, Jemiolo B, Weinheimer EM, Hollon CJ. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol. 2011;300:R655–662. doi: 10.1152/ajpregu.00611.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trappe TA, Standley RA, Jemiolo B, Carroll CC, Trappe SW. Prostaglandin and myokine involvement in the cyclooxygenase-inhibiting drug enhancement of skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol. 2012 doi: 10.1152/ajpregu.00245.2012. Epub Dec 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Hall G, Steensberg A, Fischer C, Keller C, Moller K, Moseley P, Pedersen BK. Interleukin-6 markedly decreases skeletal muscle protein turnover and increases nonmuscle amino acid utilization in healthy individuals. J Clin Endocrinol Metab. 2008;93:2851–2858. doi: 10.1210/jc.2007-2223. [DOI] [PubMed] [Google Scholar]
- 9.Barbieri M, Ferrucci L, Ragno E, Corsi A, Bandinelli S, Bonafe M, Olivieri F, Giovagnetti S, Franceschi C, Guralnik JM, Paolisso G. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab. 2003;284:E481–487. doi: 10.1152/ajpendo.00319.2002. [DOI] [PubMed] [Google Scholar]
- 10.de Gonzalo-Calvo D, de Luxan-Delgado B, Rodriguez-Gonzalez S, Garcia-Macia M, Suarez FM, Solano JJ, Rodriguez-Colunga MJ, Coto-Montes A. Interleukin 6, soluble tumor necrosis factor receptor I and red blood cell distribution width as biological markers of functional dependence in an elderly population: A translational approach. Cytokine. 2012;58:193–198. doi: 10.1016/j.cyto.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 12.Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, Colbert LH, Pahor M, Rubin SM, Tylavsky FA, Visser M. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Geron. 2009;64:1183–1189. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bodell PW, Kodesh E, Haddad F, Zaldivar FP, Cooper DM, Adams GR. Skeletal muscle growth in young rats is inhibited by chronic exposure to IL-6 but preserved by concurrent voluntary endurance exercise. J Appl Physiol. 2009;106:443–453. doi: 10.1152/japplphysiol.90831.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98:911–917. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 15.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 16.Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, Lecker SH, Goldberg AL. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 2007;21:140–155. doi: 10.1096/fj.06-6604com. [DOI] [PubMed] [Google Scholar]
- 17.Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 2008;23:160–170. doi: 10.1152/physiol.00041.2007. [DOI] [PubMed] [Google Scholar]
- 18.Dendorfer U, Oettgen P, Libermann TA. Multiple regulatory elements in the interleukin-6 gene mediate induction by prostaglandins, cyclic AMP, and lipopolysaccharide. Mol Cell Biol. 1994;14:4443–4454. doi: 10.1128/mcb.14.7.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiebich BL, Hull M, Lieb K, Gyufko K, Berger M, Bauer J. Prostaglandin E2 induces interleukin-6 synthesis in human astrocytoma cells. J Neurochem. 1997;68:704–709. doi: 10.1046/j.1471-4159.1997.68020704.x. [DOI] [PubMed] [Google Scholar]
- 20.St-Jacques B, Ma W. Role of prostaglandin E2 in the synthesis of the pro-inflammatory cytokine interleukin-6 in primary sensory neurons: an in vivo and in vitro study. J Neurochem. 2011;118:841–854. doi: 10.1111/j.1471-4159.2011.07230.x. [DOI] [PubMed] [Google Scholar]
- 21.Wang P, Zhu F, Konstantopoulos K. Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-kappaB activation. Am J Physiol Cell Physiol. 2010;298:C1445–1456. doi: 10.1152/ajpcell.00508.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delmonico MJ, Harris TB, Lee JS, Visser M, Nevitt M, Kritchevsky SB, Tylavsky FA, Newman AB. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55:769–774. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 23.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 25.Bergstrom J. Muscle electrolytes in man. Scand J Clin Lab Invest. 1962;14:7–110. [Google Scholar]
- 26.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scandinavian journal of clinical and laboratory investigation. 1975;35:609–616. [PubMed] [Google Scholar]
- 27.Fiebich BL, Schleicher S, Spleiss O, Czygan M, Hull M. Mechanisms of prostaglandin E2-induced interleukin-6 release in astrocytes: possible involvement of EP4-like receptors, p38 mitogen-activated protein kinase and protein kinase C. J Neurochem. 2001;79:950–958. doi: 10.1046/j.1471-4159.2001.00652.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang P, Zhu F, Konstantopoulos K. Interleukin-6 synthesis in human chondrocytes is regulated via the antagonistic actions of prostaglandin (PG)E2 and 15-deoxy-Delta(12,14)-PGJ2. PLoS One. 2011;6:e27630. doi: 10.1371/journal.pone.0027630. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol. 2007;103:1744–1751. doi: 10.1152/japplphysiol.00679.2007. [DOI] [PubMed] [Google Scholar]
- 30.Weinheimer EM, Jemiolo B, Carroll CC, Harber MP, Haus JM, Burd NA, Lemoine JK, Trappe SW, Trappe TA. Resistance exercise and cyclooxygenase (COX) expression in human skeletal muscle: implications for COX-inhibiting drugs and protein synthesis. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2241–2248. doi: 10.1152/ajpregu.00718.2006. [DOI] [PubMed] [Google Scholar]
- 31.Burd NA, Dickinson JM, Lemoine JK, Carroll CC, Sullivan BE, Haus JM, Jemiolo B, Trappe SW, Hughes GM, Sanders CE, Jr, Trappe TA. Effect of a cyclooxygenase-2 inhibitor on postexercise muscle protein synthesis in humans. Am J Physiol Endocrinol Metab. 2010;298:E354–E361. doi: 10.1152/ajpendo.00423.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jemiolo B, Trappe S. Single muscle fiber gene expression in human skeletal muscle: validation of internal control with exercise. Biochem Biophys Res Commun. 2004;320:1043–1050. doi: 10.1016/j.bbrc.2004.05.223. [DOI] [PubMed] [Google Scholar]
- 33.Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol A Biol Sci Med Sci. 2007;62:1407–1412. doi: 10.1093/gerona/62.12.1407. [DOI] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Dickinson JM, Lee JD, Sullivan BE, Harber MP, Trappe SW, Trappe TA. A new method to study in vivo protein synthesis in slow- and fast-twitch muscle fibers and initial measurements in humans. J Appl Physiol. 2010;108:1410–1416. doi: 10.1152/japplphysiol.00905.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henriksson J, Reitman JS. Quantitative measures of enzyme activities in type I and type II muscle fibres of man after training. Acta Physiol Scand. 1976;97:392–397. doi: 10.1111/j.1748-1716.1976.tb10279.x. [DOI] [PubMed] [Google Scholar]
- 37.Raue U, Trappe TA, Estrem ST, Qian HR, Helvering LM, Smith RC, Trappe S. Transcriptome signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. J Appl Physiol. 2012;112:1625–1636. doi: 10.1152/japplphysiol.00435.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saltin B, Gollnick PD. Skeletal muscle adaptability: significance for metabolism and performance. In: Peachey LD, Adrian RH, Geiger SR, editors. Handbook of Physiology-Skeletal Muscle. American Physiological Society; Bethesda, MD: 1983. pp. 555–631. [Google Scholar]
- 39.Yang Y, Jemiolo B, Trappe S. Proteolytic mRNA expression in response to acute resistance exercise in human single skeletal muscle fibers. J Appl Physiol. 2006;101:1442–1450. doi: 10.1152/japplphysiol.00438.2006. [DOI] [PubMed] [Google Scholar]
- 40.Hiscock N, Chan MH, Bisucci T, Darby IA, Febbraio MA. Skeletal myocytes are a source of interleukin-6 mRNA expression and protein release during contraction: evidence of fiber type specificity. FASEB J. 2004;18:992–994. doi: 10.1096/fj.03-1259fje. [DOI] [PubMed] [Google Scholar]
- 41.Wang P, Zhu F, Lee NH, Konstantopoulos K. Shear-induced interleukin-6 synthesis in chondrocytes: roles of E prostanoid (EP) 2 and EP3 in cAMP/protein kinase A- and PI3-K/Akt-dependent NF-kappaB activation. J Biol Chem. 2010;285:24793–24804. doi: 10.1074/jbc.M110.110320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 43.Vella L, Caldow MK, Larsen AE, Tassoni D, Della Gatta PA, Gran P, Russell AP, Cameron-Smith D. Resistance exercise increases NF-kappaB activity in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2012;302:R667–673. doi: 10.1152/ajpregu.00336.2011. [DOI] [PubMed] [Google Scholar]