Abstract
Keratan sulfate (KS) is an important glycosaminoglycan that is found in cartilage, reproductive, and neural tissues. Corneal KS glycosaminoglycan is found N-linked to lumican, keratocan, and mimecan proteoglycans and has been widely studied by investigators interested in corneal development and diseases. Recently, the availability of corneal KS has become severely limited due to restricted the shipment of bovine central nervous system by-products across international borders in efforts to prevent additional cases of mad cow disease. We report a simple method for the purification of multi-milligram quantities of bovine corneal KS and characterize its structural properties. We also examined its protein-binding properties and discovered that corneal KS bound with high affinity to fibroblast growth factor-2 and sonic hedgehog, a growth factor and a morphogen involved in corneal development and healing.
Keywords: keratan sulfate, cornea, sonic hedgehog, fibroblast growth factor, interaction
Introduction
Proteoglycans (PGs), proteins modified by attachment of glycosaminoglycan (GAG) chains, are important to the growth and development of normal tissue [1], and regulate several key biological processes [2]. The structure of the attached GAGs, long, highly sulfated, polysaccharide chains, are typically responsible for the signaling properties of PGs. For this reason, the analysis of the changing GAG structures in development and disease states has become a prominent area of research.
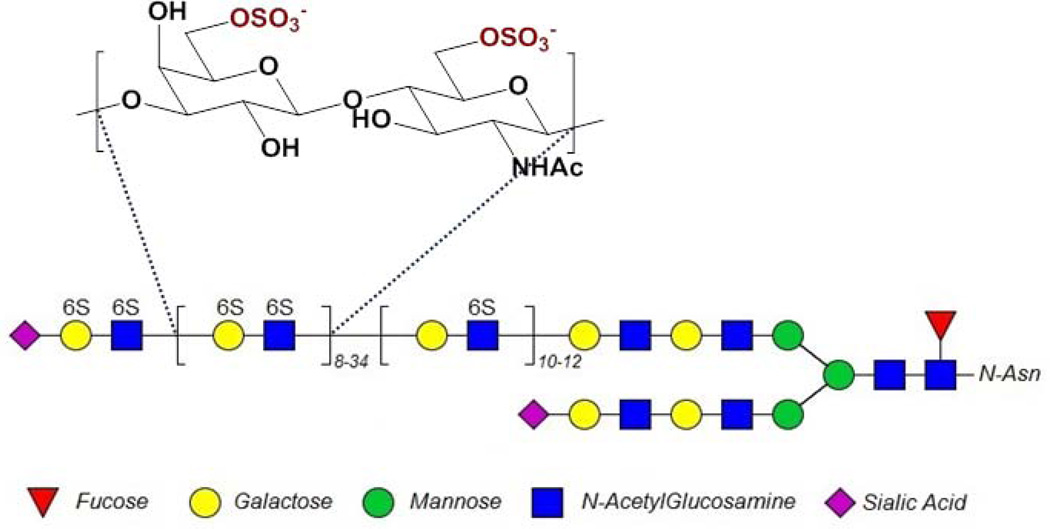
Keratan sulfate (KS) represents an important family of GAGs. Named after the corneal tissue it was first isolated from, different forms of KS are now recognized as present in several tissues, including articular cartilage, reproductive tissue, and neural tissue [3]. KS is composed of a β-1-4-galactose-β-1-3-N-acetylglucosamine disaccharide repeating structure, as shown in Fig. 1 [4–7]. The structure shown in Fig. 1 is based on a composite of several studies, including work by Oeben et al. [4] identifying the placement and relative lengths of the di-, mono- and non-sulfated disaccharide repeating units, work by Stuhlsatz et al. [5] to identify the structure of the linkage region, and work done by Tai et al. [6] to identify various end chain capping structures. KS is classified into three different groups (I-III) based on protein linkage structure and type I, having an N-asparagine linkage (Fig. 1), is found in the cornea [3]. Corneal KS is attached to three different core proteins, resulting in three different kinds of KSPGs, lumican, keratocan, and mimecan [3, 8]. Each core protein typically carries 2–3 chains that are 10–15 kDa long [8, 9].
Figure 1.
Typical structure of corneal KS. The repeating galactose-glucosamine disaccharide structure shown here may be modified with 6-O-sulfo groups (as shown). KS chains are known to have a section of low sulfation (a single 6-O-sulfo group on the N-acetylglucosamine) close to the protein backbone and a section of higher sulfation (6-O-sulfo group on both N-acetylglucosamine and galactose) towards the chain’s non-reducing terminus (See references [4–7]), and are often capped by sialic acid (see reference [6]). In the cornea, KS is N-linked through an asparagine to one of three protein core structures, lumican, keratocan or mimecan.
Several methods of isolating and analyzing KS and KS-PGs have been developed as research into KS structure has focused on its influence on corneal health and developmental biology [10–15]. This research has produced sufficient demand to support commercial KS production, a KS isolated from bovine cornea. However, rising concerns over pathological viruses and prions, such as bovine spongiform encephalopathy or mad cow disease, have restricted the shipment of bovine central nervous system by-products across international borders, making this standardized material inaccessible to many research laboratories.
In the current study we describe a convenient method for the isolation of multi-milligram quantities of KS from bovine cornea and characterize the structure of this KS by nuclear magnetic resonance (NMR) spectroscopy, high performance liquid chromatography-mass spectrometry (MS), and its chain length by gel permeation chromatography (GPC) and polyacrylamide gel electrophoresis (PAGE). This corneal KS was identical to a commercially obtained KS standard. In addition, protein binding of KS was measured by surface plasmon resonance (SPR) to assess its bioactivity. We describe the interaction of corneal KS GAG with sonic hedgehog (SHH), fibroblast growth factor-1 (FGF1), and fibroblast growth factor-2 (FGF2). These are critically important proteins for corneal development and healing, and these interactions should shed light on possible involvement of KS PGs in the growth and development of corneal tissue.
Results
Approximately 180 mg of KS GAG was isolated from 50 bovine corneas, each having a dry weight of 17.5 g. In these studies commercial bovine corneal KS from Seikagaku Kogyo (no longer available outside of Japan) was used as a standard for comparison of structural properties and bioactivities.
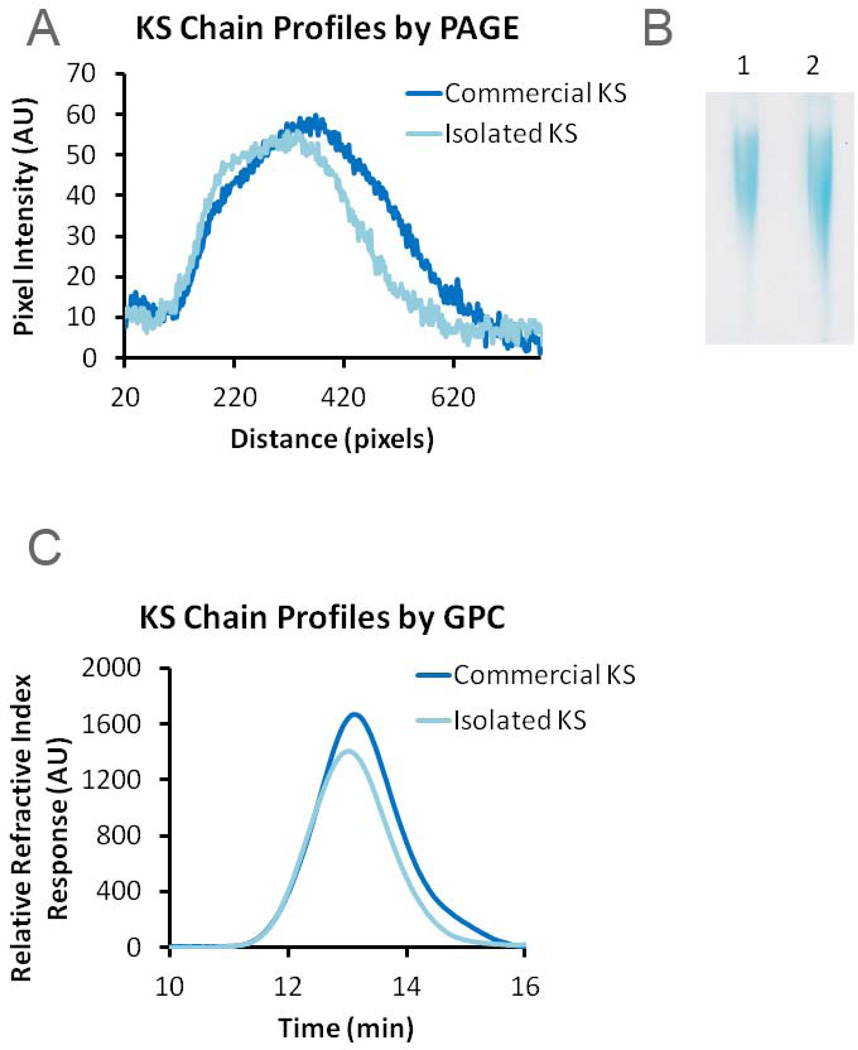
The chain length distributions of KS GAGs were first analyzed and by PAGE and by GPC. Both KS samples afforded similar profiles when analyzed by PAGE, with the commercial KS showing a slightly larger distribution of chain lengths and a lower average chain length (Fig. 2A and B). Chain length analysis by GPC also showed similar chain length profiles for both KS samples (Fig. 2C). The calculated weight average and number average molecular weights of the KS sample prepared in this study, 14.3 and 11.6 kDa, were within ~15% of those obtained for the commercial KS sample (Table 1) and both KS samples exhibited similar polydispersities.
Figure 2.
Molecular weight analysis of isolated and commercial KS. A. Plot of KS chain profile distributions determined by PAGE; B. Alcian blue stained gel obtained on PAGE analysis of isolated (lane 1) and commercial (lane 2) KS GAGs. C. Plot of KS chain profile distributions of the isolated and commercial KS measured by GPC-HPLC.
Table 1.
Molecular Weight Profiles of KS by GPC
| MN | MW | Polydispersity | |
|---|---|---|---|
| Commercial KS | 9800 | 12900 | 1.23 |
| Isolated KS | 11600 | 14300 | 1.32 |
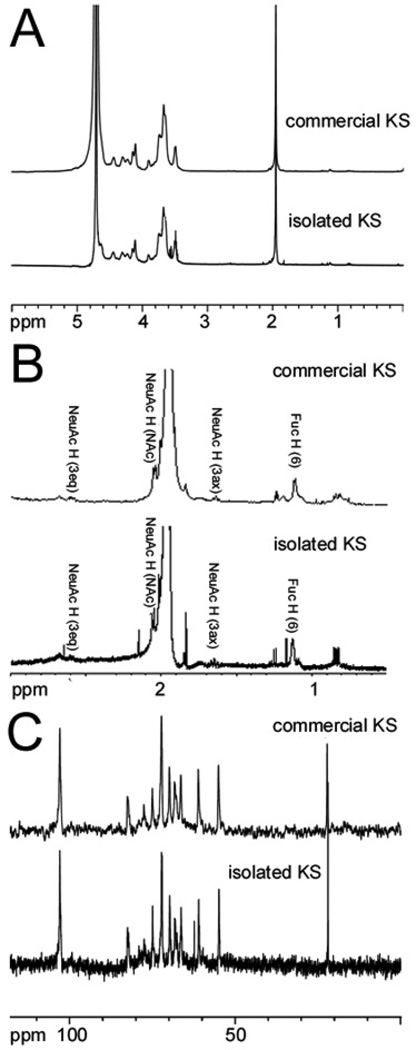
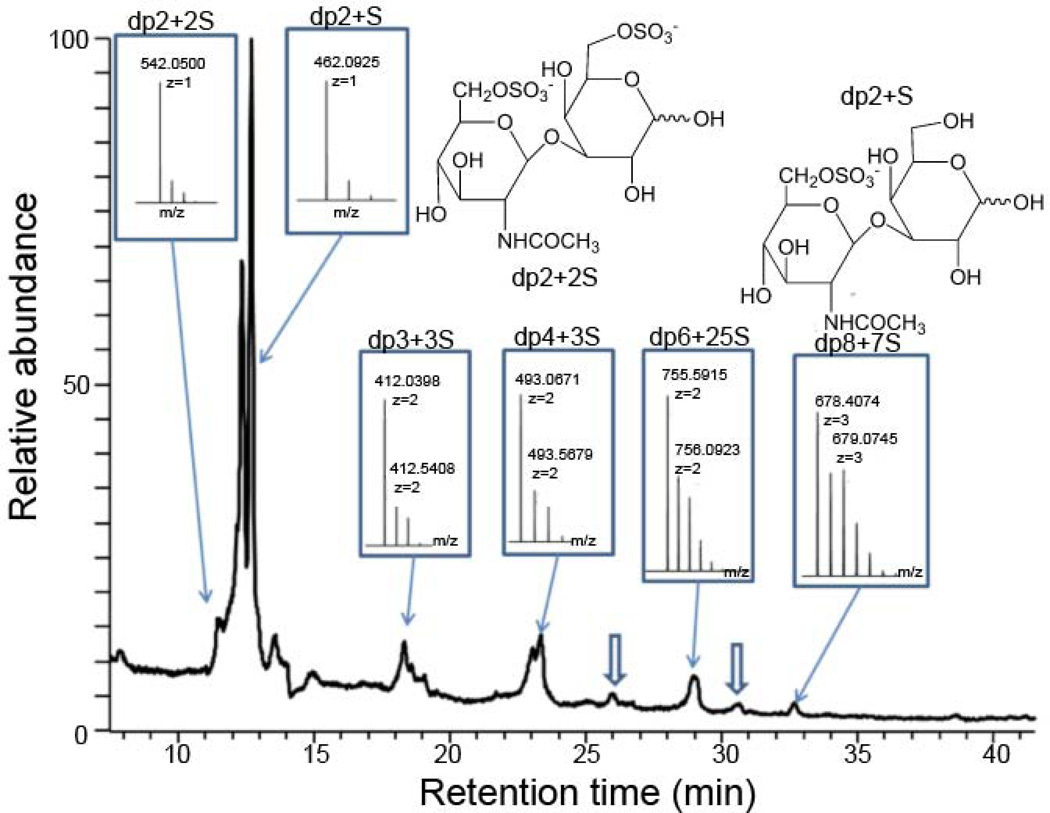
Next, KS samples were analyzed by 1H-NMR and 13C-NMR (Fig. 3). The NMR profiles of both KS samples were identical and consistent with the known structure of KS. The KS sample prepared in the current study was subjected to digestion with keratanase 2, an endo-β-galactosidase, and then analyzed by both reverse-phase ion-pairing (RPIP)-HPLC-MS and hydrophilic interaction chromatography (HILIC)-MS [16]. Past studies have used keratanase II and an endo-β-galactosidase to study the structures of cartilage and corneal KS oligosaccharides [17–19]. RPIP-HPLC-MS confirmed the major disaccharide formed on keratinase 2-treatment of the isolated KS was GlcNAc6S-Gal6S (See Supplementary material, Fig. S1) consistent with the known structure of corneal KS. HILIC-MS revealed a consistent motif of undigestible, highly sulfated (nearly 2-O-sulfo groups/disaccharide repeating unit), oligosaccharide domains clearly (Fig. 4 and Fig. S2) also consistent with the known structure of corneal KS. Highly sulfated sialic acid-capped KS oligomers with one sialic acid per chain (Fig. 1) were also detected (Fig. 4) and are similar to structures were found in KS articular cartilage [18]. Moreover, the linkage region (Fig. 1) was confirmed by MS analysis (Fig. S3).
Figure 3.
NMR analysis of commercial and isolated KS. Shown are A. Full 1H-NMR spectrum (0 to 6 ppm); B. Expanded 1H-NMR spectrum (0.5 to 3 ppm), minor peaks labeled correspond to signals assignable to fucose (Fuc) and neuraminic acid (NeuAc), other minor peaks observed in both commercial and isolated KS were impurities that could not be assigned; and C. 13C-NMR. Peaks at 3.55 ppm in the 1H-NMR and at 63 ppm in the 13C-NMR in the isolated KS sample not seen in the commercial KS correspond to a small glycerol impurity.
Figure 4.
HILIC-HPLC-FT-MS profiling of fully digested KS domain structures. Some of the oligomers contain highly sulfated domain (~1 sulfo group/saccharide), which are not digested by keratanase. Based on the accurate FT-MS (spectra inserted) we can assign the major compositions of the oligomers eluted at specific retention time. The minor component of sialic acid capped oligomers has also been detected (hollow arrow indicates degree of polymerization (dp)6 + 3S and dp8 + 5S with one sialic acid residue at the non-reducing end).
Finally, the bioactivities, of commercial and isolated KS samples, were compared by measuring their protein binding responses using surface plasmon resonance (SPR). The responses of the two KS samples were compared for three proteins, the morphogen SHH and the growth factors, FGF1 and FGF2. These proteins are known to be important in corneal development and healing. The binding responses of the two KS samples were compared with a commercial heparin sulfate (HS) standard known to interact with all three proteins and thus serving as a positive control (Fig. 5).
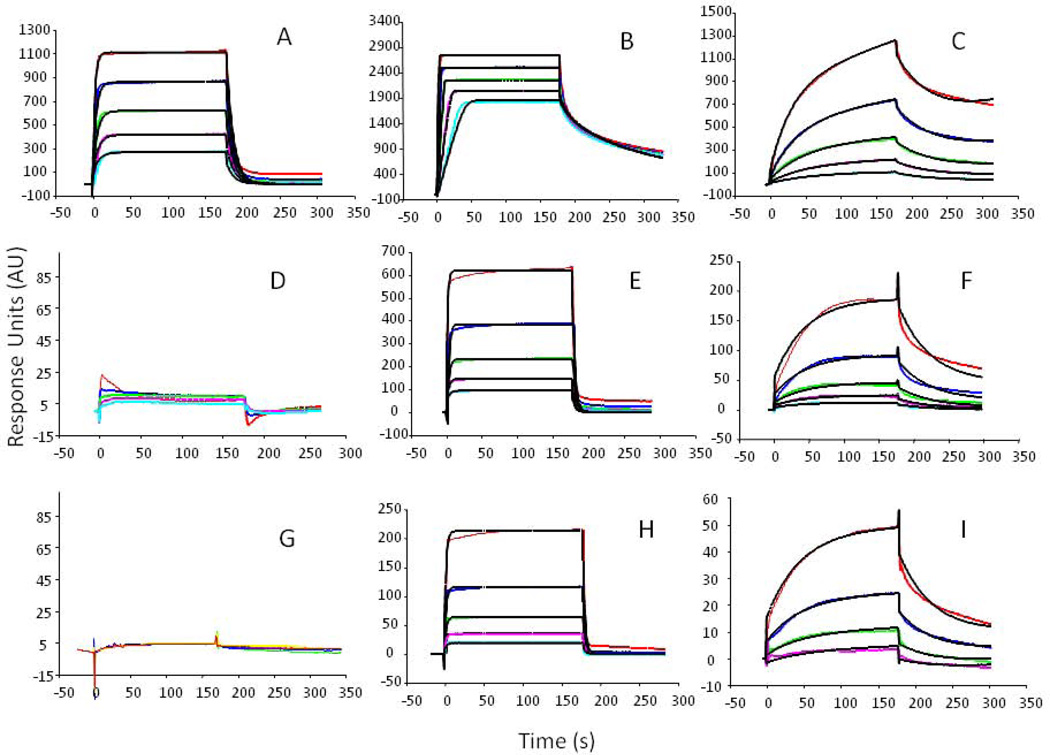
Figure 5.
SPR binding curves of KS and HS with SHH, FGF2 and FGF1 proteins. Top row: commercial HS bound to: A. FGF1; B. FGF2; C. SHH; middle row: commercial KS bound to: D. FGF1; E. FGF2; F. SHH; bottom row: isolated KS bound to: G. FGF1; H. FGF2; I. SHH. Concentrations of each protein used are delineated by line color, red, 1000 nM; navy, 500 nM; green, 250 nM, pink, 125 nM; light blue 63 nM.
The isolated and commercial KS showed similar, but not identical binding curves for all three proteins, which were markedly different from the HS protein binding interactions (Fig. 5). Using the accompanying BIAevaluation software, each series of protein binding sensograms was fitted to establish binding models used to quantify the parameters of the GAG-protein binding interactions (Table 2). Both the observed protein binding sensograms and their accompanying modeled binding interactions were remarkably similar for the two KS samples. The isolated and commercial KS showed weak but similar binding responses to SHH (KD of 6.5×10−5 and 5.0×10−5 respectively), had similar binding interactions to FGF2 (KD of 7.4×10−7 and 9.7×10−7respectively) and neither bound to FGF1. In contrast, HS showed strong binding responses to all three proteins.
Table 2.
Protein Binding Constants Derived from SPR Analysis.
| GAGs | Protein Interaction |
kon (1/MS) | koff (1/S) | KD (M) |
|---|---|---|---|---|
| HS | SHH | 1.5 × 104 | 0.017 | 1.2 × 10−6 |
| FGF2 | 7.4 × 107 | 0.53 | 7.2 × 10−9 | |
| FGF1 | 2.2 × 105 | 0.11 | 4.9 × 10−7 | |
| Commercial KS | SHH | 437 | 0.022 | 5.0 × 10−5 |
| FGF2 | 3.0 × 105 | 0.29 | 9.7 × 10−7 | |
| FGF1 | N.B. | N.B. | N.B. | |
| Isolated KS | SHH | 344 | 0.022 | 6.5 × 10−5 |
| FGF2 | 6.2 × 105 | 0.46 | 7.4 × 10−7 | |
| FGF | N.B. | N.B. | N.B. | |
N.B., no binding detected.
Discussion
The isolated KS and commercial KS were analyzed by GPC, PAGE, NMR, HPLC-MS and SPR. These KS samples were found to exhibit nearly identical structural properties suggesting that the simplified method of preparing multi-milligram amounts of KS that were used in the current study gave a high purity bovine corneal KS. Analysis of the protein binding interactions of the two KS samples by SPR also gave similar responses, suggesting that the bioactivity of these bovine corneal KS samples were identical.
SPR, a highly sensitive analytical technique used to measure the strength of molecular binding interactions, showed for the first time that bovine corneal KS bound two biologically important proteins, FGF2 and SHH. Previous binding studies had shown KS demonstrated no binding interactions with either FGF1 or FGF2 [16]. We can find no previous reports of KS interaction with SHH. The morphogen SHH and the FGFs are signaling factors that have been shown to play a wide range of roles in tissue development, especially in the nervous system [17, 18].
The FGFs encourage and regulate growth and development in many key cellular roles throughout the body. Research into FGFs and their receptors have shown that they are involved in a wide range of developmental roles in the eye, including retinal development [19]. FGF2 has been found in human tears, where in the event of corneal damage it can migrate into the stroma and act on keratocytes, fibroblast cells responsible for helping to maintain the optical transparency of the cornea [4, 20]. FGF2 has also been shown be disregulated by the corneal epithelium during corneal wounding, suggesting it plays a role in repairing tissue damage during wound healing [21].
Similarly, studies on SHH have implicated this morphogen in a wide range of biological functions, including roles in corneal health, development, and wound healing. SHH has been found to integral to the prevention of myopia [22], essential for normal retinal development [23], to be unregulated during corneal wound healing [24]. SHH (and FGF) are also implicated in the genesis of eye and retinal development [19]. However, SHH’s role in upregulating cellular growth may also have unwanted side effects in corneal wound healing, as it has also been implicated in the development of unfavorable corneal vascularization during wound healing [25].
Taken together, these studies indicate SHH and FGF2 play significant roles in many aspects of eye health and development. The novel KS binding interactions with sonic hedgehog (SHH) and fibroblast growth factor 2 (FGF2) reported here suggest that KS GAGs and KS PGs may play important roles in GAG-protein binding interactions that guide the development, wound healing, and structural properties of the cornea.
Materials and Methods
Materials
Bovine corneas were obtained from Pel-freeze Biological Inc (Rodgers, AR, USA). Actinase E was obtained from Kaken Biochemicals (Tokyo, Japan). Commercial KS and keratanase (endo-1, 4-β-D-galactohydrolase E.C. 3.2.1.103) were obtained from Seikagaku (Tokyo, Japan). Chondroitin lyases ABC and ACII were purchased from Associates of Cape Cod, Inc. (East Falmouth, MA). Hyaluronan standards were purchased from Caisson LLC (Oklahoma City, OK, USA). Vivapure Maxi and Mini Q-H strong anion exchange (SAX) columns were obtained from Sartorius (Goettingen, Germany). Biotin-PEG3-NH2 was purchased from Sigma Aldrich (St. Louis, MO, USA). Amicon centrifugal filter molecular weight cut-off membranes were purchased from Millipore (Billerica, MA, USA). Cloning, E. coli expression, and purification of the recombinant heparin lyase I (EC 4.2.2.7), heparin lyase II (no EC assigned), and heparin lyase III (EC 4.2.2.8) from F. heparinum were performed in our laboratory as described [26–28]. All other chemicals were of reagent grade.
Isolation of KS from Corneal Tissue
The opaque corneal tissue was isolated from the excess tissue in the bovine corneas received. The corneal tissue was manually dissected into roughly 3 mm square pieces and proteolyzed with actinase E (2% solution, amount per wet tissue weight) at 55 °C for 24–48 h. The resulting solution was centrifuged (5000×g for 10 min) to remove large particulates and passed through a 0.22-µm cellulose filter. The filtered solution was loaded onto a pre-washed (1-column volume of 50 mM sodium acetate, 50 mM sodium chloride pH 4.5, hereafter Buffer A) strong anion exchange spin column (Vivapure Maxi Q-H). The loaded column was then washed (3-column volumes of Buffer A) and a high salt buffer was used to elute the isolated corneal GAGs (1-column volume of 16% sodium chloride w/v). Methanol was added to the eluted GAG solution to bring the total methanol volume to 80 % (v/v), and the methanol-water-GAG mixture was allowed to precipitate over night at 4 °C and then pelleted using centrifugation (5000×g for 20 min) to give a solid white pellet.
The isolated pellet was then digested with chondrotinase enzymes (chondrotinase ABC and ACII) and heparinase enzymes (heparinase I, II, and III), digested CS and HS disaccharides were removed by washing through 3000 kDa molecular weight cut-off (MWCO) spin column. The GAG solution was lyophilized to yield a fluffy, fibrous keratan sulfate isolate.
Chain Length Analysis by PAGE
The isolated and commercial KS were analyzed by native polyacrylamide gel electrophoresis (PAGE). The KS samples were analyzed on a 0.75 mm×6.8 cm×8.6 cm mini-gel cast with 10% (9.36% acrylamide and 0.61% bis-acrylamide) resolving gel monomer solution and 5% stacking gel monomer solution as previously described [29, 30]. KS samples (5 µg) were applied to each lane (5 µL of a 1 mg/mL solution combined with 5 µL of a 50% w/v sucrose solution) and then subjected to electrophoresis at 200 V for 20 min. KS was visualized in the gels using an Alcian blue stain (0.5% w/v, with 2% .v/v aqueous acetic acid). The gel was stained for 30 min and de-stained completely with deionized water. The scanned gel image was analyzed by Image J software (http://imagej.nih.gov/ij/) [31]. Pixel intensity was measured along the center of the each gel lane to give a plot of each KS chain average distribution.
Chain Length Analysis by GPC
The average chain lengths of the isolated and commercial KS were measured using GPC performed on a HPLC system. KS chain size was correlated to sample elution time by comparison with a set of known hyaluronan molecular weight standards (30.6 kDa, 54 kDa, 128 kDa and 262 kDa). Each KS sample was injected (20 µL, containing 20 µg) at a flow rate of 0.6 mL / min onto an apparatus composed of a Shimadzu LC-10Ai pump, a Shimadzu CBM-20A controller, a TSK-GEL G4000PWxl size exclusion column and a Shimadzu RID-10A refractive index detector. The mobile phase was 0.1 M NaNO3 and the column was maintained at 40 °C with an Eppendorf column heater. The GPC chromatograms were recorded and analyzed using the LC Solution Software GPC Postrun function (Version 1.25).
Structural Analysis by NMR
The purified keratan sulfate from bovine cornea and the commercial keratan sulfate standard were prepared for NMR analysis by removing exchangeable protons. Both of samples were dissolved in 0.4 ml of 99.996 atom % deuterium oxide (2H2O,) and then freeze-dried replacing exchangeable protons with deuterium. All NMR data were acquired on Bruker Avance II Ultrashield 600 MHz (14.1-Tesla) and 800 MHz (18.8 Tesla) NMR instruments equipped with an ultrasensitive HCN cryoprobe with a z-axis gradient. The 13C NMR spectra were recorded at 150 MHz. The spectra were acquired at a probe temperature of 298K. A sweep width of 20.5 ppm and acquisition time of 2.66 s were employed.
Structural Analysis by RPIP-HPLC-MS
The isolated and commercial KS were digested with keratanase 2 (10 µg KS, 30 mU enzyme, in 100 mL of 10 mM TRIS-HCL buffer, pH 7.4) and the resulting disaccharides were isolated from the digestion mixture by passing through a 30 kDa MWCO filter and analyzed onto an Agilent 1200 LC/MSD instrument (Agilent Technologies Inc., Wilmington, DE). The Agilent LC-MS was equipped with a 6300 ion trap and a binary pump followed by an ultraviolet (UV) detector equipped with a high-pressure cell. The isolated disaccharides were injected on a Poroshell 120 C18 column (2.1 × 150 mm, 2.6 µm, Agilent) heated to 45°C. The disaccharides were separated using a gradient of buffers. Solution A was an 85:15 v/v (water:acetonitrile, respectively) and solution B was a 35:65 v/v (water:acetonitrile) mixture. Both eluents contained 12 mM tributyl amine and 38 mM NH4OAc with pH adjusted to 6.5 with acetic acid. The buffer gradient used was a 10 min gradient of solution A (100%), followed by a linear gradient (0–50% solution B) from 10 to 40 min, with a 150 µL/min flow rate. The column effluent entered the source of the ESI-MS for continuous detection by MS. To obtain the maximum abundance of ions in a full-scan spectrum (200–1500 Da), the electrospray interface was set in negative ionization mode, with a skimmer potential of −40.0 V, a capillary exit of −40.0 V, and a source temperature of 350 °C with nitrogen (8 L/min, 40 psi) used as a drying and nebulizing gas.
HILIC- ESI-LTQ-Orbitrap-FT-MS analysis of KS
A HILIC-FT-MS based method was used to analyze the keratinase 2-digested KS disaccharides and oligosaccharides to obtain domain structures. Briefly, the digested KS were separated by a Luna HILIC column (2.0×150 mm, 200 A, Phenomenex, Torrance, CA, USA) and detected by an LTQ-Orbitrap XL FT MS (Thermo Fisher Scientific, San-Jose, CA, USA) running at negative-ion mode. HPLC binary pump was used to deliver the gradient starting from 10%A to 35%A in 40 min at a flow rate of 150 µL/min. Mobile phase A is HPLC grade water with 5 mM ammonium acetate. Mobile B is HPLC grade 98% acetonitrile with 2% water and 5mM ammonium acetate. FT-MS detector operating at negative-ion mode with optimized parameters used to prevent in-source fragmentation including spray voltage 4.2 kV, capillary voltage −40 V, tube lens voltage −50 V, capillary temperature 275 °C, sheath flow rate 30, and auxiliary gas flow rate 6. External calibration of mass spectra was used to routinely produce a mass accuracy of better than 3 ppm. All FT mass spectra were acquired at a resolution 60,000 with 400–2000 Da mass range.
Protein Binding Comparisons by SPR
Binding interactions between GAGs and proteins were measured on a Biacore 3000 SPR instrument (GE Healthcare, Waukesha, WI, USA). Both KS and HS samples were immobilized on a streptavidin coated sensor chip (Sensor Chip SA, GE Healthcare, Uppsala, Sweden). Four separate flow-cells were constructed – one containing a biotinylated commercial KS, one containing a biotinylated KS isolated from bovine cornea, and two control cells, one containing a biotinylated HS and one containing only biotin were constructed. KS and HS samples were biotinylated by reductive amination. The isolated KS was digested by PNGase to remove residual amino acid residues following a previously published procedure [32]. Briefly, PNGase (5 U) was added to 500 µg of isolated KS in 150 µL of a digestion buffer (fresh 50 mM ammonium bicarbonate, pH 8.3) and digested overnight at 37 °C. The digested KS was then isolated and purified using a strong anion exchange spin column (Vivapure Mini Q-H, see isolation of KS from bovine corneas above). The eluted, purified KS was precipitated by methanol precipitation (80 % v/v methanol) and centrifugation (5000×g for 10 min). KS and HS were biotinylated by reductive amination. A typical procedure was as follows: 500 µg of the GAG was dissolved in 1 mL formamide. To a stirring KS solution, 500 µg of the amine-biotin (100 µL of a 5 mg/mL dimethylsulfoxide solution) and 600 µg of NaBH3CN (50 µL of a 12 mg/mL dimethylsulfoxide solution) were added drop wise and allowed to react at 60 °C for 12 h. After 12 h, NaBH3CN was added a second time (600 µg, 50 µL of a fresh 12 mg/mL DMSO solution), and left stirring to react for a further 12 h at 60 °C. After this, 0.5 mL of water was added to the vial to quench any remaining unreacted NaBH3CN. KS was isolated from the reaction mixture using a strong anion exchange spin column (Vivapure Mini Q-H) following the previously outlined procedure (see above).
The prepared samples (biotinylated HS, KS, or pure biotin) were then immobilized on a streptavidin (SA) coated CM5 sensor chip (GE Heathcare, Uppsala, Sweden) using the manufacturer’s protocol. In brief, a 20 mL solution of the GAG-biotin conjugate (0.1 mg/mL) in HBS-EP buffer (10 mM HEPES (4-(2-hydoxyethyl)-1-piperazineethanesulfonic acid), 150 mM NaCl, 3 mM EDTA (ethylenediaminetetraacetic acid), and 0.005% surfactant P20, pH 7.4) was injected over the flow-cell of the sensor chip at a flow-rate of 10 mL/min. The biotin control flow-cell was prepared with a 1 min injection with saturated biotin in HBS-EP buffer.
Different dilutions of protein samples were injected at a flow rate of 40 µL/min for 3 min. At the end of the sample injection, the same HBS-EP buffer was passed over the sensor surface to facilitate dissociation. After a 2 min dissociation time, the sensor surface was regenerated by injection with 40 µL of 2 M NaCl to obtain a fully regenerated surface. The response was monitored as a function of time (sensorgram) at 25 °C. The parameters of binding kinetics were determined by globally fitting the sensorgram curves to a 1:1 Langmuir model from BIAevaluation, the accompanying Biacore software (GE Healthcare, Uppsala, Sweden).
Supplementary Material
Acknowledgements
The authors gratefully acknowledge support for the National Institutes of Health in the form of grant # GM38060.
Abbreviations
- EP
ethylenediaminetetraacetic acid P20 surfactant
- ESI
electrospray ionization
- FGF
fibroblast growth factor
- GAG
glycosaminoglycan
- GPC
gel permeation chromatography
- HBS
HEPES buffered saline
- HILIC
hydrophilic interaction chromatography
- HS
heparan sulfate
- HPLC
high-performance liquid chromatography
- KS
keratan sulfate
- MS
mass spectrometry
- MWCO
molecular weight cut-off
- NMR
nuclear magnetic resonance
- PG
proteoglycan
- PAGE
polyacrylamide gel electrophoresis
- RPIP
reverse-phase ion-pairing
- SA
streptavidin
- SHH
sonic hedgehog
- SPR
surface plasmon resonance
- UV
ultraviolet
References
- 1.Bülow HE, Hobert O. The molecular diversity of glycosaminoglycans shapes animal development. Ann Rev Cell Develop Biol. 2006;22:375–407. doi: 10.1146/annurev.cellbio.22.010605.093433. [DOI] [PubMed] [Google Scholar]
- 2.Linhardt RJ, Toida T. Role of glycosaminoglycans in cellular communication. Acc Chem Res. 2004;37:431–438. doi: 10.1021/ar030138x. [DOI] [PubMed] [Google Scholar]
- 3.Funderburgh J. Keratan sulfate biosynthesis. IUBMB Life. 2002;54:187–194. doi: 10.1080/15216540214932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oeben M, Keller R, Stuhlsatz HW, Greiling H. Constant and variable domains of different disaccharide structure in corneal keratan sulphate chains. Biochem J. 1987;248:85–93. doi: 10.1042/bj2480085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stuhlsatz HW, Keller R, Becker G, Oeben M, Lennartz L, Fischer DC, Greiling H. Structure of Keratan Sulfate Proteoglycans: Core Proteins, Linkage Regions, Carbohydrate Chains. Keratan Sulfate: Chemistry, Biology, Chemical Pathology. 1989:1–11. [Google Scholar]
- 6.Tai GH, Huckerby TN, Nieduszynski Ia. Multiple non-reducing chain termini isolated from bovine corneal keratan sulfates. The Journal of Biological Chemistry. 1996;271:23535–23546. doi: 10.1074/jbc.271.38.23535. [DOI] [PubMed] [Google Scholar]
- 7.Quantock AJ, Young RD, Akama TO. Structural and biochemical aspects of keratan sulphate in the cornea. CMLS. 2010;67:891–906. doi: 10.1007/s00018-009-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassell JR, Birk DE. The molecular basis of corneal transparency. Exper Eye Res. 2010;91:326–335. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funderburgh JL. Keratan sulfate: structure, biosynthesis, and function. Glycobiology. 2000;10:951–958. doi: 10.1093/glycob/10.10.951. [DOI] [PubMed] [Google Scholar]
- 10.Mccarthy MM, Bakers JR. Isolation and desulfation of keratan sulfates. Carbohydr Res. 1979;69:151–164. [Google Scholar]
- 11.Meyer K, Linker A, Davidson EA, Weissmann B. The Mucopolysaccharides of the Bovine Cornea. J Biol Chem. 1953;205:611–616. [PubMed] [Google Scholar]
- 12.Young RD, Akama TO, Liskova P, Ebenezer ND, Allan B, Kerr B, Caterson B, Fukuda MN, Quantock AJ. Differential immunogold localisation of sulphated and unsulphated keratan sulphate proteoglycans in normal and macular dystrophy cornea using sulphation motif-specific antibodies. Histochem Cell Biol. 2007;127:115–1120. doi: 10.1007/s00418-006-0228-8. [DOI] [PubMed] [Google Scholar]
- 13.Dunlevy JR, Beales MP, Berryhill BL, Cornuet PK, Hassell JR. Expression of the keratan sulfate proteoglycans lumican, keratocan and osteoglycin/mimecan during chick corneal development. Exper Eye Ress. 2000;70:349–362. doi: 10.1006/exer.1999.0789. [DOI] [PubMed] [Google Scholar]
- 14.Blochberger TC, Cornuet PK, Hassell JR. Isolation and Partial Characterization of Lumican and Decorin from Adult Chicken Corneas. J Biol Chem. 1992;267:20613–20619. [PubMed] [Google Scholar]
- 15.Beales MP, Funderburgh JL, Jester J V, Hassell JR. Proteoglycan Synthesis by Bovine Keratocytes and Corneal Fibroblasts : Maintenance of the Keratocyte Phenotype in Culture. Invest Ophthalmol Vis Sci. 1999;40:1658–1663. [PubMed] [Google Scholar]
- 16.Li L, Zhang F, Zaia J, Linhardt RJ. Top-Down Approach for the Direct Characterization of Low Molecular Weight Heparins Using LC-FT-MS. Anal Chem. 2012;84:8822–8829. doi: 10.1021/ac302232c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huckerby TN, Tai GH, Nieduszynski Ia. Oligosaccharides derived by endo-beta-galactosidase digestion of bovine corneal keratan sulphate--characterisation of tetrasaccharides with incomplete sulphation and containing unsulphated N-acetylglucosamine residues. FEBS. 1998;253:499–506. doi: 10.1046/j.1432-1327.1998.2530499.x. [DOI] [PubMed] [Google Scholar]
- 18.Brown GM, Huckerby TN, Morris HG, Abram BL, Nieduszynski Ia. Oligosaccharides derived from bovine articular cartilage keratan sulfates after keratanase II digestion: implications for keratan sulfate structural fingerprinting. Biochemistry. 1994;33:4836–4846. doi: 10.1021/bi00182a012. [DOI] [PubMed] [Google Scholar]
- 19.Brown GM, Huckerby TN, Nieduszynski Ia. Oligosaccharides derived by keratanase II digestion of bovine articular cartilage keratan sulphates. FEBS. 1994;224:281–308. doi: 10.1111/j.1432-1033.1994.00281.x. [DOI] [PubMed] [Google Scholar]
- 20.Conrad AH, Zhang Y, Tasheva ES, Conrad GW. Proteomic analysis of potential keratan sulfate, chondroitin sulfate A, and hyaluronic acid molecular interactions. Invest Ophthalmol Vis Sci. 2010;51:4500–4515. doi: 10.1167/iovs.09-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martí E, Bovolenta P. Sonic hedgehog in CNS development: one signal, multiple outputs. Trends Neurosci. 2002;25:89–96. doi: 10.1016/s0166-2236(02)02062-3. [DOI] [PubMed] [Google Scholar]
- 22.Mason I. Initiation to end point: the multiple roles of fibroblast growth factors in neural development. Nature Rev Neurosci. 2007;8:583–596. doi: 10.1038/nrn2189. [DOI] [PubMed] [Google Scholar]
- 23.Esteve P, Bovolenta P. Secreted inducers in vertebrate eye development: more functions for old morphogens. Current Opinion in Neurobiology. 2006;16:13–19. doi: 10.1016/j.conb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Van Setten GB. Basic fibroblast growth factor in human tear fluid: detection of another growth factor. Graefe’s Arch Clin ExperOpthalmol. 1996;234:275–277. doi: 10.1007/BF00430422. [DOI] [PubMed] [Google Scholar]
- 25.Gan L, Fagerholm P, Palmblad J. Expression of basic fibroblast growth factor in rabbit corneal alkali wounds in the presence and absence of granulocytes. Acta Ophthalmol Scand. 2005;83:374–378. doi: 10.1111/j.1600-0420.2005.00439.x. [DOI] [PubMed] [Google Scholar]
- 26.Qian Y, Chu R, Hu M, Hoffman M. Sonic hedgehog expression and its role in formdeprivation myopia in mice. Curr Eye Res. 2009;34:623–635. doi: 10.1080/02713680903003492. [DOI] [PubMed] [Google Scholar]
- 27.Vinothkumar S, Rastegar S, Takamiya M, Ertzer R, Strähle U. Sequential and cooperative action of Fgfs and Shh in the zebrafish retina. Develop Biol. 2008;314:200–214. doi: 10.1016/j.ydbio.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 28.Saika S, Muragaki Y, Okada Y, Miyamoto T, Ohnishi Y, Ooshima A, Kao WW-Y. Sonic hedgehog expression and role in healing corneal epithelium. Invest Ophthalmol Vis Sci. 2004;45:2577–2585. doi: 10.1167/iovs.04-0001. [DOI] [PubMed] [Google Scholar]
- 29.Fujita K, Miyamoto T, Saika S. Sonic hedgehog: its expression in a healing cornea and its role in neovascularization. Molec Vis. 2009;15:1036–1044. [PMC free article] [PubMed] [Google Scholar]
- 30.Shaya D, Tocilj A, Li Y, Myette J, Venkataraman G, Sasisekharan R, Cygler M. Crystal structure of heparinase II from Pedobacter heparinus and its complex with a disaccharide product. J Biol Chem. 2006;281:15525–15535. doi: 10.1074/jbc.M512055200. [DOI] [PubMed] [Google Scholar]
- 31.Godavarti R, Davis M, Venkataraman G, Cooney C, Langer R, Sasisekharan R, Sas Heparinase III from Flavobacterium heparinum: cloning and recombinant expression in Escherichia coli. Biochem Biophys Res Commun. 1996;225:751–758. doi: 10.1006/bbrc.1996.1246. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida E, Arakawa S, Matsunaga T, Toriumi S, Tokuyama S, Morikawa K, Tahara Y. Cloning, sequencing, and expression of the gene from bacillus circulans that codes for a heparinase that degrades both heparin and heparan sulfate. Biosci Biotechnol Biochem. 2002;66:1873–1879. doi: 10.1271/bbb.66.1873. [DOI] [PubMed] [Google Scholar]
- 33.Laremore TN, Ly M, Zhang Z, Solakyildirim K, McCallum Sa, Owens RT, Linhardt RJ. Domain structure elucidation of human decorin glycosaminoglycans. Biochem J. 2010;431:199–205. doi: 10.1042/BJ20100788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laremore TN, Ly M, Solakyildirim K, Zagorevski DV, Linhardt RJ. High-resolution preparative separation of glycosaminoglycan oligosaccharides by polyacrylamide gel electrophoresis. Anal Biochem. 2010;401:236–241. doi: 10.1016/j.ab.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abràmoff MDM, Hospitals I, Magalhães PJ. Image Processing with Image J. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- 36.Babu P, North SJ, Jang-Lee J, Chalabi S, Mackerness K, Stowell SR, Cummings RD, Rankin S, Dell A, Haslam SM. Structural characterisation of neutrophil glycans by ultra sensitive mass spectrometric glycomics methodology. Glycoconj J. 2009;26:975–986. doi: 10.1007/s10719-008-9146-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.