Abstract
Induction of molecular chimerism through genetic modification of bone marrow is a powerful tool for the induction of tolerance. Here we demonstrate for the first time that expression of an allogeneic MHC class II gene in autologous bone marrow cells, resulting in a state of molecular chimerism, induces tolerance to MHC class II mismatched skin grafts, a stringent test of transplant tolerance. Reconstitution of recipients with syngeneic bone marrow transduced with retrovirus encoding H-2I-Ab (I-Ab) resulted the long-term expression of the retroviral gene product on the surface of MHC class II-expressing bone marrow derived cell types. Mechanistically, tolerance was maintained by the presence of regulatory T cells, which prevented proliferation and cytokine production by alloreactive host T cells. Thus, the introduction of MHC class II genes into bone marrow derived cells through genetic engineering results in tolerance. These results have the potential to extend the clinical applicability of molecular chimerism for tolerance induction.
Keywords: Tolerance, molecular chimerism, T cells, Treg
INTRODUCTION
Transplantation tolerance, defined as long-term allograft survival without ongoing immunosuppression, remains a major goal in the field of transplantation medicine. Bone marrow derived hematopoietic cells have been shown to be capable of inducing transplantation tolerance (1, 2), and tolerance to allogeneic transplants in adults can be established by inducing a state of mixed hematopoietic chimerism through allogeneic bone marrow transplantation (2, 3). Mixed chimerism leads to specific tolerance, and permits transplantation of organs matched to the donor bone marrow without immunosuppression (4). However, the use of allogeneic bone marrow transplantation to induce tolerance is associated with serious complications, (5-11). In addition, it has been difficult to reliably establish a stable state of mixed host-donor hematopoietic chimerism in primates (12-15). Further decreasing the clinical applicability of bone marrow chimerism as an approach to tolerance induction, it has been reported that induction of mixed chimerism through bone marrow transplantation is unable to prevent chronic allograft rejection (16), currently the major factor limiting long-term survival of transplants (17-19).
As an alternative to mixed chimerism, gene therapy approaches can be used to express alloantigens in syngeneic bone marrow cells, resulting in molecular rather than cellular chimerism. The induction of molecular chimerism avoids several of the serious complications associated with mixed cellular chimerism. Because genetically engineered bone marrow cells are of host origin, there is no possibility risk of graft vs. host disease in which allogeneic T cells attack the host tissues (20). The possibility of generating full donor chimerism, which results in immuno-incompetence because developing T cells are selected on host thymic epithelium, but must respond to pathogens in a donor-MHC restricted fashion (11), does not exist in molecular chimeras since all engineered bone marrow derived cells express both host and donor MHC molecules. Finally, the difficulty in obtaining a suitable bone marrow donor is avoided since syngeneic bone marrow cells are modified to induce tolerance. These advantages suggest that the induction of molecular chimerism may have clinical applicability in the induction of tolerance.
Tolerance to self is maintained through central mechanisms involving negative selection of self-reactive T cells in the thymus and peripheral mechanisms involving regulatory T cells (Tregs) (21-23). Indeed, it is clear that tolerance to self requires both a central and peripheral component (21). We have previously demonstrated that MHC class I molecular chimerism can induce permanent tolerance to MHC class I mismatched heart and skin allografts (24-26) through both central and peripheral mechanisms (24, 26-29). Notably, chronic rejection of cardiac allografts was not observed in this model. Furthermore, we have demonstrated that tolerance to cytoplasmically expressed antigens can also be induced through molecular chimerism (30). However, to date there have been no reports of successfully inducing tolerance to MHC class II alloantigens through molecular chimerism. To extend the applicability of molecular chimerism for tolerance induction, we examined whether retrovirally mediated expression of MHC class II could induce durable tolerance.
RESULTS
Efficient transduction of bone marrow with retroviruses encoding MHC class II I-Ab
To generate retroviral vectors encoding H-2I-Ab (I-Ab), constructs were prepared in which the cytoplasmic tail of the beta chain of I-Ab was fused to enhanced GFP (GFP) to facilitate tracking of MHC class II chains in hematopoietic cells, as previously described (31). I-Ab beta chain–GFP fusion genes were then cloned into the pMMP retroviral vector. Next, the full-length cDNA encoding the alpha chain of I-Ab was cloned into the pMMP-I-Ab vector, downstream of an IRES sequence, to generate a bicistronic vector (Figure 1A). This vector encodes the I-Ab beta chain fused to GFP, and the alpha chain of I-Ab. The pMMP (32) vector encoding GFP alone was used as a control in all experiments (Figure 1A). VSV-G protein enveloped retroviruses were generated in 293T cells by transient transfection of constructs as previously described (24), hereafter referred to as VSV-IAb, and VSV-GFP respectively.
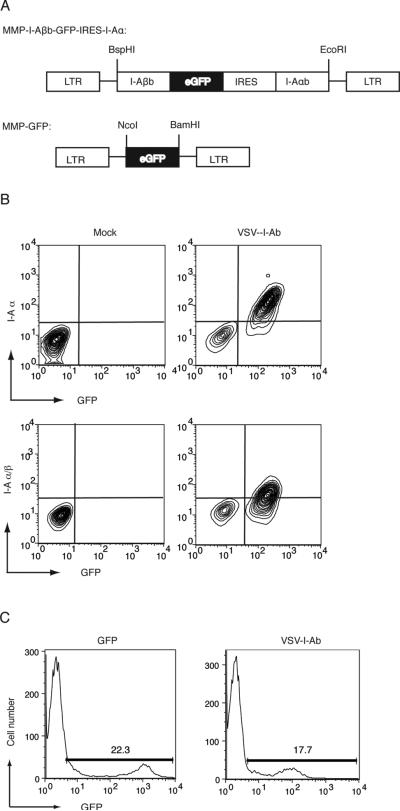
Figure 1. Bicistronic retroviral vector encoding MHC class II confers cell surface expression of I-Ab, and transduces murine bone marrow.
(A) Schematic of the bicistronic retroviral construct containing the I-Ab β gene fused to GFP, an internal ribosomal entry site and I-Ab α. Control virus encodes GFP alone (B) A20 cells were transduced with VSV-I-Ab (right panels) or mock transduced (left panels) and cell surface expression of the I-Ab α chain (upper panels) or the I-Ab/ peptide complex (lower panels) was measured by cell surface staining and flow cytometry. Mock transduced A20 cells were used as staining controls. (C) Bone marrow cells were harvested from mice treated 7 days prior with 5-FU, and transduced with VSV-GFP control virus (left panel) or VSV-I-Ab(right panel). 72 hours later, transduction was monitored via GFP fluorescence and flow cytometry. Shown is one representative of three independent experiments.
To validate the ability of VSV-IAb to confer expression of I-Ab, A20 (H-2d) cells were transduced with VSV-IAb. After 48 hours, cells were stained with a monoclonal antibody specific for I-Ab (AF6-120.1) and analyzed by flow cytometry. Expression of GFP was used as a marker of viral transduction. We observed that GFP+ A20 cells expressed detectable levels of I-Ab α chain on the cell surface (Figure 1B). We next stained A20 cells transduced with VSV-IAb with a monoclonal antibody that specifically recognizes the I-Ab α/β - peptide complex (KH74 (33)). We observed significant expression of the I-Ab complex on the surface of A20 cells that were GFP+ (Figure 1B). These results indicate that VSVIAb confers expression of both the alpha and beta chains of I-Ab, and that these retrovirally-encoded proteins are expressed on the cell surface. To determine the ability of VSV-IAb retrovirus to infect primary murine bone marrow cells, we used GFP as a pseudomarker of I-Ab expression. Bone marrow from 5-FU treated B10.MBR mice was isolated and transduced with either VSV-IAb or VSVGFP retrovirus. After 72 hours, GFP expression was analyzed by flow cytometry. Mock transduced bone marrow was used as a staining control. Transduction of B10.MBR bone marrow with VSV-IAb led to expression of GFP in 12.7±3% of cells (Figure 1C). Transduction of bone marrow with VSV-GFP led to expression of GFP in 29.7±14.7% of cells (Figure 1C). GFP was expressed in cells following transduction of bone marrow with either VSV-IAb or control virus VSV-GFP at a consistently high level suggesting that I-Ab is not inherently unstable in these cells as has been previously reported (34). However, the median fluorescent intensity of GFP in bone marrow cells transduced with VSV-GFP was higher than in VSV-IAb transduced cells, reflecting lower expression of GFP when expressed as a fusion with I-Ab β chain as observed previously (31).
Mice reconstituted with bone marrow transduced with VSV-IAb exhibit long-term expression of retrovirally-encoded MHC class II in multiple hematopoietic lineages
We next examined expression of virally encoded MHC class II in the peripheral blood of B10.MBR mice reconstituted with VSV-IAb, or mock transduced syngeneic bone marrow eight weeks after bone marrow transplantation. We observed GFP expression in 20±6% in CD4+ cells, 24±9% of CD8+ cells, 41±4% of Mac-1+ cells, 29±6% of B220+ cells and 43±8% of CD11c+ cells (Figure 2A) in mice that received VSV-IAb transduced bone marrow. As expected there was no GFP expression in mock transduced controls. We next examined the expression of I-Ab on the cell surface of transduced GFP+ PBMC. 10 weeks after reconstitution, PBMC were harvested and treated with LPS for 48 hours to increase MHC class II expression. Cells were then stained with antibodies specific for B220, I-Ab and endogenous I-Ak. In GFP+ transduced B220+ B cells from mice that received VSV-IAb transduced bone marrow, detectable levels of I-Ab were observed (Figure 2B), suggesting that the products of the retrovirally encoded MHC class II genes were expressed on the cell surface. GFP negative cells from the same animals did not express I-Ab. Moreover, we did not observe expression of I-Ab on cells from control mice reconstituted with VSV-GFP transduced bone marrow. Thus, VSV-IAb specifically confers I-Ab expression in progeny of transduced progenitors. Expression of retrovirally encoded MHC class II I-Ab did not measurably affect expression of the endogenous I-Ak chain. GFP+ and GFP- cells from the same animal expressed similar levels of I-Ak chain expression regardless of expression of I-Ab (Figure 2B). When CD4 and CD8 T cells were examined, we were unable to detect I-Ab expression on the cell surface, as expected, since in mice T cells do not express MHC class II (Figure 2B). Thus, our retroviral construct conveyed cell surface expression of allogeneic MHC class II only in MHC class II-expressing cell types.
Figure 2. Long-term multi-lineage expression of retrovirally encoded proteins.
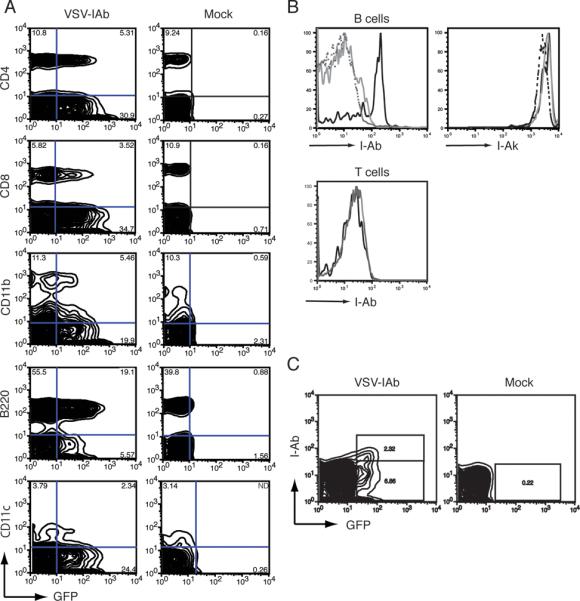
(A) 4 weeks after reconstitution with bone marrow transduced with VSV-I-Ab (left column) or mock transduced (right column), B10.MBR mice were bled, and peripheral blood mononuclear cells (PBMC) were examined by cell surface staining with the lineage markers CD4, CD8, B220, CD11b and CD11c. Cells were then analyzed by flow cytometry. Shown is one representative of three independent experiments. (B) 11 weeks mice were reconstituted with bone marrow transduced with VSV-I-Ab, PBMC were harvested and stimulated with LPS. After 72 hours, cells were stained with antibodies specific for IAb, IAk, B220 and CD3 prior to analysis by flow cytometry. Shown are B220+ cells (upper panels) gated on transduced GFP+ cells (solid black line) or GFP- cells (solid gray line. Cells from naïve B10.MBR stimulated with LPS were used as a control (dashed line). Also shown are GFP+ (black line) and GFP- (gray line) CD3+ T cells (lower panel). (C) 24 weeks after bone marrow reconstitution, recipients of VSV-I-Ab (left panel) or mock (right panel) transduced bone marrow were bled, and GFP and IAb expression in lymphocytes was analyzed by flow cytometry. Shown is one representative of three independent experiments.
To analyze long-term long term expression of I-Ab in recipients of VSV-IAb transduced bone marrow cells, B10.MBR recipients were reconstituted with 4×106 VSV-IAb or mock transduced bone marrow cells. After 24 weeks animals were sacrificed, and expression of GFP and I-Ab was analyzed by flow cytometry. Detectable levels of GFP were observed in the spleen of animals reconstituted with bone marrow transduced with VSV-IAb (Figure 2C). Mice reconstituted with VSV-IAb transduced bone marrow also expressed GFP in bone marrow, thymus, blood and lymph nodes (data not shown). As expected, GFP was not expressed in cells from animals reconstituted with mock transduced bone marrow cells. Taken together, these data indicate that long-term, cell surface expression of retrovirally-encoded MHC class II was achieved.
Expression of retrovirally encoded I-Ab induces tolerance to MHC class II mismatched skin allografts
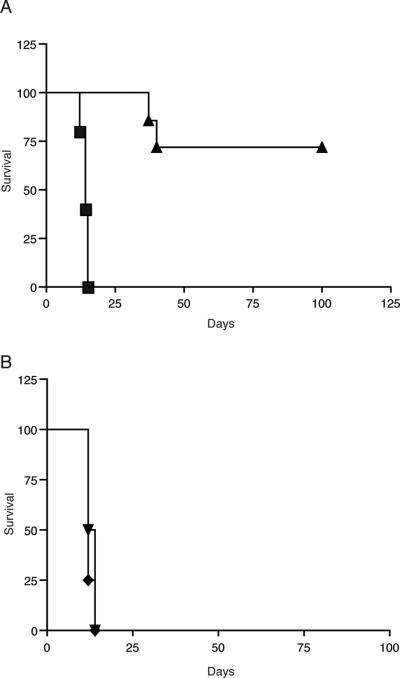
To determine the ability of retrovirally encoded I-Ab to induce immunologic tolerance, B10.MBR (Kb I-Ak, I-Ek Dq) mice were reconstituted with VSV-IAb or VSV-GFP transduced bone marrow. After eight to ten weeks, mice reconstituted with either VSV-IAb or VSV-GFP transduced bone marrow received both a MHC class II mismatched B10.QBR (Kb, I-Ab,I-Enull, Dq) and a third-party BALB/c (H-2d) skin graft. B10.MBR mice reconstituted with VSV-GFP transduced bone marrow rapidly rejected both B10.QBR (Figure 3A, median survival time (MST)= 14 days, n=12) and BALB/c skin grafts (Figure 3B, MST= 16 days, n=12). In contrast mice reconstituted with VSV-IAb transduced bone marrow accepted B10.QBR skin grafts long term (Figure 3A, MST>100 days, n=9, p < 0.006), but rejected third party BALB/c skin grafts rapidly (Figure 3B, MST= 14 days, n=9) indicating that tolerance was specific to grafts expressing I-Ab.
Figure 3. Expression of retrovirally encoded MHC class II proteins induces specific tolerance to skin grafts.
(A) 8-10 weeks after bone marrow reconstitution, B10.MBR recipients of VSV-I-Ab (triangles) or VSV-GFP (squares) transduced bone marrow received MHC class II mismatched B10.QBR skin grafts. (B) B10.MBR recipients of VSV-I-Ab (triangles) or VSV-GFP (diamonds) transduced bone marrow also received BALB/c skin grafts. Shown are the combined results of 3 independent experiments.
Decreased T cell proliferation in response to allogeneic stimulators in mice expressing retrovirally-encoded allogeneic MHC class II
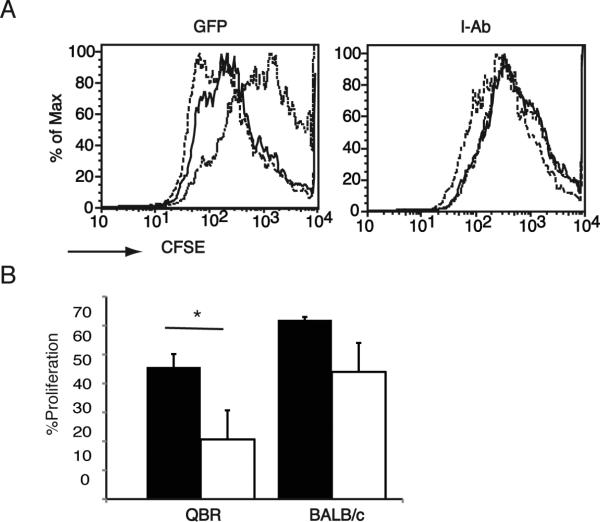
We next examined the effect of expressing retrovirally encoded I-Ab on proliferation of alloreactive T cells. B10.MBR mice reconstituted with VSV-IAb or control VSV-GFP transduced bone marrow were immunized with 107 irradiated B10.QBR splenocytes. After 10 days, splenocytes were harvested, stained with CFSE and cultured with irradiated B10.QBR or BALB/c splenocytes for 72 hours. T cells derived from mice reconstituted with VSV-GFP transduced bone marrow proliferated in response to either B10.QBR or third party BALB/c splenocytes (Figure 4, p <0.001 in comparison to syngeneic stimulators). In contrast, T cells derived from mice reconstituted with bone marrow transduced with VSV-IAb did not proliferate when cultured with B10.QBR stimulators, although they did proliferate in response to third party BALB/c splenocytes (Figure 4, p <0.05 in comparison to syngeneic stimulators), suggesting that suppression of T cell proliferation is specific. The response of splenocytes from mice reconstituted with bone marrow transduced with VSV-IAb to third party BALB/c splenocytes appeared to be lower than that of mice reconstituted with VSV-GFP transduced bone marrow, although this did not reach statistical significance (Figure 4, p > 0.05). However, mice reconstituted with bone marrow transduced with VSV-IAb were able to rapidly reject skin grafts from BALB/c mice (Figure 3B), suggesting that responses to third party antigens remain intact in these mice.
Figure 4. Expression of retrovirally encoded MHC class II prevents T cell proliferation in response to MHC class II mismatched splenocytes.
B10.MBR mice were reconstituted with VSV-I-Ab (I-Ab) or control VSV-GFP (GFP) transduced bone marrow. Recipients were immunized with 107 irradiated B10.QBR splenocytes and then sacrificed 10 days later. (A) Splenocytes were CFSE labeled and stimulated with irradiated syngeneic B10.MBR (dotted line), or allogeneic B10.QBR (solid line)or BALB/c dashed line. Proliferation was monitored by flow cytometry after 72 hours. (B). The results of the experiment in panel A expressed percent proliferation. VSV-I-Ab (white bars) or VSV-GFP (black bars). Shown is one representative experiment of two. Each experiment assayed 2-3 individual mice per group.
Induction of molecular chimerism prevents cytokine production by alloreactive T cells
We next determined the ability of T cells from B10.MBR mice reconstituted with VSV-IAb or VSV-GFP transduced bone marrow to produce effector cytokines following stimulation with irradiated MHC class II mismatched B10.QBR splenocytes. B10.MBR mice were reconstituted with VSV-IAb or VSV-GFP transduced bone marrow as described above and then sacrificed eight to ten weeks after reconstitution. Splenocytes were harvested and cultured for 48 hours together with irradiated B10.QBR splenocytes. To measure the alloreactive response, production of IL-2, IL-4, or IFN-γ was analyzed by performing cytokine ELISPOT assays. We observed relatively few IL-2 (13 ± 4 vs. 661 ± 52 per 106), IL-4 (28 ± 10 vs. 208 ± 20 per 106) and IFN-γ (0.67± 0.58 vs. 88 ± 60 per 106) producing cells (Figure 5A) in the spleens of mice reconstituted with VSV-IAb transduced bone marrow when compared to the number observed in the spleens of mice reconstituted with VSV-GFP transduced bone marrow (p <0.05 between groups).
Figure 5. Expression of retrovirally encoded MHC class II prevents T cell cytokine production in response to MHC class II mismatched splenocytes.
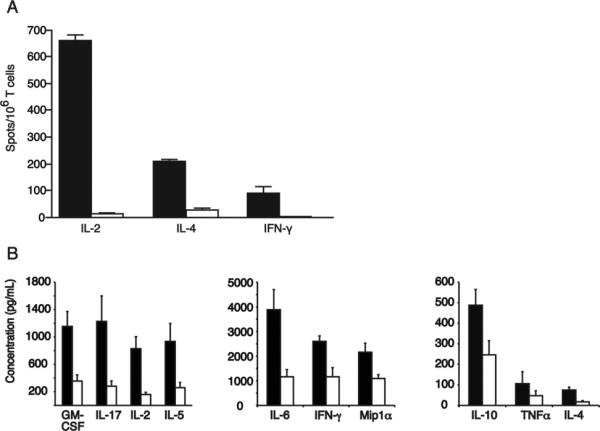
B10.MBR mice were reconstituted with VSV-I-Ab (white bars) or control VSV-GFP (black bars) transduced bone marrow. 8-10 weeks after reconstitution, mice were immunized with 107 irradiated B10.QBR splenocytes and sacrificed 10 days later. (A) Splenocytes were re-stimulated with irradiated B10.QBR splenocytes and analyzed by ELISPOT for IL-2, IL-4 or IFN-γ. Data are presented as spots per 106 T cells for each cytokine examined. Shown are the cumulative mean values and standard deviations obtained from three separate experiments. (B) Splenocytes were re-stimulated with B10.QBR or syngeneic B10.MBR splenocytes, and after 48 hours, supernatants were collected and analyzed for cytokine expression by Luminex assay. Background response to B10.MBR has been subtracted from the results shown. Shown are the cumulative mean values and standard deviations obtained from three separate experiments.
We next analyzed cytokine production using multiplex Luminex assays. B10.MBR mice were reconstituted with VSV-IAb or VSV-GFP transduced bone marrow as described above. Eight to ten weeks after reconstitution, animals were sacrificed, and splenocytes were cultured for 48 hours with irradiated B10.QBR splenocytes. After 48 hours, cell culture supernatants were harvested and production of GM-CSF, IFNγ, IL12 (p70), IL-17, IL-2, IL-4, IL-5, IL-6, IL-10, MIP-1α, RANTES, and TNFα examined by LUMINEX. When supernatants from cells derived from mice reconstituted with VSV-IAb transduced bone marrow were examined, significantly lower levels of GM-CSF, IFNγ, IL-17, IL-2, IL-4, IL-5, IL-6, IL-10, MIP-1α, and TNFα were produced when compared with supernatants from cells derived from mice reconstituted with VSV-GFP transduced bone marrow (p <0.05 between groups for each cytokine pair, Figure 5B). Taken together these data suggest that expression of retrovirally encoded I-Ab in molecular chimeras significantly impairs the production of cytokines in response to I-Ab-expressing allogeneic stimulators.
Regulatory T cells are required for long-term skin graft acceptance
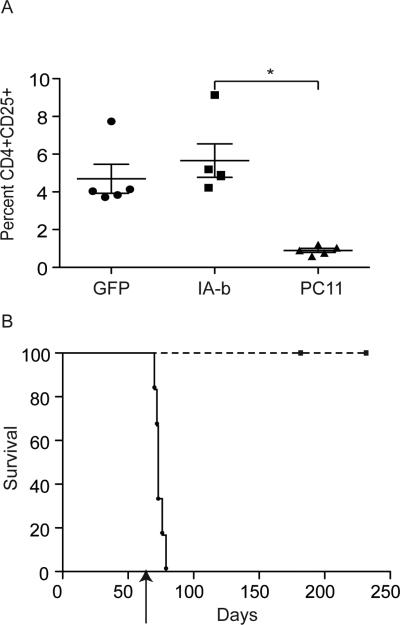
We have previously shown that tolerance induction through molecular chimerism has both a deletional and a regulatory component (24, 25, 27). To test the degree to which regulatory T cells were involved in tolerance to MHC class II mismatched skin grafts, B10.MBR mice were reconstituted with syngeneic bone marrow transduced with VSV-IAb as described. Eight weeks after reconstitution mice were challenged with a B10.QBR skin graft. As previously observed, B10.MBR mice reconstituted with bone marrow transduced with VSV-IAb accepted B10.QBR skin grafts indicating they were tolerant to I-Ab. 55 days after skin grafting, tolerant mice expressing retrovirally encoded I-Ab were then injected with the anti-CD25 antibody PC61. Antibody treatment effectively reduced the frequency of CD4+CD25+ Treg cells in the blood of B10.MBR reconstituted with bone marrow transduced with VSV-IAb (Figure 6A). We also found that FoxP3+ cells were effectively depleted from blood of these animals (11%±1% of CD4 T cells were FoxP3+ prior to administration of PC61, compared with 6%±2% after administration P<0.02). No significant difference was observed in the total frequency of CD4+CD25+ Treg in mice reconstituted with bone marrow transduced with VSV-IAb when compared with bone marrow transduced with VSV-GFP (Figure 6A). We confirmed that in our system >95% of CD4+CD25+ T cells were also positive for FoxP3 by intracellular staining and flow cytometry (data not shown). B10.MBR mice reconstituted with bone marrow transduced with VSV-IAb rejected their skin grafts rapidly following treatment with anti-CD25 antibody (Figure 6B MST= 17 days relative to the initiation of antibody treatment, n=6 p < 0.01 in relation to animals that did not receive PC61). These data suggest that regulatory T cells are involved in maintenance of tolerance to MHC class II mismatched skin grafts. In the absence of anti-CD25 antibody treatment, skin grafts are accepted for the life of the animal, suggesting that MHC class II gene therapy prevents chronic rejection of skin.
Figure 6. Tolerance induced by retrovirally encoded MHC class II is dependent on regulatory T cells.
A. Frequency of CD4+CD25+ T cells in mice that received VSV-GFP (GFP) or VSV-I-Ab before and after administration of PC61. Immediately prior to injection with PC61, recipients of bone marrow transduced with VSV-GFP (GFP) or VSV-I-Ab were bled, and PBMC were examined by cell surface staining and flow cytometry for expression of CD4 and CD25. N=5 for all groups B. 4 weeks after bone marrow reconstitution, B10.MBR recipients of VSV-I-Ab transduced bone marrow received MHC class II mismatched B10.QBR skin grafts. 56 days after skin grafting, mice were treated with anti-CD25 antibody PC61 (0.125 mg/mouse administered every other day), circles. N=6 Control mice that did not receive antibody are represented by squares. N=8. Shown are combined results of three independent experiments.
DISCUSSION
There has been a great deal of interest in using gene therapy to induce a state of tolerance in the context of restoring normal immune responses in autoimmune models (35-46), in eliminating immune responses to introduced transgenes in the context of replacement gene therapy (47-49), and in inducing tolerance to allergens (50). These approaches focus on the introduction of a single antigen which is then presented to the immune system on host MHC molecules in genetically modified cells. The presentation of these single antigens on bone marrow derived cells, B cells (43, 44, 51, 52), T cells or dendritic cells (53) has been shown to reproducibly induce tolerance in many models. However, in the transplantation setting, rejection of an allogeneic organ is not mediated by a single antigen difference, but rather through the recognition of a multitude of self-antigens and alloantigens in the context of allogeneic MHC. This can occur directly on donor cells (direct presentation), or through antigen processing and presentation on host cells (indirect presentation). The combination of direct and indirect presentation results in a high precursor frequency of cells capable of responding to the allogeneic organ, thus presenting a particular challenge to the induction of tolerance.
We have previously shown that the induction of molecular chimerism can be used to induce tolerance (24, 31, 54). In the setting of transplantation, we have shown that inducing molecular chimerism can be used to induce tolerance to MHC class I mismatched allografts (24, 25, 27). Mechanistically, tolerance to MHC class I induced through molecular chimerism occurs through both central deletion of alloreactive T cells (27) and through the induction of regulatory T cells (25). These results demonstrate that molecular chimerism is a powerful tool for the induction of tolerance without the need for extensive conditioning (55), and without the possibility of inducing complications such as GVHD since the bone marrow is syngeneic. However, for clinical transplantation it would be highly desirable to induce tolerance to both MHC class I and MHC class II mismatched organs. We therefore set out to determine whether molecular chimerism with donor MHC class II molecules had an equal capacity to induce long-term tolerance in an allogeneic system.
Our bicistronic retroviral construct encoding MHC class II α and β chains confers cell surface expression of I-Ab on transduced A20 cells without reducing cell surface expression of endogenous I-Ak α chain. The levels of endogenous I-Ak detected on transduced cells was higher than the level of I-Ab. While it is possible that retrovirally encoded MHC class II is less efficiently expressed than the endogenous chains, we suggest that it is more likely that the observed effect is due to the presence of k/b heterodimers on the cell surface as has previously been reported when α/β chain expression is driven by a strong exogenous promoter (56). Our I-Ab specific antibody recognizes I-Ab when complexed with peptide, while the antibody used to detect I-Ak α recognizes both b/k and k/k dimers present on the cell surface. Thus we suggest that increased levels of I-Ak observed may be due to increased detection.
Unlike MHC class I which is expressed on all nucleated cells, MHC class II expression is restricted to a subset of cells including antigen presenting cells such as B cells, macrophages, and dendritic cells (reviewed in (57)). Cell surface expression of MHC class II in these cells is tightly regulated (reviewed in (58)). When bone marrow transduced with VSV-IAb was used to reconstitute conditioned recipients, we observed expression of GFP in all cell lineages examined. However, cell surface expression of I-Ab was observed only on MHC class II expressing cell types. We suggest that the restriction of I-Ab expression to antigen presenting cells is due to the requirement for the cellular machinery that allows for MHC class II expression. Furthermore, we found that expression of the retrovirally encoded MHC class II was inducible. When cells from mice reconstituted with bone marrow transduced with VSV-IAb were examined directly ex vivo, only low levels of retrovirally encoded MHC class II were detected on the surface. However, when cells were stimulated with LPS, expression of MHC class II was up-regulated in fashion similar to endogenous MHC class II. This suggests that the induction of MHC class II in primary cells is controlled by cellular mechanisms unrelated to prevalence of mRNA encoding MHC class II. Taken together, these data suggest that retrovirally encoded MHC class II genes are expressed on the cell surface in a physiologically relevant manner.
Our data demonstrate that reconstitution of mice with VSV-IAb transduced bone marrow results in long-term multi-lineage expression of retrovirally-encoded genes. This point is of particular importance, since we have previously demonstrated that long-term expression of antigen is critical for tolerance induction in molecular chimeras (59). Indeed, long-term multilineage expression of retrovirally encoded I-Ab resulted in long-term acceptance of I-Ab expressing B10.QBR skin allografts. Acceptance of skin grafts is a robust measurement of tolerance induction, and therefore this finding indicates that inducing expression of MHC class II molecules through genetic engineering of autologous bone marrow results is an effective and robust approach that can result in tolerance to allografts.
In contrast to our results, LeGuern et al. have recently reported that expression of MHC class II on bone marrow derived cells is not sufficient to induce tolerance (34). These authors found that the primary T cell response to MHC class II was unchanged in mice receiving MHC class II transduced bone syngeneic marrow. However, they were able to observe prolongation of heart allografts following depletion of CD8 T cells in mice reconstituted with bone marrow transduced with virus encoding I-A, similar to what had previously been observed following CD4 T cell depletion(60). In contrast, we were able to observe long-term survival of skin grafts without the requirement for T cell depletion, a much more rigorous test of tolerance. Thus while we observed tolerance induction, the LeGuern et al. posited only an increase in the generation of regulatory T cells. Notably LeGuern et al. failed to obtain cell surface expression of MHC class II, and expression of MHC class II intracellularly (as measured by GFP as a pseudomarker) was rapidly extinguished. We therefore suggest that, since long-term antigen expression is required for the induction of tolerance (61), the failure to achieve tolerance in this study is related to a failure to achieve substantial long-term MHC class II expression. Our study shows that it would be incorrect to interpret the results of LeGuern et al. (34), and previous work by the same group in large animal models (62) to demonstrate that molecular chimerism with MHC class II fails to induce tolerance, but only that as we have demonstrated, long-term expression of retrovirally encoded products is required.
We have previously shown that induction of tolerance to MHC class I antigens through molecular chimerism results in deletion of alloreactive T cells in the thymus, and the generation of regulatory T cells (25). Depletion of regulatory T cells did not disrupt skin graft tolerance, indicating that central deletion is dominant in the case of mice rendered tolerant to MHC class I following induction of molecular chimerism. Here we have shown induced skin graft tolerance to MHC class II can be reversed through the depletion of CD25+ cells, suggesting that in an MHC class II model of molecular chimerism, regulatory T cells are required for tolerance. In contrast to the assertion by LeGuern et al. that donor specific transfusion is required to “activate” Treg in vivo in mice receiving MHC class II transduced bone marrow (34), our data show that in molecular chimeras, Tregs develop which are directly involved in controlling alloreactive T cell responses. We note that the level of MHC surface expression we were able to achieve may facilitate more efficient Treg generation.
CD4+ regulatory T cells can be either Foxp3+ regulatory T-cells (Tregs) and IL-10-producing regulatory type I (Tr1) cells. Activated Tr1 cells can express CD25, and are characterized by a unique pattern of cytokine expression consisting primarily of IL-10, TGF-β and IL-5 (63-65). While no specific markers allow us to unambiguously distinguish Tr1 cells, we would suggest that Tr1 cells are probably not involved in this model, as we do not detect significant amounts of IL-10.
Our results demonstrate that genetic modification of syngeneic hematopoietic stem cells to express allogeneic MHC genes can be used to facilitate tolerance to allografts, essentially resulting in matching at MHC class I and II. Given that MHC matching results in profound improvements in long-term survival of allogeneic transplants we suggest that the use of gene therapy may represent one way to improve organ allograft survival.
MATERIALS AND METHODS
Mice
B10.MBR (Kb, I-Ak,I-Ek, Dq), and BALB/c (H-2d) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B10.QBR (Kb, I-Ab,I-Enull, Dq) mice were bred in our facility. Mice were housed under microisolator conditions in autoclaved cages and maintained on irradiated feed and autoclaved acidified drinking water. Four to six week old female mice were used in all experiments. All experiments were performed in accordance with the humane use and care policies of our Institution.
Monoclonal antibodies and Flow Cytometry
All cell surface staining and flow cytometry was performed as described previously (66, 67). Monoclonal antibodies (mAbs) specific for CD4 (L3T4), CD8 (52-6.7), I-Ab (KH74 or AF6-120.1), I-Ak (11.5-2), CD25 (7D4), CD11b (Mac-1, M1/70), CD11c (HL3) and B220 (RA3-6B2) were obtained from BD PharMingen (San Diego, CA). FoxP3 staining kit was obtained from eBioscience (San Diego, CA). Depleting/inactivating anti-CD25 (PC61) antibody was the kind gift of Dr. Mohamed Sayegh (Brigham and Women's Hospital, Boston).
Retroviruses
The full-length cDNA encoding H-2 I-Ab alpha chain was cloned into the MMP retroviral vector containing the I-Ab beta chain fused to enhanced green fluorescent protein (eGFP) previously described (31) to generate pMMP-I-Ab. VSV-G envelope protein pseudotyped viruses were prepared by packaging the pMMP-I-Ab vector in 293T cells by transient transfection, and titered as described (31).
Retroviral transduction of bone marrow cells
Bone marrow cells were harvested from B10.MBR mice and transduced as described previously (67). Briefly, bone marrow cells from mice treated 7 days before with 150 mg/kg 5-Fluorouracil were cultured in tissue culture plates coated with Retronectin (Takara Biomedicals, Shiga, Japan) and transduced in Dulbecco minimum essential medium containing 15% lot-tested fetal calf serum and cytokines to achieve a final concentration of 100 ng/ml human interleukin 6 (IL-6; R&D Systems, Minneapolis, MN), 100 ng/ml recombinant mouse stem cell factor (R&D Systems), 50 ng/ml recombinant mouse thrombopoietin (R&D Systems), and 50 ng/ml recombinant mouse Flt-3 ligand (R&D Systems). All transductions were performed at 37°C with 5% CO2 for 96 h. Viral supernatants and transduction media were replaced after 72 h. After 96 hours, the cells were harvested, washed twice in Hanks’ balanced salt solution, and counted.
Bone marrow transplantation
Mice were conditioned with 11Gy whole-body irradiation 1 day prior to bone marrow transplantation. On the day of reconstitution, 4×106 transduced B10.MBR bone marrow cells were injected into the tail vein of recipient mice.
Mixed Lymphocyte Reaction
B10.MBR mice reconstituted with transduced bone marrow as above were immunized intraperitoneally (i.p.) with an injection of 107 irradiated (25 Gy) B10.QBR splenocytes. Ten days after immunization, animals were sacrificed and splenocytes were harvested. Splenocytes were stained with 0.1μM CFSE (Invitrogen, Carlsbad CA) for 8 min at room temperature and cultured in the presence of irradiated (25 Gy) B10.QBR splenocytes or third party BALB/c splenocytes for 96 hrs. Cell proliferation of CD4 and CD8 T cells was assessed by flow cytometry. Percent proliferation was calculated using FloJo software proliferation platform.
ELISPOT Assays
Eight to 12 weeks after B10.MBR mice were reconstituted with transduced bone marrow, recipients were immunized i.p. with 107 irradiated (25 Gy) B10.QBR splenocytes. Ten days later animals were sacrificed and single-cell suspensions of splenocytes were prepared and used in a standard cytokine ELISPOT assay as described (66). ELISPOT data are presented as the number of cytokine producing cells per 106 T cells.
Luminex
Luminex assays were performed using the Millipore (Billerica, MA) Milliplex Mouse 12 Cytokine/Chemokine Panel kit according to the manufacturer's instructions. Cytokine profile was analyzed on Luminex 100™ (Luminex Corporation, Austin, TX) based on manufacturer's instructions. Duplicate wells were used for each sample.
Skin grafting
Tail skin grafting was performed by a modification of a method previously described (67). Full-thickness tail skin grafts were harvested from the tails of B10.QBR or BALB/c mice. Recipients were anesthetized with 250 mg/kg Avertin and grafted without suturing onto prepared sites on the flanks. Skin grafts were dressed with Vaseline-impregnated gauze and an adhesive bandage for the first 7 days after surgery. Thereafter, skin grafts were assessed three times weekly, and rejection was defined as the first day on which the entire graft surface appeared necrotic.
Statistical Analysis
All statistical calculations were preformed using GraphPad Prism 4.0a software (Graphpad Software, San Diego, CA). The Kaplan and Meier method with a 95% CI was used for the calculation of survival curves. Survival curves were compared using the log rank test. P values <0.05 using Student's t test were considered statistically significant.
ACKNOWLEDGEMENTS
Peter Jindra was supported in part by NIH training grant T32 AI070085 and an AST/Wyeth Basic Science Fellowship Grant. Jessamyn Bagley is supported by an American Heart Association Scientist Development Grant. This work was also supported by NIH grants R01 AI53666, R01 AI043619 and R01 AI070601 awarded to JI.
Footnotes
Highest degree received: PTJ, Ph.D., ST Ph.D., CT M.D., JI Ph.D., JB, Ph.D.
CONFLICT OF INTEREST: The authors have no conflicts of interest.
References
- 1.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172(4379):603–6. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 2.Slavin S, Strober S, Fuks Z, Kaplan HS. Induction of specific tissue transplantation tolerance using fractionated total lymphoid irradiation in adult mice: long-term survival of allogeneic bone marrow and skin grafts. J Exp Med. 1977;146(1):34–48. doi: 10.1084/jem.146.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307(5947):168–70. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 4.Wekerle T, Sykes M. Mixed chimerism and transplantation tolerance. Annu Rev Med. 2001;52:353–70. doi: 10.1146/annurev.med.52.1.353. [DOI] [PubMed] [Google Scholar]
- 5.Socie G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009;114(20):4327–36. doi: 10.1182/blood-2009-06-204669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daly A, McAfee S, Dey B, Colby C, Schulte L, Yeap B, et al. Nonmyeloablative bone marrow transplantation: Infectious complications in 65 recipients of HLA-identical and mismatched transplants. Biol Blood Marrow Transplant. 2003;9(6):373–82. doi: 10.1016/s1083-8791(03)00100-9. [DOI] [PubMed] [Google Scholar]
- 7.Parkman R, Weinberg KI. Immunological reconstitution following bone marrow transplantation. Immunol Rev. 1997;157:73–8. doi: 10.1111/j.1600-065x.1997.tb00975.x. [DOI] [PubMed] [Google Scholar]
- 8.Williams MA, Adams AB, Walsh MB, Shirasugi N, Onami TM, Pearson TC, et al. Primary and secondary immunocompetence in mixed allogeneic chimeras. J Immunol. 2003;170(5):2382–9. doi: 10.4049/jimmunol.170.5.2382. [DOI] [PubMed] [Google Scholar]
- 9.Clift RA, Storb R. Histoincompatible bone marrow transplants in humans. Annu Rev Immunol. 1987;5:43–64. doi: 10.1146/annurev.iy.05.040187.000355. [DOI] [PubMed] [Google Scholar]
- 10.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–70. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 11.Zinkernagel RM, Althage A, Waterfield E, Kindred B, Welsh RM, Callahan G, et al. Restriction specificities, alloreactivity, and allotolerance expressed by T cells from nude mice reconstituted with H-2-compatible or -incompatible thymus grafts. J Exp Med. 1980;151(2):376–99. doi: 10.1084/jem.151.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knechtle SJ. Knowledge about transplantation tolerance gained in primates. Curr Opin Immunol. 2000;12(5):552–6. doi: 10.1016/s0952-7915(00)00137-0. [DOI] [PubMed] [Google Scholar]
- 13.Fehr T, Sykes M. Tolerance induction in clinical transplantation. Transpl Immunol. 2004;13(2):117–30. doi: 10.1016/j.trim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Toh HC, Spitzer TR, Preffer F, Alexander SI, McAfee S, Dombkowski D, et al. Fluctuating lymphocyte chimerism, tolerance and anti-tumor response in a patient with refractory lymphoma receiving nonmyeloablative conditioning and a haploidentical related allogeneic bone marrow transplant. Cytokines Cell Mol Ther. 2002;7(2):43–7. doi: 10.1080/13684730412331302054. [DOI] [PubMed] [Google Scholar]
- 15.Dey BR, McAfee S, Colby C, Cieply K, Caron M, Saidman S, et al. Anti-tumour response despite loss of donor chimaerism in patients treated with non-myeloablative conditioning and allogeneic stem cell transplantation. Br J Haematol. 2005;128(3):351–9. doi: 10.1111/j.1365-2141.2004.05328.x. [DOI] [PubMed] [Google Scholar]
- 16.Russell PS, Chase CM, Sykes M, Ito H, Shaffer J, Colvin RB. Tolerance, mixed chimerism, and chronic transplant arteriopathy. J Immunol. 2001;167(10):5731–40. doi: 10.4049/jimmunol.167.10.5731. [DOI] [PubMed] [Google Scholar]
- 17.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4(3):378–83. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 18.Womer KL, Lee RS, Madsen JC, Sayegh MH. Tolerance and chronic rejection. Philos Trans R Soc Lond B Biol Sci. 2001;356(1409):727–38. doi: 10.1098/rstb.2001.0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood KJ. Induction of tolerance to cardiac allografts. Curr Opin Cardiol. 1996;11(2):208–13. doi: 10.1097/00001573-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Bagley J, Bracy JL, Tian C, Kang ES, Iacomini J. Establishing immunological tolerance through the induction of molecular chimerism. Front Biosci. 2002;7:d1331–7. doi: 10.2741/bagley. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101(5):455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49(2):273–80. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 24.Bagley J, Tian C, Sachs DH, Iacomini J. Induction of T-cell tolerance to an MHC class I alloantigen by gene therapy. Blood. 2002;99(12):4394–9. doi: 10.1182/blood.v99.12.4394. [DOI] [PubMed] [Google Scholar]
- 25.Forman D, Kang ES, Tian C, Paez-Cortez J, Iacomini J. Induction of alloreactive CD4 T cell tolerance in molecular chimeras: a possible role for regulatory T cells. J Immunol. 2006;176(6):3410–6. doi: 10.4049/jimmunol.176.6.3410. [DOI] [PubMed] [Google Scholar]
- 26.Tian C, Yuan X, Bagley J, Blazar BR, Sayegh MH, Iacomini J. Induction of transplantation tolerance by combining non-myeloablative conditioning with delivery of alloantigen by T cells. Clin Immunol. 2008;127(2):130–7. doi: 10.1016/j.clim.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang ES, Iacomini J. Induction of central deletional T cell tolerance by gene therapy. J Immunol. 2002;169(4):1930–5. doi: 10.4049/jimmunol.169.4.1930. [DOI] [PubMed] [Google Scholar]
- 28.Tian C, Bagley J, Forman D, Iacomini J. Induction of central tolerance by mature T cells. J Immunol. 2004;173(12):7217–22. doi: 10.4049/jimmunol.173.12.7217. [DOI] [PubMed] [Google Scholar]
- 29.Tian C, Bagley J, Iacomini J. Homeostatic expansion permits T cells to re-enter the thymus and deliver antigen in a tolerogenic fashion. Am J Transplant. 2007;7(8):1934–41. doi: 10.1111/j.1600-6143.2007.01891.x. [DOI] [PubMed] [Google Scholar]
- 30.Tian C, Bagley J, Kaye J, Iacomini J. Induction of T cell tolerance to a protein expressed in the cytoplasm through retroviral-mediated gene transfer. J Gene Med. 2003;5(5):359–65. doi: 10.1002/jgm.363. [DOI] [PubMed] [Google Scholar]
- 31.Tian C, Bagley J, Cretin N, Seth N, Wucherpfennig KW, Iacomini J. Prevention of type 1 diabetes by gene therapy. J Clin Invest. 2004;114(7):969–78. doi: 10.1172/JCI22103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riviere I, Brose K, Mulligan RC. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc Natl Acad Sci U S A. 1995;92(15):6733–7. doi: 10.1073/pnas.92.15.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fung-Leung WP, Surh CD, Liljedahl M, Pang J, Leturcq D, Peterson PA, et al. Antigen presentation and T cell development in H2-M-deficient mice. Science. 1996;271(5253):1278–81. doi: 10.1126/science.271.5253.1278. [DOI] [PubMed] [Google Scholar]
- 34.LeGuern C, Akiyama Y, Germana S, Tanaka K, Fernandez L, Iwamoto Y, et al. Intracellular MHC class II controls regulatory tolerance to allogeneic transplants. J Immunol. 2010;184(5):2394–400. doi: 10.4049/jimmunol.0803664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen XT, Chan ST, Hosseini H, Layton D, Boyd R, Alderuccio F, et al. Transplantation of retrovirally transduced bone marrow prevents autoimmune disease in aged mice by peripheral tolerance mechanisms. Autoimmunity. 2011;44(5):384–93. doi: 10.3109/08916934.2010.541173. [DOI] [PubMed] [Google Scholar]
- 36.Ally BA, Hawley TS, McKall-Faienza KJ, Kundig TM, Oehen SU, Pircher H, et al. Prevention of autoimmune disease by retroviral-mediated gene therapy. J Immunol. 1995;155(11):5404–8. [PubMed] [Google Scholar]
- 37.French MB, Allison J, Cram DS, Thomas HE, Dempsey-Collier M, Silva A, et al. Transgenic expression of mouse proinsulin II prevents diabetes in nonobese diabetic mice. Diabetes. 1997;46(1):34–9. doi: 10.2337/diab.46.1.34. [DOI] [PubMed] [Google Scholar]
- 38.Jaeckel E, Lipes MA, von Boehmer H. Recessive tolerance to preproinsulin 2 reduces but does not abolish type 1 diabetes. Nat Immunol. 2004;5(10):1028–35. doi: 10.1038/ni1120. [DOI] [PubMed] [Google Scholar]
- 39.Xu H, Wawrousek EF, Redmond TM, Nickerson JM, Wiggert B, Chan CC, et al. Transgenic expression of an immunologically privileged retinal antigen extraocularly enhances self tolerance and abrogates susceptibility to autoimmune uveitis. Eur J Immunol. 2000;30(1):272–8. doi: 10.1002/1521-4141(200001)30:1<272::AID-IMMU272>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 40.Steptoe RJ, Ritchie JM, Harrison LC. Transfer of hematopoietic stem cells encoding autoantigen prevents autoimmune diabetes. J Clin Invest. 2003;111(9):1357–63. doi: 10.1172/JCI15995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan J, Ban EJ, Chun KH, Wang S, Backstrom BT, Bernard CC, et al. Transplantation of bone marrow transduced to express self-antigen establishes deletional tolerance and permanently remits autoimmune disease. J Immunol. 2008;181(11):7571–80. doi: 10.4049/jimmunol.181.11.7571. [DOI] [PubMed] [Google Scholar]
- 42.Alderuccio F, Toh BH, Tan SS, Gleeson PA, van Driel IR. An autoimmune disease with multiple molecular targets abrogated by the transgenic expression of a single autoantigen in the thymus. J Exp Med. 1993;178(2):419–26. doi: 10.1084/jem.178.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu B, Haviernik P, Wolfraim LA, Bunting KD, Scott DW. Bone marrow transplantation combined with gene therapy to induce antigen-specific tolerance and ameliorate EAE. Mol Ther. 2006;13(1):42–8. doi: 10.1016/j.ymthe.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Melo ME, Qian J, El-Amine M, Agarwal RK, Soukhareva N, Kang Y, et al. Gene transfer of Ig-fusion proteins into B cells prevents and treats autoimmune diseases. J Immunol. 2002;168(9):4788–95. doi: 10.4049/jimmunol.168.9.4788. [DOI] [PubMed] [Google Scholar]
- 45.Hosseini H, Oh DY, Chan ST, Chen XT, Nasa Z, Yagita H, et al. Non-myeloablative transplantation of bone marrow expressing self-antigen establishes peripheral tolerance and completely prevents autoimmunity in mice. Gene Ther. 2011 doi: 10.1038/gt.2011.179. [DOI] [PubMed] [Google Scholar]
- 46.Eixarch H, Espejo C, Gomez A, Mansilla MJ, Castillo M, Mildner A, et al. Tolerance induction in experimental autoimmune encephalomyelitis using non-myeloablative hematopoietic gene therapy with autoantigen. Mol Ther. 2009;17(5):897–905. doi: 10.1038/mt.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003;111(9):1347–56. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lei TC, Scott DW. Induction of tolerance to factor VIII inhibitors by gene therapy with immunodominant A2 and C2 domains presented by B cells as Ig fusion proteins. Blood. 2005;105(12):4865–70. doi: 10.1182/blood-2004-11-4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Evans GL, Morgan RA. Genetic induction of immune tolerance to human clotting factor VIII in a mouse model for hemophilia A. Proc Natl Acad Sci U S A. 1998;95(10):5734–9. doi: 10.1073/pnas.95.10.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baranyi U, Linhart B, Pilat N, Gattringer M, Bagley J, Muehlbacher F, et al. Tolerization of a type I allergic immune response through transplantation of genetically modified hematopoietic stem cells. J Immunol. 2008;180(12):8168–75. doi: 10.4049/jimmunol.180.12.8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Satpute SR, Soukhareva N, Scott DW, Moudgil KD. Mycobacterial Hsp65-IgG-expressing tolerogenic B cells confer protection against adjuvant-induced arthritis in Lewis rats. Arthritis Rheum. 2007;56(5):1490–6. doi: 10.1002/art.22566. [DOI] [PubMed] [Google Scholar]
- 52.Liang W, Karabekian Z, Mattapallil M, Xu Q, Viley AM, Caspi R, et al. B-cell delivered gene transfer of human S-Ag-Ig fusion protein protects from experimental autoimmune uveitis. Clin Immunol. 2006;118(1):35–41. doi: 10.1016/j.clim.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Ko HJ, Chung JY, Nasa Z, Chan J, Siatskas C, Toh BH, et al. Targeting MOG expression to dendritic cells delays onset of experimental autoimmune disease. Autoimmunity. 2011;44(3):177–87. doi: 10.3109/08916934.2010.515274. [DOI] [PubMed] [Google Scholar]
- 54.Bracy JL, Chase CM, Russell PS, Mauiyyedi S, Colvin RB, Iacomini J. Induction of molecular chimerism by gene therapy prevents antibody-mediated heart transplant rejection. Gene Ther. 2001;8(22):1738–44. doi: 10.1038/sj.gt.3301581. [DOI] [PubMed] [Google Scholar]
- 55.Forman D, Tian C, Iacomini J. Induction of donor-specific tolerance in sublethally irradiated recipients by gene therapy. Mol Ther. 2005;12(2):353–9. doi: 10.1016/j.ymthe.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 56.Sant AJ, Braunstein NS, Germain RN. Predominant role of amino-terminal sequences in dictating efficiency of class II major histocompatibility complex alpha beta dimer expression. Proc Natl Acad Sci U S A. 1987;84(22):8065–9. doi: 10.1073/pnas.84.22.8065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drukker M, Katz G, Urbach A, Schuldiner M, Markel G, Itskovitz-Eldor J, et al. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99(15):9864–9. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Handunnetthi L, Ramagopalan SV, Ebers GC, Knight JC. Regulation of major histocompatibility complex class II gene expression, genetic variation and disease. Genes Immun. 11(2):99–112. doi: 10.1038/gene.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian C, Bagley J, Forman D, Iacomini J. Inhibition of CD26 peptidase activity significantly improves engraftment of retrovirally transduced hematopoietic progenitors. Gene Ther. 2006;13(7):652–8. doi: 10.1038/sj.gt.3302695. [DOI] [PubMed] [Google Scholar]
- 60.Wong W, Billing JS, Stranford SA, Hyde K, Fry J, Morris PJ, et al. Retroviral transfer of donor MHC class I or MHC class II genes into recipient bone marrow cells can induce operational tolerance to alloantigens in vivo. Hum Gene Ther. 2003;14(6):577–90. doi: 10.1089/104303403764539350. [DOI] [PubMed] [Google Scholar]
- 61.Tian C, Bagley J, Iacomini J. Persistence of antigen is required to maintain transplantation tolerance induced by genetic modification of bone marrow stem cells. Am J Transplant. 2006;6(9):2202–7. doi: 10.1111/j.1600-6143.2006.01455.x. [DOI] [PubMed] [Google Scholar]
- 62.Sonntag KC, Emery DW, Yasumoto A, Haller G, Germana S, Sablinski T, et al. Tolerance to solid organ transplants through transfer of MHC class II genes. J Clin Invest. 2001;107(1):65–71. doi: 10.1172/JCI11015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 64.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389(6652):737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 65.Bacchetta R, de Waal Malefijt R, Yssel H, Abrams J, de Vries JE, Spits H, et al. Host-reactive CD4+ and CD8+ T cell clones isolated from a human chimera produce IL-5, IL-2, IFN-gamma and granulocyte/macrophage-colony-stimulating factor but not IL-4. J Immunol. 1990;144(3):902–8. [PubMed] [Google Scholar]
- 66.Bagley J, Sawada T, Wu Y, Iacomini J. A critical role for interleukin 4 in activating alloreactive CD4 T cells. Nat Immunol. 2000;1(3):257–61. doi: 10.1038/79811. [DOI] [PubMed] [Google Scholar]
- 67.Sawada T, Wu Y, Sachs DH, Iacomini J. CD4+ T cells are able to reject class I disparate allografts. Transplantation. 1997;64(2):335–40. doi: 10.1097/00007890-199707270-00027. [DOI] [PubMed] [Google Scholar]