Abstract
Background
Intrauterine exposure to gestational diabetes mellitus (GDM) increases risk for obesity. Obesity is associated with a blunted postprandial gut hormone response, which may impair satiety and thereby contribute to weight gain. The postprandial response of gut hormones among children of women with GDM has not previously been investigated.
Objective
To examine whether children of women with GDM have suppressed peptide-tyrosine-tyrosine (PYY) and glucagon-like-peptide-1 (GLP-1), and higher concentrations of ghrelin, following a meal challenge. A secondary objective was to investigate associations of these hormones with children’s free-living energy intake.
Methods
Children (N=42) aged 5-10 years were stratified into 2 groups: offspring of GDM mothers (OGD), and of non-diabetic mothers (CTRL). Body composition was measured by dual-energy X-ray absorptiometry, and circulating PYY, GLP-1, and total ghrelin were measured during a liquid meal challenge. Energy intake was assessed by three 24-hr diet recalls.
Results
Between groups analyses of fasting and incremental area under the curve (AUC) found no differences in ghrelin. Incremental AUC for GLP-1 was greater among the CTRL versus OGD (P<0.05), and fasting PYY, but not incremental AUC, was higher among OGD versus CTRL (P<0.01). Associations of fasting and incremental AUC for each gut hormone with children’s usual energy intake did not differ significantly by group.
Conclusions
Further research is needed to more fully examine the potential role of postprandial GLP-1 suppression and high fasting PYY concentrations on the feeding behavior and risk for obesity among children exposed to GDM in utero.
Keywords: PYY, ghrelin, GLP-1, satiety, obesity
Children exposed to gestational diabetes mellitus (GDM) in utero have greater risk for obesity and comorbid health problems as compared to children from non-diabetic mothers (1-3). This effect is believed to be at least partially attributable to altered intrauterine programming of metabolism and body weight regulation. Gut hormones play an important role in the regulation of body weight homeostasis via signaling of hunger and satiety. To date, however, there are no published studies that have examined the concentrations and activity of gut hormones among offspring of women with gestational diabetes (OGD) versus those from a non-diabetic intrauterine environment (CTRL).
Ghrelin is a hormone secreted in the stomach and to date, is the only peripheral signal that is known to stimulate food intake (4). Secretion of ghrelin increases prior to a meal and is suppressed following a meal (5). Among obese adults, fasting concentrations of ghrelin tend to be lower, and the post-prandial suppression of ghrelin is blunted (6; 7). Although it appears paradoxical for obese individuals to have lower fasting ghrelin, it has been hypothesized that this pattern is consequent to increased insulin, which is inversely associated with ghrelin, or is an adaptive response to positive energy balance in obese adults (6). Despite consistent findings in studies of adults, studies of lean versus obese children, have yielded less consistent results (8; 9).
Glucagon-like peptide 1 (GLP-1) and peptide-tyrosine-tyrosine (PYY) are anorectic hormones that are secreted from L-cells in the ileum of the small intestine in response to feeding. Circulating concentrations of GLP-1 tend to be lower among obese adults and children (10; 11), and the acute increase in GLP-1 following meal consumption may be suppressed in obese states (12). There are two endogenous forms of PYY: 1-36 and 3-36; the latter of these has been more strongly implicated in feeding. Some, but not all (9), studies have shown lower fasting concentrations of PYY3-36 among obese individuals (13; 14). Furthermore, obesity has been consistently associated with suppression of the usual increase in PYY3-36 concentration that occurs following a meal (9; 13; 14). Previous research has not examined whether the response of GLP-1 and PYY to a meal challenge is perturbed among children of women with GDM.
The main objective of this study was to compare fasting and postprandial concentrations of ghrelin, GLP-1 and PYY3-36 among children with and without intrauterine exposure to maternal GDM. The secondary objective was to examine whether associations of these hormones with children’s usual food intake differed by group. Differences in body composition, energy intake, physical activity, insulin sensitivity and secretion among OGD versus CTRL from this cohort have been reported previously (2). We hypothesized that, similar to obese individuals, OGD would have lower fasting concentrations of ghrelin, GLP-1, and PYY3-36, and an attenuated postprandial response of these gut hormones. These hypotheses were tested among 5-10 year-old children with and without intrauterine exposure to GDM.
METHODS
Participants
Children aged 5-10 years were recruited to fill two groups: offspring of GDM (OGD) and offspring on non-diabetic women (CTRL). Mothers of the OGD children were required to have been diagnosed with gestational diabetes (GDM) during the target pregnancy, and when available, prenatal medical records were reviewed for confirmation of GDM versus non-GDM status. Children were eligible for inclusion if they were singletons, were born at ≥37 weeks gestation, and for girls, menstruation had not started. Children who were growth restricted in utero (<2500g at birth), had congenital defects, type 1 diabetes, or a current weight of <11kg (precluding adequate blood sampling), were excluded. Informed consent/assent was obtained from the mothers and children, and the study was approved by the Institutional Review Board at the University of Alabama at Birmingham (UAB).
Procedure
Mother-child pairs reported to the Participant and Clinical Interactions Resource at UAB at approximately 6:30 am following a 12-hour overnight fast. Resting energy expenditure of children was obtained, and then children underwent a liquid meal tolerance test during which gut hormones were measured. Body composition was measured by dual-energy X-ray absorptiometry at UAB’s Webb Nutrition Sciences Building.
Liquid Meal Tolerance Test
The meal was designed to provide ~1.75 g of carbohydrate per kg of lean body mass and consisted of Carnation Instant Breakfast (Nestlé USA, Inc., Glendale, CA) mixed with whole milk. For each kilogram of lean mass, the liquid meal provided 11 kilocalories (kcals), and 27%, 55%, and 18% of total kcals from fat, carbohydrate, and protein, respectively. A flexible intravenous catheter was placed in the antecubital space of one arm for frequent blood sampling. Two blood samples were drawn over a 15 minute period prior to meal consumption and averaged to provide fasting values (time = 0). Children consumed the meals within 5 minutes and subsequent blood samples were collected at 15, 60, 90, 120, 180, and 240 minutes. The total amount of blood drawn during this test was 41 ml (~2 tablespoons). DPP-IV inhibitor was added to whole blood in order to preserve the sample. Sera were separated and stored at −85°C until analyzed for total ghrelin, active GLP-1, and PYY3-36.
Body composition
Body weight was measured to the nearest 0.1 kg (Scale-tronix 6702W; Scale-tronix, Carol Stream, IL) in minimal street clothing without shoes. Height, to the nearest 0.01 cm, was measured with a digital stadiometer (Heightronic 235; Measurement Concepts, Snoqualmie, WA).
Body composition was measured by dual-energy X-ray absorptiometry (Lunar iDXA, GE Healthcare, General Electric Company, Madison, WI). Scans were analyzed for fat and lean mass, and for total % fat using the encore software package (version 1.33; GE Healthcare, General Electric Company, Madison, WI).
Resting energy expenditure
Resting energy expenditure was obtained by indirect calorimetry using a Deltatrac Metabolic Monitor (Sensorimedics Corp., Yorba Linda, CA), and methods previously conducted at this facility (15). The time at which any large body movements, yawning, or coughing occurred was noted and that particular 1-minute period subsequently removed from the data. The remaining values were averaged to provide resting energy expenditure.
Serum assays
Total ghrelin, active GLP-1, and PYY3-36, were analyzed by the Core laboratory of the UAB Nutrition Obesity Research Center, Diabetes Research and Training Center, and Center for Clinical and Translational Science. Enzyme-linked immunoabsorbent assay (ELISA; Linco Research) was used to measure concentrations of total ghrelin and active GLP-1 (kits manufactured by Millipore Corp., Billerica, MA). Total ghrelin was measured in duplicate 20μl aliquots, and in our laboratory, this assay has a mean intra-assay coefficient of variation (CV) of 7.10% and a mean inter-assay CV of 5.98%. Active GLP-1 was measured in duplicate 100 μl aliquots; with a mean intra-assay CV of 7.32% and inter-assay CV of 6.25%. PYY3-36 was measured from duplicate 100 μl aliquots by radioimmunoassay (Millipore Corp., Billerica, MA). The intra- and inter-assay CV for this assay were 7.91% and 10.87%, respectively. Minimum sensitivities for ghrelin, PYY, and GLP-1 were 100 pg/ml, 21.1 pg/ml, and 1.0 pM, respectively.
Maternal medical records
Upon enrollment in the study, mothers provided permission for study investigators to request prenatal care and delivery records from physicians and hospitals. Subsequently, records from ~80% of the participants were obtained and GDM status verified.
Energy intake
Children’s total energy intake was measured by three 24-hour diet recalls using the multi-pass method (16). Both mothers and children provided information regarding children’s intake over the previous 24-hour period. In all cases, recalls were obtained for 1 weekend day and 2 week days. Data were entered into the Nutrition Data System for Research (version 2007; University of Minnesota, MN) and average daily total energy intake (kcal) was calculated for each participant.
Statistical analysis
Fifty-five children were enrolled in the study. Nine children were unable to provide blood samples or had incomplete blood sampling and so were excluded from final analyses. Four children were found to be at Tanner stage 2 of pubertal development after examination by a nurse practitioner, and because puberty influences ghrelin and PYY concentrations (17; 18), data from these children were also excluded. The final analyses for the ghrelin and GLP-1 outcomes were conducted on 42 children (22 CTRL, 20 OGD). A further 12 children were excluded from the final PYY analyses due to technical issues with one assay kit and a lack of excess serum with which to re-run the assay (13 CTRL, 17 OGD included in PYY analyses). The children who were included in the PYY analyses did not differ from those excluded from these analyses in terms of age, body fat, energy intake, resting energy expenditure, ghrelin, or GLP-1.
The distribution of race and gender across each group was assessed with chi-square tests. Between-groups differences in children’s body composition, REE, and energy intake were examined with independent groups t-tests.
The fasting (time = 0) concentration for each gut hormone was calculated as the average of the two fasting blood draws. Total area under the curve (AUC) for each gut hormone was calculated using the trapezoid method (19). Incremental AUC for GLP-1 and PYY were calculated as the AUC above fasting concentrations. Given that the normal postprandial response of ghrelin is to drop below fasting concentrations, incremental AUC for ghrelin was calculated as the area below fasting ghrelin concentrations. Due to violations of normality, data for each of the gut hormones were log transformed prior to analysis. Group differences in fasting and incremental AUC concentration of each gut hormone were compared using between groups analysis of covariance with adjustment for race and total body % fat.
To examine whether intrauterine exposure to GDM influenced the association between children’s usual energy intake and their gut hormone concentrations, analyses of covariance (ANCOVA) were used to test for an interactive effect of group and gut hormone concentration, on children’s usual energy intake, after adjusting for children’s resting energy expenditure as an estimate of children’s energy needs. We explored potential interactions with both fasting concentrations and incremental AUC for each gut hormone. Alpha was set at 0.05 for statistical significance, and all analyses were performed using the Statistical Package for the Social Sciences, version 18 (SPSS; SPSS Inc., Chicago, IL).
RESULTS
Characteristics of the children are shown in Table 1. Across the whole sample, fifty percent of the sample was male and the gender distribution did not differ between groups. Seventy-four percent of the sample was African American, with a trend for a higher proportion of African American children among the CTRL group versus the OGD group (P=0.052). Those in the OGD group had greater total body % fat as compared to CTRL (P<0.05).
Table 1.
Characteristics of the study population.
| CTRL | OGD | |
|---|---|---|
| N | 22 | 20 |
| Ethnicity (%AA)□ | 86% | 60% |
| Gender (%male) | 52% | 45% |
| Age (yr) | 7.3 ± 1.5 | 7.6 ± 1.7 |
| Weight (kg) | 29.8 ± 9.5 | 32.0 ± 9.7 |
| Height (cm) | 129.2 ± 11.2 | 127.2 ± 11.4 |
| BMI percentile | 63.1 ± 27.9 | 77.5 ± 28.0 |
| Body fat (%)* | 24.5 ± 9.1 | 31.6 ± 9.2 |
| Daily energy intake (kcals) | 1669 ± 376 | 1532 ± 340 |
| Resting energy expenditure (kcals/day) |
1040 ± 238 | 1109 ± 236 |
P<0.05
P=0.05
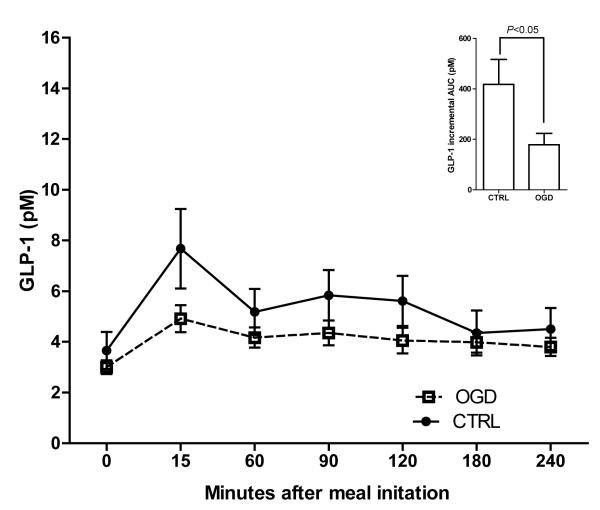
The response of each gut hormone to the meal challenge is shown in Figures 1-3. Ghrelin concentrations (Figure 1) did not differ between the groups at fasting (P=0.67) or during the postprandial period (incremental AUC: P=0.18). Fasting GLP-1 (Figure 2) also did not differ between groups (P=0.35), but incremental AUC for GLP-1 was higher among the CTRL versus OGD after adjustment for total body % fat and race (P<0.05). PYY concentrations (Figure 3) were significantly greater among OGD versus CTRL at fasting (P<0.01) but there was no difference in incremental AUC (P=0.77).
Figure 1.
Fasting and postprandial response of ghrelin did not differ among OGD (□) versus CTRL (•) children. Data shown in the main panel are unadjusted mean ± SEM. Insert shows the incremental AUC, below fasting concentrations, after adjustment for race and total body % fat (mean ± SEM).
Figure 3.
Fasting and postprandial response of PYY in OGD (□) versus CTRL (•). Data shown in the main panel are unadjusted mean ± SEM. **Fasting PYY was higher among OGD versus CTRL, P<0.01. Insert shows the incremental AUC, above fasting concentrations and after adjustment for race and total body % fat (mean ± SEM).
Figure 2.
Fasting and postprandial response of GLP-1 in OGD (□) versus CTRL (•). Data shown in the main panel are unadjusted mean ± SEM. Insert shows the incremental AUC, above fasting concentrations and after adjustment for race and total body % fat (mean ± SEM), which was greater among CTRL versus OGD (P<0.05).
Results of ANCOVA to examine whether the associations between each gut hormone and children’s usual energy intake differed by maternal GDM status, showed no statistically significant group by hormone interactions. There were trends however, for an association of group with incremental AUC for ghrelin (P=0.17) and of group with fasting PYY (P=0.16). Given that this study may have been insufficiently powered to detect interactions, partial correlation coefficients were calculated within each group to further explore these trends. In OGD, but not CTRL, incremental AUC for ghrelin was inversely associated with children’s average daily intake, after adjusting for their resting energy expenditure (partial r = −0.461, P<0.05; Figure 4). Also found exclusively in OGD, was a positive association between fasting PYY and average daily energy intake, adjusted for resting energy expenditure (partial r = 0.595, P<0.05; Figure 5). The results were comparable when children’s BMI percentile was used in these analyses instead of resting energy expenditure (not shown).
Figure 4.
Partial correlation plots showing association between incremental AUC for ghrelin and average daily energy intake, adjusted for children’s REE, in CTRL (A) and OGD (B). Postprandial ghrelin was not associated with energy intake among CTRL children, but was inversely associated with energy intake among OGD (partial r = −461, P<0.05).
Figure 5.
Partial correlation plots showing association between fasting PYY and average daily energy intake, adjusted for children’s REE, in CTRL (A) and OGD (B). PYY was not associated with energy intake among CTRL children, but was positively associated for OGD (partial r = 0.595, P<0.05).
DISCUSSION
The purpose of this study was to compare the gut hormone response to a standardized meal among children with and without intrauterine exposure to GDM, and to examine the associations of gut hormones with children’s usual energy intake. Although there were no between-group differences in ghrelin, the postprandial response of GLP-1 was suppressed, and fasting PYY concentrations were higher, among OGD versus CTRL. Furthermore, there was some evidence that postprandial ghrelin and fasting PYY may be associated with energy intake among only OGD, but these results should be viewed with caution given that tests to examine whether the association of each gut hormone with children’s energy intake differed by group failed to reach statistical significance. Together, these results suggest that intrauterine exposure to GDM may result in changes to the gut hormone profile, with possible implications for the regulation of energy intake.
Consistent with previous research (5), circulating ghrelin concentrations dropped following the meal challenge, and although there was a trend for this suppression to be greater among OGD versus CTRL, the between group difference failed to reach statistical significance. Results of exploratory analyses also suggested that among OGD only, children with less postprandial ghrelin suppression may have greater free-living energy intake. It is not clear, however, why this pattern was only seen in the OGD group and it may simply be consequent to the trend for a greater overall meal response in this group. To our knowledge, only one previous study has compared ghrelin concentrations among offspring of diabetic versus non-diabetic mothers. In this previous study, ghrelin concentrations measured during the first 2 hours following birth and prior to feeding did not differ among neonates of women with diettreated GDM versus neonates of non-diabetic women (20). Although the ghrelin results shown in the current study are novel and are useful to generate hypotheses for future research, these findings need to be replicated in a larger sample to more adequately assess the influence of maternal GDM on children’s postprandial ghrelin response. It should also be noted that a limitation of the current study was that only total ghrelin was measured, as opposed to active ghrelin. Recent literature in this field suggests that active, not total, ghrelin, is more likely to differ among obese versus lean children (21-23). Unfortunately, no additional serum from this cohort was available with which to examine active ghrelin, but it would be of interest in future research to measure active ghrelin among OGD vs. CTRL children.
Both CTRL and OGD groups experienced a transient increase in GLP-1 following the meal; a pattern consistent with previous literature (12); but the magnitude of this increase was significantly greater among the CTRL versus OGD. Importantly, this difference was independent of the greater adiposity among those in those OGD group, suggesting that differences in GLP-1 response to a meal may exist prior to the onset of obesity among children exposed to GDM in utero. This pattern of suppressed GLP-1 response is of concern not only because of GLP-1’s role in inhibiting gastric emptying and promoting satiety (24), but also because GLP-1 stimulates glucose-dependent insulin secretion from pancreatic β-cells (25) and thereby plays an important role in glucose homeostasis. In previous research, low GLP-1 concentrations have been associated with relative insulin resistance among non-diabetic adults (26). Consequently, a chronic impairment of the GLP-1 response may contribute to the development of insulin resistance and ultimately type 2 diabetes among children of women with GDM. To our knowledge, this is the first time that GLP-1 concentrations have been examined among children of women with and without GDM, and it would be of great interest to replicate this study in a larger cohort, and also to examine whether GLP-1 function among children of women with GDM is compromised from birth.
Fasting PYY concentrations were significantly higher among the OGD versus CTRL groups, but the response of PYY to the meal did not differ. This result is contrary to the a priori hypothesis and is contradictory to studies among first degree relatives of type 2 diabetics, who exhibit low circulating PYY and a suppressed postprandial response (27). To our knowledge, this is the first time that such a result has been reported, but is similar in magnitude to the higher pancreatic polypeptide concentrations found among Pima Indians who are at significant risk for obesity and type 2 diabetes (28). PYY is a member of the pancreatic polypeptide family, and secretion of both PYY and pancreatic polypeptide increases following the ingestion of food. PYY, in its 36-amino-acid form, is secreted from intestinal L-cells (29) and then is cleaved in circulation to the 3-36 form by DPP-IV (30). Pancreatic polypeptide is secreted from the islets of Langerhans in the pancreas (31). Although there is some evidence that increased parasympathetic tone may contribute to hypersecretion of pancreatic polypeptide in Pima Indians (28), the origin of excess PYY among OGD is less clear. Given that neither PYY1-36 nor DPP-IV were measured in the current study, and have not, to our knowledge, been measured in other studies of offspring from diabetic versus non-diabetic women, it is not possible to speculate as to whether exaggerated secretion or excess cleaving of the PYY1-36 form is responsible for the higher PYY3-36 concentrations. There is some evidence however, to support the possibility that excess PYY is synthesized among OGD. In mice, treatment with insulin-like growth factor 1 increases intestinal PYY mRNA and content (32), and insulin-like growth factor 1 concentrations are higher both among neonates of women with GDM (33; 34), and among children of women with type 1 diabetes as compared to healthy controls (35). It would be of interest in future research to examine concentrations of growth factors and gut hormones in the same cohort of children with exposure to GDM.
Irrespective of the mechanism by which PYY concentrations are higher among OGD, the implications of this difference are of interest given the association of maternal diabetes and childhood obesity. Given that peripheral PYY slows gut motility and enhances satiety, high circulating PYY concentrations should protect against obesity. Indeed, PYY concentrations increase following gastric bypass surgery (36; 37), and there is evidence to suggest that this change in PYY is necessary for successful weight loss (36). Furthermore, in some (13; 38), but not all (39) studies, PYY treatment has been shown to suppress intake and facilitate weight loss. Aside from its effect to slow gastrointestinal motility, PYY crosses the blood brain barrier and may have either anorectic or orexigenic effects in the brain, depending upon the site of action. In animal models, PYY infused into the arcuate nucleus of the hypothalamus suppresses energy intake (40), while PYY infused into the ventricles or paraventricular nucleus of the hypothalamus increases energy intake (41; 42), and is a particularly potent stimulator of reward-related feeding (43). Given that evidence from animal models suggests that hypothalamic satiety signaling may be compromised among offspring of diabetic mothers (44), it is possible that the ability of PYY to signal satiety in the arcuate nucleus is impaired among OGD. It is not clear from animal model research whether hedonic regulation of feeding remains intact among OGD. With the positive association of PYY with total energy intake among only the OGD children in this study, it is tempting to hypothesize that high circulating PYY may be driving hedonic feeding, against a backdrop of inadequate homeostatic regulation of feeding. Although there is little evidence at the current time to support this hypothesis, one functional neuroimaging study among healthy adults found that hypothalamic activation predicted energy intake when circulating PYY concentrations were low, while activity in the orbitofrontal region (involved in hedonic regulation) predicted energy intake when circulating PYY concentrations were high (45). Taken together with previous research, data from the current study suggest that a more thorough examination of the activity of PYY, and its function in feeding behavior, could uncover important differences among OGD versus CTRL.
Strengths of this study included the comprehensive examination of gut hormone and insulin secretion profile during a standardized meal test, along with the assessment of children’s body composition, resting energy expenditure, and their usual energy intake. The study was limited by the small sample size, particularly for the PYY measures, which emphasizes the need to interpret the PYY results with caution until they are replicated in a larger sample. The study was also limited by the lack of data regarding maternal weight status during pregnancy which may have influenced the children’s outcomes.
To conclude, results of this study contribute novel information to the literature regarding consequences of intrauterine exposure to GDM. Specifically, GDM-exposed children had significantly greater circulating PYY concentrations that were positively associated with their usual energy intake, and a trend towards a suppressed GLP-1 response to a meal that was independent of their greater adiposity. Although more research is needed to confirm these findings and to understand the mechanisms that underlie these differences, the results imply that subtle abnormalities in the activity of gut hormones may contribute to obesity among children exposed to GDM in utero.
ACKNOWLEDGEMENTS
This work was supported by the Thrasher Research Fund (NR-0025) and the National Institutes of Health (F32 DK-082028, UL-1RR025777, P30 DK-056336, P60 DK-079626). The authors thank Rachel Copper and Mickey Parks from the University of Alabama (UAB) Center for Women’s Reproductive Health for administrative, nursing, and data collection support. The authors also thank Maryellen Williams and Cindy Zeng from the Core laboratory of the UAB Diabetes Research and Training Center, the Nutrition and Obesity Research Center, and the Center for Clinical and Translational Science, for conducting the laboratory analyses, and Dr. Robert Oster of the Biostatistics Core of the Center for Clinical and Translational Science for assistance with the data analyses.
Funding: This work was supported by the Thrasher Research Fund (NR-0025) and the National Institutes of Health (F32 DK-082028, UL-1RR025777, P30 DK-056336, P60 DK-079626).
Footnotes
Disclosure summary: The authors have nothing to disclose.
REFERENCES
- 1.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 2.Chandler-Laney P, Bush N, Granger W, Rouse D, Mancuso M, Gower B. Overweight status and intrauterine exposure to gestational diabetes are associated with children’s metabolic health. Pediatric Obesity. 2012;7:44–52. doi: 10.1111/j.2047-6310.2011.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics. 2003;111:e221–226. doi: 10.1542/peds.111.3.e221. [DOI] [PubMed] [Google Scholar]
- 4.Wiedmer P, Nogueiras R, Broglio F, D’Alessio D, Tschöp MH. Ghrelin, obesity and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:705–712. doi: 10.1038/ncpendmet0625. [DOI] [PubMed] [Google Scholar]
- 5.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 6.Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 7.English PJ, Ghatei MA, Malik IA, Bloom SR, Wilding JP. Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metab. 2002;87:2984. doi: 10.1210/jcem.87.6.8738. [DOI] [PubMed] [Google Scholar]
- 8.Lomenick J, Clasey J, Anderson J. Meal-related changes in ghrelin, peptide YY, and appetite in normal weight and overweight children. Obesity (Silver Spring) 2008;16:547–552. doi: 10.1038/oby.2007.129. [DOI] [PubMed] [Google Scholar]
- 9.Stock S, Leichner P, Wong AC, et al. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab. 2005;90:2161–2168. doi: 10.1210/jc.2004-1251. [DOI] [PubMed] [Google Scholar]
- 10.Chanoine JP, Mackelvie KJ, Barr SI, Wong AC, Meneilly GS, Elahi DH. GLP-1 and appetite responses to a meal in lean and overweight adolescents following exercise. Obesity (Silver Spring) 2008;16:202–204. doi: 10.1038/oby.2007.39. [DOI] [PubMed] [Google Scholar]
- 11.Tomasik PJ, Sztefko K, Malek A. GLP-1 as a satiety factor in children with eating disorders. Horm Metab Res. 2002;34:77–80. doi: 10.1055/s-2002-20519. [DOI] [PubMed] [Google Scholar]
- 12.Verdich C, Toubro S, Buemann B, Lysgård Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety--effect of obesity and weight reduction. Int J Obes Relat Metab Disord. 2001;25:1206–1214. doi: 10.1038/sj.ijo.0801655. [DOI] [PubMed] [Google Scholar]
- 13.Batterham R, Cohen M, Ellis S, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 14.le Roux CW, Batterham RL, Aylwin SJ, et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147:3–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- 15.Higgins P, Fernández J, Goran M, Gower B. Early ethnic difference in insulin-like growth factor-1 is associated with African genetic admixture. Pediatr Res. 2005;58:850–854. doi: 10.1203/01.PDR.0000182583.92130.08. [DOI] [PubMed] [Google Scholar]
- 16.Johnson RK, Driscoll P, Goran MI. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. J Am Diet Assoc. 1996;96:1140–1144. doi: 10.1016/S0002-8223(96)00293-3. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd B, Ravi P, Mendes N, Klibanski A, Misra M. Peptide YY levels across pubertal stages and associations with growth hormone. J Clin Endocrinol Metab. 2010;95:2957–2962. doi: 10.1210/jc.2009-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellone S, Prodam F, Savastio S, et al. Acylated and unacylated ghrelin levels in normal weight and obese children: influence of puberty and relationship with insulin, leptin and adiponectin levels. J Endocrinol Invest. 2011 doi: 10.3275/7761. [DOI] [PubMed] [Google Scholar]
- 19.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng PC, Lee CH, Lam CW, Wong E, Chan IH, Fok TF. Plasma ghrelin and resistin concentrations are suppressed in infants of insulin-dependent diabetic mothers. J Clin Endocrinol Metab. 2004;89:5563–5568. doi: 10.1210/jc.2004-0736. [DOI] [PubMed] [Google Scholar]
- 21.Mittelman SD, Klier K, Braun S, Azen C, Geffner ME, Buchanan TA. Obese adolescents show impaired meal responses of the appetite-regulating hormones ghrelin and PYY. Obesity (Silver Spring) 2010;18:918–925. doi: 10.1038/oby.2009.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misra M, Tsai PM, Mendes N, Miller KK, Klibanski A. Increased carbohydrate induced ghrelin secretion in obese vs. normal-weight adolescent girls. Obesity (Silver Spring) 2009;17:1689–1695. doi: 10.1038/oby.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maffeis C, Surano MG, Cordioli S, Gasperotti S, Corradi M, Pinelli L. A high-fat vs. a moderate-fat meal in obese boys: nutrient balance, appetite, and gastrointestinal hormone changes. Obesity (Silver Spring) 2010;18:449–455. doi: 10.1038/oby.2009.271. [DOI] [PubMed] [Google Scholar]
- 24.Wettergren A, Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ. Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci. 1993;38:665–673. doi: 10.1007/BF01316798. [DOI] [PubMed] [Google Scholar]
- 25.Nauck MA, Bartels E, Orskov C, Ebert R, Creutzfeldt W. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J Clin Endocrinol Metab. 1993;76:912–917. doi: 10.1210/jcem.76.4.8473405. [DOI] [PubMed] [Google Scholar]
- 26.Rask E, Olsson T, Söderberg S, et al. Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care. 2001;24:1640–1645. doi: 10.2337/diacare.24.9.1640. [DOI] [PubMed] [Google Scholar]
- 27.Viardot A, Heilbronn LK, Herzog H, Gregersen S, Campbell LV. Abnormal postprandial PYY response in insulin sensitive nondiabetic subjects with a strong family history of type 2 diabetes. Int J Obes (Lond) 2008;32:943–948. doi: 10.1038/ijo.2008.24. [DOI] [PubMed] [Google Scholar]
- 28.Weyer C, Salbe AD, Lindsay RS, Pratley RE, Bogardus C, Tataranni PA. Exaggerated pancreatic polypeptide secretion in Pima Indians: can an increased parasympathetic drive to the pancreas contribute to hyperinsulinemia, obesity, and diabetes in humans? Metabolism. 2001;50:223–230. doi: 10.1053/meta.2001.20170. [DOI] [PubMed] [Google Scholar]
- 29.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89:1070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- 30.Grandt D, Schimiczek M, Beglinger C, et al. Two molecular forms of peptide YY (PYY) are abundant in human blood: characterization of a radioimmunoassay recognizing PYY 1-36 and PYY 3-36. Regul Pept. 1994;51:151–159. doi: 10.1016/0167-0115(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 31.Larsson LI, Sundler F, Håkanson R. Immunohistochemical localization of human pancreatic polypeptide (HPP) to a population of islet cells. Cell Tissue Res. 1975;156:167–171. doi: 10.1007/BF00221800. [DOI] [PubMed] [Google Scholar]
- 32.Lee HM, Udupi V, Englander EW, Rajaraman S, Coffey RJ, Greeley GH. Stimulatory actions of insulin-like growth factor-I and transforming growth factor-alpha on intestinal neurotensin and peptide YY. Endocrinology. 1999;140:4065–4069. doi: 10.1210/endo.140.9.6969. [DOI] [PubMed] [Google Scholar]
- 33.Grissa O, Yessoufou A, Mrisak I, et al. Growth factor concentrations and their placental mRNA expression are modulated in gestational diabetes mellitus: possible interactions with macrosomia. BMC Pregnancy Childbirth. 2010;10:7. doi: 10.1186/1471-2393-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindsay RS, Westgate JA, Beattie J, et al. Inverse changes in fetal insulin-like growth factor (IGF)-1 and IGF binding protein-1 in association with higher birth weight in maternal diabetes. Clin Endocrinol (Oxf) 2007;66:322–328. doi: 10.1111/j.1365-2265.2006.02719.x. [DOI] [PubMed] [Google Scholar]
- 35.Manderson JG, Mullan B, Patterson CC, Hadden DR, Traub AI, McCance DR. Cardiovascular and metabolic abnormalities in the offspring of diabetic pregnancy. Diabetologia. 2002;45:991–996. doi: 10.1007/s00125-002-0865-y. [DOI] [PubMed] [Google Scholar]
- 36.le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beckman LM, Beckman TR, Earthman CP. Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass procedure: a review. J Am Diet Assoc. 2010;110:571–584. doi: 10.1016/j.jada.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 39.Tschöp M, Castañeda TR, Joost HG, et al. Physiology: does gut hormone PYY3-36 decrease food intake in rodents? Nature. 2004;430:1. doi: 10.1038/nature02665. following 165; discussion 162 p following 165. [DOI] [PubMed] [Google Scholar]
- 40.Batterham R, Cowley M, Small C, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 41.Morley JE, Levine AS, Grace M, Kneip J. Peptide YY (PYY), a potent orexigenic agent. Brain Res. 1985;341:200–203. doi: 10.1016/0006-8993(85)91490-8. [DOI] [PubMed] [Google Scholar]
- 42.Corp ES, Melville LD, Greenberg D, Gibbs J, Smith GP. Effect of fourth ventricular neuropeptide Y and peptide YY on ingestive and other behaviors. Am J Physiol. 1990;259:R317–323. doi: 10.1152/ajpregu.1990.259.2.R317. [DOI] [PubMed] [Google Scholar]
- 43.Hagan MM, Moss DE. Effect of peptide YY (PYY) on food-associated conflict. Physiol Behav. 1995;58:731–735. doi: 10.1016/0031-9384(95)00100-w. [DOI] [PubMed] [Google Scholar]
- 44.Franke K, Harder T, Aerts L, et al. ‘Programming’ of orexigenic and anorexigenic hypothalamic neurons in offspring of treated and untreated diabetic mother rats. Brain Res. 2005;1031:276–283. doi: 10.1016/j.brainres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Batterham R, Ffytche D, Rosenthal J, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]