Abstract
Smooth muscle cells are ultimately responsible for determining vascular luminal diameter and blood flow. Dynamic changes in intracellular calcium are a critical mechanism regulating vascular smooth muscle contractility. Processes influencing intracellular calcium are therefore important regulators of vascular function with physiological and pathophysiological consequences. In this review we discuss the major dynamic calcium signals identified and characterized in vascular smooth muscle cells. These signals vary with respect to their mechanisms of generation, temporal properties, and spatial distributions. The calcium signals discussed include calcium waves, junctional calcium transients, calcium sparks, calcium puffs, and L-type calcium channel sparklets. For each calcium signal we address underlying mechanisms, general properties, physiological importance, and regulation.
Introduction
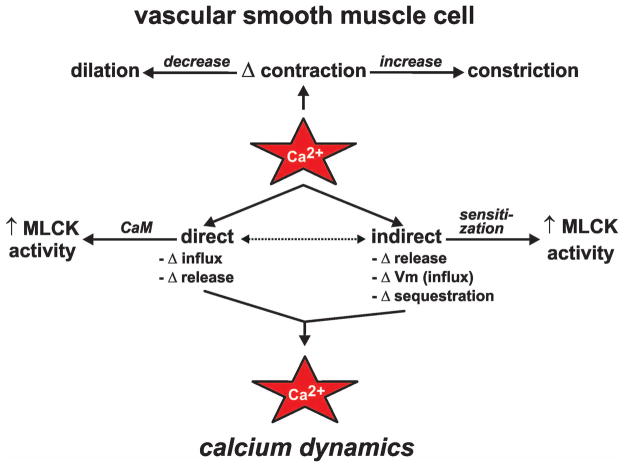
The otherwise unassuming inorganic divalent cation calcium (Ca2+) is the most important signaling molecule in mammalian cells. Cellular responses to changes in intracellular Ca2+ ([Ca2+]i) often underlie our definition of what a given cell “is” (22). For example, following an increase in [Ca2+]i, endocrine cells secrete hormones and neurons release neurotransmitters. Muscle cells, including vascular smooth muscle cells, generally contract when [Ca2+]i rises. Ca2+ induces contraction in these cells by complexing with the ubiquitous Ca2+ binding protein calmodulin and subsequently increasing the activity of myosin light-chain kinase (MLCK; see Figure 1). Changes in the contractile state of vascular smooth muscle either increases or decreases vascular diameter, which in turn increases or decreases blood flow through the vessel and subsequently the vascularized tissue. Thus, events which influence [Ca2+]i are critical regulators of vascular function with clear physiological (and pathophysiological) ramifications.
Figure 1. Overview of calcium dynamics in vascular smooth muscle.
Ca2+ influences the state of vascular smooth muscle contraction directly and indirectly. Direct mechanisms include changes (Δ) in Ca2+ influx and release from intracellular Ca2+ stores leading to increased Ca2+ within the cell, binding of Ca2+ to calmodulin (CaM), and activation of myosin light chain kinase (MLCK). Indirect mechanisms include changes in Ca2+ influx via alterations in plasma membrane potential (Vm), Ca2+ release from intracellular stores, Ca2+ sequestration, and Ca2+ sensitization of the contractile apparatus.
Due to the importance of Ca2+, it is not unexpected that changes in vascular smooth muscle [Ca2+]i (i.e., “Ca2+ dynamics”) are governed by a complex array of cellular processes. Indeed, to arrive at the appropriate physiological response, vascular smooth muscle cells must integrate multiple signaling events that influence [Ca2+]i either directly or indirectly (see Figure 1). In this paper we provide an overview of the major Ca2+ signals that contribute to changes in vascular smooth muscle [Ca2+]i. Note that many excellent and more extensive reviews on the individual topics discussed here are readily found in the published literature.
Calcium signals in vascular smooth muscle
Sources of Ca2+ available for cytoplasmic signaling in vascular smooth muscle cells include Ca2+ influx through ion-permeable channels located in the plasma membrane and Ca2+ release from intracellular Ca2+ stores (e.g., the sarcoplasmic reticulum). Ca2+ influx in vascular smooth muscle is mediated primarily by voltage-dependent L-type Ca2+ channels (39) with contributions from other channels including but not limited to voltage-dependent T-type Ca2+ channels, Ca2+-permeable members of the transient receptor potential (TRP) superfamily of cation channels, and Ca2+-permeable ligand-gated cation channels. Ca2+ release from intracellular stores is mediated by two types of Ca2+-permeable ion channels located in sarcoplasmic reticular membranes: 1) Ryanodine receptors and 2) inositol 1,4,5-trisphosphate (IP3) receptors.
Cytoplasmic signals produced by these Ca2+ fluxes can be loosely classified as either more or less localized with respect to total cell volume (see Figure 2). Here we have grouped the “less than global” subcellular Ca2+ signals observed in vascular smooth muscle into five categories: 1) Ca2+ waves, 2) junctional Ca2+ transients, 3) Ca2+ sparks, 4) Ca2+ puffs, and 5) L-type Ca2+ channel sparklets. Note that IP3-receptor-mediated Ca2+ puffs have not been definitively isolated and visualized in vascular smooth muscle cells to the best of our knowledge. This non-arbitrary categorization of subcellular Ca2+ signaling events is based on a wealth of experimental evidence obtained by numerous research groups over the past few decades. The general biophysical characteristics (when known) of each of these subcellular Ca2+ signals is presented in Table 1.
Figure 2. Overview of calcium signaling events in vascular smooth muscle.
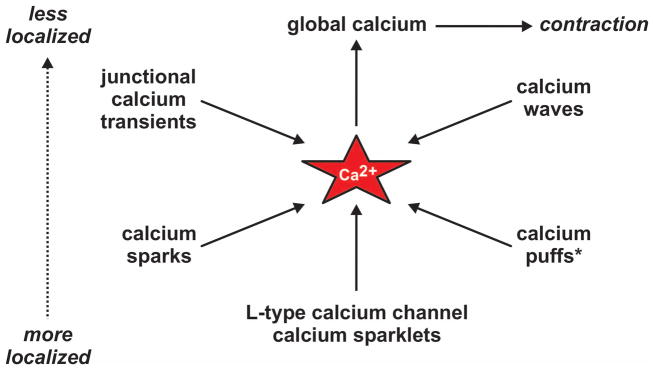
Discrete subcellular Ca2+ signaling events (junctional Ca2+ transients, Ca2+ waves, Ca2+ sparks, Ca2+ puffs, and Ca2+ sparklets) can be classified as either more or less localized with respect to total cell volume. * Direct visualization of Ca2+ puffs in arterial smooth muscle is lacking; however, abundant indirect evidence supports their existence.
Table 1.
Properties of vascular smooth muscle subcellular Ca2+ signals (approximate values). NA = not available.
In contrast to increased global [Ca2+]i, which is invariably associated with contraction (29), the role of specific subcellular Ca2+ signals is not always apparent. For instance, while Ca2+ events such as junctional Ca2+ transients are inherently contractile (32, 63, 65), Ca2+ microdomains such as Ca2+ sparks may induce vasodilation (see below; (46)). This highlights the complexity of Ca2+ dynamics in vascular smooth muscle and the need for further investigation of subcellular Ca2+ signaling in these cells.
Calcium waves
As suggested by the moniker, Ca2+ waves are propagating [Ca2+]i elevations produced by sequential series of Ca2+ release events from the sarcoplasmic reticulum that extend from one end of the cell to the other (23, 38, 66). Ca2+ waves have been shown to be produced by Ca2+ release via IP3 and ryanodine receptors located in the sarcoplasmic reticulum (20, 26). By their nature Ca2+ waves inherently require a regenerative Ca2+ release mechanism that promotes propagation of the wavefront. This is thought to occur by a Ca2+-induced Ca2+-release (CICR) mechanism between adjacent IP3 and/or ryanodine receptors (7, 9, 25, 26). A schematic representation of an IP3 receptor-dependent Ca2+ wave is shown in Figure 3. In this scenario, the Ca2+ wave is initiated by localized SR Ca2+ release via IP3-dependent opening of IP3 receptors. A self-perpetuating Ca2+ wave arises when the initiating Ca2+ induces Ca2+-dependent opening of adjacent IP3 receptors (i.e., Ca2+-induced Ca2+-release). Ca2+ release via ryanodine receptors has also been implicated in Ca2+ wave generation via frequent discharge sites in rat and rabbit portal vein (7, 20, 64). In addition, application of the alkaloid ryanodine eliminated Ca2+ waves in pressurized rat cerebral arteries (25, 62). This suggests that in some arterial smooth muscle cells Ca2+ waves involve SR Ca2+ release via ryanodine receptors.
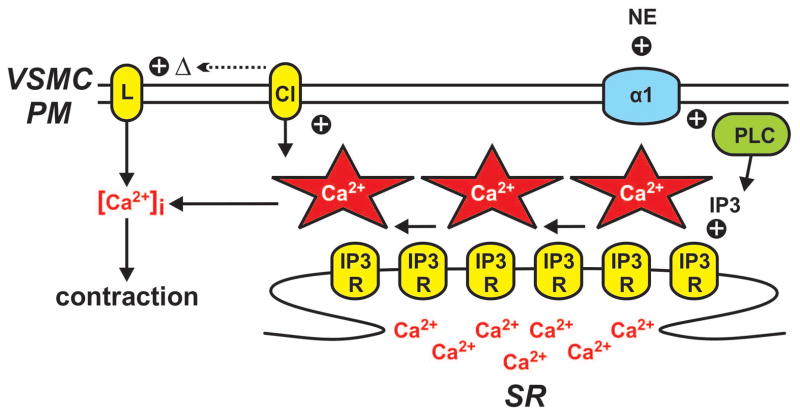
Figure 3. Vascular smooth muscle calcium waves.
Norepinephrine (NE)-dependent stimulation of α1 adrenergic receptors (α1R) on the vascular smooth muscle cell plasma membrane (VSMC PM) leads to activation of phospholipase C (PLC) and the production of inositol 1,4,5-trisphosphate (IP3). IP3 in turn promotes the opening of IP3 receptors (IP3R) on the sarcoplasmic reticulum (SR) resulting in a propagating series of Ca2+ release events (i.e., a Ca2+ wave). Ca2+ waves increase contraction directly by elevating global intracellular Ca2+ ([Ca2+]i). Ca2+ waves also modulate contraction indirectly by altering plasma membrane potential (and thus voltage-dependent Ca2+ influx through Cav1.2 L-type Ca2+ channels) by stimulating plasmalemmal Ca2+-activated chloride channels.
The occurrence of Ca2+ waves in excised vessel preparations is variable to moderate under resting conditions. For example, smooth muscle cells in mouse cremaster feed arteries (62) and rat retinal arterioles exhibit moderate basal Ca2+ wave activity (56) while mouse and rat mesenteric arterial smooth muscle cells often possess minimal Ca2+ wave activity in the absence of stimulation (38, 65). The occurrence of basal Ca2+ waves appears to be dependent on phospholipase C (PLC) activity (62). Consistent with a role of PLC in regulating Ca2+ waves, increased production of IP3 by PLC following stimulation of α1 adrenergic receptors by norepinephrine in mesenteric arteries (31) and ETA receptors by endothelin-1 in retinal arterioles (56) increases Ca2+ wave frequency.
Ca2+ waves are postulated to contract vascular smooth muscle by at least two distinct mechanisms (21). Ca2+ released into the cytosol during the wave event can contribute to global Ca2+ thus increasing MLCK activity and promoting contraction (33). This is perhaps the most common interpretation of Ca2+ wave function. However, in contrast to this direct mechanism, Ca2+ waves can also influence contraction indirectly by interacting with and changing the activity of plasmalemmal Ca2+-activated ion channels. Ca2+ waves in rat retinal arteriole smooth muscle cells elicit Ca2+-activated chloride (ClCa) currents (56). Stimulation of Ca2+-activated chloride channels results in a depolarization of the plasma membrane via Cl− efflux (an inward current by convention; see Figure 3), thus enhancing voltage-dependent L-type Ca2+ channel activity, and consequently, Ca2+ influx, global [Ca2+]i, and ultimately contraction.
As noted above, Ca2+ waves are associated with increased vascular smooth muscle contraction in excised vessels. For example, in rat mesenteric arteries, asynchronous Ca2+ waves (with respect to adjacent smooth muscle cells) following neurogenic activation of α1 receptors by norepinephrine contribute to gradually-developing contractions (31). Contractile responses to bath applied α1 agonists (e.g., phenylephrine) also include a rapid initial component due to synchronous Ca2+ wave activity. This is likely an experimental artifact resulting from strong uniform (i.e., non-physiological) activation of α1 receptors on a large population of smooth muscle cells (31, 67). Despite a substantial body of experimental data, the physiological significance of Ca2+ waves is poorly understood. For example, Ca2+ waves in mouse mesenteric arteries appear to cease following the development of tone (65) and evidence suggests that Ca2+ waves, which are readily observed in numerous excised vessels (as described above), are not apparent in corresponding in vivo experiments (36). Thus, much work is required to understand the physiological role of Ca2+ waves in the vasculature.
Junctional Calcium Transients
Short-lived Ca2+ influx events evoked by neural (i.e., sympathetic) stimulation of vascular smooth muscle cells are defined as junctional Ca2+ transients (34, 63). In contrast to Ca2+ waves, junctional Ca2+ transients remain localized, as they do not possess a regenerating or propagating mechanism. Action potentials arriving at perivascular sympathetic nerve terminals culminate in release of neurotransmitters, including ATP and norepinephrine. ATP activates postjunctional vascular smooth muscle P2X1 purinergic receptors and norepinephrine stimulates α1 adrenergic receptors giving rise to Ca2+ waves as described above (31, 32, 35) (see Figure 4).
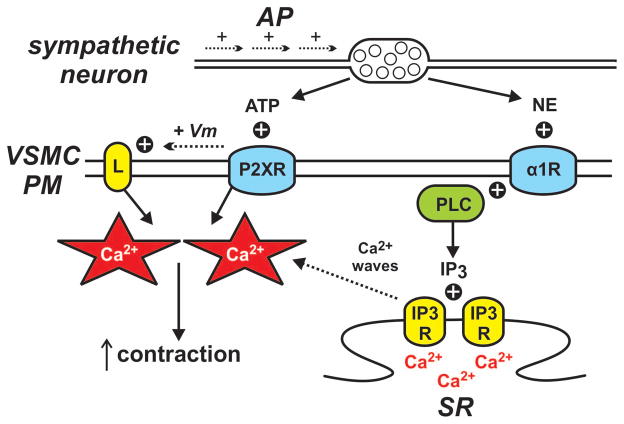
Figure 4. Vascular smooth muscle junctional calcium transients.
Neuronal action potentials (AP) promote the release of ATP and norepinephrine (NE) from perivascular nerve terminals. Norepinephrine stimulates α1 adrenergic receptors (α1R) giving rise to Ca2+ waves as described in Figure 3 while ATP opens Ca2+-permeable purinergic receptors (P2XR) on the vascular smooth muscle cell plasma membrane (VSMC PM) producing localized junctional Ca2+ transients. Purinergic receptor activation also leads to plasma membrane depolarization (+ Vm) and increased opening of voltage-dependent Cav1.2 L-type Ca2+ channels (L). Ca2+ influx from junctional Ca2+ transients and L-type Ca2+ channels summate to increase contraction.
P2X1 receptors are ligand-gated Ca2+-permeable nonselective cation channels (16). In vascular beds innervated by the sympathetic nervous system (e.g., mesenteric arteries), stimulation of vascular smooth muscle P2X1 receptors by ATP generates localized junctional Ca2+ transients on account of the relatively high Ca2+ permeability of these channels (32, 34). Temporally mirroring the kinetics of the junctional Ca2+ transients, the Ca2+ influx associated with these events induces a rapid but brief increase in contraction (31, 32). Non-neuronal stimulation of P2X1 receptors has also been described. In mouse renal afferent arterioles, increasing intraluminal pressure promotes local release of ATP, stimulation of smooth muscle P2X1 receptors, and contraction (24). This paracrine signaling mechanism is thought to contribute to autoregulation of blood flow in the kidney.
In addition to evoking local junctional Ca2+ transients, activation of P2X1 receptors by ATP induces depolarizing excitatory junctional potentials as a result of Na+ and (to a lesser extent) Ca2+ influx (16, 67). Depolarization increases the opening of L-type Ca2+ channels resulting in increased Ca2+ influx and elevated global [Ca2+]i. Thus, stimulation of P2X1 receptors with ATP increases vascular smooth muscle Ca2+ influx by two independent mechanisms: 1) Local junctional Ca2+ transients via Ca2+ entry through the P2X1 receptors and 2) non-localized Ca2+ influx through L-type Ca2+ channels following depolarizing excitatory junction potentials. These two complimentary Ca2+ influx mechanisms summate to underlie the initial component of sympathetic-mediated contraction of rat mesenteric arteries (67).
Calcium Sparks
Ca2+ sparks are localized Ca2+ microdomains produced specifically by the opening of the ryanodine receptor class of intracellular Ca2+ release channels located in sarcoplasmic reticular membranes (12). In vascular smooth muscle Ca2+ sparks have minimal direct impact on global [Ca2+]i and contraction as a consequence of their limited spatial spread (46). However, Ca2+ sparks can have substantial influence on global [Ca2+]i and contraction through indirect mechanisms. Accordingly, regions of sarcoplasmic reticulum are often found in close juxtaposition to the plasma membrane of vascular smooth muscle cells (≈10–20 nm; (14, 28, 57)). As noted above, Ca2+-activated plasmalemmal ion channels (e.g., KCa and ClCa) are expressed in various vascular smooth muscle cells. Ca2+ sparks occurring in close proximity to these channels can increase local [Ca2+]i to levels sufficient (i.e., μM; (48)) for channel activation (see Figure 5). Thus, the contractile response of vascular smooth muscle to Ca2+ sparks depends on the expression and coupling of the sparks to Ca2+-activated plasmalemmal ion channels.
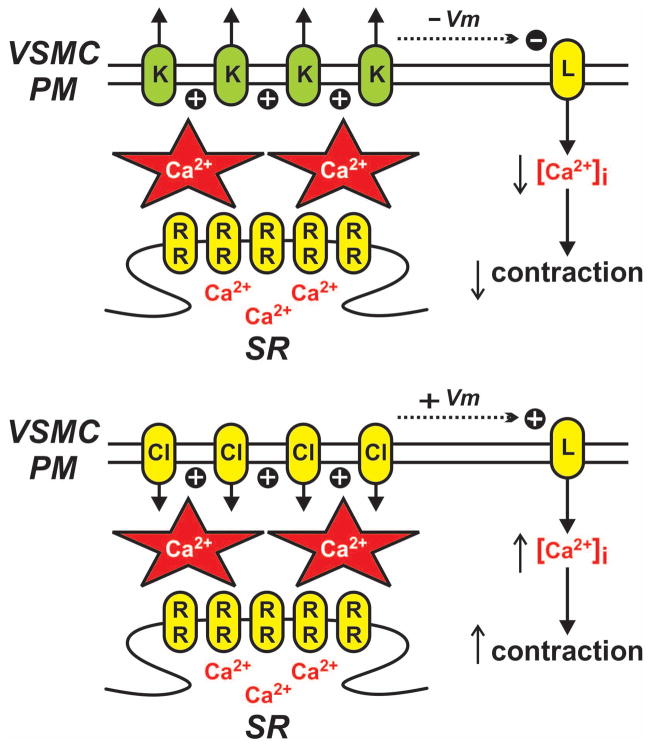
Figure 5. Vascular smooth muscle calcium sparks.
Ca2+-permeable ryanodine receptors (RR) on the sarcoplasmic reticulum (SR) produce highly localized sites of elevated Ca2+ (i.e., Ca2+ sparks). Ca2+ sparks near the vascular smooth muscle cell plasma membrane (VSMC PM) increase the activity of plasmalemmal ion channels including Ca2+ activated K+ (K) and Ca2+-activated Cl− (Cl) channels. Ca2+ spark-dependent activation of K+ channels generates hyperpolarizing outward currents (via K+ efflux) resulting in decreased opening of voltage-dependent Cav1.2 L-type Ca2+ channels (L) and decreased contraction. Conversely, Ca2+ spark-dependent activation of Cl− channels generates depolarizing (+ Vm) inward currents (via Cl− efflux) resulting in increased opening of L-type Ca2+ channels and increased contraction.
In rat and mouse cerebral arteries, large-conductance, Ca2+-activated K+ (BK; maxi K) channels are a primary KCa target for Ca2+ sparks (27, 46). Ca2+ spark activation of BK channels results in a stereotypical temporal pattern of hyperpolarizing K+ currents called spontaneous transient outward currents (STOCs) (6, 46). Simultaneous recording of intracellular Ca2+ and membrane potential has provided definitive evidence that Ca2+ sparks give rise to STOCs as the two events correlate not only in time but also in magnitude (1, 10, 46, 48). In rat cerebral arteries elimination of Ca2+ sparks eliminates contractile responses to BK channel inhibition (30, 46) thus indicating that Ca2+ sparks are the physiological activators of BK channels in these vascular smooth muscle cells.
In rabbit portal vein, Ca2+ sparks are coupled to ClCa channels (59). In contrast to Ca2+ spark activation of BK channels, which results in hyperpolarization, Ca2+ spark activation of Ca2+-activated Cl− channels causes plasma membrane depolarizing outward currents (via Cl− efflux; see Figure 5). Consequently, similar to Ca2+-activated K+ channels, activation of Ca2+-activated Cl− channels by Ca2+ sparks produces a signature pattern of inward current called spontaneous transient inward currents (STICs) that correlate in time and magnitude with each other. Depolarization induced by Ca2+-activated Cl− channel activity results in activation of voltage dependent L-type Ca2+ channels and increased contraction (see Figure 5) (51).
The dependence of Ca2+-activated ion channels on underlying Ca2+ sparks provides an opportunity for regulation of vascular smooth muscle function by vasodilators and vasoconstrictors. In rat cerebral arteries, vasodilators increase the hyperpolarizing influence of BK channels by increasing the sensitivity of the BK channels to Ca2+ and by increasing Ca2+ spark (thus STOC) frequency (49, 54). These effects are mediated by activation of adenosine 3′, 5′-cyclic monophosphate (cAMP)/protein kinase A (PKA) and guanosine 3′,5′-cyclic monophosphate (cGMP)/protein kinase G (PKG) signaling cascades. Thus, vasodilators can increase BK channel activity by increasing the coupling strength between the channel and the Ca2+ sparks (increasing STOC amplitude) and increasing the occurrence of Ca2+ sparks (increasing STOC frequency). Conversely, vasoconstrictors associated with protein kinase C (PKC) decrease the hyperpolarizing influence of BK channels by decreasing BK channel sensitivity to Ca2+ (reducing STOC amplitude) and by reducing the occurrence of Ca2+ sparks (reducing STOC frequency) (8, 54).
The coupling strength between Ca2+ sparks and BK channels is also associated with vascular dysfunction during diseases such as hypertension. The sensitivity of vascular smooth muscle BK channels to Ca2+ is greatly dependent on expression of BK channel β1 subunits (10). Genetic ablation of the β1 subunit decreases the sensitivity of cerebral arterial smooth muscle BK channels to Ca2+. As a consequence, Ca2+ sparks in these cells do not efficiently generate corresponding STOCs (10). This results in increased contraction of excised cerebral arterial segments and increased mean arterial blood pressure. Similarly, in rat models of hypertension, the function and expression of BK channel β1 subunits in cerebral arteries is decreased and contributes to vascular dysfunction (1, 4).
Calcium Puffs
Ca2+ puffs are localized Ca2+ microdomains produced by the opening of sarcoplasmic reticulum IP3 receptors (58). Although Ca2+ puffs have been observed in colonic smooth muscle cells (5), they have not been visualized in vascular smooth muscle cells. However, indirect experimental evidence in rat cerebral arterial smooth muscle cells indicates that physiologically-relevant localized IP3 receptor-dependent Ca2+ release does occur (17, 18). Indeed, in these smooth muscle cells, IP3-dependent Ca2+ release has been shown to promote the opening of transient receptor potential melastatin 4 (TRPM4) channels. Opening of these Ca2+-activated, Na+-permeable cation channels causes arterial smooth muscle membrane depolarization, opening of voltage-dependent L-type Ca2+ channels, Ca2+ influx, and ultimately contraction (see Figure 6) (17–19). A more detailed explanation of these findings can be found in the accompany paper by Gonzales and Earley in this special issue of Microcirculation.
Figure 6. Localized IP3 receptor-dependent SR Ca2+ release in vascular smooth muscle.
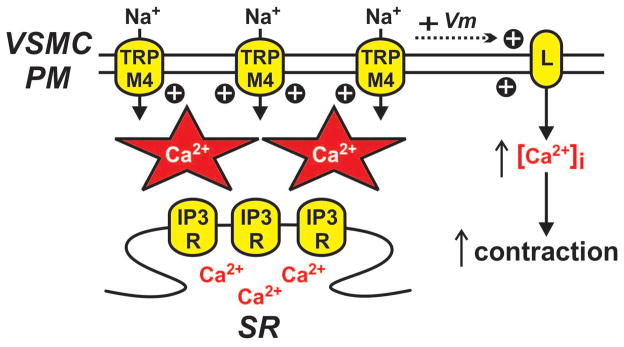
Ca2+-permeable inositol 1,4,5-trisphosphate receptors (IP3R) on the sarcoplasmic reticulum (SR) can produce non-propagating localized sites of elevated Ca2+. IP3 receptor Ca2+ release events near the vascular smooth muscle cell plasma membrane (VSMC PM) increase the activity of plasmalemmal ion channels such as transient receptor potential melastatin 4 (TRPM4). Ca2+ puff-dependent activation of TRPM4 channels generates depolarizing (+ Vm) inward currents (via Na+ influx) resulting in increased opening of voltage-dependent Cav1.2 L-type Ca2+ channels (L) and increased contraction.
Calcium Sparklets
Ca2+ sparklets are distinct Ca2+ microdomains produced by plasmalemmal Ca2+-permeable channels. Despite the name, Ca2+ sparklets are not small Ca2+ sparks. Ca2+ sparklets arise from Ca2+ influx through plasmalemmal ion channels (see below) and (as discussed above) Ca2+ sparks arise from the release of Ca2+ from the sarcoplasmic reticulum through ryanodine receptors (see Figure 7).
Figure 7. Vascular smooth muscle L-type calcium channel calcium sparklets.
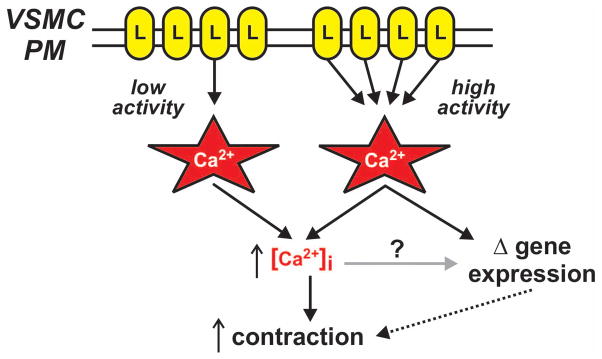
Cav1.2 L-type Ca2+ channels (L) in the vascular smooth muscle cell plasma membrane (VSMC PM) produce highly localized Ca2+ influx events (i.e., Ca2+ sparklets). Brief stochastic opening of single L-type Ca2+ channels produce low activity Ca2+ sparklet sites while prolonged opening of one or more clustered L-type Ca2+ channels produce high activity Ca2+ sparklet sites. Ca2+ influx from low and high activity Ca2+ sparklet sites contribute to global intracellular Ca2+ ([Ca2+]i) resulting in contraction. Elevated Ca2+ concentrations at high activity Ca2+ sparklet sites (and perhaps global intracellular Ca2+ as well) contribute to changes in gene expression associated with increased contraction.
The original definition of a “Ca2+ sparklet” was a visualized Ca2+ influx event produced by a voltage-dependent L-type Ca2+ channel (60). Since that time, Ca2+ influx events produced by other Ca2+-permeable channels have been visualized and referred to as “Ca2+ sparklets” (e.g., (55). To remove ambiguity we suggest that that the term “Ca2+ sparklet” be refined to specifically designate the visualization of a Ca2+ influx event through a Ca2+-permeable plasmalemmal ion channel. The need for different terms denoting Ca2+ influx through different channels can then be eliminated by preceding “sparklets” with the name of the underlying channel (e.g., “L-type Ca2+ channel sparklets”, “TRPV4 sparklets”, etc…).
L-Type Calcium Channel Sparklets
Cav1.2 L-type Ca2+ channels are the main source of Ca2+ influx in vascular smooth muscle cells (39). Conventional electrophysiological recordings of steady-state L-type Ca2+ channel activity (50) provide no information with regard to potential spatial heterogeneity of L-type Ca2+ channel activity throughout the smooth muscle plasma membrane. However, when conventional electrophysiology and advanced Ca2+ imaging techniques (e.g., total internal reflection fluorescence (TIRF) microscopy) are used together this experimental limitation is overcome (41,60).
Using a combinatorial approach of electrophysiology and TIRF microscopy, Ca2+ influx through single L-type Ca2+ channels (i.e., L-type Ca2+ channel sparklets) is readily observed in smooth muscle cells isolated from rat and mouse cerebral and mesenteric arteries (2, 3, 40–42). Data from experiments using this approach have confirmed the importance of Cav1.2 channels in vascular smooth muscle and yielded unexpected information with regard to spatial organization and regulation of L-type Ca2+ channels (40, 41)). Previous conventional electrophysiological data suggested that, under steady-state conditions, Ca2+ influx in vascular smooth muscle cells was the end result of stochastic opening of L-type Ca2+ channels dispersed broadly throughout the plasma membrane (50). However, the exceptional temporal and spatial resolution provided by TIRF microscopy revealed that Ca2+ influx in vascular smooth muscle cells through L-type Ca2+ channels is not stochastic but rather segregates strikingly into sites of low and high activity (see Figure 7).
Consistent with conventional electrophysiological data, sites of low activity L-type Ca2+ channel influx arise from stochastic opening of randomly dispersed L-type Ca2+ channels. In contrast, sites of high activity L-type Ca2+ channel function result from non-stochastic, apparently coordinated opening of clustered channels (15, 43). High activity L-type Ca2+ channel sparklet sites require AKAP150-targeted kinase activity (i.e., PKC and PKA), account for approximately 50 % of the steady-state Ca2+ entry through L-type Ca2+ channels in isolated rat and mouse cerebral arterial smooth muscle cells. In addition, High activity L-type Ca2+ channel sparklets contribute to myogenic tone in mouse mesenteric arteries and are necessary for contractile responses of mouse mesenteric and rat cerebral arteries to angiotensin II (3, 11, 44, 45, 47). Microdomains of elevated Ca2+ generated by high-activity L-type Ca2+ channel sparklet sites also induce changes in gene expression via specific activation of the Ca2+/calcineurin/NFAT signaling pathway (47, 52, 53). A more detailed explanation of these concepts can be found in the accompany paper by Navedo and Amberg in this special issue of Microcirculation.
Other Considerations and Future Directions
The Ca2+ signals described above are defined by the molecular mechanisms underlying their initiation and are classified as different “less than global” Ca2+ events with unique properties. While mechanisms underlying Ca2+ entry into vascular smooth muscle cytosol clearly distinguish one Ca2+ signal from another, termination of these events is also critical. Ca2+ signals in vascular smooth muscle are terminated by no less than five distinct mechanisms: 1) Diffusion into the surrounding cytosol, 2) extrusion via the Na+/Ca2+ exchanger, 3) extrusion via the plasma membrane Ca2+-ATPase, 4) sequestration via the sarcoplasmic reticular Ca2+-ATPase (SERCA), and 5) sequestration and redistribution via mitochondria. Detailed discussion of these important mechanisms in regulating Ca2+ signaling in vascular smooth muscle is beyond the scope of this review. However, these mechanisms clearly influence the vascular smooth muscle Ca2+ dynamics. For example, mitochondria are known to be important modulators of IP3-dependent Ca2+ signaling (37), the Na+/Ca2+ exchanger regulates L-type Ca2+ channel function (68), and sarcoplasmic reticular Ca2+-ATPase function influences ryanodine receptor-dependent Ca2+ sparks (13, 61).
Three major areas of future research are necessary to further our understanding of Ca2+ dynamics in vascular smooth muscle. First, cryptic Ca2+ signals such as Ca2+ puffs (see above) need to be visualized directly. Similarly, novel, discrete Ca2+ signals associated with store operated Ca2+ entry (SOCE) are in need of identification and characterization. Second, the relationship between local Ca2+ signals (e.g., Ca2+ waves and Ca2+ sparklets) and global Ca2+ needs to be clarified. Third and finally, future investigations should explore Ca2+ dynamics with respect to mechanisms regulating intracellular and intercellular signaling in the intact vascular syncytium. Experiments along these lines are necessary not only to assess the validity of experiments performed on isolated smooth muscle cells, but also to examine the influence of other cell types (i.e., endothelial cells) on Ca2+ signaling in vascular smooth muscle. Advances in Ca2+ imaging techniques such as high-speed confocal microscopy and availability of cell-specific Ca2+ indicators are already and will continue to be critical in advancing these areas of research.
Acknowledgments
This work was supported by grants from the Pew Charitable Trusts and the Colorado State University College Research Council (to G.C.A.), and the American Heart Association-Scientist Development Grant 0735251N and National Institute of Health 1R01HL098200 (to M.F.N.).
References
- 1.Amberg GC, Bonev AD, Rossow CF, Nelson MT, Santana LF. Modulation of the molecular composition of large conductance, Ca+ channels in vascular smooth muscle during hypertension. J Clin Invest. 2003;112:717–724. doi: 10.1172/JCI18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amberg GC, Earley S, Glapa SA. Local regulation of arterial L-type calcium channels by reactive oxygen species. Circ Res. 2010;107:1002–1010. doi: 10.1161/CIRCRESAHA.110.217018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amberg GC, Navedo MF, Nieves-Cintron M, Molkentin JD, Santana LF. Calcium sparklets regulate local and global calcium in murine arterial smooth muscle. J Physiol. 2007;579:187–201. doi: 10.1113/jphysiol.2006.124420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amberg GC, Santana LF. Downregulation of the BK channel β1 subunit in genetic hypertension. Circ Res. 2003;93:965–971. doi: 10.1161/01.RES.0000100068.43006.36. [DOI] [PubMed] [Google Scholar]
- 5.Bayguinov O, Hagen B, Bonev AD, Nelson MT, Sanders KM. Intracellular calcium events activated by ATP in murine colonic myocytes. Am J Physiol Cell Physiol. 2000;279:C126–135. doi: 10.1152/ajpcell.2000.279.1.C126. [DOI] [PubMed] [Google Scholar]
- 6.Benham CD, Bolton TB. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boittin FX, Macrez N, Halet G, Mironneau J. Norepinephrine-induced Ca2+ waves depend on InsP3 and ryanodine receptor activation in vascular myocytes. Am J Physiol. 1999;277:C139–151. doi: 10.1152/ajpcell.1999.277.1.C139. [DOI] [PubMed] [Google Scholar]
- 8.Bonev AD, Jaggar JH, Rubart M, Nelson MT. Activators of protein kinase C decrease Ca2+ spark frequency in smooth muscle cells from cerebral arteries. Am J Physiol. 1997;273:C2090–2095. doi: 10.1152/ajpcell.1997.273.6.C2090. [DOI] [PubMed] [Google Scholar]
- 9.Bootman MD, Berridge MJ. Subcellular Ca2+ signals underlying waves and graded responses in HeLa cells. Curr Biol. 1996;6:855–865. doi: 10.1016/s0960-9822(02)00609-7. [DOI] [PubMed] [Google Scholar]
- 10.Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the b1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 11.Chaplin NL, Amberg GC. Hydrogen peroxide mediates oxidant-dependent stimulation of arterial smooth muscle L-type calcium channels. Am J Physiol Cell Physiol. 2012;302:C1382–1393. doi: 10.1152/ajpcell.00222.2011. [DOI] [PubMed] [Google Scholar]
- 12.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 13.Cheranov SY, Jaggar JH. Sarcoplasmic reticulum calcium load regulates rat arterial smooth muscle calcium sparks and transient KCa currents. J Physiol. 2002;544:71–84. doi: 10.1113/jphysiol.2002.025197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devine CE, Somlyo AV, Somlyo AP. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol. 1972;52:690–718. doi: 10.1083/jcb.52.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixon RE, Yuan C, Cheng EP, Navedo MF, Santana LF. Ca2+ signaling amplification by oligomerization of L-type Cav1.2 channels. Proc Natl Acad Sci U S A. 2012;109:1749–1754. doi: 10.1073/pnas.1116731109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans RJ, Lewis C, Virginio C, Lundstrom K, Buell G, Surprenant A, North RA. Ionic permeability of, and divalent cation effects on, two ATP-gated cation channels (P2X receptors) expressed in mammalian cells. J Physiol. 1996;497 ( Pt 2):413–422. doi: 10.1113/jphysiol.1996.sp021777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzales AL, Amberg GC, Earley S. Ca2+ release from the sarcoplasmic reticulum is required for sustained TRPM4 activity in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2010;299:C279–288. doi: 10.1152/ajpcell.00550.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzales AL, Earley S. Endogenous cytosolic Ca2+ buffering is necessary for TRPM4 activity in cerebral artery smooth muscle cells. Cell calcium. 2012;51:82–93. doi: 10.1016/j.ceca.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzales AL, Garcia ZI, Amberg GC, Earley S. Pharmacological inhibition of TRPM4 hyperpolarizes vascular smooth muscle. American journal of physiology Cell physiology. 2010;299:C1195–1202. doi: 10.1152/ajpcell.00269.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordienko DV, Bolton TB. Crosstalk between ryanodine receptors and IP3 receptors as a factor shaping spontaneous Ca2+-release events in rabbit portal vein myocytes. J Physiol. 2002;542:743–762. doi: 10.1113/jphysiol.2001.015966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill-Eubanks DC, Werner ME, Heppner TJ, Nelson MT. Calcium signaling in smooth muscle. Cold Spring Harb Perspect Biol. 2011;3:a004549. doi: 10.1101/cshperspect.a004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hille B. Ion channels of excitable membranes. 3. Suderland, Massachusetts: Sinauer Associates, Inc; 2001. [Google Scholar]
- 23.Iino M, Kasai H, Yamazawa T. Visualization of neural control of intracellular Ca2+ concentration in single vascular smooth muscle cells in situ. EMBO J. 1994;13:5026–5031. doi: 10.1002/j.1460-2075.1994.tb06831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest. 2003;112:1895–1905. doi: 10.1172/JCI18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaggar JH. Intravascular pressure regulates local and global Ca2+ signaling in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2001;281:C439–448. doi: 10.1152/ajpcell.2001.281.2.C439. [DOI] [PubMed] [Google Scholar]
- 26.Jaggar JH, Nelson MT. Differential regulation of Ca2+ sparks and Ca2+ waves by UTP in rat cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2000;279:C1528–1539. doi: 10.1152/ajpcell.2000.279.5.C1528. [DOI] [PubMed] [Google Scholar]
- 27.Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278:C235–256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- 28.Jaggar JH, Wellman GC, Heppner TJ, Porter VA, Perez GJ, Gollasch M, Kleppisch T, Rubart M, Stevenson AS, Lederer WJ, Knot HJ, Bonev AD, Nelson MT. Ca2+ channels, ryanodine receptors and Ca2+-activated K+ channels: a functional unit for regulating arterial tone. Acta Physiol Scand. 1998;164:577–587. doi: 10.1046/j.1365-201X.1998.00462.x. [DOI] [PubMed] [Google Scholar]
- 29.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508 (Pt 1):199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knot HJ, Standen NB, Nelson MT. Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels. J Physiol. 1998;508 ( Pt 1):211–221. doi: 10.1111/j.1469-7793.1998.211br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamont C, Vainorius E, Wier WG. Purinergic and adrenergic Ca2+ transients during neurogenic contractions of rat mesenteric small arteries. J Physiol. 2003;549:801–808. doi: 10.1113/jphysiol.2003.043380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamont C, Vial C, Evans RJ, Wier WG. P2X1 receptors mediate sympathetic postjunctional Ca2+ transients in mesenteric small arteries. Am J Physiol Heart Circ Physiol. 2006;291:H3106–3113. doi: 10.1152/ajpheart.00466.2006. [DOI] [PubMed] [Google Scholar]
- 33.Lamont C, Wier WG. Different roles of ryanodine receptors and inositol (1,4,5)-trisphosphate receptors in adrenergically stimulated contractions of small arteries. Am J Physiol Heart Circ Physiol. 2004;287:H617–625. doi: 10.1152/ajpheart.00708.2003. [DOI] [PubMed] [Google Scholar]
- 34.Lamont C, Wier WG. Evoked and spontaneous purinergic junctional Ca2+ transients (jCaTs) in rat small arteries. Circ Res. 2002;91:454–456. doi: 10.1161/01.res.0000035060.98415.4b. [DOI] [PubMed] [Google Scholar]
- 35.Lewis CJ, Ennion SJ, Evans RJ. P2 purinoceptor-mediated control of rat cerebral (pial) microvasculature; contribution of P2X and P2Y receptors. J Physiol. 2000;527(Pt 2):315–324. doi: 10.1111/j.1469-7793.2000.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mauban JR, Zacharia J, Zhang J, Wier WG. Vascular tone and Ca2+ signaling in murine cremaster muscle arterioles in vivo. Microcirculation. 2012 doi: 10.1111/micc.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarron JG, Olson ML, Chalmers S. Mitochondrial regulation of cytosolic Ca2+ signals in smooth muscle. Pflugers Arch. 2012;464:51–62. doi: 10.1007/s00424-012-1108-9. [DOI] [PubMed] [Google Scholar]
- 38.Miriel VA, Mauban JR, Blaustein MP, Wier WG. Local and cellular Ca2+ transients in smooth muscle of pressurized rat resistance arteries during myogenic and agonist stimulation. J Physiol. 1999;518 ( Pt 3):815–824. doi: 10.1111/j.1469-7793.1999.0815p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moosmang S, Schulla V, Welling A, Feil R, Feil S, Wegener JW, Hofmann F, Klugbauer N. Dominant role of smooth muscle L-type calcium channel Cav1.2 for blood pressure regulation. EMBO J. 2003;22:6027–6034. doi: 10.1093/emboj/cdg583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Navedo MF, Amberg GC, Nieves M, Molkentin JD, Santana LF. Mechanisms underlying heterogeneous Ca2+ sparklet activity in arterial smooth muscle. J Gen Physiol. 2006;127:611–622. doi: 10.1085/jgp.200609519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navedo MF, Amberg GC, Votaw VS, Santana LF. Constitutively active L-type Ca2+ channels. Proc Natl Acad Sci U S A. 2005;102:11112–11117. doi: 10.1073/pnas.0500360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navedo MF, Amberg GC, Westenbroek RE, Sinnegger-Brauns MJ, Catterall WA, Striessnig J, Santana LF. Cav1.3 channels produce persistent calcium sparklets, but Cav1.2 channels are responsible for sparklets in mouse arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2007;293:H1359–1370. doi: 10.1152/ajpheart.00450.2007. [DOI] [PubMed] [Google Scholar]
- 43.Navedo MF, Cheng EP, Yuan C, Votaw S, Molkentin JD, Scott JD, Santana LF. Increased coupled gating of L-type Ca2+ channels during hypertension and Timothy syndrome. Circ Res. 2010;106:748–756. doi: 10.1161/CIRCRESAHA.109.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Navedo MF, Nieves-Cintron M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, McKnight GS, Santana LF. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res. 2008;102:e1–e11. doi: 10.1161/CIRCRESAHA.107.167809. [DOI] [PubMed] [Google Scholar]
- 45.Navedo MF, Takeda Y, Nieves-Cintron M, Molkentin JD, Santana LF. Elevated Ca2+ sparklet activity during acute hyperglycemia and diabetes in cerebral arterial smooth muscle cells. Am J Physiol Cell Physiol. 2010;298:C211–220. doi: 10.1152/ajpcell.00267.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 47.Nieves-Cintron M, Amberg GC, Navedo MF, Molkentin JD, Santana LF. The control of Ca2+ influx and NFATc3 signaling in arterial smooth muscle during hypertension. Proc Natl Acad Sci U S A. 2008;105:15623–15628. doi: 10.1073/pnas.0808759105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perez GJ, Bonev AD, Nelson MT. Micromolar Ca2+ from sparks activates Ca2+-sensitive K+ channels in rat cerebral artery smooth muscle. Am J Physiol Cell Physiol. 2001;281:C1769–1775. doi: 10.1152/ajpcell.2001.281.6.C1769. [DOI] [PubMed] [Google Scholar]
- 49.Porter VA, Bonev AD, Knot HJ, Heppner TJ, Stevenson AS, Kleppisch T, Lederer WJ, Nelson MT. Frequency modulation of Ca2+ sparks is involved in regulation of arterial diameter by cyclic nucleotides. Am J Physiol. 1998;274:C1346–1355. doi: 10.1152/ajpcell.1998.274.5.C1346. [DOI] [PubMed] [Google Scholar]
- 50.Rubart M, Patlak JB, Nelson MT. Ca2+ currents in cerebral artery smooth muscle cells of rat at physiological Ca2+ concentrations. J Gen Physiol. 1996;107:459–472. doi: 10.1085/jgp.107.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saleh SN, Greenwood IA. Activation of chloride currents in murine portal vein smooth muscle cells by membrane depolarization involves intracellular calcium release. Am J Physiol Cell Physiol. 2005;288:C122–131. doi: 10.1152/ajpcell.00384.2004. [DOI] [PubMed] [Google Scholar]
- 52.Santana LF, Navedo MF. Molecular and biophysical mechanisms of Ca2+ sparklets in smooth muscle. J Mol Cell Cardiol. 2009;47:436–444. doi: 10.1016/j.yjmcc.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santana LF, Navedo MF. Natural inequalities: why some L-type Ca2+ channels work harder than others. The Journal of general physiology. 2010;136:143–147. doi: 10.1085/jgp.200910391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schubert R, Nelson MT. Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci. 2001;22:505–512. doi: 10.1016/s0165-6147(00)01775-2. [DOI] [PubMed] [Google Scholar]
- 55.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stewart M, Needham M, Bankhead P, Gardiner TA, Scholfield CN, Curtis TM, McGeown JG. Feedback via Ca2+-activated ion channels modulates endothelin 1 signaling in retinal arteriolar smooth muscle. Invest Ophthalmol Vis Sci. 2012;53:3059–3066. doi: 10.1167/iovs.11-9192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takeda Y, Nystoriak MA, Nieves-Cintron M, Santana LF, Navedo MF. Relationship between Ca2+ sparklets and sarcoplasmic reticulum Ca2+ load and release in rat cerebral arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2011;301:H2285–2294. doi: 10.1152/ajpheart.00488.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tovey SC, de Smet P, Lipp P, Thomas D, Young KW, Missiaen L, De Smedt H, Parys JB, Berridge MJ, Thuring J, Holmes A, Bootman MD. Calcium puffs are generic InsP3-activated elementary calcium signals and are downregulated by prolonged hormonal stimulation to inhibit cellular calcium responses. Journal of cell science. 2001;114:3979–3989. doi: 10.1242/jcs.114.22.3979. [DOI] [PubMed] [Google Scholar]
- 59.Wang Q, Hogg RC, Large WA. Properties of spontaneous inward currents recorded in smooth muscle cells isolated from the rabbit portal vein. J Physiol. 1992;451:525–537. doi: 10.1113/jphysiol.1992.sp019177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang SQ, Song LS, Lakatta EG, Cheng H. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 2001;410:592–596. doi: 10.1038/35069083. [DOI] [PubMed] [Google Scholar]
- 61.Wellman GC, Santana LF, Bonev AD, Nelson MT. Role of phospholamban in the modulation of arterial Ca2+ sparks and Ca2+-activated K+ channels by cAMP. Am J Physiol Cell Physiol. 2001;281:C1029–1037. doi: 10.1152/ajpcell.2001.281.3.C1029. [DOI] [PubMed] [Google Scholar]
- 62.Westcott EB, Goodwin EL, Segal SS, Jackson WF. Function and expression of ryanodine receptors and inositol 1,4,5-trisphosphate receptors in smooth muscle cells of murine feed arteries and arterioles. J Physiol. 2012;590:1849–1869. doi: 10.1113/jphysiol.2011.222083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wier WG, Zang WJ, Lamont C, Raina H. Sympathetic neurogenic Ca2+ signalling in rat arteries: ATP, noradrenaline and neuropeptide Y. Exp Physiol. 2009;94:31–37. doi: 10.1113/expphysiol.2008.043638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wray S, Burdyga T. Sarcoplasmic reticulum function in smooth muscle. Physiological reviews. 2010;90:113–178. doi: 10.1152/physrev.00018.2008. [DOI] [PubMed] [Google Scholar]
- 65.Zacharia J, Zhang J, Wier WG. Ca2+ signaling in mouse mesenteric small arteries: myogenic tone and adrenergic vasoconstriction. Am J Physiol Heart Circ Physiol. 2007;292:H1523–1532. doi: 10.1152/ajpheart.00670.2006. [DOI] [PubMed] [Google Scholar]
- 66.Zang WJ, Balke CW, Wier WG. Graded α1-adrenoceptor activation of arteries involves recruitment of smooth muscle cells to produce ‘all or none’ Ca2+ signals. Cell calcium. 2001;29:327–334. doi: 10.1054/ceca.2000.0193. [DOI] [PubMed] [Google Scholar]
- 67.Zang WJ, Zacharia J, Lamont C, Wier WG. Sympathetically evoked Ca2+ signaling in arterial smooth muscle. Acta Pharmacol Sin. 2006;27:1515–1525. doi: 10.1111/j.1745-7254.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 68.Zhang J, Ren C, Chen L, Navedo MF, Antos LK, Kinsey SP, Iwamoto T, Philipson KD, Kotlikoff MI, Santana LF, Wier WG, Matteson DR, Blaustein MP. Knockout of Na+/Ca2+ exchanger in smooth muscle attenuates vasoconstriction and L-type Ca2+ channel current and lowers blood pressure. Am J Physiol Heart Circ Physiol. 2010;298:H1472–1483. doi: 10.1152/ajpheart.00964.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]