Abstract
Aims/hypothesis
Insulin clearance is a highly heritable trait, for which few quantitative trait loci have been discovered. We sought to determine whether validated type 2 diabetes and/or glycaemic trait loci are associated with insulin clearance.
Methods
Hyperinsulinaemic–euglycaemic clamps were performed in two Hispanic-American family cohorts totalling 1329 participants in 329 families. The Metabochip was used to fine-map about 50 previously identified loci for type 2 diabetes, fasting glucose, fasting insulin, 2 h glucose or HbA1c. This resulted in 17,930 variants, which were tested for association with clamp-derived insulin clearance via meta-analysis of the two cohorts.
Results
In the meta-analysis, 38 variants located within seven loci demonstrated association with insulin clearance (p < 0.001). The top signals for each locus were rs10241087 (DGKB/TMEM195 [TMEM195 also known as AGMO]) (p = 4.4 × 10−5); chr1:217605433 (LYPLAL1) (p = 3.25 × 10−4); rs2380949 (GLIS3) (p = 3.4 × 10−4); rs55903902 (FADS1) (p = 5.6 × 10−4); rs849334 (JAZF1) (p = 6.4 × 10−4); rs35749 (IGF1) (p = 6.7 × 10−4); and rs9460557 (CDKAL1) (p = 6.8 × 10−4).
Conclusions/interpretation
While the majority of validated loci for type 2 diabetes and related traits do not appear to influence insulin clearance in Hispanics, several of these loci do show evidence of association with this trait. It is therefore possible that these loci could have pleiotropic effects on insulin secretion, insulin sensitivity and insulin clearance.
Keywords: Association, Insulin clearance, Meta-analysis, Type 2 diabetes mellitus
Introduction
Type 2 diabetes arises when increases in insulin secretion become insufficient to overcome tissue insulin resistance. However, reduced insulin clearance (removal of insulin from the circulation) is another physiological response to insulin resistance that might delay the development of diabetes [1]. Reduced insulin clearance at baseline was found to predict incident diabetes after 5 years of follow-up independently of baseline insulin secretion [2]. Further studies are needed to characterise the role of insulin clearance in the genesis of diabetes.
We and others have established that there is a genetic basis for insulin clearance. Early evidence came from studies that found racial and/or ethnic differences in insulin clearance. For example, compared with non-Hispanic whites, African-Americans and Hispanics have lower insulin clearance [3, 4]. Furthermore, non-diabetic first-degree relatives of diabetic individuals have lower insulin clearance than individuals without a family history of diabetes [5]. We were the first to report the heritability of insulin clearance; in a study of Mexican-Americans, insulin clearance had a higher heritability (h2 = 0.58) than either fasting insulin (h2 = 0.38) or insulin sensitivity (h2 = 0.44) [6]. We confirmed the high heritability of insulin clearance in an independent Hispanic cohort (h2 = 0.73) [7]. Heritability studies in other ethnic groups are needed.
Despite this evidence for genetic regulation, few quantitative trait loci for insulin clearance have been identified. Validated genes for type 2 diabetes and related glucose and insulin traits are a logical source of candidate genes for insulin clearance. The most obvious of these is IDE, encoding insulin degrading enzyme, which cleaves insulin in cells that clear it. Genome-wide association studies (GWAS) of type 2 diabetes identified single nucleotide polymorphisms (SNPs) (rs1111875, rs5015480) linked with a region encompassing IDE and the neighbouring genes HHEX and KIF11 [8–11]. Subsequently, rs1111875 was found to be associated with reduced insulin clearance [12]. Another study found an association between the IDE SNP rs1887922 and decreased hepatic insulin degradation [13]; this SNP is not in linkage disequilibrium with the type 2 diabetes SNPs noted above [14]. The Ala allele of the Pro12Ala variant (rs1801282) in the peroxisome proliferator activated receptor gamma (PPARG) gene is associated with reduced risk of type 2 diabetes mellitus [9–11, 15]. In carriers of the Ala allele, insulin clearance was found to be significantly greater, with lower NEFA levels [16].
It is clear that genes for diabetes and related traits that have been discovered and validated by GWAS have been inadequately studied as loci for insulin clearance, as there are now over 100 such loci for type 2 diabetes, fasting glucose, fasting insulin, 2 h glucose and HbA1c. Our goal, therefore, was to systematically evaluate such loci for association with insulin clearance, using the Metabochip [17], which contains fine-mapping content for a large number of these loci.
Methods
Participants and phenotyping
The current study was conducted in the two independent family cohorts in which we had previously documented the heritability of insulin clearance [6, 7]. The Hypertension-Insulin Resistance (HTN-IR) cohort consists of Los Angeles Hispanic-American families ascertained via a proband with essential hypertension. The recruitment and phenotyping of this cohort have been described previously [18]. After data cleaning as described below, we studied 638 participants from 148 HTN-IR families. The participants had undergone a euglycaemic clamp with steady-state insulin levels as a means of phenotyping insulin clearance.
The second cohort is the Mexican-American Coronary Artery Disease (MACAD) study [6]. After data cleaning as described below, 181 families were included in the current study, comprising 691 participants who were drawn from the offspring generation (adult offspring of probands with coronary artery disease and the spouses of those offspring) and underwent phenotyping for insulin clearance.
By design, participants undergoing detailed phenotyping in both cohorts were free of known diabetes and clinically manifest cardiovascular disease, thus avoiding secondary changes in phenotype caused by overt disease. A small percentage of participants in both cohorts were newly diagnosed with diabetes as a result of their participation in the studies. These participants were not taking any glucose-lowering medications when they were phenotyped.
Participants in the HTN-IR and MACAD cohorts underwent the hyperinsulinaemic–euglycaemic clamp procedure performed with the same techniques and assays. Insulin was measured using a kit (Human Insulin Specific RIA; Linco Research, St Charles, MO, USA) in which the cross-reactivity with proinsulin was <0.2%. During the clamp [19], a priming dose of human insulin (Novolin, Clayton, NC, USA) was followed by a constant-rate infusion of insulin (60 mU m−2min−1) for 120 min, the aim being to achieve a plasma insulin concentration of 600 pmol/l or greater. Blood was sampled every 5 min and the rate of co-infused 20% (20 g/100 ml) dextrose was adjusted to maintain plasma glucose concentrations at 5.27 to 5.55 mmol/l. Blood samples were drawn for glucose and insulin measurement at −30, −20, −10, 100, 110 and 120 min. The metabolic clearance rate of insulin (ml m−2 min−1) is calculated as the insulin infusion rate divided by the final steady-state plasma insulin level (SSPI) (average of insulin levels at 100, 110 and 120 min) during the euglycaemic clamp. Because all our participants received the same insulin infusion rate (60 mU m−2min−1), the SSPI is a direct measure of insulin clearance at steady state and was the trait used in genetic analyses in this study [6].
All studies were approved by the Institutional Review Boards at the participating institutions. All participants gave informed consent before participation.
Metabochip genotyping and quality control in HTN-IR and MACAD cohorts
Both cohorts were genotyped using the Metabochip, which was designed to provide high-throughput genotyping for replication and fine-mapping of GWAS results for cardiac (e.g. myocardial infarction, QT interval, blood pressure), metabolic (e.g. diabetes, fasting glucose, fasting insulin), anthropometric (e.g. BMI, WHR) and lipid traits [17].
Genotyping was performed at the Medical Genetics Institute at Cedars-Sinai Medical Center, using custom Infinium II technology, following the manufacturer’s protocol (Illumina, San Diego, CA, USA) [20]. Quality control sampling led to 16 samples being removed for low genotyping rates (<98%) or low p10GC scores. Another 26 participants were removed for sex mismatch, which was determined using Genome Studio (Illumina).
Following these quality control steps, 640 HTN-IR and 693 MACAD participants with SSPI data were included in the study. The genotyping rate in these samples was 99.98% (HTN-IR) and 99.96% (MACAD). Across the two projects, 22 pairs of sample duplicates were run (representing 1% of the entire sample run as either within-plate or across-plate duplicates), yielding an average reproducibility of 99.99%.
For the quality control pipeline, 196,475 SNPs were available from Genome Studio (Illumina). Of these, 37,337 SNPs were excluded due to quality control variables that included poor cluster formation and a SNP failure rate of >2%. Further quality control excluded SNPs with: (1) a minor allele frequency (MAF) of <1% (HTN-IR: 37,238; MACAD: 35,992); (2) Hardy–Weinberg equilibrium at p < 1 × 10−7 (HTN-IR: three SNPs; MACAD: seven SNPs); or (3) observed heterozygosity of >53% (HTN-IR: 1,135; MACAD: 709 SNPs). The final number of SNPs available after quality control was about 120,000.
In this study, we first used the fine-mapping content selected for the Metabochip by the DIAGRAM (DIAbetes Genetics Replication And Meta-analysis) and MAGIC (Meta-Analyses of Glucose and Insulin-related traits Consortium) consortia. DIAGRAM selected content to fine-map loci for type 2 diabetes; MAGIC content fine-mapped loci for fasting glucose, fasting insulin, 2 h glucose and HbA1c. The two consortia selected 50 loci for fine-mapping (Electronic supplementary material [ESM] Table 1). Of these, five loci (ADCY5, G6PC2, GCK, KCNQ1 and MTNR1B) were selected for multiple traits; thus, the number of unique loci examined in this study was 45. Of the approximately 200,000 SNPs available on the Metabochip [17], the 45 loci fine-mapped for type 2 diabetes and related glycaemic traits encompass a total of 33,110 SNPs. Of these, 14,473 passed quality control in both cohorts and were analysed as part of the present study (the majority of SNPs failed quality control due to low MAF).
In 2012, the DIAGRAM and MAGIC consortia conducted large-scale association meta-analyses using the Metabochip, leading to the discovery of over 50 additional loci for type 2 diabetes, fasting glucose, 2 h glucose and fasting insulin [21, 22]. Of these, nine were fine-mapped on the Metabochip to investigate signals for traits including WHR, BMI, and HDL- and LDL-cholesterol (ESM Table 2). Given that two of these loci overlapped (GRB14/COBLL1), we analysed these eight unique fine-mapped loci in addition to the 45 fine-mapped loci described above, making for a total of 53 loci. The eight loci were fine-mapped by 7,848 SNPs, 3,457 of which passed quality control in both cohorts and were analysed. Therefore, the total number of SNPs analysed was 17,930 (14,473 + 3,457).
Population stratification
A potential pitfall in association analysis is population stratification, in which systematic differences in ancestry are associated with phenotypes that might be associated with disease. The Hispanic-American population is significantly substructured [23]; it is therefore important to address the possible confounding effect of stratification. We computed principal components (PCs) of ancestry for unrelated founders and then projected them to all family members using SMARTPCA, which is distributed with the software package EIGENSTRAT [24]. The PC analysis was performed using 43,000 autosomal SNPs in the HTN-IR project and 43,000 SNPs in the MACAD project, with SNPs selected for minimal linkage disequilibrium (r2 < 0.2). Two outliers (defined as >10 standard deviations) were identified in each of the two cohorts; these participants were excluded from association analyses, yielding sample sizes of 638 for the HTN-IR and 691 for the MACAD cohorts (for clinical characteristics, see Table 1). In the HTN-IR cohort, the top PC explained around 1.35% and the second PC around 0.5% of genetic variance; the remaining PCs each explained less than 0.4% of variance. We therefore adjusted for the top two PCs in the HTN-IR association analysis. In the MACAD cohort, the top three PCs explained 1.11%, 0.55%, and 0.43% respectively, with the remaining PCs each explaining less than 0.4%. Therefore, the top three PCs were adjusted for in the MACAD association analyses.
Table 1.
Characteristics of the participants in the two cohorts
| Trait | HTN | MACAD | p value |
|---|---|---|---|
| Families (n) | 148 | 181 | n/a |
| Participants (n) | 638 | 691 | n/a |
| Male sex (%) | 40.3 | 42.8 | 0.37 |
| Age (years) | 37.4±14.4 | 34.6±8.9 | <0.0001 |
| BMI (kg/m2) | 28.9±5.7 | 28.9±5.0 | 0.99 |
| Diabetes (%) | 6.9 | 2.5 | <0.0001 |
| Fasting insulin (pmol/l) | 91.7±57.0 | 84.6±49.2 | 0.014 |
| Fasting glucose (mmol/l) | 5.39±0.50 | 5.12±0.63 | <0.0001 |
| SSPI (pmol/l) | 829.4±187.1 | 810.6±233.4 | 0.11 |
Unless otherwise indicated, data are mean±SD (quantitative traits) or per cent (qualitative traits)
n/a, not applicable
Association analysis
Association between individual candidate SNPs and insulin clearance measures was evaluated using the general estimating equations (GEE1) method implemented in the Genome-Wide Association Analyses with Family Data (GWAF) program [25]. GWAF uses functions in existing R packages to properly model the residual correlations in families in the test of genotype–phenotype association. The additive genetic model was used in both cohorts. In all analyses, log10-transformed SSPI was the dependent variable, with potential confounding factors, including age, sex, BMI and diabetes status being included, together with the top PCs, as covariates. We combined the results of the HTN-IR and MACAD cohorts using fixed-effects, inverse-variance weighting meta-analysis as implemented in METAL [26].
The power of this two-cohort meta-analysis to detect association is shown in ESM Table 3.With the two cohorts studied, we had 85% power to detect SNPs that explain 1.4% of SSPI variance. Even for a SNP explaining as little as 1%of variance, we had a power of 64% to detect association.
The Metabochip comprises a large number of SNPs and therefore a large number of statistical tests. Given the chip’s focus on fine-mapping, and the fact that it contains a large number of variants in linkage disequilibrium, a simple correction for the total number of SNPs is not appropriate. Instead, we adjusted for the number of loci examined (53 loci) and defined the significance cut-off as p < 10−3 after Bonferroni’s correction (p = 0.05/53, i.e. approximately 1 × 10−3). Only loci containing SNPs that met this level of significance in the meta-analysis were evaluated further.
Results
Characteristics of the cohorts are given in Table 1. Participants in the HTN-IR cohort were slightly older and had slightly higher fasting glucose and insulin values than participants in the MACAD cohort. They were comparable in BMI, sex distribution and SSPI.
In the HTN-IR and MACAD cohorts, 17,930 SNPs passed quality control measures and were used for association testing. The meta-analysis results did not show significant genomic inflation, with minimal deviation from what would have been expected by chance (λgenomic control = 1.015). Of the 45 original diabetes and related trait loci, six harboured 29 SNPs associated with SSPI at p < 0.001 in the meta-analysis of both cohorts. These consisted of 19 SNPs in the DGKB/TMEM195 locus, and of four, two, two, one and one SNPs in IGF1, JAZF1, FADS1, CDKAL1 and GLIS3, respectively. None of these SNPs were identical to or in linkage disequilibrium with the index SNP used by DIAGRAM or MAGIC to select the loci for fine-mapping (ESM Table 4). Table 2 shows the associations of the lead SNP within each of the six loci. The beta values are very similar in each cohort, indicating that the associations are not driven by one study. The similar MAFs suggest that there were no unrecognised ethnic differences between the two cohorts.
Table 2.
Lead SNPs in seven loci associated with insulin clearance
| Genetic variant | HTN-IR | MACAD | Meta-analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chr | BP | SNP | Locus | Selection category | MAF | Beta | p value | MAF | Beta | p value | p value |
| 6 | 20,902,604 | rs9460557 | CDKAL1 | T2D | 0.015 | 0.04 | 0.1 | 0.018 | 0.072 | 0.0016 | 6.81E-04 |
| 7 | 14,240,868 | rs10241087 | DGKB/TMEM195 | Fasting glucose | 0.19 | 0.025 | 0.0007 | 0.15 | 0.019 | 0.016 | 4.43E-05 |
| 7 | 28,190,367 | rs849334 | JAZF1 | T2D | 0.067 | 0.031 | 0.0092 | 0.059 | 0.028 | 0.025 | 6.37E-04 |
| 9 | 4,279,809 | rs2380949 | GLIS3 | Fasting glucose | 0.075 | −0.024 | 0.03 | 0.098 | −0.03 | 0.004 | 3.42E-04 |
| 11 | 61,288,567 | rs55903902 | FADS1 | Fasting glucose | 0.38 | 0.014 | 0.015 | 0.35 | 0.015 | 0.014 | 5.59E-04 |
| 12 | 101,464,313 | rs35749 | IGF1 | Fasting insulin | 0.073 | −0.026 | 0.021 | 0.078 | −0.028 | 0.013 | 6.71E-04 |
| 1a | 217,605,433 a | chr1:217605433a | LYPLAL1a | WHR | 0.068 | 0.022 | 0.058 | 0.067 | 0.037 | 0.0016 | 3.25E-04 |
The insulin clearance measure in these analyses was log10-transformed SSPI during the euglycaemic clamp
The locus in the last line is a new locus from Metabochip studies [21, 22]; all others are original loci from GWAS
BP, base position (NCBI Genome Build 36.3, www.ncbi.nlm.nih.gov/genome/guide/human/release_notes.html, accessed 26 November, 2012); Chr, chromosome; T2D, type 2 diabetes
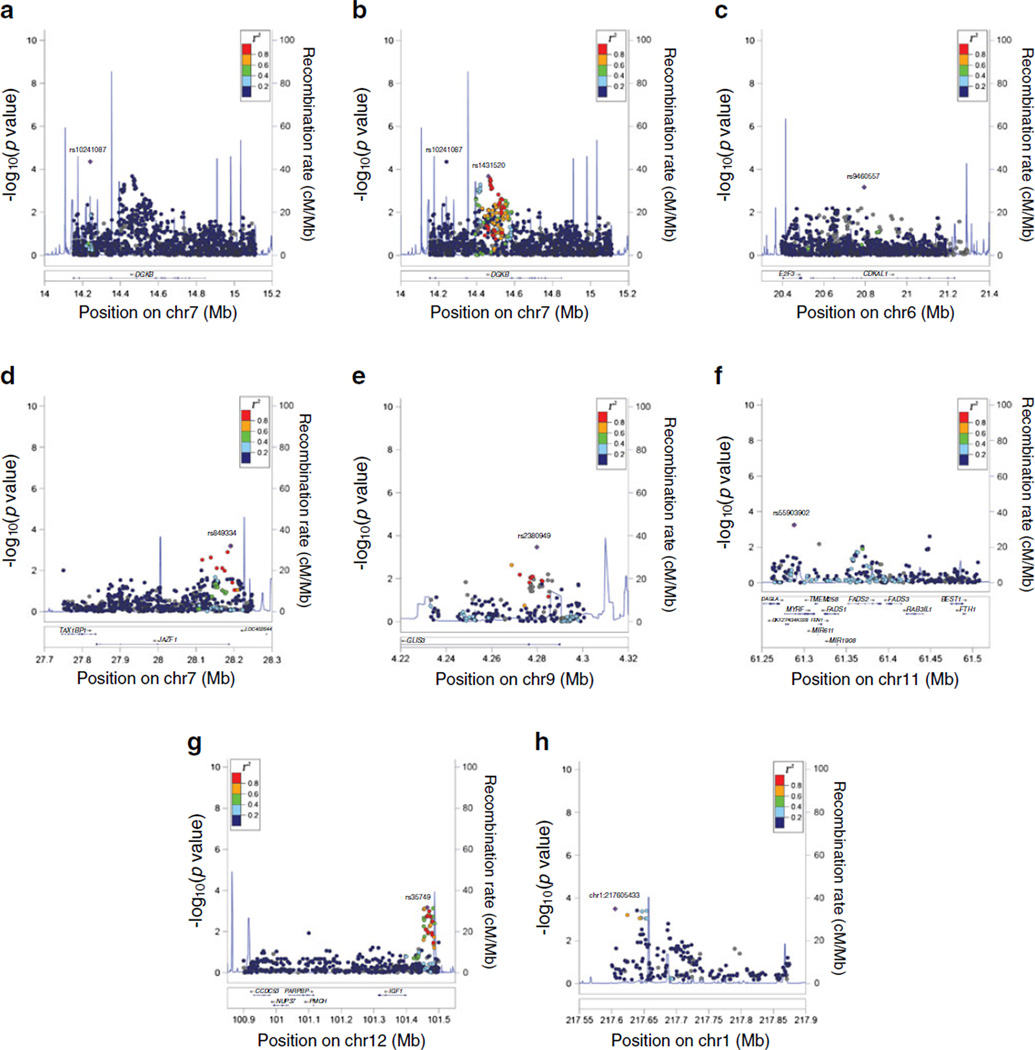
Regional association plots for these six loci are given in Fig. 1. Regarding the signal at the DGKB/TMEM195 locus, we found that the variant with the lowest p value, rs10241087 (p = 4.43 × 10−5), had little supporting evidence of nearby associated variants with which it was in linkage disequilibrium (Fig. 1a). On the other hand, the variant with the second best p value, rs1431520 (p = 2.1 × 10−4), manifested a robust locus of associated SNPs in the area between 14.4 and 14.6Mb; this SNP was therefore selected as the index SNP for the regional plot in Fig. 1b. Relatively robust signals were also observed at JAZF1 (Fig. 1d) and IGF1 (Fig. 1g).
Fig. 1.
Regional association plots of association with insulin clearance. The plots display genes and recombination rates in each region. SNP associations with insulin clearance are plotted as −log10(p value). The SNP used as the index SNP in each plot is plotted as a purple diamond and labelled. Linkage disequilibrium of nearby SNPs with the index SNP is indicated by colors: dark blue for r2 < 0.2; light blue for 0.2 < r2 < 0.4; green for 0.4 < r2 < 0.6; orange for 0.6 < r2 < 0.8; and red for r2 > 0.8. (a) DGKB (based on rs10241087), (b) DGKB (based on rs1431520), (c) CDKAL1 (based on rs9460557), (d) JAZF1 (based on rs849334), (e) GLIS3 (based on rs2380949), (f) FADS1 (based on rs55903902), (g) IGF1 (based on rs35749), (h) LYPLAL1 (based on chr1:217605433)
Of the loci recently associated with type 2 diabetes and related glycaemic traits by Metabochip studies in DIAGRAM and MAGIC [21, 22], eight were fine-mapped on the Metabochip. One of these, nearest to the LYPLAL1 gene, contained nine SNPs associated with SSPI at p < 0.001 in the meta-analysis of both cohorts. None of these SNPs were identical to or in linkage disequilibrium with the index SNP (rs4846567) used by the Genetic Investigation of ANthropometric Traits (GIANT) consortium to select the locus for fine-mapping of a WHR signal, nor were they in linkage disequilibrium with the SNP (rs4846565) associated with fasting insulin adjusted for BMI in MAGIC [22] (ESM Table 4). Table 2 displays the association of the lead SNP in this locus; its regional association plot is given in Fig. 1h.
We examined the SNPs in IDE and PPPARG, which have previously been reported to be associated with insulin clearance [12, 13, 16]. Neither the IDE SNPs rs1887922 (p = 0.10) and rs1111875 (p = 0.40), nor the PPARG SNP rs1801282 (p = 0.93) were associated with SSPI in the meta-analysis.
Discussion
In this study, we examined 53 fine-mapped loci for type 2 diabetes, fasting glucose, 2 h glucose, fasting insulin and HbA1c for association with insulin clearance. We found that seven loci (DGKB/TMEM195, IGF1, JAZF1, FADS1, CDKAL1, GLIS3, LYPLAL1) harboured SNPs associated with euglycaemic clamp-derived insulin clearance in a meta-analysis of two Hispanic cohorts. An understanding of genetic regulators of insulin clearance is important because altered insulin levels characterise several common metabolic disorders, including diabetes, metabolic syndrome, polycystic ovary syndrome and non-alcoholic fatty liver disease.
We had previously conducted a microsatellite linkage scan for insulin clearance, which identified loci on chromosomes 15 and 20 [7]. In that prior study, we used all of the Metabochip content under the −1 linkage of the odds intervals of the two linkage peaks to identify positional candidate genes for insulin clearance. Six associated loci on chromosome 15 and two loci on chromosome 20 were identified, none of which were selected to fine-map signals for diabetes or related traits [7]. They were, however, selected to fine-map systolic and diastolic blood pressure, myocardial infarction/coronary artery disease and LDL-cholesterol. Therefore, in the current study we directly examined the diabetes and related glycaemic trait loci that were fine-mapped on the Metabochip.
None of the SNPs associated with insulin clearance in the current study were identical to or in linkage disequilibrium with the SNPs identified to be associated with diabetes or related quantitative traits in the original GWAS that identified these loci (ESM Table 4). This raises the possibility that different variants in these loci associate with diabetes-related traits and with insulin clearance. Alternatively, the insulin clearance-associated SNPs might represent diabetes signals in Hispanics. We could not test this hypothesis in the current study because our cohorts are largely free of diabetes. Published studies have examined the European SNPs of only two of these genes in Hispanics, with findings including: (1) mixed results regarding the association between CDKAL1 and type 2 diabetes [27–29]; (2) positive association between CDKAL1 variation and reduced insulin secretion [30]; and (3) negative association between JAZF1 variation and diabetes [27].
Of the seven loci identified, five have been found to be associated with physiological measurements of insulin secretion derived from: (1) OGTTs (FADS1 [31, 32], DGKB [31, 32], GLIS3 [32], CDKAL1 [33], JAZF1 [34]); (2) intravenous glucose tolerance tests (CDKAL1 [35]); and (3) hyperglycaemic clamps (CDKAL1 [36]). IGF1 is thought to influence insulin sensitivity, based on its association with fasting insulin and OGTT-based insulin sensitivity assessment [31, 37]. Interestingly, none of these physiological studies examined whether variants in these loci were associated with insulin clearance. Reduced insulin response to oral glucose administration has been the most common finding used to conclude that a diabetes gene acts through insulin secretion; however, hepatic insulin clearance may significantly impact on insulin levels during an OGTT, as the liver removes around 50% of insulin at first pass [38]. Regarding insulin secretion, the most compelling functional studies have been performed for CDKAL1, which has been found to encode a methylthiotransferase that modifies a transfer RNA for lysine; murine beta cells deficient in CDK5 regulatory subunit associated protein 1-like 1 (CDKAL1) exhibited misreading of the lysine codon during proinsulin translation, resulting in an accumulation of aberrant proinsulin, endoplasmic reticulum stress, decreased insulin secretion and hyperglycaemia [39]. GLIS3 codes for a transcription factor that plays a role in pancreatic development; it also transactivates the insulin gene in beta cells [40]. For the remaining loci, strong functional evidence that they affect pancreatic beta cell development or function has yet to be published. The primary effect of these loci may be on insulin secretion, with secondary effects on insulin clearance, as the pulsatility of insulin secretion may regulate the rate of hepatic insulin extraction [41].
Alternatively, it is possible that these loci (or a subset of them) exert pleiotropic influences on insulin secretion (insulin sensitivity for IGF1) and insulin clearance. For example, GLIS3 also affects development of the liver and kidney [42], two organs that clear insulin. Pleiotropic effects of many of these loci on other traits have already been suggested by genetic association studies. For example, GLIS3 was identified in a GWAS for type 1 diabetes [43]. Variation in CDKAL1 has been associated with type 2 diabetes, Crohn’s disease and psoriasis, the variant that affects diabetes being independent of those implicated in the other two [44].
The liver is the main organ that clears insulin. In obesity and insulin resistance, insulin clearance is reduced, possibly related to the hepatic steatosis that commonly occurs in these states. Three loci, DGKB, FADS1 and LYPLAL1, modulate fatty acid and lipid metabolism, and may contribute to hepatic insulin resistance. Insulin action and insulin clearance are intimately linked in the liver, as the first event for both is binding of insulin to the insulin receptor, with receptor binding triggering internalisation of the insulin-insulin receptor complex by endocytosis [38]. DGKB encodes a diacylglycerol kinase, which regulates cellular levels of diacylglycerol by converting it to phosphatidic acid. Diacylglycerol accumulation in liver has been found to impair insulin signalling by activating protein kinase C epsilon [45]. Diacylglycerol kinase activity may also regulate the amount of diacylglycerol available for conversion to triacylglycerol by diacylglycerol acyltransferases. FADS1 codes for delta 5 fatty acid desaturase, which adds double bonds to fatty acids, playing a central role in the generation of highly unsaturated long-chain fatty acids. Depressed delta 5 saturation has been associated with obesity and insulin resistance in multiple studies [46, 47]. Interestingly, the FADS1 locus was identified in a recent GWAS for liver enzyme concentrations, which are often abnormal in hepatic steatosis [48]. We note that our insulin clearance signal at the FADS1 locus encompasses several other genes, including two other related factors, FADS2 and FADS3 (Fig. 1f), along with a number of unrelated genes (e.g. DAGLA, which encodes a DAG lipase), any of which could ultimately contribute to the insulin clearance signal. LYPLAL1 codes for a lipase whose function is not well understood. Several variants in linkage disequilibrium near LYPLAL1 have been associated with WHR, fasting insulin, triacylglycerol and adiponectin levels; independent variants have also been associated with non-alcoholic fatty liver disease and visceral fat [22, 49–53]. An association between this WHR locus and insulin clearance is consistent with the finding that waist circumference was correlated with insulin clearance independently of BMI and insulin sensitivity [54]. The observations above raise the possibility that DGKB, FADS1 and LYPLAL1 affect insulin clearance via their effects on hepatic lipid metabolism, steatosis and/or insulin responsiveness.
IGF1 codes for insulin-like growth factor 1, which mediates the action of growth hormone. IGF-1 and growth hormone excess (acromegaly, gigantism) are known to induce insulin resistance, diabetes and other features of the metabolic syndrome. Little is known about whether IGF-1 influences insulin clearance. A small study in which six adults with severe growth hormone deficiency were treated with low-dose growth hormone found no change in insulin clearance as determined by the clamp procedure [55]. Receptors for IGF-1 and insulin are known to form hybrids [56]. We speculate that IGF-1 could affect insulin clearance by binding to hybrid receptors or by its cross-reactivity with insulin receptors, which might influence the availability of receptors for insulin internalisation.
We used HaploReg (www.broadinstitute.org/mammals/haploreg/haploreg.php, accessed 26 November 2012) to conduct a preliminary assessment of the potential functional impact of the lead SNPs in Table 2 (as well as of rs1431520 from the DGKB locus). This revealed strong conservation of the sequence encompassing four SNPs (rs1431520, DGKB; rs2380949, GLIS3; rs849334, JAZF1; rs9460557, CDKAL1). Each of these SNPs was found to alter the binding sites for several transcription factors. In particular, the CDKAL1 SNP alters binding of forkhead family transcription factors; the DGKB SNP alters binding of homeobox family transcription factors.
We did not replicate the previously noted associations between both PPARG and the HHEX/IDE locus and insulin clearance [12, 13, 16]. One possible explanation is that those previous studies were conducted in German European individuals, while ours were conducted in Hispanic-Americans; differences in the genetic architecture of insulin clearance and/or linkage disequilibrium patterns between the ethnic groups may thus explain the non-replication. Furthermore, the German studies mainly [16] or exclusively [12, 13] used OGTT-derived measures of insulin clearance, which primarily reflect hepatic insulin clearance [16]. On the other hand, we used the euglycaemic clamp, which quantifies whole-body (hepatic and peripheral) insulin clearance. This difference in phenotyping may also have contributed to the non-replication.
One limitation of our study concerns the accuracy of SSPI as a measurement of the metabolic clearance rate of insulin. Because we did not measure C-peptide levels during the euglycaemic clamps to document suppression of endogenous insulin secretion, it is possible that our estimates of insulin clearance may underestimate the true values. However, because the proportion of SSPI represented by residual insulin secretion is expected to be small during hyperinsulinaemic infusion, we are confident that this had a minimal effect on our association results.
In conclusion, this study identified additional quantitative trait loci for insulin clearance by focusing on fine-mapping of 53 loci for diabetes and related traits. Approximately 20 additional loci for type 2 diabetes and related quantitative traits were identified by GWAS published after the design of the Metabochip. Furthermore, Metabochip studies by the DIAGRAM and MAGIC consortia have recently validated over 50 additional loci for these traits [21, 22], only eight of which we were able to study in detail, given the available fine-mapping content. Additional work will be needed in future to determine whether variants in the remaining newer loci are also associated with insulin clearance. Finally, our studies in genetic determinants of insulin clearance were conducted exclusively in Hispanics; studies in other ethnic groups are now needed to shed light on this trait, which is a major contributor to circulating insulin levels [54].
Supplementary Material
Acknowledgments
Funding This study was supported by National Institutes of Health (NIH) grants R01-DK079888, R01-HL067974, P50-HL055005 and P30-DK063491, and General Clinical Research Center grants M01-RR000425 and M01-RR000043. The study was also supported by the National Center for Advancing Translational Sciences, Grant UL1-TR000124. Further support came from the Cedars-Sinai Winnick Clinical Scholars Award (to M.O. Goodarzi) and the Cedars-Sinai Board of Governors’ Chair in Medical Genetics (to J.I. Rotter).
Abbreviations
- DIAGRAM
DIAbetes Genetics Replication And Meta-analysis
- GWAF
Genome-Wide Association Analyses with Family Data
- GWAS
Genome-wide association study
- HTN-IR
Hypertension-Insulin Resistance
- MACAD
Mexican-American Coronary Artery Disease
- MAF
Minor allele frequency
- MAGIC
Meta-Analyses of Glucose and Insulin-related traits Consortium
- PC
Principal component
- SNP
Single nucleotide polymorphism
- SSPI
Steady-state plasma insulin
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00125-013-2880-6) contains peer-reviewed but unedited supplementary material, which is available to authorised users.
Duality of interest The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement All authors made substantial contributions to the conception and design of the study, the acquisition of data, or the analysis and interpretation of data; they also participated in drafting the article or revising it critically for important intellectual content, and gave final approval of the version to be published.
Contributor Information
M. O. Goodarzi, Email: mark.goodarzi@cshs.org, Division of Endocrinology, Diabetes and Metabolism, Cedars-Sinai Medical Center, 8700 Beverly Blvd., Room B-131, Los Angeles, CA 90048, USA; Medical Genetics Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA; Department of Biomedical Sciences, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
X. Guo, Medical Genetics Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA Department of Biomedical Sciences, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
J. Cui, Medical Genetics Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA
M. R. Jones, Division of Endocrinology, Diabetes and Metabolism, Cedars-Sinai Medical Center, 8700 Beverly Blvd., Room B-131, Los Angeles, CA 90048, USA Department of Biomedical Sciences, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
T. Haritunians, Medical Genetics Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA
A. H. Xiang, Department of Research and Evaluation, Kaiser Permanente Southern California Medical Group, Pasadena, CA, USA
Y.-D. I. Chen, Medical Genetics Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA Department of Biomedical Sciences, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
K. D. Taylor, Medical Genetics Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA Department of Biomedical Sciences, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
T. A. Buchanan, Department of Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA, USA
W. A. Hsueh, Diabetes Research Center, Division of Diabetes, Obesity and Lipids, Methodist Hospital Research Institute, Houston, TX, USA
L. J. Raffel, Medical Genetics Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA Department of Biomedical Sciences, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
J. I. Rotter, Medical Genetics Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA Department of Biomedical Sciences, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
References
- 1.Kim SP, Ellmerer M, Kirkman EL, Bergman RN. Beta-cell "rest" accompanies reduced first-pass hepatic insulin extraction in the insulin-resistant, fat-fed canine model. Am J Physiol Endocrinol Metab. 2007;292:E1581–E1589. doi: 10.1152/ajpendo.00351.2006. [DOI] [PubMed] [Google Scholar]
- 2.Lee CC, Haffner SM, Wagenknecht LE, et al. Insulin clearance and the incidence of type 2 diabetes in Hispanics and African Americans: the IRAS family study. Diabetes Care. 2012 doi: 10.2337/dc12-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haffner SM, Stern MP, Watanabe RM, Bergman RN. Relationship of insulin clearance and secretion to insulin sensitivity in non-diabetic Mexican Americans. Eur J Clin Invest. 1992;22:147–153. doi: 10.1111/j.1365-2362.1992.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 4.Osei K, Schuster DP. Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white Americans. Diabet Med. 1994;11:755–762. doi: 10.1111/j.1464-5491.1994.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 5.Rudovich NN, Rochlitz HJ, Pfeiffer AF. Reduced hepatic insulin extraction in response to gastric inhibitory polypeptide compensates for reduced insulin secretion in normal-weight and normal glucose tolerant first-degree relatives of type 2 diabetic patients. Diabetes. 2004;53:2359–2365. doi: 10.2337/diabetes.53.9.2359. [DOI] [PubMed] [Google Scholar]
- 6.Goodarzi MO, Taylor KD, Guo X, et al. Variation in the gene for muscle-specific AMP deaminase is associated with insulin clearance, a highly heritable trait. Diabetes. 2005;54:1222–1227. doi: 10.2337/diabetes.54.4.1222. [DOI] [PubMed] [Google Scholar]
- 7.Guo X, Cui J, Jones MR, et al. Insulin clearance: confirmation as a highly heritable trait, and genome-wide linkage analysis. Diabetologia. 2012;55:2183–2192. doi: 10.1007/s00125-012-2577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sladek R, Rocheleau G, Rung J, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 9.Zeggini E, Weedon MN, Lindgren CM, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saxena R, Voight BF, Lyssenko V, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 12.Pivovarova O, Nikiforova VJ, Pfeiffer AF, Rudovich N. The influence of genetic variations in HHEX gene on insulin metabolism in the German MESYBEPO cohort. Diabetes Metab Res Rev. 2009;25:156–162. doi: 10.1002/dmrr.926. [DOI] [PubMed] [Google Scholar]
- 13.Rudovich N, Pivovarova O, Fisher E, et al. Polymorphisms within insulin-degrading enzyme (IDE) gene determine insulin metabolism and risk of type 2 diabetes. J Mol Med. 2009;87:1145–1151. doi: 10.1007/s00109-009-0540-6. [DOI] [PubMed] [Google Scholar]
- 14.Johnson AD, Handsaker RE, Pulit SL, Nizzari MM, O'Donnell CJ, de Bakker PI. SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics. 2008;24:2938–2939. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altshuler D, Hirschhorn JN, Klannemark M, et al. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet. 2000;26:76–80. doi: 10.1038/79216. [DOI] [PubMed] [Google Scholar]
- 16.Tschritter O, Fritsche A, Stefan N, et al. Increased insulin clearance in peroxisome proliferator-activated receptor gamma2 Pro12Ala. Metabolism. 2003;52:778–783. doi: 10.1016/s0026-0495(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 17.Voight BF, Kang HM, Ding J, et al. The Metabochip, a custom genotyping array for genetic studies of metabolic, cardiovascular, and anthropometric traits. PLoS Genet. 2012;8:e1002793. doi: 10.1371/journal.pgen.1002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiang AH, Azen SP, Raffel LJ, et al. Evidence for joint genetic control of insulin sensitivity and systolic blood pressure in Hispanic families with a hypertensive proband. Circulation. 2001;103:78–83. doi: 10.1161/01.cir.103.1.78. [DOI] [PubMed] [Google Scholar]
- 19.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 20.Gunderson KL, Steemers FJ, Ren H, et al. Whole-genome genotyping. Methods Enzymol. 2006;410:359–376. doi: 10.1016/S0076-6879(06)10017-8. [DOI] [PubMed] [Google Scholar]
- 21.Morris AP, Voight BF, Teslovich TM, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44:981–990. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott RA, Lagou V, Welch RP, et al. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44:991–1005. doi: 10.1038/ng.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bryc K, Velez C, Karafet T, et al. Colloquium paper: genome-wide patterns of population structure and admixture among Hispanic/Latino populations. Proc Natl Acad Sci U S A. 2010;107(Suppl 2):8954–8961. doi: 10.1073/pnas.0914618107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 25.Chen MH, Yang Q. GWAF: an R package for genome-wide association analyses with family data. Bioinformatics. 2010;26:580–581. doi: 10.1093/bioinformatics/btp710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gamboa-Melendez MA, Huerta-Chagoya A, Moreno-Macias H, et al. Contribution of common genetic variation to the risk of type 2 diabetes in the Mexican Mestizo population. Diabetes. 2012;61:3314–3321. doi: 10.2337/db11-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cruz M, Valladares-Salgado A, Garcia-Mena J, et al. Candidate gene association study conditioning on individual ancestry in patients with type 2 diabetes and metabolic syndrome from Mexico City. Diabetes Metab Res Rev. 2010;26:261–270. doi: 10.1002/dmrr.1082. [DOI] [PubMed] [Google Scholar]
- 29.Campbell DD, Parra MV, Duque C, et al. Amerind ancestry, socioeconomic status and the genetics of type 2 diabetes in a Colombian population. PLoS One. 2012;7:e33570. doi: 10.1371/journal.pone.0033570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer ND, Goodarzi MO, Langefeld CD, et al. Quantitative trait analysis of type 2 diabetes susceptibility loci identified from whole genome association studies in the Insulin Resistance Atherosclerosis Family Study. Diabetes. 2008;57:1093–1100. doi: 10.2337/db07-1169. [DOI] [PubMed] [Google Scholar]
- 31.Ingelsson E, Langenberg C, Hivert MF, et al. Detailed physiologic characterization reveals diverse mechanisms for novel genetic Loci regulating glucose and insulin metabolism in humans. Diabetes. 2010;59:1266–1275. doi: 10.2337/db09-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boesgaard TW, Grarup N, Jorgensen T, Borch-Johnsen K, Hansen T, Pedersen O. Variants at DGKB/TMEM195, ADRA2A, GLIS3 and C2CD4B loci are associated with reduced glucose-stimulated beta cell function in middle-aged Danish people. Diabetologia. 2010;53:1647–1655. doi: 10.1007/s00125-010-1753-5. [DOI] [PubMed] [Google Scholar]
- 33.Kirchhoff K, Machicao F, Haupt A, et al. Polymorphisms in the TCF7L2, CDKAL1 and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia. 2008;51:597–601. doi: 10.1007/s00125-008-0926-y. [DOI] [PubMed] [Google Scholar]
- 34.Grarup N, Andersen G, Krarup NT, et al. Association testing of novel type 2 diabetes risk alleles in the JAZF1, CDC123/CAMK1D, TSPAN8, THADA, ADAMTS9, and NOTCH2 loci with insulin release, insulin sensitivity, and obesity in a population-based sample of 4,516 glucose-tolerant middle-aged Danes. Diabetes. 2008;57:2534–2540. doi: 10.2337/db08-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stancakova A, Pihlajamaki J, Kuusisto J, et al. Single-nucleotide polymorphism rs7754840 of CDKAL1 is associated with impaired insulin secretion in nondiabetic offspring of type 2 diabetic subjects and in a large sample of men with normal glucose tolerance. J Clin Endocrinol Metab. 2008;93:1924–1930. doi: 10.1210/jc.2007-2218. [DOI] [PubMed] [Google Scholar]
- 36.Groenewoud MJ, Dekker JM, Fritsche A, et al. Variants of CDKAL1 and IGF2BP2 affect first-phase insulin secretion during hyperglycaemic clamps. Diabetologia. 2008;51:1659–1663. doi: 10.1007/s00125-008-1083-z. [DOI] [PubMed] [Google Scholar]
- 37.Dupuis J, Langenberg C, Prokopenko I, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev. 1998;19:608–624. doi: 10.1210/edrv.19.5.0349. [DOI] [PubMed] [Google Scholar]
- 39.Wei FY, Suzuki T, Watanabe S, et al. Deficit of tRNA(Lys) modification by Cdkal1 causes the development of type 2 diabetes in mice. J Clin Invest. 2011;121:3598–3608. doi: 10.1172/JCI58056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang HS, Kim YS, ZeRuth G, et al. Transcription factor Glis3, a novel critical player in the regulation of pancreatic beta-cell development and insulin gene expression. Mol Cell Biol. 2009;29:6366–6379. doi: 10.1128/MCB.01259-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes. 2005;54:1649–1656. doi: 10.2337/diabetes.54.6.1649. [DOI] [PubMed] [Google Scholar]
- 42.Kim YS, Nakanishi G, Lewandoski M, Jetten AM. GLIS3, a novel member of the GLIS subfamily of Kruppel-like zinc finger proteins with repressor and activation functions. Nucleic Acids Res. 2003;31:5513–5525. doi: 10.1093/nar/gkg776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barrett JC, Clayton DG, Concannon P, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quaranta M, Burden AD, Griffiths CE, et al. Differential contribution of CDKAL1 variants to psoriasis, Crohn's disease and type II diabetes. Genes Immun. 2009;10:654–658. doi: 10.1038/gene.2009.51. [DOI] [PubMed] [Google Scholar]
- 45.Kumashiro N, Erion DM, Zhang D, et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. 2011;108:16381–16385. doi: 10.1073/pnas.1113359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Araya J, Rodrigo R, Pettinelli P, Araya AV, Poniachik J, Videla LA. Decreased liver fatty acid delta-6 and delta-5 desaturase activity in obese patients. Obesity (Silver Spring) 2010;18:1460–1463. doi: 10.1038/oby.2009.379. [DOI] [PubMed] [Google Scholar]
- 47.Vessby B, Gustafsson IB, Tengblad S, Boberg M, Andersson A. Desaturation and elongation of fatty acids and insulin action. Ann N Y Acad Sci. 2002;967:183–195. doi: 10.1111/j.1749-6632.2002.tb04275.x. [DOI] [PubMed] [Google Scholar]
- 48.Chambers JC, Zhang W, Sehmi J, et al. Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nat Genet. 2011;43:1131–1138. doi: 10.1038/ng.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fox CS, Liu Y, White CC, et al. Genome-wide association for abdominal subcutaneous and visceral adipose reveals a novel locus for visceral fat in women. PLoS Genet. 2012;8:e1002695. doi: 10.1371/journal.pgen.1002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bille DS, Banasik K, Justesen JM, et al. Implications of central obesity-related variants in LYPLAL1, NRXN3, MSRA, and TFAP2B on quantitative metabolic traits in adult Danes. PLoS One. 2011;6:e20640. doi: 10.1371/journal.pone.0020640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Speliotes EK, Yerges-Armstrong LM, Wu J, et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7:e1001324. doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heid IM, Jackson AU, Randall JC, et al. Meta-analysis identifies 13 new loci associated with waist–hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet. 2010;42:949–960. doi: 10.1038/ng.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dastani Z, Hivert MF, Timpson N, et al. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 2012;8:e1002607. doi: 10.1371/journal.pgen.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goodarzi MO, Cui J, Chen YD, Hsueh WA, Guo X, Rotter JI. Fasting insulin reflects heterogeneous physiological processes: role of insulin clearance. Am J Physiol Endocrinol Metab. 2011;301:E402–E408. doi: 10.1152/ajpendo.00013.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arafat AM, Mohlig M, Weickert MO, Schofl C, Spranger J, Pfeiffer AF. Improved insulin sensitivity, preserved beta cell function and improved whole-body glucose metabolism after low-dose growth hormone replacement therapy in adults with severe growth hormone deficiency: a pilot study. Diabetologia. 2010;53:1304–1313. doi: 10.1007/s00125-010-1738-4. [DOI] [PubMed] [Google Scholar]
- 56.Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.