Abstract
Depressive symptoms are considered to have evolutionary social functions to reduce social risks with peers and family members. However, social processes and their relationship to depressive symptoms have been understudied in adolescent boys. Low engagement in social contexts may predict depressive symptoms in adolescent boys, as it may signify efforts to reduce social risks. To address these issues, this study focused on 160 boys at risk for affective problems based on low socioeconomic status. We evaluated how behavioral and physiological engagement in peer and family contexts, respectively, in late childhood predicted depressive symptoms at age 12 and age 15. Social withdrawal was measured across late childhood (ages 9 to 12) in a camp setting using a latent variable of the teacher ratings of withdrawn behavior, peer nominations of withdrawn behavior, and camp counselor ratings of withdrawn behavior. Physiological reactivity was measured during a provocative parent-child conversation using respiratory sinus arrhythmia (RSA) at age 12. Social withdrawal in late childhood predicted depressive symptoms at age 12. The combination of high levels of social withdrawal with peers from ages 9 to 12 and low RSA reactivity with a parent at age 12 predicted higher depressive symptoms at age 15. Withdrawal in multiple social contexts may place boys at risk for depressive symptoms during the vulnerable period of adolescence.
Keywords: Depression, Adolescence, Social Withdrawal, RSA, Engagement
Introduction
Recent conceptual models have characterized depression as a disorder of problematic social functioning, with social frustrations (e.g., peer rejection) frequently preceding the onset of first episodes (Allen & Badcock, 2003). The current study examined how two components of low engagement in social contexts, diminished physiological arousal and greater behavioral withdrawal, may specifically predict depressive symptoms in adolescence. We propose that trait-like levels of low arousal and engagement in social situations may function as an endophenotype for depression (Hasler, Drevets, Manji, & Charney, 2004), in that withdrawing in social situations may be an effort to reduce the threat of social disappointment associated with depression. As rates of depressive symptoms in adolescence rise even in the absence of clinical diagnosis, and as even subthreshold levels of depressive symptoms have been associated with social and cognitive impairments and increased risk for clinical depression later in development (Lewinsohn, Solomon, Seeley, & Zeiss, 2000), we use the term depression throughout this article to signify both subthreshold depressive symptoms and clinical depression.
Low Engagement in Social Contexts: Threat-Related Withdrawal
Low engagement in social contexts may capture low approach and positive affect tendencies. Lack of pleasurable engagement in social activities appears to be a stronger predictor of depression than negative affect or distress in adolescents and adults (Clark & Watson, 1991; Joiner, Lewinsohn, & Seeley, 2002). An examination of behavioral withdrawal and physiological arousal in youth provides further evidence to support the claim that less engagement in social contexts is associated with concurrent depressive symptoms (Compas, Connor-Smith, & Jaser, 2004) and clinical diagnoses of depression (Rubin, Coplan, & Bowker, 2009; Silk et al., 2007).
Interestingly, there is also some support for the claim that these differences in behavioral and physiological engagement emerge prior to the onset of clinical depression. In regard to behavioral responses, social withdrawal in contexts associated with novel and rewarding experiences appears to be both a precursor to later clinical depression and present with concurrent depressive symptoms (Rubin et al., 2009). The association between low behavioral engagement and depressive symptoms appears to emerge as early as the preschool years, as less socially interactive preschoolers demonstrate an increased risk for internalizing problems (Rubin, Coplan, Fox & Calkins, 1995). In middle childhood and adolescence, youth who utilize coping strategies based on disengagement also tend to show higher levels of depressive symptoms (Compas et al., 2004). Behavioral social withdrawal also may diminish youth’s opportunity to experience and enjoy rewarding experiences (e.g., pursuing new relationships), a key risk factor of depression (Forbes & Dahl, 2005).
In addition to low levels of behavioral engagement in social contexts, children with low approach and engagement tendencies may exhibit low physiological arousal in social contexts. Low physiological arousal in both challenging and rewarding contexts has been associated with risk for depression as measured by multiple physiological indices (e.g., blunted cortisol-stress reactivity, Burke, Davis, Otte, & Mohr, 2005; diminished late pupil dilation, Silk et al., 2007). One measure of physiological arousal, respiratory sinus arrhythmia (RSA), has been used widely as a measure of engagement in social situations and trait-like regulation in children and adolescents (Calkins & Keane, 2004). Respiratory sinus arrhythmia (RSA) is advantageous as a physiological measure as it is postulated to reflect flexible modulation of heart rate by the parasympathetic nervous system in response to challenges (Gentzler, Santucci, Kovacs, & Fox, 2009). Low RSA appears to be related to depression (Rottenberg, Clift, & Bowker, 2007) and low physiological arousal during social experiences may be an additional mechanism of withdrawing arousal during challenging situations. Paired with behavioral withdrawal, children and adolescents with low physiological engagement may not fully activate autonomic resources to cope with challenging situations.
RSA reactivity, or changes in vagal tone from baseline when exposed to provocative or challenging experiences, putatively captures one’s ability to control physiologic responses appropriately. High RSA reactivity, characterized by greater decreases in RSA and increased heart rate in response to stressors, should reflect greater ability to respond during stressful situations. Whereas research findings on behavioral correlates of RSA reactivity have been varied, research has indicated that high RSA reactivity is adaptive among youth and is linked to lower levels of depressive symptoms (Gentzler et al., 2009; Shannon, Beauchaine, Brenner, Neuhaus, Gatzke-Kopp, 2007). In contrast, low RSA reactivity could reflect poor flexibility in responding to challenging situations and a greater tendency to withdraw (rather than engage or maintain emotion arousal) during challenging social contexts. Indeed, low baseline RSA and low RSA reactivity have been associated with clinical depression in adult populations (Rottenberg et al., 2007) and increased risk for depressive symptoms in 8 to 12 year old children (Shannon et al., 2007). Adolescents who show low RSA reactivity during challenging social contexts could be at greater risk for emerging depression, as this would signify a physiological pattern of withdrawing arousal in response to socially challenging situations.
Combination of Risk Factors and Specificity to Depression
Although individually behavioral withdrawal and low physiological arousal may convey risk for multiple types of problem behavior, the combination of these behavioral and physiological responses in social contexts could be particularly important to the development of depression given the postulated role of threat-related withdrawal as an endophenotype for depression (Hasler et al., 2004).Hasler et al. (2004) describes an endophenotype as a behavioral manifestation of a gene associated with a disorder that is present in those at risk for the disorder and before manifestation of the disorder. Social withdrawal and low physiological reactivity likely represent two independent, but related factors of an endophenotype. As some empirical evidence indicates that both RSA reactivity and social withdrawal may be stable and trait-like (Calkins & Keane, 2004; El-Sheikh, 2005; Rubin et al., 2009), low RSA reactivity and high levels of social withdrawal may be correlates of depressive symptoms prior to the onset of clinical depression (Rubin et al., 2009). Identification of endophenotypes can be used to determine which youth are at greater risk for development of the disorder (i.e., depression) later in life.
Research Questions and Hypotheses
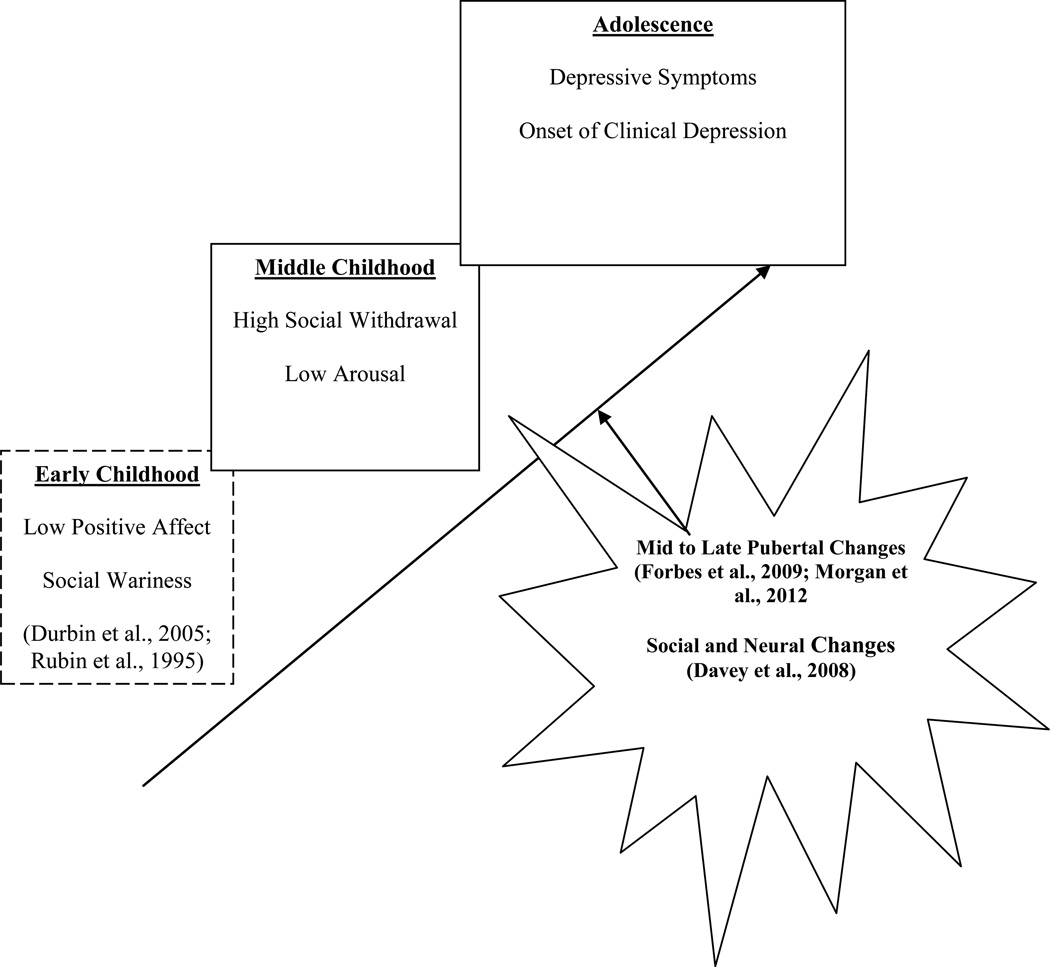
We suggest that boys who demonstrate stable tendencies toward social withdrawal (via both behavioral and physiological indices) during challenging social situations (both with peers and family) may be at greater risk for developing depression during adolescence, when they may be more likely to employ these threat-reduction responses due to the increased salience of social rewards. Indeed, depression may emerge increasingly during adolescence because failure to achieve social rewards becomes more salient in the face of greater concern about social goals and increasing awareness of one’s success in achieving such goals during adolescence (Davey, Yucel, & Allen, 2008). It may be that those with social behavioral traits in childhood that are indicative of disengagement in social contexts may be at greater risk for developing depression in adolescence, once these social contexts become more developmentally important. We propose that these social behavioral traits (putatively influenced by an endophenotype) may play a causal role for one pathway leading to adolescent depression, such that these social behavioral traits may be present for some children prior to depressive symptoms becoming manifest in adolescence (see Figure 1 for theoretical model).
Figure 1.
Hypothesized model of social behavioral traits on adolescent depression
Note: Dashed boxes reflect parts of theoretical model not tested in current study but demonstrated in prior research.
We expected that adolescents who show both behavioral and physiological patterns of withdrawal in social contexts (i.e., behavioral withdrawal and low RSA reactivity) would be at risk for higher levels of depressive symptoms in adolescence than those who do not show those patterns. This vulnerability may result from greater likelihood of missing out on potentially rewarding and positive experiences (e.g., forming new friendships, strengthening parent-child relationships). We evaluated this research question in a sample of adolescent boys at high socioeconomic risk for various behavioral problems, including depression. Despite boys’ being an understudied population for research of depression, boys also experience increases in depressive symptoms during adolescence and risk factors associated with depressive symptoms in boys are less known (Cicchetti & Toth, 1998). Internal processes may affect depression differently in adolescent boys. For example, social withdrawal appears to be associated with greater social costs for boys compared with girls (Coplan, Prakash, O’Neil, & Armer, 2004). An evaluation of these social behavioral traits as risk factors for adolescent depression in boys seems necessary.
We utilized data from multiple settings and informants, specifically social withdrawal as reported by peers and camp counselors in a camp setting and teachers from a classroom setting across late childhood (ages 9 to 12). As an assessment of relevant social contexts in adolescence, we evaluated withdrawal of physiological arousal in a discussion task with parents, as parent-child interactions represent an important means of capturing adolescent engagement and arousal in response to socially challenging, yet rewarding interactions and the family context is considered an important one for the development of depression (Morris, Silk, Steinberg, Myers, & Robinson, 2007). We chose to measure behavioral and physiological engagement in social contexts in late childhood and depressive symptoms in late childhood and adolescence, as we were interested in how social behavioral traits present during childhood may be associated with the emergence of depressive symptoms in adolescence. Depressive symptoms were measured at age 12 and at age 15 to test the independent and additive effects of these social behavioral traits on concurrent and later depressive symptoms. We chose age 15 for our longitudinal measurement of depressive symptoms based on evidence suggesting that rates of depression begin to peak at this age (Hankin et al., 1998; Lewinsohn, Clarke, Seeley, & Rohde, 1994). We also expected that because of the social and neural changes occurring during mid-adolescence (Davey et al., 2008), the increasing rates of depression during mid-adolescence (Lewinsohn et al., 1994), and the strong association between depressive symptoms and mid-to-late puberty (Morgan, Olino, McMakin, Ryan, & Forbes, 2012), boys’ vulnerability to these social behavioral traits would increase risk for depressive symptoms at age 15 but not at age 12.
Thus, we hypothesized that high levels of social withdrawal in late childhood (9 to 12) would be associated with depressive symptoms at age 15 but not at age 12. We also predicted that low RSA reactivity at age 12 would relate to higher levels of depressive symptoms at age 15, but not at age 12. Additionally, we expected that both high levels of social withdrawal and low RSA reactivity would contribute independent variance to the prediction of age 15 depressive symptoms when examined within a multivariate framework. Finally, we predicted that the interaction between high social withdrawal and low RSA reactivity would predict higher levels of adolescent depressive symptoms at age 15. We explored whether this moderating effect would be additive (e.g., presence of both social withdrawal and low RSA reactivity would both independently and in combination predict higher depressive symptoms) or interactive (i.e., the effect of one on depressive symptoms depends on the presence of the other).
Method
Participants and Procedure
Participants were 160 boys from an ongoing longitudinal project on vulnerability and resilience in lowincome families, the Pitt Mother and Child Project (PMCP). Boys were recruited to the original study between the ages of 7 and 17 months of age from the Women, Infants, and Children (WIC) program in the greater Pittsburgh area. Data from this larger project have been published previously (e.g., Forbes, Shaw, & Dahl, 2007; Trentacosta & Shaw, 2009). All participants were boys because of the project’s original focus on developmental pathways to antisocial behavior in low-income boys, for which rates are higher than for girls (Keenan & Shaw, 1994). However, boys were expected to be at elevated risk for various other behavioral problems, including depression, based on their low SES status (Wadsworth & Achenbach, 2005). Boys in the sample showed variable levels of depressive symptoms (1.9% with clinical diagnoses of depression based on the KSADS at age 12; 8.6% with clinical diagnoses of depression based on the KSADS at age 15). Boys in the sample also showed relatively high levels of family histories of depression. For example, at age 10, 26% of boys had mothers with a BDI score in the mild depression range, 7% in the moderate depression range, and <1% in the severe depression range. The sample consisted of 52% European-American boys, 40% African American boys, and 8% of other ethnicity (e.g., Biracial, Hispanic). Average family income was $1,952 per month (SD = $1,324) and the mean SES score was 30.7 (SD = 9.6) using the Hollingshead Index, indicating working-class status (Hollingshead, 1975).
Originally, 310 boys and their families were recruited to participate in the longitudinal project (PMCP). At age 9/10, the families of all 310 boys were invited to have their sons participate in a two week summer camp study (SCS). The SCS was a sub-component of the larger PMCP and was not intended to serve as an intervention but as a vehicle for measuring children’s behavior with peers in a naturalistic setting. Because of the time commitment required of the SCS, only 145 of the 310 boys participated. Boys’ teachers also reported on their socially withdrawn behavior at ages 11 and 12. At age 12, RSA reactivity was measured while boys participated in a “hot topics” discussion with a parent. Only 160 of the original 310 boys completed the Hot Topics interaction. Analyses in the present paper are derived from this smaller sample of boys (n = 160 with RSA reactivity data). Boys with RSA data but not camp data (n = 20) were retained in the structural equation modeling (SEM) analyses as SEM allows for estimation of missing data. At age 15, boys rated their depressive symptoms during a semi-structured interview and on a questionnaire. There were no significant differences in mother’s education level, family income, or socioeconomic status at study entry (18 months old) or in teacher-rated withdrawn behavior and depressive symptoms at age15 for boys who participated in the SCS relative to those who did not participate in the SCS. There were also no significant differences in these variables for boys with RSA data and those without these data.
Social withdrawal
At age 9/10, boys attended one of three sessions of the SCS. Each group (‘huddle’) of boys within a session was comprised of 10–12 boys and lasted for 10 days across a two-week period. Groups were led by undergraduate level counselors and were heterogeneous with respect to child age. Boys were placed in camp groups with other boys whom they had not previously met (Trentacosta & Shaw, 2009).
At the end of the two-week camp, boys’ camp counselors completed a 32-item behavioral ratings measure based on the Playground Observer Impressions (POI; Reid, Fetrow, & Mayne, 1991) on each child. We used the four item shy/withdrawn subscale of this measure as part of our social withdrawal composite. Sample items include “did the child seem detached or distant?” and “is the child ignored by peers?” The internal consistency of this subscale was .81.
Additionally, at the end of both weeks of a session boys were told to nominate as many peers as they would like on four items associated with social withdrawal. These items were “are usually ignored”, “are very shy”, “are picked on by other kids” and “are afraid to stand up for themselves” (α = .84). Each boy then received a score of peer nominated social withdrawal based on the total number of nominations they received on these four items.
Boys’ teachers also completed Achenbach’s (1992) Teacher Report Form (TRF) Withdrawn behavior subscale when boys were 11 and 12 years old. Sample items from the Withdrawn subscale include “would rather be alone than with others”. The internal consistencies for these teacher ratings were .83 and .78, respectively.
We created a latent variable for behavioral social withdrawal using MPLUS 5 (Muthen & Muthen, 1998) using teacher-reported withdrawn behavior on the TRF at ages 11 and 12, peer nominations of withdrawn behavior from the SCS at age 9/10, and camp counselor rating of withdrawn behavior from the SCS at age 9/10. A confirmatory factor analysis indicated that model fit (CFI = .98) and factor loadings (β = .35 to .96; ps < .01) were good for this latent variable.
RSA Reactivity
At age 12, physiological data were collected during a “hot topics” discussion task, in which parents and target youth discussed issues in the parent-child relationship that parents had identified as being of concern in their relationship (e.g., discussion of household chores or grades in school) based on a list completed earlier in the assessment (Hetherington et al., 1992). This task is designed to engage children and parents in an interaction focused on a topic of strong mutual interest. It typically elicits negative emotions in children and parents but provides an opportunity to resolve disagreements and to regulate emotions. To assess RSA we used the 3992/2-ER Biolog system, which provides ambulatory electrocardiograph (ECG) recording sampled at 1000 Hz and respiration sampled at 5 Hz. Boys were attached to the Biolog system using three electrodes placed on the upper chest just below the right and left shoulders and on the left abdomen near the bottom of the ribs, respectively. Physiological data were recorded continuously throughout a 5-min baseline period when boys were reading a magazine, and during the first 5 min of the “hot topics” task (Vanderbilt & Shaw, 2007). We used a program for spectral analysis of point events (PSPAT) to process the data, after correcting artifact R-wave occurrences in the ECG signals (Weber, Molenaar, & van der Molen, 1988). Following standard recommendations, oscillations in heart period occurring within the high-frequency (HF) range of .15–.40 Hz were used as an estimate of parasympathetic (vagal) activity (Berntson et al., 1997).
Baseline RSA was measured during the initial five minutes when boys read a magazine. RSA reactivity was computed by subtracting task HF from baseline HF, with positive change scores indicating greater vagal withdrawal (high RSA reactivity) or, in other words, greater cardiac activity in response to the challenging task. On the other hand, negative change scores indicate less withdrawal of the vagal brake (i.e., low RSA reactivity, Calkins, Blandon, Williford, & Keane, 2007). As a behavioral check of task participation, independent observers globally coded each boy’s engagement with his parent during the hot topics task as a measure of behavioral participation in the task. RSA reactivity was not significantly correlated with boys’ level of engagement during the hot topics task (r = −.11, ns), indicating that low RSA reactivity did not simply reflect failure to participate as instructed. Furthermore, engagement in the Hot Topics task was not associated with social withdrawal (t = 1.14, ns).
Depressive symptoms
Boys completed the short form of the Child Depression Inventory (CDI; Kovacs, 1978) at ages 12 and 15. The short form of the CDI is a 10-item scale that asks boys to self-report depressive symptoms. Sample items include “Nothing will ever work out for me” and “I am sad all the time.” Also at both the age 12 and 15 assessments, boys participated in a semi-structured interview, the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS; Kaufman et al., 1997). We totaled the scores on the first three items of the K-SADS depressive disorders module to measure current depressive symptoms. We chose to use only the first three items of the depressive disorders module because these items are used as a screen for continuing with the modules and were therefore administered to all participants. Using additional items after the screening items would have resulted in an incomplete data set. We aggregated boys’ self-report CDI scores and the screening items from the K-SADS (rs = .21 and.26, p < .01 for ages 12 and 15 respectively) to create a depressive symptoms composite score (α = .58 for age 12, α = .69 for age 15). We opted to use an aggregate of CDI depressive symptoms and KSADS depressive symptoms, rather than creating a latent variable as it is recommended that latent variables have three or more indicators for adequate loading (Kline, 2005).
Results
Preliminary Analyses
In Table 1 are means, standard deviations and intercorrelationships of the study variables, including means and standard deviations (SD) of the measures assessing boys’ depressive symptoms (i.e., CDI and KSADS). RSA reactivity did not correlate significantly with depressive symptoms at age 15. Baseline RSA was correlated with higher RSA reactivity (r = .37, p < .01). Teacher ratings of withdrawn behavior at age 12, peer nominations at age 9/10 during week 1 and at age 9/10 during week 2 of camp were correlated with age 12 depressive symptoms (r = .25, p < .01; r = . 20, p < .03; r = .19, p < .04, respectively). None of our social withdrawal indicators (peer nominations, camp counselor ratings, and teacher ratings of withdrawn behavior) were correlated with RSA reactivity or our age 15 depressive symptoms aggregate variable.
Table 1.
Means, Standard Deviations, and Intercorrelationships Among Social Withdrawal, Baseline RSA, RSA Reactivity, and Depressive Symptoms
| Variable | M | SD | R | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Baseline RSA (age 12) | .24 | .24 | .00–1.59 | -- | -- | -- | -- | -- | -- | -- | -- |
| 2. | RSA Reactivity (age 12) | .00 | .19 | −1.24–1.05 | .37** | -- | -- | -- | -- | -- | -- | -- |
| 3. | SW Week 1 Peer Rating (age 9/10) | 5.57 | 4.62 | .00–25.00 | −.07 | -.16 | -- | -- | -- | -- | -- | -- |
| 4. | SW Week 2 Peer Rating (age 9/10) | 4.84 | 4.18 | .00–20.00 | −.13 | .00 | .75** | -- | -- | -- | -- | -- |
| 5. | SW Camp Counselor (age 9/10) | 2.21 | .94 | 1.00–5.00 | .03 | .01 | .32** | .25** | -- | -- | -- | -- |
| 6. | SW Teacher Ratings (age 11) | 3.28 | 3.57 | .00–17.00 | −.11 | .02 | .37** | .28* | .13 | -- | -- | -- |
| 7. | SW Teacher Ratings (age 12) | 2.59 | 2.83 | .00–13.00 | −.07 | −.10 | .45** | .37** | .15 | .48** | -- | -- |
| 8. | Depressive Symptoms (age 12) | .01 | 2.63 | −1.30–15.79 | .08 | .07 | .20* | .19* | .12 | .16 | .25** | -- |
| 9. | Depressive Symptoms (age 15) | .01 | 1.59 | −1.14–7.22 | .08 | .04 | .02 | −.09 | .00 | .08 | −.04 | .22** |
| 10. | CDI (age 12) | .96 | 1.39 | .00–6.00 | -- | -- | -- | -- | -- | -- | -- | -- |
| 11. | CDI (age 15) | 1.27 | 1.79 | .00–10.00 | -- | -- | -- | -- | -- | -- | -- | -- |
| 12. | KSADS (age 12) | .12 | .43 | .00–3.00 | -- | -- | -- | -- | -- | -- | -- | -- |
| 13. | KSADS (age 15) | .31 | .72 | .00–3.00 | -- | -- | -- | -- | -- | -- | -- | -- |
Note:
p < .01.
p < .05.
p < .10.
SW = social withdrawal. RSA = respiratory sinus arrhythmia.
Structural Equation Modeling
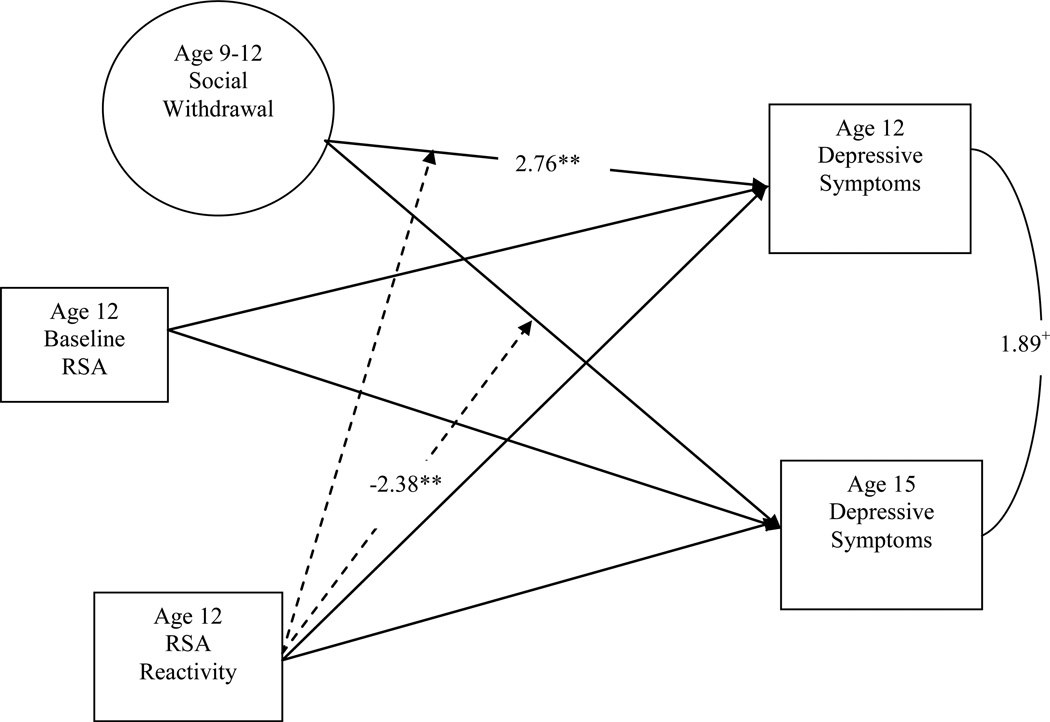
Structural equation modeling (SEM) in MPLUS 5 (Muthen & Muthen, 1998) was used to evaluate our hypothesis that the combination of high levels of social withdrawal and low RSA reactivity would relate to more depressive symptoms. We used SEM as it allows for modeling of latent variables and simultaneous evaluation of two dependent variables (Kline, 2005).
Our model consisted of baseline RSA at age 12, the latent variable of social withdrawal across late childhood (ages 9 to 12), RSA reactivity at age 12, and the interaction of RSA reactivity and social withdrawal on depressive symptoms at age 12 and depressive symptoms at age 15 (Figure 1). We included depressive symptoms at both age 12 and age 15 as dependent variables to test both the concurrent and longitudinal effects of these social behavioral traits on adolescent depressive symptoms. The path from age 12 to age 15 depressive symptoms was also freed in the model, allowing us to account for the effects of early depressive symptoms (at age 12) on later depressive symptoms (age 15) and statistically adjust for continuity in depression. Baseline RSA measured at age 12 was entered as a covariate in the model as initial levels of RSA may influence reactivity, by limiting the amount of change that is possible (Santucci et al., 2008).
As shown in Table 2, greater social withdrawal was positively related to boys’ depressive symptoms at age 12 (t = 2.76, p < .01, d = .44). Age 12 depressive symptoms approached significance in correlating with age 15 depressive symptoms (t = 1.89, p < .06, d = .30). RSA reactivity and the interaction between RSA reactivity and social withdrawal did not predict depressive symptoms at age 12. Contrary to our hypothesis, neither social withdrawal nor RSA reactivity was directly related to depressive symptoms at age 15. However, the interaction between RSA reactivity and social withdrawal was significant in predicting depressive symptoms at age 15 (t = −2.38, p < .01, d = .38).
Table 2.
Structural Equation Model of Depressive Symptoms at Age 12 and Age 15 on Baseline RSA, RSA Reactivity, Social Withdrawal, and the Interaction between RSA Reactivity and Social Withdrawal
| Unstandardized Model Effects | β | t | p | Cohen’s d |
|---|---|---|---|---|
| Age 15 Depressive Symptoms (N = 160) | ||||
| Baseline RSA | .20 | −.35 | .73 | |
| Social Withdrawal | −.23 | −1.06 | .29 | |
| RSA Reactivity | 1.10 | 1.08 | .28 | |
| Social Withdrawal X RSA Reactivity | −3.31 | −2.38 | .02 | −.38 |
| Age 12 Depressive Symptoms (N = 160) | ||||
| Baseline RSA | .65 | .85 | .40 | |
| Social Withdrawal | .68 | 2.76 | .01 | .44 |
| RSA Reactivity | 1.00 | 1.32 | .19 | |
| Social Withdrawal X RSA Reactivity | −.68 | −.61 | .54 |
Note. Effect sizes (Cohen’s d) are reported for values significant at p < .10.
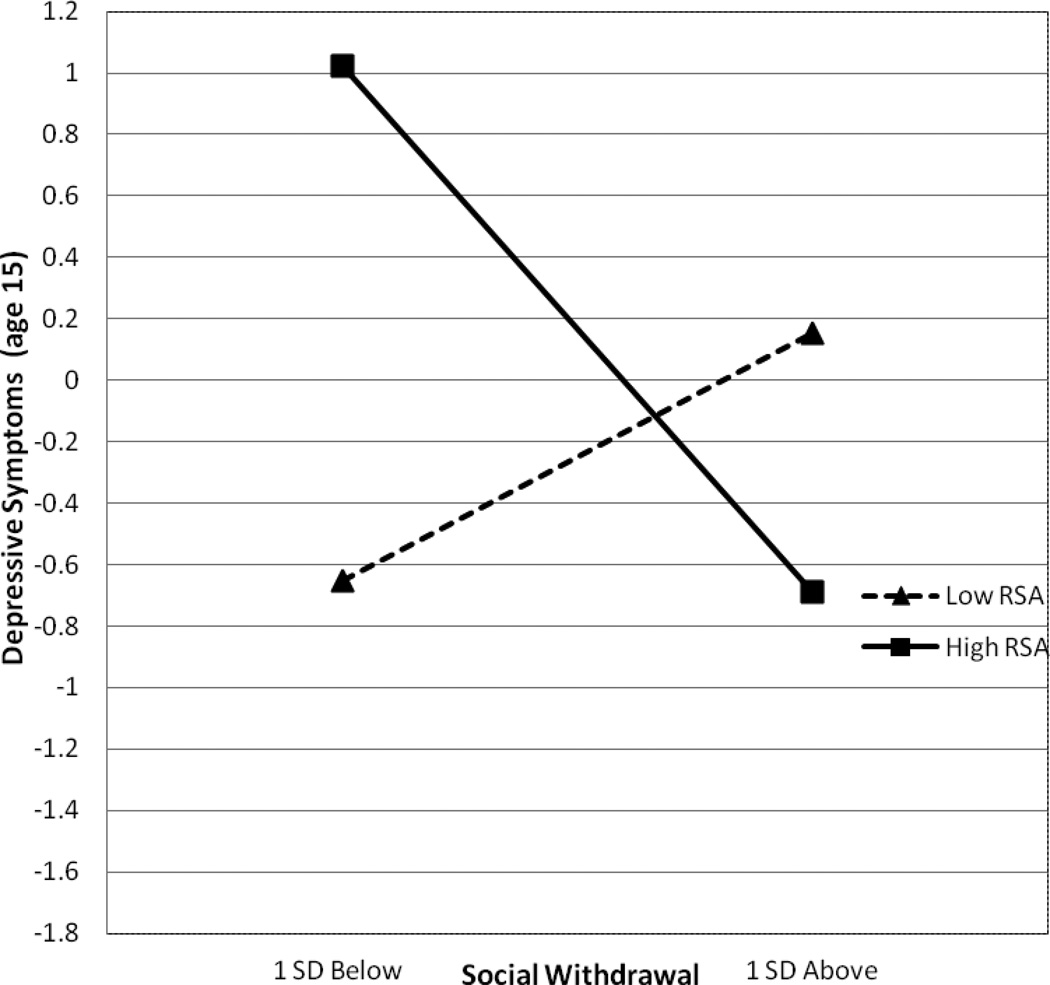
Post-hoc simple slope analyses were conducted to examine the nature of the interaction (Bauer & Curran, 2005). Figure 2 illustrates the interaction between RSA and social withdrawal in relationship to depressive symptoms. Consistent with our hypotheses, more social withdrawal predicted more depressive symptoms, but only in the context of low RSA reactivity (t = 2.37, p < .01, d = .38 from simple slopes). Indeed, boys who were both low on RSA reactivity and high on social withdrawal reported the highest levels of depressive symptoms at age 15 compared to all other groups of boys. Unexpectedly, greater social withdrawal related to fewer depressive symptoms at age 15 for boys high on RSA reactivity, however this association only approached significance (t = −1.91, p < .06, d = −.30 from simple slopes).
Figure 2.
Structural Equation Model of the Interactive Effect of Social Withdrawal and RSA Reactivity on Depressive Symptoms at age 15.
Note. Variables in circles (i.e., age 9 to 12 social withdrawal) represent latent variables. T-values are reported only for paths significant at p < .10
Discussion
A large body of research has evaluated risk factors for depression in girls (e.g., Gotlib et al., 2010; Keenan & Hipwell, 2005; Kercher, Rapee, & Schniering, 2009; Stice, Presnell, & Bearman, 2001), but less attention has been given to social behavioral characteristics that may place boys at risk for depression. Low engagement in social contexts appears to be associated with social costs in boys (Coplan et al., 2004) and theoretical models indicate that social behavioral characteristics, such as threat-related withdrawal, may be particularly relevant to the onset of depression in adolescence for both males and females (Davey et al., 2008). Based on this theory, we hypothesized that boys who show behavioral and physiological characteristics of low social engagement would have higher levels of depressive symptoms during mid-adolescence. Indeed, we found that the combination of high social withdrawal and low RSA reactivity predicted higher levels of depressive symptoms at age 15, but not at age 12. The use of multiple methods and multiple informants (i.e., teacher-report, peer nominations, RSA reactivity) of social engagement across two settings (i.e., naturalistic camp, laboratory observation) and assessed with two important social influences (i.e., peers, parent) added clinical and ecological relevance to the study. Our findings suggest that boys who show multiple forms of low social engagement across social contexts are at particular risk for developing depressive symptoms during mid-adolescence.
Thus, we were able to identify two trait-like risk factors (low RSA reactivity during a family interaction and high behavioral withdrawal with peers) that, in combination, were associated with depressive symptoms during mid-adolescence but not late childhood. Indeed, our hypothesized findings that adolescents who show both higher social withdrawal in contexts with novel peers and lower RSA reactivity (less physiological arousal) during a potentially conflictual social interaction with a parent are at greater risk for depressive symptoms at age 15 (but not at age 12) lend behavioral and physiological support to conceptual models on depression, adolescent social and neural changes, and efforts to reduce social risk (Allen & Badcock, 2003; Davey et al., 2008). Boys who show both less behavioral and physiological engagement in social contexts that appear challenging may be withdrawing to reduce social threat and, as a result of their low engagement, may be less likely to pursue and experience rewarding social interactions, increasing their risk for depression. This finding may indicate that multiple forms of low engagement during challenging situations in late childhood may amplify risk for later depressive symptoms, although the combination of these factors may not be associated with concurrent depressive symptoms in late childhood.
Our hypothesis that the direct effects of social withdrawal and low RSA reactivity would predict depressive symptoms at age 15 but not at age 12 was not supported. Neither social withdrawal nor RSA reactivity individually predicted depressive symptoms at age 15. This finding indicates perhaps that risk for depressive symptoms becomes manifest only when markers of withdrawal are evident in multiple domains (i.e., behaviorally and physiologically). Only when boys showed low RSA reactivity did social withdrawal predict boys’ depressive symptoms at age 15. Further, this finding may suggest that the presence of both of these social behavioral factors (low RSA reactivity and social withdrawal) could represent an endophenotype for depression (Hasler et al. 2004), that is present prior to the onset of the clinical disorder and increases vulnerability during the sensitive period of mid-adolescence. Indeed, exploratory analyses indicated that multiple forms of low social engagement across late childhood still predicted higher depressive symptoms at age 15, even when boys with clinical diagnoses at age 12 (n =3) were removed from the analyses.
Greater social withdrawal independently predicted depressive symptoms at age 12. Whereas we purport that high social withdrawal and low physiological arousal represent an endophenotype of low social engagement indicative of risk for later depression, social withdrawal in isolation may also be troublesome for boys at the transition from late childhood to early adolescence (age 12) when concerns about social status and reputation start to become more salient (Rose & Rudolph, 2006). We did not find that low RSA reactivity independently predicted adolescent depressive symptoms at age 12 or age 15. Instead, we found that low RSA reactivity served as a moderator on the effect of social withdrawal on depressive symptoms in middle adolescence (age 15). Our use of a latent variable of social withdrawal in multiple settings (classroom, camp setting) with multiple informants (teachers, peers, camp counselor) across time (ages 9 to 12) allowed us to more comprehensively measure social withdrawal than our one-time measurement of RSA reactivity. It may be possible that a more comprehensive measurement of physiological reactivity (across multiple settings and with multiple techniques) may have produced more robust findings of low physiological reactivity on depressive symptoms.
Finally, although we hypothesized that we would find an additive effect of low RSA reactivity and high social withdrawal on age 15 depressive symptoms, our results provide evidence of an interactive effect of these characteristics on adolescent depressive symptoms. Greater social withdrawal predicted higher levels of depressive symptoms at age 15, but only for boys with low RSA reactivity. It appears that greater social withdrawal may be associated with less depressive symptoms at age 15 for boys with high RSA reactivity. Although much research has indicated that high RSA reactivity is adaptive (Gentzler et al., 2009; Shannon et al., 2007), some evidence suggests that there is an optimal level of RSA reactivity for adaptive functioning, with extreme levels of high or low RSA reactivity associated with emotional and behavioral problems (Calkins et al., 2007). For boys with levels of RSA reactivity one SD above the mean, social withdrawal may serve as a protective factor against emotional problems, such as depressive symptoms. Another possibility is that boys with adaptive levels of high RSA reactivity and social withdrawal may simply prefer solitude to spending time with peers (Rubin et al., 2009). However, as this interactive finding was not hypothesized and was only marginally significant, corroboration of this interaction would be needed in future research before drawing firm conclusions about its validity.
The timing of our measurements allowed us to understand how behavioral and physiological engagement responses to social challenges in late childhood may relate to the development of depressive symptoms at age 15, a vulnerable period for the development of depression. We measured social withdrawal at the cusp of late childhood and early adolescence, when social rewards start to become increasingly important to the individual (Davey et al., 2008; Morris et al., 2007). By evaluating RSA reactivity during a parent-child provocative task at age 12, when emotions during parent-child difficulties may be intensified during the emergence of adolescence (Morris et al., 2007), we were able to obtain a snapshot of boys’ physiological regulation abilities during a developmentally-salient time point. Furthermore, previous literature has indicated that both behavioral social withdrawal and RSA reactivity appear to be relatively stable (Gentzler et al., 2009; Rubin et al., 2009). In this case, behavioral and physiological traits of low engagement during challenging social contexts that may be rewarding may both mark the presence of genetic vulnerability to depression and set children up to develop depression during adolescence when social rewards and affiliative bonds become more valued.
Previous literature has indicated a greater tendency for depression in females relative to males, perhaps as a result of females’ greater tendency to be socially oriented and affiliative relative to males (Compas et al., 2004). However, although the types of social risks that affect adolescent boys may be different in nature than those that affect adolescent girls, they also may increase risk for depression. Adolescent boys tend to value social status among their peers and are more likely than girls to work towards creating relationships that promote such status (Rose & Rudolph, 2006). Boys high on social withdrawal in our study were deemed so in part by peer nominations, indicating some possibility that these boys were viewed as having lower social status than other boys in the camp setting. Understanding how social processes may relate to depression in adolescent boys is important as boys pursue these types of social rewards during this developmental period.
Strengths and Limitations
Our study included multiple methods and informants (self-report, camp counselor report, peer nominations, physiological measures) to measure our constructs of interest. As noted earlier, the use of peer nominations and camp counselors to assess social withdrawal was one of the study’s important methodological strengths. This method of data collection allowed us to obtain important information on peer perception regarding which peers do not engage with others in social settings. We also were able to incorporate responses to two different but important social contexts in adolescence: the parent-child context and the peer context. An additional strength was our evaluation of adolescent engagement in social situations in a controlled laboratory setting and in a naturalistic, real-world setting. Furthermore, the study’s longitudinal design allowed us to examine risk factors in late childhood and early adolescence that were predictive of depressive symptoms during mid-adolescence. Overall, a longitudinal design that evaluated how multiple indices of social withdrawal across settings predicted depressive symptoms in adolescence was a major strength of our study.
The current study is one of few to focus on development of depressive symptoms in adolescent boys. Understandably, much research has focused on depression in adolescents in general or on how depression emerges in adolescent girls because of the gender differences in rates of depression beginning after puberty (Cicchetti & Toth, 1998). Research is also needed to understand how depression emerges in boys, especially populations of boys at heightened risk for psychopathology because of genetic, family, and/or socioeconomic risk factors. As all of our participants were boys from primarily low-income and urban settings, we are unable to generalize our findings to girls, and boys and girls from higher socioeconomic strata and living in rural or suburban communities. It is possible that interactions between social withdrawal and RSA reactivity during late childhood may affect the emergence of depressive symptoms in adolescence differently than in girls, and boys and girls from higher-SES backgrounds living in non-urban settings. As this is a novel finding, future research should replicate our findings in other samples of boys and girls across socioeconomic strata from rural, suburban, and urban communities. It also would be important to evaluate how these social behavioral traits predict clinical depression in adolescents. Only 8.6% of boys in our sample had clinical diagnoses of depression on the KSADS at age 15, limiting our ability to examine this question.
Another limitation was our one-time measurement of RSA at age 12. As the purpose of our study was to evaluate how RSA reactivity and social withdrawal act in tandem as trait-like characteristics to increase risk for depression, measurement of these two constructs at the same time is recommended. Whereas we measured social withdrawal using multiple methods in multiple settings across late childhood, our evaluation of RSA reactivity at a single time point resulted in our inability to confirm the stability of RSA reactivity. We do believe that, because our measurement of social withdrawal and RSA reactivity overlapped at age 12, we have captured their interaction in daily life in adolescent boys. However, clearly more research is needed to replicate our finding. Multiple measurements of RSA may provide more robust findings in the evaluation of physiological withdrawal on depressive symptoms in adolescence.
Conclusion
Our findings are important and have the potential to inform prevention efforts, as they provide information on identification of boys at risk for depression based on observable trait-like behavioral and physiological patterns. Boys who show multiple forms of low social engagement showed higher levels of depressive symptoms during mid-adolescence, a developmental period when neural and social changes increase the value and salience of social contexts. Boys with these social behavioral traits may be protected from depressive symptoms during middle childhood prior to the onset of these social developmental changes, as the combination of these traits was not associated with depressive symptoms during middle childhood. Boys with high levels of withdrawn behavior and low arousal during different social contexts may benefit from preventive interventions focused on increasing their engagement and enjoyment with peers and family members in middle childhood and during earlier developmental periods.
Figure 3.
Interaction of Social Withdrawal and RSA Reactivity on Depressive Symptoms at age 15.
Acknowledgements
This research was funded by Grants MH50907 and MH46925 from the National Institute of Mental Health. We thank the staff and families of the Pitt Mother and Child Project for making this research possible.
References
- Achenbach TM. Manual for the Child Behavior Checklist and Revised Child Behavior Profile. Burlington: University of Vermont, Department of Psychiatry; 1992. [Google Scholar]
- Allen NB, Badcock PBT. The social risk hypothesis of depressed mood: Evolutionary, psychosocial, and neurobiological perspectives. Psychological Bulletin. 2003;129:887–913. doi: 10.1037/0033-2909.129.6.887. [DOI] [PubMed] [Google Scholar]
- Bauer DJ, Curran PJ. Probing interactions in fixed and multilevel regression: Inferential and graphical techniques. Multivariate Behavioral Research. 2005;40:373–400. doi: 10.1207/s15327906mbr4003_5. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Keane SP. Cardiac vagal regulation across the preschool period: Stability, continuity, and implications for childhood adjustment. Developmental Psychobiology. 2004;31:125–135. doi: 10.1002/dev.20020. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Blandon AY, Williford AP, Keane SP. Biological, behavioral, and relationshipal levels of resilience in the context of risk for early childhood behavior problems. Development and Psychopathology. 2007;19:675–700. doi: 10.1017/S095457940700034X. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Keane SP. Cardiac vagal regulation differentiates among children at risk for behavior problems. Biological Psychology. 2007;74:144–153. doi: 10.1016/j.biopsycho.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. The development of depression in children and adolescents. American Psychologist. 1998;53:221–240. doi: 10.1037//0003-066x.53.2.221. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;1000:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Compas BE, Connor-Smith J, Jaser SS. Temperament, stress reactivity, and coping: Implications for depression in childhood and adolescence. Journal of Clinical Child and Adolescent Psychology. 2004;33:21–31. doi: 10.1207/S15374424JCCP3301_3. [DOI] [PubMed] [Google Scholar]
- Coplan RJ, Prakash K, O’Neil K, Armer M. Do you “want” to play? Distinguishing between conflicted-shyness and social disinterest in early childhood. Developmental Psychology. 2004;40:244–258. doi: 10.1037/0012-1649.40.2.244. [DOI] [PubMed] [Google Scholar]
- Davey CG, Yucel M, Allen NB. The emergence of depression in adolescence: Development of prefrontal cortex and the representation of reward. Neuroscience and Biobehavioral Reviews. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Durbin CE, Klein DN, Hayden EP, Buckley ME, Moerk KC. Temperamental emotionality in preschoolers and parental mood disorders. Journal of Abnormal Psychology. 2005;114:28–37. doi: 10.1037/0021-843X.114.1.28. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M. Stability of respiratory sinus arrhythmia in children and young adolescents: A longitudinal examination. Developmental Psychobiology. 2005;46:66–74. doi: 10.1002/dev.20036. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Neural systems of positive affect: Relevance to understanding child and adolescent depression? Developmental Psychopathology. 2005;17:827–850. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Shaw DS, Dahl RE. Alterations in reward-related decision making in boys with recent and future depression. Biological Psychiatry. 2007;61:633–639. doi: 10.1016/j.biopsych.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Gentzler AL, Santucci AK, Kovacs M, Fox N. Respiratory sinus arrhythmia reactivity predicts emotion regulation and depressive symptoms in at-risk and control children. Biological Psychology. 2009;82:156–163. doi: 10.1016/j.biopsycho.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joorman J. Neural processing of reward and loss in girls at risk for major depression. Archives of General Psychiatry. 2010;67:380–387. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson L, Moffitt TE, Silva P, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmocology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Hetherington EM, Clingempeel WG, Anderson ER, Deal JE, Stanley Hagan M, Hollier AE, Lindner MS. Coping with marital transitions: A family systems perspective. Monographs of the Society for Research in Child Development. 1992;57(Nos. 12-3):227. [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale University: Department of Sociology; 1975. Unpublished manuscript, [Google Scholar]
- Joiner TE, Lewinsohn PM, Seeley JR. The core of loneliness: Lack of pleasurable engagement— more so than painful disconnection—predicts social impairment, depression onset, and recovery from depressive disorders among adolescents. Journal of Personality Assessment. 2002;79:472–491. doi: 10.1207/S15327752JPA7903_05. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keenan K, Hipwell A. Preadolescent cues to understanding depression in girls. Clinical Child and Family Psychology Review. 2005;8:89–105. doi: 10.1007/s10567-005-4750-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan K, Shaw DS. The development of aggression in toddlers: A study of low income families. Journal of Abnormal Child Psychology. 1994;22:53–77. doi: 10.1007/BF02169256. [DOI] [PubMed] [Google Scholar]
- Kercher AJ, Rapee RM, Schniering CA. Neuroticism, life events, and negative thoughts in the development of depression in adolescent girls. Journal of Abnormal Child Psychology. 2009;37:903–915. doi: 10.1007/s10802-009-9325-1. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. New York: Guilford Press; 2005. [Google Scholar]
- Kovacs M. Children’s depression inventory (CDI) University of Pittsburgh; 1978. Unpublished manuscript. [Google Scholar]
- Lewinsohn PM, Clarke GN, Seeley JR, Rohde P. Major depression in community adolescents: Age at onset, episode duration, and time to recurrence. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:809–818. doi: 10.1097/00004583-199407000-00006. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Solomon A, Seeley JR, Zeiss A. Clinical implications of “subthreshold” depressive symptoms. Journal of Abnormal Psychology. 2000;109:345–351. [PubMed] [Google Scholar]
- Morgan JK, Olino TM, McMakin DL, Ryan ND, Forbes EE. Neural response to reward as a predictor of increases in depressive symptoms in adolescence. Neurobiology of Disease. 2012 doi: 10.1016/j.nbd.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AS, Silk JS, Steinberg L, Myers SS, Robinson LR. The role of the family context in the development of emotion regulation. Social Development. 2007;16(2):361–388. doi: 10.1111/j.1467-9507.2007.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen L, Muthen B. Mplus: User’s guide 6.0. Los Angeles, CA: Muthen & Muthen; 1998–2010. [Google Scholar]
- Reid JB, Fetrow RA, Mayne T. LIFT Playground Observer Impressions. Oregon Social Learning Center; 1991. Unpublished measure. [Google Scholar]
- Rose AJ, Rudolph KD. A review of sex differences in peer relationship processes: Potential trade-offs for the emotional and behavioral development of girls and boys. Psychological Bulletin. 2006;132:98–131. doi: 10.1037/0033-2909.132.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J, Clift A, Bolden S, Saloman K. RSA fluctuation in major depressive disorder. Psychophysiology. 2007;44:450–458. doi: 10.1111/j.1469-8986.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- Rubin KH, Coplan RJ, Bowker JC. Social withdrawal in childhood. Annual Review of Psychology. 2009;60:141–171. doi: 10.1146/annurev.psych.60.110707.163642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin KH, Coplan RJ, Fox NA, Calkins SD. Emotionality, emotion regulation, and preschoolers’ social adaptation. Development and Psychopathology. 1995;7:49–62. [Google Scholar]
- Santucci AK, Silk JS, Shaw DS, Gentzler A, Fox NA, Kovacs M. Vagal tone and temperament as predictors of emotion regulation strategies in young children. Developmental Psychobiology. 2008;50:205–216. doi: 10.1002/dev.20283. [DOI] [PubMed] [Google Scholar]
- Shannon KE, Beauchaine TP, Brenner SL, Neuhaus E, Gatzke-Kopp L. Familial and temperamental predictors of resilience in children at risk for conduct disorder and depression. Development and Psychopathology. 2007;19:701–727. doi: 10.1017/S0954579407000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk J, Dahl RE, Ryan ND, Forbes EE, Axelson DA, Birmaher B, Siegle GJ. Pupillary reactivity to emotional information in child and adolescent depression: Links to clinical and ecological measures. American Journal of Psychiatry. 2007;164:1873–1880. doi: 10.1176/appi.ajp.2007.06111816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Presnell K, Bearman SK. Relation of early menarche to depression, eating disorders, substance abuse, and comorbid psychopathology among adolescent girls. Developmental Psychology. 2001;37:608–619. doi: 10.1037//0012-1649.37.5.608. [DOI] [PubMed] [Google Scholar]
- Trentacosta CJ, Shaw DS. Emotional self-regulation, peer rejection, and antisocial behavior: Developmental associations from early childhood to early adolescence. Journal of Applied Developmental Psychology. 2009;30:356–365. doi: 10.1016/j.appdev.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderbilt E, Shaw D. Psychosocial, cognitive, and physiological protective factors and the absence of antisocial behavior in a longitudinal study of low income boys. 2007 Unpublished manuscript. [Google Scholar]
- Weber EJM, Molenaar PCM, van der Molen MW. PSPAT: A program for spectral analysis of point events including a test for stationarity. In: Mulder LJM, Maarse FJ, Sjouw WPB, Akkerman AE, editors. Computers in psychology: Applications in education, research, and psychodiagnostics. Amsterdam: Swets and Zeitlinger; 1988. [Google Scholar]
- Wadsworth ME, Achenbach TM. Explaining the link between low socioeconomic status and psychopathology: Testing two mechanisms of the social causation hypothesis. Journal of Consulting and Clinical Psychology. 2005;73:1146–1153. doi: 10.1037/0022-006X.73.6.1146. [DOI] [PubMed] [Google Scholar]