Opinion statement
Merkel cell carcinoma (MCC) is a rare but aggressive neuroendocrine skin cancer with a disease-specific mortality of approximately 40 %. The association of MCC with a recently discovered polyomavirus, combined with the increased incidence and mortality of MCC among immunocompromised patients, highlight the importance of the immune system in controlling this cancer. Initial management of MCC is summarized within the NCCN guidelines and in recently published reviews. The high rate of recurrent and metastatic disease progression in MCC, however, presents a major challenge in a cancer that lacks mechanism-based, disease-specific therapies. Traditional treatment approaches have focused on cytotoxic chemotherapy that, despite frequent initial efficacy, rarely provides durable responses and has high morbidity among the elderly. In addition, the immunosuppressive nature of chemotherapy is of concern when treating a virus-associated cancer for which survival is unusually tightly linked to immune function. With a median survival of 9.6 months after development of an initial metastasis (n=179, described herein), and no FDA-approved agents for this cancer, there is an urgent need for more effective treatments. We review diverse management options for patients with advanced MCC, with a focus on emerging and mechanism-based therapies, some of which specifically target persistently expressed viral antigens. These treatments include single-dose radiation and novel immunotherapies, some of which are in clinical trials. Due to their encouraging efficacy, low toxicity, and lack of immune suppression, these therapies may offer viable alternatives to traditional cytotoxic chemotherapy.
Keywords: Merkel cell carcinoma, Skin cancer, Immunotherapy, Merkel cell polyomavirus, Pazopanib, Octreotide, Somatostatin, Neuroendocrine carcinoma, Adoptive T cell therapy, Single-dose radiation therapy, PD-1, Survival
Introduction
MCC is diagnosed in approximately 1,600 patients each year in the United States [1], a reported incidence that has grown rapidly due to both better pathologic diagnostic tools and an increase in the risk factors associated with this cancer. These factors include age greater than 50 years, Caucasian ethnicity, UV exposure, and immune suppression, although more than 90 % of MCC patients have no known immune dysfunction [2]. Primary MCCs are most frequently found on the head and neck (29 %), followed by lower (24%) and upper (21 %) extremities [2].
MCC takes its name from the Merkel cell, a part of the somatosensory system located in the basal layer of the epidermis, with which it shares characteristics, such as neuroendocrine granules and cytokeratin-20 expression. MCC is associated with Merkel cell polyomavirus (MCPyV) in approximately 80 % of cases [3]. Although MCPyV is ubiquitous, in MCC the virus has undergone two rare mutations that contribute to unchecked host cell growth. MCPyV large T-antigen binds the retinoblastoma protein, promoting E2F activity and cell cycle progression [4]. Expression of the large T-antigen also increases expression of host cell survivin, an anti-apoptosis oncogene [5]. Less is known about the biology of virus-negative MCCs. These tumors have been associated with activating mutations in PI3KCA [6], inactivating mutations in p53 [7•], and poorer MCC-specific survival [8, 9], although this point is controversial [10].
MCC generally presents as a painless nodule that is red, purple, or skin-tone (in cases of deeper presentation). The tumor typically grows rapidly in the span of a few months. In one large study of 5,823 patients, the majority (66 %) presented with localized disease, whereas 27 % had lymph node involvement and 7 % had metastatic disease at presentation [11].
There is no “gold standard” for the diagnosis of MCC, but the triad of MCPyV and cytokeratin-20 (CK20) positivity plus location in a sun-exposed area is diagnostic. CK20 is positive in 88–100 % of MCCs, whereas CK7 and TTF-1, markers of small cell lung cancer, are typically negative [12, 13].
The American Joint Committee on Cancer TNM Staging Classification for MCC should be used for a comprehensive staging reference [11, 14]. Local disease is classified as stage I for tumors ≤2 cm and as stage II for tumors >2 cm, with A or B sub-classification based on pathologic versus clinical evaluation of lymph nodes. Regional nodal disease is stage IIIA when nodes are examined by pathology only and stage IIIB when clinically apparent by examination or radiologic study. Stage IV denotes distant metastatic disease. Sentinel lymph node biopsy (SLNB) is a useful staging tool; multiple studies indicate that pathologic examination is a more sensitive method of lymph node evaluation, and in several studies pathologic nodal examination detected microscopic disease in 23–32 % of clinically negative nodes [15–17].
There are no clear data-driven consensus guidelines for how patients should be tracked to detect disease progression early. Clues for detecting subclinical disease progression can be taken from data relating to initial workup. Several imaging modalities can be used to monitor disease progression. (F-18-FDG)-PET scan is more sensitive than CT for detecting positive lymph nodes (sensitivity of 83 % vs. 47 %) [18] and bone metastases [19]. FDG-PET also was found to be more sensitive than radiolabeled octreotide scintigraphy (111In-Pentetreotide, OctreoScan), which labels somatostatin receptor expressing tumors [20]. However in our experience, PET scans performed without contrast-enhanced diagnostic CT can miss liver metastases, perhaps because of the higher baseline glucose metabolism in the liver.
Serology can be used to detect IgG against the MCPyV T antigen in 40.5 % of MCC cases (n=205). Viral antigen titers track closely with disease burden, decreasing eightfold per year in patients without recurrence and increasing rapidly in patients with progressive disease [21]. A subsequent study is ongoing in our center. This assay continues to perform well, both to reassure patients and to identify clinically occult recurrences. We anticipate routine clinical availability of this T-antigen serology study in 2013 (for details see www.merkelcell.org).
MCC-specific 5-year survival is 63–87 % for patients with local disease and 39–42 % for those with regional nodal disease but only 0–18 % for patients with distant metastatic disease [11, 22]. Among all patients with local or regional disease, two independent studies each found that 48 % of patients ultimately developed recurrent disease. Among patients who recurred, the median time between diagnosis and recurrence was 9 months [15, 22]. In a cohort of 179 MCC patients who developed distant metastatic disease, the median survival from the time of initial metastasis was approximately 9.6 months (289 days; Fig. 1). Some markers of improved outcome appear to have very strong support, including multivariate analysis in multiple larger studies. These parameters include lower-stage disease [11, 23], no chronic immunosuppression [24, 25], intratumoral infiltration by CD3 [26] (or CD8 [27]) T cells, and absence of lymphovascular invasion [23] [our unpublished data]. Other parameters are supported by multivariate analysis in a single study. These include a nodular (versus infiltrative) growth pattern [23], the absence of p63 expression [28], and increased titer of antibodies to the Merkel polyomavirus capsid protein (VP1) [25]. Another set of outcome measures remains controversial because conflicting data exist. These include a better prognosis associated with decreased Breslow tumor thickness [23, 29] and positive Merkel polyomavirus status [8–10].
Figure 1.
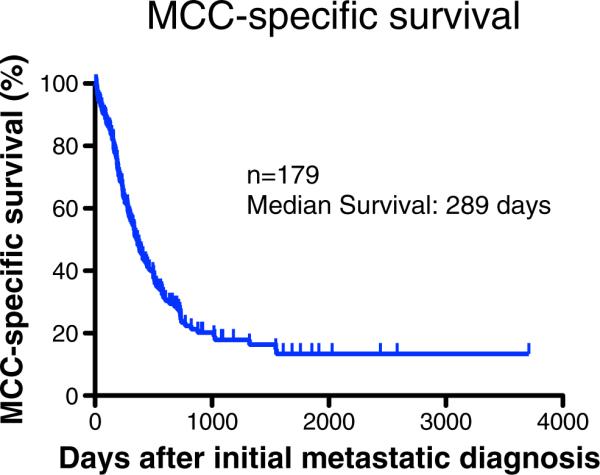
Disease-specific survival in patients who developed metastatic Merkel cell carcinoma. Survival data are shown from 179 patients with metastatic MCC who were followed through the University of Washington/Fred Hutchinson Cancer Research Center. Median survival was 289 days from initial diagnosis of metastatic disease. When measured starting at the time of developing metastatic disease, there were no significant differences in survival based on the initial stage at presentation (data not shown). However, stage greatly influenced the likelihood of and median time to developing metastatic disease [11]. Among patients who developed metastatic disease, the interval between initial diagnosis and metastasis was longer for patients presenting with less advanced stage. Overall survival is very similar to the MCC-specific survival curve shown above.
Treatment
Searches of the FDA website and CenterWatch.com for all available years (1995–2012) yielded no FDA-approved agents for this cancer. A summary of the following therapeutics can be found in Table 1.
Table 1.
Summary of therapies discussed, including mechanism of action and evaluation of evidence for use in MCC
| Treatment | Target cell | Mechanism | Strength of data |
|---|---|---|---|
| Traditional chemotherapy agents | |||
| Cisplatin/carboplatin | Tumor | Crosslinks DNA | NCCN [32, 33] |
| Etoposide | Tumor | Inhibits topoisomerase II | NCCN (IV) [32, 33], CR(4) (oral) [36] |
| Cyclophosphamide | Tumor | DNA alkylating agent | NCCN [32, 33] |
| Doxorubicin | Tumor | DNA intercalator | NCCN [32, 33] |
| Vincristine | Tumor | Inhibits microtubule assembly | NCCN [32, 33] |
| Topotecan | Tumor | Inhibits topoisomerase I | NCCN |
| Interventional procedures | |||
| Fractionated radiation | Tumor | DNA damage | NCCN |
| Single dose radiation | Tumor | DNA damage, immune stimulation (?) | CR(6) [42] |
| Mechanism-based drugs | |||
| Octreotide | Tumor, endothelium (?) | Antiproliferative, vasoconstriction and tumor necrosis (?) | CR(2) [47, 48] Ph-I in preparation [50] |
| 177Lutetium-octreotide | See above | Coupled radio-peptide; see above plus DNA damage | CR(2) [51, 52] Ph-II ongoing [53] |
| Pazopanib | Tumor, endothelium | Antiproliferative and inhibits angiogenesis | CR(1) [55] |
| PI3K inhibitors | Tumor | Antiproliferative | MCC in vitro [6, 56] Ph-II other CA ongoing |
| Lorvotuzumab mertansine (IMGN901) | Tumor | Inhibits microtubule assembly in CD56 expressing cells | Ph-I [58] |
| YM-155 | Tumor | May downregulate survivin to promote apoptosis | MCC xenograft/in vitro [5], Ph-II other CA |
| Immunotherapy | |||
| IL-12 DNA electroporation | Lymphocytes, NK cells | Promotes Th1 response; increases IFN-γ and cytolytic activity | Ph-I/II ongoing [71] |
| Interferon (intralesional) | Tumor | MHC-I upregulation, antiproliferative, antiangiogenic | CR(4) [72, 75], in vitro [73, 74] |
| Anti-PD-1 | CD8+ T cell | Blocks inhibitory/exhaustion signaling to CD8+ T cells | Ph-I other CA [60] |
| Anti-PD-L1 | Tumor, APC | Blocks inhibitory/exhaustion signaling to CD8+ T cells | Ph-I other CA [61] |
| Ipilimumab | CD8+ T cell | Blocks CTLA-4 mediated inhibition of immune activation | Ph-III other CA [63, 64] |
| 4-1BB (CD137) agonist | CD8+ T cell | Costimulatory signal for immune activation | Ph-I ongoing [67], xenografts other CA [66] |
| Transgenic T cell receptors (TCRs) | Tumor | Engineered tumor-antigen targeted T cells | Ph-II other CA [78] |
| STAR conjugates | Tumor | Drug delivery to tumor | Ph-I other CA [79] |
| Adoptive T cell therapy | Tumor | Expansion of and restoration of cytotoxic capability to tumor-targeted lymphocytes | Ph-I/II in preparation [77] |
| Agents lacking significant efficacy | |||
| Interferon (systemic) | Tumor | See above, no evidence for systemic efficacy | CR(4) [83, 84] |
| Imatinib | Tumor | Antiproliferative: blocks KIT signaling | Ph-II [80] |
| Oblimersen (Genasense) | Tumor | Downregulates Bcl-2 to promote apoptosis | Ph-II [82] |
Under each subheading, therapies are listed in order of frequency of use/strength of data NCCN, “appropriate” therapy per NCCN Consensus Guidelines for Merkel Cell Carcinoma [35]; CR, case report (number of patients reported); Ph-I, Phase I clinical trial; Ph-II, Phase II; Ph-III, Phase III; CA, cancer; NK, natural killer cell; APC, antigen-presenting cell
Surgery
In most cases of recurrent or metastatic disease, surgical management does not have a significant role due to the high probability of subclinical microscopic disease. One situation where surgery has utility is in the case of free tissue transfer reconstruction after microvascular anastomosis [30]. This approach has the advantage of allowing additional radiation therapy to anatomic areas that previously underwent significant irradiation.
Pharmacologic
MCC is generally considered to be a chemotherapy-sensitive tumor [31] with tumor regression observed in the majority of cases treated with first-line chemotherapy. Whereas cytotoxic chemotherapy is the dominant mode of treatment for advanced disease, it is virtually never curative and is associated with significant toxicity. Side effects, such as myelosuppression (including neutropenic fever), nausea/vomiting, fatigue, and hair loss are common, with therapy-related death occurring in up to 16 % of older patients [32]. Chemotherapy regimens for MCC are mostly extrapolated from those used for small cell lung cancer (SCLC), another neuroendocrine tumor. The commonly used regimens are described below:
Chemotherapy for Metastatic Disease
The data on cytotoxic chemotherapy for MCC is mostly obtained from retrospective institutional reviews or meta-analysis of small case series, and hence is subject to reporting bias. In a review of 31 patients with local recurrences, 68 % responded to first line chemotherapy (regimen unspecified), whereas 59 % of 103 patients with distant disease demonstrated responses [33]. In a retrospective study, 69 % of patients with locally advanced disease and 57 % with metastatic disease responded to first-line chemotherapy. However, survival was limited to an average of 24 or 9 months with locally advanced or metastatic disease, respectively. Death from drug toxicity was high (7.7 %) in this disease that mostly affects older patients [32].
Available data for specific chemotherapy regimens, described below, unfortunately represent a mixture of both local and advanced disease. Because of this limitation, and the fact that some patients received radiation in addition, the reported response rate is likely overly optimistic for chemotherapy alone in the setting of recurrent and metastatic disease.
Etoposide + Platinum Agent (Cisplatin or Carboplatin)
Platinum plus etoposide (PE) is the most commonly used chemotherapy regimen for MCC. For local and advanced disease, this combination gave an overall response rate of 60 % [33]. In a study of radiation plus PE in a mixture of local and advanced patients, there was 76 % 3-year survival rate [34].
Cyclophosphamide, Doxorubicin (or Epirubicin), and Vincristine (CAV)
CAV is another chemotherapy combination commonly used in MCC. A retrospective review of local and advanced MCC cases treated with CAV found an overall response rate of 76 %, with significant toxicities including death in 3.5 % of patients [33].
Topotecan
This topoisomerase I inhibitor is commonly used for small cell lung cancer and can be considered for use in older patients [35].
Oral Etoposide
In a small recent case series, oral etoposide led to durable remission or stable disease in four patients, with minimal side effects, including neutropenia in one patient [36].
Adjuvant Chemotherapy
Current data on adjuvant chemotherapy are insufficient to determine its potential usefulness. In the single largest study of 76 patients with nodal disease, there was a trend toward poorer 4-year survival in those who received adjuvant chemotherapy (42 %, n=23) compared with those who did not (60 %, n=53) [15]. Although this was not a randomized trial and comorbidities may have played a role, this certainly does not suggest a clinically meaningful benefit of adjuvant chemotherapy. It is plausible that the potential cytotoxic benefits of adjuvant chemotherapy may be offset by chemotherapy-induced immunosuppression in this immune-sensitive malignancy. The toxicity considerations in a mostly elderly population also are important when discussing the role of adjuvant chemotherapy with patients.
Interventional Procedures
Radiotherapy – General Principles
MCC has long been known to be a radiosensitive tumor [37], and radiotherapy plays an integral role in the treatment of every stage of this cancer. In the curative setting, it is used commonly in combination with surgical excision or as monotherapy when surgery cannot be performed or the morbidity of surgery is prohibitive. Optimal local treatment of MCC requires radiotherapy after a complete surgical resection. Surgery by itself is inadequate treatment in all but highly selected cases of very early stage/favorable tumors (for example, <1 cm primary, without lymphovascular invasion, and sentinel lymph node negative in a nonimmune-suppressed patient). Adjuvant radiation has been associated with improved overall survival from 45 to 63 months in a study by Mojica et al., with benefit especially noted for tumors >2 cm [38]. In another retrospective meta-analysis of 1,254 patients, adjuvant radiation was associated with a disease-specific survival benefit (hazard ratio, 0.62) compared with surgery alone [39].
Radiation monotherapy is a highly successful strategy for the treatment of MCC in our experience with local control rates exceeding 90 % [40]. The radiation dose for curative intent is 60–66 Gy to the tumor mass during monotherapy and 50 Gy when addressing residual microscopic disease. This is given at a standard fractionation of 2 Gy per fraction for 25–33 treatments over 5–6.5 weeks [35].
Radiotherapy is an effective modality in the palliative setting of incurable metastatic or recurrent MCC. It has typically been delivered in multiple fractions (5–20) and reliably provides relief from cancer symptoms with minimal side effects, thus improving the quality of life of patients. The responses to target lesions are generally durable.
Single-Dose Radiotherapy
The effects of radiation on the immune system are not fully understood. It was recently found that a single fraction of high-dose radiation stimulates lymph node priming as well as CD8 T-cell-mediated reduction of primary and metastatic tumors in a mouse model [41••]. Subsequent doses of fractionated radiation can suppress the activity of recruited lymphocytes, thus single-dose treatment may have advantages in terms of promoting immune function. In addition, RT given in a single, large-dose fraction of 8 Gy is well known to provide safe and effective palliation for bone metastases and a single fraction is logistically very convenient for patients. At our center, we treated 15 MCC tumors with a single fraction of 8-Gy radiation during a 1-year period. These included 7 chemorefractory tumors. There were 11 complete and 4 partial (>50 %) responses. No side effects were reported during a median follow-up of 5 months [42]. Although these studies are in the early stages, a single fraction of 8-Gy radiotherapy may offer a better therapeutic ratio compared with traditional treatments for MCC. In addition to its superb side-effect profile, this approach is cost effective and convenient for patients who are ill with metastatic disease for whom multiple visits to a radiotherapy center are a major burden.
Brachytherapy
Brachytherapy is the precise delivery of short-range radiation (within a few millimeters) by positioning the radioactive source within or in close proximity to the tumor, and is derived from the Greek brachy, meaning close. Its use is limited but can be effective in widely disseminated cutaneous disease. A case report showed a durable response of multiple cutaneous metastatic MCC nodules, including one untreated lesion, in the right lower extremity after delivery of 12 Gy by brachytherapy [43].
Palliation
The goal of palliative care is to provide pain control and support to patients with serious illness. In a recent randomized study of lung cancer patients, early palliative care led to significant improvements in both quality of life and mood. Unexpectedly, despite receiving less aggressive care at the end of life, patients randomized to palliative care survived longer than those who received standard care alone [44]. It is ideal to discuss palliative care options early in the treatment process for patients with high risk or advanced disease. The ASCO Palliative Care Checklist [45] and the NCCN Palliative Care Guidelines [46] provide useful tools for these discussions.
Mechanism-Based Therapies
Octreotide
Octreotide is a potent, biologically stable octapeptide analog of the naturally occurring hormone somatostatin. Somatostatin has an antiproliferative effect on neuroendocrine tumor cells and may inhibit tumor angiogenesis. mRNA expression of somatostatin receptor 2 has been demonstrated on 90 % of MCCs [47], providing rationale for treatment of MCC with this class of drugs. Imaging via radiolabeled octreotide (OctreoScan) can be a useful clinical indicator of physiologic octreotide binding to a given patient's tumor. Among two reported cases, encouraging responses were seen in both patients [48, 49]. A phase I clinical trial in MCC with pasireotide, another somatostatin analog, is upcoming [50]. Two case reports in which a therapeutic radioisotope was coupled to a peptide of this class demonstrated clinical benefit with a favorable safety profile [51, 52]. A Phase II clinical trial with such an agent is currently underway in neuroendocrine cancers, including MCC [53].
Pazopanib
Pazopanib is a receptor tyrosine kinase inhibitor that targets VEGFR-1, -2, -3, PDGFR-α,-β, and c-kit. Immunohistochemistry has detected VEGF-A, VEGF-C, VEGF-R2, and PDGF-α expression in 72–91 % of MCCs [54]. Pazopanib is hypothesized to inhibit both tumor growth and angiogenesis and is currently FDA-approved for the treatment of renal cell carcinoma and soft tissue sarcoma. The drug is generally well tolerated and is not considered to be immunosuppressive. In a recent case report of oral pazopanib used to treat a patient who had failed multiple prior treatment modalities, the patient's scalp tumor completely resolved after 2 months of pazopanib with a partial response in her pulmonary metastases that lasted 6 months [55•]. The investigators found a germline mutation in the gene for PDGFR-α in three patients, suggesting a possible role of the gene in predisposition toward MCC or as a marker for potential treatment response.
PI3K Inhibition
A recent study identified activating PI3KCA gene mutations in 10 % of MCCs analyzed, with the majority found in virus-negative cancers [6]. MCC cell lines are sensitive to PI3K inhibitors currently in clinical development [6, 56].
YM-155
Survivin is a cellular protein with antiapoptotic properties that is commonly upregulated in MCC. Its expression tracks with levels of Merkel polyomavirus large T antigen. YM-155 is a small molecule that has been suggested to downregulate survivin. This drug causes cell death in MCC cell lines in vitro and appears to be cytostatic in a mouse xenograft tumor model [5]. A previous Phase 1 trial of YM-155 in other cancers showed that it can be administered safely and is well tolerated [57].
Lorvotuzumab Mertansine (IMGN901)
IMGN901 is an antibody-drug conjugate consisting of a maytansinoid microtubule assembly inhibitor coupled with a humanized monoclonal antibody to CD56, which is expressed on nearly all MCCs. In a phase 1 trial that included 12 MCC patients, two patients experienced durable complete responses after treatment with IMGN901 given IV at either 36 or 60 mg/m2/day [58].
Emerging and Viral Antigen-Directed Immunotherapies
The three immune-stimulatory antibodies below are all being actively investigated in clinical studies for various cancers and may have benefit in MCC. Although there are no MCC-specific clinical trials ongoing for these agents, the hope is that they will be forthcoming.
PD-1/PD-L1 Inhibitors
PD-1 is an inhibitory cell surface receptor that blocks T-cell receptor (TCR) signaling on lymphocytes. Persistent, unresolved viral infections often are associated with functionally impaired T cells that have increased expression of surface PD-1 [59]. A Phase I trial of a PD-1 inhibitor demonstrated cumulative response rates of 18 % in non-small cell lung cancer patients, 28 % in melanoma patients, and 27 % in renal-cell cancer patients. Importantly, 68 % of responses were durable for at least 1 year. Response to PD-1 blockers was strongly linked to tumors expressing PD-L1 [60•]. Antibodies blocking PD-L1 also showed durable tumor regression in a recent Phase I trial with responses of 6–17 % in the same three cancers [61]. Due to the viral etiology and immunosuppression associated with MCC, it is very possible that PD-1 blockers would have efficacy in this cancer. It was recently found that PD-1 is upregulated on Merkel polyomavirus-specific CD8 T cells compared with control virus-specific cells in MCC patients [62].
Ipilimumab
Ipilimumab is a monoclonal antibody that blocks the inhibitory receptor CTLA-4, increasing T-cell activation. It has been shown to improve survival of metastatic melanoma patients [63, 64].
A case report of ipilimumab combined with radiotherapy to treat metastatic melanoma demonstrated an abscopal effect of tumor shrinkage in untreated lesions, as well as increased antibody titers to diverse melanoma antigens [65]. This may be relevant to MCC, especially with the excellent tolerability of single-dose radiation treatment that could be combined with systemic immunotherapies.
4-1BB (CD137) Agonist
4-1BB is a TNF-family costimulatory receptor expressed on activated T cells. In preclinical trials, antibodies that bind this receptor increase NF-κBactivity leading to cytokine production, increased leukocyte proliferation, and reduced tumor growth [66]. A phase I trial is currently underway in patients with advanced/metastatic solid tumors [67]. We have found that Merkel polyomavirus-specific T cells express higher levels of CD137 compared with control virus-specific cells, suggesting a role for 4-1BB agonists in treating MCC [62].
Interleukin-12 DNA Electroporation
IL-12 is a Th1 skewing cytokine that induces proliferation, cytotoxicity and IFN-γ production by preactivated natural killer and T cells. Systemic administration of rIL-12 has been limited by toxicity and temporary immune suppression [68], promoting investigation into local administration routes. In a mouse melanoma model, intratumoral injection of a plasmid encoding IL-12 followed by electroporation caused several desirable immune effects. These included IL-12 and IFN-γ induction, enhanced lymphocyte migration, reduced tumor vascularity, and tumor elimination in 47 % of treated mice [69]. A Phase 1 study of electroporated IL-12 in patients with metastatic melanoma demonstrated complete resolution of distant, nonelectroporated lesions in 10 % of patients, with partial or stable response in 42 % of patients and minimal systemic side effects [70]. A phase II trial is currently ongoing for MCC [71].
Intralesional Interferon
Whereas MCC is associated with immune suppression, more than 90 % of patients are not immunocompromised and these tumors have thus likely acquired immune evasion mechanisms to avoid detection by cytotoxic T cells. Indeed, more than half (51 %) of 114 MCC tumors demonstrated downregulation of MHC-I, an established mechanism for CD8 T-cell evasion. In vitro, interferon treatment of MCC cell lines led to reversal of this MHC-I downregulation [72] and induced apoptosis [73, 74]. In a case report, daily IFN-β injections into a patient's forearm metastases resulted in a durable (>8 years) complete response following 5 weeks of monotherapy [75]. In our pilot studies, intralesional interferon-β (3 MIU, 3× week, for 1–4 weeks) led to increased expression of MHC-I, increased CD8 T-cell infiltration, and local tumor regression among three patients with available pre- and post-treatment biopsy materials [72].
Adoptive T-Cell Therapy
This process involves the enrichment and reinfusion of autologous antitumor T cells into cancer patients. Adoptive cell transfer into metastatic melanoma patients who had been heavily pretreated with lymphodepletion and/or radiation had an objective response rate of 56 % [76]. Although not widely available, this response rate is superior to other available chemo- and immunotherapies. The persistent expression of non-self (Merkel polyomavirus) antigens in most MCC tumors makes adoptive T cell therapy for this cancer particularly attractive. At our center, we have treated one patient who developed metastatic MCC using Merkel polyomavirus-specific T cells [77] and plan to start a Phase I/II trial that will enroll 16 advanced-stage patients.
Transgenic T-Cell Receptor-Based Therapies
Lymphocytes can be genetically engineered to express transgenic T-cell receptors (TCRs) that recognize cancer antigens. Among 36 metastatic melanoma patients, autologous lymphocytes expressing transgenic TCRs targeting two melanocyte antigens persisted in vivo and elicited objective response rates of 19 % or 30 %. However, reactivity toward normal tissues led to significant side effects, such as vitiligo, uveitis, or hearing loss, in more than half of patients [78].
STAR Reagents
Soluble T-cell antigen receptor (STAR) reagents are synthesized TCRs that recognize cancer antigens and are coupled to therapeutic agents including cytokines or radioisotopes. A p53 targeted, HLA-A0201 restricted STAR reagent coupled to IL-2 was found to increase serum IFN-γ in a phase 1 trial in metastatic cancer patients. In this study, 10 of 26 subjects had stable disease after 11 weeks, with one complete remission and minimal toxicity [79]. Advantages of STAR reagents include the potential for commercial viability and “off the shelf” use, although they do need to be compatible with the patient's HLA-type.
Agents Without Apparent Clinical Efficacy
Imatinib is a tyrosine kinase inhibitor with activity against KIT, a receptor tyrosine kinase commonly expressed in MCCs. Imatinib was therefore investigated in a phase II trial with 23 MCC patients. Unfortunately, most patients showed rapid disease progression during treatment [80]. It was subsequently shown that KIT expression in MCC is less common than previously reported and activating KIT mutations are infrequent, perhaps explaining the low efficacy of imatinib in this cancer [81].
Oblimersen (Genasense) is an oligonucleotide that targets and downregulates Bcl-2 expression, increasing apoptosis. In a phase II trial with 12 advanced MCC patients, no objective responses were seen [82].
Systemic interferon has been investigated in MCC. In four metastatic MCC patients treated with systemic interferon-α, no tumor responses were seen [83, 84]. This may have been due to inadequate levels of interferon within the tumor microenvironment or compensatory systemic immune regulation.
Conclusions
While the prognosis for distant metastatic MCC is grim, recent advances in our understanding of cancer immunity have provided rational mechanism-based therapies that are entering clinical testing. The strong links between the immune system and this virus-associated malignancy provide exciting opportunities for immunotherapy, including the possibility of combining antibody-based therapeutics that stimulate the immune system globally with tumor antigen targeted treatments. Because of fundamental biological differences, virus-negative MCCs may require independent, focused attention to create effective therapeutic approaches.
Acknowledgments
This work was supported by T32 ES 7032-35, ARCS Fellowship (NM); NIH- R01CA16252, NIH- RC2CA147820, NIH-K24-CA139052, NIH-U01-CA-154967, Michael Piepkorn Endowment (PN); MCC Patient Gift Fund, David & Rosalind Bloom Fund for MCC.
Footnotes
Conflict of Interest No potential conflicts of interest relevant to this article were reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2010;37(1):20–7. doi: 10.1111/j.1600-0560.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- 2.Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58(3):375–81. doi: 10.1016/j.jaad.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319(5866):1096–100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shuda M, Feng H, Kwun HJ, et al. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc Natl Acad Sci U S A. 2008;105(42):16272–7. doi: 10.1073/pnas.0806526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arora R, Shuda M, Guastafierro A, et al. Survivin is a therapeutic target in Merkel cell carcinoma. Sci Transl Med. 2012;4(133):1–11. doi: 10.1126/scitranslmed.3003713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nardi V, Song Y, Santamaria-Barria JA, et al. Activation of PI3K signaling in Merkel cell carcinoma. Clin Cancer Res. 2012;18(5):1227–36. doi: 10.1158/1078-0432.CCR-11-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7•.Rodig SJ, Cheng J, Wardzala J, et al. Improved detection suggests all Merkel cell carcinomas harbor Merkel polyomavirus. J Clin Invest. 2012;122(12):4645–53. doi: 10.1172/JCI64116. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides new, more sensitive methods for detecting MCPyV DNA and protein. Moving toward a gold standard for MCPyV detection will improve the understanding of this cancer.
- 8.Waltari M, Sihto H, Kukko H, et al. Association of Merkel cell polyomavirus infection with tumor p53, KIT, stem cell factor, PDGFR-alpha and survival in Merkel cell carcinoma. Int J Cancer. 2011;129(3):619–28. doi: 10.1002/ijc.25720. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia K, Goedert JJ, Modali R, et al. Merkel cell carcinoma subgroups by Merkel cell polyomavirus DNA relative abundance and oncogene expression. Int J Cancer. 2010;126:2240–6. doi: 10.1002/ijc.24676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrama D, Peitsch WK, Zapatka M, et al. Merkel cell polyomavirus status is not associated with clinical course of Merkel cell carcinoma. J Investig Dermatol. 2011;131(8):1631–8. doi: 10.1038/jid.2011.115. [DOI] [PubMed] [Google Scholar]
- 11.Lemos BD, Storer BE, Iyer JG, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010;63(5):751–61. doi: 10.1016/j.jaad.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen K, Kohler S, Rouse RV. Cytokeratin staining in Merkel cell carcinoma: an immunohistochemical study of cytokeratins 5/6, 7, 17, and 20. Appl Immunohistochem Mol Morphol. 2000;8(4):310–5. [PubMed] [Google Scholar]
- 13.Cheuk W, Kwan MY, Suster S, Chan JK. Immunostaining for thyroid transcription factor 1 and cytokeratin 20 aids the distinction of small cell carcinoma from Merkel cell carcinoma, but not pulmonary from extrapulmonary small cell carcinomas. Arch Pathol Lab Med. 2001;125(2):228–31. doi: 10.5858/2001-125-0228-IFTTFA. [DOI] [PubMed] [Google Scholar]
- 14.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 15.Allen PJ. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23(10):2300–9. doi: 10.1200/JCO.2005.02.329. [DOI] [PubMed] [Google Scholar]
- 16.Gupta SG, Wang LC, Peñas PF, et al. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: the Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006;142(6):685–90. doi: 10.1001/archderm.142.6.685. [DOI] [PubMed] [Google Scholar]
- 17.Fields RC, Busam KJ, Chou JF, et al. Recurrence and survival in patients undergoing sentinel lymph node biopsy for Merkel cell carcinoma: analysis of 153 patients from a single institution. Ann Surg Oncol. 2011;18(9):2529–37. doi: 10.1245/s10434-011-1662-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colgan MB, Tarantola TI, Weaver AL, et al. The predictive value of imaging studies in evaluating regional lymph node involvement in Merkel cell carcinoma. J Am Dermatol. 2012;3(18):1–7. doi: 10.1016/j.jaad.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Hawryluk EB, O'Regan KN, Sheehy N, et al. Positron emission tomography/computed tomography imaging in Merkel cell carcinoma: a study of 270 scans in 97 patients at the Dana-Farber/Brigham and Women's Cancer Center. J Am Acad Dermatol. 2012:1–8. doi: 10.1016/j.jaad.2012.08.042. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y, Fleming SE, Fields RC, et al. Comparison of 18F-FDG PET/CT and 111In pentetreotide scan for detection of Merkel cell carcinoma. Clin Nucl Med. 2012;37(8):759–62. doi: 10.1097/RLU.0b013e31825ae8e7. [DOI] [PubMed] [Google Scholar]
- 21.Paulson KG, Carter JJ, Johnson LG, et al. Antibodies to Merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in Merkel cell carcinoma patients. Cancer Res. 2010;70(21):8388–97. doi: 10.1158/0008-5472.CAN-10-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santamaria-Barria J, Boland G, Yeap B, et al. Merkel cell carcinoma: 30-year experience from a single institution. Ann Surg Oncol. 2012:1–9. doi: 10.1245/s10434-012-2779-3. [DOI] [PubMed] [Google Scholar]
- 23.Andea AA, Coit DG, Amin B, Busam KJ. Merkel cell carcinoma. Cancer. 2008;113(9):2549–58. doi: 10.1002/cncr.23874. [DOI] [PubMed] [Google Scholar]
- 24.Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. 2012:1–5. doi: 10.1038/jid.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Touze A, Le Bidre E, Laude H, et al. High levels of antibodies against Merkel cell polyomavirus identify a subset of patients with Merkel cell carcinoma with better clinical outcome. J Clin Oncol. 2011;29(12):1612–9. doi: 10.1200/JCO.2010.31.1704. [DOI] [PubMed] [Google Scholar]
- 26.Sihto H, Joensuu H. Tumor-infiltrating lymphocytes and outcome in Merkel cell carcinoma, a virus-associated cancer. Oncoimmunology. 2012;1(8):1420–1. doi: 10.4161/onci.21120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulson KG, Iyer JG, Tegeder AR, et al. Transcriptome-wide studies of Merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival. J Clin Oncol. 2011;29(12):1539–46. doi: 10.1200/JCO.2010.30.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asioli S, Righi A, de Biase D, et al. Expression of p63 is the sole independent marker of aggressiveness in localised (stage I–II) Merkel cell carcinomas. Mod Pathol. 2011;24(11):1451–61. doi: 10.1038/modpathol.2011.100. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg SR, Neifeld JP, Frable WJ. Prognostic value of tumor thickness in patients with Merkel cell carcinoma. J Surg Oncol. 2007;95:618–22. doi: 10.1002/jso.20737. [DOI] [PubMed] [Google Scholar]
- 30.Londino AV, III, Miles BA. The role of free tissue transfer in Merkel cell carcinoma of the head and neck. J Skin Cancer. 2012:1–6. doi: 10.1155/2012/742303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wynne CJ, Kearsley JH. Merkel cell tumor. A chemosensitive skin cancer. Cancer. 1988;62(1):28–31. doi: 10.1002/1097-0142(19880701)62:1<28::aid-cncr2820620107>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 32.Voog E, Biron P, Martin JP, Blay JY. Chemotherapy for patients with locally advanced or metastatic Merkel cell carcinoma. Cancer. 2000;85(12):2589–95. doi: 10.1002/(sici)1097-0142(19990615)85:12<2589::aid-cncr15>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 33.Tai PT, Yu E, Winquist E, et al. Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: case series and review of 204 cases. J Clin Oncol. 2000;18(12):2493–9. doi: 10.1200/JCO.2000.18.12.2493. [DOI] [PubMed] [Google Scholar]
- 34.Poulsen M. High-risk Merkel cell carcinoma of the skin treated with synchronous carboplatin/etoposide and radiation: a Trans-Tasman Radiation Oncology Group study-TROG 96:07. J Clin Oncol. 2003;21(23):4371–6. doi: 10.1200/JCO.2003.03.154. [DOI] [PubMed] [Google Scholar]
- 35.Miller SJ, Alam M, Andersen J, et al. NCCN guidelines version 1.2012 Merkel cell carcinoma. National Comprehensive Cancer Network. 2012 [Google Scholar]
- 36.Schlaak M, Podewski T, Von Bartenwerffer W, et al. Induction of durable responses by oral etoposide monochemotherapy in patients with metastatic Merkel cell carcinoma. Eur J Dermatol. 2012;22(2):187–91. doi: 10.1684/ejd.2011.1634. [DOI] [PubMed] [Google Scholar]
- 37.Leonard JH, Ramsay JR, Kearsley JH, Birrell GW. Radiation sensitivity of Merkel cell carcinoma cell lines. Int J Radiat Oncol Biol Phys. 1995;32(5):1401–7. doi: 10.1016/0360-3016(94)00610-W. [DOI] [PubMed] [Google Scholar]
- 38.Mojica P, Smith D, Ellenhorn JDI. Adjuvant radiation therapy is associated with improved survival in Merkel cell carcinoma of the skin. J Clin Oncol. 2007;25(9):1043–7. doi: 10.1200/JCO.2006.07.9319. [DOI] [PubMed] [Google Scholar]
- 39.Lewis KG, Weinstock MA, Weaver AL, Otley CC. Adjuvant local irradiation for Merkel cell carcinoma. Arch Dermatol. 2006;142(6):693–700. doi: 10.1001/archderm.142.6.693. [DOI] [PubMed] [Google Scholar]
- 40.Parvathaneni U, Iyer J, Nagase K, et al. The safety and efficacy of primary radiation therapy without upfront surgery for Merkel cell carcinoma [abstract] Int J Radiat Oncol Phys Biol. 2012;84(3):s168. [Google Scholar]
- 41••.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114(3):589–95. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that high dose radiation stimulates anti-tumor immune function in a mouse model. These findings challenge the current treatment model of fractionated radiotherapy plus chemotherapy and propose that a single high dose of ionizing radiation combined with immunotherapy may be more clinically efficacious.
- 42.Parvathaneni U, Iyer J, Nagase K, et al. Effective and durable palliation using a novel single fraction radiation therapy approach for Merkel cell carcinoma metastatic lesions [abstract] Int J Radiat Oncol Phys Biol. 2012;84(3):S631. [Google Scholar]
- 43.Cotter SE, Devlin PM, Sahni D, et al. Treatment of cutaneous metastases of Merkel cell carcinoma with surface-mold computer-optimized high-dose-rate brachytherapy. J Clin Oncol. 2010;28(27):464–6. doi: 10.1200/JCO.2010.29.0635. [DOI] [PubMed] [Google Scholar]
- 44.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–42. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 45. [Accessed November 2012];American Society of Clinical Oncology: Palliative Care Checklist. Available at http://www.asco.org/pco/palliativecare.
- 46.Levy MH, Adolph MD, Back A, et al. Palliative care. J Natl Compr Cancer Netw. 2012;10(10):1284–309. doi: 10.6004/jnccn.2012.0132. [DOI] [PubMed] [Google Scholar]
- 47.Papotti M, Macri L, Pagani A, et al. Quantitation of somatostatin receptor type 2 in neuroendoctine (Merkel cell) carcinoma of the skin by competitive RT-PCR. Endocr Pathol. 1999;10(1):37–46. [Google Scholar]
- 48.Cirillo F, Filippini L, Lima GF, et al. Merkel cell tumor. Report of case and treatment with octreotide [abstract] Minerva Chir. 1997;52(11):1359–65. [PubMed] [Google Scholar]
- 49.Fakiha M, Letertre P, Vuillez JP, Lebeau J. Remission of Merkel cell tumor after somatostatin analog treatment. J Cancer Res Ther. 2010;6(3):382. doi: 10.4103/0973-1482.73352. [DOI] [PubMed] [Google Scholar]
- 50.National Institutes of Health [Accessed December 2012];Dose escalation study to investigate safety, PK and anti-tumor activity of pasireotide in patients with metastatic melanoma or Merkel cell carcinoma (MACS1670) Available at http://www.clinicaltrials.gov/ct2/show/NCT01652547?term=Merkel+Cell+Carcinoma&rank=1.
- 51.Meier G, Waldherr C, Herrmann R, et al. Successful targeted radiotherapy with 90Y-DOTATOC in a patient with Merkel cell carcinoma. Oncology. 2004;66(2):160–3. doi: 10.1159/000077443. [DOI] [PubMed] [Google Scholar]
- 52.Salavati A, Prasad V, Schneider C-P, et al. Peptide receptor radionuclide therapy of Merkel cell carcinoma using 177lutetium-labeled somatostatin analogs in combination with radiosensitizing chemotherapy: a potential novel treatment based on molecular pathology. Ann Nucl Med. 2012;26(4):365–9. doi: 10.1007/s12149-012-0578-3. [DOI] [PubMed] [Google Scholar]
- 53.National Institutes of Health [Accessed December 2012];177Lutetium-DOTA-octreotate therapy in somatostatin receptor-expressing neuroendocrine neoplasms. Available at http://www.clinicaltrials.gov/ct2/show/NCT01237457?term=Merkel+Cell+Carcinoma&recr=Open&rank=7.
- 54.Brunner M, Thurnher D, Pammer J, et al. Expression of VEGF-A/C, VEGF-R2, PDGF-α/β, c-kit, EGFR, Her-2/Neu, Mcl-1 and Bmi-1 in Merkel cell carcinoma. Mod Pathol. 2008;21(7):876–84. doi: 10.1038/modpathol.2008.63. [DOI] [PubMed] [Google Scholar]
- 55•.Davids MS, Davids M, Charlton A, et al. Response to a novel multitargeted tyrosine kinase inhibitor pazopanib in metastatic Merkel cell carcinoma. J Clin Oncol. 2009;27(26):97–100. doi: 10.1200/JCO.2009.21.8149. [DOI] [PubMed] [Google Scholar]; This case report of oral pazopanib in MCC reports responses in both the primary tumor and pulmonary metastases to treatment with this agent. We are currently using pazopanib in our clinic for patients who have failed other therapies.
- 56.Hafner C, Houben R, Baeurle A, et al. Activation of the PI3K/AKT pathway in Merkel cell carcinoma. PLoS One. 2012;7(2):e31255. doi: 10.1371/journal.pone.0031255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tolcher AW, Mita A, Lewis LD, et al. Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of survivin. J Clin Oncol. 2008;26(32):5198–203. doi: 10.1200/JCO.2008.17.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woll PJKM, Bhatia S, et al. Efficacy results from a phase I study of lorvotuzumab mertansine (IMGN901) in patients with CD56-positive solid tumors [abstract] J Clin Oncol. 2011;29(Suppl):e.13582. [Google Scholar]
- 59.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2005;439(7077):682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 60•.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this Phase I trial, a PD-1 inhibitor generated durable responses in 18–28 % of non-small cell lung cancer, renal cell carcinoma, and melanoma patients. PD-1 blockers have promising potential efficacy in MCC and a clinical trial will hopefully be forthcoming.
- 61.Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Afanasiev O, Yelistratova L, Miller N, et al. Merkel cell carcinoma-targeted T cells increase with disease and express therapeutically reversible PD-1 and Tim-3 exhaustion markers [abstract]. the Keystone Symposium on Cancer Immunology and Immuno-therapy; Vancouver, Canada. Jan 27 1, Feb 27 1, 2013. [Google Scholar]
- 63.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 64.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Postow MA, Callahan MK, Barker CA, et al. Immuno-logic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fisher TS, Kamperschroer C, Oliphant T, et al. Targeting of 4-1BB by monoclonal antibody PF-05082566 enhances T-cell function and promotes anti-tumor activity. Cancer Immunol Immunother. 2012;61(10):1721–33. doi: 10.1007/s00262-012-1237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.National Institutes of Health [Accessed December 2012];Safety, tolerability, pharmacokinetics, and immunoregulatory study of BMS-663513 in subjects with advanced and/or metastatic solid tumors. Available at http://www.clinicaltrials.gov/ct2/show/NCT01471210?term0CD137&rank03.
- 68.Atkins MB, Robertson MJ, Gordon M, et al. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin Cancer Res. 1997;3(3):409–17. [PubMed] [Google Scholar]
- 69.Lucas M. IL-12 plasmid delivery by in vivo electro-poration for the successful treatment of established subcutaneous B16.F10 melanoma. Mol Ther. 2002;5(6):668–75. doi: 10.1006/mthe.2002.0601. [DOI] [PubMed] [Google Scholar]
- 70.Daud AI, DeConti RC, Andrews S, et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008;26(36):5896–903. doi: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.National Institutes of Health [Accessed December 2012];Interleukin-12 gene and in vivo electroporation-mediated plasmid DNA vaccine therapy in treating patients with Merkel cell cancer. Available at http://www.clinicaltrials.gov/ct2/show/NCT01440816?term0Merkel+Cell+Carcinoma&rank04.
- 72.Paulson KG, Tegeder AR, Willmes C, et al. Reversal of local immune evasion mechanisms and regression of human Merkel cell carcinoma by intralesional injection of interferon-beta [abstract] J Investig Dermatol. 2011;131(Suppl1):e. s92. [Google Scholar]
- 73.Krasagakis K, Krüger-Krasagakis S, Tzanakakis GN, et al. Interferon-alpha inhibits proliferation and induces apoptosis of merkel cell carcinoma in vitro. Cancer Invest. 2008;26(6):562–8. doi: 10.1080/07357900701816477. [DOI] [PubMed] [Google Scholar]
- 74.Willmes C, Adam C, Alb M, et al. Type I and II IFNs inhibit Merkel cell carcinoma via modulation of the Merkel cell polyomavirus T antigens. Cancer Res. 2012;72(8):2120–8. doi: 10.1158/0008-5472.CAN-11-2651. [DOI] [PubMed] [Google Scholar]
- 75.Nakajima H, Takaishi M, Yamamoto M, et al. Screening of the specific polyoma virus as diagnostic and prognostic tools for Merkel cell carcinoma. J Dermatol Sci. 2009;56(3):211–3. doi: 10.1016/j.jdermsci.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 76.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immuno-therapy. Clin Cancer Res. 2011;17(13):4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Afanasiev O, Chapui A, Iyer J, et al. Merkel cell carcinoma therapy with viral oncoprotein-specific T cells in combination with immunostimulatory adjuvants [abstract] J Investig Dermatol. 2012;132(S1):S96. [Google Scholar]
- 78.Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114(3):535–46. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fishman MN, Thompson JA, Pennock GK, et al. Phase I trial of ALT-801, an interleukin-2/T-cell receptor fusion protein targeting p53 (aa264-272)/HLA-A*0201 complex, in patients with advanced malignancies. Clin Cancer Res. 2011;17(24):7765–75. doi: 10.1158/1078-0432.CCR-11-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Samlowski WE, Moon J, Tuthill RJ, et al. A phase II trial of imatinib mesylate in Merkel cell carcinoma (Neuroendocrine carcinoma of the skin) Am J Clin Oncol. 2010;33(5):495–9. doi: 10.1097/COC.0b013e3181b9cf04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang Q, Hornick JL, Granter SR, Wang LC. Merkel cell carcinoma: lack of KIT positivity and implications for the use of imatinib mesylate. Appl Immunohistochem Mol Morphol. 2009;17(4):276–81. doi: 10.1097/PAI.0b013e318194da49. [DOI] [PubMed] [Google Scholar]
- 82.Shah MH, Varker KA, Collamore M, et al. G3139 (Genasense) in patients with advanced Merkel cell carcinoma. Am J Clin Oncol. 2009;32(2):174–9. doi: 10.1097/COC.0b013e31817eebf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bajetta E, Zilembo N, Di Bartolomeo M, et al. Treatment of metastatic carcinoids and other neuroendocrine tumors with recombinant interferon-alpha-2a. A study by the Italian trials in Medical Oncology Group. Cancer. 1993;72(10):3099–105. doi: 10.1002/1097-0142(19931115)72:10<3099::aid-cncr2820721035>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 84.Biver-Dalle C, Nguyen T, Touzé A, et al. Use of interferon-alpha in two patients with Merkel cell carcinoma positive for Merkel cell polyomavirus. Acta Oncol. 2011;50(3):479–80. doi: 10.3109/0284186X.2010.512924. [DOI] [PubMed] [Google Scholar]


