Abstract
Cardiac pacemaker cells autonomously generate electrical impulses that initiate and maintain the rhythmic contraction of the heart. Although the majority of the heart is thought to originate from the primary and secondary heart fields, we report that chick pacemaker cells arise from a discrete region of mesoderm outside of these fields. Shortly after gastrulation, canonical Wnts promote the recruitment of mesodermal cells within this region into the pacemaker lineage. These findings identify the ontogeny of cardiac pacemaker cells, suggesting that pacemaker cells are physically segregated and molecularly programmed in a tertiary heart field, prior to the onset of cardiac morphogenesis.
The rhythm of the heart is maintained by a specialized sub-class of myocytes known as cardiac pacemaker cells (PCs). These cells generate action potentials (APs) in a cyclic manner to stimulate cardiac contractions. The anatomic position of mature PCs, the sinoatrial node (SAN), was described more than 100 years ago (1), however, little is known regarding the ontogeny or molecular mechanisms that specify PCs during development. This study was designed to address the timing, location, and mechanisms of PC cell fate acquisition.
Electrophysiological studies (2, 3, 4) have mapped cells that initiate cardiac APs to the inflow region at heart tube, looping, and septation stages. However, recent evidence indicates that as the heart matures, it continually expands with cells being added to both the inflow and outflow segments (reviewed 5). To determine which, if any, of the previously identified developmental pacing centers give rise to the mature SAN, whole embryonic chick hearts were imaged using optical mapping (4). Coincident with the heart’s first contractions at Stage 10 (Hambuger and Hamilton staging (6)) the AP initiation site was preferentially associated with the left posterior inflow segment of the heart (Figure 1A (red region), F). Left sided pacing remained dominant through the process of dextral looping, (Figure 1B,F). By late heart looping (St18), the AP initiation site shifted to the ventral surface of the right inflow, juxtaposed to and outside of the forming atria (Figure 1C). From this stage on, all hearts displayed a right-sided AP initiation site (Figure 1D, E, F, see also Supplementary Movies 1–5).
Figure 1.
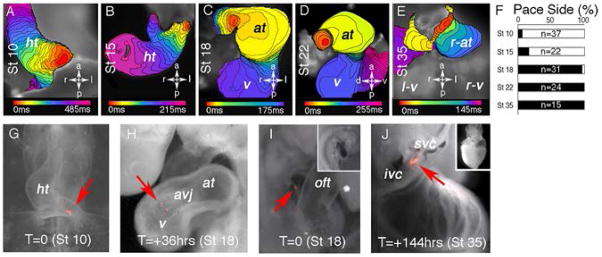
PCs begin pacing heart rhythm during late looping stages: A–E) Isochronal maps denoting AP initiation site (red) and propagation pattern, St10–St35. F) Pacemaker side spanning 5 progressive embryonic stages (white = left-sided, black = right-sided pacemaker). G, H) Labeling of St 10 AP initiation site (arrow) developed for 36hrs to St 18. I, J) Labeling of St 18 AP initiation site (arrow) developed 144hrs to St 35. Insets show low magnification images of labeled embryo. a-anterior, p-posterior, r-right, l-left, ht- heart tube, v-ventricle, at- atria, avj- atrioventricular junction, r-at- right atria, lv- left ventricle, rv- right ventricle, oft - outflow tract, svc- superior vena cava, ivc-inferior vena cava.
As the AP initiation site shifted from left to right, AP morphology changed substantially, displaying pronounced slow diastolic depolarization and shorter AP duration than earlier pacing centers (Supplemental figure 1). Right sided pacemakers additionally exhibited a unique expression profile, becoming enriched for genes associated with mature PC AP generation including Hcn4, Serca2, and Ryr2 (7,8,9,10), and co-expressing the atrial and ventricular muscle markers Amhc1 and Vmhc1 (Supplementary Fig 2). To determine whether these differences were due to the maturation of migrating earlier pacing cells, or were caused by the differentiation of a new cell population, vital lipophilic fluorescent dyes were used to trace the fates of early pacing cells. In no case did dye labeled cells from left sided AP initiation sites contribute to older pacing centers (Figure 1G, H, Supplementary Fig 3). In contrast, cells from the post looping, right inflow remained associated with the heart’s pacing region at all subsequent stages examined, eventually integrating into the SAN region at the back of the right atria (Figure 1I, J, Supplementary Fig 4). These data demonstrate that PC precursors emerge from a population of cells that is electrically inactive during the initial stages of heart development and begin pacing the heart within about an 8 hour developmental window coinciding with late dextral looping.
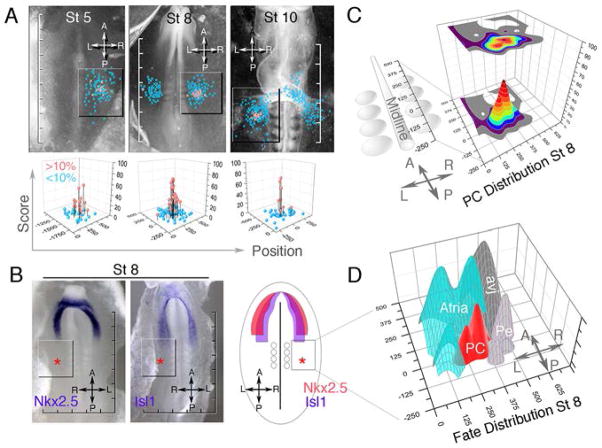
To identify when the assignment of PC fate occurs, we determined the location of PC precursors at stages prior to their electrical activation. Since none of the above markers displayed similar enrichment at earlier time points (Supplementary Fig 2), we utilized a non-marker, direct cell labeling approach to create a series of geometric fate maps. Fate maps were scored based on labeled cell incorporation into the late looping stage PC region, and verified by whole mount in situ hybridization for Hcn4 (see Supplemental Figures 5,6). At each of the stages examined, including gastrulation stages (St5), neurula stages (St8), and heart tube stages (St10), PC precursors were mapped to a discreet region of the right lateral plate mesoderm (Figure 2A Blue = all tagged sites, Red =>10% PC). This region was posterior to the classical primary and secondary heart fields, as previously defined by cell tracing experiments and Nkx2.5 and Isl-1 expression (Figure 2B) (11–17). Due to the limitations of vital dye labeling, we cannot rule out that at each of these stages other regions contribute to the PCs. However, since labeled cells occupied the majority of the available area (Supplementary Figure 5) when present at the ventral right inflow outside contributions would have to be minor.
Figure 2.
PCs originate from mesoderm posterior to the heart fields. A) Fate maps depicting the location of PC progenitors at St 5, St 8, and St 10 (St 5 and 8 dorsal view, St 10 ventral view). Scale = 250um/division. Quantification of labels in boxed region is shown below (see materials and methods/supplemental Figure 5). B) In situ hybridization (dorsal view) and schematic (ventral vie) for heart field markers Nkx2.5 and Isl1 at St 8. asterisks = PC region. C) Best fit contour plot of data from St 8 fate mapping. D) Overlays of surface plots indicating location of atrial, atrioventricular junction, proepicardial, and pacemaker precursors. A-Anterior, P-Posterior, R-Right, L-Left.
PC precursors mapped to a region of the embryo that had not previously been identified as cardiogenic. Given the relatively large distance between PC precursors and the Nkx2.5/Isl1 expression domains currently associated with cardiac mesoderm, we wanted to determine the distribution of cell fates within this region where Nkx2.5/Isl1 were undetecable. A contour plot of the St 8 labeling data revealed that the region with the highest probability of PC fate was 100um in diameter centered 300um lateral to somite 3 (Figure 2C). The surrounding Nkx2.5/Isl1 negative mesoderm generated atria, atrioventricular junction, and the proepicardium (Figure 2D, Supplementary Figure 5), indicating large portions of the cardiogenic mesoderm does not express detectable levels Nkx2.5 or Isl1 at St 8. PC precursors did not substantially overlap with adjacent cardiac cell types, suggesting that heart precursors spatially segregate very early during lateral plate mesoderm formation.
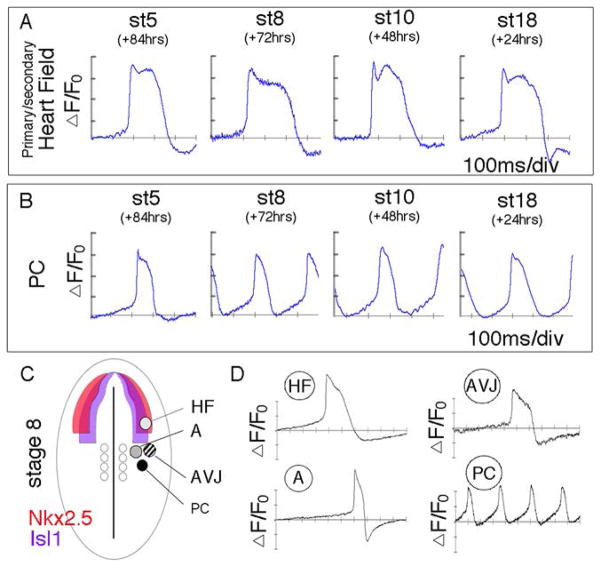
Using the above fate mapping information, we examined when PC fate became specified. PC precursors and primary/secondary heart field cells (Nkx2.5/Isl1 positive domain) were isolated at the embryonic stages outlined above and allowed to differentiate ex vivo. The physiological and molecular identity of explants was then monitored. St 18 explants spontaneously initiated APs with a periodicity of 256 +/− 29ms and displayed phase 4 (diastolic) depolarization (Figure 3A,B, Supplementary Figure 7, Movie 7) distinct from stage matched working myocardial explants. PC AP characteristics were not present in St 5 explants (Figure 3A, B, Supplementary Figure 7, Movie 7). Explants from St 5 had large variations in interbeat intervals, slow beating rates, lacked phase 4 depolarization, and displayed an expression profile inconsistent with St18 PCs (Supplementary Figure 8). PC precursors from both St 8 and St 10, however, did differentiate into PC-like cells following culture. Both stages displayed clear phase 4 depolarization, which was absent in age matched primary/secondary heart field derived cells, (Figure 3A, B, Supplementary Figure 7, Movie 7), and spontaneously depolarized at intervals of 303 +/− 53 ms and 294 +/− 45 ms, respectively. Expression profiles of explants from St 8 were consistent with in vivo PC as described above, showing enrichment for Hcn4, Serca2, Ryr2, as well as co-expressing Amhc1 and Vmhc1 (Supplementary Figure 8).
Figure 3.
PC fate is specified by St 8. A) Membrane depolarization recorded from heart field and (B) PC precursors isolated from indicated stages. C) Schematic indicating sites of mesodermal isolation from St 8 embryos relative to the Nkx2.5 and the Isl1 expression. D) Representative optical tracings of membrane potential from regions indicated in (C) following 72 hrs of culture.
These data suggest that by St 8, PC fate is already established in the Nkx2.5/Isl1 negative lateral plate mesoderm. To determine the spatial restrictions of PC specification, mesoderm directly adjacent to the PC precursors was isolated from the presumptive atrial, atrioventricular junction, and proepicardium (see Figure 3C). Only the PC region displayed elevated phase 4 depolarization and high rate AP production (Figure 3D, Supplementary Figure 7). These findings suggest that by St8, PC fate is specified in a highly restricted sub-domain of the right lateral plate mesoderm, and that the initial events dictating the functional divergence of PC-fate from the adjacent working myocardium must occur prior to this stage.
Additionally, by this stage, our data indicate a large region of mesoderm outside of the primary and secondary heart fields is already specified into working myocardial and PC fates. In order to delineate this mesodermal subdomain from the more classically defined heart fields, we refer to it as a tertiary heart field in chick. Although the precise boundaries of the primary and secondary heart fields remain controversial (18), our high resolution fate mapping reveals the distribution and boundaries of several subtypes of cardiac precursors within the tertiary heart field. Conservation of this field in other models systems will require further validation.
Many studies have identified factors necessary for inducing myocyte specification in the primary and secondary heart fields. To determine factors that may play a role in inducing PC fate within the tertiary heart field, we examined the expression of several factors thought to positively or negatively influence myocyte specification during the developmental window outlined above. Expression of a canonical Wnt, Wnt8c, was detected in the region of pre-specified PCs but not the more anterior heart fields (Supplementary Fig 9). Additionally, PC precursors, but not heart field cells, displayed nuclear accumulation of beta-catenin suggesting active Wnt signaling (Supplementary Figure 9I–L). We found this surprising since Wnts have previously been identified as inhibitory for heart field specification (19, 20).
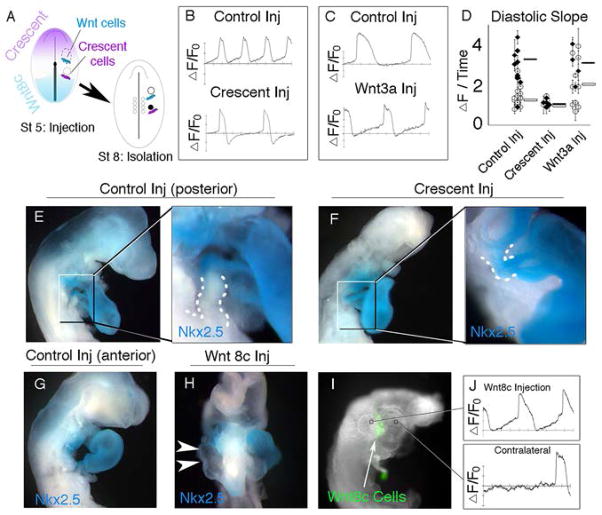
To determine whether Wnt signaling is required to promote PC fate, cells expressing the soluble Wnt antagonist Crescent (19, 20) were microinjected adjacent to PC precursors prior to their specification. Following 8hrs, PC precursors were explanted and allowed to differentiate ex vivo. Exposure to Crescent decrease the slope of PC phase 4 depolarization by 65% when compared to control injections (Figure 4B, D). These experiments cannot rule out that Crescent is interacting with factors not associated canonical Wnt signaling, however. Therefore, to further demonstrate that Wnt signaling was capable of inducing PC fate, we injected Wnt expressing cells into the presumptive heart fields. This resulted in a a 69% increase in phase 4 slope (Figure 4C, D). Further we activated Wnt signaling in the heart field using a pharmacological GSK3 inhibitor, Bio, which has previously been shown to stabilize beta-catenin (21,22). Consistent with above, 10uM Bio increased diastolic slope in heart field explants when compared to control (Supplementary Figure 10).
Figure 4.
Canonical Wnt signaling promotes PC fate. A) Schematic of stage 5 embryo indicating Crescent and Wnt8c expression domains. B, C) Following Crescent or Wnt expressing cell injections, embryos were developed to St 8, when PC or 1/2 Heart field precursors were isolated and placed in culture. Optical Membrane potential recordings from PC or heart field cultures following culture. D) Quantification of diastolic slope from PC (black diamonds) or heart field cells (white circles). E–H) Nkx2.5 expression is expanded in to the PC region following Crescent expressing cell injection (dashed area of high magnification insets from E–F ), and is lost in the heart following Wnt cell injection (arrow in H) compared to control cells injection. I) Location of Wnt8C expression relative to ectopic PC-like AP generation (J).
When we allowed injected embryos to develop to late looping stages, aberrant Wnt signaling led to severe morphological defects, consistent with previous reports (Figure 4F, H) (23). Crescent injection adjacent to PC precursors led to the ectopic expression of Nkx2.5 in PC at St 18, which is in agreement with a conversion of PC into a more working myocardial fate (24) (Figure 4E, F). Approximately 35% of Wnt injected embryos survived to heart looping stages. Wnt introduction into the primary and secondary heart field mesoderm, resulted in irregularly contracting hearts, with decreased Nkx2.5 expression on the injected side of the embryo (Figure 4G, H). To confirm that these Nkx2.5 negative regions were still electrically active, we preformed optical mapping. Consistent with a Wnt-based conversion of working myocardium into PC-like cells, retrograde propagation (outflow towards inflow) as well as ectopic pacemaker sites were detected (Supplementary Movie 8). These ectopic sites, were restricted to the Wnt injected side of the embryo and demonstrated AP shapes similar to control PCs (Figure 4I, J, Supplementary Movie 8).
These findings suggest that early mesodermal, Wnt-mediated, cues are sufficient to induce pacemaker-like fates that do not manifest until late looping stages. However, since Wnts are broadly and bilaterally expressed in the posterior mesoderm, additional cues are most likely required to restrict PC fate, including laterality genes (25, 26). The early diversification of PC fate from the working myocardium, however, suggests that fate specification is assigned directly in the lateral plate mesoderm, and is not the result of the specialization of an already functional embryonic myocyte. These data establish a framework through which PC development should be viewed providing a foundation for tissue engineering and stem cell-based approaches for PC generation.
Supplementary Material
Supplementary Fig 1. Maturation of AP morphology in the developing heart. A) Optical traces (change in fluorescence/initial fluorescence) from the St 10 AP initiation site, Heart tube, and outflow region depicted in figure 1A. Red dashed line indicates diastolic slope at AP initiation site. B) As in (A), for the St 15 heart depicted in Figure 1B. C) Optical traces from the AP initiation site, Atria, and Ventricle of the St 18 heart depicted in Figure 1C. D) As in (C), for the St 35 heart depicted in figure 1E. Red dashed line indicates diastolic slope at AP initiation site. E) Comparison of Diastolic slope between AP initiation sites recorded in St 10 hearts vs St 18 heart. F) Comparison of action potential duration at the AP initiation site in hearts recorded at St 10 vs St 18.
Supplementary Fig 2. Gene enrichment in newly functional PCs. A) Time series of action potential initiation and propagation in a St 18 heart. Green signal indicates depolarization, red arrows indicate initiation site (See also Supplementary movie 3). B) Diagram of St 18 heart, red arrow depicts action potential initiation site. 200x200 μm fragments of cardiac tissue denoted by asterisks were removed from areas corresponding to the ventral surface of the right inflow (V R Inflow), atria, atrioventricular junction (avj), and ventricle (also referred to as heart field in text), and processed for Real-Time PCR analysis. C) In situ hybridization for Hcn4 at St 18. C′) Relative Expression of Hcn4 in cells isolated from regions indicated in (B). Expression levels were normalized to GAPDH, and are reported as fold change relative to ventricular cells. D,D′) In situ hybridization and Real-time PCR analysis of Serca2. E,E′) In situ hybridization and Real-time PCR analysis of Ryr2. F,F′) In situ hybridization and Real-time PCR analysis of Ncx1. G) Double In situ hybridization for Amhc1 (blue) and Vmhc1 (purple) at St 18. G′) Real-Time PCR analysis of Amhc1(black) and Vmhc1(white), note their co-expression in in the ventral right inflow corresponding to the initial excitable area shown in (A). H–M) In situ hybridization for indicated genes at St 10.
Supplementary Fig 3. Regional shifting of early pacemaker function. A–D) Isochronal maps from hearts at St 10, St 11, St 12, and St 13 (8ms/div). E) Zero time point of a St 10 heart labelled with DiI (red) at pacing region (replicated from figure 1I). E′) Heart from (E) following 36 hrs of additional incubation (replicated from Figure 1J). F) Zero time point of a St 11 heart labelled with DiI at pacing region. F′) Heart from (F) following 36hrs of incubation. G) Zero time point of a St 12 heart labelled with DiI at pacing region. G′) Heart from (G) following 36hrs of incubation. H) Zero time point of a St 13 heart labelled with DiI at pacing region. H′) Heart from (H) following 36hrs of incubation. I) Zero time point of a St 10 heart in which pacing region is labelled with DiI (red) and contralateral position labelled with DiO (green). As Indicated in inset 2/37 hearts imaged at this stage paced from the right-sided position marked by DiO. J) Heart from (I) after 36 hrs of incubation. Note neither the DiI (red) tagged region nor the DiO (green) tagged region remains associated with the hearts inflow, instead both contributed to the atrioventricular junction (avj). ht- heart tube, at- atria, avj-atrioventricular junction, v-ventricle.
Supplementary Fig 4. Right inflow PC progenitors maintain pacemaker function throughout the termination of heart looping and septation. A, E, I) In Ovo DiI (red) labeling of primary pacing site in St 18 Embryos (see Figure 1). B) Heart isolated from (A) following 72 hrs of incubation to St 29. C) Boxed region from (B), note DiI labelled cells are present in the bulbous right inflow attached to the back of the right atria (r-at). D) Isochronal Map (1ms/div) of the heart from (A,B,C). The action potential initiates from a position in the right inflow overlapping with the DiI labeled cells (red arrows). F) Heart from (E) following 108 hrs of incubation to St 32. G) Boxed region from (F), DiI labelled cells are present in the remnant of the right inflow as it incorporates into the right atria. H) Isochronal Map (1ms/div) of heart from (E,F,G). Action potential initiation site again overlaps with DiI labeled cells (red arrows). J) Heart from (I) following Incubation to St 35. K) Boxed region from (J), DiI label has incorporated into the r-at between the superior vena cava (svc) and inferior vena cava (ivc). L) Isochronal Map (1ms/dv) of heart from (I,J,K), action potential initiation site overlaps with DiI labeling (red arrows - see Supplementary movie 6). cs- coronary sinus.
Supplementary Fig 5. Quantitative fate map construction. A) St 8 embryo labelled with DiI (red) and DiO (green). B) Higher magnification image of region from (A). The position of each label was measured relative to the center of the right third somite (boxed area). C) Following incubation to St 18, Bright field images of each heart and fluorescent images of labelled cells were taken and overlaid. D) Each region of the heart was traced on the bright field image using Image J (V1.42q, NIH). This template was then superimposed over florescent images as in (E, F). Fluorescent images were thresholded, highlighting the brightest 75% of the image. G) The distribution of fluorescence derived from each tagged region was then assigned back to the starting position determined in (B). This position was plotted against its percent contributions to the St 18 heart. Thin Plate Spline interpolation was used to construct contour plots for the distribution of (H) PC precursors, (I) atria precursors, (J) atrioventricular junction (AVJ) precursors, and (K) proepicardial precursors at St 8. L–O) Examples of embryos labelled at St 8 with DiI (red), DiO (green), and DiD (blue), showing non-pacemaker, Nkx2.5, Islet-1 negative mesodermal populations, (see Figure 2B) incorporating into the heart.
Supplementary Fig 6. Labelled cell incorporation into enriched Hcn4 expression domain. A) Region labeled at St 6 with DiO. B) Embryo from (A) developed to late looping stages. C) Heart from (B), following whole mount in situ hybridization for Hcn4. D) DiO labeling at St 8. E) Embryo from (D) developed to St 24. F) Heart from (E), following whole mount in situ hybridization for Hcn4. G) DiO labeling at St 10. H) Embryo from (G) developed to St 29. I) Heart from (H), following whole mount in situ hybridization for Hcn4.
Supplementary Fig 7. Quantification of in vitro PC Differentiation. A) Quantification of phase 4 (diastolic) slope in cultures isolated from PC precursor of Heart field precursor regions at St 5, St 8, St 10, and St 18. Black diamonds = PC precursors, White circles = heart field cells. Averages are indicated by lines. B) Quantification of beat rate from cultures in (A). C) Schematic of the St 8 regions explanted for culture relative to Nkx2.5 and Isl1 expression. D) Quantification of phase 4 slope from regions from (C) following 72hrs culture. E) Quantification of beat rate from cultures in (D).
Supplementary Fig 8. St 8 but not St 5 explant cultures give rise to PC-like cells. A) Diagram of St 5 embryo indicating PC region (light red) and heart field (HF) (light blue) isolated for culture. B) Diagram of St 8 embryo indicating PC region (dark red) and heart field region (dark blue) isolated for culture. C) Percentage of explants beating through 96 hrs of cultures. St 5 heart field - light blue, St 8 heart field - dark blue, St 5 PC - light red, St 8 PCs - dark red. D) 10 second recoding of culture beating (spikes correspond to culture contraction) from St 5 and St 8 cultures following 72 hrs of culture. E) Rate of culture beating (beats per minute) from regions described in (A,B) through 96 hrs of culture. St 5 heart field - blue dashed line, St 8 heart field - blue solid line, St 5 PC - red dashed line, St 8 PCs - red solid line. F) Rhythmicity of St 5 and St 8 contraction following 72 hrs of culture measured as average variation between contractions. G) Expression of ion channels associated with Pc AP production in St 5 vs St 8 PC precursors following 80 and 72 hrs of culture respectively (fold change relative to heart field cultures). H) As for (G), expression of Amhc1 and Vmhc1.
Supplementary Fig 9. Canonical Wnt8c expression overlaps with the St 5 PC region. A, B) St 5 and St 8 fate maps from Figure 2A. C) Whole mount in situ hybridization for Crescent at St 5. Embryo is viewed from the ventral surface and approximate position of PCs is indicated by red arrow. D) As in (C) for Wnt8c. E) Whole mount in situ hybridization for Crescent viewed from the ventral surface in a St 8 embryo. Approximate position of PCs is indicated by red arrow. F) As in (E) for Wnt8c. G) Real Time PCR analysis of Crescent expression from mesoderm isolated from St 5 and St 8 heart field (HF), pacemaker region (PC), and mesoderm located 200 μm posterior to PC region (P). Values were normalized to GAPDH and are presented as fold change relative to St 5 heart field. H) As in (G), Real-Time PCR analysis of Wnt8c expression. I,J) Transverse sections through a St 5 embryo as indicated in (A) stained for B-Catenin (green). Nuclei were counter-stained with Dapi and pseudo-colored red. K,L) Transverse section from a St 8 embryo at levels indicated in (B), stained for B-catenin (green) and Dapi (red).
Supplementary Fig 10. Stabilization of B-Catenin induces pacemaker-like phenotype. A) Embryos were isolated at St 5 and treated with vehicle (DMSO) or 10 μM GSK3 inhibitor IX, BIO. B) These ebryos were allowed to develop for 8 hrs to St 8, when heart field mesoderm (circle), was excised for culture. C) Optical mapping of Vehicle and Bio treated explants following 72hrs of culture. D) quantification of diastolic slope of cultured explants from vehicle of BIO treated embryos, averages are indicated by white bars.
Supplementary Movie 1. Optical mapping of a St 10 heart viewed from its ventral surface (see Figure 1). Green signal corresponds to membrane depolarization presented as change in fluorescence/over original florescent intensity (ΔF/Fo ). Note action potential initiates in the left posterior inflow.
Supplementary Movie 2. Optical mapping of a St 15 heart viewed from ventral/anterior surface (see Figure 1). Green signal corresponds to membrane depolarization presented as change in fluorescence/over original florescent intensity (ΔF/Fo ). As for St 10 heart, action potential initiates in the left posterior inflow.
Supplementary Movie 3. Optical mapping of a St 18 heart viewed from ventral surface (see Figure 1). Green signal corresponds to membrane depolarization presented as change in fluorescence/over original florescent intensity (ΔF/Fo ). Action potential initiates has shifted to the ventral surface of the right inflow.
Supplementary Movie 4. Optical mapping of a St 22 heart viewed from the right (see Figure 1). Green signal corresponds to membrane depolarization presented as change in fluorescence/over original florescent intensity (ΔF/Fo ). As for the St 18 heart, action potential initiates in the right inflow.
Supplementary Movie 5. Optical mapping of a mature embryonic heart at St 35. Heart is viewed from its dorsal surface. Green signal corresponds to membrane depolarization presented as change in fluorescence/over original florescent intensity (ΔF/Fo ). Action potential initiates from a region along the back of the right atria, between the insertions of the superior and inferior vena cava.
Supplementary Movie 6. Optical Mapping of the DiI labeled heart in Figure 1K,L. Position of DiI labeled cells is shown in red. Note action potential initiation site overlaps with labeled cells.
Supplementary Movie 7. Optically mapped primary cultures following isolation and ex vivo differentiation. As described for Figure 3, St 5 heart field mesoderm (A), St 8 heart field mesoderm (B), St 5 heart tube cells (C), and St 18 ventricular cells (D), were isolated and optically mapped following indicated periods of culture. Culture action potential wave forms indicate very similar electrophysiological characteristics are present regardless of stage of isolation. E) St 5, (F), St 8, (G), St 10, and (H) St 18 CPC progenitors were likewise isolated and allowed to differentiate ex vivo. Unlike heart field cells CPC progenitors isolated from St 5 gave rise to cultures with varied physiological characteristics, however CPC features were consistently present in explants from St 8 onward.
Supplementary Movie 8. Wnt8c expressing cells were injected into embryos prior to St 5, A) Indicates position of Wnt8c expression cells (green) following incubation to late looping stages. Heart looping is stunted compared to equivalently incubated control embryos. B) optical mapping of heart from (A). Action potential initiates at position adjacent to Wnt8c expressing cells [compare with (A)], and propagate both towards in flow and out flow components. C) Tracings of signal from ectopic pacing site and adjacent myocardium. oft-out flow tract, l-in- left inflow, r-in- right inflow.
Acknowledgments
We thank T, Kornberg, D. Stainier, S. Coughlin, and R. Shaw for their comments on this manuscript. Our thanks extend to Mikawa lab members for their suggestions. All data reported in this paper can be found in the main text or supplementary materials. This work has been funded in part by grants from the NIH (R01HL093566, R01HL112268 to TM., M.B. was also supported by T32HL007544).
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
References and Notes
- 1.Keith A, Flack M. J Ant Physiol. 1907;41:172–189. [PMC free article] [PubMed] [Google Scholar]
- 2.Hoff EC, Kramer TC, DuBois D, Patten BM. American Heart J. 1939;17:470–488. [Google Scholar]
- 3.Van Mierop LHS. Am J Physiol. 1967;212:407–415. doi: 10.1152/ajplegacy.1967.212.2.407. [DOI] [PubMed] [Google Scholar]
- 4.Kamino K, Hirota A, Fujii S. Nature. 1981;290:595–597. doi: 10.1038/290595a0. [DOI] [PubMed] [Google Scholar]
- 5.Buckingham M, Meilhac S, Zaffran S. Nature Reviews Genetics. 2005;6:826–834. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 6.Hamburger V, Hamilton HL. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 7.Lieberman M, Paes de Carvalho A. J Genral Physiol. 1965;49:351–363. doi: 10.1085/jgp.49.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stieber J, et al. PNAS. 2003;100:15235–15240. doi: 10.1073/pnas.2434235100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinogradova TM, et al. Circ Research. 2012;107:767–775. doi: 10.1161/CIRCRESAHA.110.220517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogdanov KY, Vinogradova TM, Lakatta EG. Circ Research. 2001;88:1254–1258. doi: 10.1161/hh1201.092095. [DOI] [PubMed] [Google Scholar]
- 11.Rawles ME. Physiological Zool. 1943;16:22–43. [Google Scholar]
- 12.Stalsberg H, DeHaan RL. Developmental Biol. 1969;19:128–159. doi: 10.1016/0012-1606(69)90052-9. [DOI] [PubMed] [Google Scholar]
- 13.Abu-Issa R, Kirby ML. Developmental Biol. 2008;319:223–233. doi: 10.1016/j.ydbio.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lints TJ, Parsons LM, Hartley L, Lyons I, Harvey RP. Development. 1993;119:419–431. doi: 10.1242/dev.119.2.419. [DOI] [PubMed] [Google Scholar]
- 15.Lyons I, et al. Genes Dev. 1995;9:1654–1666. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- 16.Yuan S, Schoenwolf GC. Anat Rec. 2000;260:204–207. doi: 10.1002/1097-0185(20001001)260:2<204::AID-AR90>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Cai C, et al. Dev Cell. 2003;5:877–899. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Q, Zhou B, Pu WT. Dev Biol. 2007;304:286–296. [Google Scholar]
- 19.Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider VA, Mercola M. Genes Dev. 2000;15:304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meijer L, et al. Chem Biol. 2003;10:1255–1266. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Sato N, et al. Nature Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 23.Manisastry SM, Han M, Linask KK. Dev Dyn. 2006;235:2160–2174. doi: 10.1002/dvdy.20878. [DOI] [PubMed] [Google Scholar]
- 24.Mommersteeg M, et al. Circ Research. 2007;100:354–362. doi: 10.1161/01.RES.0000258019.74591.b3. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, et al. PNAS. 2010;107:9753–9758. [Google Scholar]
- 26.Ammirabile G, et al. Cardiovascular Research. 2012;93:291–301. doi: 10.1093/cvr/cvr314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoff EC, Kramer TC, DuBois D, Patten BM. American Heart J. 1939;17:470–488. [Google Scholar]
- 28.Chapman SC, Collignon J, Schoenwolf GC, Lumsden A. Dev Dyn. 2001;220:284–289. doi: 10.1002/1097-0177(20010301)220:3<284::AID-DVDY1102>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Trichas G, Begbie J, Srinivas S. BMC Biol. 2008;6:1–13. doi: 10.1186/1741-7007-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig 1. Maturation of AP morphology in the developing heart. A) Optical traces (change in fluorescence/initial fluorescence) from the St 10 AP initiation site, Heart tube, and outflow region depicted in figure 1A. Red dashed line indicates diastolic slope at AP initiation site. B) As in (A), for the St 15 heart depicted in Figure 1B. C) Optical traces from the AP initiation site, Atria, and Ventricle of the St 18 heart depicted in Figure 1C. D) As in (C), for the St 35 heart depicted in figure 1E. Red dashed line indicates diastolic slope at AP initiation site. E) Comparison of Diastolic slope between AP initiation sites recorded in St 10 hearts vs St 18 heart. F) Comparison of action potential duration at the AP initiation site in hearts recorded at St 10 vs St 18.
Supplementary Fig 2. Gene enrichment in newly functional PCs. A) Time series of action potential initiation and propagation in a St 18 heart. Green signal indicates depolarization, red arrows indicate initiation site (See also Supplementary movie 3). B) Diagram of St 18 heart, red arrow depicts action potential initiation site. 200x200 μm fragments of cardiac tissue denoted by asterisks were removed from areas corresponding to the ventral surface of the right inflow (V R Inflow), atria, atrioventricular junction (avj), and ventricle (also referred to as heart field in text), and processed for Real-Time PCR analysis. C) In situ hybridization for Hcn4 at St 18. C′) Relative Expression of Hcn4 in cells isolated from regions indicated in (B). Expression levels were normalized to GAPDH, and are reported as fold change relative to ventricular cells. D,D′) In situ hybridization and Real-time PCR analysis of Serca2. E,E′) In situ hybridization and Real-time PCR analysis of Ryr2. F,F′) In situ hybridization and Real-time PCR analysis of Ncx1. G) Double In situ hybridization for Amhc1 (blue) and Vmhc1 (purple) at St 18. G′) Real-Time PCR analysis of Amhc1(black) and Vmhc1(white), note their co-expression in in the ventral right inflow corresponding to the initial excitable area shown in (A). H–M) In situ hybridization for indicated genes at St 10.
Supplementary Fig 3. Regional shifting of early pacemaker function. A–D) Isochronal maps from hearts at St 10, St 11, St 12, and St 13 (8ms/div). E) Zero time point of a St 10 heart labelled with DiI (red) at pacing region (replicated from figure 1I). E′) Heart from (E) following 36 hrs of additional incubation (replicated from Figure 1J). F) Zero time point of a St 11 heart labelled with DiI at pacing region. F′) Heart from (F) following 36hrs of incubation. G) Zero time point of a St 12 heart labelled with DiI at pacing region. G′) Heart from (G) following 36hrs of incubation. H) Zero time point of a St 13 heart labelled with DiI at pacing region. H′) Heart from (H) following 36hrs of incubation. I) Zero time point of a St 10 heart in which pacing region is labelled with DiI (red) and contralateral position labelled with DiO (green). As Indicated in inset 2/37 hearts imaged at this stage paced from the right-sided position marked by DiO. J) Heart from (I) after 36 hrs of incubation. Note neither the DiI (red) tagged region nor the DiO (green) tagged region remains associated with the hearts inflow, instead both contributed to the atrioventricular junction (avj). ht- heart tube, at- atria, avj-atrioventricular junction, v-ventricle.
Supplementary Fig 4. Right inflow PC progenitors maintain pacemaker function throughout the termination of heart looping and septation. A, E, I) In Ovo DiI (red) labeling of primary pacing site in St 18 Embryos (see Figure 1). B) Heart isolated from (A) following 72 hrs of incubation to St 29. C) Boxed region from (B), note DiI labelled cells are present in the bulbous right inflow attached to the back of the right atria (r-at). D) Isochronal Map (1ms/div) of the heart from (A,B,C). The action potential initiates from a position in the right inflow overlapping with the DiI labeled cells (red arrows). F) Heart from (E) following 108 hrs of incubation to St 32. G) Boxed region from (F), DiI labelled cells are present in the remnant of the right inflow as it incorporates into the right atria. H) Isochronal Map (1ms/div) of heart from (E,F,G). Action potential initiation site again overlaps with DiI labeled cells (red arrows). J) Heart from (I) following Incubation to St 35. K) Boxed region from (J), DiI label has incorporated into the r-at between the superior vena cava (svc) and inferior vena cava (ivc). L) Isochronal Map (1ms/dv) of heart from (I,J,K), action potential initiation site overlaps with DiI labeling (red arrows - see Supplementary movie 6). cs- coronary sinus.
Supplementary Fig 5. Quantitative fate map construction. A) St 8 embryo labelled with DiI (red) and DiO (green). B) Higher magnification image of region from (A). The position of each label was measured relative to the center of the right third somite (boxed area). C) Following incubation to St 18, Bright field images of each heart and fluorescent images of labelled cells were taken and overlaid. D) Each region of the heart was traced on the bright field image using Image J (V1.42q, NIH). This template was then superimposed over florescent images as in (E, F). Fluorescent images were thresholded, highlighting the brightest 75% of the image. G) The distribution of fluorescence derived from each tagged region was then assigned back to the starting position determined in (B). This position was plotted against its percent contributions to the St 18 heart. Thin Plate Spline interpolation was used to construct contour plots for the distribution of (H) PC precursors, (I) atria precursors, (J) atrioventricular junction (AVJ) precursors, and (K) proepicardial precursors at St 8. L–O) Examples of embryos labelled at St 8 with DiI (red), DiO (green), and DiD (blue), showing non-pacemaker, Nkx2.5, Islet-1 negative mesodermal populations, (see Figure 2B) incorporating into the heart.
Supplementary Fig 6. Labelled cell incorporation into enriched Hcn4 expression domain. A) Region labeled at St 6 with DiO. B) Embryo from (A) developed to late looping stages. C) Heart from (B), following whole mount in situ hybridization for Hcn4. D) DiO labeling at St 8. E) Embryo from (D) developed to St 24. F) Heart from (E), following whole mount in situ hybridization for Hcn4. G) DiO labeling at St 10. H) Embryo from (G) developed to St 29. I) Heart from (H), following whole mount in situ hybridization for Hcn4.
Supplementary Fig 7. Quantification of in vitro PC Differentiation. A) Quantification of phase 4 (diastolic) slope in cultures isolated from PC precursor of Heart field precursor regions at St 5, St 8, St 10, and St 18. Black diamonds = PC precursors, White circles = heart field cells. Averages are indicated by lines. B) Quantification of beat rate from cultures in (A). C) Schematic of the St 8 regions explanted for culture relative to Nkx2.5 and Isl1 expression. D) Quantification of phase 4 slope from regions from (C) following 72hrs culture. E) Quantification of beat rate from cultures in (D).
Supplementary Fig 8. St 8 but not St 5 explant cultures give rise to PC-like cells. A) Diagram of St 5 embryo indicating PC region (light red) and heart field (HF) (light blue) isolated for culture. B) Diagram of St 8 embryo indicating PC region (dark red) and heart field region (dark blue) isolated for culture. C) Percentage of explants beating through 96 hrs of cultures. St 5 heart field - light blue, St 8 heart field - dark blue, St 5 PC - light red, St 8 PCs - dark red. D) 10 second recoding of culture beating (spikes correspond to culture contraction) from St 5 and St 8 cultures following 72 hrs of culture. E) Rate of culture beating (beats per minute) from regions described in (A,B) through 96 hrs of culture. St 5 heart field - blue dashed line, St 8 heart field - blue solid line, St 5 PC - red dashed line, St 8 PCs - red solid line. F) Rhythmicity of St 5 and St 8 contraction following 72 hrs of culture measured as average variation between contractions. G) Expression of ion channels associated with Pc AP production in St 5 vs St 8 PC precursors following 80 and 72 hrs of culture respectively (fold change relative to heart field cultures). H) As for (G), expression of Amhc1 and Vmhc1.
Supplementary Fig 9. Canonical Wnt8c expression overlaps with the St 5 PC region. A, B) St 5 and St 8 fate maps from Figure 2A. C) Whole mount in situ hybridization for Crescent at St 5. Embryo is viewed from the ventral surface and approximate position of PCs is indicated by red arrow. D) As in (C) for Wnt8c. E) Whole mount in situ hybridization for Crescent viewed from the ventral surface in a St 8 embryo. Approximate position of PCs is indicated by red arrow. F) As in (E) for Wnt8c. G) Real Time PCR analysis of Crescent expression from mesoderm isolated from St 5 and St 8 heart field (HF), pacemaker region (PC), and mesoderm located 200 μm posterior to PC region (P). Values were normalized to GAPDH and are presented as fold change relative to St 5 heart field. H) As in (G), Real-Time PCR analysis of Wnt8c expression. I,J) Transverse sections through a St 5 embryo as indicated in (A) stained for B-Catenin (green). Nuclei were counter-stained with Dapi and pseudo-colored red. K,L) Transverse section from a St 8 embryo at levels indicated in (B), stained for B-catenin (green) and Dapi (red).
Supplementary Fig 10. Stabilization of B-Catenin induces pacemaker-like phenotype. A) Embryos were isolated at St 5 and treated with vehicle (DMSO) or 10 μM GSK3 inhibitor IX, BIO. B) These ebryos were allowed to develop for 8 hrs to St 8, when heart field mesoderm (circle), was excised for culture. C) Optical mapping of Vehicle and Bio treated explants following 72hrs of culture. D) quantification of diastolic slope of cultured explants from vehicle of BIO treated embryos, averages are indicated by white bars.
Supplementary Movie 1. Optical mapping of a St 10 heart viewed from its ventral surface (see Figure 1). Green signal corresponds to membrane depolarization presented as change in fluorescence/over original florescent intensity (ΔF/Fo ). Note action potential initiates in the left posterior inflow.
Supplementary Movie 2. Optical mapping of a St 15 heart viewed from ventral/anterior surface (see Figure 1). Green signal corresponds to membrane depolarization presented as change in fluorescence/over original florescent intensity (ΔF/Fo ). As for St 10 heart, action potential initiates in the left posterior inflow.
Supplementary Movie 3. Optical mapping of a St 18 heart viewed from ventral surface (see Figure 1). Green signal corresponds to membrane depolarization presented as change in fluorescence/over original florescent intensity (ΔF/Fo ). Action potential initiates has shifted to the ventral surface of the right inflow.
Supplementary Movie 4. Optical mapping of a St 22 heart viewed from the right (see Figure 1). Green signal corresponds to membrane depolarization presented as change in fluorescence/over original florescent intensity (ΔF/Fo ). As for the St 18 heart, action potential initiates in the right inflow.
Supplementary Movie 5. Optical mapping of a mature embryonic heart at St 35. Heart is viewed from its dorsal surface. Green signal corresponds to membrane depolarization presented as change in fluorescence/over original florescent intensity (ΔF/Fo ). Action potential initiates from a region along the back of the right atria, between the insertions of the superior and inferior vena cava.
Supplementary Movie 6. Optical Mapping of the DiI labeled heart in Figure 1K,L. Position of DiI labeled cells is shown in red. Note action potential initiation site overlaps with labeled cells.
Supplementary Movie 7. Optically mapped primary cultures following isolation and ex vivo differentiation. As described for Figure 3, St 5 heart field mesoderm (A), St 8 heart field mesoderm (B), St 5 heart tube cells (C), and St 18 ventricular cells (D), were isolated and optically mapped following indicated periods of culture. Culture action potential wave forms indicate very similar electrophysiological characteristics are present regardless of stage of isolation. E) St 5, (F), St 8, (G), St 10, and (H) St 18 CPC progenitors were likewise isolated and allowed to differentiate ex vivo. Unlike heart field cells CPC progenitors isolated from St 5 gave rise to cultures with varied physiological characteristics, however CPC features were consistently present in explants from St 8 onward.
Supplementary Movie 8. Wnt8c expressing cells were injected into embryos prior to St 5, A) Indicates position of Wnt8c expression cells (green) following incubation to late looping stages. Heart looping is stunted compared to equivalently incubated control embryos. B) optical mapping of heart from (A). Action potential initiates at position adjacent to Wnt8c expressing cells [compare with (A)], and propagate both towards in flow and out flow components. C) Tracings of signal from ectopic pacing site and adjacent myocardium. oft-out flow tract, l-in- left inflow, r-in- right inflow.