Abstract
The prevalence of obesity and type 2 diabetes increases with age. Despite this, few studies have examined these conditions simultaneously in aged animals, and fewer studies have measured the impact of these conditions on brain function. Using an established animal model of brain aging (F344 rats), we investigated whether high fat diet (HFD) exacerbates cognitive decline and the hippocampal calcium-dependent afterhyperpolarization (a marker of age-dependent calcium dysregulation). Young and mid-aged animals were maintained on control or HFD for 4.5 months and peripheral metabolic variables, cognitive function, and electrophysiological responses to insulin in the hippocampus were measured. HFD increased lipid accumulation in the periphery, though overt diabetes did not develop, nor was spatial learning and memory altered. Hippocampal adiponectin levels were reduced in aging animals but unaffected by HFD. For the first time, however, we show that the AHP is sensitive to insulin, and that this sensitivity is reduced by HFD. Interestingly, although peripheral glucose regulation was relatively insensitive to HFD, the brain appeared to show greater sensitivity to HFD in F344 rats.
Keywords: adiponectin, AHP, calcium, learning, aging, hippocampus, metabolism
1. Introduction
Multiple components of metabolic syndrome including obesity and diabetes correlate with, and even predict a higher incidence of Alzheimer’s disease [14,36,62]. Importantly, considerable evidence also indicates that metabolic syndrome plays a critical role in cognitive decline during normal aging [4,31,38,43]. Although metabolic dysregulation and cognitive decline appear to be distinct pathological processes, some common aspects of both conditions include brain insulin resistance, vascular disease, and/or inflammation [39,58]. Still, little is known about how or whether changes in peripheral glucose or lipid metabolism impact neuronal function and brain aging.
Despite considerable attention focusing on the links between the periphery and the brain regarding food intake and energy metabolism, it is not yet clear how peripheral hormones/peptides (e.g., insulin, adiponectin) that regulate these processes change with age. It is also unclear how or to what extent peripheral metabolic dysregulation influences cognitive decline or neuronal vulnerability in disease states [56]. Interestingly, caloric restriction and exercise, two manipulations that slow aging and associated cognitive decline [27], are also able to enhance adiponectin levels, which improve insulin sensitivity [15,22].
Animal models have been used extensively for studies of diabetes and obesity but have some limitations for studies of brain aging and cognitive decline. Genetic models of diabetes (Zucker diabetic fatty rat and the db/db mouse) show decreased learning and altered synaptic plasticity [34], although this is not always the case [3]. Irrespective of the results seen with these and other genetic models, one critical limitation is their short lifespan which precludes studies of aging. Experimentally-induced diabetes using streptozotocin (STZ) decreases hippocampal-dependent learning in young animals [10,47], but only a few studies have been conducted in aged animals using this model [25]. Furthermore, the STZ model, while valuable for studies of the impact of type 1 diabetes on learning and memory, does not recapitulate the condition most commonly seen in the aging population, including the clinically silent period of hyperinsulinemia that precedes type 2 diabetes (T2DM).
An alternative approach to study how peripheral metabolic dysregulation might influence cognitive decline in aging is to use a high fat diet (HFD). Numerous rodent studies indicate that HFD decreases insulin sensitivity while increasing cholesterol levels and body weight [7]. HFD increases visceral fat mass and circulating free fatty acids (FFA), resulting in widespread inflammation via cytokine/adipokine secretion [63]. A FFA-mediated reduction in insulin receptor signaling is a recognized pathway linking obesity to insulin resistance in liver, muscle and fat. Indeed, several reports indicate that elevated plasma FFAs induce insulin resistance through inhibition of glucose transport, mediated, in part, by a decrease in phosphotidyl inositol 3-kinase (PI3K) and its interaction with insulin receptor substrate 1 (IRS1) [8,16].
Among the most commonly used strains for aging research is the F344 rat. While this model has been characterized and is routinely used in studies of brain aging, the response to HFD has not been studied as extensively as in other strains. This is important, because the impact of dietary manipulations is clearly sensitive to rat strain [2,45,50]. Further, most studies of HFD in aging rodents focus solely on effects in the periphery and largely ignore the impact of the diet on the brain [6,27,42]. To address this gap, we compared long-term HFD in young and mid-aged F344s. Mid-aged rats were used to parallel the age at which the initial rise in peripheral metabolic dysregulation is typically observed in the human population. We assessed effects of HFD on peripheral metabolic variables and cognitive acuity in the Morris Water Maze (MWM). At the cellular level, we measured an electrophysiological marker of age-related cognitive decline, the Ca2+-dependent afterhyperpolarization (AHP), in area CA1 pyramidal neurons [5,18,44,60]. In the same brain area, we also measured insulin sensitivity, insulin signaling and adiponectin. In both age groups, HFD was associated with robust dyslipidemia and mild obesity but surprisingly did not induce diabetes or alter spatial memory. This is unlike other models and perhaps is related to the comparatively higher peripheral levels of adiponectin we observed in F344s. Electrophysiological measures in the hippocampus show for the first time that the AHP is sensitive to insulin, and that this sensitivity is reduced by HFD. Together, these results suggest the intriguing possibility that the brain, may in some instances, be more sensitive to the effects of HFD than the periphery.
2. Methods
2.1. Subjects
All experiments presented here were conducted under an approved IACUC protocol granted by the University of Kentucky. Sixty six male F344/NIA rats were maintained single-housed on a control diet (CD) for 3 weeks and baseline values for the glucose tolerance test (GTT), insulin tolerance test (ITT) and glycated hemoglobin (HbA1c) were obtained (see blood and serum analyses). After 3 weeks on CD, animals were separated into 4 groups as follows: young-adult (2.5–4 months old) on CD (n =13); young-adult on HFD (n = 13); mid-aged (12.5–13.5 months old) on CD (n =20) and mid-aged on HFD (n = 20). HFD was initiated on the fourth week of the study. GTT, ITT and HbA1c tests were repeated after 4.5 months. Food consumption and body weight measures were monitored 2–3 times a week for the duration of the study and averaged per week per animal. Four mid-aged animals on HFD, 3 mid-aged animals on CD, and 1 young-adult on CD were excluded due to poor health.
2.2. Diets
Purified diets were provided ad libitum (Harlan Teklad, Madison, WI). The CD consisted of the following (in % Kcal) 19.2 protein, 67.9 carbohydrate and 13 fat [TD. 08485]. The HFD, a “Western-style” diet, consisted of the following (in % Kcal) 15.2 protein, 42.7 carbohydrate and 42 fat [TD. 88137].
2.3. Glucose and insulin tolerance tests
In order to test for differences between weight-adjusted and standard single dose glucose or insulin tolerance tests (GTT, ITT, respectively), we monitored blood glucose levels in a series of pilot animals (n = 5–6/group; 2 months and 13 months old). These animals were exposed to a weight-adjusted glucose (2g/Kg body weight of 60% glucose), a weight-adjusted insulin dose (2mU/g body weight of humulin®), and a standard glucose/insulin dose (670 mg glucose/animal, or 450 mU humulin®/animal). The data are presented in supplemental Figure 1 and supplemental Table 1 and show that in the weight-adjusted condition glucose levels were significantly increased in older animals (F(1,26) = 21.6, p < 0.0001), an effect mediated mostly by greater glucose levels seen at the 30 min time point (post-hoc). This result was likely due to the greater mean group weight seen in older animals, and disappeared entirely under standard single dose glucose injection conditions (supplemental Fig. 1B1). No significant differences were found in measures of area-under-the-curve (AUC) for both glucose and insulin tolerance tests using either approach (supplemental Table 1). Following a 6 hr fast, glucose levels were monitored (mg/dL using a FreeStyle Lite glucometer; Abott Diabetes Care Inc., Alameda, CA) from tail prick blood samples taken at 30 min intervals over a 2 hour period in response to an intraperitoneal glucose or insulin injection [5]. For some analyses, glucose responses over time were integrated to determine the area-under-the-curve (AUC) and normalized to the initial time point value. Tests of glucose or insulin sensitivity were separated by one week to limit stress and carry over effects. Animals that did not show at least a 9 % change in glucose levels at the 30 min time point following glucose or insulin injection were removed from the analysis.
2.4. Tissue collection
Twenty-four animals were anesthetized with pentobarbital (100 mg/Kg body weight) and blood was collect from the right ventricle immediately prior to perfusion for 10 minutes with ice cold saline (0.9 %). Brains were post-fixed in 4% paraformaldehyde overnight and cryoprotected in 30% sucrose solution at −20°C until sectioning for immunohistochemistry. Heart, liver, and epididymal and retroperitoneal fat pads were collected and weighed (Table 1).
Table 1.
Normalized tissue weights
| Young CD | Young HFD | Mid-Aged CD | Mid-Aged HFD | ||
|---|---|---|---|---|---|
| Heart | # | 0.289 ± 0.008 | 0.274 ± 0.008 | 0.284 ± 0.003 | 0.274 ± 0.005 |
| Liver | # * | 3.42 ± 0.10 | 3.80 ± 0.06 | 3.30 ± 0.10 | 3.50 ± 0.07 |
| Epididymal Fat | # | 3.79 ± 0.12 | 4.43 ± 0.10 | 3.94 ± 0.08 | 4.16 ± 0.06 |
| Retroperitoneal Fat | # * | 3.89 ± 0.11 | 5.02 ± 0.17 | 4.65 ± 0.20 | 5.92 ± 0.19 |
Tissue weights at time of sacrifice were normalized to individual body weight and multiplied by 100.
Asterisks indicate significance by age, and # indicates significance by diet.
Abbreviations: control diet (CD), high fat diet (HFD). Means ± SEM are shown.
2.5. Blood serum analyses
Serum was isolated from whole blood and frozen for chemical analyses (Comparative Pathology Laboratory, University of California, Davis, CA). Adiponectin levels were determined by the Vanderbilt Hormone Assay Core Lab (Vanderbilt University, Nashville, TN). Because glycated hemoglobin levels are considered the “gold standard” for clinical monitoring of diabetes, prior to blood separation, a drop was used for HbA1c measurement using a DCA Vantage ™ Analyzer (Siemens Healthcare Diagnostics Inc. Tarrytown, NY). Serum insulin levels were determined using Millipore rat/mouse insulin Elisa kit (Billerica, MA) according to the manufacturer’s instructions.
2.6. Western blotting
Hippocampal dorsal and ventral quarters were combined and used to quantify protein levels. Insulin receptor (IR) and insulin receptor substrate 1 (IRS-1) were quantified with a standard Western blot protocol. Subcellular fractions (membrane and cytosolic) were obtained from homogenates following a protocol adapted from Dou and colleagues (2005), and quantified for proteins using a Bradford assay. Samples were run on 10 % Mini-PROTEAN® TGX™ gels (Bio-Rad, Hercules, CA, USA). After transfer to nitrocellulose membranes, samples were immunostained overnight at 4 °C (anti-IRS-1 antibody, Santa Cruz Biotechnology, sc-81466; 1:800; anti-IR β antibody, Santa Cruz Biotechnology, sc-560; 1:600; and anti-β-actin antibody, Sigma-Aldrich, A1978, 1:2000). Following secondary antibody exposure, membranes were developed with ECL plus (GE Healthcare, Pittsburgh, PA, USA) and visualized using a phosphoimager (Storm 840; Molecular Dynamics, Sunnyvale, CA). ImageJ was used for signal intensity measures (NIA, Bethesda, MD, USA; http://rsb.info.nih.gov/ij/), and each sample was normalized to β-actin. All experiments were run in duplicate.
2.7. Morris water maze
As in prior work [5], temperature was maintained at 25–26°C and one quadrant contained a 15 cm diameter escape platform. Videomex-V (Columbus Instrument, Columbus, OH) was used to monitor animal movements. For all training days, three trials were run (each with a random start location). Animals were placed in the pool and given 60 s to find the platform, otherwise, they were guided to the platform. All animals were allowed to stay on the platform for 30 s. Following this 30 s period, animals were taken outside the MWM enclosure for 2 min, and returned to the MWM for a second, and then a third trial. On day 1, three cue trials were run while a hanging white cup was positioned over the partially visible platform. The next 3 days of training/learning (days 4, 5, and 6) animals continued to receive 3 trials/day (without cue and a submerged platform). Twenty-four hours after the last training day, a 1 min probe test with the platform removed was conducted. The next day, animals were trained to learn a new platform location (reversal training - opposite quadrant from previous position) also using 3 trials/day. Seventy-two hours later a reversal probe was conducted. Performance was evaluated using measures of path length to platform and a cumulative search error index. The index is representative of how far each animal deviated from an ideal path to goal [5,17].
2.8. Immunohistochemistry
Coronal sections (30 μm) cut on a freezing-sliding microtome were exposed to the following primary antibodies: adiponectin (rabbit, 1:500; Abbiotec, San Diego, CA), insulin receptor-α antibody (rabbit IR-α, 1:200, Abbiotec, San Diego, CA) and insulin receptor subtype-1 antibody (rabbit IRS-1, 1:200, LSBio, Seattle, WA). For adiponectin and IRS-1 staining, sections were pretreated 30 min at 85°C in sodium citrate (pH = 6.5). All tissues were pretreated 30 min in 2% H202. Following incubation with primary antibody in TBS-T overnight at room temperature, tissues were rinsed 3x and incubated with the appropriate biotinylated secondary antibody for 2 h at room temperature. Sections were rinsed 3x, incubated 2 h with the tertiary antibody Extra-Avidinperoxidase, rinsed 3x, and exposed to nickel-enhanced DAB staining for visualization. Slides were air-dried, dehydrated in xylene and coverslipped with DPX. For each animal, 2 sections were measured using an area drawn around the pyramidal layer of area CA1 (NIH Scion Image program) and averaged. Images of hippocampal areas were acquired with an Olympus DP70 digital camera. For each antibody all sections were processed in parallel.
2.9. Electrophysiology
Electrophysiological data were collected from 31 animals at least one week after the last day of Morris water maze to limit the impact of learning and arousal on transient hippocampal excitability changes [44]. Hippocampal slices were obtained according to previously published protocols [5]. Animals were briefly anesthetized in a CO2 chamber and decapitated. Hippocampi were removed and transverse slices were prepared (350 μm) in ice-cold low-calcium artificial cerebrospinal fluid (ACSF) composed of the following (in mM): 128 NaCl, 1.25 KH2PO4, 10 Glucose, 26 NaHCO3, 3 KCl, 0.1 CaCl2, 2 MgCl2) using a Vibratome® (TPI, Saint Louis, MO). Slices were transferred to a heated (32°C) interface-type chamber and maintained in oxygenated (95% O2, 5% CO2) normal-calcium ACSF containing 2 mM CaCl2 and 2 mM MgCl2 for at least 2 h prior to recording. Each hippocampal slice was placed in a recording chamber (RC22C, Warner Instruments, Co., Hamden, CT) and maintained in a continuous flow of oxygenated normal-calcium ACSF pre-heated at 32°C using a TC2Bip/HPRE2 in-line heating system (Cell Micro Controls, Norfolk, VA). The recording chamber was mounted on the stage of a Nikon E600FN inverted microscope. Cells were impaled with sharp microelectrodes filled with 2M KmeSO4 and 10mM HEPES, pH 7.4, pulled from borosilicate glass capillaries (World Precision Instruments, Sarasota, FL).
To generate an afterhyperpolarization (AHP) cells were held at −65 mV (baseline) and depolarized with a 100 ms intracellular current injection sufficient to generate 3 Na+ action potentials. AHPs were elicited every 30 s and between 2 to 10 sweeps were averaged for each cell. The medium AHP (mAHP) was measured as the peak hyperpolarization immediately after the offset of the depolarizing current injection, the slow AHP (sAHP) was measured 800 ms after the end of the current injection. AHP duration was measured from the end of the depolarizing step until return to baseline. Neurons with input resistance >35 MΩ, holding current <250 pA, and overshooting action potentials were included in this analysis. Action potential height, input resistance, current injection to elicit the AHPs, and recording electrode resistance were quantified (Table 3). Data were acquired using pClamp 8.0 (Molecular Devices) software through a Digidata 1320A A/D converter and an Axoclamp2B (Molecular Devices).
Table 3.
Electrophysiological properties
| Young CD | Young HFD | Mid-Age CD | Mid-Age HFD | |
|---|---|---|---|---|
| Electrode Resistance (MΩ) | 128.3 ± 10.3 | 121.6 ± 3.56 | 114.6 ± 8.06 | 113.5 ± 5.61 |
| Input Resistance (MΩ) | 47.67 ± 3.81 | 48.00 ± 3.55 | 48.88 ± 2.84 | 44.42 ± 1.67 |
| Action Potential Height (mV) | 12.58 ± 2.41 | 15.94 ± 2.62 | 11.92 ± 1.64 | 14.69 ± 1.25 |
| Current Injection (pA) | 251.7 ± 18.7 | 233.3 ± 14.2 | 231.2 ± 16.7 | 296.7 ± 28.4 |
No significant differences (2-way ANOVA, p > 0.05) were found in any of the electrophysiological properties: electrode resistance, input resistance, action potential height (measure at peak), and intracellular current injection required to elicit 3 action potentials. Mean ± SEM are shown.
Some hippocampal slices were exposed to acute insulin to determine its effect on the AHP. Humalog® was perfused into the flow of oxygenated recording ACSF at a concentration of 4 uM (diluted in ACSF from the 600 uM stock) and subject to an eight fold dilution resulting in a final concentration at 500 nM insulin. Another set of cells were recorded (n = 5) to control for the impact of time and vehicle application. In these cells, and following a 15 min perfusion with ACSF, the AHP was not altered.
2.10. Statistics
Body weight and food consumption were analyzed from weeks 4 to 23 using a 2-way repeated measure ANOVA (for time and diet effects) in young and mid-aged animals separately. A 2-way ANOVA across diet and age was also used at week 23. Two-way RM ANOVAs were used for all other measures with a primary focus on diet and age effects. Post-hoc analyses used the Bonferroni test. For all variables measured, outliers (> 2 SD from group mean) were removed from the analysis.
The peripheral lipid index (PLI) for each animal in the study was constructed from measures of retroperitoneal fat, the HDL to LDL ratio, and cholesterol. Each measure obtained from each animal was ranked. The average of each animal’s rank from these 3 measures was defined as that subject’s PLI value (higher values represent poorer lipid profiles). A similar procedure was used to obtain a spatial learning and memory index (SLMI) using measures of cumulative search errors during learning, and during the probe and reversal probe (high values represent poorer spatial memory). Correlations report the Pearson r values.
3. Results
3.1. Body, organ weights and food consumption
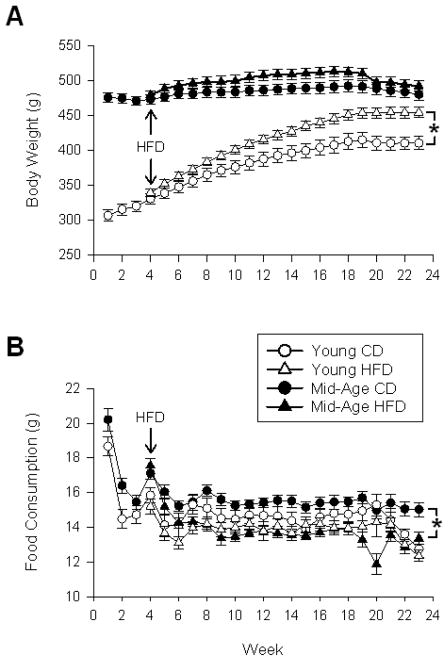
Body weights increased significantly from weeks 4 to 23 in young (F(19,23) = 213.1; p < 0.0001) and mid-aged animals (F(19,31) = 28.0 p < 0.0001) on a control diet (CD). As shown in figure 1A, HFD increased body weights more robustly in young animals (F(1,23) = 9.2; p < 0.01) compared to mid-aged animals (F(1,31) = 2.0; p = 0.17). By the end of the study on week 23, significant aging (F(1,54) = 44.0; p < 0.0001) and diet effects (F(1,54) = 11.9; p = 0.001) were still present, with a greater impact of the diet seen on measures of body weight in younger animals. Table 1 provides analyses of peripheral tissue weights normalized to body weight. Epididymal and retroperitoneal fat pad weights were increased by HFD (epididymal F(1,54) = 23.8; p < 0.0001, retroperitoneal F(1,54) = 42.0; p < 0.0001), but only retroperitoneal was also increased by age (F(1,54) = 20.0; p < 0.0001). Liver weights were increased by diet (F(1,54) = 10.9; p < 0.005) and decreased by age (F(1,54) = 5.5; p < 0.05), while heart weights were significantly decreased by diet (F(1,54) = 4.2; p < 0.05). The decrease in normalized liver and heart weights is likely due to the increase in body weights in the mid-aged and HFD groups (analysis on non weight-corrected data did not reveal decreases in absolute tissue weights). As shown in figure 1B, across weeks 4 to 23, HFD caused a significant reduction in food consumption, but only in older animals (F(1,31) = 29.9; p = 0.0001). Analysis of food consumption by the end of the study, on week 23, shows significant aging (F(1,54) = 17.6; p < 0.0001) and diet effects (F(1,54) = 6.8; p < 0.05). The greater decrease in food consumption seen in the older animals is consistent with their lower weight gain in comparison to the younger animals.
Figure 1. Body weight and food consumption across 23 weeks of HFD.
A shows mean body weights across weeks of treatment. B shows amounts of food consumed (averaged on a weekly basis). Data analysis includes the period from 4–23 weeks during which the HFD was in effect. Mean ± SEM are shown. Asterisks indicate significant difference @ p < 0.05 level.
3.2. Blood measures
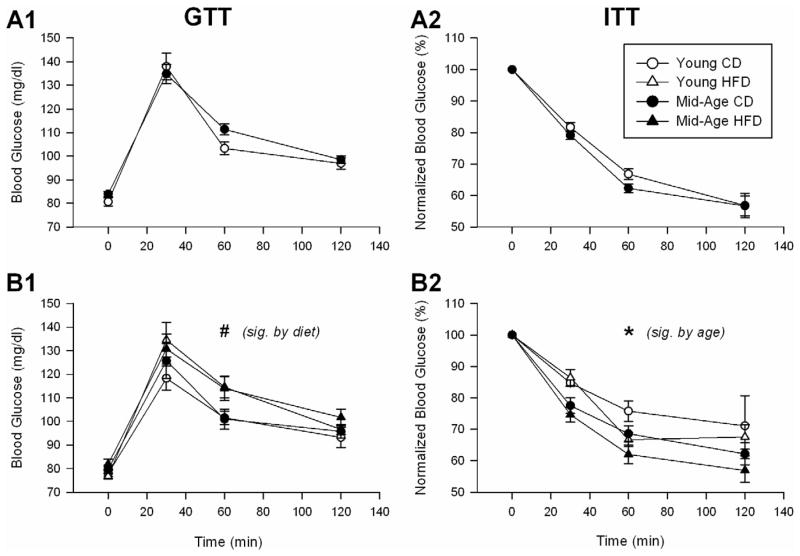
Consistent with prior reports in aging rats [2,24,35], and based on blood levels of glucose and insulin, our animals did not show signs of hyperglycemia either initially, or after 4.5 months of HFD or CD (Table 2). We complemented these static measures with assessment of dynamic glucose responses to glucose or insulin injections (measured as AUC, Table 2). As the conventional clinical approach for the GTT uses a single dose irrespective of weight, and because of the results from our pilot study (see methods), we report data using the standard single dose method. Animals were subjected to the GTT and ITT experiments initially, and after 4.5 months on either diet (Fig. 2). A slightly greater response to glucose injection over time was seen in animals on HFD when compared to CD (Fig. 2B1), as determined by a greater AUC (F(1,51) = 6.15; p < 0.05; Table 2). Surprisingly, an enhanced insulin response was seen (decreased glucose levels) in older animals after 4.5 months on either diet (Table 2 and Fig. 2B2), again as determined by a greater AUC (F(1,41) = 8.7; p = 0.005), which suggests greater insulin sensitivity in older animals. While these data are normalized to baseline glucose levels, analyses of non-normalized data at the 30 min time point also yielded significant age-dependent decreases in glucose levels (F(1,41) = 13.3; p = 0.0008). To complement the analysis of glucoregulation, we measured glycated hemoglobin levels (HbA1c). HbA1c were significantly reduced in mid-aged animals (F(1,53) = 40.2; p < 0.0001), and HFD caused a small (6–9 %), yet significant increase in glycated hemoglobin levels in both age groups (F(1,53) = 24.3; p < 0.0001; Table 2). The diet-mediated increase in HbA1c levels is consistent with the greater AUC seen in response to the GTT (Table 2), and may indicate longer postprandial glucose excursions in the HFD group. Nevertheless, together, these data indicate that neither animal group showed signs of frank diabetes.
Table 2.
In vivo and in vitro serum lipid- and glucose-related markers
| Young CD | Young HFD | Mid-Aged CD | Mid-Aged HFD | ||
|---|---|---|---|---|---|
| Glucose (initial - mg/dl) | 80.7 ± 1.7 | 83.7 ± 1.0 | |||
| Glucose (post - mg/dl) | 78.4 ± 1.3 | 76.8 ± 1.1 | 80.6 ± 1.8 | 81.9 ± 2.2 | |
| Insulin (post - ng/mL) | 6.05 ± 0.71 | 4.51 ± 0.38 | 5.84 ± 1.05 | 4.41 ± 0.76 | |
| AUC GTT (initial) | 3230 ± 293 | 3175 ± 215 | |||
| AUC GTT (post) | # | 2735 ± 352 | 4030 ± 408 | 2816 ± 384 | 3503 ± 407 |
| AUC ITT (initial) | −2693 ± 120 | −3063 ± 158 | |||
| AUC ITT (post) | * | −1819 ± 319 | −2257 ± 168 | −2545 ± 185 | −3107 ± 305 |
| HbA1c (initial) | 3.87 ± 0.03 | 3.63 ± 0.02 | |||
| HbA1c (post) | # * | 3.67 ± 0.04 | 3.92 ± 0.06 | 3.29 ± 0.06 | 3.59 ± 0.05 |
| Cholesterol (mg/dL) | * | 147.4 ± 3.9 | 142.1 ± 4.7 | 237.4 ± 14.4 | 223.5 ± 8.9 |
| HDL (mg/dL) | # * | 94.4 ± 2.1 | 47.7 ± 4.4 | 180.4 ± 16.1 | 82.6 ± 4.8 |
| LDL (mg/dL) | * | 17.7 ± 0.7 | 14.0 ± 1.4 | 47.0 ± 6.6 | 42.2 ± 3.6 |
| HDL: LDL | # * | 6.02 ± 0.30 | 3.79 ± 0.28 | 4.50 ± 0.61 | 2.23 ± 0.27 |
| NEFA (mEq/L) | 0.90 ± 0.07 | 0.96 ± 0.04 | 0.84 ± 0.06 | 0.91 ± 0.06 | |
| Triglycerides (mg/dL) | # * | 117.7 ± 9.5 | 213.3 ± 16.4 | 50.0 ± 4.5 | 125.0 ± 13.0 |
| Glucagon (pg/mL) | 101.7 ± 10.5 | 87.6 ± 4.8 | 111.4 ± 9.0 | 101.4 ± 7.0 | |
| Adiponectin (μg/mL) | 19.5 ± 1.1 | 18.0 ± 0.5 | 19.7 ± 1.5 | 20.4 ± 0.7 |
Some data were obtained prior to the initiation of the diet change (initial) as well as towards the end of the study after 4.5 months on the diets (post). If not specified, numbers were obtained at the end of the study.
Abbreviations: control diet (CD), high fat diet (HFD), AUC (area-under-the-curve), GTT (glucose tolerance test), ITT (insulin tolerance test), HbA1c (percent glycated hemoglobin), HDL (high density lipoprotein), LDL (low density lipoprotein), NEFA (non-esterified fatty acids).
Asterisks (*) indicate significance by age, and pound signs (#) indicates significance by diet. Means ± SEM are shown.
Figure 2. Glucose and insulin tolerance tests over a 2 hour period using a single dose approach.
A shows mean glucose levels during both GTT (A1) and ITT (A2) prior to initiation of the high fat diet (HFD). Similar results during GTT (B1) and ITT (B2) and taken after 19 weeks of the HFD are shown in B. Pound sign shows a significant effect of diet on area-under-the-curve (AUC) data (see Table 2). A main effect of age was noted at 30 min following insulin injection (B2; asterisk sign), indicating greater insulin sensitivity with age. Mean ± SEM are shown. Significant differences between age and diet groups was tested at the @ p < 0.05 level.
In contrast to the small HFD-induced responses in glucoregulation, HFD had a robust impact on lipid homeostasis (Table 2). Total cholesterol was significantly increased in mid-aged animals (F(1,52) = 57.3; p < 0.0001), but levels were not altered by the diet. High density lipoprotein (HDL) levels were significantly increased with age (F(1,52) = 32.6; p < 0.0001) but decreased by HFD (F(1,52) = 46.6; p < 0.0001). Low density lipoprotein (LDL) levels were significantly elevated in mid-aged animals (F(1,52) = 42.6; p < 0.0001), but were not sensitive to the HFD. HDL to LDL ratio, a relevant clinical index was computed and found to be significantly reduced by age (F(1,54) = 12.8; p = 0.0007) and HFD (F(1,54) = 27.3; p < 0.0001). Triglyceride levels were significantly increased by HFD (F(1,52) = 53.2; p < 0.0001), but mid-aged animals had lower levels compared to younger animals (F(1,52) = 44.6; p < 0.0001). Non-esterified fatty acids (NEFA) and adiponectin levels were not changed by either diet or age (Table 2).
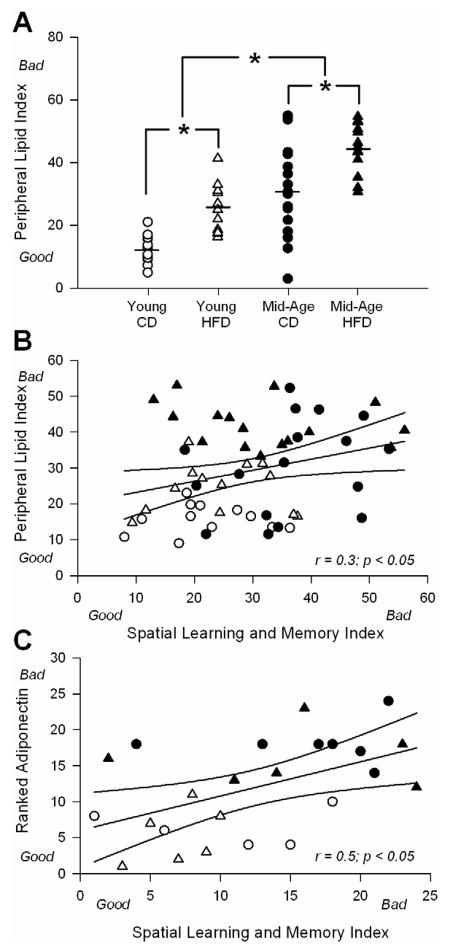
To summarize these data, we compiled a peripheral lipid index (PLI) for each animal using measures of retroperitoneal fat, HDL to LDL ratio and cholesterol. This approach revealed significant differences by both age (F(1,54) = 53.7; p < 0.0001) and diet (F(1,54) = 29.3; p < 0.0001), effectively separating the 4 animal groups (Fig. 3A). The value of each animal using the PLI was then correlated with a learning and memory index (described in 3.3).
Figure 3. Correlations across peripheral lipid index (PLI), spatial learning and memory index (SLMI), and adiponectin levels.
A for each animal, an index was created from cholesterol levels, HDL: LDL, and retroperitoneal fat weight (higher number is associated with greater dyslipidemia). Lower (better) scores on the PLI are seen in younger animals on control diet (CD). Main effects of both diet and age were seen and clearly separate the animal groups. Asterisks represent significant differences between treatment groups @ p < 0.05 level. B shows the correlation between the PLI and the SLMI for all animals in the study. Ninety-five percent confidence intervals are shown along with trendline and results of the analysis. C shows a similar analysis between ranked adiponectin staining levels and the SLMI for twenty-four animals from which both data sets were available. Ninety-five percent confidence intervals are shown along with trendline and results of the analysis.
3.3. Behavior
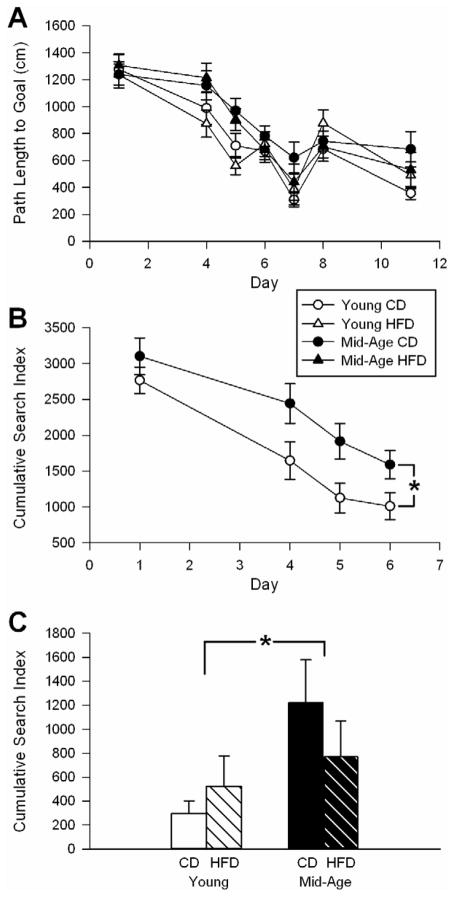
We monitored learning and memory performance using the Morris water maze spatial task. As previously reported [13,52], mid-aged animals on CD showed a significant decrease in performance (Fig. 4). This was evidenced by age-dependent decreases in time to reach the platform during the training/learning phase (F(1,54) = 7.2; p < 0.05 – data not shown). Performance was also analyzed using a cumulative search error index for both the learning phase (Fig. 4B – F(1,54) = 9.24; p < 0.01) and the memory phase of the task - probe (Fig. 4C). During the probe trial, a significant age-dependent decrease in memory performance was seen (F(1,54) = 4.1; p < 0.05). During the reversal probe, 72 h after reversal training (new platform location), similar results were observed (F(1,54) = 5.8; p < 0.05 – data not shown).
Figure 4. Morris water maze learning and memory task.
A day 1 was cue day. Days 4, 5, and 6 were training days, followed by a probe on day 7. Day 8 was reversal training, followed 72 h later by a reversal probe on day 11. B Based on the cumulative search index, a significant age-dependent decrease in the learning phase of the task (training days 1–6) was seen in control diet (CD) animals. C The cumulative index of the probe trial is shown. As shown in A and C, HFD had no impact on learning and memory performance. Means ± SEM are shown. * Significant aging differences @ p < 0.05.
In comparison to the effects of aging on learning and memory, the HFD had no impact on maze performance (Fig. 4). Nevertheless, we tested for the presence of an association between the peripheral lipid index (PLI) and learning/memory. For each animal, individual values obtained from the cumulative search errors during learning, the probe and the reversal probe, were ranked and averaged to yield a single index. This provided us with a spatial learning and memory index (SLMI) for each animal. We correlated PLI data with SLMI data for all 58 animals and obtained a weak, albeit significant correlation (r = 0.3; p < 0.05 – Fig. 3B). We interpret this as supporting evidence for a PNS-CNS link, however, the association appears mostly mediated by strong changes in peripheral variables as opposed to behavioral variables (HFD had no significant impact on learning or memory).
3.4. Physiology
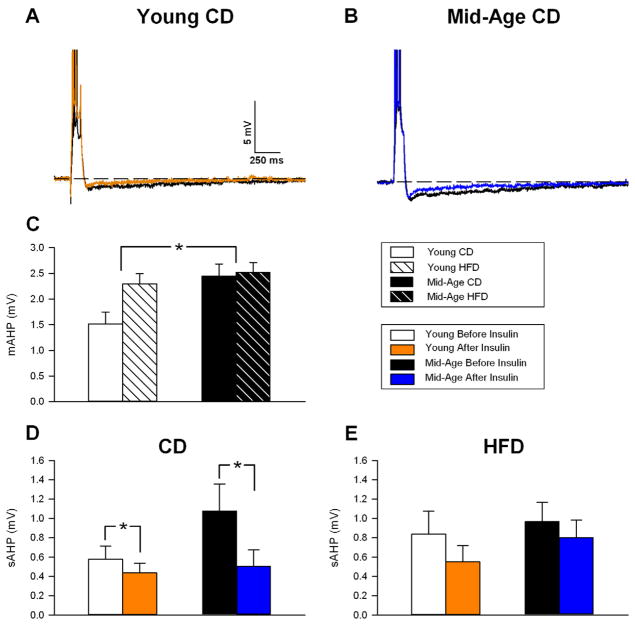
We also evaluated the potential effects of HFD on hippocampal physiology in area CA1 neurons ex-vivo. We focused on the afterhyperpolarization (AHP) because of its sensitivity to the aging process and learning [54,60]. Electrophysiological cell properties and electrode characteristics were unaffected by age or diet (Table 3). As previously reported, the AHP was larger in mid-aged animals (Fig. 5C) [9,18]. The medium AHP (mAHP) was significantly increased with age (F(1,31) = 6.81; p < 0.05), and trends were seen for increases in the slow AHP amplitude (F(1,31) = 2.9; p < 0.1) and duration (F(1,31) = 3.0; p < 0.1). HFD, however, was unable to significantly alter the AHP, albeit a strong trend for an increase in the mAHP was seen in young animals (Fig. 5C, F(1,31) = 3.8; p < 0.1). Given the relationship between the AHP and learning, the lack of a diet effect on the AHP is consistent with the lack of a diet effect on spatial learning and memory.
Figure 5. Insulin’s acute effects on the afterhyperpolarization (AHP).
A shows a representative AHP recorded in a young animal on the control diet (CD) before (black) and after (red) insulin administration. Action potentials are truncated to emphasize the AHP. B shows a representative AHP from a mid-aged CD animal before (black) and after (red) insulin administration. C shows the average medium AHP (mAHP) and reveals a significant aging effect, but no diet effect. D and E show the degree of insulin-mediated AHP reduction, and hence, insulin sensitivity in CD and HFD animals (respectively). While insulin reduced the sAHP in both age groups, the degree of inhibition appeared greater in older animals on CD (D). HFD was able to reduce insulin sensitivity in older animals (E). Asterisks indicate significant difference @ p < 0.05 level.
Because prior clinical evidence shows that aging and diabetes can downregulate insulin transporters at the blood brain barrier [11,38] and may be responsible for decreased insulin sensitivity in the aged brain [20,55], we hypothesized that aging and/or HFD may alter insulin sensitivity in the hippocampus. In a subset of cells (Fig. 5D and E) we quantified insulin sensitivity by measuring the AHP before, and after a 15 min insulin perfusion (500 nM Humalog®, Eli Lilly, IN). Irrespective of age (Fig. 5D), the sAHP from animals on CD showed a significant reduction in response to acute insulin (F(1,10) = 7.1; p < 0.05). While there was no significant interaction term in the 2-way RM ANOVA (p = 0.14), the degree of insulin-mediated reduction in the sAHP appears larger in aged, compared to younger animals (Fig. 5D). Importantly, in HFD animals, this reduction was eliminated (Fig. 5E), suggesting the HFD caused a decrease in insulin sensitivity.
3.5. Westerns and Immunohistochemistry from Hippocampal tissue
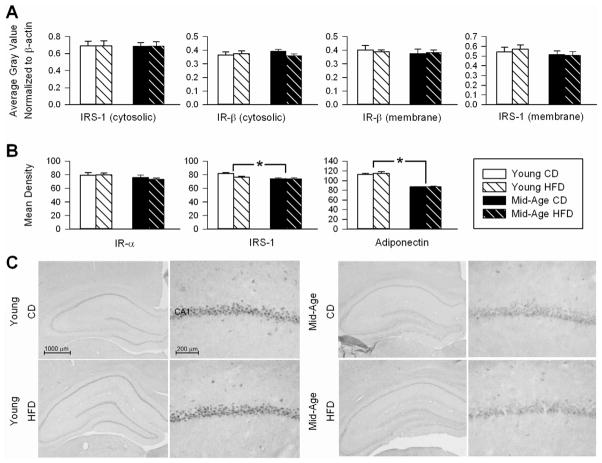
As an additional measure of insulin signaling in the brain, and because insulin signaling is dependent on IRS-1 in its early stage [58], we quantified protein levels for IR and IRS-1 using Western and immunohistochemical techniques. Prior studies have reported decreases in brain IR signaling in aging and AD, as well as in response to dietary manipulations AD [48,58,66]. However, these effects may be dependent on animal strain, treatment duration [40,51], and cognitive status [10,66]. Western blot analysis showed no significant age or diet effects on the upstream portions of the insulin pathway (IR and IRS-1 levels; Fig. 6A), however, IRS-1 staining showed a small, yet significant age-dependent reduction in area CA1 immunoreactivity (F(1,20) = 11.55; p <0.005; Figure 6B middle panel). A robust age-dependent reduction in adiponectin staining was seen in the pyramidal layer of area CA1 (F(1,20) = 165.1; p < 0.0001; Fig. 6B and C). Given that this reduction was seen in the same animals that displayed decreases in cognitive function (Fig. 4C), this suggested CNS adiponectin may modulate cognition with age. To test this possible association, we correlated the SLMI index for each animal to ranked area CA1 adiponectin staining and found a significant association was indeed present (Fig. 3C; r = 0.5, p < 0.05).
Figure 6. Insulin receptor and insulin receptor substrate-1 expression and adiponectin immunostaining.
A shows results of Western blot analyses on hippocampal insulin receptor substrate-1 (IRS-1; 175 kDa) and insulin receptor β subunit (IR-β; 95 kDa) separated into membrane and cytosolic fractions. Data represent means ± SEM. B shows semiquantitative mean density measures of immunostained brain sections for IR-α, IRS-1 and adiponectin in stratum pyramidale of area CA1. Asterisks indicate significant difference @ p < 0.05 level. C shows representative photomicrographs of two adiponectin immunostained sections with different magnifications of the hippocampus area, obtained from young-adult (left panel) and mid-aged animals (right panel). While no diet effect was seen, note the significant age-dependent reduction in immunostained neurons in stratum pyramidale.
4. Discussion
In recent years increasing attention has focused on determining whether peripheral metabolic dysregulation and associated weight gain and diabetes pose a risk for accelerated brain aging. The F344 rat has been used extensively to study aging, however, few studies have examined the impact of peripheral dysregulation in this animal model. Here, we treated aging F344 rats with a “Western-style” high fat diet (HFD) to induce peripheral dysregulation and test whether CNS markers of aging (e.g., impaired learning/memory and larger Ca2+-dependent AHPs) were exacerbated. Prior results with F344s are inconsistent, with some studies showing these animals to be resistant to obesity [32] or the impact of a high fructose diet with aging [41]. On the other hand, other studies report that signs of insulin resistance are seen as early as 2–3 months of age on a regular diet [33] or with aging [2,6,24,35]. Our results showed that a chronic HFD in both young and aged F344s caused significant lipid dysregulation, but surprisingly these animals appeared to maintain normal peripheral glucose regulation. While HFD did not exacerbate spatial learning and memory, it did reduce insulin sensitivity in the brain as shown by electrophysiological analyses of the AHP. Importantly, these results provide the first demonstration of the sensitivity of the AHP to insulin and suggest that the consequences of HFD can be observed in the brain even in the absence of overt peripheral metabolic dysregulation.
4.1. Peripheral effects
The HFD had some of the intended effects in the periphery, at least on measures of lipid homeostasis, increasing triglycerides and lowering the HDL/LDL ratio (Table 1). This was supported by other peripheral variables including increased fat pads and liver weight (Fig. 1 and Table 1). Interestingly, the diet-induced reduction in food consumption (Fig. 1B) was larger in older animals and mirrors the reduced weight gain seen in this group (Fig. 1A), suggesting greater age-dependent accommodation to the higher caloric density of the HFD. Nevertheless, the effects of the HFD on peripheral lipids as well as in three key metabolic hormones (insulin, glucagon and adiponectin), were similar in both young and aging rats (Table 2).
Sustained blood glucose levels during a 2 hour glucose tolerance test (GTT) are reflective of poor glucose uptake into cells (i.e., insulin resistance) and, together with high insulin levels between meals, are indicative of a prediabetic/diabetic condition. Even though we show evidence for a small diet-mediated increase in the area-under-curve of the glucose response during the GTT (Table 2), it does not appear that F344s on HFD were prediabetic because insulin levels were not significantly changed and even tended to decrease with HFD. Furthermore, analyses of insulin sensitivity using the insulin tolerance test (ITT) showed lower glucose levels at 30 min in mid-aged animals (Fig. 2B2). While this suggests older animals are more sensitive to insulin, reductions in glucagon levels and glycogenolysis (altered counter regulation) could also have accounted for lower glucose levels. Serum glucagon levels, however, were not significantly different by either age, or diet (Table 2). Finally, even though HFD increased HbA1c slightly, it is likely that such small changes have little physiological impact, given the absence of elevated fasting glucose levels (Fig. 2A1 and 2B1). Together, these results highlight a clear effect of HFD on peripheral lipids, supportive of the adipogenic nature of this diet. However, in the F344, neither frank diabetes not prediabetes appear to develop. In fact, we present some evidence for an increase in insulin sensitivity in older animals (Fig. 2B2).
Compared to other rodent models, the level of circulating adiponectin in the F344 appears elevated. Reported total adiponectin levels typically range between 10 ng/mL and ~ 7 ug/mL [19,37,65,67]. Here we found adiponectin levels that are higher and reaching ~20 ug/mL (Table 2). These levels are comparable to those induced by 12 weeks of medium intensity exercise seen in young Wistar rats [19]. Increased adiponectin levels have been shown to protect from metabolic dysregulation associated with HFD [1] and are inversely correlated with insulin resistance [22]. Thus, it seems reasonable to propose that the increased levels of adiponectin in the F344 may have protected against some of the impact of HFD in the periphery, thereby maintaining glucoregulation. Consistent with this possibility, one of the strongest predictors of insulin sensitivity is the circulating adiponectin level, with insulin-sensitive obese subjects showing greater adiponectin levels when compared to insulin-resistant and diabetic obese patients [30].
4.2. Brain effects
As previously reported [5], we observed impaired learning and memory performance in aging F344s (Fig. 4). However, the peripheral lipid dysregulation caused by the HFD was only weakly correlated with memory performance in young or aged animals (Fig. 3B). There is considerable inconsistency in the literature regarding the impact of HFD on cognition. In young Long-Evans rats, increased fat intake has been shown to impair spatial learning and memory [21], particularly when a lard-type diet is used [23]. While a high fat/high sucrose diet causes insulin resistance and reduction in learning and memory in Sprague-Dawley rats [57] and CD1 mice [12], this is not the case for C57BL/6 mice [40,46]. In our study, HFD did not impair learning and memory in young rats or exacerbate decrements in performance in the older rats. Perhaps earlier or longer HFD exposure may be required to negatively alter learning or memory in this model. Alternatively, the age-dependent decline in cognition may have been too extensive and, therefore, less likely to be impacted by HFD. Importantly, the F344s appears to be resistant to the potential negative consequences of HFD on cognition, and could well serve as a valid model to identify factors that confer protection.
At the cellular level, and to complement data from Western blots and immunohistochemistry, we used electrophysiological analyses and monitored brain insulin response and sensitivity directly. This allowed us to determine the impact of HFD on a key biomarker of brain aging associated with impaired cognition, namely the Ca2+-dependent AHP, which is larger with aging. Importantly, reductions in the AHP have been shown to improve memory [44,54]. We show for the first time that the AHP was significantly reduced by acute insulin exposure to hippocampal slices. Furthermore, the insulin-mediated inhibition appeared greater in older compared to the younger animals on the control diet (Fig. 5D), providing some support for increased insulin sensitivity with age in this animal model. Notably, the sensitivity of the AHP to insulin was lost by exposure to HFD in both age groups (Fig. 5E). These results may have relevance for clinical studies examining the potential benefits of intranasal insulin for cognitive decline in AD [53]. Thus, in addition to other beneficial effects of insulin, including those on metabolism, vascularization, neurotransmission, and Aβ clearance [29,39], the reduction in the AHP may represent a novel neuroprotective mechanism. Our results also demonstrate that neurons can respond to acute insulin relatively quickly and that long-term intraventricular insulin infusion can adversely affect hippocampal synaptic physiology [26]. Finally, it is interesting to note that hormones that oppose insulin actions such as glucocorticoids, increase the AHP [28] and drugs that restore insulin sensitivity (e.g., thiazolidinediones) reduce the AHP [5]. Thus, the hippocampal AHP appears to be an insulin-sensitive target, whose modulation could well represent a mechanism by which insulin improves cognition in aging/AD.
Adiponectin staining in the brain revealed a robust age-dependent decrease in the hippocampus that did not show sensitivity to HFD (Figs. 6B and C). Given that peripheral adiponectin levels did not change with age, this result suggests reduced adiponectin transport into the brain with aging. There is clear evidence for adiponectin transport into the CNS [49,61] where it is able to increase peripheral energy metabolism [49] and stimulate neurogenesis [64]. Because a decrease in adiponectin levels is associated with mild cognitive impairment [59], this led us to examine the relationship between hippocampal adiponectin staining and the spatial learning and memory index (SLMI) for each animal. The correlation was significant (Fig. 3C), and provides further support for the potential role of CNS adiponectin in modulating learning and memory processes. Future studies directly manipulating adiponectin levels in the aged brain should clarify this relationship.
4.3. Conclusions
Human studies are implicating the peripheral metabolic dysregulation that occurs with T2DM as a risk factor for accelerated aging-related cognitive decline yet little is known regarding possible underlying mechanisms as the corresponding studies in aging animal models have not been performed. We treated young and aging F344s with a HFD and surprisingly found that they did not display peripheral signs of glucose/insulin dysregulation or overt diabetes, unlike what is seen in other animal models. However, the effects in the brain appeared to differ from those in the periphery and we found that: 1) in rats fed the control diet, the AHP, which determines the extent of neuronal excitability, was modulated by acute insulin and, 2) the response to insulin was lost in F344s on the HFD. As we show for the first time that the AHP is sensitive to insulin, our results appear to have important implications for neuronal function, irrespective of age, and show that a common Western style diet can impact the brain even in the absence of overt diabetes-like changes in the periphery. Because of the rather resilient nature of the F344 to diabetes, our studies also suggest that the F344 may represent a unique model to better understand mechanisms which may help to prevent diabetes and associated complications.
Supplementary Material
Supplemental Figure 1: Weight adjusted vs. single dose glucose or insulin injections on the glucose tolerance (GTT) and the insulin tolerance tests (ITT). Because of the nearly two-fold increase in body weight between young and mid-aged animals, glucose and insulin tolerance tests were monitored over a 2 hour period using either a weight adjusted (WA) or a single dose (SD) approach. A1 and A2 show mean glucose levels in young and mid-aged animals on control diet (CD) during both GTT and ITT using the WA approach. B1 and B2 show similar graphs, but obtained using the SD approach. The only significant difference was seen in the peak response 30 min after injection (A1). Mean ± SEM are shown. Asterisks indicate significant differences between age groups @ p < 0.05 level.
Supplemental Table 1: Area-under-the-curve data on glucose and insulin tolerance tests.
Acknowledgments
The authors thank Drs. Hadley and Piascik for critical reading and reviewing of the manuscript.
Footnotes
Disclosure statement
None of the authors on the manuscript has an actual or potential conflict of interest to declare. The work was supported by NIH/NIA grant AG033649. The data presented in this manuscript has not been submitted or published elsewhere. We will not submit the manuscript elsewhere while it is under review at Neurobiology of Aging. All authors approve of the procedures and the content of the manuscript and are able to validate the accuracy of the data presented.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Tristano Pancani, Email: tristano.pancani@vanderbilt.edu.
Katie L. Anderson, Email: Katie.anderson2@uky.edu.
Lawrence D. Brewer, Email: lawrencebrewer@uky.edu.
Inga Kadish, Email: ikadisha@uab.edu.
Chris DeMoll, Email: chris.demoll@uky.edu.
Philip W. Landfield, Email: pwland@uky.edu.
Eric M. Blalock, Email: emblal@uky.edu.
Nada M. Porter, Email: nadap@uky.edu.
References
- 1.Asterholm IW, Scherer PE. Enhanced metabolic flexibility associated with elevated adiponectin levels. Am J Pathol. 2010;176(3):1364–76. doi: 10.2353/ajpath.2010.090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barzilai N, Rossetti L. Relationship between changes in body composition and insulin responsiveness in models of the aging rat. Am J Physiol. 1995;269(3 Pt 1):E591–7. doi: 10.1152/ajpendo.1995.269.3.E591. [DOI] [PubMed] [Google Scholar]
- 3.Belanger A, Lavoie N, Trudeau F, Massicotte G, Gagnon S. Preserved LTP and water maze learning in hyperglycaemic-hyperinsulinemic ZDF rats. Physiol Behav. 2004;83(3):483–94. doi: 10.1016/j.physbeh.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 4.Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurol. 2008;7(2):184–90. doi: 10.1016/S1474-4422(08)70021-8. [DOI] [PubMed] [Google Scholar]
- 5.Blalock EM, Phelps JT, Pancani T, Searcy JL, Anderson KL, Gant JC, Popovic J, Avdiushko MG, Cohen DA, Chen KC, Porter NM, Thibault O. Effects of long-term pioglitazone treatment on peripheral and central markers of aging. PLoS ONE. 2010;5(4):e10405. doi: 10.1371/journal.pone.0010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bracho-Romero E, Reaven GM. Effect of age and weight on plasma glucose and insulin responses in the rat. J Am Geriatr Soc. 1977;25(7):299–302. doi: 10.1111/j.1532-5415.1977.tb00641.x. [DOI] [PubMed] [Google Scholar]
- 7.Buettner R, Scholmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity (Silver Spring) 2007;15(4):798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- 8.Curtis R, Geesaman BJ, DiStefano PS. Ageing and metabolism: drug discovery opportunities. Nat Rev Drug Discov. 2005;4(7):569–80. doi: 10.1038/nrd1777. [DOI] [PubMed] [Google Scholar]
- 9.Disterhoft JF, Oh MM. Alterations in intrinsic neuronal excitability during normal aging. Aging Cell. 2007;6(3):327–36. doi: 10.1111/j.1474-9726.2007.00297.x. [DOI] [PubMed] [Google Scholar]
- 10.Dou JT, Chen M, Dufour F, Alkon DL, Zhao WQ. Insulin receptor signaling in long-term memory consolidation following spatial learning. Learn Mem. 2005;12(6):646–55. doi: 10.1101/lm.88005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duelli R, Maurer MH, Staudt R, Heiland S, Duembgen L, Kuschinsky W. Increased cerebral glucose utilization and decreased glucose transporter Glut1 during chronic hyperglycemia in rat brain. Brain Res. 2000;858(2):338–47. doi: 10.1016/s0006-8993(00)01942-9. [DOI] [PubMed] [Google Scholar]
- 12.Farr SA, Yamada KA, Butterfield DA, Abdul HM, Xu L, Miller NE, Banks WA, Morley JE. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149(5):2628–36. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol Aging. 1995;16(2):149– 60. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- 14.Frisardi V, Solfrizzi V, Seripa D, Capurso C, Santamato A, Sancarlo D, Vendemiale G, Pilotto A, Panza F. Metabolic-cognitive syndrome: a cross-talk between metabolic syndrome and Alzheimer’s disease. Ageing Res Rev. 2010;9(4):399–417. doi: 10.1016/j.arr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98(4):2005–10. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furuhashi M, Tuncman G, Gorgun CZ, Makowski L, Atsumi G, Vaillancourt E, Kono K, Babaev VR, Fazio S, Linton MF, Sulsky R, Robl JA, Parker RA, Hotamisligil GS. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447(7147):959–65. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107(4):618–26. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- 18.Gant JC, Sama MM, Landfield PW, Thibault O. Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+ release. J Neurosci. 2006;26(13):3482–90. doi: 10.1523/JNEUROSCI.4171-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garekani ET, Mohebbi H, Kraemer RR, Fathi R. Exercise training intensity/volume affects plasma and tissue adiponectin concentrations in the male rat. Peptides. 2011;32(5):1008–12. doi: 10.1016/j.peptides.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 20.Gasparini L, Netzer WJ, Greengard P, Xu H. Does insulin dysfunction play a role in Alzheimer’s disease? Trends Pharmacol Sci. 2002;23(6):288–93. doi: 10.1016/s0165-6147(02)02037-0. [DOI] [PubMed] [Google Scholar]
- 21.Greenwood CE, Winocur G. Learning and memory impairment in rats fed a high saturated fat diet. Behav Neural Biol. 1990;53(1):74–87. doi: 10.1016/0163-1047(90)90831-p. [DOI] [PubMed] [Google Scholar]
- 22.Gustafson DR. Adiposity hormones and dementia. J Neurol Sci. 2010;299(1–2):30–4. doi: 10.1016/j.jns.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 23.Jurdak N, Lichtenstein AH, Kanarek RB. Diet-induced obesity and spatial cognition in young male rats. Nutr Neurosci. 2008;11(2):48–54. doi: 10.1179/147683008X301333. [DOI] [PubMed] [Google Scholar]
- 24.Kalant N, Stewart J, Kaplan R. Effect of diet restriction on glucose metabolism and insulin responsiveness in aging rats. Mech Ageing Dev. 1988;46(1–3):89–104. doi: 10.1016/0047-6374(88)90117-0. [DOI] [PubMed] [Google Scholar]
- 25.Kamal A, Biessels GJ, Duis SE, Gispen WH. Learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: interaction of diabetes and ageing. Diabetologia. 2000;43(4):500–6. doi: 10.1007/s001250051335. [DOI] [PubMed] [Google Scholar]
- 26.Kamal A, Ramakers GM, Gispen WH, Biessels GJ. Effect of chronic intracerebroventricular insulin administration in rats on the peripheral glucose metabolism and synaptic plasticity of CA1 hippocampal neurons. Brain Res. 2012;1435:99–104. doi: 10.1016/j.brainres.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 27.Keenan KP, Soper KA, Hertzog PR, Gumprecht LA, Smith PF, Mattson BA, Ballam GC, Clark RL. Diet, overfeeding, and moderate dietary restriction in control Sprague-Dawley rats: II.Effects on age-related proliferative and degenerative lesions. Toxicol Pathol. 1995;23(3):287–302. doi: 10.1177/019262339502300306. [DOI] [PubMed] [Google Scholar]
- 28.Kerr DS, Campbell LW, Hao SY, Landfield PW. Corticosteroid modulation of hippocampal potentials: increased effect with aging. Science. 1989;245(4925):1505–9. doi: 10.1126/science.2781293. [DOI] [PubMed] [Google Scholar]
- 29.Kim B, Feldman EL. Insulin resistance in the nervous system. Trends Endocrinol Metab. 2012;23(3):133–41. doi: 10.1016/j.tem.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kloting N, Fasshauer M, Dietrich A, Kovacs P, Schon MR, Kern M, Stumvoll M, Bluher M. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 2010;299(3):E506–15. doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- 31.Launer LJ. Diabetes and brain aging: epidemiologic evidence. Curr Diab Rep. 2005;5(1):59–63. doi: 10.1007/s11892-005-0069-1. [DOI] [PubMed] [Google Scholar]
- 32.Levin BE, Triscari J, Sullivan AC. Relationship between sympathetic activity and diet-induced obesity in two rat strains. Am J Physiol. 1983;245(3):R364–71. doi: 10.1152/ajpregu.1983.245.3.R364. [DOI] [PubMed] [Google Scholar]
- 33.Levy JR, Davenport B, Clore JN, Stevens W. Lipid metabolism and resistin gene expression in insulin-resistant Fischer 344 rats. Am J Physiol Endocrinol Metab. 2002;282(3):E626–33. doi: 10.1152/ajpendo.00346.2001. [DOI] [PubMed] [Google Scholar]
- 34.Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113(3):607–15. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 35.Li YM, Steffes M, Donnelly T, Liu C, Fuh H, Basgen J, Bucala R, Vlassara H. Prevention of cardiovascular and renal pathology of aging by the advanced glycation inhibitor aminoguanidine. Proc Natl Acad Sci U S A. 1996;93(9):3902–7. doi: 10.1073/pnas.93.9.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luchsinger JA, Cheng D, Tang MX, Schupf N, Mayeux R. Central Obesity in the Elderly is Related to Late-onset Alzheimer Disease. Alzheimer Dis Assoc Disord. 2011 doi: 10.1097/WAD.0b013e318222f0d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malloy VL, Krajcik RA, Bailey SJ, Hristopoulos G, Plummer JD, Orentreich N. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell. 2006;5(4):305–14. doi: 10.1111/j.1474-9726.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- 38.McNay EC. The impact of recurrent hypoglycemia on cognitive function in aging. Neurobiol Aging. 2005;26 (Suppl 1):76–9. doi: 10.1016/j.neurobiolaging.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 39.McNay EC, Recknagel AK. Brain insulin signaling: a key component of cognitive processes and a potential basis for cognitive impairment in type 2 diabetes. Neurobiol Learn Mem. 2011;96(3):432–42. doi: 10.1016/j.nlm.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mielke JG, Nicolitch K, Avellaneda V, Earlam K, Ahuja T, Mealing G, Messier C. Longitudinal study of the effects of a high-fat diet on glucose regulation, hippocampal function, and cerebral insulin sensitivity in C57BL/6 mice. Behav Brain Res. 2006;175(2):374–82. doi: 10.1016/j.bbr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Mooradian AD, Albert SG. The age-related changes in lipogenic enzymes: the role of dietary factors and thyroid hormone responsiveness. Mech Ageing Dev. 1999;108(2):139–49. doi: 10.1016/s0047-6374(99)00007-x. [DOI] [PubMed] [Google Scholar]
- 42.Mooradian AD, Wong NC, Shah GN. Apolipoprotein A1 expression in young and aged rats is modulated by dietary carbohydrates. Metabolism. 1997;46(10):1132–6. doi: 10.1016/s0026-0495(97)90204-3. [DOI] [PubMed] [Google Scholar]
- 43.Morley JE. The metabolic syndrome and aging. J Gerontol A Biol Sci Med Sci. 2004;59(2):139–42. doi: 10.1093/gerona/59.2.m139. [DOI] [PubMed] [Google Scholar]
- 44.Moyer JR, Jr, Thompson LT, Disterhoft JF. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J Neurosci. 1996;16(17):5536–46. doi: 10.1523/JNEUROSCI.16-17-05536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narimiya M, Azhar S, Dolkas CB, Mondon CE, Sims C, Wright DW, Reaven GM. Insulin resistance in older rats. Am J Physiol. 1984;246(5 Pt 1):E397–404. doi: 10.1152/ajpendo.1984.246.5.E397. [DOI] [PubMed] [Google Scholar]
- 46.Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, Bruce-Keller AJ. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010;219(1–2):25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Popovic M, Biessels GJ, Isaacson RL, Gispen WH. Learning and memory in streptozotocin-induced diabetic rats in a novel spatial/object discrimination task. Behav Brain Res. 2001;122(2):201–7. doi: 10.1016/s0166-4328(01)00186-3. [DOI] [PubMed] [Google Scholar]
- 48.Pratchayasakul W, Kerdphoo S, Petsophonsakul P, Pongchaidecha A, Chattipakorn N, Chattipakorn SC. Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sci. 2011;88(13–14):619–27. doi: 10.1016/j.lfs.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10(5):524–9. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 50.Reaven E, Wright D, Mondon CE, Solomon R, Ho H, Reaven GM. Effect of age and diet on insulin secretion and insulin action in the rat. Diabetes. 1983;32(2):175–80. doi: 10.2337/diab.32.2.175. [DOI] [PubMed] [Google Scholar]
- 51.Ross AP, Bruggeman EC, Kasumu AW, Mielke JG, Parent MB. Non-alcoholic fatty liver disease impairs hippocampal-dependent memory in male rats. Physiol Behav. 2012;106(2):133–41. doi: 10.1016/j.physbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 52.Rowe WB, Blalock EM, Chen KC, Kadish I, Wang D, Barrett JE, Thibault O, Porter NM, Rose GM, Landfield PW. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J Neurosci. 2007;27(12):3098–110. doi: 10.1523/JNEUROSCI.4163-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schioth HB, Craft S, Brooks SJ, Frey WH, 2nd, Benedict C. Brain insulin signaling and Alzheimer’s disease: current evidence and future directions. Mol Neurobiol. 2012;46(1):4–10. doi: 10.1007/s12035-011-8229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song C, Detert JA, Sehgal M, Moyer JR., Jr Trace Fear Conditioning Enhances Synaptic and Intrinsic Plasticity in Rat Hippocampus. J Neurophysiol. 2012 doi: 10.1152/jn.00692.2011. [DOI] [PubMed] [Google Scholar]
- 55.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? J Alzheimers Dis. 2005;7(1):63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 56.Stranahan AM, Mattson MP. Metabolic Reserve as a Determinant of Cognitive Aging. J Alzheimers Dis. 2011 doi: 10.3233/JAD-2011-110899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18(11):1085–8. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122(4):1316–38. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teixeira AL, Diniz BS, Campos AC, Miranda AS, Rocha NP, Talib LL, Gattaz WF, Forlenza OV. Decreased Levels of Circulating Adiponectin in Mild Cognitive Impairment and Alzheimer’s Disease. Neuromolecular Med. 2012 doi: 10.1007/s12017-012-8201-2. [DOI] [PubMed] [Google Scholar]
- 60.Thibault O, Landfield PW. Increase in single L-type calcium channels in hippocampal neurons during aging. Science. 1996;272(5264):1017–20. doi: 10.1126/science.272.5264.1017. [DOI] [PubMed] [Google Scholar]
- 61.Une K, Takei YA, Tomita N, Asamura T, Ohrui T, Furukawa K, Arai H. Adiponectin in plasma and cerebrospinal fluid in MCI and Alzheimer’s disease. Eur J Neurol. 2011;18(7):1006–9. doi: 10.1111/j.1468-1331.2010.03194.x. [DOI] [PubMed] [Google Scholar]
- 62.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008 doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 63.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of clinical investigation. 2003;112(12):1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang D, Guo M, Zhang W, Lu XY. Adiponectin stimulates proliferation of adult hippocampal neural stem/progenitor cells through activation of p38 mitogen-activated protein kinase (p38MAPK)/glycogen synthase kinase 3beta (GSK-3beta)/beta-catenin signaling cascade. J Biol Chem. 2011;286(52):44913–20. doi: 10.1074/jbc.M111.310052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang W, Liu CQ, Wang PW, Sun SY, Su WJ, Zhang HJ, Li XJ, Yang SY. Puerarin improves insulin resistance and modulates adipokine expression in rats fed a high-fat diet. Eur J Pharmacol. 2010;649(1–3):398–402. doi: 10.1016/j.ejphar.2010.09.054. [DOI] [PubMed] [Google Scholar]
- 66.Zhao WQ, Chen H, Quon MJ, Alkon DL. Insulin and the insulin receptor in experimental models of learning and memory. Eur J Pharmacol. 2004;490(1–3):71–81. doi: 10.1016/j.ejphar.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 67.Zhu M, Miura J, Lu LX, Bernier M, DeCabo R, Lane MA, Roth GS, Ingram DK. Circulating adiponectin levels increase in rats on caloric restriction: the potential for insulin sensitization. Exp Gerontol. 2004;39(7):1049–59. doi: 10.1016/j.exger.2004.03.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Weight adjusted vs. single dose glucose or insulin injections on the glucose tolerance (GTT) and the insulin tolerance tests (ITT). Because of the nearly two-fold increase in body weight between young and mid-aged animals, glucose and insulin tolerance tests were monitored over a 2 hour period using either a weight adjusted (WA) or a single dose (SD) approach. A1 and A2 show mean glucose levels in young and mid-aged animals on control diet (CD) during both GTT and ITT using the WA approach. B1 and B2 show similar graphs, but obtained using the SD approach. The only significant difference was seen in the peak response 30 min after injection (A1). Mean ± SEM are shown. Asterisks indicate significant differences between age groups @ p < 0.05 level.
Supplemental Table 1: Area-under-the-curve data on glucose and insulin tolerance tests.