Abstract
The ability to perceive and produce speech undergoes important changes in late adulthood. The goal of the present study was to characterize functional and structural age-related differences in the cortical network supporting speech perception and production using magnetic resonance imaging, as well as the relationship between functional and structural age-related changes occurring in this network. We asked young and older adults to (1) observe videos of a speaker producing single words (perception), and (B) observe and repeat the words produced (production). Results show a widespread bilateral network of brain activation for Perception and Production that was uncorrelated with age. In addition, several regions did show age-related change (auditory cortex, planum temporale, superior temporal sulcus, premotor cortices, SMA-proper). Examination of the relationship between brain signal and regional and global gray matter volume and cortical thickness revealed a complex set of relationships between structure and function, with some regions showing a relationship between structure and function and not. The present results provide novel findings about the neurobiology of aging and verbal communication.
Keywords: Normal aging, speech perception, speech production, brain reserve capacity, surface-based cortical thickness, gray matter volume, MRI
Introduction
The ability to communicate verbally through language critically depends upon the ability to recognize and articulate speech sounds (i.e. phonemes and syllables). Like many other aspects of human behaviour, these abilities are vulnerable to senescence, which may lead to communication difficulties (e.g. more difficult sentence comprehension or word recognition) and, ultimately, to a social participation that is less diverse, is more restricted to home settings and involves fewer social relationships (Law, 2002). As the proportion of the world’s population in the older ages continues to grow, the prevalence of disorders of speech, voice and language increases. Because these disorders pose a major challenge to patients, families, and health care systems, particularly in this time of significant global population aging, there is increasing need for reliable neurobiological data about expected (normal) age-related changes. In this paper we focus on the relation between functional and structural changes that accompany aging, and whether and how these changes affect behaviour.
At the behavioural level, numerous age-related changes have been documented in both speech perception and production. In terms of speech perception, which refers to the extraordinary ability of the human brain to recognize the phonemes (the smallest unit in a language, such as/p/) and syllables that form a speakers’ language and to parse the continuous speech stream, the most common change is an age-related loss of auditory sensitivity to high frequency sounds, known as presbycusis, which affects approximately 20–40% of adults over 65 (Gates & Mills, 2005; Ries, 1994). Presbycusis reduces the ability to recognize speech sounds and, consequently, to comprehend words and sentences; it mainly affects consonants with energy in the high frequency range (Nilsson et al., 1994; Plomp & Mimpen, 1979). In addition to peripheral hearing loss, the ability to perceive speech in noisy environments also declines with age (Cooper & Gates, 1991; Frisina & Frisina, 1997; Plath, 1991; Working Group on Speech Understanding and Aging and Committee on Hearing, 1988). Because communication often occurs in the presence of background noise (e.g. at social gatherings, in public transportation, on the phone), this can have dramatic ecological consequences. Decreased hearing-in-noise capacity appears independent of peripheral hearing loss (Frisina & Frisina, 1997). Other changes affecting speech perception include a reduced ability to discriminate between pairs of minimally different syllables (e.g./pa-ba/) (Strouse et al., 1998) and to read lips (Sommers et al., 2005). In addition to changes affecting speech perception, and by extension, language comprehension and communication, age-related changes in speech production have also been described, and include a reduction in speech rate (Duchin & Mysak, 1987; Fozo & Watson, 1998; Hartman & Danahuer, 1976), an increase in the duration of individual speech sounds (Morris & Brown, 1987; Ryan & Burk, 1974), a decrease in voice amplitude (Baker et al., 2001) and a change (either increase or decrease) in the fundamental frequency of voice (Decoster & Debruyne, 1997; Linville, 1996; Mueller, 1997; Ramig, 1983a, 1983b). These changes are likely to reduce communication efficiency by reducing intelligibility, that is, the ability for a person to be understood by another.
Speech perception and production abilities are vulnerable to aging as are many aspects of human behaviour such as processing speed, episodic memory, and attention. However, several important questions remain unanswered with regards to aging of the speech system, and we try to address a few of these in the present study. First, the extent to which behavioural changes affecting speech perception and production are associated with normal age-related structural changes at the level of the central nervous system (CNS), a phenomenon often referred to as “brain senescence” (Dickstein et al., 2009; Raz & Kennedy, 2009), is undetermined. Some of the behavioural changes may be related to peripheral age-related changes in the speech system, such as ossification of the laryngeal cartilages and reduced facial muscle strengths, rather than functional and structural changes in the brain. Second, it is unclear whether age-related difficulties in processing and producing speech sounds are related to general aging mechanisms that affect the brain globally, or to changes affecting specific regions involved in speech processing and production. Age-related changes at the level of the prefrontal cortex, for instance, have been abundantly documented in relation to cognitive decline. Are these prefrontal changes relevant to the behavioural decline in speech perception and production? Third, we don’t know whether age-related behavioural speech perception and production changes have overlapping origins in the brain. Closed-loop accounts of motor control suggest that movements, including speech (Nasir & Ostry, 2008; Tremblay et al., 2003), are under the constant control of sensory feedback. Because aging is associated with a decline in hearing acuity, this account predicts that the precision and stability of the speech movements will decrease with age as a consequence of decreased sensory acuity (so too will speech perception).
Consistent with this idea, it has been shown that sudden deafness leads to a rapid deterioration of several aspects of speech production (Gould et al., 2001; Matthies et al., 1994; Perkell et al., 1992). Moreover, several studies have shown that online acoustical and somatosensory manipulation of a person’s auditory/tactile feedback triggers immediate compensatory changes in speech production (Burnett et al., 1998; Houde & Jordan, 1998; Jones & Munhall, 2000; Tremblay et al., 2003), supporting the notion that motor control relies on online feedback processing. Consistent with this behavioural evidence, neuroimaging and neurostimulation studies have shown overlap in the neural representation of speech perceptual and motor mechanisms, in particular at the level of the ventral premotor cortex (PMv) (Callan, Callan, et al., 2006; Callan et al., 2010; Callan, Tsytsarev, et al., 2006; D’Ausilio et al., 2009; Pulvermüller et al., 2006; Tremblay et al., 2012; Wilson et al., 2004) and in auditory association areas in particular in the planum temporale (PT) (Tremblay et al., 2011; Tremblay & Small, 2011c). Because the neural system underlying speech perception and speech production are overlapping, it is possible that age-related alterations to this system affects speech behaviour globally, independent of the specific speech task.
A few groups have begun to investigate the neurobiological changes that are related to decline in speech perception and found age differences, especially at the level of the supratemporal cortex, but also in the frontal lobe (Frisina & Frisina, 1997; Harkrider et al., 2005; Peelle et al., 2011; Sheppard et al., 2011; Tremblay et al., 2002; Wong et al., 2010). For example, using fMRI, Wong et al. (Wong et al., 2009) showed that speech perception in aging is associated with lower activation magnitude in the left auditory cortex in older compared to younger adults, most likely reflecting neuronal under-recruitment (Gazzaley & D’Esposito, 2005). Results also showed higher activation magnitude in the left middle temporal gyrus and right precuneus, two areas associated with higher order cognitive processing, which may indicate compensatory neural mechanisms. In a follow-up study, Wong et al. (Wong et al., 2010) further demonstrated a link between cognition and speech in aging using structural MRI. In this study, the ability to perceive speech in noise was examined against structural changes occurring in the frontal lobe. A significant relationship was found between the volume of left pars triangularis inferior frontal gyrus (IFG) and behavioural performance. Other studies however have found lower brain activation in older compared to younger adults, without concomitant increases in brain activation. For example, Peelle et al. (Peelle et al., 2011), reported lower activation magnitude in the primary auditory cortices (A1) bilaterally during a speech perception task in older compared to younger adults, which was accompanied by smaller grey matter volume in this area. This result is consistent with high-resolution MRI morphometric studies showing structural vulnerability to age in A1 (Allen et al., 2005). Sheppard et al. (Sheppard et al., 2011) recently showed an age-related regional decrease in network efficiency at the level of the supratemporal cortex, including A1, during speech perception in quiet and in noise. There was no evidence of compensatory network changes.
One fMRI study has examined age-related neural changes in overt speech production (Soros et al., 2011) and found increased activation in several areas in elderly compared to young adults, including A1, the ventral premotor cortex (PMv), the supplementary motor area (SMA-proper) and the prefrontal cortex. There was also decreased activation in posterior supratemporal areas including STG. Increased activation was interpreted as evidence of neural compensation, that is, neural reorganization used to counteract age-related neural decline and resulting in maintained high performance (Cabeza et al., 2002). Taken together, results of these previous brain-imaging studies on speech in aging reveal age-related functional and structural alterations in the CNS that appear to affect the ability to process, and perhaps also produce, speech.
The general objective of the present study is to further current understanding of the neurobiology of speech in aging. First, we examine the direction of magnitude changes (higher vs. lower), context relevance (task-dependent vs. task-independent) and scope (regional vs. global) of age-related differences in the Blood Oxygenation Level Dependent (BOLD) during speech perception and production using functional magnetic resonance imaging (fMRI). Second, we examine the relationship between age-related functional and structural alterations, with focus on grey matter volume and surface-based cortical thickness, two measures widely used to quantify structural change of the cortex (Fischl & Dale, 2000; Han et al., 2006; Walhovd et al., 2005). We hypothesize that areas involved in processing and producing speech, in particular the planum temporale (PT) and the ventral premotor cortex (PMv), would show lower activation in older adults compared to younger adults reflecting less efficient neural processes. Further, we expect that these age-related functional differences will be dependent upon regional structural brain changes in PT and PMv, with thinner cortical thickness and smaller gray matter volume predicting regional changes in BOLD signal. We used a multiple mediation approach to better characterize whether and how structural changes mediate the relation between age and changes in the BOLD response. Our results provide important new insights into normative age-related brain changes.
Methods
Participants
The young adult group was comprised of twenty healthy right-handed (Oldfield, 1971) native speakers of American English (mean age 23.7 ± 5.5; range: 18–38 years; 11 females), with a mean of 15.1 ± 2 years of education (range: 12–18). The data from one additional participant could not be used because of a technical problem with the stimulus presentation. The older adult group was comprised of nineteen healthy right-handed (Oldfield, 1971) native speakers of American English (mean age 62.05 ± 3.84; range: 57–70 years; 11 females), with a mean of 16.2 ± 2 years of education (range: 13–22). Participants in both groups had normal or corrected-to-normal vision and no self-reported history of speech, voice, language or neurological disorder. Participants were screened for depression using the Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977) and cognitive functioning using the Mini Mental State Examination (MMSE) (Folstein et al., 1975). There were no differences across groups on these tests. Participants’ characteristics are reported in Table 1. The study was approved by the Institutional Review Board of the Biological Sciences Division of The University of Chicago.
Table 1.
Group description
| Variable | Young (N = 20) | Older (N = 19) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | ||
| Age | 23.7 | 5.5 | 18–38 | 62.1 | 3.8 | 13–22 |
| Education (years) | 15.1 | 2.0 | 12–18 | 16.4 | 2.5 | 57–70 |
| MMSE | 29.3 | .7 | 28–30 | 28.8 | 1.3 | 26–30 |
| Depression | 10.5 | 7.1 | 0–31 | 6.9 | 7.2 | 0–20 |
| Left ear PTA | 8.3 | 4.3 | 2–18 | 17.8 | 7.5 | 5–32 |
| Right Ear PTA | 8.1 | 6.8 | −2–28 | 19.3 | 8.6 | 3–43 |
| Better Ear PTA | 4.7 | 4.1 | −2–17 | 15.8 | 7.4 | 3–32 |
Behavioural tests
Participants’ hearing sensitivity was assessed using a standard audiometric testing procedure (pure-tone air conduction thresholds for the following frequencies: 250, 500, 1000 , 2000, 3000, 4000, 6000, and 8000Hz). A standard speech discrimination test (Northwestern University auditory test number six, form A) was also used to evaluate participants’ ability to identify speech sounds. Speech discrimination procedures measure a person’s ability not only to hear words but also to identify them. For each participant, a standard pure tone average (PTA – average of threshold at 1, 2 and 4KHz) was computed for the left and right ear, as well as a better ear PTA.
Stimuli and Procedures
The experiment consisted of two tasks: (1) observation of a set of short video clips showing a female actor producing words (Perception), and (2) observation of a set of similar videos followed by repetition of the word produced by the speaker (Production). A resting condition (crosshair fixation) was also included as a baseline condition and interleaved with rest trials; the order of the conditions and the number of rest trials were jittered and optimized using Optseq2 http://surfer.nmr.mgh.harvard.edu/optseq/). Participants always completed the Perception task first. Moreover, they did not know that they would be required to produce words until the beginning of the Production task. This was done to avoid covert rehearsal during Perception. The stimuli were 120 short video clips of a native English-speaking female actor articulating bisyllabic words (nouns). These words were either simple or complex, as measured in terms of the presence or absence of a consonant cluster. The complexity manipulation is reported elsewhere (Tremblay & Small, 2011c). In the present study, stimuli were collapsed across complexity levels. All videos were presented using Presentation Software (Neurobehavioral System, CA, USA). Visual stimuli were delivered to a custom rear projection screen placed inside the bore of the magnet approximately 24″ from the participant, who viewed the stimuli via a mirror attached to the head coil. Auditory stimuli were delivered via a high quality full frequency range auditory amplifier (Avotec Inc., FL, USA).
Image acquisition
The data were acquired on a whole-body Siemens 3.0 Tesla Tim Trio MRI scanner (Siemens Medical Solutions, Erlangen, Germany) at Northwestern University (Chicago, IL, USA). Thirty-two axial slices (3×1.7×1.7 mm, no gap) were acquired in interleaved order using a multislice EPI sequence (TR = 2sec, TE = 20ms; FOV = 200×207×127mm; 128×128 matrix; Flip angle: 75). This covered the entire cortex, but, for most participants, the cerebellum was only partially covered. For this reason we do not report group results for the cerebellum. Two experimental runs (6.3 minutes each) resulted in the acquisition of 380 T2*-weighted BOLD images (120 experimental trials and 60 baseline trials). High-resolution T1-weighted volumes were acquired for anatomical localization (176 sagittal slices, 1×1×1 mm resolution, TR = 23ms, TE = 2.91, FOV = 256×256×176 mm). Throughout the procedure, each participant’s head was immobilized by means of a set of cushions and pads.
Image analysis
Pre-processing
All time series were spatially registered, motion-corrected, time-shifted, de-spiked and mean-normalized using AFNI (Cox, 1996). All time points occurring during excessive motion, defined as >1 mm, were excluded from the analyses as part of the regression (Johnstone et al., 2006). Separate regressors for Perception and Production were created for each participant; additional regressors were the mean, linear, and quadratic trend components, and the 6 motion parameters (x, y, z and roll, pitch and yaw). To remove additional sources of spurious variance unlikely to represent signal of interest, we also included in the regression signal from the lateral ventricles (Dick et al., 2010; Fox et al., 2005), which was identified using the automated subcortical segmentation from Freesurfer to mask the ventricles. A linear least squares model was used to establish a fit to each time point of the hemodynamic response function for each condition. Event-related signals were deconvolved by linear interpolation, beginning at two seconds post stimulus onset and continuing at 2-sec intervals for 10 sec, using AFNI’s tent function (i.e. a piecewise linear spline model). This resulted in regression weights (beta values) indexing percent signal change at each 2-sec interval. All analyses focused on the beta values averaged across the 4–8 sec post-stimulus onset time lag. The FreeSurfer software package (Dale et al., 1999; Fischl et al., 1999; Fischl et al., 2004) was used to create surface representations of each participant’s anatomy by inflating each hemisphere of the anatomical volumes to a surface representation and aligning it to a template of average curvature. SUMA was then used to import the surface representations into the AFNI 3D space and to project the functional data from the 3-dimensional volumes onto the 2-dimensional surfaces. Data were smoothed on the surface to achieve a target smoothing value of 6mm using a Gaussian full width half maximum (FWHM) filter. Smoothing on the surface as opposed to smoothing on the volume ensures that white matter values are not included, and that functional data situated in anatomically distant locations on the cortical surface are not averaged across sulci (Argall et al., 2006; Desai et al., 2005).
Whole-brain analyses
Whole-brain, group analyses were performed using SUMA on the participants’ smoothed beta values resulting from the first-level analysis. For each analysis, we controlled for sex, education and PTA. A series of vertex-wise directional t-tests was computed for the contrasts of (1) Conjunction of Perception against rest for the young and Perception against rest for the older adults, yielding a map of age-independent Perception-related activation, and (2) Conjunction of Production against rest for the young and Production against rest for the older adults, yielding a map of age-independent Production-related activation. Next, we examined (3) Age-dependent activation within Perception; (4) Age-dependent activation within Production; and (5) Task x Age interactions. Conjunction analyses were conducted using the procedure outlined in Nichols et al., 2005 (Nichols et al., 2005), which is essentially an intersection of vertices that are significantly active relative to resting baseline. A Monte Carlo simulation conducted in the surface space, based on Forman et al., 1995 (Nyberg et al., 2010), was used to identify significant clusters of activated vertices, with an individual vertex threshold of p < .005, corrected for multiple comparisons to achieve a family-wise error (FWE) rate of p < 0.05 (clusters ≥ 163 vertices).
ROI
An analysis of regions of interests (ROI) was conducted on all the age-sensitive cortical areas (bilateral PMv, right SMA-proper, left pSFG, left auditory cortex, right PT, right middle superior temporal sulcus (STS), right anterior occipito-temporal sulcus (aOTS) and right occipital pole (Opole)) identified through the whole-brain analysis (contrasts #3, 4 and 5) to determine the direction of the age effects. The group images were used as masks to extract the percentage of change in these regions for each condition (Perception, Production) and each participant. Using Freesurfer, for each ROI, we computed two morphometric measures: surface-based cortical thickness, and gray matter volume. This process involves normalizing intensity from a high resolution T1-weighted anatomical MRI, removing non-brain voxels using a skull-stripping procedure (the result of which was visually inspected and manually adjusted when necessary), segmentation of the gray and white matter and CSF as well as surface deformation and topological corrections (Dale et al., 1999). Cortical thickness measures were obtained by calculating the distance between white matter and pial surfaces at each vertex across the cortical mantle (Fischl & Dale, 2000). The cortical volumes were computed using the statistical module of FreeSurfer, which integrates the area of each surface triangle and multiplies it by the mean thickness over the entire ROI. Two summary measures were also computed for the whole hemispheres: mean cortical thickness (in mm) and overall gray matter volume (calculated as the sum of the volumes for all cortical and subcortical gray and white matter ROIs, in cubic cm). To examine whether age-related changes in BOLD signal are mediated by regional volume and thickness, a series of multiple mediations were conducted. These analyses were conducted using the INDIRECT macro for SPSS (http://www.afhayes.com/) (Preacher & Hayes, 2004; Preacher & Hayes, 2008b).
Mediation analyses provide a very powerful analytical framework for testing predictions about mechanisms to explain age effects. Indeed, mediation models allow researchers to explain the mechanisms by which one variable affect another (Baron & Kenny, 1986; MacKinnon et al., 2007; Preacher & Hayes, 2004, 2008a; Shrout & Bolger, 2002). Multiple mediation analyses estimate the path coefficients in a multiple mediator model and generate bootstrap confidence intervals for total and specific indirect effects of X on Y through multiple mediator variables (M). The model that was used is illustrated in Supplementary Material S1. In this multiple (parallel) mediation model, the dependent (y) variables were the signal magnitude in Perception and in Production (each tested separately) for each of our age-sensitive ROIs, while the independent (x) variable was the categorical variable Age (young, older). Four mediators (bilateral volume, bilateral thickness) and three covariates (PTA, sex and education) were used. For each ROI, a linear regression was used to test for a direct effect of Age on brain structure --the a path in the model. A linear regression was also conducted to test for an effect of brain structure on BOLD signal (the b path). Next, a series of multiple regressions was conducted, each including one of the mediators to examine (1) whether there was a direct effect of Age on BOLD independent of brain structure (the c′ path), and (2) whether there was an indirect effect of Age on BOLD signal through regional brain structure (the ab path). An indirect effect indicates that a change in brain structure affects BOLD signal while Age is kept constant. A bootstrapping approach was used to test for the significance of the indirect effects (Shrout & Bolger, 2002) (P < 0.05, using bootstrapping with 20,000 samples). Bootstrapping involves the repeated extraction of samples, with replacement, from a dataset and the estimation of the indirect effect in each resampled data set.
Results
Hearing assessment
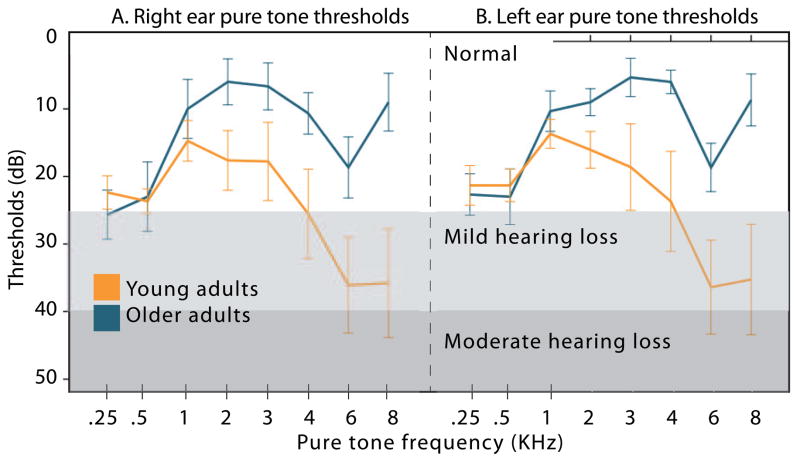
Examination of hearing sensitivity across groups, as measured by better ear PTA, revealed significant between-group differences (t(37df) = −5.843, p = .000001). Hearing results are presented in Table 1 and illustrated in Figure 1. As can be seen in the figure, some of our older participants had mild hearing loss; thus in all analyses, PTA was used as a covariate to remove potential effects of hearing sensitivity on BOLD signal.
Figure 1.
Pure tone thresholds (in dB) for the young (orange line) and older adults (blue line) for different frequencies, provided separately for the right ear (A) and the left ear (B).
Behavioural data
Participants’ performance in the repetition task approached ceiling. The young participants committed a total of 15 errors in over 960 trials. The older participants committed a total of 14 errors in over 960 trials.
Imaging data
Whole-brain analysis
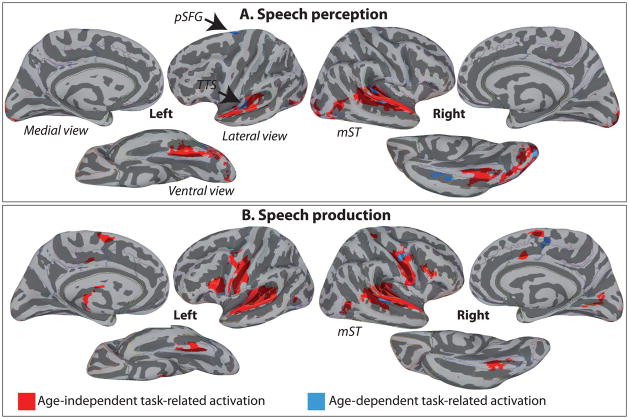
We first examined the magnitude of age-independent activation at the whole-brain level (see Table 2). For Perception (Figure 2A, red), age-independent activation included a large bilateral cluster covering the supratemporal planes, part of the occipital pole and fusiform gyrus. For Production, (Figure 2B, red) age-independent activation included additional bilateral clusters in ventral primary motor cortex (M1v), PMv, and SMA-proper. For the contrast of age-independent Production against age-independent Perception, Figure 3 (red) shows activation in bilateral M1v, PMv and along the supratemporal cortices, including the transverse temporal gyrus (TTG), transverse temporal sulcus (TTS) and planum temporale (PT). No region showed stronger response for Perception than Production.
Table 2.
FWE-corrected group-level whole brain conjunctions across groups for A. Perception, and B. Production. Coordinates are in Talairach space. Regions significantly active represent age-independent task-related activation. Cluster size is calculated in number of surface vertices, and area is in mm2.
| Contrast | Region | Hemi | x | y | z | Number of nodes | Surface area |
|---|---|---|---|---|---|---|---|
| Perception Young ∩ Perception Older | Supratemporal plane, including the transverse temporal gyrus and sulcus, the planum temporale and the anterior half of the STG. | Left | −50 | −22 | 2 | 4197 | 1318.85 |
| Activation also extend onto the middle STS. Occipito-temporal cortex, including the fusiform gyrus, OTS, inferior and middle occipital gyri and occipital pole. | Left | −39 | −74 | −13 | 2505 | 833.29 | |
| Occipital pole | Left | −10 | −102 | 4 | 237 | 81.46 | |
| Lingual gyrus | Left | −8 | −95 | −12 | 201 | 46.25 | |
| Supratemporal plane, including the transverse temporal gyrus and sulcus, the planum temporale and the entire STG. Activation also runs along most of the STS. | Right | 48 | −26 | 9 | 8652 | 2484.92 | |
| Occipito-temporal cortex, including the fusiform gyrus, OTS, inferior and middle occipital gyri and occipital pole. | Right | 28 | −80 | −11 | 3804 | 1176.91 | |
|
| |||||||
| Production Young ∩ Production Older | Supratemporal plane, including the transverse temporal gyrus and sulcus, the planum temporale and the entire STG. Activation also extend onto the middle STS. | Left | −50 | −27 | 5 | 6584 | 2041.05 |
| Ventral primary motor area in the central sulcus, extending into the ventral precentral gyrus (PMv). | Left | −46 | −8 | 34 | 3965 | 1302.74 | |
| Ventral precentral sulcus, extending into the pars opercularis of the inferior frontal gyrus. | Left | −43 | 9 | 19 | 1156 | 420.49 | |
| Fusiform gyrus, extending into the OTS. | Left | −35 | −58 | −18 | 627 | 235.76 | |
| SMA-proper. | Left | −6 | 0 | 61 | 418 | 122.62 | |
| Anterior occipital sulcus. | Left | −39 | −67 | 5 | 163 | 52.73 | |
| Posterior cingulate gyrus. | Left | −11 | −16 | 38 | 187 | 47.23 | |
| Supratemporal plane, including the transverse temporal gyrus and sulcus, the planum temporale and the entire STG. Activation also extend onto the posterior half of the STS. | Right | 48 | −26 | 9 | 8748 | 2497.38 | |
| Ventral primary motor area in the central sulcus, extending into the ventral precentral gyrus (PMv). | Right | 49 | −12 | 30 | 4640 | 1367.79 | |
| Calcarine sulcus, cuneus and lingual gyrus. | Right | 11 | −68 | 12 | 473 | 251.41 | |
| Fusiform gyrus. | Right | 35 | −55 | −17 | 440 | 200.57 | |
| Dorsal central sulcus (M1d) | Right | 20 | −32 | 57 | 495 | 145.58 | |
| Pars opercularis of the inferior frontal gyrus, extending onto the ventral precentral sulcus. | Right | 42 | 15 | 23 | 466 | 129.8 | |
| Pre-SMA/SMA-proper. | Right | 7 | 5 | 61 | 311 | 104.91 | |
| Anterior occipital sulcus. | Right | 41 | −67 | 3 | 433 | 104.2 | |
| Posterior cingulate gyrus. | Right | 8 | 13 | 34 | 253 | 84.3 | |
Figure 2.
Regions significantly active, at the group-level, corrected for multiple comparisons, A. for the conjunction of Perception Young and Perception Older (top row – in red), and B. for the conjunction of Production young and Production Older (bottom row – in red), shown on lateral, medial and ventral views of the cerebral hemispheres. Red-coloured activation represents the age-independent task-related activation, while blue-coloured activation represent age-dependent activation in speech perception and speech production. Activations are shown on the group average smoothed white matter inflated surface.
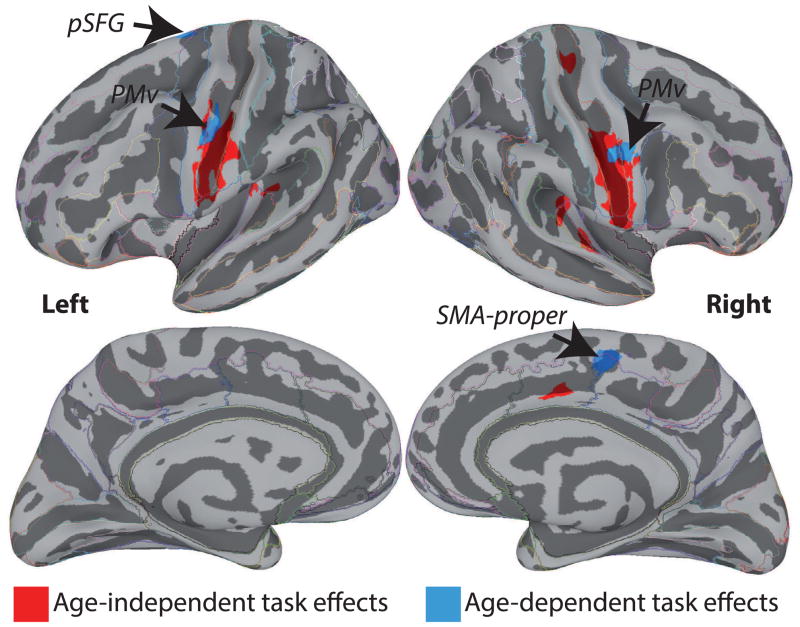
Figure 3.
Regions significantly modulated by task (Production > Perception), at the group-level, corrected for multiple comparisons, for the conjunction of Task Young and Task Older (in red). Red-coloured activation represents the age-independent task-related activation, while blue-coloured activation represent age-dependent activation in speech perception and speech production. Activation is shown on the group average smoothed white matter inflated surface.
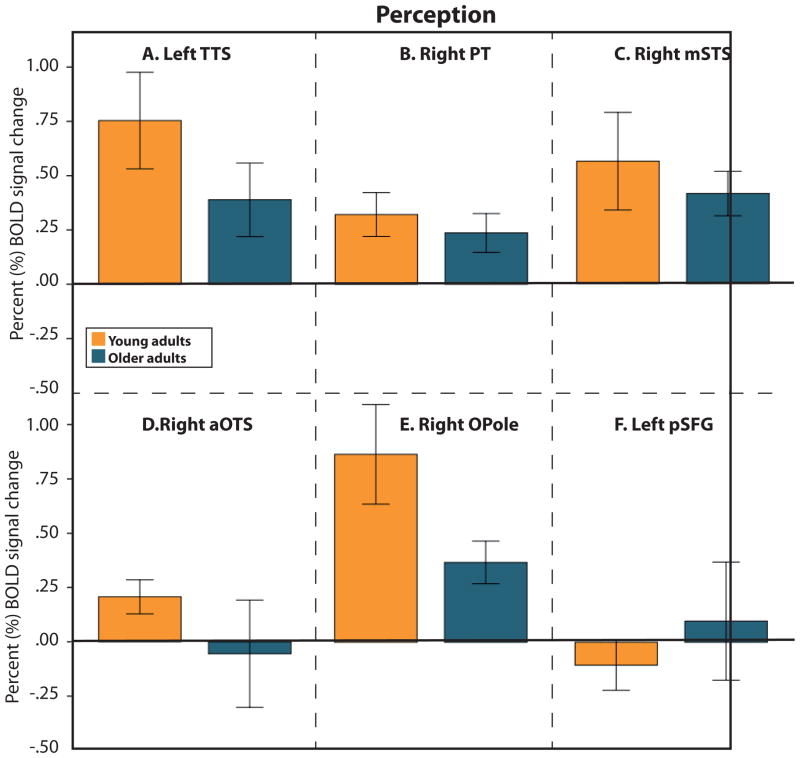
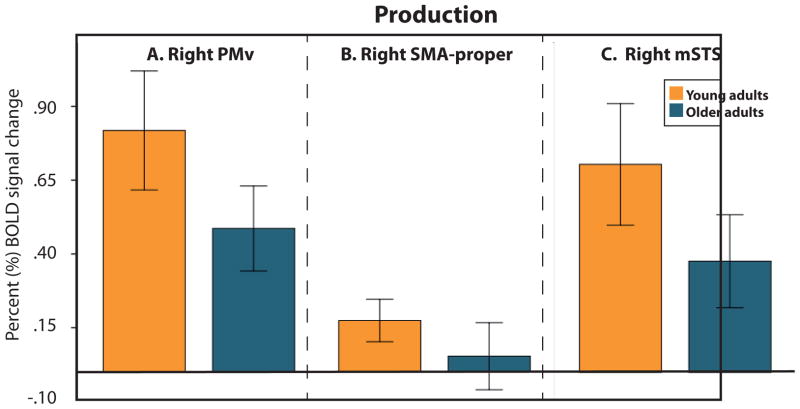
We next examined age-dependent task-related activation (Table 3). As shown in Figure 2A (blue), For Perception, we found cortical regions sensitive to Age in the left TTS, right PT, in the right STS, approximately in the middle of the sulcus (along the anterior/posterior dimension) (mSTS), left superior frontal gyrus (pSFG), right occipital pole (OPole) and right anterior occipito-temporal sulcus (aOTS; Figure 2A, blue). In all these regions except pSFG, the activation magnitude during Perception was significantly weaker for older compared to younger adults (Figure 4). For Production, we found cortical regions sensitive to Age in the right PMv, right mSTS and right SMA-proper (Figure 2B, blue). In all these ROIs, the amplitude of the BOLD signal was lower for older compared to younger adults (Figure 5).
Table 3.
FWE-corrected age-dependent whole brain effects in A. Perception, B. Production, and C. Production > Perception. Coordinates are in Talairach space. Cluster size is calculated in number of surface nodes, cluster area is calculated in mm2.
| Contrast | Region | Hemi | x | y | z | t | p | Cluster size | Cluster area |
|---|---|---|---|---|---|---|---|---|---|
| A. Perception | TTS, extending into TTG | Left | −49 | −19 | 4 | −3.340218 | 0.002 | 312 | 97.61 |
| Dorsal precentral sulcus/superior frontal gyrus. | Left | −13 | −7 | 69 | 3.239507 | 0.002 | 184 | 68.69 | |
| PT extending into TTS. | Right | 49 | −32 | 11 | −4.613151 | 0.00004 | 306 | 80.13 | |
| Occipital pole. | Right | 16 | −99 | −7 | −4.180524 | 0.0002 | 169 | 80.03 | |
| Lateral occipital-temporal sulcus extending into the fusiform gyrus. | Right | 39 | −19 | −22 | −3.818996 | 0.0005 | 203 | 54.02 | |
| Middle STG. | Right | 58 | −24 | 2 | −3.885855 | 0.0004 | 205 | 75.14 | |
|
| |||||||||
| B. Production | Ventral precentral gyrus (posterior PMv). | Right | 41 | −10 | 38 | −4.383471 | 0.00009 | 281 | 123.7 |
| Middle STG/STS. | Right | 50 | −31 | 3 | −4.381458 | 0.00009 | 384 | 118.66 | |
| SMA-proper. | Right | 8 | −4 | 54 | −3.99374 | 0.0003 | 171 | 38.83 | |
|
| |||||||||
| C. Production > Perception | Ventral central sulcus and precentral gyrus (PMv). | Left | −42 | −11 | 36 | −3.79114 | 0.0005 | 347 | 144.42 |
| Dorsal precentral sulcus/posterior superior frontal gyrus (pSFG). | Left | −14 | −9 | 68 | −3.096119 | 0.004 | 163 | 63.98 | |
| Ventral precentral gyrus (PMv) | Right | 49 | −3 | 46 | −3.946285 | 0.0003 | 347 | 160.42 | |
| SMA-proper. | Right | 8 | −3 | 54 | −4.225055 | 0.0001 | 285 | 69.56 | |
Figure 4.
Bar graphs illustrating the pattern of activation in regions exhibiting a significant Age effect during Perception (left TTS, right PT, right STS, right aOTS and, right Opole and left pSFG). The error bars represent the 95% confidence intervals.
Figure 5.
Bar graphs illustrating the pattern of activation in regions exhibiting a significant Age effect during Production (left right PMv, Right SMA-proper, and right mSTS). The error bars represent the 95% confidence intervals.
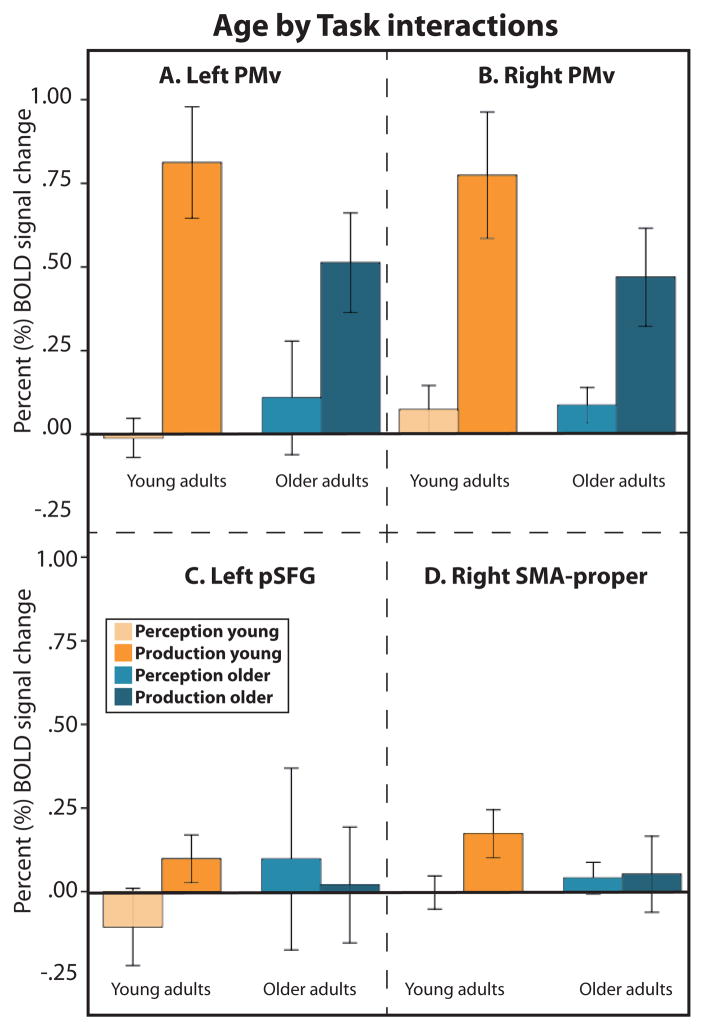
Finally, we examined regions exhibiting an Age by Task interaction. As shown in Figure 3 (blue), an interaction was found in the bilateral PMv, left pSFG, and right SMA-proper. In both PMv regions and in the right SMA-proper, the interaction was driven by lower BOLD signal for Production in older compared to younger adults without an effect of Age on Perception (Figure 6A, B, and C). In the left pSFG, there was no significant effect of Age on Production, but the amplitude of the BOLD signal was stronger for older compared to younger participants during Perception (Figure 6D).
Figure 6.
Bar graphs illustrating the pattern of activation in regions exhibiting a significant Task by Age interaction (bilateral PMv, left pSFG and right SMA-proper). The error bars represent the 95% confidence intervals.
Age-related structural changes
Global brain senescence
The left hemisphere gray matter volume was 266 cc (±21.2 cc) for the young and 226.3 cc (±21.39 cc) for the older adults; this difference was significant (t(34) = 4.60, p < .0001). The right hemisphere gray matter volume was 267.4 cc (±2.1 cc) for the young and 227.9 cc (±22.54 cc) for the older adults; this difference was significant (t(34) = 4.49, p < .0001). The left hemisphere thickness was 2.46 mm (±.077 mm) for the young and 2.34 mm (±.059 mm) for the older adults (t (34) = 4.32, p < .001). The right hemisphere thickness was 2.47 mm (±.069 mm) for the young and 2.35 mm (±.063 mm) for the older adults (t (34) = 4.037, p < .001).
Regional brain senescence
First, we conducted a set of FDR-corrected independent t-tests (q = .05, i = 8 tests per hemisphere) to examine the volume of gray matter in young and older adults in age-sensitive ROIs identified through whole-brain analyses (PMv, SMA-proper, pSFG, TTS, PT, mSTS, aOTS and Opole). Since all available evidence points towards a lower volume and thickness in older ages (see for example Drachman, 2006; Raz & Kennedy, 2009), directional tests were conducted. The PMv, SMA-proper, pSFG and the aOTS had smaller gray matter volume (bilaterally) for older compared to younger adults. The left mSTS and right Opole had smaller gray matter volume, and PT and TTS showed no age difference. These results are presented in Table 4. Next, we examined regional differences in cortical thickness. The results revealed a difference in cortical thickness (thinner cortical mantle for older compared to younger adults) in SMA-proper, pSFG, TTS and mSTS bilaterally, left PMv, and left PT, and no difference in thickness in the occipital ROIs. These results are presented in Table 5.
Table 4.
Age-related decline in gray matter volume.
| ROI | Hemi | GID | Gray matter volume (cc) | Percent change | p value (difference) | FDR |
|---|---|---|---|---|---|---|
| Ventral PM | Left | Younger | 1.86 | −22.47 | 0.00037 | * |
| Left | Older | 1.44 | ||||
| Right | Younger | 1.64 | −19.69 | 0.00239 | * | |
| Right | Older | 1.32 | ||||
|
| ||||||
| SMA- proper | Left | Younger | 3.05 | −20.18 | 0.00128 | * |
| Left | Older | 2.44 | ||||
| Right | Younger | 3.47 | −21.96 | 0.00016 | * | |
| Right | Older | 2.71 | ||||
|
| ||||||
| Posterior SFG | Left | Younger | 2.38 | −24.85 | 0.00007 | * |
| Left | Older | 1.78 | ||||
| Right | Younger | 2.13 | −21.22 | 0.00236 | * | |
| Right | Older | 1.68 | ||||
|
| ||||||
| PT | Left | Younger | 1.12 | −7.01 | 0.19119 | n.s. |
| Left | Older | 1.04 | ||||
| Right | Younger | .80 | −3.94 | 0.34774 | n.s. | |
| Right | Older | .77 | ||||
|
| ||||||
| TTS | Left | Younger | .55 | −7.12 | 0.13462 | n.s. |
| Left | Older | .51 | ||||
| Right | Younger | .46 | −11.07 | 0.05231 | n.s. | |
| Right | Older | .41 | ||||
|
| ||||||
| Middle STS | Left | Younger | 2.89 | −18.82 | 0.00276 | * |
| Left | Older | 2.35 | ||||
| Right | Younger | 3.41 | −7.64 | 0.11521 | n.s. | |
| Right | Older | 3.15 | ||||
|
| ||||||
| aOTS | Left | Younger | 2.05 | −24.98 | 0.00802 | * |
| Left | Older | 1.54 | ||||
| Right | Younger | 2.05 | −23.32 | 0.00229 | * | |
| Right | Older | 1.57 | ||||
|
| ||||||
| OPole | Left | Younger | 3.22 | −0.08 | 0.49458 | n.s. |
| Left | Older | 3.22 | ||||
| Right | Younger | 5.38 | −10.87 | 0.02360 | * | |
| Right | Older | 4.79 | ||||
Table 5.
Age-related decline in surface-based cortical thickness.
| ROI | Hemi | GID | Cortical thickness (mm) | Percent change | p value (difference) | FDR |
|---|---|---|---|---|---|---|
| Ventral PM | Left | Younger | 2.91 | −6.59 | 0.00042 | * |
| Left | Older | 2.72 | ||||
| Right | Younger | 2.82 | −3.81 | 0.05921 | n.s. | |
| Right | Older | 2.71 | ||||
|
| ||||||
| SMA-proper | Left | Younger | 2.90 | −6.05 | 0.00017 | * |
| Left | Older | 2.73 | ||||
| Right | Younger | 2.92 | −7.60 | 0.00013 | * | |
| Right | Older | 2.70 | ||||
|
| ||||||
| Posterior SFG | Left | Younger | 2.98 | −7.31 | 0.00138 | * |
| Left | Older | 2.76 | ||||
| Right | Younger | 2.90 | −5.93 | 0.00129 | * | |
| Right | Older | 2.72 | ||||
|
| ||||||
| PT | Left | Younger | 2.56 | −4.42 | 0.03620 | * |
| Left | Older | 2.45 | ||||
| Right | Younger | 2.61 | −3.29 | 0.09122 | n.s. | |
| Right | Older | 2.52 | ||||
|
| ||||||
| TTS | Left | Younger | 2.47 | −6.95 | 0.03766 | * |
| Left | Older | 2.30 | ||||
| Right | Younger | 2.67 | −9.80 | 0.00008 | * | |
| Right | Older | 2.41 | ||||
|
| ||||||
| Middle STS | Left | Younger | 2.54 | −6.04 | 0.00019 | * |
| Left | Older | 2.38 | ||||
| Right | Younger | 2.70 | −6.92 | 0.00015 | * | |
| Right | Older | 2.51 | ||||
|
| ||||||
| aOTS | Left | Younger | 2.70 | −1.23 | 0.35579 | n.s. |
| Left | Older | 2.67 | ||||
| Right | Younger | 2.65 | −1.20 | 0.27132 | n.s. | |
| Right | Older | 2.62 | ||||
|
| ||||||
| OPole | Left | Younger | 2.09 | −1.24 | 0.28350 | n.s. |
| Left | Older | 2.07 | ||||
| Right | Younger | 2.13 | −2.38 | 0.11829 | n.s. | |
| Right | Older | 2.08 | ||||
Mediation analyses
A main focus of this study was to examine the relationship between group differences in regional structural measures and regional task-related BOLD signal magnitude. To this aim, we conducted a series of multiple mediation analyses on the age-sensitive ROIs identified in the whole-brain analysis (PMv, SMA-proper, pSFG, TTS, PT, mSTS, aOTS and Opole). In the occipital lobe ROIs (right Opole and right aOTS) there was no direct or indirect relationship between structure and BOLD signal (Supplementary material S2; statistics are presented in Supplementary material S3).
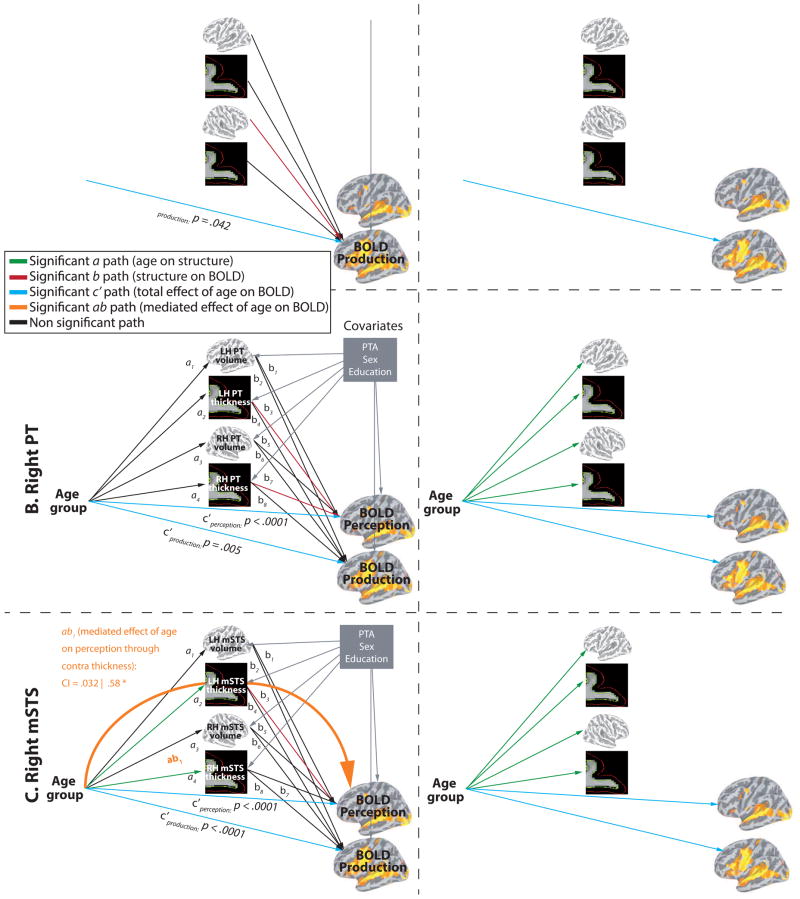
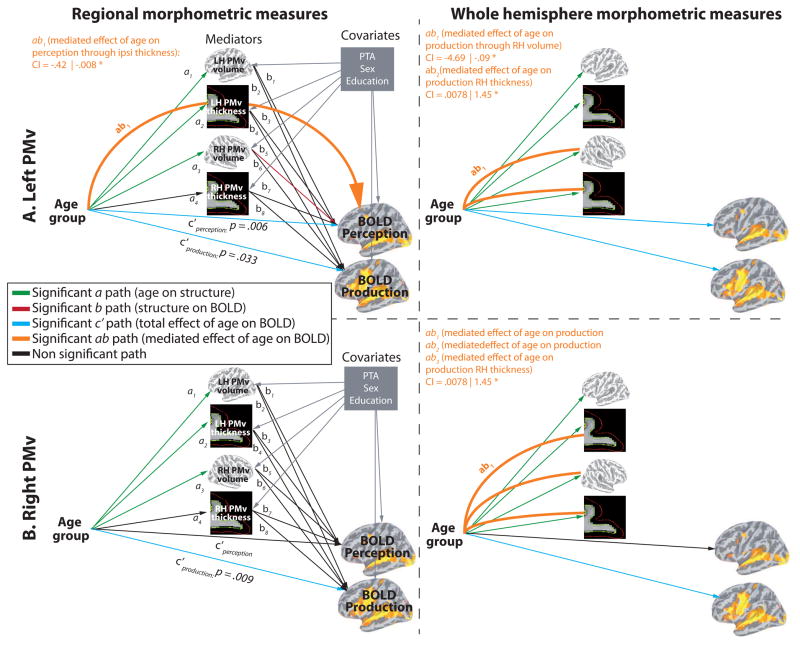
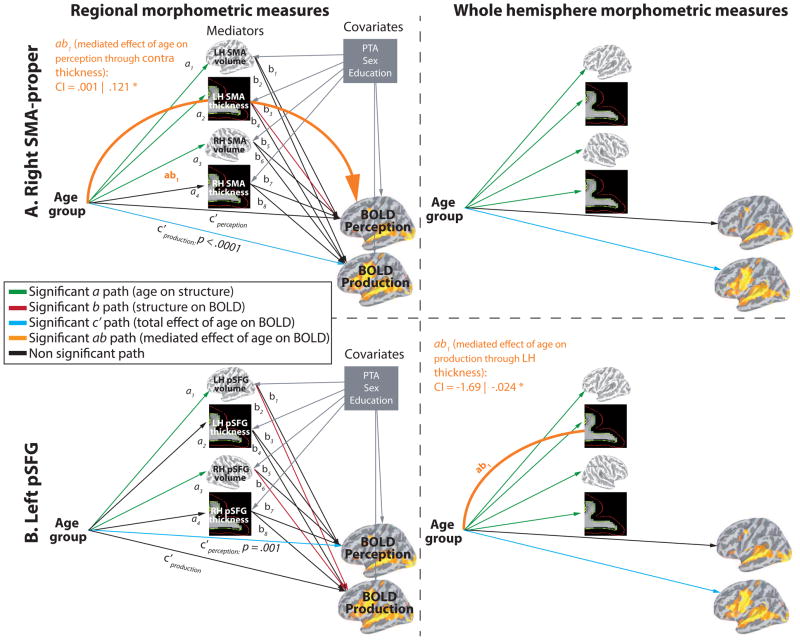
In the temporal ROIs, BOLD signal changes were predicted by age in both Perception and Production, and all ROIs showed a direct relationship between structure and BOLD (see Fig. 7A). In the left TTS, there was a direct effect of contralateral TTS volume on BOLD signal during Production. In the right PT, there was a direct effect of bilateral PT thickness on BOLD signal during Perception. In the right mSTS, there was a direct effect of contralateral mSTS thickness on BOLD signal magnitude during Perception. A partial indirect effect of Age on Perception through contralateral thickness was also found (ab path). Partial mediation indicates that the mediation effect is smaller than total effect (c path), meaning that the direct effect (c′ path) and the indirect effect (ab path) are both significantly different from zero. The frontal ROIs (bilateral PMv, right SMA-proper, left pSFG) showed various response patterns. As show in figure 8A (top row) the left PMv BOLD signal was modulated by age in both Perception and Production. There was a direct effect of contralateral volume on BOLD signal magnitude during Perception and a partial indirect effect of Age on Perception through contralateral volume. As show in figure 8A (bottom row) the right PMv BOLD signal was modulated by age only in Production. There was no direct or indirect effect of structure on BOLD. In the right SMA-proper (see Fig. 9A, top row), BOLD signal was modulated by age only in Production. There was a direct effect of contralateral thickness on BOLD signal magnitude during Perception and an indirect effect (complete mediation) of Age on Perception through contralateral SMA thickness. As show in figure 9A (bottom row) the left pSFG BOLD signal was modulated by age only in Perception, consistent with whole-brain results. In contrast to all other ROIs, pSFG showed stronger activation in older compared to younger adults; this effect was not influenced, directly or indirectly, by regional structural differences. There was, however, a direct effect of bilateral thickness on BOLD signal magnitude during Production.
Figure 7.
Result of the multiple mediation analysis for the temporal ROIs. A. Mediations analyses with regional morphometric measurements as mediator variables. B. Mediations analyses with global (whole hemisphere) morphometric measurements as mediator variables. Significant paths are coloured (see legend on the figure).
Figure 8.
Result of the multiple mediation analysis for the left and right PMv. A. Mediations analyses with regional morphometric measurements as mediator variables. B. Mediations analyses with global (whole hemisphere) morphometric measurements as mediator variables.
Figure 9.
Result of the multiple mediation analysis for the other frontal ROIs. A. Mediations analyses with regional morphometric measurements as mediator variables. B. Mediations analyses with global (whole hemisphere) morphometric measurements as mediator variables.
An additional set of mediation analyses was conducted to determine whether examining the effect of regional structural changes onto BOLD signal provides information different from using global structural measures. The result are presented in Figures 7B, 8B and 9B. As can be seen in the figures, the results of this analysis are quite different from those obtained using regional measures. Most of the relations (direct and indirect) between brain structure and BOLD signal were mediated exclusively through either regional or global structural measurements. Only in the left TTS we found that the relation of right hemisphere volume to BOLD was significant using both right TTS volume and total right hemisphere volume. In terms of the ability of each method to detect relationships, we found significant direct structure/BOLD relationships (b paths) in 6 ROIs using regional values and in 4 using global measures. For indirect (mediated) structure/BOLD relationships (ab paths), they were found in 3 ROIs using regional values and in 4 using global measures.
Discussion
The unprecedented aging of the population worldwide has triggered a renewed interest in understanding how the brain ages, both functionally and structurally. Modern brain imaging techniques such as functional MRI and PET offer powerful and non-invasive tools to study, in vivo, the structure and function of the human brain throughout the lifespan. At the functional level, imaging studies have consistently documented age-related alterations, though the pattern of observed change has varied from one study to the other, with some studies showing a decline in functional activation with aging, and others demonstrating co-occurring increases and decreases in functional activation with aging. One difficulty in interpreting these apparently divergent findings derives from the fact that most studies have either focused on changes in the BOLD signal, or changes in brain structure, but not both concurrently. Only a small number of studies have examined the relationship between structural and functional changes in aging (see for example Nyberg et al., 2002; Sheppard et al., 2011). Here we explore this issue in the context of speech perception and production. The four most important findings of this study are (1) age-related, gender-independent changes are regional, largely task-dependent, and essentially consisting of lower activation in older compared to younger adults; (2) neural compensation, defined as increased brain activation serving to counteract age-related neural decline, was generally not evident, with the exception of the left pSFG; (3) global decreases in cortical thickness and gray matter volume were present with important regional differences; (4) the relation between brain structure and function is heterogeneous and complex. In some regions there is a significant relationship between structure and function, while in others functional changes are independent of regional ipsi- and contralateral structural changes. These findings are discussed in the following paragraphs.
Aging and the BOLD signal
We tackle first the general aging effects on the BOLD signal. The comparison of BOLD responses in individuals of different ages relies on the assumption of comparable cerebrovascular coupling and cerebral blood flow (CBF) since the BOLD signal depends on the integrity of neurovascular coupling and is believed to depend on CBF. Changes in the cerebrovascular system frequently occur in older ages (e.g. reduced vascular reactivity, arteriosclerosis), which are likely to affect (reduce) the BOLD signal (D’Esposito et al., 2003). One way to circumvent this problem is to focus on age by condition interactions in the BOLD signal rather than absolute age differences in BOLD signal across age groups. In the same way that a receding tide lowers all boats, reduced BOLD signal will occur across conditions within age. Focusing on the interaction—the difference of the condition differences across age—is a strategy that helps alleviate main effects of aging such as vascular changes (D’Esposito et al., 2009). In the present study, we focused on Age by Task (Perception, Production) interactions; that is, we identified brain regions in which the BOLD signal varied as a function of both Task and Age group.
Functional changes in the speech network: nature, context-relevance and scope
In the present study, we examined age-independent and age-dependent activation in the cortical system involved during simple audiovisual speech perception and production tasks in healthy adults. Our results demonstrate a large bilateral network of cortical areas not affected by age, which included primary motor and premotor areas of the frontal lobe, most of the supratemporal cortex, the fusiform gyrus and parts of the occipital lobe. In addition, our results demonstrate lower activation magnitude in frontal, temporal and occipital cortices during audiovisual perception and production of single bisyllabic words for older compared to younger adults. The only exception to this pattern was found in the left pSFG, which showed stronger activation for older compared to younger adults during Perception. Most regions showing age-related effects in BOLD signal magnitude in the present study - which included the TTS, PT, mSTS, PMv, SMA-proper and pSFG - are known to be part of the cortical speech network, which suggests that age-related changes in speech skills have a cortical correlate. For example, the right PT exhibited a decrease in functional activation in both perception and production, suggesting a role in both processes. Consistent with this observation, several studies have shown that PT is involved in both speech perception and overt speech production (Tremblay et al., 2011; Tremblay & Small, 2011c). It is also active during silent speech production, which does not involve self-generated auditory feedback, suggesting that its role in speech production goes beyond the processing of self-generated feedback (e.g. Hickok et al., 2003; Huang et al., 2002; Okada et al., 2003; Pa & Hickok, 2008; Wise et al., 2001). Our results demonstrate that the biological function of this core speech area is vulnerable to normal aging. The bilateral PMv, which is known to be involved in both perceptual and motor aspects of speech, was among the only areas showing a Task by Age interaction, revealing stronger functional decline in speech production than perception, consistent with prior evidence for a role in both perception and production, with greater involvement in production (Tremblay & Small, 2011a, 2011c). A similar pattern was found in the SMA-proper, consistent with its the role in motor preparation for speech production (Alario et al., 2006; Bohland & Guenther, 2006; Tremblay & Gracco, 2006, 2009, 2010; Tremblay & Small, 2011b). In both left PMv and right SMA-proper, the age to BOLD relationship was not directly or indirectly mediated by regional structural changes. Interestingly, in the left PMv, age-related BOLD signal change in Production was mediated, not by regional but by global brain shrinkage. It has recently been suggested that BOLD signal in PMv during speech perception is dependent upon working memory skills—i.e., the ability to maintain and manipulate information, skills that are necessary to perceive speech and understand language (Szenkovits et al., 2012). Given the well-documented decline in working memory skills with age (for a review, see for example Bopp & Verhaeghen, 2005), it is possible that our finding of lower activation in PMv in older adults compared to younger adults is subsequent to decrease in functional efficiency in working memory network. However, since we did not collect a measure of working memory, future studies looking at the relation between cognitive and speech decline will be necessary to uncover the nature of the senescence mechanisms occurring in the cortical network supporting speech processes. Understanding the aetiology of changes in this system (cognitive, motor, sensory, etc) may provide avenues of behavioural treatments for age-related speech difficulties.
Another area vulnerable to aging was a region of mSTS, a region that has been described as a core phonological processing area (Hickok, 2009; Hickok & Poeppel, 2007) for speech perception and production. In the present study, the mSTS bilaterally was jointly active for speech perception and production; its activation magnitude was lower for older compared to younger adults in both tasks in the right hemisphere but not in the left. Though we did not specifically evaluate phonological skills, core phonological processing skills were necessary to succeed in the production task, which requires transformation of an auditory or audiovisual code into a code that the motor system can understand. This suggests that despite neural decline in this area, participants were still capable of sustaining basic speech repetition mechanisms.
The left posterior SFG, corresponding to the rostral sector of the dorsal PM (area F2 in monkey) showed a unique response pattern. In contrast to all other age-sensitive ROIs who exhibited lower BOLD signal in older compared to younger adults, the pSFG showed higher activation for older compared to younger adults during speech perception but not speech production. This region is often found during speech and non-speech orofacial movement execution (Grabski et al., 2012; Tatsumi et al., 1999; Tremblay & Small, 2011b) as well as execution of hand and arm movements (Colebatch et al., 1991). In monkeys, parietal-premotor loops involving area F2 appear to be involved in planning and controlling movements on the basis of sensory information (Rizzolatti et al., 1998); F2 is a source of corticospinal projections and is connected with the primary motor area. Parietal afferents are very rich (Luppino & Rizzolatti, 2000). It is possible that this region is involved in the planning and execution of speech movements, which too rely on sensory information, including somatosensory and auditory feedback. The fact that it is more strongly active in older compared to younger adults during perception but not production suggests that it may be providing additional sensorimotor information that could be used to constrain speech sound identification. The Motor Theory of Speech Perception (Liberman et al., 1967; Liberman & Mattingly, 1985) contends that speech perception and production are intimately linked, and that each speech sound is associated with a specific combination of motor commands, such as “tongue retraction” and “jaw opening”. According to this view, the ability to categorize the speech sounds forming the incoming speech stream is accomplished by tracking the intended articulatory patterns, and thus, the intended articulatory patterns represent the ultimate objects of speech perception. Given the well-known decline in speech perception skills that occurs with age, the finding of increased premotor activation during speech perception is consistent with the Perception-for-Action-Control Theory (PACT) (Schwartz et al., 2010), which posits that speech production mechanisms have two functions in speech perception: (1) co-structure auditory categories with learned motor routines, which leads to the integration of articulatory information into perceptual categories, and, (2) mediate speech perception through a predictive mechanism, which comes into play when there is perceptual ambiguity, to recover the missing information. Loss of sensitivity to speech acoustical features, as it occurs in older ages, may represent one such situation.
Lower efficiency of the speech system in aging or age-related expertise?
Lower BOLD signal in aging, given appropriate experimental design and analysis (‘see section on aging and the BOLD signal’), can be interpreted in at east two ways. One hypothesis is that lower BOLD reflects a reduced level of neural functioning, especially if it is accompanied with poorer behavioural performance. Such a pattern may or may not be associated with age-related reduction in brain tissue. An alternative possibility is that lower BOLD signal magnitude, especially if accompanied with similar or better behavioural performance, reflects expertise (increased neural efficiency), rather than reduced neural functioning. Prior work has shown that expertise is associated with regional decrease in brain activation in young adults during object recognition (Wiesmann & Ishai, 2011) and auditory perception (Berkowitz & Ansari, 2010; Petrini et al., 2011), though there is also evidence of increased activation associated with action observation in expert dancers (Berkowitz & Ansari, 2010). Expertise, however, has never been associated with brain tissue reduction. In contrast, it has been associated with increased thickness in STS in athletes (Wei et al., 2011), and with increased gray matter volume in the prefrontal cortex in car experts (Gilaie-Dotan et al., 2012). While some forms of expertise do not correlate with increase in cortical volume and/or thickness, such as medical expertise (Woollett et al., 2008), we have not found evidence for an association between lower volume or thickness and expertise.
In the present study, lower BOLD signal magnitude in regions involved in perceiving or producing speech was found in several cortical areas (PMv, SMA-proper, TTS, PT, mSTS, aOTS and OPole), reflecting both lower functioning (PMv, SMA-proper, TTS, PT, mSTS) and expertise (OPole, aOTS). For the PMv, SMA-proper, TTS, PT, mSTS, the patterns of results suggest lower functioning level because the age difference in BOLD signal was mediated by regional changes in cortical thickness or volume, with lower thickness and/or volume associated with lower BOLD signal. This pattern is more consistent with the hypothesis of decreased neural functioning, despite the absence of a difference in behavioural performance between groups; it is possible that, while decreased, neural functioning level was still sufficient to maintain performance especially during such a low difficulty speech repetition task.
In the occipital ROIs (OPole and aOTS), we suggest that expertise rather than lower functional efficiency accounts for the lower BOLD signal. Indeed, the occipital ROIs exhibited lower BOLD signal magnitude in older adults during speech perception (which involved hearing and seeing a person producing words) uncorrelated with regional morphometric differences. Recall that our right aOTS ROI encompassed both the occipito-temporal sulcus and the fusiform gyrus. This areas has been shown to be involved in human movement perception as well as face perception (Grill-Spector et al., 2004; Kanwisher et al., 1997; Rossion et al., 2012; Yovel & Kanwisher, 2004) and facial motion (Puce et al., 2003). The finding of lower BOLD signal in older adults in areas involved in vision and face perception is interesting because empirical evidence shows that visual speech information enhances speech perception (Binnie et al., 1974; MacLeod & Summerfield, 1987; Summerfield, 1979) particularly for older adults (Winneke & Phillips, 2011). Rehabilitation strategies designed to compensate for an impoverished auditory signal often encourage lip-reading (Alpiner, 1986). If older adults rely more heavily on visual information, one might expect to see increased BOLD signal in visual areas, but we found no visual area showing such pattern. Unlike the other ROIs, the BOLD signal in the occipital ROIs showed no relationship to regional structural measures, which may indicate that despite increased reliance on visual information, expertise rather than lower functional efficiency accounts for the lower BOLD signal found in these areas. Lower thickness or lower volume associated with lower BOLD signal, as discussed above, suggests decreased functional efficiency rather than expertise, because expertise has been associated with increased morphometric indices rather than decreases. In sum, our data reveal patterns of BOLD/structure relationship suggesting of both lower functioning (temporal and frontal ROIs with the exception of the pSFG) and expertise (occipital areas) in healthy older adults.
Regional structural senescence in the speech network vs. global brain senescence
In addition to expected global trends, our data indicate regional differences in the relationship between age and structure, consistent with the hypothesis that age-related structural changes are not uniform across the whole brain, with some regions showing heightened vulnerability (Cabeza, 2001). Global age-related brain senescence is a well-established phenomenon. Multiple autopsy studies have revealed global age-related decreases in brain volume and weight (Dekaban, 1978; Ho et al., 1980; Skullerud, 1985). Consistent with these findings, more recent MRI and CT experiments have shown age-related linear decline in several morphometric measures such as global gray matter volume (Allen et al., 2005; Carmichael et al., 2007; Giorgio et al., 2010; Raz et al., 1998; Takeda & Matsuzawa, 1984; Takeda et al., 1984; Yamaura et al., 1980). Based on the literature, we thus expected to find global senescence in both cortical thickness and gray matter volume, which we found, while controlling for gender.
Compared to the frontal ROIs, the temporal and occipital ROIs showed little sensitivity to aging. In a recent longitudinal MRI study of 38 healthy elderly participants (average of 66 years) many regions, including the right inferior frontal gyrus and bilateral postcentral gyrus showed a structural decline, while other regions, including the precentral gyrus and the auditory cortex, showed no structural decline, consistent with our findings (Nyberg et al., 2010). In another longitudinal study in a large group (N = 142) of healthy adults with mean age of 75.6 years (Fjell et al., 2009), regional differences in the rate of atrophy were examined at one and two-years intervals. While most regions showed structural decline, some regions were well preserved such as the precentral and postcentral regions. Hence, our results, in keeping with previous findings, demonstrate different patterns of cortical vulnerability, with some regions, such as the supratemporal cortex, beginning to decay only later in life.
Another important finding of the present study is that different morphometric measures reveal distinct patterns of structural vulnerability to aging. Our results show that only 6 unilateral ROIs (representing fewer than 40% of all ROIs examined) showed significant reduction in both gray matter volume and cortical thickness. All other ROIs were vulnerable to aging as measured in only one of these measures. These results emphasize the importance of looking at multiple measures of brain morphology in aging as they have various sensitivity profiles, each of which potentially providing complementary information on the course of brain structural decline, and perhaps, aging, more generally.
Structural to functional decline
A central objective of this study was to examine the relationship between age, BOLD signal, and anatomy in other to further current understanding of brain senescence mechanisms. Despite the repeated observation that the degree of brain atrophy is generally a poor predictor of behaviour, whether normal or impaired (Katzman et al., 1988), only a handful of studies (Nyberg et al., 2002; Tremblay et al., Submitted) have examined aging of both brain structure and brain function, which may be a more sensitive and more representative measure of brain senescence, and perhaps also a better predictor of speech performance, than any measure (functional, anatomical) taken individually. As was discussed in the previous sections, we found significant functional and structural regional differences in multiple sensory and motor areas involved in producing and perceiving speech in healthy older adults. In addition to these changes, our results also revealed direct or indirect relationship between structure and BOLD in all but one age-sensitive functionally-defined ROI (aOTS). As described in the methods section, these regions were identified based on the group results. When we examined their homologues in the other hemisphere, we noted that all homologues expect for the Opole showed a significant age difference in either thickness or volume; yet none exhibited age-related BOLD signal magnitude difference, indicating that, at this age, structural and functional changes are not necessarily connected, but that functional changes, when present, appear to co-occur with regional structural changes.
Brain/behaviour relationship and the brain reserve hypothesis
In the present study, speech performance (repetition of audiovisual words in noise) was similar across groups, which suggests that the neural system supporting speech production is capable of coping with a certain amount of functional and structural brain changes. The only other study that examined speech production in aging using fMRI found both decreased and increased functional activations (in PMv, SMA-proper and prefrontal cortex) in older adults (Soros et al., 2011). Though these increased activations were interpreted as neural compensation, since no behavioural measure of speech production was reported, it is difficult to determine whether these hyperactivations really reflected compensatory mechanisms associated with better functional outcome, or instead neural dedifferentiation (Heuninckx et al., 2008), that is, failure to recruit specialized neural mechanism, a phenomenon that isn’t associated with better functional outcome. Resistance to cortical loss may indeed be the product of neural compensation/reorganization strategies (D’Esposito et al., 2009; Grady, 2009; Steffener & Stern, 2012), which involve the use of additional brain mechanisms or alternative brain networks to counteract age-related structural decline and maintain successful performance (Heuninckx et al., 2008; Stern, 2003). One possible explanation for these apparently divergent results is that, because participants were on average 10 years older in the study by Soros et al. compared to the present one, it is possible that they had already begun using compensatory strategies to counteract protracted structural decline that could no longer be sustained by the CNS. In contrast, our participants were, on average, 62 years old; hence they were only just entering older adulthood and their CNS may still have been capable of coping with early effect of brain senescence. While further studies are necessary to explore factors that may trigger behavioural decline (e.g. age, global health factors, expertise), our results are generally consistent with the hypothesis of a ‘brain reserve capacity’ (Satz, 1993; Steffener & Stern, 2012; Stern, 2002, 2003). This hypothesis stems from the repeated observation that degree of brain atrophy is generally a poor predictor of behaviour, whether normal or impaired (Katzman et al., 1988). Indeed, functional brain systems appear to be able to sustain different levels of normal age-related brain atrophy. In the present study, we found only limited evidence of compensatory processes, and no behavioural disadvantage for older compared to younger adults, consistent with the notion of brain reserve.
Conclusion
The present results provide novel and exciting findings about normative neurobiological aging processes occurring in the cortical network involved in verbal communication. Our results show regional variations in brain structure, task-specific decrease and increase in the magnitude of functional activation in the cortical network that supports speech functions, and a complex relationship between structural and functional mechanisms. Further studies are needed to identify the factors, such as global health, life style, environment and genetics, age-related cognitive and motor decline that contribute to the aetiology of age-related cortical and behavioural changes affecting the speech system; this knowledge is critical to understand normative brain aging mechanisms and inter-individual differences in the course and course and quality of aging. Knowledge about the functional and neurobiological factors contributing to the aetiology of speech difficulties in older ages is necessary to lay the foundation for the development of new interventions to prevent, slow down or reverse age-related speech difficulties.
Supplementary Material
Panel A. Illustration of the process of measuring cortical thickness. Panel B. The conceptual model that was used for the multiple mediation analyses. In this model, the dependent (y) variables were the signal magnitude in Perception and in Production (each tested separately) for each ROIs, while the independent (x) variable was the categorical variable Age (young, older). Four mediator and three covariates (PTA, sex and education) were also included in the model. The mediators were the ROI left and right average gray matter volume and surface based cortical thickness.
Result of the multiple mediation analysis for the occipital ROIs. A. Mediations analyses with regional morphometric measurements as mediator variables. B. Mediations analyses with global (whole hemisphere) morphometric measurements as mediator variables. Significant paths are coloured (see legend on the figure).
Acknowledgments
We thank Blythe Buchholz, Margaret Flynn, and Michael Andric for their help collecting the data, and Matthew Schiel for his help in pre-processing the fMRI data. Thanks also to all participants. This study was supported by the National Institutes of Health under NIDCD grants R33 DC008638 and R01 DC003378 to S.L. Small, and by a postdoctoral fellowship from the CIHR to P. Tremblay. Their support is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alario FX, Chainay H, Lehericy S, Cohen L. The role of the supplementary motor area (SMA) in word production. Brain Res. 2006;1076(1):129–143. doi: 10.1016/j.brainres.2005.11.104. [DOI] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26(9):1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. discussion 1279–1282. [DOI] [PubMed] [Google Scholar]
- Alpiner JG. Rehabilitation concepts with the cochlear implant. Otolaryngol Clin North Am. 1986;19(2):259–265. [PubMed] [Google Scholar]
- Argall BD, Saad ZS, Beauchamp MS. Simplified intersubject averaging on the cortical surface using SUMA. Human Brain Mapping. 2006;27(1):14–27. doi: 10.1002/hbm.20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KK, Ramig LO, Sapir S, Luschei ES, Smith ME. Control of vocal loudness in young and old adults. J Speech Lang Hear Res. 2001;44(2):297–305. doi: 10.1044/1092-4388(2001/024). [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Berkowitz AL, Ansari D. Expertise-related deactivation of the right temporoparietal junction during musical improvisation. Neuroimage. 2010;49(1):712–719. doi: 10.1016/j.neuroimage.2009.08.042. [DOI] [PubMed] [Google Scholar]
- Binnie CA, Montgomery AA, Jackson PL. Auditory and visual contributions to the perception of consonants. Journal of Speech and Hearing Research. 1974;17:619–630. doi: 10.1044/jshr.1704.619. [DOI] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32(2):821–841. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- Bopp KL, Verhaeghen P. Aging and verbal memory span: a meta-analysis. J Gerontol B Psychol Sci Soc Sci. 2005;60(5):P223–233. doi: 10.1093/geronb/60.5.p223. [DOI] [PubMed] [Google Scholar]
- Burnett TA, Freedland MB, Larson CR, Hain TC. Voice F0 responses to manipulations in pitch feedback. J Acoust Soc Am. 1998;103(6):3153–3161. doi: 10.1121/1.423073. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Cognitive neuroscience of aging: contributions of functional neuroimaging. Scand J Psychol. 2001;42(3):277–286. doi: 10.1111/1467-9450.00237. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17(3):1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Callan AM, Callan DE, Tajima K, Akahane-Yamada R. Neural processes involved with perception of non-native durational contrasts. Neuroreport. 2006;17(12):1353–1357. doi: 10.1097/01.wnr.0000224774.66904.29. [DOI] [PubMed] [Google Scholar]
- Callan D, Callan A, Gamez M, Sato MA, Kawato M. Premotor cortex mediates perceptual performance. Neuroimage. 2010;51(2):844–858. doi: 10.1016/j.neuroimage.2010.02.027. [DOI] [PubMed] [Google Scholar]
- Callan DE, Tsytsarev V, Hanakawa T, Callan AM, Katsuhara M, Fukuyama H, Turner R. Song and speech: brain regions involved with perception and covert production. Neuroimage. 2006;31(3):1327–1342. doi: 10.1016/j.neuroimage.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Carmichael OT, Kuller LH, Lopez OL, Thompson PM, Dutton RA, Lu A, Becker JT. Ventricular volume and dementia progression in the Cardiovascular Health Study. Neurobiol Aging. 2007;28(3):389–397. doi: 10.1016/j.neurobiolaging.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebatch JG, Deiber MP, Passingham RE, Friston KJ, Frackowiak RS. Regional cerebral blood flow during voluntary arm and hand movements in human subjects. Journal of Neurophysiology. 1991;65(6):1392–1401. doi: 10.1152/jn.1991.65.6.1392. [DOI] [PubMed] [Google Scholar]
- Cooper JC, Jr, Gates GA. Hearing in the elderly--the Framingham cohort, 1983–1985: Part II. Prevalence of central auditory processing disorders. Ear Hear. 1991;12(5):304–311. doi: 10.1097/00003446-199110000-00002. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- D’Ausilio A, Pulvermüller F, Salmas P, Bufalari I, Begliomini C, Fadiga L. The motor somatotopy of speech perception. Current Biology. 2009;19:381–385. doi: 10.1016/j.cub.2009.01.017. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4(11):863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Jagust WJ, Gazzaley A. Methodological and Conceptual Issues in the Study of the Aging Brain. In: Jagust WJ, D’Esposito M, editors. Imaging the aging brain. Oxford University Press; 2009. pp. 11–25. [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based Analysis: I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Decoster W, Debruyne F. The ageing voice: changes in fundamental frequency, waveform stability and spectrum. Acta Otorhinolaryngol Belg. 1997;51(2):105–112. [PubMed] [Google Scholar]
- Dekaban AS. Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol. 1978;4(4):345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- Desai R, Liebenthal E, Possing ET, Waldron E, Binder JR. Volumetric vs. surface-based alignment for localization of auditory cortex activation. NeuroImage. 2005;26(4):1019–1029. doi: 10.1016/j.neuroimage.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Dick AS, Solodkin A, Small SL. Neural development of networks for audiovisual speech comprehension. Brain Lang. 2010;114(2):101–114. doi: 10.1016/j.bandl.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DL, Miorrison JH, Hof PR. The neuropathology of aging. In: Jagust WJ, D’Esposito M, editors. Imaging the aging brain. Oxford: Oxford University Press; 2009. pp. 27–40. [Google Scholar]
- Drachman DA. Aging of the brain, entropy, and Alzheimer disease. Neurology. 2006;67(8):1340–1352. doi: 10.1212/01.wnl.0000240127.89601.83. [DOI] [PubMed] [Google Scholar]
- Duchin SW, Mysak ED. Disfluency and rate characteristics of young adult, middle-aged, and older males. J Commun Disord. 1987;20(3):245–257. doi: 10.1016/0021-9924(87)90022-0. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Dale AM. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Dale AM. One-year brain atrophy evident in healthy aging. J Neurosci. 2009;29(48):15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozo MS, Watson BC. Task complexity effect on vocal reaction time in aged speakers. J Voice. 1998;12(4):404–414. doi: 10.1016/s0892-1997(98)80049-0. [DOI] [PubMed] [Google Scholar]
- Frisina DR, Frisina RD. Speech recognition in noise and presbycusis: relations to possible neural mechanisms. Hear Res. 1997;106(1–2):95–104. doi: 10.1016/s0378-5955(97)00006-3. [DOI] [PubMed] [Google Scholar]
- Gates GA, Mills JH. Presbycusis. Lancet. 2005;366(9491):1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- Gazzaley AH, D’Esposito M. BOLD Functional MRI and Cognitive Aging. In: Cabeza R, Nyberg L, Park D, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. Oxford: Oxford University Press; 2005. pp. 107–131. [Google Scholar]
- Gilaie-Dotan S, Harel A, Bentin S, Kanai R, Rees G. Neuroanatomical correlates of visual car expertise. Neuroimage. 2012;62(1):147–153. doi: 10.1016/j.neuroimage.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, Johansen-Berg H. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 2010;51(3):943–951. doi: 10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould J, Lane H, Vick J, Perkell JS, Matthies ML, Zandipour M. Changes in speech intelligibility of postlingually deaf adults after cochlear implantation. Ear Hear. 2001;22(6):453–460. doi: 10.1097/00003446-200112000-00002. [DOI] [PubMed] [Google Scholar]
- Grabski K, Lamalle L, Vilain C, Schwartz JL, Vallee N, Tropres I, Sato M. Functional MRI assessment of orofacial articulators: neural correlates of lip, jaw, larynx, and tongue movements. Hum Brain Mapp. 2012;33(10):2306–2321. doi: 10.1002/hbm.21363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL. Compensatory Reorganization of Brain Networks in Older Adults. In: Jagust WJ, D’Esposito M, editors. Imaging the aging brain. Oxford: Oxford University Press; 2009. pp. 105–113. [Google Scholar]
- Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nat Neurosci. 2004;7(5):555–562. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32(1):180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Harkrider AW, Plyler PN, Hedrick MS. Effects of age and spectral shaping on perception and neural representation of stop consonant stimuli. Clin Neurophysiol. 2005;116(9):2153–2164. doi: 10.1016/j.clinph.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Hartman DE, Danahuer JL. Perceptual features of speech for males in four perceived age decades. J Acoust Soc Am. 1976;59(3):713–715. doi: 10.1121/1.380894. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci. 2008;28(1):91–99. doi: 10.1523/JNEUROSCI.3300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G. The functional neuroanatomy of language. Phys Life Rev. 2009;6(3):121–143. doi: 10.1016/j.plrev.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Buchsbaum B, Humphries C, Muftuler T. Auditory-motor interaction revealed by fMRI: speech, music, and working memory in area Spt. J Cogn Neurosci. 2003;15(5):673–682. doi: 10.1162/089892903322307393. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Ho KC, Roessmann U, Straumfjord JV, Monroe G. Analysis of brain weight. I. Adult brain weight in relation to sex, race, and age. Arch Pathol Lab Med. 1980;104(12):635–639. [PubMed] [Google Scholar]
- Houde JF, Jordan MI. Sensorimotor adaptation in speech production. Science. 1998;279(5354):1213–1216. doi: 10.1126/science.279.5354.1213. [DOI] [PubMed] [Google Scholar]
- Huang J, Carr TH, Cao Y. Comparing cortical activations for silent and overt speech using event-related fMRI. Hum Brain Mapp. 2002;15(1):39–53. doi: 10.1002/hbm.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, Ores Walsh KS, Greischar LL, Alexander AL, Fox AS, Davidson RJ, Oakes TR. Motion correction and the use of motion covariates in multiple-subject fMRI analysis. Human Brain Mapping. 2006;27:779–788. doi: 10.1002/hbm.20219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JA, Munhall KG. Perceptual calibration of F0 production: evidence from feedback perturbation. J Acoust Soc Am. 2000;108(3 Pt 1):1246–1251. doi: 10.1121/1.1288414. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Peck A. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23(2):138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- Law M. Participation in the occupations of everyday life. Am J Occup Ther. 2002;56(6):640–649. doi: 10.5014/ajot.56.6.640. [DOI] [PubMed] [Google Scholar]
- Liberman AM, Cooper FS, Shankweiler DP, Studdert-Kennedy M. Perception of the speech code. Psychol Rev. 1967;74(6):431–461. doi: 10.1037/h0020279. [DOI] [PubMed] [Google Scholar]
- Liberman AM, Mattingly IG. The motor theory of speech perception revised. Cognition. 1985;21(1):1–36. doi: 10.1016/0010-0277(85)90021-6. [DOI] [PubMed] [Google Scholar]
- Linville SE. The sound of senescence. J Voice. 1996;10(2):190–200. doi: 10.1016/s0892-1997(96)80046-4. [DOI] [PubMed] [Google Scholar]
- Luppino G, Rizzolatti G. The Organization of the Frontal Motor Cortex. News and Views. 2000;15:219–224. doi: 10.1152/physiologyonline.2000.15.5.219. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod A, Summerfield Q. Quantifying the contribution of vision to speech perception in noise. British Journal of Audiology. 1987;21:131–141. doi: 10.3109/03005368709077786. [DOI] [PubMed] [Google Scholar]
- Matthies ML, Svirsky MA, Lane HL, Perkell JS. A preliminary study of the effects of cochlear implants on the production of sibilants. J Acoust Soc Am. 1994;96(3):1367–1373. doi: 10.1121/1.410281. [DOI] [PubMed] [Google Scholar]
- Morris R, Brown WS. Age-related voice measures among adult women. Journal of voice. 1987;1(1):43. [Google Scholar]
- Mueller PB. The aging voice. Semin Speech Lang. 1997;18(2):159–168. doi: 10.1055/s-2008-1064070. quiz 168–159. [DOI] [PubMed] [Google Scholar]
- Nasir SM, Ostry DJ. Speech motor learning in profoundly deaf adults. Nat Neurosci. 2008;11(10):1217–1222. doi: 10.1038/nn.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Soli SD, Sullivan JA. Development of the Hearing in Noise Test for the measurement of speech reception thresholds in quiet and in noise. J Acoust Soc Am. 1994;95(2):1085–1099. doi: 10.1121/1.408469. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Forkstam C, Petersson KM, Cabeza R, Ingvar M. Brain imaging of human memory systems: between-systems similarities and within-system differences. Brain Res Cogn Brain Res. 2002;13(2):281–292. doi: 10.1016/s0926-6410(02)00052-6. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Salami A, Andersson M, Eriksson J, Kalpouzos G, Kauppi K, Nilsson LG. Longitudinal evidence for diminished frontal cortex function in aging. Proc Natl Acad Sci U S A. 2010;107(52):22682–22686. doi: 10.1073/pnas.1012651108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Smith KR, Humphries C, Hickok G. Word length modulates neural activity in auditory cortex during covert object naming. Neuroreport. 2003;14(18):2323–2326. doi: 10.1097/00001756-200312190-00007. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pa J, Hickok G. A parietal-temporal sensory-motor integration area for the human vocal tract: evidence from an fMRI study of skilled musicians. Neuropsychologia. 2008;46(1):362–368. doi: 10.1016/j.neuropsychologia.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Grossman M, Wingfield A. Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci. 2011;31(35):12638–12643. doi: 10.1523/JNEUROSCI.2559-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkell J, Lane H, Svirsky M, Webster J. Speech of cochlear implant patients: a longitudinal study of vowel production. J Acoust Soc Am. 1992;91(5):2961–2978. doi: 10.1121/1.402932. [DOI] [PubMed] [Google Scholar]
- Petrini K, Pollick FE, Dahl S, McAleer P, McKay LS, Rocchesso D, Puce A. Action expertise reduces brain activity for audiovisual matching actions: an fMRI study with expert drummers. Neuroimage. 2011;56(3):1480–1492. doi: 10.1016/j.neuroimage.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Plath P. Speech recognition in the elderly. Acta Otolaryngology. 1991;476(Suppl):127–130. doi: 10.3109/00016489109127266. [DOI] [PubMed] [Google Scholar]
- Plomp R, Mimpen AM. Speech-reception threshold for sentences as a function of age and noise level. J Acoust Soc Am. 1979;66(5):1333–1342. doi: 10.1121/1.383554. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008a;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008b;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Puce A, Syngeniotis A, Thompson JC, Abbott DF, Wheaton KJ, Castiello U. The human temporal lobe integrates facial form and motion: evidence from fMRI and ERP studies. Neuroimage. 2003;19(3):861–869. doi: 10.1016/s1053-8119(03)00189-7. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Huss M, Kherif F, Moscoso del Prado Martin F, Hauk O, Shtyrov Y. Motor cortex maps articulatory features of speech sounds. Proceedings of the National Academy of Sciences. 2006;103(20):7865–7870. doi: 10.1073/pnas.0509989103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(385):385–401. [Google Scholar]
- Ramig LA. Effects of physiological aging on speaking and reading rates. J Commun Disord. 1983a;16(3):217–226. doi: 10.1016/0021-9924(83)90035-7. [DOI] [PubMed] [Google Scholar]
- Ramig LA. Effects of physiological aging on vowel spectral noise. J Gerontol. 1983b;38(2):223–225. doi: 10.1093/geronj/38.2.223. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12(1):95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Raz N, Kennedy KM. A systems approach to the aging brain: neuroanatomic changes, thir modifiers, and cognitive correlates. In: Jagust WJ, D’Esposito M, editors. Imaging the aging brain. Oxford: Oxford University Press; 2009. pp. 43–70. [Google Scholar]
- Ries PW. Prevalence and characteristics of persons with hearing trouble: United States, 1990–91. Vital Health Stat. 1994;10(188):1–75. [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalography and Clinical Neurophysiology. 1998;106:283–296. doi: 10.1016/s0013-4694(98)00022-4. [DOI] [PubMed] [Google Scholar]
- Rossion B, Hanseeuw B, Dricot L. Defining face perception areas in the human brain: a large-scale factorial fMRI face localizer analysis. Brain Cogn. 2012;79(2):138–157. doi: 10.1016/j.bandc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Ryan WJ, Burk KW. Perceptual and acoustic correlates of aging in the speech of males. J Commun Disord. 1974;7(2):181–192. doi: 10.1016/0021-9924(74)90030-6. [DOI] [PubMed] [Google Scholar]
- Satz P. Brain reserve capacity on symptom onset after brain injury: A formulation and review of evidence for threshold theory. Neuropsychology. 1993;7(3):273–295. [Google Scholar]
- Schwartz J, Basirat ALM, Sato M. The Perception-for-Action-Control Theory (PACT): A perceptuo-motor theory of speech perception. Journal of Neurolinguistics 2010 [Google Scholar]
- Sheppard JP, Wang JP, Wong PC. Large-scale cortical functional organization and speech perception across the lifespan. PLoS ONE. 2011;6(1):e16510. doi: 10.1371/journal.pone.0016510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7(4):422–445. [PubMed] [Google Scholar]
- Skullerud K. Variations in the size of the human brain. Influence of age, sex, body length, body mass index, alcoholism, Alzheimer changes, and cerebral atherosclerosis. Acta Neurol Scand Suppl. 1985;102:1–94. [PubMed] [Google Scholar]
- Sommers MS, Tye-Murray N, Spehar B. Auditory-visual speech perception and auditory-visual enhancement in normal-hearing younger and older adults. Ear Hear. 2005;26(3):263–275. doi: 10.1097/00003446-200506000-00003. [DOI] [PubMed] [Google Scholar]
- Soros P, Bose A, Sokoloff LG, Graham SJ, Stuss DT. Age-related changes in the functional neuroanatomy of overt speech production. Neurobiol Aging. 2011;32(8):1505–1513. doi: 10.1016/j.neurobiolaging.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffener J, Stern Y. Exploring the neural basis of cognitive reserve in aging. Biochim Biophys Acta. 2012;1822(3):467–473. doi: 10.1016/j.bbadis.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–460. [PubMed] [Google Scholar]
- Stern Y. The concept of cognitive reserve: a catalyst for research. J Clin Exp Neuropsychol. 2003;25(5):589–593. doi: 10.1076/jcen.25.5.589.14571. [DOI] [PubMed] [Google Scholar]
- Strouse A, Ashmead DH, Ohde RN, Grantham DW. Temporal processing in the aging auditory system. J Acoust Soc Am. 1998;104(4):2385–2399. doi: 10.1121/1.423748. [DOI] [PubMed] [Google Scholar]
- Summerfield Q. Use of visual information for phonetic perception. Phonetica. 1979;36(4–5):314–331. doi: 10.1159/000259969. [DOI] [PubMed] [Google Scholar]
- Szenkovits G, Peelle JE, Norris D, Davis MH. Individual differences in premotor and motor recruitment during speech perception. Neuropsychologia. 2012;50(7):1380–1392. doi: 10.1016/j.neuropsychologia.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Takeda S, Matsuzawa T. Brain atrophy during aging: a quantitative study using computed tomography. J Am Geriatr Soc. 1984;32(7):520–524. doi: 10.1111/j.1532-5415.1984.tb02237.x. [DOI] [PubMed] [Google Scholar]
- Takeda S, Matsuzawa T, Ito H. Atrophy of gray and white matters in the brain during aging: a quantitative study with computed tomography. Sci Rep Res Inst Tohoku Univ Med. 1984;31(1–4):38–41. [PubMed] [Google Scholar]
- Tatsumi IF, Fushimi T, Sadato N, Kawashima R, Yokoyama E, Kanno I, Senda M. Verb generation in Japanese--A multicenter PET activation study. Neuroimage. 1999;9(1):154–164. doi: 10.1006/nimg.1998.0382. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Piskosz M, Souza P. Aging alters the neural representation of speech cues. Neuroreport. 2002;13(15):1865–1870. doi: 10.1097/00001756-200210280-00007. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Baroni M, Hasson U. Processing of speech and non-speech sounds in the supratemporal plane: Auditory input preference does not predict sensitivity to statistical structure. Neuroimage. 2012;66C:318–332. doi: 10.1016/j.neuroimage.2012.10.055. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Deschamps I, Gracco VL. Regional heterogeneity in the processing and the production of speech in the human planum temporale. Cortex. 2011 doi: 10.1016/j.cortex.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Dick AS, Small SL. Functional and structural aging of the speech sensorimotor neural system: fMRI evidence. doi: 10.1016/j.neurobiolaging.2013.02.004. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay P, Gracco VL. Contribution of the frontal lobe to externally and internally specified verbal responses: fMRI evidence. Neuroimage. 2006;33(3):947–957. doi: 10.1016/j.neuroimage.2006.07.041. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Gracco VL. Contribution of the pre-SMA to the production of words and non-speech oral motor gestures, as revealed by repetitive transcranial magnetic stimulation (rTMS) Brain Res. 2009;1268:112–124. doi: 10.1016/j.brainres.2009.02.076. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Gracco VL. On the selection of words and oral motor responses: evidence of a response-independent fronto-parietal network. Cortex. 2010;46(1):15–28. doi: 10.1016/j.cortex.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Small SL. From language comprehension to action understanding and back again. Cerebral Cortex. 2011a;21(5):1166–1177. doi: 10.1093/cercor/bhq189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay P, Small SL. Motor response selection in overt sentence production: a functional MRI study. Frontiers in psychology. 2011b;2:253. doi: 10.3389/fpsyg.2011.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay P, Small SL. On the context-dependent nature of the contribution of the ventral premotor cortex to speech perception. Neuroimage. 2011c;57(4):1561–1571. doi: 10.1016/j.neuroimage.2011.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S, Shiller DM, Ostry DJ. Somatosensory basis of speech production. Nature. 2003;423(6942):866–869. doi: 10.1038/nature01710. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26(9):1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. discussion 1275–1268. [DOI] [PubMed] [Google Scholar]
- Wei G, Zhang Y, Jiang T, Luo J. Increased cortical thickness in sports experts: a comparison of diving players with the controls. PLoS ONE. 2011;6(2):e17112. doi: 10.1371/journal.pone.0017112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesmann M, Ishai A. Expertise reduces neural cost but does not modulate repetition suppression. Cogn Neurosci. 2011;2(1):57–65. doi: 10.1080/17588928.2010.525628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Saygin AP, Sereno MI, Iacoboni M. Listening to speech activates motor areas involved in speech production. Nature Neuroscience. 2004;7(7):701–702. doi: 10.1038/nn1263. [DOI] [PubMed] [Google Scholar]
- Winneke AH, Phillips NA. Does audiovisual speech offer a fountain of youth for old ears? An event-related brain potential study of age differences in audiovisual speech perception. Psychol Aging. 2011;26(2):427–438. doi: 10.1037/a0021683. [DOI] [PubMed] [Google Scholar]
- Wise RJ, Scott SK, Blank SC, Mummery CJ, Murphy K, Warburton EA. Separate neural subsystems within ‘Wernicke’s area’. Brain. 2001;124(Pt 1):83–95. doi: 10.1093/brain/124.1.83. [DOI] [PubMed] [Google Scholar]
- Wong PC, Ettlinger M, Sheppard JP, Gunasekera GM, Dhar S. Neuroanatomical characteristics and speech perception in noise in older adults. Ear Hear. 2010;31(4):471–479. doi: 10.1097/AUD.0b013e3181d709c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PC, Jin JX, Gunasekera GM, Abel R, Lee ER, Dhar S. Aging and cortical mechanisms of speech perception in noise. Neuropsychologia. 2009;47(3):693–703. doi: 10.1016/j.neuropsychologia.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollett K, Glensman J, Maguire EA. Non-spatial expertise and hippocampal gray matter volume in humans. Hippocampus. 2008;18(10):981–984. doi: 10.1002/hipo.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Working Group on Speech Understanding and Aging and Committee on Hearing B Biomechanics. Speech understanding and aging. J Acoust Soc Am. 1988;83(3):859–895. [PubMed] [Google Scholar]
- Yamaura H, Ito M, Kubota K, Matsuzawa T. Brain atrophy during aging: a quantitative study with computed tomography. J Gerontol. 1980;35(4):492–498. doi: 10.1093/geronj/35.4.492. [DOI] [PubMed] [Google Scholar]
- Yovel G, Kanwisher N. Face perception: domain specific, not process specific. Neuron. 2004;44(5):889–898. doi: 10.1016/j.neuron.2004.11.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Panel A. Illustration of the process of measuring cortical thickness. Panel B. The conceptual model that was used for the multiple mediation analyses. In this model, the dependent (y) variables were the signal magnitude in Perception and in Production (each tested separately) for each ROIs, while the independent (x) variable was the categorical variable Age (young, older). Four mediator and three covariates (PTA, sex and education) were also included in the model. The mediators were the ROI left and right average gray matter volume and surface based cortical thickness.
Result of the multiple mediation analysis for the occipital ROIs. A. Mediations analyses with regional morphometric measurements as mediator variables. B. Mediations analyses with global (whole hemisphere) morphometric measurements as mediator variables. Significant paths are coloured (see legend on the figure).