Abstract
Crizotinib (PF02341066) is a tyrosine kinase inhibitor of ALK that has been shown to selectively inhibit growth of cancer cells that harbor the EML4-ALK fusion, found in a subset of patients with non-small cell lung cancer (NSCLC). While in clinical trials PF02341066 has shown a significant therapeutic benefit as a single agent, the effectiveness of combining it with other therapeutic modalities including ionizing radiation remains unknown. To further elucidate the role of PF02341066 in tumor inhibition, we examined its effects alone and in combination with radiation on downstream signaling, apoptosis, and radiosensitivity in two NSCLC cell lines in vitro: H3122, which harbors the EML4-ALK fusion; and H460, which does not. We also examined the in vivo effects of PF02341066 in H3122 mouse xenografts. In the H3122 cell line, PF02341066 inhibited phosphorylation of ALK and its downstream effectors: AKT, ERK, and STAT3. H3122 cells treated with a combination of PF02341066 and radiation showed an increase in cellular apoptosis and were sensitized to radiation therapy (dose enhancement ratio, 1.43; p < 0.0001). Moreover, in a H3122 xenograft model, the combined treatment resulted in greater tumor growth inhibition than either treatment alone (p < 0.05). None of these effects was observed in the EML4-ALK-negative H460 cells. Our findings indicate that PF02341066 acts as a radiation sensitizer in cells harboring the EML4-ALK fusion, providing a rationale for a clinical trial combining ALK inhibitor with radiation in the NSCLC expressing ALK.
Keywords: Lung cancer, Tyrosine kinase inhibitor, PF02341066, EML4-ALK, radiation sensitizer
Introduction
Lung cancer is the leading cause of cancer-related mortality in the United States and the world (1). Despite recent advances in the treatment of this disease, the 5-year overall survival rate in the United States remains only 15%. Therefore, novel therapeutic strategies are urgently needed. Recently, the focus of new drug development has concentrated on target-based therapies as potential alternatives to conventional chemotherapeutic agents. For example, activating mutations in the tyrosine kinase domain of the epidermal growth factor receptor (EGFR) are associated with tumor responsiveness to EGFR tyrosine kinase inhibitors (TKIs) in a subset of individuals with non–small cell lung cancer (NSCLC) (2–4). These findings suggest that molecular targeted therapy may prove to be an effective strategy in other genetically-defined subsets of NSCLC patients. Treatment of these relatively small subpopulations of patients harboring genetic abnormalities translates into a large number of overall patients treated because of the high prevalence of the disease.
The echinoderm microtubule-associated protein-like 4 – anaplastic lymphoma kinase (EML4-ALK) is an oncoprotein found in 4 to 7% of NSCLCs (5–7) that leads to constitutive activation of the ALK tyrosine kinase. Constitutive ALK activation results in the development of tumorigenic activity through activation of downstream signaling targets, including Akt, signal transducer and activator of transcription 3 (STAT3), and extracellular regulated kinase (ERK1/2). In NSCLC, EML4-ALK fusion has been shown to be mutually exclusive with EGFR or Kirsten rat sarcoma (KRAS) mutations (8), but not mutations in human epidermal growth factor receptor 2 (HER2) (9). The EML4-ALK fusion is typically detected in young patients without a significant smoking history (i.e. ≤ 10 pack years). Moreover, EML4-ALK positive NSCLC is more commonly classified as adenocarcinoma with signet ring cells, providing methods to possibly preselect patients both clinically and histologically for targeted ALK therapy (10). Thus, EML4-ALK is a unique biomarker for diagnosis and treatment of certain NSCLCs.
In a recent retrospective study, patients with EML4-ALK fusion showed similar response rates to platinum-based combination chemotherapy and no difference in overall survival when compared to patients without EML4-ALK (10). ALK inhibitors have been found to suppress the growth and to induce apoptosis in EML4-ALK–positive lung cancer cells, suggesting that ALK inhibition is a potential strategy for the treatment of NSCLC patients with this fusion protein (9, 11, 12). A selective inhibitor of the kinase activity of ALK, PF02341066 (crizotinib/Xalkori), is currently undergoing clinical trials and has demonstrated significant clinical efficacy in NSCLC patients with the EML4-ALK fusion (13). However, the exact effects of PF02341066 on the downstream signaling pathways that regulate the proliferation or survival of EML4-ALK–positive lung cancer cells remain to be established, and the combination of effects from ALK inhibitors and ionizing radiation has not been addressed. Given the therapeutic potential of the ALK inhibitor PF02341066, we hypothesized that combining this agent with radiation would result in increased tumor inhibition compared to either agent alone. We used the EML4-ALK-positive H3122 human lung cancer cell line in vitro and a xenograft model in vivo to examine how PF02341066 affects EML4-ALK downstream signaling and its potential as a novel radiosensitizing agent in NSCLC.
Materials and Methods
Cell culture and reagents
The human NSCLC cell line NCI-H460 (H460) was obtained from the American Type Culture Collection (Manassas, VA) and were authenticated by STR assay two months before experiments. The H3122 and H2288 cell lines were kindly provided by Dr. William Pao at Vanderbilt University (Nashville, TN); these cell lines were not authenticated, but purchased from the American Type Culture Collection (Manassas, VA) within six months of the experiments. The cells were cultured in an environment of 5% CO2 at 37°C in RPMI 1640 (Invitrogen; Carlsbad, CA) supplemented with 10% fetal bovine serum. PF02341066 (ChemieTek, Inc.; Indianapolis, IN.) was dissolved in DMSO.
Cell viability assay
MTS assays were performed using tetrazolium based CellTiter 96® AQueous One Solution Cell Proliferation assay (Promega; Fitchburg, WI). H3122, H460, and H2228 cells were seeded in 96 well plates at 3,000 cells/well. Cells were treated with various concentrations of PF02341066 one day after plating. MTS assay was performed at 24 h, 48 h, and 72 h after treatment with PF02341066.
Western blot analysis
Cells were washed twice with ice-cold PBS and then lysed in M-Per mammalian lysis buffer (Thermo Scientific; Waltham, MA). The protein concentration of the lysates was determined with the Bradford reagent (Bio Rad; Hercules, CA) and equal amounts of protein were subjected to SDS-PAGE of a 10% or 15% gel. Separated proteins were transferred to a nitrocellulose membrane, which was then exposed to 5% non-fat dried milk in TBS containing 0.1% Tween 20 (0.1% TBST) for 1 h at room temperature and incubated overnight at 4 °C with antibodies against caspase-3, phospho-ALK (Tyr1278/1282/1283; p-ALK), total ALK (T-ALK), phospho-AKT (p-AKT), total AKT (T-AKT), phospho-STAT3 (p-STAT3), C-Met, phospho-C-met (phospho-C-Met [p-Met]) (Cell Signaling Technology; Danvers, MA), phospho-STAT3 (p-STAT3), total STAT3 (T-STAT3), phospho-ERK (p-ERK), total ERK (T-ERK; Santa Cruz Biotechnology; Santa Cruz, CA), actin, or tubulin (Sigma-Aldrich; St. Louis, MO). The membranes were then washed with 0.1% TBST before incubation with horseradish peroxidase–conjugated goat antibodies to rabbit or mouse (Santa Cruz Biotechnology). Immune complexes were detected with chemiluminescence reagents (Perkin-Elmer; Waltham, MA Life Science).
Clonogenic survival assay
Exponentially growing cells in a 100 mm dish were trypsinized and counted. Cells were diluted serially to appropriate densities and plated in triplicate in 60 mm dishes containing 5 mL of complete medium, in the presence of 0.4 µM PF02341066 or vehicle (final DMSO concentration of 0.1%; we confirmed that this DMSO concentration did not affect the proliferation of NSCLC cell lines). After incubation for 2 h, the cells were irradiated using a 137Cs irradiator (J.L. Shepherd and Associates, Glendale, CA, USA) at room temperature. The dose rate was 1.8 Gy/min and dose range was 0 to 6 Gy. After irradiation for 48 hours, the cells were washed with PBS, cultured in drug-free medium for 7 to 8 d, fixed with 70% ethanol, and stained with 0.5% crystal violet (Sigma). Colonies containing > 50 cells were counted. After correcting for drug toxicity, the dose enhancement ratio (DER) was calculated as the radiation dose that yielded a surviving fraction of 0.3 for vehicle-treated cells divided by that for PF02341066-treated cells.
Animals and tumor xenograft assay
All animal studies were approved and handled following Institutional Animal Care and Use Committee (IACUC) guidelines (IACUC-approved protocol M/08/095). Female athymic nude mice (5–6 weeks old) were purchased from Harlan Laboratories (Indianapolis, IN). Exponentially growing parental H3122 cells were trypsinized and washed with PBS and diluted into 1 × 106 cells per 100 µL PBS. The cell suspension was injected subcutaneously into the right flank of mice using a 1-cc syringe with 27½-gauge needle. Tumors were grown for 6 to 8 days until the average tumor volume reached 0.25 cm3. Treatment groups consisted of vehicle control, PF02341066, radiation, and PF02341066 combined with radiation. Each treatment group contained 5 mice. PF02341066 was administered orally at doses of 100 mg/kg for 7 consecutive days. Mice in radiation groups were irradiated 1 hr after PF02341066 treatment with 2 Gy daily over 5 consecutive days. Tumors on the flanks of the mice were irradiated using an X-ray irradiator (Therapax, Agfa NDT, Inc., Lewis Town, PA). The non-tumor parts of the mice were shielded with lead blocks. Tumors were measured two to three times weekly in three perpendicular dimensions using a Vernier caliper. Tumor volumes were calculated using the modified ellipse volume formula (Volume = (Height × Width × Depth)/2).
Histological sections, Ki-67 and active caspase-3
Mice were implanted with H3122 lung cancer cells as described above in the tumor volume studies. After 6 to 8 days, mice in the drug treatment group were orally treated with 100 mg/kg PF02341066 daily for 7 days, with or without radiation as described above in the tumor volume studies. After 7 days of daily treatments all tumors were resected and fixed in paraffin. Staining for Ki67 (a marker of proliferation) and active caspase-3 was performed in the Vanderbilt University Pathology Core laboratory using standard protocols. The number of positive cells per 400X field were scored and graphed by averaging three repeated assessments.
Statistical Analysis
Student’s t-test was utilized to determine the significance between groups. Statistical analysis was performed with Microsoft Excel. Standard error in all measured biological parameters is displayed in the appropriate figures. Significance was defined at the level of P < 0.05.
Results
PF02341066 inhibits ALK phosphorylation and downstream signaling in H3122 cells
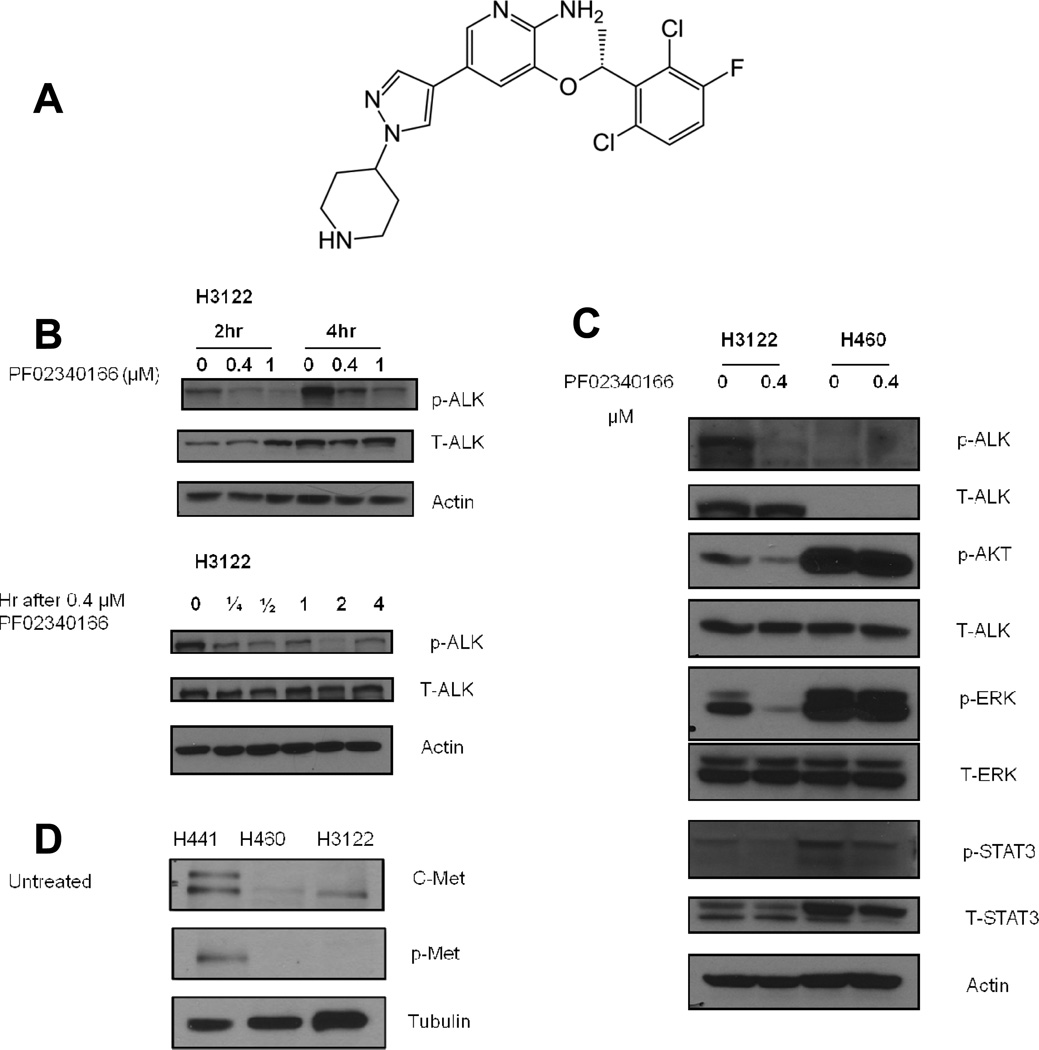
In order to establish the effects of PF02341066 (structure in Figure 1A) on downstream signaling of EML4-ALK, H3122 cells (which harbor the EML4-ALK fusion) and H460 cells (which are EML4-ALK negative (14–16) and therefore act as a negative control) were treated with PF02341066. Following the treatment, cell lysates from both cell lines were analyzed by Western blot. In H3122 cells a reduction in ALK autophosphorylation was observed in a dose dependent manner at 2 and 4 hours following treatment whereas no ALK protein was detected in H460 cells (data not shown). H3122 cells treated with 0.4 µM PF02341066 exhibited a decrease in phosphorylated ALK as early as 15 minutes following treatment (Figure 1B). Since AKT, ERK and STAT3 have been reported as downstream effectors of ALK, their levels were also analyzed following treatment with PF02341066. In the H3122 cell line, substantial inhibition of AKT, ERK1/2 and STAT3 phosphorylation was observed compared to control; in contrast, none of these molecules was inhibited by PF02341066 in H460 cells (Figure 1C).
Figure 1. PF02341066 inhibits ALK signaling in a dose- and time- dependent manner in H3122 cells but not H460 cells.
(1A) The molecular structure of the ALK inhibitor PF02341066 (crizotinib).
(1B) H3122 cells were treated with 0.4 and 1 µM PF02340122. The results of Western blots at 2 and 4 hours using labeled antibodies to phospho- and total ALK are shown. Next, H3122 cells were treated with 0.4 µM PF02340122. Cells were collected at the indicated times. The results of Western blots using labeled antibodies to phospho- and total ALK are shown.
(1C) H3122 cells and H460 cells were treated with 0.4 µM PF02340122. Cells were collected 2 hours after treatment. Western blots using labeled antibodies to phospho- and total ALK, phospho- and total STAT3, phospho- and total Akt, phospho- and total ERK are shown. Actin was probed to demonstrate equal loading.
(1D) PF02341066 causes radiosensitization of H3122 and H460 cells through ALK inhibition. Cell lysates of untreated H441, H460, and H3122 cells were analyzed by Western blot using labeled antibodies to phospho-Met and C-Met. Tubulin was probed to demonstrate equal loading.
Radiosensitization of H3122 cells by PF02341066 is due to ALK inhibition
PF02341066 is an inhibitor of the C-Met proto-oncogene, which encodes the hepatocyte growth factor receptor (HGFR) and can drive invasive growth of cancer cells (17). To investigate whether the radiosensitizing activity of PF0234016 was due to its effects as an ALK inhibitor or as a C-Met inhibitor, cell lysates of untreated H3122 and H460 cells were analyzed by Western blot. The cell line H441, which demonstrates high expression of C-Met and does not harbor the EML4-ALK fusion, served as the positive control. In both H3122 and H460 cells there was little expression of C-Met and no expression of p-Met before treatment with PF02341066 (Figure 1D).
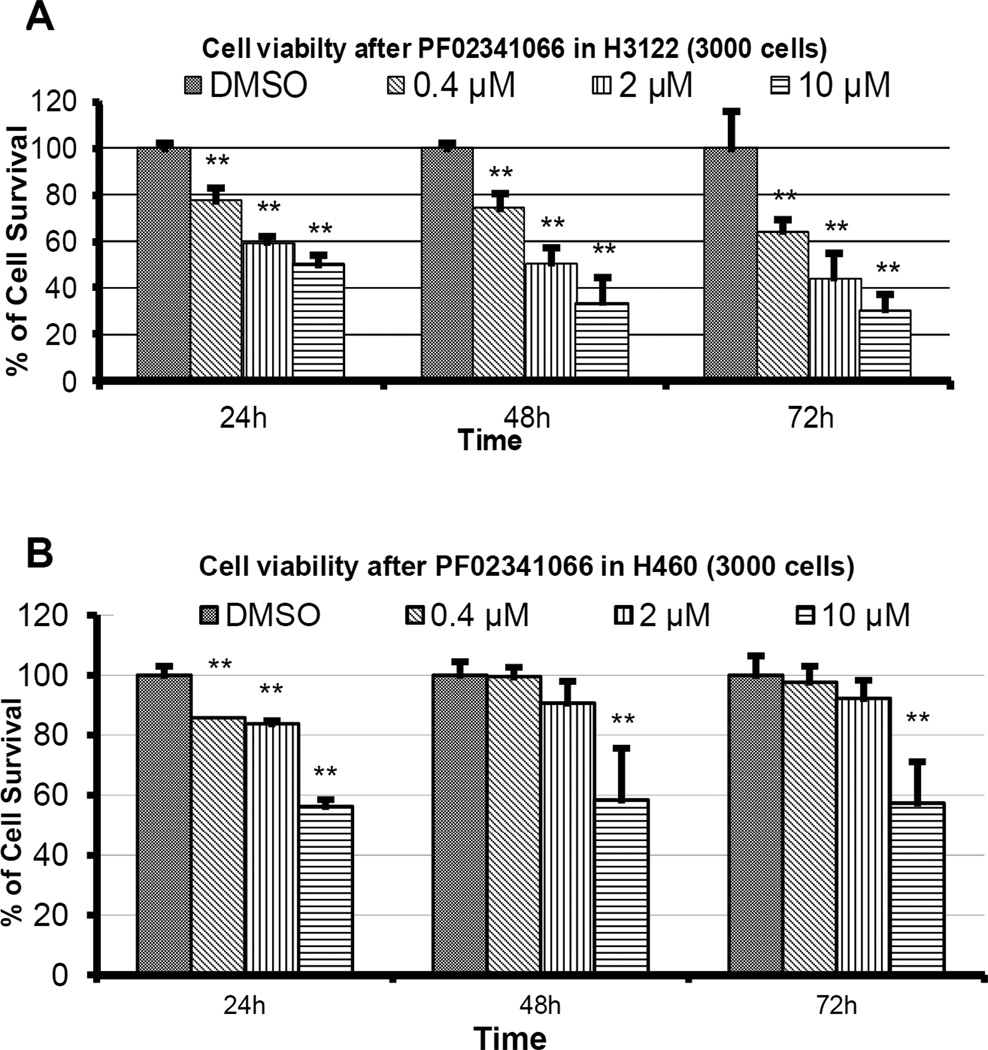
PF02341066 inhibits H3122 growth in a dose-dependent manner
To determine the cellular response to PF02341066, a cell viability MTS assay was performed on H3122 and H460 cells previously treated with increasing concentrations of PF02341066. The viability of H3122 cells decreased significantly in a dose-dependent manner at 24h, 48h, and 72h following treatment (p < 0.05). At 72 h, cell viabilities of H3122 cells treated with 0.4, 2, and 10 µM PF02341066 were 64.4%, 44.7%, and 30.9%, respectively (Figure 2A). At 72h, H460 cells had a 90% viability at 0.4 and 2 µM PF02341066 and showed a significant decrease in cell viability at 58%, the highest concentration of PF02341066 (p < 0.01; Figure 2B).
Figure 2. PF02341066 inhibits H3122 growth in a dose-dependent manner and it inhibits H460 growth to a lesser extent.
H3122 (2A) and H460 (2B) lung cancer cells were seeded at 3,000/well in 96-well plate and allowed to attach overnight in triplicate. Cells were then exposed to various concentrations of PF02341066. MTS assay was performed after 24 hr, 48 hr and 72 hr. Bar graphs of mean survival fractions (with respective confidence intervals) of each treatment group relative to control are shown. Note: ** denotes p < 0.01 compared to control.
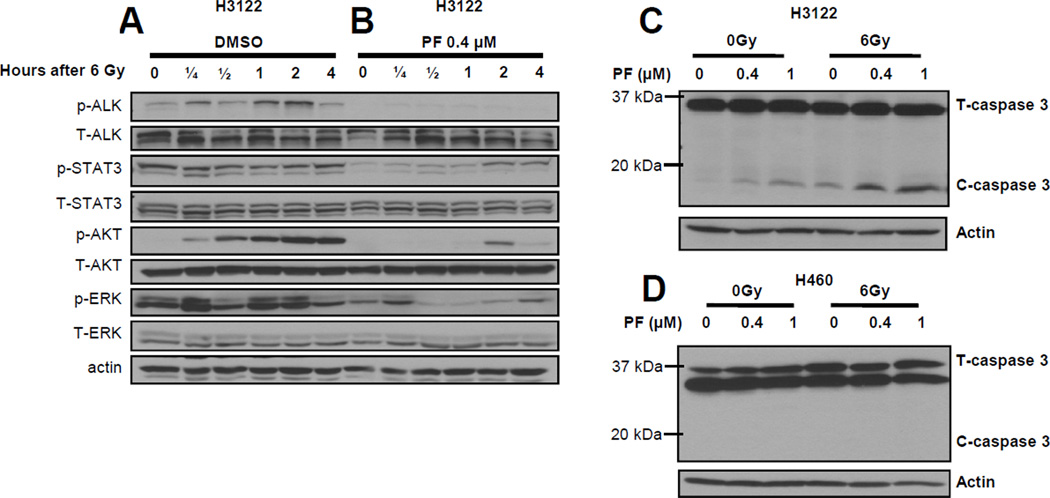
PF02341066 and radiation induce apoptosis in H3122 cells
We next examined the effects of radiation on ALK downstream effectors and on apoptosis marker caspase 3. Following treatment with 6 Gy radiation, lysates from H3122 cells were probed with antibodies to the active p-ALK, p-AKT, p-STAT3 and p-ERK. In the presence of PF02341066, following RT, we found that ALK was no longer activated, and the activation of AKT and ERK were attenuated (Figure 3B). Following radiation, H3122 cells treated with PF02341066 showed increased levels of cleaved caspase 3 compared to cells that were not treated with PF02341066, indicating a combined induction of apoptosis in these cells (Figure 3C). In H460 cells, no cleaved caspase 3 was detected when treated with PF02341066 alone or in combination with radiation (Figure 3D).
Figure 3. Combined treatment of PF02341066 and radiation induces more apoptosis in H3122 cells than either agent alone.
H3122 and H460 cells were treated with 6 Gy radiation. Western blots at 0, 0.25, 0.5, 1, 2, and 4 hours after radiation treatment using labeled antibodies to phospho- and total ALK, phospho- and total STAT3, phospho- and total Akt, phospho- and total ERK are shown for H3122 cells (3A) in DMSO and (3B) in 0.4 µM PF02341066. H3122 (3C) and H460 (3D) cells were treated with 0, 0.4, and 1 µM PF02341066 for 2 hours followed by radiation treatment with 0 or 6 Gy. Expression of total caspase-3, and cleaved caspase-3 was determined by immunoblotting 48 h post radiation treatment. For all experiments, actin was probed to demonstrate equal loading.
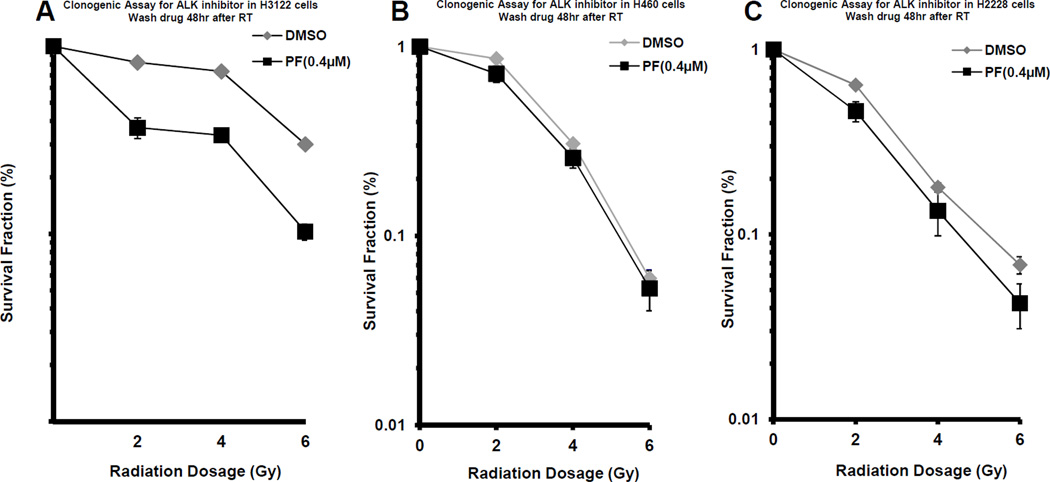
PF02341066 sensitizes H3122 cells to radiation treatment
To determine whether the inhibition of ALK signaling sensitizes cells to radiation, a clonogenic assay was used to investigate the combined effects of PF02341066 and radiation on H3122 and H460 cells. H3122 cells exposed to 0.4 µM PF02341066 were more sensitive to radiation (DER 1.43; p < 0.0001; Figure 4A). H460 cells treated with PF02341066 did not exhibit radiation sensitization (DER = 1.11; p>0.05; Figure 4B). H2228 cells, which are also positive for EML4-ALK (17), were exposed to 0.4 µM PF02341066 and were more sensitive to radiation (DER 1.23; p < 0.008; Figure 4C). This suggests that ALK signaling blockade leads to sensitization to radiation treatment in EML4-ALK mutant cell lines.
Figure 4. Radiosensitization of H3122, H460, and H2228 lung cancer cells by PF02341066.
H3122 (4A), H460 (4B), and H2228 (4C) cells were treated with 0.4 µM PF02341066 or DMSO for 2 hours followed by irradiation with the indicated doses. Forty-eight hours after radiation, drug-containing media was replaced with fresh media. After 8 days, surviving colonies were stained and scored. Shown are survival curves containing the mean ± standard deviation of three separate, repeated experiments.
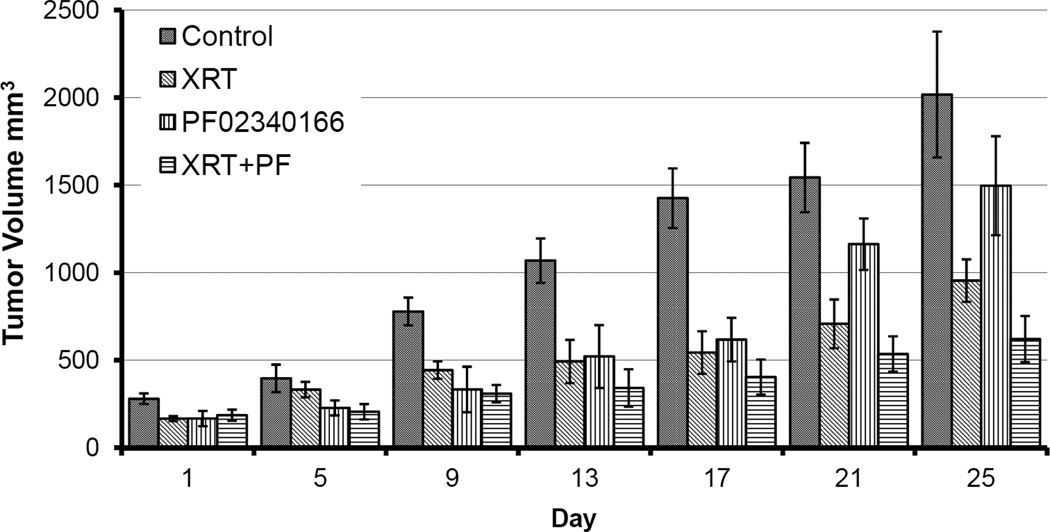
Combination of PF02341066 and radiation inhibits growth of H3122 xenograft tumors
To test whether the radiosensitizing effects of PF02341066 on lung cancer cells in vitro are translated in vivo, tumor growth was assessed in a mouse xenograft model. Mice receiving combination therapy with PF02341066 and radiation showed significant tumor growth inhibition compared with mice receiving radiation alone. Mice treated with PF02341066 alone also exhibited a significant inhibition in tumor growth when compared to no treatment (Figure 5); specifically, the major difference for xenograft tumor growth occurred at day 25. Mice body weights were monitored to assess the tolerability of systemic PF02341066 therapy. Body weight changes over the course of the experiment were minimal in all treatment groups, suggesting that PF02341066 is well tolerated (Supplemental Figure 1).
Figure 5. Combination of PF02341066 and radiation inhibits tumor growth in lung cancer xenograft model.
H3122 lung cancer cells (1 ×106 cells in 100 µl PBS) were injected subcutaneously into athymic nude mice. After 6 to 8 days, mice were treated with vehicle control, PF02341066 (100 mg/kg twice a day for 7 consecutive days), radiotherapy, or combined PF02341066 and radiotherapy. Mice were irradiated 1 hour after PF02341066 treatment with 2 Gy daily for 5 consecutive days. Tumors were measured regularly and were graphed by the days.
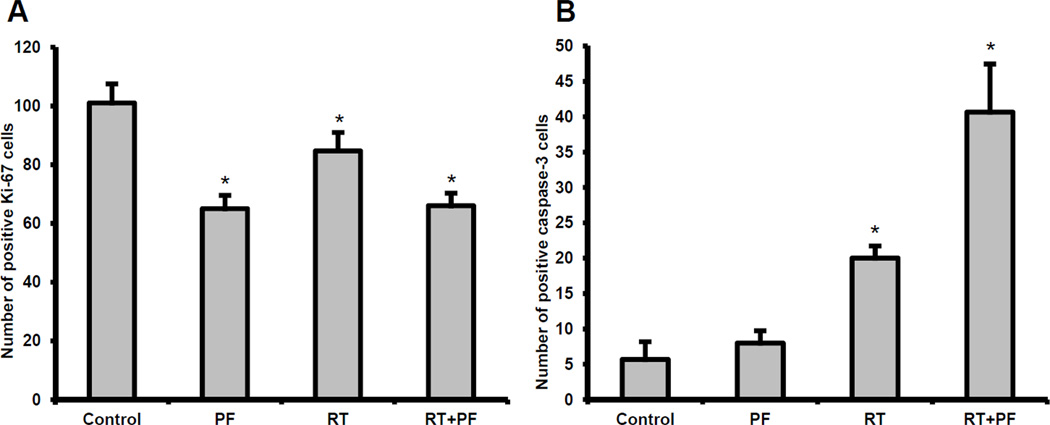
PF02341066 reduces tumor proliferation and increases apoptosis in irradiated lung cancer mouse xenografts
To determine the mechanism that contributes to tumor growth inhibition after combined modality treatment with radiation and PF02341066 in vivo, the Ki-67 proliferation index was used to quantify cellular proliferation in fixed tumor sections. Combined PF02341066 and radiation treatment resulted in an approximately 34.7% reduction of proliferating cells as compared to the untreated control (66 cells versus 101 cells, p < 0.05) and a 34.1% reduction compared with radiation alone (66 cells versus 85 cells, p < 0.05) (Figure 6A). Apoptosis was assessed using active caspase-3 staining. Given that lymphocytes expressing caspase-3 can infiltrate tumor tissue, we cannot exclude a subpopulation of caspase-3-expressing lymphocytes, although we believe that it would be small to negligible. Combined PF02341066 and radiation treatment resulted in 41 apoptotic cells per microscopic high power field, compared to 20 in radiation alone (p < 0.05) and 5.7 in control tumors (p < 0.05) (Figure 6B). Representative photographs for fixed tumor sections stained for cellular proliferation (Ki-67) and apoptosis (active caspase-3) are presented in Supplemental Figures 2 and 3.
Figure 6. PF02341066 with radiation reduces Ki-67 proliferative marker and increases apoptosis in H3122 lung tumor xenograft model.
Histological sections were obtained from the tumors of the mice in each treatment group from the tumor volume study. Staining for Ki-67 was performed to assess cell proliferation and the number of positive cells was scored and graphed by averaging three repeated experiments (6A). Staining for active caspase-3 was performed to measure apoptosis and the number of positive cells was scored and graphed by averaging three repeated experiments (6B). Note: * denotes p < 0.05 compared to control.
Discussion
In the present study, we examined the effects of PF02341066 on ALK signaling and tumor growth used either alone and in combination with ionizing radiation in the H3122 lung cancer cell line expressing the EML4-ALK fusion protein; and in H460 cells, which have no detectable EML4-ALK. To our knowledge, this is the first study investigating the combined effects of radiation and PF02341066. In addition to inhibition of ALK autophosphorylation, PF02341066 inhibited phosphorylation of AKT, STAT3, and ERK1/2 (Figure 1). In a previous study (9), a similar effect was observed with the ALK inhibitor TAE684. These results are consistent with previous evidence and suggest that overexpression of oncogenic EML4-ALK tyrosine kinase in NIH3T3 cells activates ERK and STAT3 signaling pathways; contrarily, knock down of oncogenic EML4-ALK by siRNA abrogates ERK and STAT3 signaling pathways (18).
On a functional level, inhibition of EML4-ALK signaling by PF02341066 led to a decrease in cell survival in treated H3122 cells (Figure 2). While H3122 cells exhibited a significant decrease in viability at all concentrations of PF02341066, cell viability of H460 cells only decreased at the highest concentration of PF02341066 examined (10 µM). H3122 cells in DMSO were irradiated, the levels of p-ALK and p-AKT increased gradually over hours, suggesting that RT activates ALK, AKT, and STAT-3. In the presence of PF02341066, following RT, we found that ALK was no longer activated, and the activation of AKT and ERK were attenuated. This suggests that other signaling activation following radiation may also be involved.
Nonetheless, since all three pathways appear were attenuated by PF02341066 in H3122 cells and since there was no activation of downstream ALK effectors in the H460 cells (Figure 1C), we believe that the EML4-ALK fusion protein is indeed a selective target of PF02341066, while cells that do not express the fusion protein are likely not sensitive to PF02341066 treatment.
Since PF02341066 inhibits both C-Met and ALK, we also investigated if the effects seen in PF02341066-treated H3122 and H460 cells were due to either C-Met inhibition or ALK inhibition. From Western blot analysis, H3122 and H460 cells showed very little C-Met expression and no p-Met expression compared to H441 cells, which had a high level of C-Met expression. Because of the low levels of C-Met expression in H3122 and H460, these two cell lines are good candidates for studying the EML4-ALK gene and the effects of PF02341066. The effects of PF02341066 on H3122 and H460 were due to inhibition of EML4-ALK and not C-Met.
We have previously reported that radiation activates ERK and AKT signaling pathways in H460 cells (19). In the current study, we observed similar activation of these signaling pathways together with the STAT3 pathway in irradiated H3122 cells. Interestingly, we observed that the active basal levels of all three pathways (p-STAT3, p-ERK, and p-AKT) were higher in H460 cells than in H3122 cells (Figure 1C). Because of a higher basal activation of these pathways, one would expect the H460 cells to have a higher resistance to radiation; instead, H460 cells are more sensitive to radiation. Further experimentation will be necessary to elucidate the biological mechanisms responsible for cell signaling in these cell lines.
Since PF02341066 inhibits these pro-survival signaling pathways in H3122 cells, we wanted to investigate whether combined treatment with PF02341066 and ionizing radiation would result in combined inhibition of H3122 tumor cell growth (Figure 4). Administration of PF02341066 and radiation yielded greater inhibition of growth in H3122 cells relative to radiation alone. Additionally, we detected increased levels of cleaved caspase-3 in treated H3122 cells, indicating higher levels of apoptosis (Figure 3C/D). These findings indicate that PF02341066 acts as a radiation sensitizer in vitro in cells harboring EML4-ALK via inhibition of pro-survival signaling pathways and, consequently, induces cell death. Moreover, because H3122 and H460 have such low levels of C-Met expression (Figure 1D), the radiosensitizing effect of PF02341066 is likely due to EML4-ALK inhibition, rather than C-Met inhibition. Given that some of the aforementioned ALK effectors have been implicated in resistance to chemotherapy, it would be interesting to examine whether the combined effect of PF02341066 and radiation would also be observed with platinum-based chemotherapy.
To test whether the combined effects of PF02341066 and radiation observed in vitro would translate to an in vivo model, we examined effects of PF02341066 and radiation in H3122 mouse xenografts. In our in vivo model, PF02341066 alone inhibited tumor growth compared to control, but PF02341066 alone was not as potent as radiation alone in inhibiting tumor growth. Combination of PF02341066 and radiation yielded greater inhibition of tumor growth than either agent did alone; moreover, the major difference for tumor growth occurred at day 25 (Figure 5). These results suggest that PF02341066 is an in vivo radiation sensitizer and provides a rationale for combined PF02341066 and radiation therapy as a strategy to inhibit tumor growth in vivo.
To elucidate the potential mechanisms by which combined PF02341066 and radiation inhibit tumor growth in vivo, we examined tumor sections for markers of cell proliferation and apoptosis (Figure 6). Consistent with our in vitro results, tumors treated with combined PF02341066 and radiation exhibited lower cell proliferation indices and increased levels of apoptosis.
As combined PF02341066 and radiation resulted in increased apoptosis, it would be interesting to examine the effect of PF02341066 on proteins directly controlling cell viability. Tanizaki et al. reported that PF02341066 up-regulated the expression of Bim, a proapoptotic member of the Bcl-2 family, and down-regulated expression of survivin, a member of the inhibitor of apoptosis protein family, in H3122 cells with MET amplification (18). Notably, PF02341066 was initially designed to target C-Met, and was later found to have a dual role in inhibiting m-met/ALK. It would be interesting to see whether the addition of radiation to PF02341066 would modulate these proteins in the apoptotic pathway.
The treatment of lung cancer continues to pose a challenge to physicians because the majority of cases are diagnosed as advanced, non-curable disease; further, certain early-stage patients relapse after local therapy. Although combinations of surgery, radiation, and chemotherapy are successful for certain patients, it is not always clear which patient subsets benefit most from them. Thus, novel personalized therapies that are tailored to the tumor-specific molecular characteristics are urgently needed. Clinical trials have shown a survival benefit of PF02341066 treatment in NSCLC patients with the EML4-ALK fusion. The in vitro and in vivo data from the present study supports the radiosensitizing role of PF02341066 in certain NSCLC and provides rationale for the clinical trial in ALK-expressing NSCLC combining PF02341066 with radiation therapy. Further experiments in different cell lines are necessary to investigate if ALK inhibition is a radiosensitizing approach for other ALK amplified tumors. Additionally, it is necessary further elucidate the mechanisms of how EML4-ALK may be suppressing DNA damage-induced apoptosis (20).
In conclusion, our study shows that PF02341066-mediated EML4-ALK inhibition combined with radiation suppress in vitro and in vivo growth of certain tumor cells expressing the EML4-ALK fusion protein. This data supports the use of combined treatment with PF02341066 and radiation to selectively target a subset of NSCLC expressing the EML4-ALK fusion and suggests that the combined treatment may prove effective in future clinical trials in NSCLC patients.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. William Pao and Dr. Christina Lovly for the cell line and reagents.
Financial support: Bo Lu: NCI 1R01 CA125842-01A1, NCI P30 CA56036. Yunguang Sun: National Center for Research Resources Vanderbilt CTSA Grant UL1 RR024975
Footnotes
Conflicts of interest: The authors report that they have no conflicts of interest.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 3.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 4.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horn L, Pao W. EML4-ALK: honing in on a new target in non-small-cell lung cancer. J Clin Oncol. 2009;27:4232–4235. doi: 10.1200/JCO.2009.23.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martelli MP, Sozzi G, Hernandez L, Pettirossi V, Navarro A, Conte D, et al. EML4-ALK rearrangement in non-small cell lung cancer and non-tumor lung tissues. Am J Pathol. 2009;174:661–670. doi: 10.2353/ajpath.2009.080755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 8.Inamura K, Takeuchi K, Togashi Y, Nomura K, Ninomiya H, Okui M, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol. 2008;3:13–17. doi: 10.1097/JTO.0b013e31815e8b60. [DOI] [PubMed] [Google Scholar]
- 9.Koivunen JP, Mermel C, Zejnullahu K, Murphy C, Lifshits E, Holmes AJ, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14:4275–4283. doi: 10.1158/1078-0432.CCR-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247–4253. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soda M, Takada S, Takeuchi K, Choi YL, Enomoto M, Ueno T, et al. A mouse model for EML4-ALK-positive lung cancer. Proc Natl Acad Sci U S A. 2008;105:19893–19897. doi: 10.1073/pnas.0805381105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakamoto H, Tsukaguchi T, Hiroshima S, Kodama T, Kobayashi T, Fukami TA, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell. 2011;19:679–690. doi: 10.1016/j.ccr.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Kwak EL, Bang Y-J, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic Lymphoma Kinase Inhibition in Non,ÄìSmall-Cell Lung Cancer. New England Journal of Medicine. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Ye X, Liu J, Zha J, Pei L. Evaluation of EML4-ALK fusion proteins in non-small cell lung cancer using small molecule inhibitors. Neoplasia. 2011;13:1–11. doi: 10.1593/neo.101120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katayama R, Khan TM, Benes C, Lifshits E, Ebi H, Rivera VM, et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proceedings of the National Academy of Sciences. 2011;108:7535–7540. doi: 10.1073/pnas.1019559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDermott U, Iafrate AJ, Gray NS, Shioda T, Classon M, Maheswaran S, et al. Genomic Alterations of Anaplastic Lymphoma Kinase May Sensitize Tumors to Anaplastic Lymphoma Kinase Inhibitors. Cancer Research. 2008;68:3389–3395. doi: 10.1158/0008-5472.CAN-07-6186. [DOI] [PubMed] [Google Scholar]
- 17.Lovly CM, Heuckmann JM, de Stanchina E, Chen H, Thomas RK, Liang C, et al. Insights into ALK-driven cancers revealed through development of novel ALK tyrosine kinase inhibitors. Cancer Res. 2011;71:4920–4931. doi: 10.1158/0008-5472.CAN-10-3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanizaki J, Okamoto I, Okamoto K, Takezawa K, Kuwata K, Yamaguchi H, et al. MET Tyrosine Kinase Inhibitor Crizotinib (PF-02341066) Shows Differential Antitumor Effects in Non-small Cell Lung Cancer According to MET Alterations. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6:1624–1631. doi: 10.1097/JTO.0b013e31822591e9. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Moretti L, Giacalone NJ, Schleicher S, Speirs CK, Carbone DP, et al. Inhibition of JAK2 signaling by TG101209 enhances radiotherapy in lung cancer models. J Thorac Oncol. 2011;6:699–706. doi: 10.1097/JTO.0b013e31820d9d11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z, Sasaki T, Tan X, Carretero J, Shimamura T, Li D, et al. Inhibition of ALK, PI3K/MEK, and HSP90 in murine lung adenocarcinoma induced by EML4-ALK fusion oncogene. Cancer Res. 2010;70:9827–9836. doi: 10.1158/0008-5472.CAN-10-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.