Abstract
AIMS
To assess the effects of adding motivational interviewing (MI) counseling to nicotine patch for smoking cessation among homeless smokers.
DESIGN
Two-group randomized controlled trial with 26-week follow-up.
PARTICIPANTS AND SETTING
430 homeless smokers from emergency shelters and transitional housing units in Minneapolis/St. Paul, Minnesota, USA.
INTERVENTION AND MEASUREMENTS
All participants received 8-week treatment of 21mg nicotine patch. In addition, participants in the intervention group received six individual sessions of MI counseling which aimed to increase adherence to nicotine patch and to motivate cessation. Participants in the Standard Care control group received one session of brief advice to quit smoking. Primary outcome was seven-day abstinence from cigarette smoking at 26 weeks as validated by exhaled carbon monoxide and salivary cotinine.
FINDINGS
Using intention-to-treat analysis, verified seven-day abstinence rate at week 26 for the intervention group was non-significantly higher than for the control group (9.3% vs. 5.6%, p=0.15). Among participants that did not quit smoking, reduction in number of cigarettes from baseline to week 26 was equally high in both study groups (−13.7 ±11.9 for MI vs. −13.5 ±16.2 for Standard Care).
CONCLUSIONS
Adding motivational interviewing counseling to nicotine patch did not significantly increase smoking rate at 26-week follow-up for homeless smokers.
Background
The prevalence of cigarette smoking among homeless adults remains an alarming 70%–80% or greater,(1, 2) which is 2–3 times that of the general adult population in the United States. Because homeless individuals are faced with meeting competing basic survival needs such as finding food and shelter, it is often assumed that smoking cessation is not a priority for this population. However, recent cross-sectional surveys showed homeless smokers reported a similar level of interest in smoking cessation and quit attempts compared to the general population of smokers.(3, 4) Nicotine replacement alone or in combination with other treatments was the most preferred treatment (42.2%), followed by counseling alone or in combination (24.6%).
Homeless smokers face multiple barriers to accessing and adhering to treatments(5) such as the daily need to find food, clothing and shelter; as well as practical limits on accessing and storing medicines. Furthermore, high rates of psychiatric and other substance abuse co-morbidity conditions(6) within homeless populations could create additional challenges to adherence to smoking cessation treatment and ultimately to smoking cessation.
While studies on motivational interviewing (MI) for smoking cessation have yielded mixed results, a recent meta-analysis (n=23 studies) suggest that MI significantly outperformed comparison conditions at long-term follow-up points.(7) Also, MI has been shown to improve treatment adherence and retention.(8) In a pilot study of nicotine patch among homeless smokers, MI was shown to be a feasible and acceptable intervention, however MI was not used to address adherence in that study.(9) To date there are no controlled trials of interventions to improve adherence to self-administered medications that specifically target homeless persons. To address the gap we conducted a smoking cessation randomized clinical trial (RCT) among smokers experiencing homelessness, called Power To Quit (PTQ). We tested the hypothesis that MI addressing smoking and NRT adherence will result in higher quit rates among homeless smokers compared to standard care. The current paper describes the smoking cessation (primary) and NRT adherence (secondary) outcomes of the study. Understanding the effectiveness of smoking cessation treatment for this underserved population will assist researchers and healthcare providers in developing and implementing smoking cessation interventions for homeless and other vulnerable populations.
Methods
Study design
This study was a community-based RCT of 430 homeless adult cigarette smokers that assessed the effectiveness of MI for smoking cessation. Participants were randomized to either the intervention arm (nicotine patch + MI) or to the control arm (nicotine patch + standard care). At baseline, participants in both groups received a two-week supply of 21-mg nicotine patches, and every two weeks they received an additional two-week supply of 21 mg nicotine patches. Participants randomized to the Intervention arm also received six individual MI counseling sessions each lasting 15 to 20 minutes, while participants randomized to the Standard Care arm received a one-time brief (10–15 minutes) advice to quit smoking. Participants provided written informed consent before they were enrolled into the study. The study procedures which have been published elsewhere,(10, 11) were approved and monitored by the University of Minnesota’s Institutional Review Board.
Participant Recruitment and Randomization
Recruitment occurred from May 2009 to August 2010 at a total of eight homeless shelters in Minneapolis/St. Paul, Minnesota. Recruitment was conducted at health fairs and via staff informational sessions and posted flyers at the study sites. Study eligibility criteria included being currently homeless and having lived in the Twin Cities for ≥6 months, having smoked at least one cigarette per day in the past 7 days and at least 100 cigarettes in their lifetime, aged ≥18 years, and willing to use nicotine patch for 8 weeks and participate in counseling sessions. Participants were classified as homeless based upon the Stewart B. McKinney Act passed by the US congress in 1987 in which homelessness was defined as anyone lacking “a fixed, regular and adequate nighttime residence;” or anyone staying at “a supervised publicly or privately operated shelter designed to provide temporary living accommodations, transitional housing, or other supportive housing program or a public or private place not meant for human habitation.(12) Smoking status was confirmed with exhaled carbon monoxide (CO) monitor using a cut-off of 5ppm. Exclusion criteria included: pregnancy, use of another tobacco cessation aid in the previous 30 days, severe cognitive impairment, suicidal ideation in the last 14 days, a major medical condition within the prior month, or scoring >5 on items assessing psychotic symptoms from the nine-item Mini International Neuropsychiatric Interview (M.I.N.I.).(13)
At the baseline visit, pre-assigned randomization numbers prepared by the study statistician determined which study arm the participant would be enrolled. The assignment to MI versus standard care was not blinded to participants. Sequential enrollment continued until a total of 430 participants were randomized into the study.
Treatment period
Eligible participants were scheduled for the baseline appointment which was 7–10 days after the initial contact. At the baseline visit participants were randomly assigned to either the MI intervention arm or to the Standard Care control arm. All participants received a health educational resource called, “The Power to Quit: A Quit Smoking Guide,” a 23-page guide developed by the project investigators. The guide included messages on the health risks of smoking, common reasons for smoking, and cognitive exercises to improve self-directed quit attempts.
Intervention Components
Motivational Interviewing (MI)
Participants randomized to the MI intervention arm received six individual MI counseling sessions each lasting 15 to 20 minutes. The MI counseling sessions were conducted by trained counselors and occurred at baseline and weeks 1, 2, 4, 6, and 8 follow-up. The focus of the MI sessions was encouraging cessation and NRT adherence. Although MI has typically been used to build motivation to quit(7) we also applied the principles and strategies to encourage adherence to the patch..
Standard Care (SC)
Participants in the Standard Care control condition received a onetime session of brief advice to quit smoking lasting approximately 10–15 minutes and delivered by trained study counselors(10). Topics covered in the standard care session included smoking history, current smoking, direct advice about the health risks of smoking and the health benefits of quitting, affirmation of the participant’s decision to quit, an assessment of preparedness to quit, and addressing strategies for coping with smoking cues.
Retention
To minimize attrition, study staff made reminder calls to participants during the week prior to each appointment, both pending and missed, until the window for completing appointments closed. Calls were placed from the project office and made either to each participant’s cell phone or to the shelter identified as the most recent nighttime residence in the participant’s file. At each of the 15 visits, participants received incentives. For participants who attended all 15 sessions, the monetary incentives totaled $275 over six months.(10, 11)
Measures
All questionnaire items were read to, or along with, the participants by trained research assistants that included master’s level public health students, medical students, or community mobilizers. Community mobilizers were individuals who had experienced homelessness either themselves or with a family member. At the baseline visit, we assessed demographic and smoking behaviors, psychosocial variables, environmental factors, and biological measurements. Demographic variables included age, ethnicity, gender, income, education level, marital status, and employment status and homelessness history including duration and type of homelessness. Psychosocial variables included social support(14, 15) and self-efficacy to refrain from smoking using the Smoking Self-Efficacy Questionnaire (SEQ-12).(16) Psychiatric co-morbidities of depression and anxiety were assessed with the 4-item Rost-Burnham screener for depression,(17) the patient health questionnaire (PHQ-9) for depression,(18) the 4-item perceived stress scale for stress in past 30 days,(19) and the M.I.N.I. generalized anxiety disorder assessment.(13) Further, study participants were asked questions about lifetime drug treatment history and drug and alcohol use and dependence.(17) Adherence to nicotine patch was measured by direct observation by study staff at weeks 2, 4, 6, and 8. Participants were asked if they had the patch on; for those who answered in the affirmative, study staff then asked to see the patch. Motivation and confidence for adherence to NRT patch was assessed with the Motivation/Confidence to Adhere Scale.(20) which is a 5-item scale with score range of 1–10 for each item and reflects participants’ level of commitment, desire, need, and readiness to adhere to smoking cessation. Higher totals indicate higher levels of motivation for adherence (Cronbach’s alpha=0.84); Self-Efficacy to Adhere(21) is a modified 10-item Adult AIDS Clinical Trials Group (AACTG) measure which asks participants to indicate their level of confidence in performing specific adherence tasks relating to treatment. Responses range from 0 (cannot do at all) to 10 (certain I can do). Higher scores indicate higher adherence self-efficacy.
The primary outcome was biochemically-verified self-reported 7-day point prevalence abstinence from smoking assessed at week 26 post-randomization defined as having smoked no cigarettes (not even a puff) during the previous seven days. Those who self-reported abstinence were verified using with an expired carbon monoxide (≤10ppm) test. Salivary cotinine testing was done if the expired CO was greater than 10ppm for those who self-reported abstinence. A cut-off of ≤20ng/ml for salivary cotinine was used to verify abstinence. The secondary outcome was adherence to the nicotine patch, measured by direct observation at in-person appointments during the treatment period.
Analysis
The sample size was determined a priori assuming a two-tailed type I error of 0.05, a power of at least 80% and a week 26 biochemically verified quit rate of 18% and 8% for MI intervention and Standard Care conditions, respectively based on previous research.(9) The primary analysis was a Yates-corrected chi-square test of the difference between the proportions quit in the two groups. With these assumptions, using the Chi Square test we needed 214 participants per study arm. With the final sample achieved of 430 participants we had 83% power (at a 5% significance level) to detect statistically significant main effects. Following intention-to-treat analyses, participants who did not attend the 26-week visits were assumed to be smokers. We also compared CO-verified repeated 7-day abstinence at weeks 8 and 26 using the Chi Square test as a secondary outcome measure. The repeated point prevalence abstinence was defined as participants who self-reported and verified by CO or cotinine that they were abstinent both at week 8 and week 26. All the other participants were treated as smokers. We also performed longitudinal analysis using repeated measures logistic regression with generalized estimating equations for the CO verified abstinence at weeks 1, 2, 4, 6, 8 and 26 including time (continuous) and intervention group as predictors using PROC GENMOD in SAS v9.2.{SAS Institute Inc., 2009 #6990}.
Results
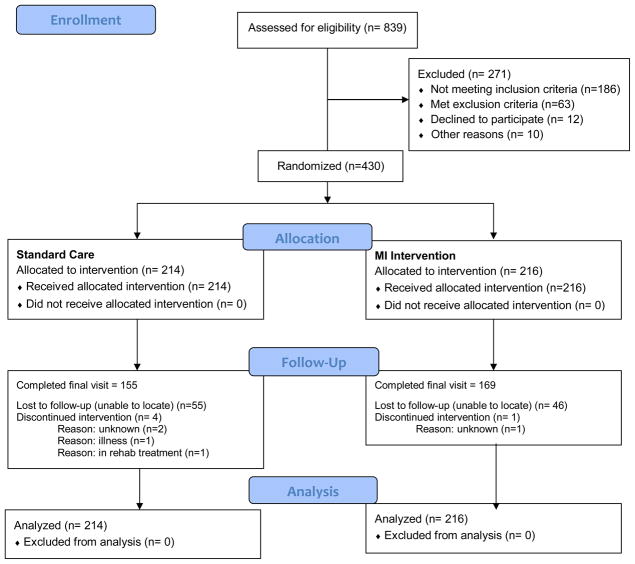
Of the 839 individuals screened for study eligibility, 568 were eligible and 430 were randomized, 216 to the MI intervention and 214 to the control group (Figure 1). Eligible participants who returned for randomization were older and more likely to have a phone number compared to eligible participants who were not enrolled in the study. Of the 430 enrolled, 76.1% completed their week 8 visit (end of treatment) and 75.4% completed the final week 26 visit. There were no significant differences in attrition rates (Table 4) between the two study groups.
Figure 1.
CONSORT(22) flow diagram for the study
Table 4.
Summary of Attendance at study visits of 430 Study Participants enrolled in the PTQ Study
| Study Visit Time Points | Study Visit Completed | |||
|---|---|---|---|---|
| SC (n= 214) | MI (n= 216) | |||
| N | % | N | % | |
| Week 1 | 191 | 89.3 | 189 | 87.5 |
| Week 2 | 190 | 88.8 | 172 | 79.6 |
| Week 4 | 184 | 86.0 | 174 | 80.6 |
| Week 6 | 171 | 79.9 | 159 | 73.6 |
| Week 8 | 168 | 78.5 | 162 | 75.0 |
| Week 10 | 146 | 68.2 | 145 | 67.1 |
| Week 12 | 143 | 66.8 | 155 | 71.8 |
| Week 14 | 140 | 65.4 | 145 | 67.1 |
| Week 16 | 137 | 64.0 | 143 | 66.2 |
| Week 18 | 142 | 66.4 | 146 | 67.6 |
| Week 20 | 136 | 63.6 | 146 | 67.6 |
| Week 22 | 139 | 65.0 | 155 | 71.8 |
| Week 24 | 141 | 65.9 | 156 | 72.2 |
| Week 26 | 155 | 72.4 | 169 | 78.2 |
There were no significant differences in baseline characteristics of participants between treatment groups (Table 1). Participants’ mean age was 44 years and the majority were male; African American or White; unemployed; high school graduate or equivalent; with a monthly income of less than US$400. Nearly two-thirds reported sleeping in emergency shelters and 15% slept in transitional housing often in past 6 months. Participants smoked about a pack of cigarettes a day and 87% smoked their first cigarette of the day within 30 minutes of awakening. More than 80% of the sample screened positive for lifetime history of drug abuse or dependence. Self-reported and verified 7-day point prevalence abstinence rates for the MI and control study groups at various assessment points are shown in Table 2.
Table 1.
Baseline characteristics of 430 Study Participants enrolled in the PTQ Study
| Total N=430 | Motivational Interviewing Arm N=216 | Standard Care Arm N=214 | |||
|---|---|---|---|---|---|
|
| |||||
| Mean (SD) | N | Mean (SD) | N | ||
|
| |||||
| Demographic Variables | |||||
|
| |||||
| Age, mean ± SD, years | 44.4 (9.9) | 44.5 (9.7) | 216 | 44.2 (10.1) | 214 |
|
| |||||
| Male, n (%) | 321 (74.7) | 158 (73.1) | 216 | 163 (76.2) | 214 |
|
| |||||
| Race/Ethnicity, n (%) | 216 | 214 | |||
| African American/Black | 242 (56.3) | 130 (60.2) | 112 (52.3) | ||
| White, non-Hispanic | 153 (35.6) | 69 (31.9) | 84 (39.3) | ||
| Hispanic/Latino | 10 (2.3) | 5 (2.3) | 5 (2.3) | ||
| Native American/Alaska Native | 10 (2.3) | 5 (2.3) | 5 (2.3) | ||
| Other | 14 (2.3) | 7 (3.3) | 7 (3.3) | ||
|
| |||||
| Monthly family income < $400, n (%) | 273 (63.5) | 140 (64.8) | 216 | 133 (62.1) | 214 |
|
| |||||
| Education ≥ High school, n (%) | 330 (76.8) | 166 (76.9) | 216 | 164 (76.6) | 214 |
|
| |||||
| BMI, mean (SD) | 30.1 (7.6) | 30.3 (7.8) | 215 | 30.0 (7.5) | 211 |
|
| |||||
| Psychosocial Variables | |||||
|
| |||||
| Depression (PHQ9, in past 2 weeks), mean (SD) | 8.5 (6.4) | 8.8 (6.7) | 216 | 8.1 (6.1) | 212 |
|
| |||||
| PHQ9 ≥ 10 in past 2 weeks, n (%) | 148 (34.6) | 92 (42.5) | 216 | 81 (38.2) | 212 |
|
| |||||
| Stress (PSS-4, in past 30 days), mean (SD) | 8.4 (2.3) | 8.5 (2.5) | 215 | 8.3 (2.1) | 213 |
|
| |||||
| Tobacco Related Variables | |||||
|
| |||||
| Serum cotinine in ng/ml., mean (SD) | 213.7 (159.0) | 229.2 (179.9) | 204 | 198.3 (133.8) | 205 |
|
| |||||
| Exhaled carbon monoxide in ppm., mean (SD)* | 15.6 (9.0) | 15.0 (8.3) | 202 | 16.2 (9.65) | 207 |
|
| |||||
| Cigarettes per day, mean (SD) | 19.3 (13.7) | 19.1 (11.1) | 215 | 19.4 (16.0) | 212 |
|
| |||||
| Time to first cigarette, ≤30 minutes, n (%) | 374 (87.0) | 188 (87.0) | 216 | 186 (87.0) | 270 |
|
| |||||
| Smoke menthol cigarettes, n (%) | 268 (62.6) | 138 (64.2) | 216 | 130 (61.0) | 214 |
|
| |||||
| Number of 24 hour quit attempts in the past year, mean (SD) | 2.5 (5.2) | 2.5 (5.3) | 212 | 2.6 (5.1) | 212 |
|
| |||||
| Age started smoking regularly, mean (SD) | 16.2 (5.9) | 16.3 (6.0) | 215 | 16.1 (5.7) | 214 |
|
| |||||
| Motivation to quit, mean (SD) | 9.1 (1.6) | 9.0 (1.8) | 216 | 9.1 (1.5) | 214 |
|
| |||||
| Confidence to quit, mean (SD) | 7.3 (2.4) | 7.3 (2.4) | 216 | 7.3 (2.5) | 214 |
|
| |||||
| Substance Abuse Variables | |||||
|
| |||||
| Ever used illicit drug* more than 5 times in lifetime, n (%) | 355 (82.8) | 182 (84.3) | 216 | 173 (81.2) | 213 |
|
| |||||
| Ever needed larger amount of illicit drugs to get an effect, n (%) | 170 (39.6) | 80 (37.0) | 216 | 90 (42.3) | 213 |
|
| |||||
| Ever had emotional or Psychological problems from using illicit drugs, n (%) | 140 (32.6) | 65 (30.1) | 216 | 75 (35.2) | 213 |
|
| |||||
| Ever thought you were an excessive drinker, n (%) | 195 (45.5) | 95 (44.0) | 216 | 100 (46.9) | 213 |
|
| |||||
| Ever drank one-fifth of liquor in one day, n (%) | 179 (41.7) | 90 (41.7) | 216 | 89 (41.8) | 213 |
|
| |||||
| Ever drank 7 or more alcoholic drinks daily for 2 weeks, n (%) | 174 (40.7) | 83 (38.6) | 216 | 91 (42.9) | 213 |
Abbreviation: MI= Motivational Interviewing; SC-Standard Care; SD, standard deviation; BMI, body mass index; PHQ9, Patient Health Questionaire-9 Depression Scale; PSS-4=Perceived Stress Scale-4 items.
List of drugs included Marijuana (Hashish, Pot, Grass); Amphetamines (Stimulants, Uppers, Speed); Barbiturates (Sedatives, Downers, Sleeping Pills, Seconal, Quaaludes); Tranquilizers (Valium, Librium); Cocaine (Coke, Crack); Heroine; Opiates (Codeine, Demerol, Morphine, Methadone, Darvon, Opium), Psychedelics (LSD, Mescaline, Peyote, Psilocybin, DMT, PCP).
Table 2.
Self-report and biochemically verified 7-day point-prevalence abstinence rates of 430 Study Participants enrolled in the PTQ Study *
| MI | Standard Care | P value | |
|---|---|---|---|
| Self-report | |||
| Quit at Week 8, n (%) | 33 (15.28%) | 26 (12.15%) | 0.350 |
| Quit at Week 26, n (%) | 36 (16.67%) | 25 (11.68%) | 0.140 |
| Verified | |||
| Quit at Week 8, n (%) | 20 (9.26%) | 19 (8.88%) | 0.890 |
| Quit at Week 26 n (%)** | 20 (9.26%) | 12 (5.61%) | 0.150 |
Those lost to follow-up were treated as smokers;
CO (<=10 ppm) or Cotinine (<= 20ng/ml) verified
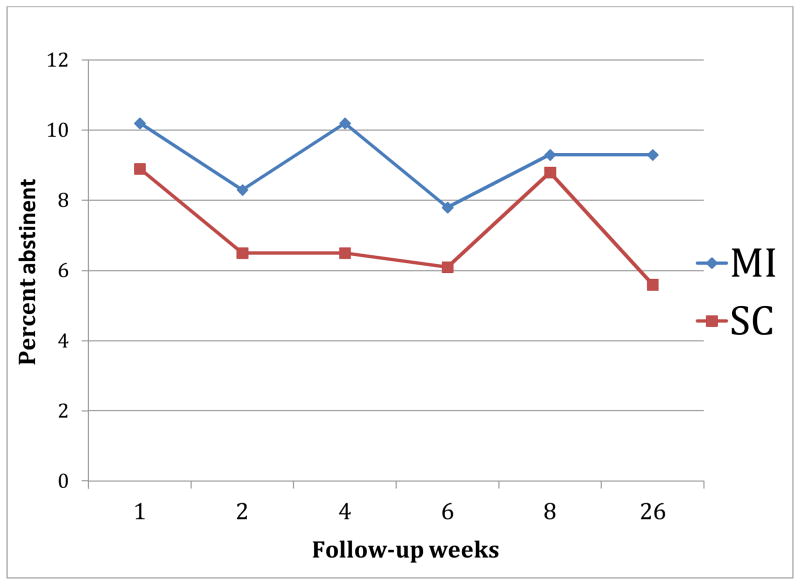
Using intention-to-treat analysis, 7-day verified smoking abstinence rates at week 8 were 9.3% vs. 8.9% (p=0.89) and at week 26 were 9.3% vs. 5.6% (p=0.15) for the intervention and control groups respectively. The repeated point prevalence abstinence rate was 3.24% for MI group and 1.40% for SC group (Fisher exact p= 0.338). We used repeated measures logistic regression with generalized estimating equations (Figure 2) for the CO verified abstinence at weeks 1, 2, 4, 6, 8 and 26 while treating those lost to follow-up as smokers and included time (continuous) and intervention group as predictors. This yielded an odds ratio for the MI versus SC group of 1.33 (95% CI=0.88, 2.02; p= 0.17). If those lost to follow-up were treated as missing, the odds ratio for the MI versus SC group was 1.40 (95% CI: 0.93, 2.11; p=0.11.
Figure 2.
Verified Abstinence by time
Table 3 shows results of various measures of adherence. Motivation for adherence scores at week 6 were marginally higher for participants in the intervention group than those in the control group (45.8 ±6.9 for MI vs. 44.4 ±7.4 for Control, p=0.08). Table 3 also shows results of “patch checks”, i.e. proportion of participants who had their nicotine patches on at various study visits. There were no differences between study groups in the proportion of participants who had their nicotine patches on at various study visits. Table 4 shows the attendance for various study contact points.
Table 3.
Adherence measures of 430 Study Participants enrolled in the PTQ Study
| Variables | Total (n=430) | MI (n=216) | Standard Care (n=214) | p-value |
|---|---|---|---|---|
|
| ||||
| Baseline Motivation to adhere, mean (SD) | 45.4 (6.5) | 45.4 (6.3) | 45.3 (6.7) | 0.77 |
|
| ||||
| Week 6 Motivation to adhere, mean (SD) | 45.1 (7.3) | 45.8 (7.0) | 44.4 (7.5) | 0.08 |
|
| ||||
| Motivation to adhere change in scores from Baseline to Week 6, mean (SD) | 0.02 (8.2) | 0.4 (8.4) | −0.4 (8.1) | 0.40 |
|
| ||||
| Baseline Self-efficacy to adhere, mean (SD) | 78.4 (17.6) | 78.2 (18.0) | 78.7 (17.1) | 0.76 |
|
| ||||
| Week 6 Self-efficacy to adhere, mean (SD) | 84.1 (18.3) | 85.4 (19.1) | 82.9 (17.5) | 0.22 |
|
| ||||
| Self-efficacy to adhere change in scores from Baseline to Week 6, mean (SD) | 5.7 (20.8) | 5.7 (22.5) | 5.7 (19.2) | 0.99 |
|
| ||||
| Had nicotine patch on at visit (“patch check”), % yes | ||||
| Week 1 | 52.8 | 49.1 | 56.5 | 0.12 |
| Week 2 | 52.6 | 49.1 | 56.1 | 0.15 |
| Week 4 | 44.7 | 46.8 | 42.5 | 0.38 |
| Week 6 | 38.6 | 40.7 | 36.5 | 0.36 |
| Week 8 | 33.7 | 33.8 | 33.7 | 0.97 |
Discussion
Results from this study show that verified quit rates at week 8 and week 26 for MI were not significantly better than those for Standard Care. Although the quit rates for MI were consistently higher at all study time-points, the magnitude of the effects was small.
The 9.3% verified quit rate at week 26 is comparable to findings from a cluster-randomized trial that tested nicotine gum plus five MI sessions for smoking cessation in 20 low-income housing developments (n=173).(23) For that study, biochemically-verified 7-day abstinence rates were 6.1% vs. 5.6% at week 8 and 7.6% vs. 9.3% at week 26 for the intervention and comparison groups respectively. However, the quit rates in our current study are lower than rates reported from two pilot studies with homeless smokers.(9, 24) In one study (n=46)(9) that utilized five individual MI, six group meetings, and a choice of NRT, CO-verified abstinence rates at week 26 were 17.4% vs. 8.7% for intervention and comparison groups respectively. The second study(24) (n=58) which had no control group tested the effects of a 12-week group Cognitive Behavioral Therapy and choice of NRT, bupropion, or varenicline, reported a CO-verified quit rate of 13.6% at 24 weeks. These pilot studies had more intensive counseling interventions that may have contributed to higher quit rates. There are other reasons that could contribute to the low quit rates in our current study. In addition to multiple competing challenges that being homeless could pose to smoking cessation, our study sample had characteristics suggestive of high nicotine dependence including factors such as smoking a pack of cigarettes per day on average and nearly all participants smoked their first cigarette of the day within 30 minutes of awakening.
In addition, our study sample showed high rates of co-morbidities with depression, alcohol, and other substance abuse with nearly 40% having PHQ-9 scores in the moderate or worse depression range and nearly half considered themselves as alcoholic or chemically dependent. Studies in other populations have shown that these co-morbidities make quitting smoking more challenging.(2, 25, 26) In essence, our study had a lower dose of counseling and higher rate of co-morbidities than the two studies described above. Unlike the protocol of most smoking cessation studies in the general population that excludes smokers with these co-morbid conditions, smokers with these conditions were allowed to enroll in this study provided they were medically stable as determined by a psychiatrist. This protocol decision was made to ensure that the study sample was similar to homeless smokers in general which would enhance the study’s external validity.
Given the challenges to smoking cessation in homeless populations, it could be argued that even these low cessation rates are likely higher than secular trends in this population and are therefore encouraging. These results highlight that homeless smokers are interested in quitting smoking and will enroll in a smoking cessation trial. Also, 75% of eligible participants returned for randomization and 75% of those enrolled completed their final week 26 visit. These results about interest within homeless populations in smoking cessation are consistent with findings from earlier studies.(3, 27, 28) However, these findings are in direct contrast to the presumption by some that homeless persons would not be interested in smoking cessation due to many competing daily challenges or that follow-up for longitudinal studies would be nearly impossible because of their transient housing situation.
This study also found that contrary to expectations MI did not improve adherence measures among participants who received MI. The lack of effect of MI to promote treatment adherence is contrary to several studies(8) that have reported large effects of MI in promoting treatment adherence. It should be noted however that despite not requiring motivation to quit or adhere to treatment as study enrollment criteria in current study, participants reported high motivation to quit smoking as well as high motivation and self-efficacy to adhere to nicotine patch use. Having less favorable outcomes with MI is consistent with other studies in non-homeless settings that have shown that MI works better among people who are resistant, angry or demonstrate low motivation to change a particular health behavior(29) and therefore may be contraindicated for patients who are ready for action. Another study,(30) in a non-homeless sample found that MI was less effective than health education for smoking cessation among a sample of African American light smokers who were highly motivated to quit smoking at study enrollment.
This study has many strengths. To our knowledge, this is the largest and the first adequately powered randomized smoking cessation clinical trial in homeless populations. We successfully randomized a diverse sample of 430 homeless smokers in 15 months. We were able to achieve a study sample that is reflective of the general homeless population of the same community.(1, 31) We also achieved 75% retention for 26 weeks. This suggests that smokers regardless of their housing situation want to quit and can be successful in doing so when provided with the opportunity.
This study has limitations. First, it was conducted at a single metropolitan area in the upper Midwest of the United States and there may be differences between cities, states, or regions within a country and between countries that limit external validity. However, this generalizability concern is somewhat mitigated by the fact that participants were recruited from a variety of emergency shelters and transitional housing units. Data about emergency shelters from a tri-annual statewide survey{Wilder Foundation, 2009 #5998} shows that mean age for homeless person in Hennepin and Ramsey Counties encompassing the Twin Cities were 42.4 years and 42.9 years respectively compared to 44.4 years in our study sample. Also, our study sample was 74.7% male which is comparable to that in Hennepin (72.7%) but lower than that in Ramsey (86.5%) Counties(31). Second, because this was a treatment study, the sample was self-selected and motivated to quit smoking and thus may not be representative of homeless smokers generally. The high motivation of participants may also have made MI less effective since MI is best suited for less motivated people.(7, 30) However, the sample represents the group of smokers that would seek smoking cessation treatment if it were to become available in homeless populations.
Our results reveal that despite many competing daily challenges, homeless smokers are interested in smoking cessation and that motivational interviewing and nicotine replacement showed promising effects for smoking cessation for homeless populations. The low quit rates of the study calls for more studies and programs to enhance smoking cessation rates in homeless populations. It is possible that other counseling approaches besides MI might be more effective or perhaps more intensive interventions are needed for smokers experiencing homelessness. Due to the high rates of psychiatric and substance abuse co-morbidities in this population, will intervening in these co-morbid conditions concurrently or in sequence result in improved smoking cessation rates? Because of the strikingly high prevalence of smoking and associated morbidity in homeless populations, developing and implementing programs to improve smoking cessation outcomes is critical for reducing the tobacco-related health disparities in homeless and other underserved populations.
Acknowledgments
The authors thank Jennifer Warren, PhD, and project staff Sharae Walker, Bonnie Houg, R’Gina Sellers, Casey Tuck, Abimbola Olayinka, Carolyn Borja, Carolyn Bramante, Julia Davis, Pravesh Napaul, and Brandi White for their assistance with implementation of the project. The authors further acknowledge the directors of participating shelters, Dorothy Day Center, Our Savior’s Shelter, Listening House, Union Gospel Mission, Naomi Family Center, and People Serving People and, finally, express gratitude to the members of the CAB and the study participants.
This work was supported by a grant from the National Heart Lung and Blood Institute [R01HL081522]. Lillian Gelberg received support from NIDA (DA022445), NCI (CA112441), and NIDDK (DK071065). John E. Connett and Hongfei Guo received supported from the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR000114).
Footnotes
Trial Registration: clinicaltrials.gov: NCT00786149
References
- 1.Wilder Foundation. Homeless Adults and Children in Minnesota Statewide Survey. Saint Paul, MN: Wilder Foundation; 2004. [cited 2006 September 29, 2006]; Available from: http://www.wilder.org/fileadmin/user_upload/research/Homeless2003/adult/StatewideAdultsByMetroGreaterMN_Tables_192-199.pdf. [Google Scholar]
- 2.Torchalla I, Strehlau V, Okoli CT, Li K, Schuetz C, Krausz M. Smoking and predictors of nicotine dependence in a homeless population. Nicotine Tob Res. 2011 Oct;13(10):934–42. doi: 10.1093/ntr/ntr101. [DOI] [PubMed] [Google Scholar]
- 3.Butler J, Okuyemi KS, Jean S, Nazir N, Ahluwalia JS, Resnicow K. Smoking characteristics of a homeless population. Subst Abus. 2002 Dec;23(4):223–31. doi: 10.1080/08897070209511495. [DOI] [PubMed] [Google Scholar]
- 4.Connor J, Broad J, Rehm J, Vander Hoorn S, Jackson R. The burden of death, disease, and disability due to alcohol in New Zealand. N Z Med J. 2005 Apr 15;118(1213):U1412. [PubMed] [Google Scholar]
- 5.Teeter T. Adherence: working with homeless populations. Focus. 1999;14(2):5–6. [PubMed] [Google Scholar]
- 6.el-Guebaly N, Cathcart J, Currie S, Brown D, Gloster S. Smoking Cessation Approaches for Persons With Mental Illness or Addictive Disorders. Psychiatr Serv. 2002 Sep 1;53(9):1166–70. doi: 10.1176/appi.ps.53.9.1166. [DOI] [PubMed] [Google Scholar]
- 7.Hettema JE, Hendricks PS. Motivational interviewing for smoking cessation: a meta-analytic review. J Consult Clin Psychol. 2010 Dec;78(6):868–84. doi: 10.1037/a0021498. [DOI] [PubMed] [Google Scholar]
- 8.Hettema J, Steele J, Miller WR. Annual Review of Clinical Psychology. Palo Alto: Annual Reviews; 2005. Motivational interviewing; pp. 91–111. [DOI] [PubMed] [Google Scholar]
- 9.Okuyemi KS, Thomas JL, Hall S, Nollen NL, Richter KP, Jeffries SK, et al. Smoking cessation in homeless populations: A pilot clinical trial. Nicotine & Tobacco Research [Article] 2006 Oct;8(5):689–99. doi: 10.1080/14622200600789841. [DOI] [PubMed] [Google Scholar]
- 10.Goldade K, Whembolua GL, Thomas J, Eischen S, Guo H, Connett J, et al. Designing a smoking cessation intervention for the unique needs of homeless persons: a community-based randomized clinical trial. Clin Trials. 2011 Dec;8(6):744–54. doi: 10.1177/1740774511423947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okuyemi KS, Goldade K, Whembolua GL, Thomas JL, Eischen S, Guo H, et al. Smoking Characteristics and Comorbidities in the Power To Quit Randomized Clinical Trial for Homeless Smokers. Nicotine Tob Res. 2012 May 15; doi: 10.1093/ntr/nts030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilder Research Center. Homelessness in Minnesota 2003: Key facts from survey of Minnesotans without permanent housing. Saint Paul, Minnesota: Amherst H. Foundation; 2004. [Google Scholar]
- 13.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 4–57. [PubMed] [Google Scholar]
- 14.Everson SA, Goldberg DE, Kaplan GA, Cohen RD, Pukkala E, Tuomilehto J, et al. Hopelessness and risk of mortality and incidence of myocardial infarction and cancer. Psychosom Med. 1996 Mar-Apr;58(2):113–21. doi: 10.1097/00006842-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Everson SA, Kaplan GA, Goldberg DE, Salonen JT. Hypertension incidence is predicted by high levels of hopelessness in Finnish men. Hypertension. 2000 Feb;35(2):561–7. doi: 10.1161/01.hyp.35.2.561. [DOI] [PubMed] [Google Scholar]
- 16.Etter JF, Bergman MM, Humair JP, Perneger TV. Development and validation of a scale measuring self-efficacy of current and former smokers. Addiction. 2000 Jun;95(6):901–13. doi: 10.1046/j.1360-0443.2000.9569017.x. [DOI] [PubMed] [Google Scholar]
- 17.Rost K, Burnam MA, Smith GR. Development of screeners for depressive disorders and substance disorder history. Med Care. 1993 Mar;31(3):189–200. doi: 10.1097/00005650-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity ofa brief depression severity measure. J Gen Intern Med. 2001 Sep;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983 Dec;24(4):385–96. [PubMed] [Google Scholar]
- 20.Amrhein PC, Miller WR, Yahne CE, Palmer M, Fulcher L. Client commitment language during motivational interviewing predicts drug use outcomes. J Consult Clin Psychol. 2003 Oct;71(5):862–78. doi: 10.1037/0022-006X.71.5.862. [DOI] [PubMed] [Google Scholar]
- 21.Johnson MO, Neilands TB, Dilworth SE, Morin SF, Remien RH, Chesney MA. The role of self-efficacy in HIV treatment adherence: validation of the HIV Treatment Adherence Self-Efficacy Scale (HIV-ASES) Journal of behavioral medicine. 2007 Oct;30(5):359–70. doi: 10.1007/s10865-007-9118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz KF, Altman DG, Moher D, Fergusson D. CONSORT 2010 changes and testing blindness in RCTs. Lancet. 2010 Apr 3;375(9721):1144–6. doi: 10.1016/S0140-6736(10)60413-8. [DOI] [PubMed] [Google Scholar]
- 23.Okuyemi KS, James AS, Mayo MS, Nollen N, Catley D, Choi WS, et al. Pathways to health: a cluster randomized trial of nicotine gum and motivational interviewing for smoking cessation in low-income housing. Health Educ Behav. 2007 Feb;34(1):43–54. doi: 10.1177/1090198106288046. [DOI] [PubMed] [Google Scholar]
- 24.Shelley D, Cantrell J, Wong S, Warn D. Smoking cessation among sheltered homeless: a pilot. American journal of health behavior. 2010 Sep-Oct;34(5):544–52. doi: 10.5993/ajhb.34.5.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan MA, Covey LS. Current perspectives on smoking cessation among substance abusers. Curr Psychiatry Rep. 2002 Oct;4(5):388–96. doi: 10.1007/s11920-002-0087-5. [DOI] [PubMed] [Google Scholar]
- 26.Humfleet G, Munoz R, Sees K, Reus V, Hall S. History of alcohol or drug problems, current use of alcohol or marijuana, and success in quitting smoking. Addict Behav. 1999 Jan-Feb;24(1):149–54. doi: 10.1016/s0306-4603(98)00057-4. [DOI] [PubMed] [Google Scholar]
- 27.Connor SE, Cook RL, Herbert M, Neal SM, Williams JT. Smoking cessation in a homeless population -There is a will, but is there a way? J Gen Intern Med [Article; Proceedings Paper] 2002 May;17(5):369–72. doi: 10.1046/j.1525-1497.2002.10630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnsten JH, Reid K, Bierer M, Rigotti N. Smoking behavior and interest in quitting among homeless smokers. Addict Behav. 2004 Aug;29(6):1155–61. doi: 10.1016/j.addbeh.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Heather N, Rollnick S, Bell A, Richmond R. Effects of brief counselling among male heavy drinkers identified on general hospital wards. Drug and Alcohol Review. 1996;15(1):29–38. doi: 10.1080/09595239600185641. [DOI] [PubMed] [Google Scholar]
- 30.Ahluwalia JS, Okuyemi K, Nollen N, Choi WS, Kaur H, Pulvers K, et al. The effects of nicotine gum and counseling among African American light smokers: a 2 × 2 factorial design. Addiction. 2006 Jun;101(6):883–91. doi: 10.1111/j.1360-0443.2006.01461.x. [DOI] [PubMed] [Google Scholar]
- 31.Homelessness in Minnesota: Key findings from the 2009 statewide survey [database on the Internet] Wilder Research; 2009. [cited August 2010]. Available from: http://www.wilder.org/reportsummary.0.html?tx_ttnews[tt_news]=2300. [Google Scholar]