Abstract
Homer1c has been shown to play a role in learning and memory. Overexpression of Homer1c in the hippocampus can improve memory in normal rats and can also rescue spatial learning deficits in Homer1 knockout mice. In a previous study, we found that Homer1c mRNA is upregulated after a spatial learning paradigm in aged rats that successfully learn the task, when compared to aged rats that are learning-impaired (AI). This study was designed to validate the role of Homer1c in successful cognitive aging. In this report, we find that gene delivery of Homer1c into the hippocampus of aged learning-impaired rats significantly improves individual performance on an object location memory task. The learning ability of these rats on the Morris Water Maze was also superior to that of AI control rats. In summary, using two independent spatial memory tasks, we demonstrate that Homer1c is sufficient to improve the spatial learning deficits in a rodent model of cognitive aging. These results point to Homer1c as a potential therapeutic target for improving age-related cognitive impairment.
Keywords: aged learning impaired, hippocampus, gene rescue, Homer, object location memory, Morris Water Maze, spatial learning, cognitive aging
1. Introduction
Homer1c has been implicated in successful cognitive aging (Burger et al., 2007; Ménard and Quirion, 2012). Previous research by us and others also implicates the Homer1c isoform in spatial learning and synaptic plasticity in young animals (Klugmann et al., 2005; Szumlinski et al., 2005; Ronesi and Huber, 2008; Gerstein et al., 2012)). The gene Homer1 belongs to a family of scaffolding proteins that interact with various post-synaptic density proteins including group I metabotropic glutamate receptors (mGluR1/5) (Brakeman et al., 1997), inositol 1,4,5-trisphosphate receptors (IP3Rs) (Tu et al., 1998), and Ryanodine receptors (Feng et al., 2008). The Homer1 gene codes for several proteins, including Homer1a (the short form) and Homer1b/c (long forms), each of which exerts a distinct effect on neuronal function (Kato et al., 1997; Sun et al., 1998; Xiao et al., 1998).
Aged rats display variable cognitive ability and can be segregated into groups of learning impaired (AI) and superior learner animals (SL) (Gage et al., 1984; Gage and Björklund, 1986; deToledo-Morrell et al., 1988; Markowska et al., 1989; Gallagher et al., 1993; Schulz et al., 2002; Burger et al., 2007; 2008). In a genome wide study, we found that Homer1c was upregulated in the CA1 region of hippocampus in SL relative to AI rats after completing the Morris water maze (MWM; (Burger et al., 2007)). Therefore, we hypothesized that gene targeting of Homer1c to the dorsal hippocampus of aged learning impaired rats would result in amelioration of their cognitive deficits. In this study, we investigated the role of Homer1c on the performance of aged learning impaired rats.
Our results show that expression of Homer1c in the hippocampus of AI rats significantly improves performance on the Object Location Memory test of spatial memory (OLM). Moreover, the learning abilities of AI animals injected with Homer1c are significantly better than those of AI animals injected with a control GFP during learning and on platform crossings in the probe trial of the MWM. The results support our hypothesis that Homer1c expression is sufficient for an enhancement of spatial memory in aged-learning impaired rats, as measured by two independent tests of hippocampal function, the OLM and the MWM.
2. Methods
2.1. Animals
20-month old male Fisher 344 rats were purchased from the National Institute of Aging rodent colony (NIA, Washington DC). 3-month old male Fisher 344 rats were purchased from Harlan Laboratories (Madison, WI). All animals had free access to water and food. In addition, 12 hour dark and light cycles were maintained. Behavioral tests were given during the light cycle. All procedures concerning animals were approved by the University of Wisconsin Institutional Animal Care and Use Committee and were conducted in accordance with the U.S. National Institutes of Heath ‘Guide for the Care and Use of Laboratory Animals’.
2.2 Object Location Memory
The experimental apparatus was made of clear Lucite, the outside of which was covered with dark blue construction paper, and measured 40.65 cm × 40.65 cm × 30.5 cm. Corncob bedding was spread ~2 inches deep on the floor. The box was placed in the middle of the empty MWM pool to track animals’ performance using the same video camera we use for swim tracking in the MWM. To encourage exploration, direct overhead lighting was not used. On each day of the experiment, the arena and objects were cleaned with 70% ethanol and fresh bedding was put down to limit olfactory cues. Both rounds of OLM were carried out in the same room and arena.
2.2. Object Location Memory 1 (OLM1)
On the first day (habituation day), all rats were habituated to the behavioral room and arena. There were no objects in the arena at this time and the rat was given 5 minutes to explore freely. The rat was then placed back in its home cage, any feces were removed, the bedding was mixed or stirred, smoothed down and habituation proceeded to the next animal. All rats received a total of two 5-min habituation exposures.
On Training Day, 24 hours after habituation, rats were trained on the locations of two identical objects. The arena was the same as previously described but with the addition of two identical objects (Duplo™ plastic blocks, 1.25×1.25×1 inch). Rats were allowed to explore the arena and the two objects freely over the course of a 10-minute trial. The bedding was stirred and the blocks were cleaned with 70% ethanol, before moving on to the next animal. Any animal failing to investigate both objects on the training trial, or whose total investigation time on the training trial was less than 10 s, was excluded from the analysis so as to avoid confusing very low activity with low novelty seeking behavior.
Testing of OLM occurred 24 hours after training. Spatial memory was tested by measuring preference for the object in a novel location versus a familiar location. The arena was arranged as before with the same identical objects (Duplo™ blocks) and fresh bedding. However, in each trial, one of the two objects was placed in the center of the arena instead of its original location. The experiment was run similarly as during training, with 10-minute trials.
All trials on both the training and testing days were videotaped and analyzed by an experimenter blind to the identity of the rat, using Videotrack software by ViewPoint Life Sciences (Montreal, CANADA). Total amount of time spent exploring the novel and familiar objects (Duplo™ blocks) was recorded for each animal on both the training and testing days. The relative exploration time on the test day was recorded for each object and expressed as a Novelty Index: (Time Spent (s) Investigating Object in Novel Location / Time Spent (s) Investigating Both Objects in Total)×100. Investigation times were calculated and Novelty Indices were examined in both young and aged animals.
2.3. Object Location Memory 2 (OLM2)
All animals were allowed to rest undisturbed in the animal facility for 2 weeks post-surgery before retesting in the OLM paradigm. After this rest period, the animals were again brought down prior to the first day (training) for habituation to the behavior room and arena. Habituation for OLM2 was carried out exactly as described above for OLM1 and in the same room, except the arena is placed in a different orientation (side with spatial cue is now West instead of East) and using two identical 50 mL glass vials, spray painted matte grey, and wiped down with 70% ethanol.
2.3.1. Criteria for categorizing SL and AI in the OLM
Aged animals were identified as superior learners when their novelty index on the OLM task was at or above the mean score of young rats (65% was the young mean on both OLM1 (± 2.2 S.E.M) and OLM2 (± 1.4 S.E.M). This resulted in selecting aged rats with the highest novelty indices (as they spent more time than other aged animals investigating the object in the novel location), whereas aged impaired learners were identified as animals that spent 51% (or less) of their total object investigation time attending to the novel object. All AI animals were used for either Homer1c or GFP gene delivery experiments. Note that 5 of the bottom-performing aged intermediate animals had to be included in the impaired group due to the low numbers of AI we obtained per cohort in each of the treatment groups.
2.4. Morris Water Maze
The MWM task was performed as previously described (Burger et al., 2007). Morris Water Maze testing began 2–3 days after conclusion of OLM2. Briefly, during the first two days of water maze, animals were acclimated to the task with a visible platform placed in the exact center of the pool. The visual cue training was run with full view of the spatial cues used in the hidden platform training task. These training trials also served to test for visual acuity in aged rats, as this strain of rats (Fischer 344) are prone to retinal degeneration (Markowska et al., 1990). Animals were also screened for visible cataracts.
Following the two-day visible platform training, subjects were then trained in sets of four trials per day for eight days using a hidden platform (32 trials total). The inter-trial interval was 10 seconds, and animals remained on the platform until next trial. The rat was then immediately taken to the next drop location and the next trial began. At the end a subject’s four trials for a given day, the rat was dried with a towel before being placed in a heated dry-off cage until thoroughly dry, then returned to its home cage. If a rat was not able to find the platform after 90 seconds, he was guided to the platform and allowed to sit on it for 10 seconds before being removed and dried off. Animals were given four trials a day, except for the final day (Day 10) on which they were given four trials followed by a probe trial, as follows:
For the probe trial, the platform was removed immediately after the last hidden trial; the animal was reintroduced to the pool, and allowed to swim for 90 seconds. Percent of total distance covered and time spent in the target quadrant that previously contained the platform was measured. Number of platform crossings in the probe trial was calculated by tallying the number of times each subject entered the platform zone during the 90-second trial. A rat spending approximately 25% of total swim distance in the target quadrant was performing at chance levels and was not considered to have learned the task. During hidden and visible platform trials, the length of the swim path to the escape platform (or distance traveled in cm) was analyzed and measured. Swim speed during the probe trial was measured as the distance traveled (in m) divided by the total time spent ( 90 sec).
2.4.1. Criteria for categorizing SL and AI in the MWM
Aged animals were identified as aged superior learners when 40% (or greater) of total swim distance (cm) was spent in the target quadrant that previously contained the platform. Aged impaired learners spent approximately chance percentages of their total swim distance in the target quadrant (25% of individual’s total + SEM of young performance).
2.5. Statistics
The difference between OLM1 and OLM2 novelty indices was assessed using Paired T-tests for each group. The effect of treatment group on change in novelty index, number of platform crossings, percent distance in platform annulus, percent distance in wall annulus and gene expression were all assessed using 1-way ANOVA blocked by animal cohort followed by Tukey’s honest significant difference for pairwise comparisons.
A linear mixed-effects model was used to assess the differences in the learning trajectories among the three treatment groups for the MWM task. This model included a random effect for animal and a fixed effect for animal cohort. Following a finding of significant effects in the mixed effects model, the same model was used to test for differences among all pairs of treatment groups by applying it to a reduced data set (protected pairwise test).
The number of platform crossings in the MWM data was transformed to the log scale in order to obtain constant variability over the range of responses. Since platform crossings can be zero, the transformation log(x+1) was used. All statistical analyses were performed using the R statistical analysis package (R Development Core Team, 2010). Figures show means ± SEM.
2.6. Viral Vectors
Cloning and purification of rAAV-Homer1c has been previously described (Gerstein et al., 2012). Briefly, rat Homer1c was cloned by PCR from rat hippocampal mRNA. Recombinant virus was purified by iodixanol gradient purification followed by FPLC. Vector titers were determined by dot blot assay. The titer for rAAV-Homer1c used was 3×1013 genome copies/ml (2.4×1011 total genomes injected per animal). The titer for the rAAV-GFP used was 4.5×1013 gc/ml (3.6×1011 total genomes injected per animal).
2.7. Intracerebral injections of AAV vectors
All surgical procedures were performed using aseptic techniques and isofluorane gas anesthesia. Two injections per hemisphere were performed into the hippocampus using a stereotaxic frame (Kopf Instruments, Tujunga, CA). Coordinates for hippocampal injections into rats are as follows: Temporal site (AP = −2.75, Lat = +/−2, DV = −2.6); Septal site (AP = −4.35, Lat = +/−2.5, DV = −2.6). Subjects were maintained under isofluorane anesthesia (2.5% in oxygen) during the injection procedure. Injections were performed with a 10 µl Hamilton syringe fitted with a custom-made beveled 32-gauge needle (Hamilton). Each injection consisted of 2 µl of rAAV5 virus infused at a rate of 0.5 µl/minute. The needle was then left in place for 5 minutes prior to withdrawal from the brain. Subjects were allowed two weeks for recovery before the start of OLM2.
2.8. Protein Extraction and Western Blot Analysis
Hippocampi were dissected out from the brain 24 hours after the probe trial, snap frozen in liquid nitrogen, and stored at −80°C until ready to process. Tissue was lysed in RIPA buffer (50 mM Tris [pH 7.8], 150 mM HCl, 1% NP40, 0.5% Sodium Deoxycholate, 0.1% SDS) in the presence of mammalian protease inhibitors (1:100, Sigma, St. Louis, MO) and phosphatase inhibitors [in mM] (10 NaF, 2 Na Vanadate, 4 Na pyrophosphate,10 b-glycerophosphate). Following extraction, the hippocampi were triturated with a 28.5 gauge insulin syringe to shear up DNA. Lysates were spun down at 12 krpm for 30 min and the supernatant transferred to a clean tube. Protein was quantitated using the BCA protein assay (Thermo). 40 µg of protein extract was separated using 4–15% gradient SDS-Page gels from Bio-Rad (Hercules, CA) and then transferred to nitrocellulose membranes using Trans-Blot Turbo Transfer System (Bio-Rad). Antibodies were applied to blots using the SNAPiD System (Millipore, Billerica, MA) with a filter cartridge dedicated for each primary antibody. Primary antibodies used were Homer1b/c (Santa Cruz [#SC-8923], 1:60 dilution) and GAPDH (Millipore [clone 6C5], 1:75 dilution). GAPDH was diluted in 0.1% milk, while blocking and antibody dilutions for Homer1b/c utilized the BLØK™ Noise Canceling Reagent (Millipore) and this primary antibody was passed through the system twice to maximize signal. Concentrations of antibodies were optimized for use with the SNAPiD Protein Detection System. Western blots were developed with SuperSignal West Femto Maximum Sensitivity Substrate (Pierce Protein Research, Rockford, IL) and imaged with the GelDoc-It™ Imaging Systems by UVP LLC (Upland, CA). Bands were normalized against GAPDH expression and densitometric quantitation of immuno-positive bands was performed using Image J software (NIH). Two-tailed Student’s T-test was employed for statistical analysis using Prism 5 (Graphpad Software Inc, La Jolla CA).
3. Results
3.1. Gene delivery of Homer1c into the hippocampus of aged rats results in high levels of Homer1c expression
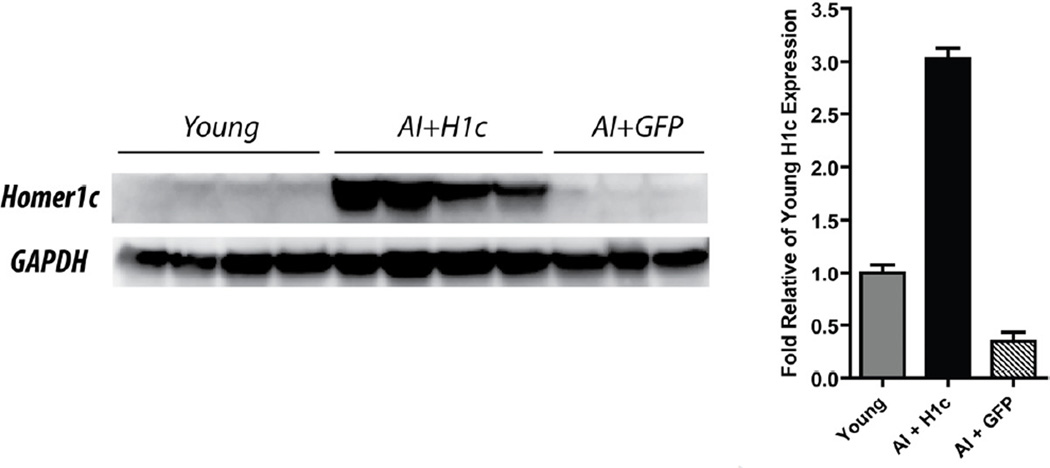
We used the OLM task to segregate a set of aged rats (n=38) into SL and AI groups, relative to young rats (n=20) (see methods). Four days after this first round of the OLM task (OLM1), AI and low performing intermediate animals were randomly assigned to two groups. Eight of these animals were injected with rAAV-Homer1c (AI+H1c) and seven animals were injected with rAAV-GFP as a control (AI+GFP). Ourselves and others have previously shown that GFP does not affect learning ability (Rex et al., 2010; Gerstein et al., 2012) and thus, is a good control for the effects of surgery and transgene expression on behavior. In order to determine the levels of recombinant Homer1c protein expressed in the AI+H1c animals, Western blot analysis was performed on hippocampal extracts at the end of behavioral testing. Densitometry analysis of the immuno-positive bands showed an 6-fold increase in Homer1c expression in the AI animals injected with rAAV-Homer1c relative to AI animals injected with rAAV-GFP, and a 3-fold increase in Homer1c expression in AI+H1c relative to young animals. AI injected with GFP show 2/3-fold lower expression levels than young rats (Fig. 1; One way ANOVA followed by Tukey’s HSD, F(2,20) = 210.4, p < 0.0001; AI+H1c vs. AI+GFP, p < 0.0001, AI+H1c vs. young, p < 0.0001; AI+GFP vs young, p = 0.0008).
Figure 1.
AI rats injected with rAAV-Homer1c show high levels of expression in the hippocampus. Left panel, representative Western blot showing the expression levels in the different experimental groups. Right panel, data presented as mean of optical density ± S.E.M (fold change relative to young expression levels after normalization to GAPDH expression [AI+H1c = 3.03 ± 0.1 fold above young; AI+GFP = 0.35 ± 0.08 fold difference from young]). Fold change was calculated from the following number of hippocampal hemispheres: AI+H1c n=8; AI+GFP n=4; young n=10. Each lane represents one hippocampal hemisphere. Lanes 1–2, 3–4, 5–6, 7–8, and 9–10 represent the left and right hemispheres from experimental animals, respectively. Lane 11 shows expression from the left hippocampal hemisphere of an aged animal.
3.2. Aged-related learning impairments on the object location memory task can be rescued by gene delivery of rAAV-Homer1c
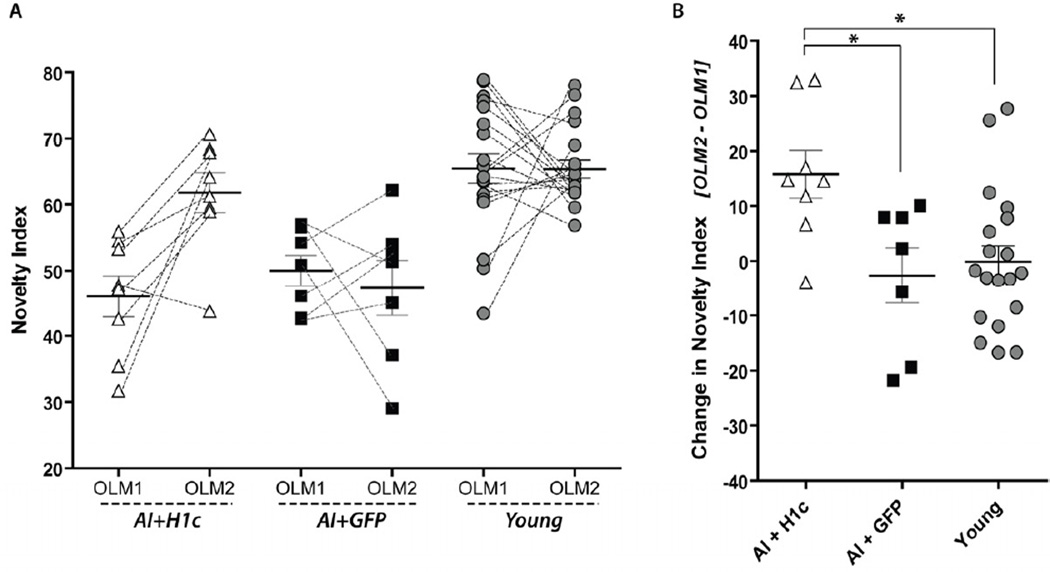
The cognitive ability of the different experimental groups (AI+H1c, AI+GFP, and young animals) was evaluated post-surgery in a second round of OLM using different objects (OLM2, see methods). We found that aged impaired learners injected with rAAV-Homer1c significantly improved their performance on the OLM2, as compared to their previous performance on this task in OLM1 (Fig. 2A: Paired t-test for AI+H1c; t(7) = 3.62, p = 0.0085). In contrast, neither the young nor the AI+GFP groups showed significant change in their novelty indices between OLM1 and OLM2 (Fig. 2A: Paired t-test for AI+GFP; t(6) = 0.53, p = 0.6154; Paired t-test for young; t(19) = 0.03, p = 0.9765). These latter results were expected, since all aged impaired animals used in this task failed to show a preference for the novel location in OLM1, and AI animals treated with rAAV-GFP as a control measure should not show a change in location preference. As the young group was already performing at high levels (with a high level of preference for the novel location) and did not undergo treatment, no change was expected.
Figure 2.
Expression of Homer1c improves performance of AI animals on the OLM task. (A) AI+H1c rats (open triangles) show a significant improvement in discrimination of novel object location post-treatment (OLM2) relative to pre-treatment (OLM1). Conversely, AI+GFP animals (black squares) performed poorly on both rounds of OLM. Young animals (grey circles) show a high level of discrimination of object location in both OLM1 and OLM2. (B) Change in Novelty Index was calculated as OLM2 - OLM1. This measure of change in performance shows that AI+H1c animals improved their performance on OLM while no change was seen in AI+GFP or young animals. (A, B): AI+H1c, n=8; AI+GFP, n=7; young, n=20.
The effect of group on the change in performance (OLM2 – OLM1) is shown in Figure 2B. Again, as young rats were already performing at a high level on the OLM, no change was expected, which is reflected with a novelty index change of approximately 0 for this group. One-way ANOVA shows a main effect of group on novelty index change (Fig. 2B: F(2,31) = 5.3264, p = 0.0105). Post-hoc comparisons using Tukey’s HSD show no difference between the Young and AI+GFP (p = 0.8521), and significant differences between AI+H1c and young subject groups (p = 0.0169), and between AI+H1c and AI+GFP (p = 0.0207), showing that AI+H1c animals improve their score on the OLM (an increase in preference for the object in the novel location) significantly more than either of the other groups.
Except for one individual, all AI and low-performing intermediate animals injected with Homer1c improved their performance on OLM2 over their OLM1 performance. AI and low-performing intermediate animals injected with GFP control vector did not uniformly change their performance on OLM2, as some show improvement while some worsen in the second round of this task.
3.3. Homer1c improves Morris Water Maze deficits found in aged-learning impaired rats
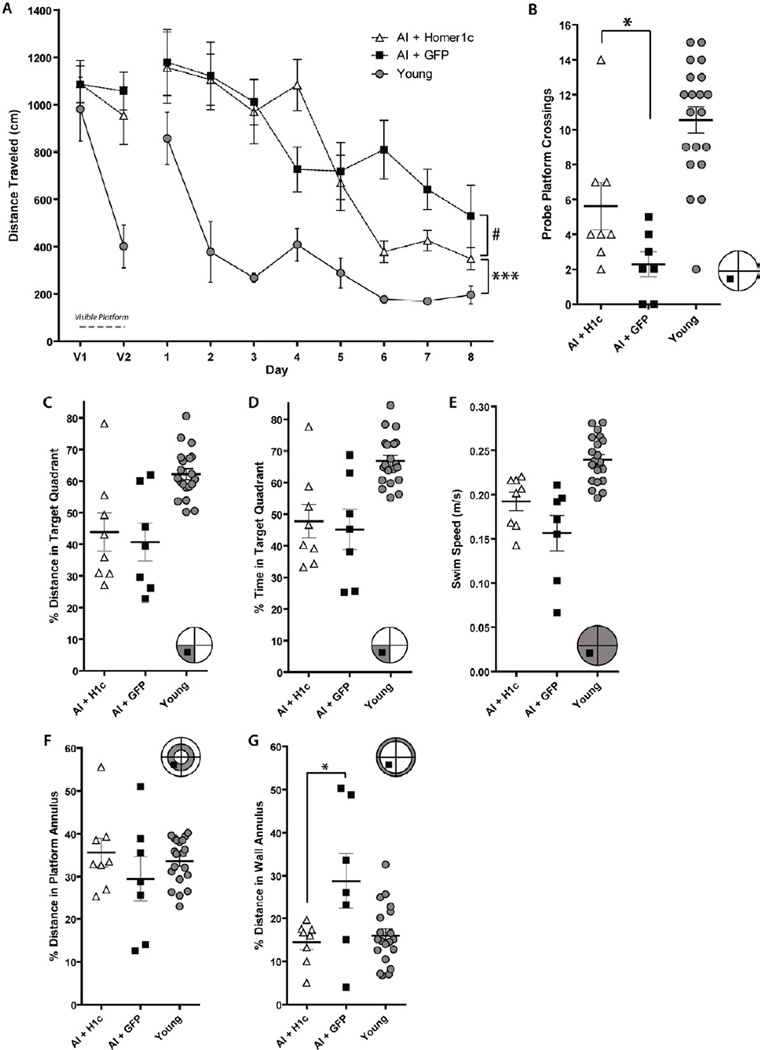
We have shown that individual performance in the OLM correlates with performance in MWM, in aged rats. Specifically, we found a significant correlation between individual OLM novelty index and percent distance spent in the target quadrant and platform crossings in the MWM probe trial when pooling all groups, young and aged, (OLM vs. probe percent distance, n = 55, r = 0.34, p = 0.0105; and OLM vs. probe platform crossings, r = 0.33, p = 0.0129; respectively, Gerstein et al. unpublished results). Therefore, we used the MWM as an additional way to test the animals’ abilities after gene transfer of Homer1c. After completion of OLM2, the three experimental groups of animals (AI+Homer1c, AI+GFP, young) were tested in the MWM. Because aged rats tend to swim slower than young rats, we analyzed path length taken to reach the escape platform (cm) instead of latency (time to find the platform) (Gage et al., 1984; Markowska et al., 1989; Clark et al., 1992; Gallagher et al., 1993). Path length was measured in each trial and data was binned by day (Fig. 3A). A significant interaction between group and time was found (F(18, 288) = 5.77, p < 0.0001) indicating that the three groups have significantly different learning paths over time on the hidden platform. Post hoc analysis via protected pairwise tests showed significant interactions between treatment group and time for all pairs of treatment group (young vs. H1c, p < 0.0001; young vs. GFP, p < 0.0001; H1c vs. GFP, p = 0.0186). Thus, superior task acquisition was seen in young animals as compared to AI rats from both treatment groups.
Figure 3.
AI rats expressing Homer1c show superior ability in MWM acquisition and probe trial performance than AI control rats. (A) MWM visible platform and acquisition curve. # indicates a significant difference between AI+H1c and AI+GFP performance in the last three data bins (Days 6–8, p < 0.05). Young animals learned the task significantly better than either aged AI groups (asterisks; p < 0.001). (B) A significant difference was seen between AI+H1c and AI+GFP groups on platform crossings in the probe trial (p < 0.05), while young perform significantly better than either of the aged groups (p < 0.01). (C) Analysis of percent swim distance in platform quadrant during the probe trial shows no difference in performance between AI+H1c and AI+GFP, with young animals performing significantly better than both aged groups (p < 0.01). (D) Analysis of percent swim time in platform quadrant during the probe trial shows no difference in performance between AI+H1c and AI+GFP, with young animals performing significantly better than both aged groups (p < 0.01). (E) Analysis of swim speed during the probe trial shows no difference in performance between AI+H1c and AI+GFP, with young animals performing significantly better than both aged groups (p < 0.01 and p < 0.001, respectively). (F) Analysis of percent swim distance in the platform annulus during the probe trial shows no significant effect of group. (G) Analysis of percent swim distance in the wall annulus during the probe trial shows that AI+GFP animals significantly preferred a thigmotaxic strategy as compared to AI+H1c or young animals (p < 0.05 for both comparisons). Young and AI+H1c groups showed similar levels of thigmotaxis. Maze schematics in the corner of (B-G); the shaded sections depict the area of the maze analyzed in that panel. The black square indicates the position of the platform during the training trials.
The AI+H1c rats decreased their distance to find the hidden platform over time relative to control AI rats late in the last three days of training, at the end of the learning phase. We have previously shown that superior aged rats show a slower task acquisition on the MWM as compared to young animals in this paradigm (Burger et al., 2007; 2008). It is during the final three days of MWM training that the performance of aged SL rats significantly diverges from AI rats based on spatial learning and memory ability. Thus, we examined the final three days of MWM training in our experiment to investigate whether there was a difference in performance between the aged AI+GFP and AI+H1c groups after initial task acquisition had been completed (Fig. 3A). Examining just these last three days showed no interaction between day and time (p = 0.1332) and a significant difference in average performance among all three groups (main effect of group, Days 6 – 8: F(2,31) = 127.32, p < 0.0001). Significant differences between all pairs of groups were seen in this same time frame via post hoc analysis (p ≤ 0.0174). Thus, towards the end of the MWM training, the learning curve of the AI+H1c animals dissociates from that of the AI+GFP controls, showing significant improvement in the rats injected with Homer1c.
After completion of training trials, the probe trial was performed in which the platform was removed and each animal was given 90 s to explore the pool (see methods). Both platform area crossings and percent distance spent in the platform quadrant were assessed. One-way ANOVA showed a significant effect of group in the number of platform crossings during the probe trial (Fig. 3B; F(2,31) = 21.07, p < 0.0001). We found that AI+H1c animals performed significantly better than AI+GFP animals, crossing the exact platform location significantly more times (p = 0.0153, Tukey’s HSD). Young animals showed a significantly higher number of platform crossings than either the AI+H1c or AI+GFP groups (p = 0.0146, p < 0.0001, respectively). No effect of group was found on percent of total distance spent in the platform quadrant (F(2,32), p = 0.3749). There was a significant effect of group on the percent of total time spent in the platform quadrant (F(2,31) = 12.78, p < 0.001). In pairwise tests there was no difference between the AI+H1c or AI+GFP groups. However, young animals performed significantly better than either of the AI groups (p ≤ 0.001 for both comparisons, Tukey’s HSD).
Additionally, swim speed (m/s) was assessed in the probe trial. A significant effect of group was found (main effect of group, F(2,31) = 17.49, p < 0.0001), however, post-hoc analysis using Tukey’s HSD showed that this was due to the faster swim speed in young animals rather than due to significant differences in swimming in the aged rat groups (Fig. 3E). Young animals swam significantly faster on the probe trial than both AI+GFP (p < 0.001) and AI+H1c (p < 0.01). No difference in swim speed was seen between AI+GFP and AI+H1c groups (p > 0.05).
To ascertain if subjects were using a spatial search strategy in the probe trial, percent swim distance in the platform annulus was assessed (Fig. 3F). All experimental groups (young, AI+H1c, AI+GFP) show similar amounts of search within the platform annulus, with no effect of group (F(2,31) = 0.9592, p = 0.3943). Thus, the use of a non-spatial egocentric search strategy (swimming a fixed distance from the perimeter) can be ruled out as the reason for the higher number of platform crossings seen in AI+H1c rats as compared to AI+GFP controls. We have previously shown that AI animals display higher levels of thigmotaxis than SL or young animals (Burger et al., 2007; 2008), therefore we also analyzed percent distance in the wall annulus. We found that AI+GFP rats showed a higher level of thigmotaxis in the probe trial (Fig. 3G) than other groups (main effect of group, F(2,31) = 5.64, p = 0.0086). Aged impaired rats injected with GFP spent a significantly higher percent of their total swim distance in the wall annulus (outer perimeter) of the pool than either the AI+H1c or the young animals, who more fully suppressed their natural preference for the pool wall in order to search for the platform (p ≤ 0.0171 for both comparisons, Tukey’s HSD).
It is important to note that, although aged rats did not perform as well as young rats in the visual version of the MWM (training days V1 and V2 in Fig. 3A), AI+H1c (as well as SL, unpublished data) show improvement in their ability to find the hidden platform over time. In addition, they also show spatial bias towards the target quadrant on the probe trial. Therefore, the initial impairments seen in the aged animals on the visible platform trials are probably not due to visual or motor deficits. Rather, this is likely to be an age-related slowness in procedural learning or task acquisition.
Based on these results, we conclude that the aged impaired animals injected with Homer1c better utilized a spatial search strategy in the MWM as compared to aged impaired animals injected with GFP as a control. Although AI+H1c animals did not perform to the level of young rats, improvement in spatial memory can be seen as compared to AI+GFP in both the OLM and MWM acquisition and probe trial platform crossing data.
4. Discussion
Our results show that the performance of aged learning-impaired rats can be improved in two tests of spatial memory by gene delivery of Homer1c to the dorsal hippocampus. By using a within-subjects design, we compared the performance of individuals on the OLM task before and after injection with Homer1c, clearly showing the effect of this treatment. The significantly enhanced group performance of AI+H1c over AI+GFP in the MWM suggests that Homer1c expression is sufficient to augment performance on this task as well.
We have previously found aged rat performance on the OLM test to show a small but significant correlation to performance on the probe trial of the MWM (OLM vs. probe percent distance, n = 55, r = 0.34, p = 0.0105; Gerstein et al., unpublished). However, it is not totally unsurprising that we found a difference between these tasks in the level of behavioral improvement with Homer1c treatment in aged impaired rats. Though the MWM and OLM tasks are both commonly used to study spatial memory and hippocampal function, these tasks differ in several ways. Disparities include the nature of the motivation, the reinforcement, the training paradigm required, and the duration of the learned spatial memory. In addition, these two tasks may not rely equally on the same molecular/cellular mechanisms and/or hippocampal subfields, and thus might be differentially sensitive to the aging process (Burger et al., 2010).
We find that expression of Homer1c in the hippocampus of AI rats can improve performance on the OLM, but shows a less robust effect on MWM training trial performance and MWM probe trial platform crossing performance. No differences were seen in percent of total distance or percent of total time spent in the platform quadrant between AI+H1c and AI+GFP groups. This could be attributed to the fact that aged rats swim slower than young rats, and that animals tend to slow down as the approach the platform (Whishaw et al., 1995); therefore platform crossings are a more accurate measurement of performance, since it measures the number of times the animal was in the exact prior location of the platform.
Due to the differences in these paradigms and the highly complex nature of the MWM, is not unexpected that a drug treatment would show different degrees of improvement on each of these spatial memory tests. In addition, the MWM is more heavily dependent on motor function, whereas the OLM has the advantage that it does not. Our MWM data is suggestive of an improvement in performance by gene delivery of Homer1c, whereas the OLM data is more conclusive in pointing towards a role for Homer1c in the enhancement of spatial memory ability in aged learning impaired animals. Thus, the OLM might be the more appropriate test for aged rats, minimizing the confounding effect of decreased motor function on tests of spatial learning.
It is important to note that the aged and young subjects were not equally active in the OLM and thus, had different levels of total exploration of the objects (both in training and testing phases). As a group, aged animals were less active in the OLM arena and spent less time exploring the objects than young rats. However, no differences were seen in total exploration time between SL and AI rats in either the training or test phases (data not shown). Thus, a difference in exploratory drive can be ruled out as a factor in the difference in performance between aged animals with different learning abilities (SL vs. AI rats).
The proportion of improvement seen in each AI animal injected with rAAV-Homer1c was not uniform. For example, the rat performing most poorly on the OLM1 became one of the very top performers in OLM2 after Homer1c treatment. Aged animals classified as impaired on the OLM1 that were then injected with rAAV-Homer1c improved their performance on OLM2 in 7 out of 8 individuals (Figure 2). Post-mortem analysis of the brain of the one individual who failed to improve shows that the rAAV-Homer1c vector did fully express in the dorsal hippocampus. It is unclear why this individual did not show improvement with Homer1c treatment, although given the age of the rats (~21 months) there are many possibilities as to the etiology of this variable response to Homer1c treatment, such as declining function of other related brain regions besides the hippocampus, the presence of tumors or other factors. Overall, treatment of AI rats with Homer1c was successful regardless of the relatively small number of animals used (n=8), showing that overexpression of Homer1c ameliorates age-related deficits in spatial memory.
The molecular mechanism by which Homer1c expression results in amelioration of AI performance needs to be explored. We have found that overexpression of Homer1c in Homer1 knockout mice results in upregulation of mGluR5 in astrocytes (Gerstein et al., 2012). Further, we have also shown that rescue of synaptic plasticity in these knockout mice is dependent on mGluR5 activation. Menard and Quirion (Ménard and Quirion, 2012) have shown an upregulation of mGluR5 and Homer1c in the hippocampal post-synaptic density of aged unimpaired rats following training in the MWM. This in turn results in activation of downstream signaling pathways, including ERK1/2 and mTOR (both pathways have been shown to cooperate in translation initiation in synaptic plasticity (Kelleher et al., 2004; Banko et al., 2006)). Therefore, mGluR5/Homer1c interactions appear to play a role in the molecular mechanism of learning and memory. Our preliminary data in Homer1 KO mice indicate that mGluR1/5 activation in the Homer1 KO expressing Homer1c results in phosphorylation of ERK1/2 and the downstream effector protein p70S6 kinase (unpublished data, Gerstein & Burger). A large number of genes have been implicated in successful cognitive aging (Burger, 2010). It will be of interest to study the pathways activated by rescue of aged impaired animals with Homer1c, to better understand the molecular mechanism involved in learning in the senescent brain.
In conclusion, our experiments show that Homer1c expression is sufficient to improve spatial memory in a rat model of age-related memory impairment. This finding emphasizes the role of Homer1c in learning and supports data from other studies in which overexpression of Homer1c improved spatial memory in young rats and in Homer1 KO mice (Klugmann et al., 2005; Gerstein et al., 2012). Together, these data implicate Homer1c in learning and memory formation, both early in life and during senescence. The significance of the role of Homer1 in cognitive aging stems from evidence showing that, in aged learning impaired rats, the levels of Homer1c mRNA in area CA1 of the hippocampus are decreased relative to those of successful aged learners after training in a MWM task (Burger et al., 2007). In addition, Homer1c protein levels are also decreased in the postsynaptic density of area CA1 in aged learning impaired animals after training in the MWM (Menard & Quirion, 2012). Thus, the results from this Homer1c gene replacement experiment validate both our microarray data (Burger et al., 2007) and the protein expression data mentioned above. Together, these studies suggest that during aging, lower levels of Homer1c in the hippocampus translate into comparatively worse spatial memory. These results point to Homer1c as a potential therapeutic target for improving cognitive function in the senescent brain.
Acknowledgments
We would like to thank Catherine Auger and Mary Behan for their critical reading of the manuscript. This research was supported by funds from the Department of Neurology, University of Wisconsin-Madison to C.B. H.G. was supported by the University of Wisconsin Neuroscience Training Program Grant NIH/NIGMS T32GM007507. M.J.L. was supported by grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources (NCRR), and now by the National Center for Advancing Translational Sciences (NCATS), grant 9U54TR000021. H.G. was also supported by the UW-Madison Graduate School. Finally, we would also like to acknowledge the rat subjects who contributed to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement for Authors
There are no actual or potential conflicts of interest.
References
- Banko JL, Hou L, Poulin F, Sonenberg N, Klann E. Regulation of eukaryotic initiation factor 4E by converging signaling pathways during metabotropic glutamate receptor-dependent long-term depression. Journal of Neuroscience. 2006;26:2167–2173. doi: 10.1523/JNEUROSCI.5196-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan A, O'Brien R, Roche KW, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Burger C. Region-Specific Genetic Alterations in the Aging Hippocampus: Implications For Cognitive Aging. Front. Ag. Neurosci. 2010;2:1–12. doi: 10.3389/fnagi.2010.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger C, López MC, Baker HV, Mandel RJ, Muzyczka N. Genome-wide analysis of aging and learning-related genes in the hippocampal dentate gyrus. Neurobiol Learn Mem. 2008;89:379–396. doi: 10.1016/j.nlm.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger C, López MC, Feller JA, Baker HV, Muzyczka N, Mandel RJ. Changes in transcription within the CA1 field of the hippocampus are associated with age-related spatial learning impairments. Neurobiol Learn Mem. 2007;87:21–41. doi: 10.1016/j.nlm.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Clark AS, Magnusson KR, Cotman CW. In vitro autoradiography of hippocampal excitatory amino acid binding in aged Fischer 344 rats: relationship to performance on the Morris water maze. Behavioral Neuroscience. 1992;106:324–335. doi: 10.1037//0735-7044.106.2.324. [DOI] [PubMed] [Google Scholar]
- deToledo-Morrell L, Geinisman Y, Morrell F. Age-dependent alterations in hippocampal synaptic plasticity: relation to memory disorders. Neurobiol Aging. 1988;9:581–590. doi: 10.1016/s0197-4580(88)80117-9. [DOI] [PubMed] [Google Scholar]
- Feng W, Tu J, Pouliquin P, Cabrales E, Shen X, Dulhunty A, Worley PF, Allen PD, Pessah IN. Dynamic regulation of ryanodine receptor type 1 (RyR1) channel activity by Homer 1. Cell Calcium. 2008;43:307–314. doi: 10.1016/j.ceca.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH, Björklund A. Cholinergic septal grafts into the hippocampal formation improve spatial learning and memory in aged rats by an atropine-sensitive mechanism. J Neurosci. 1986;6:2837–2847. doi: 10.1523/JNEUROSCI.06-10-02837.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH, Dunnett SB, Björklund A. Spatial learning and motor deficits in aged rats. Neurobiol Aging. 1984;5:43–48. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gerstein H, O'Riordan K, Osting S, Schwarz M, Burger C. Rescue of synaptic plasticity and spatial learning deficits in the hippocampus of Homer1 knockout mice by recombinant Adeno-associated viral gene delivery of Homer1c. Neurobiol Learn Mem. 2012;97:17–29. doi: 10.1016/j.nlm.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Ozawa F, Saitoh Y, Hirai K, Inokuchi K. vesl, a gene encoding VASP/Ena family related protein, is upregulated during seizure, long-term potentiation and synaptogenesis. FEBS Lett. 1997;412:183–189. doi: 10.1016/s0014-5793(97)00775-8. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, Govindarajan A, Jung H-Y, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Klugmann M, Symes CW, Leichtlein CB, Klaussner BK, Dunning J, Fong D, Young D, During MJ. AAV-mediated hippocampal expression of short and long Homer 1 proteins differentially affect cognition and seizure activity in adult rats. Mol Cell Neurosci. 2005;28:347–360. doi: 10.1016/j.mcn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Ingram DK, Barnes CA, Spangler EL, Lemken VJ, Kametani H, Yee W, Olton DS. Acetyl-1-carnitine. 1: Effects on mortality, pathology and sensory-motor performance in aging rats. Neurobiol Aging. 1990;11:491–498. doi: 10.1016/0197-4580(90)90109-d. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Stone WS, Ingram DK, Reynolds J, Gold PE, Conti LH, Pontecorvo MJ, Wenk GL, Olton DS. Individual differences in aging: behavioral and neurobiological correlates. Neurobiol Aging. 1989;10:31–43. doi: 10.1016/s0197-4580(89)80008-9. [DOI] [PubMed] [Google Scholar]
- Ménard C, Quirion R. Successful Cognitive Aging in Rats: A Role for mGluR5 Glutamate Receptors, Homer 1 Proteins and Downstream Signaling Pathways. PLoS ONE. 2012;7:e28666. doi: 10.1371/journal.pone.0028666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Gavin CF, Rubio MD, Kramar EA, Chen LY, Jia Y, Huganir RL, Muzyczka N, Gall CM, Miller CA, et al. Myosin IIb Regulates Actin Dynamics during Synaptic Plasticity and Memory Formation. Neuron. 2010;67:603–617. doi: 10.1016/j.neuron.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi JA, Huber KM. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. Journal of Neuroscience. 2008;28:543–547. doi: 10.1523/JNEUROSCI.5019-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz D, Huston JP, Jezek K, Haas HL, Roth-Härer A, Selbach O, Luhmann HJ. Water maze performance, exploratory activity, inhibitory avoidance and hippocampal plasticity in aged superior and inferior learners. Eur J Neurosci. 2002;16:2175–2185. doi: 10.1046/j.1460-9568.2002.02282.x. [DOI] [PubMed] [Google Scholar]
- Sun J, Tadokoro S, Imanaka T, Murakami SD, Nakamura M, Kashiwada K, Ko J, Nishida W, Sobue K. Isolation of PSD-Zip45, a novel Homer/vesl family protein containing leucine zipper motifs, from rat brain. FEBS Lett. 1998;437:304–308. doi: 10.1016/s0014-5793(98)01256-3. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Kleschen MJ, Oleson EB, Dehoff MH, Schwartz MK, Seeberg PH, Worley PF, Kalivas PW. Behavioral and neurochemical phenotyping of Homer1 mutant mice: possible relevance to schizophrenia. Genes Brain Behav. 2005;4:273–288. doi: 10.1111/j.1601-183X.2005.00120.x. [DOI] [PubMed] [Google Scholar]
- Tu J, Xiao B, Yuan JP, Lanahan A, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Cassel JC, Jarrad LE. Rats with fimbria-fornix lesions display a place response in a swimming pool: a dissociation between getting there and knowing where. J. Neurosci. 1995;15:5779–5788. doi: 10.1523/JNEUROSCI.15-08-05779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Tu J, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan A, Wenthold RJ, Worley PF. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]