Abstract
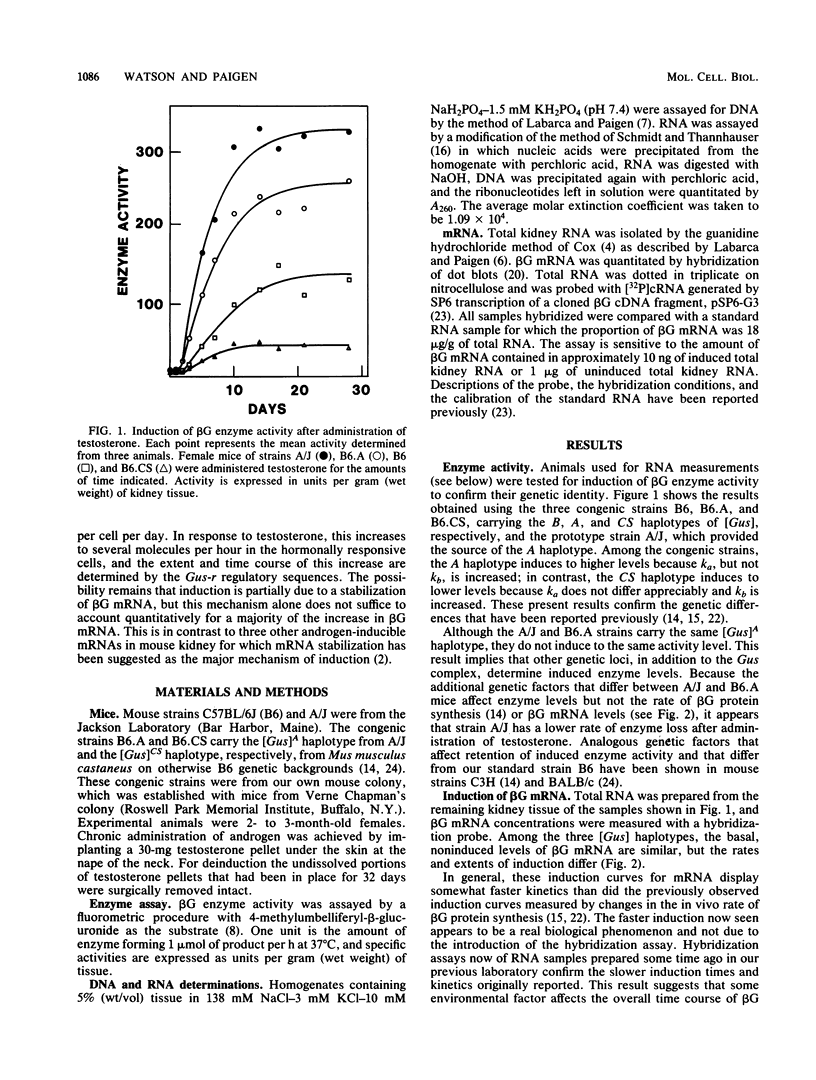
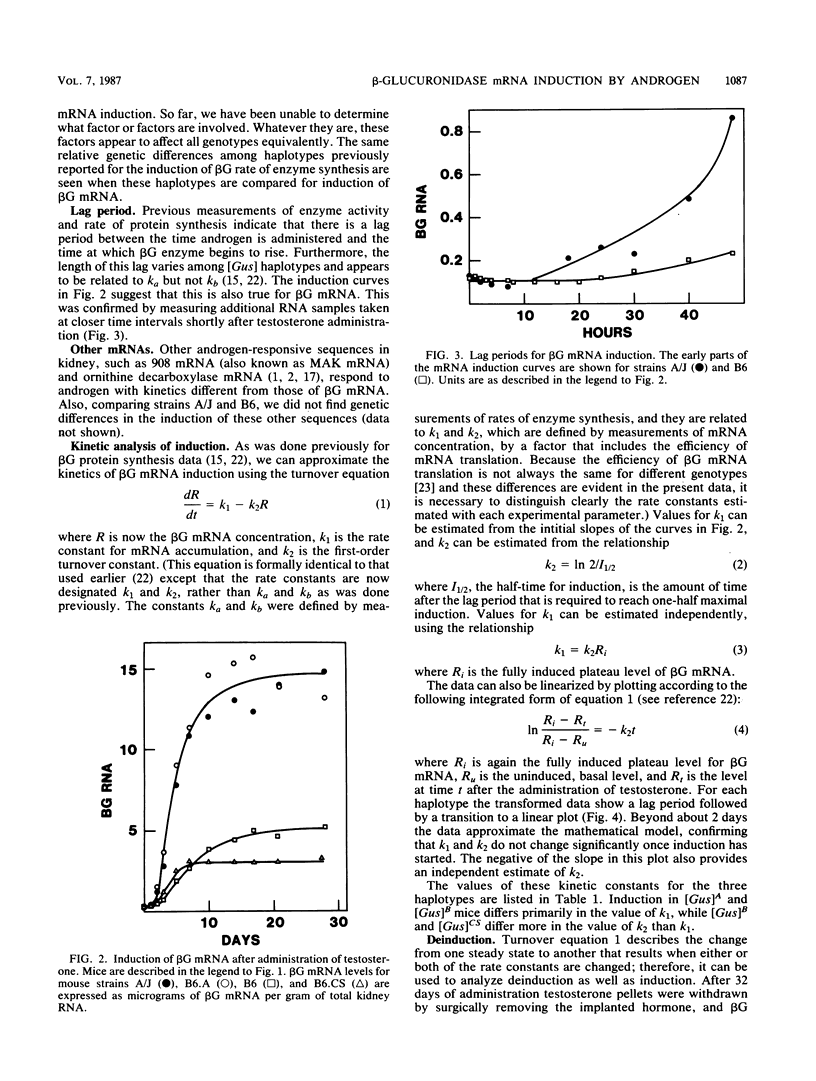
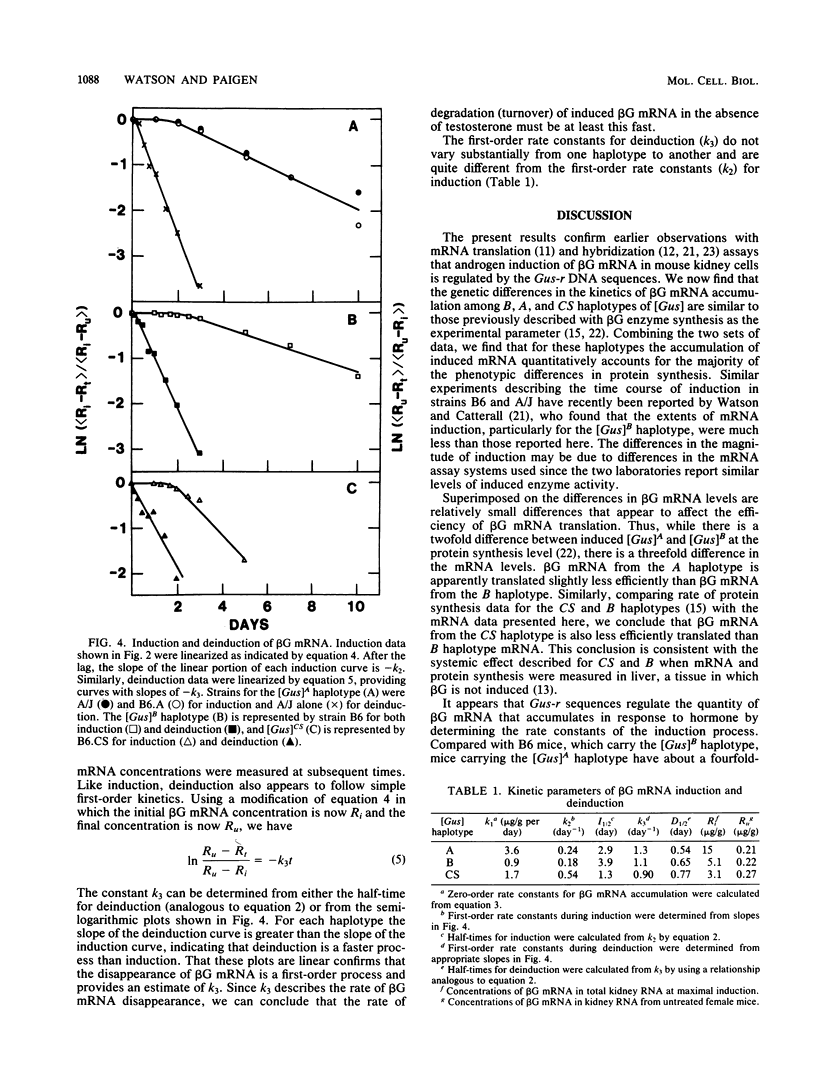
The kinetics of beta-glucuronidase mRNA induction by androgen in mouse kidney were determined for A, B, and CS haplotypes of the beta-glucuronidase gene. After a lag period, the kinetics of mRNA (R) induction are approximated by the turnover equation dR/dt = k1 - k2R. The A haplotype differs from the B primarily in the duration of the lag period and in k1, the rate constant determining the initial slope of the induction curve. The CS haplotype differs from B primarily in k2, the first-order rate constant that determines the half-time for induction. None of the haplotypes differs significantly in the half-life of beta-glucuronidase mRNA as measured by deinduction. Thus, there was no correlation between the half-time or extent of induction and the half-life of the RNA. Comparing half-times for induction with the half-life of the mRNA suggests that message stabilization can at most account for only part of the induction. We conclude that transcriptional activation of the beta-glucuronidase gene must be an important component of induction. Estimating absolute numbers of mRNA molecules and absolute rates of gene transcription, it appears that before induction there is approximately one molecule of beta-glucuronidase mRNA per cell and that each gene copy is transcribed once every 35 to 40 h. Depending on the haplotype examined, after induction, mRNA goes up to 80 to 400 molecules per induced cell. In the A haplotype, which has the highest induction, this corresponds to one transcript from each gene every 6 min if there is no induced stabilization of beta-glucuronidase mRNA, and one every 30 min if there is. Thus, it seems unlikely that more than one transcript is ever being synthesized at the same time from the beta-glucuronidase gene.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger F. G., Gross K. W., Watson G. Isolation and characterization of a DNA sequence complementary to an androgen-inducible messenger RNA from mouse kidney. J Biol Chem. 1981 Jul 10;256(13):7006–7013. [PubMed] [Google Scholar]
- Berger F. G., Loose D., Meisner H., Watson G. Androgen induction of messenger RNA concentrations in mouse kidney is posttranscriptional. Biochemistry. 1986 Mar 11;25(5):1170–1175. doi: 10.1021/bi00353a034. [DOI] [PubMed] [Google Scholar]
- Catterall J. F., Leary S. L. Detection of early changes in androgen-induced mouse renal beta-glucuronidase messenger ribonucleic acid using cloned complementary deoxyribonucleic acid. Biochemistry. 1983 Dec 20;22(26):6049–6053. doi: 10.1021/bi00295a001. [DOI] [PubMed] [Google Scholar]
- FISHMAN W. H. Beta-glucuronidase and the action of steroid hormones. Ann N Y Acad Sci. 1951 Dec;54(4):548–557. doi: 10.1111/j.1749-6632.1951.tb46612.x. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. MRNA-directed synthesis of catalytically active mouse beta-glucuronidase in Xenopus oocytes. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4462–4465. doi: 10.1073/pnas.74.10.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owerbach D., Luis A. J. Phenobarbital induction of egasyn: availability of egasyn in vivo determines glucuronidase binding to membrane. Biochem Biophys Res Commun. 1976 Apr 5;69(3):628–634. doi: 10.1016/0006-291x(76)90922-0. [DOI] [PubMed] [Google Scholar]
- Paigen K. Acid hydrolases as models of genetic control. Annu Rev Genet. 1979;13:417–466. doi: 10.1146/annurev.ge.13.120179.002221. [DOI] [PubMed] [Google Scholar]
- Paigen K., Jakubowski A. F. Progressive induction of beta-glucuronidase in individual kidney epithelial cells. Biochem Genet. 1982 Oct;20(9-10):875–881. doi: 10.1007/BF00484065. [DOI] [PubMed] [Google Scholar]
- Paigen K., Labarca C., Watson G. A regulatory locus for mouse beta-glucuronidase induction, Gur, controls messenger RNA activity. Science. 1979 Feb 9;203(4380):554–556. doi: 10.1126/science.760204. [DOI] [PubMed] [Google Scholar]
- Palmer R., Gallagher P. M., Boyko W. L., Ganschow R. E. Genetic control of levels of murine kidney glucuronidase mRNA in response to androgen. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7596–7600. doi: 10.1073/pnas.80.24.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister K., Chapman V., Watson G., Paigen K. Genetic variation for enzyme structure and systemic regulation in two new haplotypes of the beta-glucuronidase gene of Mus musculus castaneus. J Biol Chem. 1985 Sep 25;260(21):11588–11594. [PubMed] [Google Scholar]
- Pfister K., Paigen K., Watson G., Chapman V. Expression of beta-glucuronidase haplotypes in prototype and congenic mouse strains. Biochem Genet. 1982 Jun;20(5-6):519–536. doi: 10.1007/BF00484702. [DOI] [PubMed] [Google Scholar]
- Pfister K., Watson G., Chapman V., Paigen K. Kinetics of beta-glucuronidase induction by androgen. Genetic variation in the first order rate constant. J Biol Chem. 1984 May 10;259(9):5816–5820. [PubMed] [Google Scholar]
- Snider L. D., King D., Lingrel J. B. Androgen regulation of MAK mRNAs in mouse kidney. J Biol Chem. 1985 Aug 15;260(17):9884–9893. [PubMed] [Google Scholar]
- Swank R. T., Paigen K., Ganschow R. E. Genetic control of glucuronidase induction in mice. J Mol Biol. 1973 Dec 5;81(2):225–243. doi: 10.1016/0022-2836(73)90191-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C. S., Catterall J. F. Genetic regulation of androgen-induced accumulation of mouse renal beta-glucuronidase messenger ribonucleic acid. Endocrinology. 1986 Mar;118(3):1081–1086. doi: 10.1210/endo-118-3-1081. [DOI] [PubMed] [Google Scholar]
- Watson G., Davey R. A., Labarca C., Paigen K. Genetic determination of kinetic parameters in beta-glucuronidase induction by androgen. J Biol Chem. 1981 Mar 25;256(6):3005–3011. [PubMed] [Google Scholar]
- Watson G., Felder M., Rabinow L., Moore K., Labarca C., Tietze C., Vander Molen G., Bracey L., Brabant M., Cai J. D. Properties of rat and mouse beta-glucuronidase mRNA and cDNA, including evidence for sequence polymorphism and genetic regulation of mRNA levels. Gene. 1985;36(1-2):15–25. doi: 10.1016/0378-1119(85)90065-4. [DOI] [PubMed] [Google Scholar]
- Watson G., Paigen K. Segregation of genetic determinants for murine glucuronidase synthesis and loss in CXB recombinant-inbred strains. Biochem Genet. 1978 Oct;16(9-10):897–903. doi: 10.1007/BF00483741. [DOI] [PubMed] [Google Scholar]


