Abstract
The contribution of the autosomal dominant mutations to the etiology of familial Alzheimer’s disease (AD) is well characterized. However, the molecular mechanisms contributing to sporadic AD are less understood. Increased ceramide levels have been evident in AD patients. We previously reported that increased ceramide levels, regulated by increased serine palmitoyltransferase (SPT), directly mediate amyloid β (Aβ) levels. Therefore, we inhibited SPT in an AD mouse model (TgCRND8) through subcutaneous administration of L-cylcoserine (LCS). The cortical Aβ42 and hyperphosphorylated tau levels were down regulated with the inhibition of SPT/ceramide. Positive correlations were observed between cortical SPT, ceramide and Aβ42 levels. With no evident toxic effects observed, inhibition of SPT could be a safe therapeutic strategy to ameliorate the AD pathology. We previously observed that miR-137, -181c, -9 and 29a/b post-transcriptionally regulate SPT levels, and the corresponding miRNA levels in the blood sera are potential diagnostic biomarkers for AD. Here, we observe a negative correlation between cortical Aβ42 and sera Aβ42, and a positive correlation between cortical miRNA levels and sera miRNA levels suggesting their potential as non-invasive diagnostic biomarkers.
Keywords: Alzheimer’s disease, serine palmitoyltransferase, inhibition, amyloid beta, tau hyperphosphorylation, microRNA
Introduction
There is consistent evidence suggesting ceramide, a sphingolipid, is increased in Alzheimer’s disease (AD) patients (Han et al., 2002; Cutler et al., 2004; Satoi et al., 2005; He et al., 2010). Several in vitro and in vivo studies indicate associations between ceramides and Aβ, and signifying elevated ceramide levels as a possible risk factor for AD (Cutler et al., 2002; Gulbins and Kolesnick, 2003; Puglielli et al., 2003; Kalvodova et al., 2005; Mattson et al., 2005). Membrane ceramides, the major component of lipid rafts, in addition to stabilizing BACE1 (Puglielli et al., 2003; Costantini et al., 2007), facilitates Aβ production by translocating the pathogenic secretases to the primary location of amyloidogenesis (Lee et al., 1998; Vetrivel et al., 2004; Vetrivel et al., 2005; Hur et al., 2008; Haughey et al., 2010), the lipid rafts (Sisodia, 1992; Cordy et al., 2003; Ehehalt et al., 2003; Wada et al., 2003; Won et al., 2008). In a previous study we demonstrated that serine palmitoyltransferase (SPT), the first rate limiting enzyme in the de novo ceramide synthesis pathway (Merrill et al., 1985; Hanada et al., 1997; Hannun and Obeid, 2008), regulates ceramide levels through elevated serine palmitoyltransferase long chain 1 (SPTLC1) and serine palmitoyltransferase long chain 2 (SPTLC2) levels in AD (Geekiyanage and Chan, 2011). We found that SPT, post-transcriptionally regulated by miRNAs, directly regulate Aβ levels in AD (Geekiyanage and Chan, 2011).
Activation of SPT raises ceramide levels (Perry et al., 2000) while inhibition of SPT decreases ceramide levels, both in vitro and in vivo (Hojjati et al., 2005; Holland et al., 2007; Patil et al., 2007; Strettoi et al., 2010). L-cylcloserine has been established to be a potent inhibitor of SPT (Sundaram and Lev, 1984a, b; Williams et al., 1987). Long-term subcutaneous administration of LCS on alternate days for 2 months exclusively reduced brain cerebroside levels (Sundaram and Lev, 1989), which essentially consist of ceramides. The route of LCS administration, subcutaneous or intraperitoneal, not only determined the class of glycolipids inhibited, but also influenced the extent of the side effects, with minimal toxic effects observed with prolonged subcutaneous (as oppose to intraperitoneal) administration (Sundaram and Lev, 1985). In contrast, oral administration has demonstrated little reduction in brain cerebroside levels (Sundaram and Lev, 1989). Additionally, cycloserine also functions as a partial agonist of NMDA receptors and facilitate the activation of NMDA receptors in AD brains (Chessell et al., 1991). Cognitive enhancements have been observed with the treatment of cycloserine in mice (Quartermain et al., 1994) and rats (Schuster and Schmidt, 1992; Myhrer and Paulsen, 1997; Stromme Johannesen and Myhrer, 2002). Furthermore, AD patients have shown significant cognitive improvement with the treatment of cycloserine (100 mg/day for 14 days) in a double-blinded controlled clinical trial (Tsai et al., 1999).
Given that research demonstrate inhibition of SPT decreases neuronal cell death by Aβ (Cutler et al., 2004) and induces non-amyloidogenic processing of amyloid beta precursor protein (APP) (Sawamura et al., 2004), and our findings that show SPT directly regulates Aβ levels (Geekiyanage and Chan, 2011), here we investigated the inhibition of SPT as a potential therapeutic strategy for AD using the potent SPT inhibitor, cycloserine. Concomitantly, our observations show that high-fat diet increases cortical ceramide and SPT levels (Geekiyanage and Chan, 2011), and other research suggest high dietary fat intake is a potential risk factor for AD (Julien et al., 2010). Therefore, we sought to incorporate the dietary risk component into our study, with emphasis on the inhibition of SPT as a potential therapeutic strategy for AD.
Material and Methods
Mice
TgCRND8 mice, an early-onset transgenic mouse model, encoding the double mutant form of the amyloid precursor protein 695 (KM670/671NL1V717F) under the control of the PrP gene promoter was used for this study (Chishti et al., 2001). DNA was extracted for genotyping using QIAamp DNA mini kit (Qiagen) following PCR and gel electrophoresis from tail tips from mice ear-tagged for identification. The primers used were APP: 5’-AACAGAAGGACAGACAGCAC-3’ and 5’-GTTTCCGTAACTGATCCTTG-3’
12 TgCRND8 mice (7 males and 5 females) were fed a control chow diet and 13 TgCRND8 mice (5 males and 8 females) were fed a 60% kcal high-fat diet (D12492, Research Diets) starting at 4 weeks of age and continuing up to 4 months of age, while 13 other TgCRND8 mice (5 males and 8 females) were administered 10 mg/kg of LCS subcutaneously via surgically implanted osmotic pumps (ALZET, 2004) at 3 months of age, that delivered LCS at a constant rate of 0.25 uL/hour for a period of 28 days, while being fed the 60% kcal high-fat diet stating from 4 weeks to 4 months of age. Isoflurane to-effect, with the use of a vaporizer, was used as the anesthetic agent. Mice were placed into the 3 treatment groups based on availability. Mice were randomly assigned to the 3 treatment groups at the outset of the experiment (i.e. there was no strategy incorporated to exclusively include or exclude mice in to a particular treatment group). Due to the high mortality rate, mice were supplemented to each treatment group as they became available in order to maintain relatively equivalent sample sizes. 78 mice were fed a control chow diet or a high-fat diet with only 12 mice on a control chow diet and 13 mice (1 eliminated due to infection) on a high-fat diet surviving to term (4 months of age) and 14 mice survived until surgery (3 months of age) (survival rate ~50% (38 out of 78 survived)). 14 mice were administered with LCS and 13 mice survived to term while 1 mouse died of the Pasteurella pneumotropica infection in the colony (survival rate of ~90% from the time of surgery (28 days)) (see supplementary Fig. S1 for random assignment to treatment group and survival information). All mice were euthanized (12 controls, 13 high-fat diet and 13 LCS administered) at 4 months of age and their brains were sectioned for histopathology, and the brain cortices and blood sera were extracted for ceramide, protein and RNA analyses.
The colony had a severe Pasteurella pneumotropica infection which was lethal in addition to the 40% mortality rate observed in TgCRND8 mice (Chishti et al., 2001). Infected mice from the control chow diet and high-fat diet categories were treated with Baytril (Bayer) subcutaneously or orally (dissolved in their daily water consumption). (At the time in which the colony was infected, some of the animals from the control diet and high-fat diet categories were already euthanized and their brains and blood was harvested. Thus, all mice were not treated with Baytril. In attempts to maintain homogeneity within the groups only the animals infected were treated). However, all animals in the control chow and high-fat diet categories, that were treated with Baytril died from the infection, except 1 in the high-fat diet category. All mice that underwent surgical implantation were administered a single dose of the analgesic meloxicam (1–2 mg/kg) subcutaneously at the time of surgery while Baytril (0.003%) was dissolved in their daily water consumption (250 mL) from 2 days prior to surgery until the time of euthanasia (see supplementary Table S1 for information on nutrient and drug treatment on the 3 categories). The 1 animal that survived in the high-fat diet category treated with Baytril was eliminated from this study to maintain homogeneity in the group. This mouse showed increased brain cortical ceramide levels by ~21% (d18:1; 16:0) to ~37% (d18:1; 18:0) compared with the average ceramide levels in the mice fed a control chow diet, possibly suggesting that the reduced ceramide and Aβ42 observed in this study is due to inhibition of SPT and not the oral administration of Baytril. It must be noted that a placebo control treatment for LCS (i.e. saline) was not incorporated in this study due to the limited number of animals available with the increased deaths and slow breeding rates. All procedures conducted were approved by the Institutional Animal Care and Use Committee at Michigan State University.
Special staining
Tissue samples previously fixed in 10% neutral buffered formalin were processed and vacuum infiltrated with paraffin on the ThermoFisher Excelsior tissue processor followed by embedding with the Thermo Fisher Histo-Centre III embedding station. Once the blocks were cooled, excess paraffin was removed from the edges and placed on a Reichert Jung 2030 rotary microtome faced to expose the tissue sample. Once the blocks were faced they were cooled and finely sectioned at 8 µM. Sections were dried overnight at room temperature followed by incubation at 56°C to ensure adherence to the slides for 2 – 24 hours. Slides were removed from the incubator and de-paraffinized with 2 changes of xylene for 5 minutes each, 2 changes of absolute ethanol for 2 minutes each, 2 changes of 95% ethanol for 2 minutes each, and under running tap water for 2 minutes followed by a distilled water rinse.
Thioflavin-S staining was conducted according to (Klatt, 1994–2012) with 5 minutes in 1% aqueous Thioflavin-S followed by 5 minutes in 70% ethanol with several distilled water rinses and mounting with aqueous media.
Bielschowsky staining was conducted according to (Sheehan, 1980) with modifications. A preliminary 20% aqueous silver nitrate impregnation at 37°C for 10 minutes is followed by an ammonical silver treatment at 37°C for 8 minutes. 1% ammonium hydroxide stops the fiber development and silver is deposited on the neurofibrils and axons, and reduce to a visible metallic silver (black) by the actions of the formaldehyde reducing agent in a water bath at 70°C for 2–3 minutes followed by 1% ammonium hydroxide and 5% sodium thiosulfate incubations to remove any unreduced silver from the tissue. No counter-stain was employed. Dehydration and clearing was performed using 1 change of 95% ethanol for 2 minutes, 4 changes of 100% ethanol for 2 minutes each, 4 changes of xylene for 2 minutes each, followed by cover-slipping with synthetic mounting media for permanent retention and visualization. All staining were conducted double-blindedly.
Immunohistochemistry
Formalin fixed specimens were processed, embedded in paraffin and sectioned on a rotary microtome at 4 µm. Sections were placed on adhesive slides and dried at room temperature followed by 56 ° C overnight drying. The slides were subsequently de-paraffinized in xylene and hydrated through descending concentrations of ethanol to distilled water. Slides were placed in Tris buffered saline pH 7.5 for 5 minutes for pH adjustment. Heat induced (in a rice steamer for 30 minutes followed by 10 minutes at room temperature) or enzyme induced epitope retrieval (10 minutes at 37 °C) was conducted followed by subsequent rinses and blocking for endogenous peroxidase using 3% hydrogen peroxide/methanol bath (1:4 ratio) for 30 minutes at room temperature followed by running tap and distilled water rinses. Following pretreatments, standard avidin – biotin complex staining steps were performed at room temperature on the Dako Autostainer by rinsing with Tris buffered saline + Tween 20 between staining steps. Slides were blocked for non-specific protein with normal goat serum (Vector Labs) for 30 minutes. Endogenous biotin was blocked by incubation in avidin D (Vector Labs) and d-biotin (Sigma) for 15 minutes. Rabbit polyclonal antibodies were diluted in normal antibody diluent (NAD) (Scytek – Logan) and incubated for 1 hour at room temperature. Biotinylated goat anti-Rabbit and IgG H+L (Vector Labs) were diluted to 11ug/ml and incubated for 30 minutes followed by a 30 minute incubation with R.T.U. VectaStain Elite ABC Reagent (Vector Labs). The reaction was developed with Nova Red (Vector Labs) for 15 minutes followed by counterstaining in Gill 2 hematoxylin (Richard-Allan Scientific Co) for 10 seconds and differentiated in 1% aqueous glacial acetic acid and rinsed under running tap water. Slides were then dehydrated through ascending grades of ethanol; cleared through several changes of xylene and cover-slipped using Flotex permanent mounting media. The sections were pretreated with 0.03% Pronase E in TBS at 37°C for 10 minutes for β-amyloid (1:400 dilution) (Cell Signalling Technology) and NF200 (1:100 dilution) (Sigma), and Scytek citrate Plus at pH 6.0 in a steamer (30 minute), and on the bench top (10 minute) for SPTLC2 (1:100 dilution) (Abcam), with no pretreatment for SPTLC1 (1:100 dilution) (Proteintech group). All staining were conducted double-blind.
Quantification of plaques
For Thioflavin-S (fluorescence) and Aβ (transmitted light) antibody, whole-brain sections were imaged with an Olympus FluoView FV1000 Confocal Laser Scanning Microscope (Olympus America, Inc, Center Vally, PA) configured with the 4× UPlanFLN (NA 0.13). Identical imaging parameters were used for all sections. Individual images across the brain section were collected using the Multi Area Time Lapse Controller within the Olympus FluoView Advanced Software (version 3.1). The individual images were then stitched together to generate a single image of the entire brain slice using the Multi Area Time Lapse Viewer within the Olympus FluoView Advanced Software. The default threshold adjusting parameter with adjusted brightness in the NIH-imageJ particle analyses was used to determine plaque area and integrated densities with the same thresholding parameters for all the sections while the plaque numbers were counted manually. Imaging quantification and counting were conducted in a double-blinded manner.
Bright-field image generation with camera
The Olympus DP72 camera and DP2-BSW software (version 2.2) were used to generate images under bright-field. The area imaged was the right or left cortex area above the hippocampus, configured with either 10× UPlanSApo (NA 0.40), 20× UPlanSApo (NA 0.50) or 40× UPlanSApo (NA 1.00) oil immersion objective.
Protein extraction and western blot analysis
Mouse brain cortices, homogenized in 5M guanidine HCl/ 50mM Tris HCl, were lysed followed by protein extraction and western blot analyses using NuPAGE® Novex 12% Bis-Tris Gels, as described previously (Geekiyanage and Chan, 2011). Protein quantifications were conducted by normalizing to β-Actin. Western blots were quantified using Quantity One (BioRad) version 4.5.
Enzyme-linked immunosorbent assay (ELISA)
Proteins were extracted with 5M guanidine HCl/ 50mM Tris HCl from mouse cortices and blood sera. ELISA was performed for human-Aβ42 using KHB3441 (Invitrogen) according to the manufacturer’s instruction. The cortical Aβ42 levels were calculated by normalizing to the total protein levels measured by Bradford’s assay.
Quantitative RT-PCR (qRT-PCR)
Total miRNAs were extracted from brain cortices and blood sera using miRNeasy Mini Kit (Qiagen) and RNeasy MinElute Cleanup Kit (Qiagen), total RNA was quantified using ND-1000 nanodrop spectrophotometer as described previously (Geekiyanage and Chan, 2011; Geekiyanage et al., 2012). RNU6B was used as the normalizing control for cortical samples (Geekiyanage and Chan, 2011) while miR-22, which was found to be abundant and stable in blood sera of AD patients, was used as the normalizing control for sera samples as described previously (Geekiyanage et al., 2012).
Ceramide quantification
Lipids were extracted from homogenized mouse brain cortices and blood sera according to Bligh and Dyer (Bligh and Dyer, 1959) following tandem mass spectrometry (MS/MS) using Quattro Premier XE (Waters), Acquity ultra performance liquid chromatography (Waters) (LC-MS/MS) and Mass Lynx 4.1 software. External ceramide standards and C12:0 internal standards were purchased from Avanti, Polar Lipid Inc.
Lactate Dehydrogenase (LDH) Enzyme Assay
LDH assay kit specific for measuring sera LDH levels, IDTox™ LDH color endpoint assay kit was purchased from ID Labs Biotechnology Inc. The assay was conducted according manufacturer’s instructions.
Antibodies
The antibodies used were, LCB1 (BD Transduction Laboratories™), SPTLC1 (proteintech group), SPTLC2 (Abcam), β-Actin (Sigma), β-Amyloid (cell signaling), Phospho-PHF-tau pSer202/Thr205 Monoclonal Antibody (AT8) (Thermo scientific).
Statistical analysis
Statistical significances were determined by Mann-Whitney U tests and Spearman’s correlation (2 tailed-T distribution test).
Results
In order to investigate the inhibition of SPT as a therapeutic strategy for AD, we used an early onset transgenic model, TgCRND8, encoding a double mutant form of APP 695 (KM670/671NL1V717F) under the control of the PrP gene promoter (Chishti et al., 2001). TgCRND8 mice were fed a control chow diet (n=12) or a 60% kcal high-fat-diet (n=13, referred to as “fed a high-fat diet”) starting from 4 weeks to 4 months of age. Another group of mice (n=13, referred to as “administered with LCS”) were administered 10mg/kg of a SPT inhibitor, LCS, via subcutaneous surgical implantation of an osmotic pump at 3 months of age, that delivered LCS at a constant rate of 0.25uL/hr for a period of 28 days, while being fed the 60% kcal high-fat diet (starting from 4 weeks to 4 months of age). The mice were euthanized at 4 months of age and their brains were sectioned for histopathology while their brain cortices and blood sera were extracted for ceramide, protein and RNA analyses.
Inhibition of SPT/ceramide decreases cortical Aβ levels
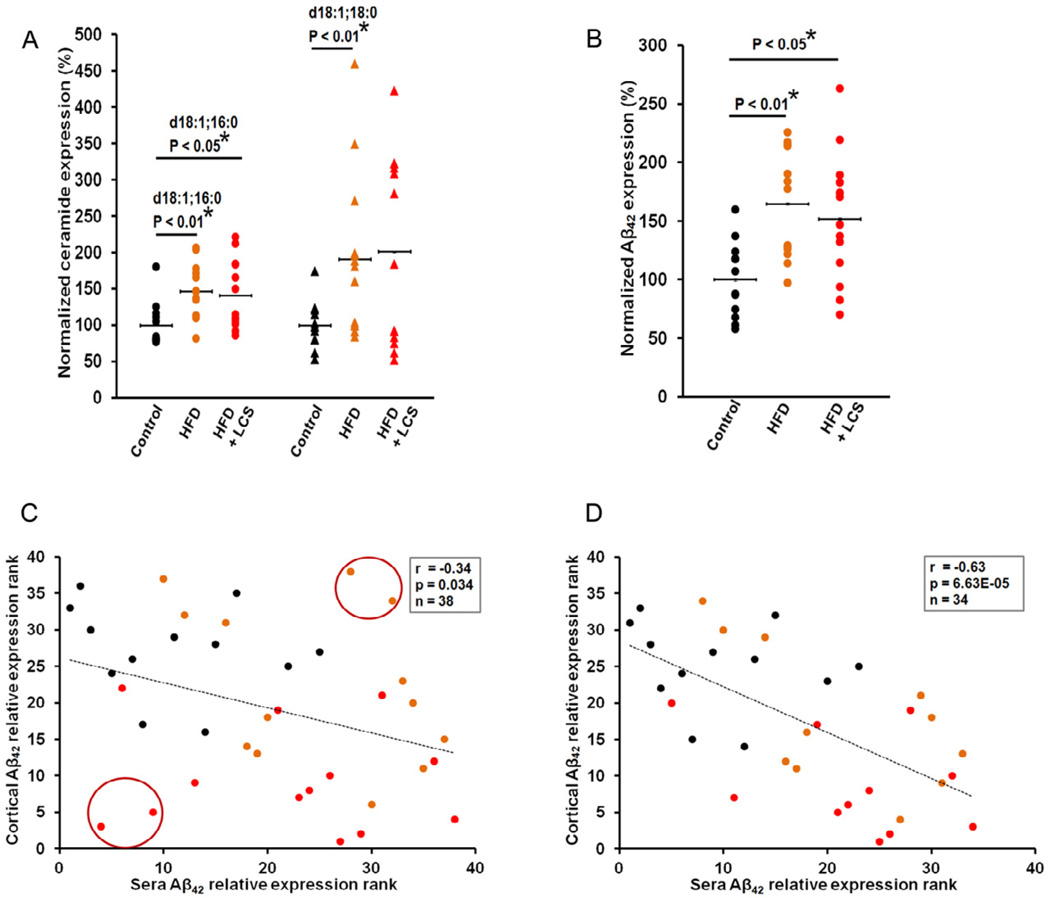
Mice administered with LCS showed decreased ceramide levels, d18:1;16:0 (P < 0.001, Mann-Whitney U test) and d18:1;18:0 (P < 0.01) (Fig. 1A), in comparison with mice fed a control chow or a high-fat-diet. In addition, mice administered with LCS showed decreased Aβ42 levels (Fig. 1B) in comparison with mice fed a control chow (P < 0.001) or a high-fat-diet (P < 0.01). Furthermore, statistically significant positive correlations were observed between Aβ42 and ceramide d18:1;16:0 (r= 0.656, p= 7.79E−06, Spearman’s correlation) (Fig. 1C), d18:1;18:0 (r= 0.56, p= 2.43E−04) (Fig. 1D) levels in all treatment groups. Mice fed a high-fat diet did not demonstrate a statistically significant change in ceramide and Aβ42 levels as compared to their control chow diet counterparts. Comparable to the cumulative (males and females together) results, significant differences were observed in ceramide and Aβ levels among the same gender, between groups while no significant differences were observed between males and females within groups (see supplementary Fig. S4, S5 and S6).
Figure 1. Aβ42 is down-regulated with LCS administration.
(A) Ceramide levels, d18:1; 16:0 and d18:1; 18:0, were significantly decreased in LCS administered mice (n=13). The brain cortices were analyzed via tandem mass spectrometry and the normalized concentrations are shown as a percentage of the average control (n=12). The samples were normalized to internal standard (d18:1, 12:0) concentration and to brain total protein concentrations. (B) Aβ42 levels were significantly decreased in LCS administered mice (n=13). The brain cortices were analyzed with ELISA and the normalized concentrations are shown as a percentage of the average control (n=12). The samples were normalized to brain total protein concentrations. The statistical significances were determined by Mann-Whitney U tests. Spearman's correlation tests demonstrate significant positive correlations between cortical ceramides, (C) d18:1; 16:0, (D) d18:1; 18:0, and Aβ42 levels in the entire sample set (control chow, high-fat, diets and LCS administration) (n=38). The statistical significance of the correlation was determined by two-tailed T distribution tests.
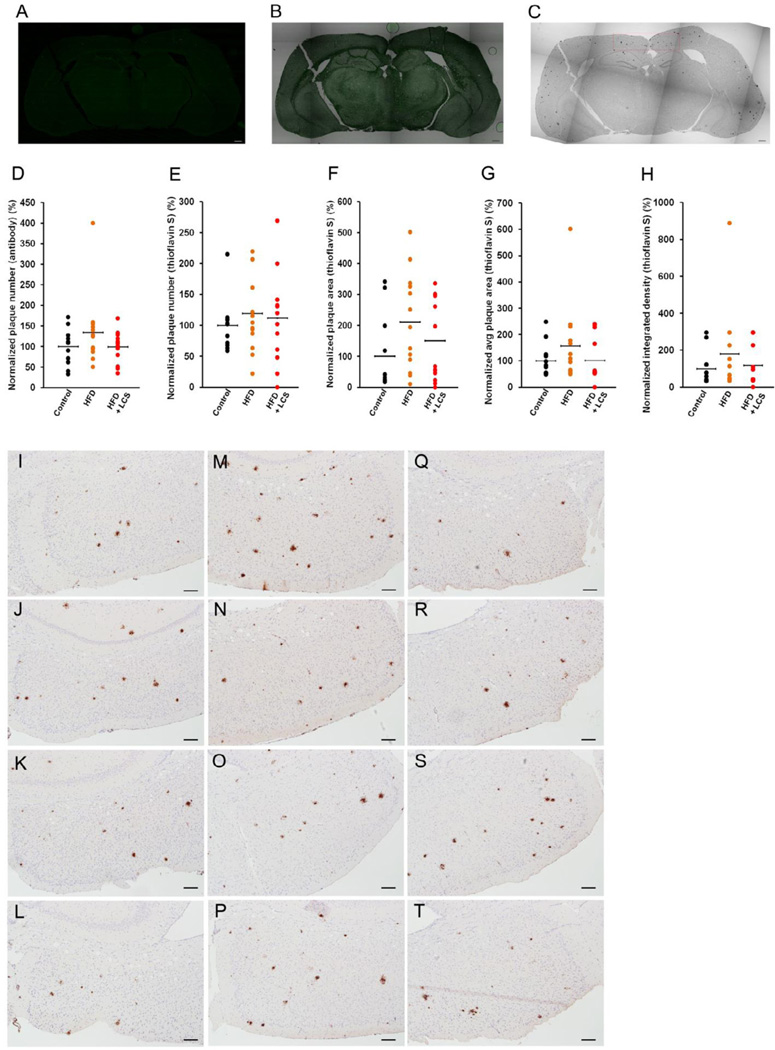
The brain sections were stained with thioflavin-S (Fig. 2A and B) and antibody (Fig. 2C) for Aβ plaques. The plaque areas and integrated densities were determined using NIH-imageJ for thioflavin-S stained sections, while the plaque numbers were manually counted in both thioflavin-S (Fig. 2A) and antibody (Fig. 2C) stained sections. The staining, imaging, NIH-imageJ data generation and counting of the plaques were all conducted in a double-blinded manner. Statistically significant differences in the plaque numbers (Fig. 2D and 2E), total plaque area (Fig. 2F), average area per plaque (Fig. 2G) and integrated density (Fig. 12H) levels were not observed in the control chow diet (Fig. 2I–L), high-fat diet (Fig. 2M–P) and LCS administered (Fig. 2Q–T) groups. TgCRND8 is an aggressive Aβ plaque model thus making them relatively insensitive to modulators of plaque levels (Pedrini et al., 2009). This provides a possible explanation for the nominal effect on the Aβ plaque levels observed in this study. In addition, the relatively small sample size in each category may also have hindered possibilities of any observable significant changes due to insufficient statistical power. On a side note, a statistically significant reduction in the plaque number was observed in a subset of animals administered with LCS (n=10) (see supplementary Fig. S7) in comparison with the control chow (n=8) (P < 0.05) (see supplementary Fig. S7) and high-fat diet fed mice (n=10, note that the 1 extreme high (Fig. 2D) was eliminated along with 2 extreme lows) (P < 0.05) (see supplementary Fig. S7).
Figure 2. Aβ plaque load is reduced in a subset of LCS administered mice.
The brain sections were stained with (A) thioflavin-S and imaged with the fluorescence laser (4×). (B) Overlay of a thioflavin-S section with its bright-field image. (C) The brain sections were stained with Aβ antibody and imaged with the transmitted laser (4×). All images were stitched together to generate a single image of the entire brain slice. The plaque numbers were manually counted for (D) antibody and (E) thioflavin-S stained entire sections and the normalized values are shown as a percentage of the average control (n=12). NIH-imageJ particle analysis was used to calculate the (F) amyloid plaque area, (G) average plaque area and (H) integrated density per brain section for thioflavin-S stained entire sections and the normalized values are shown as a percentage of the average control (n=12). The antibody stained sections were captured with the camera on bright-field at 10× at the location denoted in red in Fig. 2C for (I–L) control chow, (M–P) high-fat and (Q–T) LCS administration. The statistical significances were determined by Mann-Whitney U tests.
High-fat-diet increases blood sera ceramide and Aβ42 levels
Mice fed a high-fat-diet showed increased blood sera ceramide levels (Fig. 3A), d18:1;16:0 (P < 0.01) and d18:1;18:0 (P < 0.01), and Aβ42 levels (P <= 0.01) (Fig. 3B), and mice administered with LCS also showed statistically significant increase in ceramide levels, d18:1;16:0 (P < 0.05), with the exception of d18:1;18:0 where the difference was not statistically significant, (Fig. 3A); and Aβ42 levels (P < 0.05) (Fig. 3B) compared with their control chow diet counterparts. Mice administered with LCS did not show statistically significant differences in their sera ceramide and Aβ42 levels, as compared to mice fed a high-fat diet. However, a statistically significant negative correlation (r= −0.34, p= 0.034) (Fig. 3C) was observed between cortical Aβ42 and sera Aβ42 levels. Additionally, a stronger negative correlation (r= −0.63, p= 6.63E−05) (Fig. 3D), was observed between cortical Aβ42 and sera Aβ42 levels with the elimination of 4 subjects (Fig. 3C, circled) as “outliers".
Figure 3. Sera Aβ correlates with cortical Aβ levels.
(A) Sera ceramide levels, d18:1; 16:0 and d18:1; 18:0, were statistically increased in high-fat (n=13) and, LCS administered mice (n=13) with the exception of d18:1; 18:0. The blood sera were analyzed via tandem mass spectrometry and the normalized concentrations are shown as a percentage of the average control (n=12). The samples were normalized to internal standard (d18:1, 12:0) concentration. (B) Aβ42 levels were significantly increased in high-fat (n=13) and LCS administered mice (n=13). The blood sera were analyzed with ELISA and the normalized concentrations are shown as a percentage of the average control (n=12). The statistical significances were determined by Mann-Whitney U tests. Spearman's correlation tests demonstrate a negative correlation between sera Aβ42 and cortical Aβ42 levels in the (C) entire sample set (n=38) and a statistically stronger significant negative correlation with the (D) elimination of 4 subjects as outliers (n=34). The statistical significance of the correlation was determined by two-tailed T distribution tests.
SPT expression levels are decreased with LCS administration
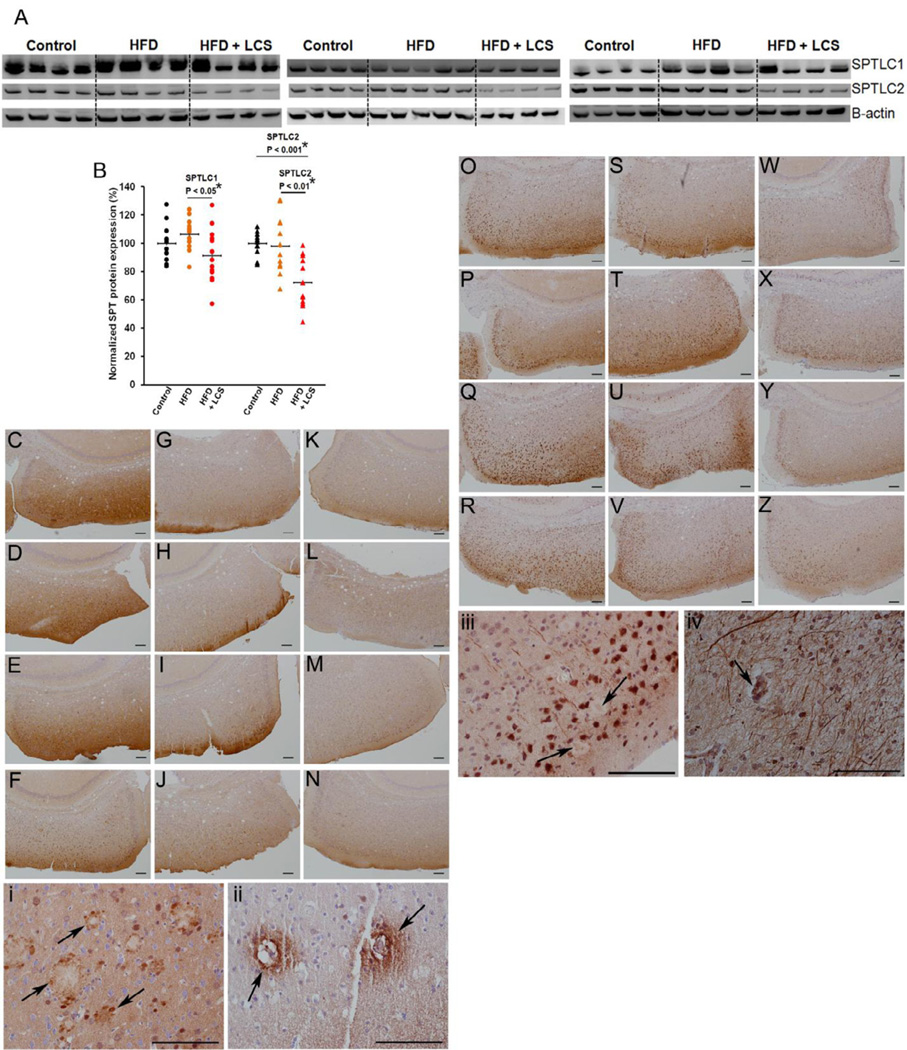
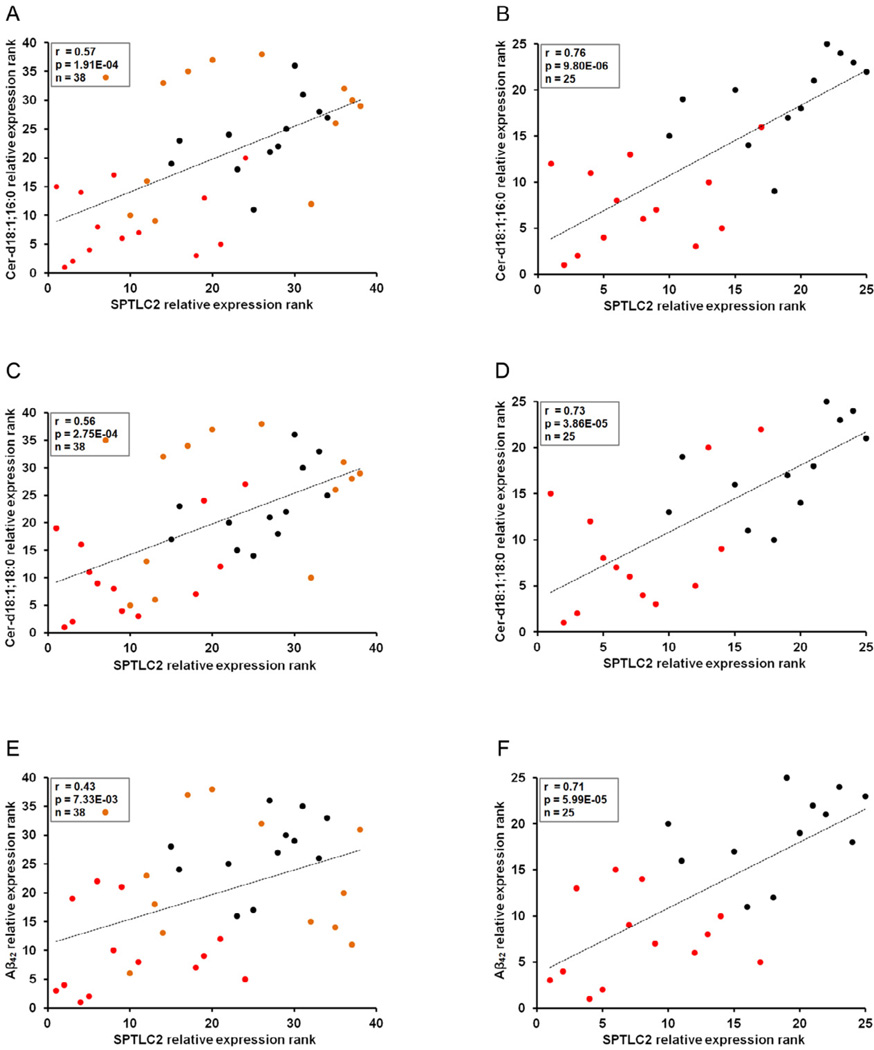
Decreased SPTLC1 (Fig. 4A, 4B) protein expression levels were observed in the brain cortices of mice administered LCS in comparison with their high-fat diet fed (Fig. 4G–J) counterparts (P < 0.05). Decreased levels of SPTLC2 (Fig. 4A, 4B) were observed in mice administered LCS (Fig. 4W–Z) in comparison with their high-fat (P < 0.01) (Fig. 4S–V) and control chow (P < 0.001) (Fig. 4O–R) diets fed counterparts. SPT consists of SPTLC1 and SPTLC2 at a 1:1 ratio (Hanada et al., 2000) and SPTLC1/SPTLC2 complex can be modulated through the regulation of SPTLC2, even without parallel regulation of SPTLC1 (Gable et al., 2000; Yasuda et al., 2003), thus reflecting the similar reductions observed in ceramide (Fig. 1A) and SPTLC2 (Fig. 4B) levels in the brain cortices of mice administered with LCS. Nevertheless, we observed statistically significant positive correlations between SPTLC1 and ceramide d18:1;16:0 (r= 0.44, p= 0.006) (data not shown), and d18:1;18:0 (r= 0.43, p= 0.007) (data not shown), however, with no statistically significant correlation with Aβ42 (r= 0.12, p= 0.47) (data not shown), levels in all treatment groups. Concomitantly, we observed statistically significant positive correlations between SPTLC2 and ceramide d18:1;16:0 (r= 0.57, p= 1.91E−04) (Fig. 5A), and d18:1;18:0 (r= 0.56, p= 2.75E−04) (Fig. 5C) levels in all treatment groups. Additionally, a statistically significant positive correlation was observed between SPTLC2 and Aβ42 (r= 0.43, p= 7.33E−03) (Fig. 5E) levels in all treatment groups. Moreover, stronger positive correlations were observed between SPTLC2 and ceramide d18:1;16:0 (r= 0.76, p= 9.80E−06) (Fig. 5B), d18:1;18:0 (r= 0.73, p= 3.86E−05) (Fig. 5D), and Aβ42 (r= 0.71, p= 5.99E−05) (Fig. 5F) levels in control chow diet and LCS administered groups, indicative of high variability in biomarker expression levels with high-fat diet possibly due to individual aberrations in food consumption and metabolic rates. Similarly, stronger positive correlations were observed between SPTLC1 and ceramide d18:1;16:0 (r= 0.53, p= 0.007) (data not shown), and d18:1;18:0 (r= 0.60, p= 0.001) (data not shown) levels in control chow diet and LCS administered groups. Furthermore, we observed that SPTLC1 protein is expressed in the core and the surroundings of the senile plaques in both mouse (Fig. 4i) and human (Fig. 4ii) brains, while SPTLC2 is present in the core of the senile plaques in the mouse (Fig. 4iii) and human (Fig. 4iv) brains alike.
Figure 4. SPT is decreased with the inhibitor.
(A) Representative and (B) quantification of western blots for SPTLC1 (probed with LCB1) and SPTLC2 proteins in control chow (n=12), high-fat (n=13) and LCS administered (n=13) mice. The expression levels were quantified by normalizing to β-actin and represented as a percentage of the control chow mice average expression. The statistical significances were determined by Mann-Whitney U tests. The brain sections were stained with SPTLC1 antibody and were captured with the camera on bright-field at 10× at the location denoted in red in Fig. 2C for (C–F) control chow, (G–J) high-fat and (K–N) LCS administration. The brain sections were stained with SPTLC2 antibody and were captured with the camera on bright-field at 10× at the location denominated in red in Fig. 2C for (O–R) control chow, (S–V) high-fat and (W–Z) LCS administration. Representation of SPTLC1 protein (40×-oil) surrounding the senile plaques and present in the core of the plaques (arrow) in (i) mice (13 month old) and (ii) humans. Representation of SPTLC2 (40×-oil) protein being present in the core of senile plaques (arrow) in (iii) mice (13 month old) and (iv) a humans.
Figure 5. SPT expression correlates with ceramide and Aβ levels.
Spearman's correlation tests demonstrate significant positive correlation between cortical SPTLC2 protein levels and (A) cortical d18:1; 16:0, (C) d18:1; 18:0, and (E) Aβ42 levels in the entire sample set (control chow, high-fat, diets and LCS administration) (n=38). Stronger positive correlations are observed between cortical SPTLC2 protein levels and (B) cortical d18:1; 16:0, (D) d18:1; 18:0, and (F) Aβ42 levels in control chow diet and LCS administered mice (n=35). The statistical significance of the correlation was determined by two-tailed T distribution tests.
Ceramide expression has been observed in neurons (Becker et al., 2008) and astrocytes (Wang et al., 2012). In addition, ceramide synthase 5 (CerS5), a palmitoyl-Co-A specific ceramide synthase, is ubiquitously expressed in most cell types within the gray and white matters of the brain (Becker et al., 2008). Similarly, we observed that SPTLC1 (Fig. 14C–F) and SPTLC2 (Fig. 14O–R) are expressed in both the gray and white matters of the brain. This in combination with the observation that ceramide co-localizes with Aβ plaques (Wang et al., 2012) and our observations that SPT (Fig. 4i–iv) also co-localizes with the Aβ plaques, further lends support to the involvement of SPT in ceramide and thus Aβ production.
Hyperphosphorylated tau expressions are reduced with LCS administration
Similarly to other transgenic APP mouse models neurofibrillary tangles (NFT) are absent in TgCRND8 mice (Chishti et al., 2001). In accordance with other research (Chishti et al., 2001) we could not observe histopathological staining for hyperphosphorylated tau. In this study, we measured the soluble phospho-PHF-tau pSer202/Thr205 (AT8) via western blotting. We anticipate similar results for other prominent extracellular and intracellular NFT staining antibodies, AT100 (pT212/pS214), and PHF-1 (pS396/pS404). The other phosphor-tau antibodies, TG3 (pT231), pS262, and pT153 are able to detect the pre-tangle state. However, serine or threonines are reported to be the most prominent hyperphosphorylated sites (Augustinack et al., 2002) and the pre-tangle state contains S199 and 202 (Kimura et al., 1996), all of which can be detected by AT8. Therefore, we used AT8 as it is reported to detect pre-NFT and intra- and extracellular NFTs suggesting that the other antibodies would possibly produce similar results.
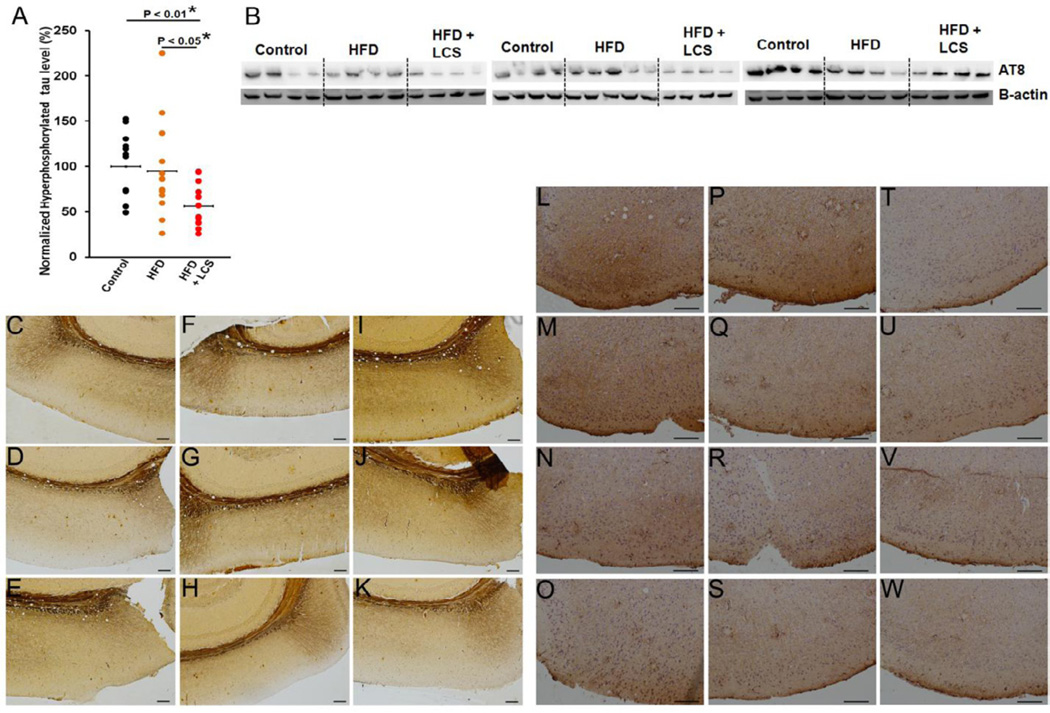
A decrease in mouse cortical hyperphosphorylated tau protein expression levels were observed with the administration of LCS (Fig. 6A and 6B) in comparison with their high-fat (P < 0.05) and control chow (P < 0.01) diet counterparts. Bielschowsky’s silver staining revealed filamentous silver-positive inclusions in control chow (Fig. 6C–E), high-fat (Fig. 6F–H) diets and LCS administered (Fig. 6I–K) mice brains, indicative of the presence of senile plaques and dystrophic neurites. In addition, anti-neurofilament antibody (NF200) staining showed the neuritic aggregations surrounding the senile plaques in control chow (Fig. 6L–O), high-fat (Fig. 4P–S) diets and LCS administrated (Fig. 6T–W) mice brains.
Figure 6. Tau hyperphosphorylation is down-regulated with LCS administration.
(A) Quantification and (B) representation of western blot for tau hyperphosphorylation (probed with AT8) in control chow (n=12), high-fat (n=13) and LCS administered (n=13) mice. The expression levels were quantified by normalizing to β-actin and represented as a percentage of the control chow mice average expression. The statistical significances were determined by Mann-Whitney U tests. The brain sections were stained for Beilschowsky’s silver staining and captured with the camera on bright-field at 10× at the location denoted in red in Fig. 2C for (C–E) control chow, (F–H) high-fat and (I–K) LCS administration. The brain sections were stained with NF200 antibody and were captured with the camera on bright-field at 20× at the location denominated in red in Fig. 2C for (L–O) control chow, (P–S) high-fat and (T–W) LCS administration.
Sections stained with Bielschowsky’s silver staining becomes difficult to quantify due to high background levels. Nevertheless, LCS administration seem to reduce filamentous silver-positive inclusions as compared with control chow and high-fat diet mice (Fig, 6C–6K). NF200 staining shows neuritic aggregations surrounding the senile plaques. The senile plaques were quantified in Figure 2. Mice administered with LCS appear to demonstrate reduced neuritic aggregations around the plaques as compared with chow and high-fat diet mice (Fig, 6L–6W).
Changes in cortical miRNA correlate with sera miRNA levels
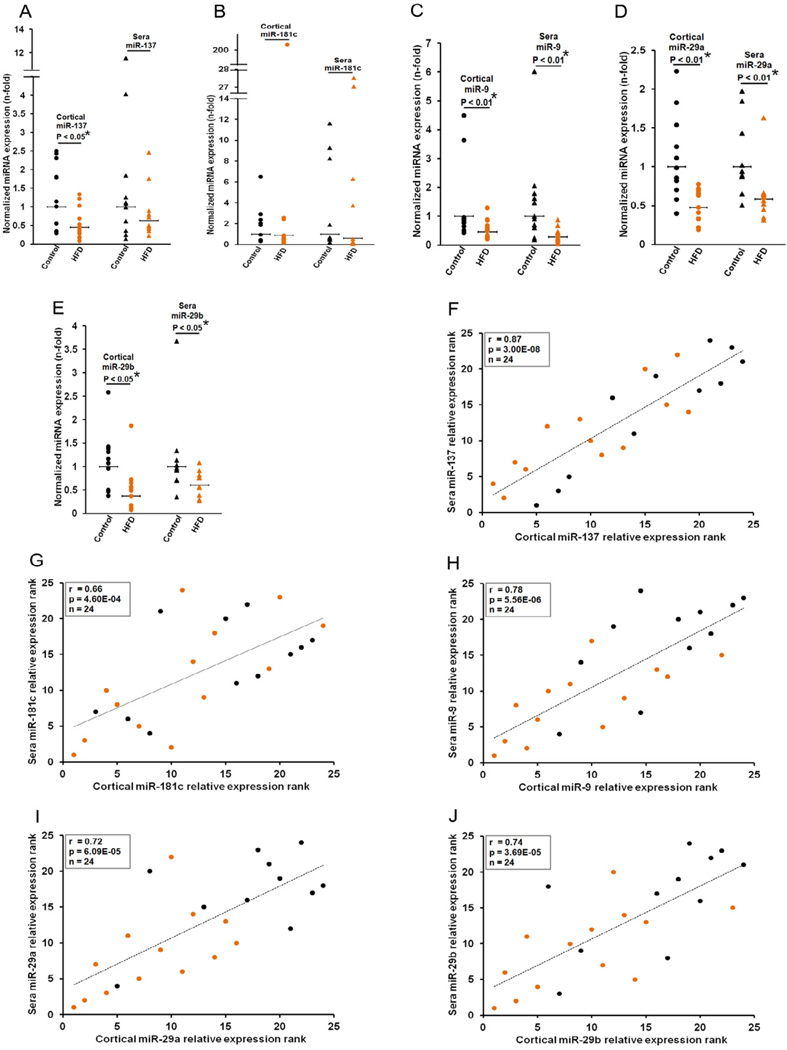
The expression levels of miR-137 (P < 0.05) (Fig. 7A), miR-9 (P < 0.01) (Fig. 7C), miR-29a (P < 0.01) (Fig. 7D) and miR-29b (P < 0.05) (Fig. 7E) were down-regulated in the brain cortices of animals fed a high-fat-diet in comparison with their control chow diet counterparts. Similarly, the expression levels of miR-9 (P < 0.01) (Fig. 7C), miR-29a (P < 0.01) (Fig. 7D) and miR-29b (P < 0.05) (Fig. 7E) were down-regulated in the blood sera of animals fed a high-fat-diet in comparison with their control chow diet counterparts. Significant differences in the expressions of cortical miR-181c (Fig. 7B), and sera miR-137 (Fig. 7A) and miR-181c (Fig. 7B) levels were not observed.
Figure 7. Misregulation of cortical miR-137,-181c,-9 and -29a/b correlate with sera miRNA levels.
(A) miR-137, (B) -181c, (C) -9, (D) -29a and (E) -29b levels were quantified by qRT-PCR in the cortices and blood sera of control chow (n=11) and high-fat (n=13) diets. Relative expressions shown are normalized to RNU6B for cortical expressions and miR-22 average for sera expressions, and average control chow diet expressions. The statistical significances between control and high-fat diets were determined by Mann-Whitney U tests. Spearman's correlation test demonstrated significant positive correlations between cortical and sera (F) miR-137, (G) -181c, (H) -9, (I) -29a and (J) -29b in control chow (n=11) and high-fat diet (n=13) fed mice. Note that sufficient serum was not available from 1 control chow diet subject. Therefore, 11 out of 12 control chow diet samples were used for analysis. The significance of the correlation was determined by two-tailed T distribution tests.
Statistically significant positive correlations were observed between cortical and sera miR-137 (r= 0.87, p= 3.00E−08) (Fig. 7F), miR-181c (r= 0656, p= 4.60E−04) (Fig. 7G), miR-9 (r= 0.78, p= 5.56E−06) (Fig. 7H), miR-29a (r= 0.72, p= 6.09E−05) (Fig. 7I), and miR-29b (r= 0.78, p= 3.69E−05) (Fig. 7J), in mice fed a control chow and a high-fat diet.
SPT is essentially a safe drug target
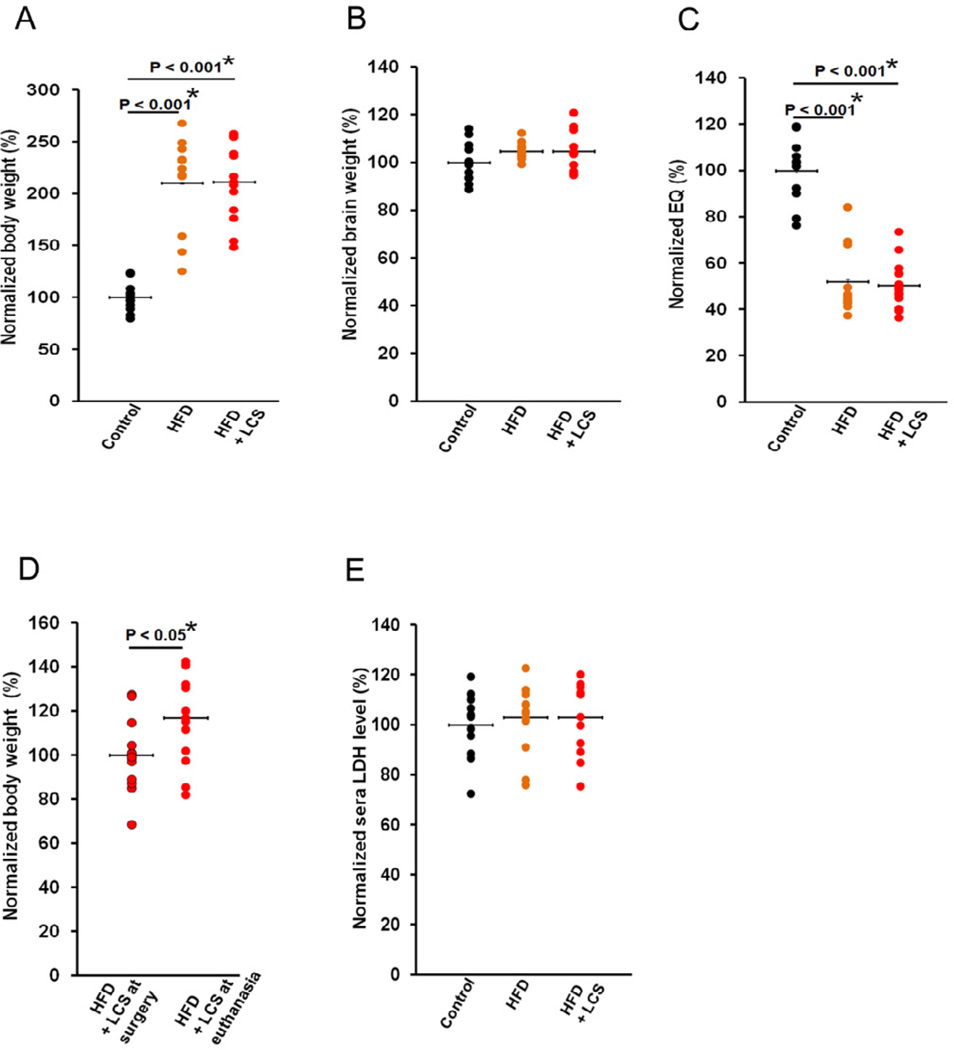
The total body weight (Fig. 8A) of the animals fed a high-fat diet (P < 0.001) and animals administered with LCS (P < 0.001) were elevated at the time of euthanasia in comparison with their chow control diet counterparts, with no differences in their total brain weights (Fig. 8B), thus decreasing the encephalization quotient (EQ) levels (Fig. 8C) in mice fed a high-fat diet (P < 0.001) and administered LCS (P < 0.001). No differences were observed in body and brain weights of mice administered LCS in comparison to mice fed a high-fat diet. In addition, a statistically significant increase (P < 0.05) in body weight was observed from the time of surgery to euthanasia in LCS administered mice (Fig. 8D), suggesting the absence of toxic effects that may exhibit alterations in growth, hormonal changes, changes in neurotransmitters that affect food consumption, or nonspecific systemic toxicity (Bailey et al., 2004).
Figure 8. Inhibition of SPT is a safe therapeutic target.
(A) Total body weight, (B) total brain weight, and (C) EQ at the time of euthanasia, in control chow (n=12), high-fat (n=13) diets and LCS administered (n=13) mice. (D) Statistically significant increase in total body weights in high fat-fed mice administered with LCS from the time of surgery to euthanasia. (E) Blood sera LDH levels at the time of euthanasia in control chow (n=12), high-fat diet (n=13) and LCS administered (n=12) mice. Note that 1 LCS administered subject is unavailable for analysis. Therefore, 12 out of 13 LCS administered samples were used for analysis. The normalized values are shown as a percentage of the average control chow diet samples (n=12). The statistical significances were determined by Mann-Whitney U tests.
The LDH levels remained unchanged in the blood sera between the 3 treatment groups (Fig. 8E) suggesting that LCS administration imposes no toxicity effects on the animals. Further, all but 1 animal administered with LCS, via the surgically implanted osmotic pump, survived to term (28 days). The mouse that did not survive to term was found dead, with vaginal discharge, a classical symptom of the aggressive Pasteurella pneumotropica infection in the colony, 6 days shy of the scheduled euthanasia date. In addition, no visual side effects were observed in the mice administered LCS with the exception of 1 mouse demonstrating convulsion on the 2nd day of administration which subsided the next day. The LDH data and physical observations indicate that a daily, 10mg/kg dose of LCS for a period of 28 days is essentially safe, with no observed toxic side-effects related to LCS or target in this limited exposure paradigm.
Discussion
Previously, we found increased SPT levels in AD directly regulate ceramide and Aβ levels (Geekiyanage and Chan, 2011). In this study, we observed that an AD mouse model administered with a SPT inhibitor, express reduced levels of cortical ceramide and Aβ42 levels not only in comparison with their high-fat-diet counterparts, but also in comparison with mice fed a control chow diet, suggesting that SPT/ceramide could be a potential therapeutic target for AD. Furthermore, post-hoc statistical power analysis conducted on the 13 animals (sample size) administered with 10 mg/kg of the SPT inhibitor demonstrates that the reduction of Aβ42 levels (~30% reduction) observed has a power of 97% to yield a significant effect with an effect size (Cohen’s d) of 2.4 (see supplementary Fig. S2).
We previously observed that SPT positively correlates with Aβ in human autopsy brain cortices and directly regulates Aβ levels (Geekiyanage and Chan, 2011). In this study we observed that the SPT inhibitor reduced cortical SPT protein levels which in turn decreased ceramide and Aβ levels in the brains of an AD mouse model. In addition, we observed that SPT levels show a significant positive correlation with ceramide and Aβ levels in all groups. Interestingly, we found that SPT encircles and resides in the core of the senile plaques in TgCRND8 mice and humans, further supporting the direct involvement of SPT in Aβ generation. Inhibition of SPT enables the assessment of target engagement, facilitating the early proof-of-concept and thus increasing the efficiency of early clinical trial development. Reduced SPT and ceramide levels observed with LCS administration provide information on the physical and biological interaction with the molecular target of the drug making them potentially good target engagement biomarkers. The decrease in SPT/ceramide levels along with the decrease in Aβ levels observed with LCS administration shows the combination of target engagement and disease-related biomarkers. This combination of target engagement and disease-related biomarkers is useful in drug development (Wagner, 2008).
The dimeric SPT enzyme contains an essential pyridoxal-5’-phosphate (PLP) cofactor bound to each of the subunits (Lowther et al., 2010). Cycloserine inhibits many PLP-dependent enzymes (alanine racemace (Fenn et al., 2003; Wu et al., 2008), ArnB aminotransferase (Noland et al., 2002), dialkylglycine decarboxylase (Malashkevich et al., 1999)) by disabling the essential PLP cofactor. Research suggest that LCS is an irreversible inhibitor of SPT. LCS inhibits the PLP-dependent SPT by transamination to form a free pyridoxamine-5’-phosphate (PMP) and β-aminoxyacetaldehyde that remains bound at the active site. LCS binds to the SPT active site and forms a PLP-LCS external aldimine complex. It has been proposed that this occurs via ring opening of the LCS ring followed by decarboxylation. Kinetic studies shows that the half-life of the SPT:LCS complex is approximately 15 minutes (Lowther et al., 2010). Bruce W. Craigs group have observed a reduction in SPT protein levels with the treatment of LCS (Choi, 2006). Similarly, in this study a reduction of SPT levels is observed with LCS administration, suggesting a possible degradation of the PLP deficient SPT complex.
In our previous study, we observed that high-fat diet increased cortical ceramide levels in wild-type mice (n=3). However, the TgCRND8 mice fed a high-fat-diet (n=13) failed to demonstrate a statistically significant increase of ceramide levels in comparison with mice fed a control chow diet. Nevertheless, 9 out of 13 mice demonstrated a statistically significant (P > 0.01) (data not shown) elevation of ceramide levels in high-fat diet fed mice in comparison with mice fed a control chow diet. The TgCRND8 mice fed a high-fat diet (n=13) failed to show a statistically significant difference in Aβ42 levels in comparison to mice fed a control chow diet. In support of this, APP/PS1 mice showed no change in cumulative Aβ40 and Aβ42 levels with a typical western diet (essentially a high-fat diet) (Oksman et al., 2006). In contrast, increased levels of cumulative Aβ40 and Aβ42 have been observed in TgCRND8 (Pedrini et al., 2009) and 3×Tg (Julien et al., 2010) mice fed a high-fat diet. In our study, 5 out of 13 mice fed a high-fat diet showed statistically significant increase in Aβ42 levels (P > 0.05) (data not shown) when compared with the control chow diet counterparts. In agreement with other research conducted with TgCRND8 (Pedrini et al., 2009) and APP/PS1 (Oksman et al., 2006) mice, we did not observe differences in Aβ plaque burden with the high-fat diet (n=13). As stated previously (Pedrini et al., 2009), TgCRND8 mice are relatively insensitive to modulators of amyloidosis due to its aggressive amyloid pathology, thus providing a possible explanation for the nominal effects. Nevertheless, a statistically significant positive correlation was observed between cortical ceramide and Aβ42 levels in mice fed a control chow diet, high-fat-diet and mice administered with the SPT inhibitor, further supporting the involvement of ceramide in Aβ production.
In addition to the reduction in Aβ levels, we observed reductions in hyperphosphorylated tau levels with the inhibition of SPT. Increased tau levels were observed in triple transgenic mice (3×Tg-AD) with the consumption of high-fat-diet from 4 to 13 months of age. However, no change in hyperphosphorylated tau levels was observed (Julien et al., 2010). Additionally, no significant effect was observed on tau hyperphosphoryalation levels and tau phosphatases (e.g. PP2A) when C57/BL6 mice were fed a high fat diet for 12–16 weeks (Becker et al., 2012). In agreement with the aforementioned studies we did not observe an effect on tau hyperphosphorylation by high-fat diet fed mice. Nevertheless, it must be noted that the mice in our study were fed a high-fat diet only for 3 months and thus, extending the duration of the high-fat diet consumption could possibly have an effect on the Aβ and hyperphosphorylated tau levels. In our previous study we observed increased SPT levels in wild-type hybrid (C57/Bl6 × C3H) mice when they were fed a high-fat diet for 5 months (starting at 4 months of age), while we did not observe an increase in SPT levels in this current study where the high-fat diet was consumed only for 3 months. This suggests that the duration of high-fat diet consumption could have an effect on biological processes including metabolic processes. LCS has not been reported to have a direct effect on tau. It also should be noted that transgenic APP mouse models are not models to study taupathy as they do not recapitulate the NFT observed in AD. Nevertheless, our group has previously observed that LCS decreases GSK3β levels, a kinase that mediates phosphorylation of tau (Patil et al., 2007). However, GSK3β levels are also activated in response to Aβ which in turn regulates tau phosphorylation (Gomez-Ramos et al., 2003). In support, a number of reports have recognized that Aβ oligomers directly regulate tau hyperphosphorylation (Resende et al., 2008; Ma et al., 2009; Tomiyama et al., 2010; Jin et al., 2011; Chabrier et al., 2012).
Ceramides contribute to Aβ pathology by facilitating the mislocation of BACE1 and γ-secretase to lipid rafts. Under non-pathological conditions the inactive BACE1 and γ-secretase reside outside of lipid rafts while under pathological settings the ceramides facilitate the trafficking of the secretases to lipid rafts where they become active to produce Aβ (Ebina et al., 2009). In addition to stabilizing BACE1, the membrane lipid raft topography affects the efficiency of γ-secretase activity (Fassbender et al., 2001; Wahrle et al., 2002; Zha et al., 2004). This coupled with the fact that over-expression of BACE1 fails to restore suppressed Aβ levels (Hebert et al., 2008), and over-expression of SPT restores Aβ levels (Geekiyanage and Chan, 2011), suggest a potential role of SPT/ceramide in the regulation of γ-secretase activity. Along the same lines, treatments with modulators of γ-secretase reduced Aβ burden, attenuated memory deficits (Schilling et al., 2008; Imbimbo et al., 2009), and reduced hyperphosphorylated tau levels (Lanzillotta et al.) in AD mouse models. Our results suggest that LCS, a SPT inhibitor, could indirectly function as a possible γ-secretase inhibitor to reduce Aβ production.
Several studies demonstrate increased plasma ceramide levels in rodents fed a high-fat diet (Ichi et al., 2007; Shah et al., 2008). Similarly, we observed an increase in blood sera ceramide levels in mice fed a high-fat diet. Increased ceramide levels have been reported in muscle, adipose tissue and livers of obese rodents and humans (Turinsky et al., 1990; Unger and Orci, 2001; Adams et al., 2004; Samad et al., 2006; Zendzian-Piotrowska et al., 2006). In this study, mice fed an obesity inducing high-fat diet show increased weight at the time of euthanasia, regardless of administration of the inhibitor, due to their continuous consumption. Therefore, the increased ceramide levels observed in the blood sera is possibly due to contributions from muscle, adipose and liver tissues.
Plasma Aβ42 levels have been observed to increase before the onset of AD, followed by a decline with disease progression (Schupf et al., 2008; Henry et al., 2012). In addition, other reports have associated low levels of plasma Aβ42 with increased dementia (van Oijen et al., 2006; Graff-Radford et al., 2007). Although it is unclear whether abnormal Aβ42 metabolism in the brain is precisely emulated by the plasma/sera Aβ42 levels (Henry et al., 2012), we nevertheless observed a statistically significant negative correlation between cortical and blood sera Aβ42 levels, providing possible connotations for the search of blood Aβ biomarkers.
Previously we observed that miR-137, miR-181c, miR-9 and miR-29a/b are down-regulated in brain cortices of AD patients, and wild-type mice fed a high fat diet (Geekiyanage and Chan, 2011). In another study we observed that the respective miRNAs were down-regulated in blood sera of AD patients and in wild-type mice fed a high-fat diet suggesting a possible role for these miRNAs as non-invasive diagnostic biomarkers (Geekiyanage et al., 2012). In this current study we show a positive correlation between the corresponding cortical and sera miRNA, further indicating that sera miRNAs may reflect upon the brain miRNA expression patterns. It is noteworthy that the miRNAs that demonstrate the highest degrees of statistically significant correlations, miR-137 and miR-9, are brain enriched miRNAs. This further strengthens the use of these miRNAs, miR-137 and miR-9, as potential sera biomarkers, i.e. directly reflecting on the brain fraction with minimal interference from other organs. Our prior results show that SPTLC1/2 are post-transcriptionally regulated by miR-137/-181c and miR-9,-29a/b respectively. In our previous study we observed decreased cortical miR-137,-181c,-9 expressions along with increased SPTLC1/2 levels in a small number (n=3) of wild-type mice, fed a high-fat diet for 5 months starting at 4 months of age (Geekiyanage and Chan, 2011). Here we observed a reduction in the cortical miR-137 with no change in miR-181c levels in the AD mouse model fed a high fat diet for 3 months, providing possible explanations for the predominantly unchanged SPTLC1 levels. Research demonstrates that unless attached to SPTLC1, SPTLC2 is unstable (Gable et al., 2000; Yasuda et al., 2003). Thus due to the 1:1 ratio between SPTLC1 and SPTLC2, unattached SPTLC2 could be degraded providing a possible explanation for the unchanged SPTLC2 levels even with reduced miR-9 and -29a/b levels. It must be noted that the strain differences, age and duration of diet consumption, and the sample size in the previous study could have contributed to the divergences in ceramide, SPT and miRNA levels observed in the 2 studies. Nevertheless both studies found strong correlations between ceramide, SPT and miRNA levels.
Finally, administration of LCS did not show toxic effects as determined by sera LDH levels, brain and body weight. Administering large doses (100mg/kg) of LCS has shown immediate reduction of brain SPT with significant weight loss (Sundaram and Lev, 1984b) whereas extended administration of lower doses (25mg/kg) imposed nominal side effects, with no changes in weight. In addition, chronic LCS administration was found not to affect brain histology, morphology, myelination or memory retention in healthy mice (Sundaram and Lev, 1989). These observations together suggest SPT inhibition could be a safe therapeutic target with LCS as a possible “drug candidate”. In contrast inhibition of ceramide synthase, another enzyme in the ceramide synthesis pathway, by Fumonisin B1 leads to growth inhibition and cytotoxicity (Schmelz et al., 1998). Further, consumption of Fumonisin B1 causes veterinary diseases and contributes to esophageal cancer in humans (Chu and Li, 1994; Yoshizawa et al., 1994). Other inhibitors of SPT, such as ISP-1 are difficult to solubilize and inflict gastrointestinal toxicity when incorporated into the diet (Hojjati et al., 2005). Additionally, in agreement with previous studies conducted in dogs (Kluepfel et al., 1972) we observed (unpublished observation) that ISP-1 is lethal to mice when administered subcutaneously. Therefore, our study suggests that inhibition of SPT and thus ceramide, through a less-invasive route, can potentially ameliorate the Aβ burden and tau hyperphosphorylation observed in AD with nominal toxicity.
Supplementary Material
Acknowledgements
We thank Amy S. Porter, HT (ASCP) QIHC and Kathleen A. Joseph, HT (ASCP) QIHC from the MSU Investigative Histopathology Laboratories. We thank Melinda K. Frame, PhD from the MSU Center for Advanced Microscopy. We thank the MSU mass spectrometry facility. This work was supported in part by the National Institute of Health (R01GM079688 and R01GM089866) and the National Science Foundation (CBET 0941055).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors disclose no conflict of interests.
Contributor Information
Hirosha Geekiyanage, Email: geekiyan@msu.edu.
Aditi Upadhye, Email: upadhyea@msu.edu.
References
- Adams JM, 2nd, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25–31. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- Augustinack JC, Schneider A, Mandelkow EM, Hyman BT. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathol. 2002;103:26–35. doi: 10.1007/s004010100423. [DOI] [PubMed] [Google Scholar]
- Bailey SA, Zidell RH, Perry RW. Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol Pathol. 2004;32:448–466. doi: 10.1080/01926230490465874. [DOI] [PubMed] [Google Scholar]
- Becker I, Wang-Eckhardt L, Yaghootfam A, Gieselmann V, Eckhardt M. Differential expression of (dihydro)ceramide synthases in mouse brain: oligodendrocyte-specific expression of CerS2/Lass2. Histochem Cell Biol. 2008;129:233–241. doi: 10.1007/s00418-007-0344-0. [DOI] [PubMed] [Google Scholar]
- Becker K, Freude S, Zemva J, Stohr O, Krone W, Schubert M. Chronic peripheral hyperinsulinemia has no substantial influence on tau phosphorylation in vivo. Neurosci Lett. 2012;516:306–310. doi: 10.1016/j.neulet.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Chabrier MA, Blurton-Jones M, Agazaryan AA, Nerhus JL, Martinez-Coria H, LaFerla FM. Soluble abeta promotes wild-type tau pathology in vivo. J Neurosci. 2012;32:17345–17350. doi: 10.1523/JNEUROSCI.0172-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessell IP, Procter AW, Francis PT, Bowen DM. D-cycloserine, a putative cognitive enhancer, facilitates activation of the N-methyl-D-aspartate receptor-ionophore complex in Alzheimer brain. Brain Res. 1991;565:345–348. doi: 10.1016/0006-8993(91)91668-q. [DOI] [PubMed] [Google Scholar]
- Chishti MA, et al. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. The Journal of biological chemistry. 2001;276:21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- Choi MD. The regulation of ceramide content and insulin resistance in skeletal muscle. Ball State University; 2006. [Google Scholar]
- Chu FS, Li GY. Simultaneous occurrence of fumonisin B1 and other mycotoxins in moldy corn collected from the People's Republic of China in regions with high incidences of esophageal cancer. Appl Environ Microbiol. 1994;60:847–852. doi: 10.1128/aem.60.3.847-852.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordy JM, Hussain I, Dingwall C, Hooper NM, Turner AJ. Exclusively targeting beta-secretase to lipid rafts by GPI-anchor addition up-regulates beta-site processing of the amyloid precursor protein. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11735–11740. doi: 10.1073/pnas.1635130100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini C, Ko MH, Jonas MC, Puglielli L. A reversible form of lysine acetylation in the ER and Golgi lumen controls the molecular stabilization of BACE1. The Biochemical journal. 2007;407:383–395. doi: 10.1042/BJ20070040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler RG, Pedersen WA, Camandola S, Rothstein JD, Mattson MP. Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress-induced death of motor neurons in amyotrophic lateral sclerosis. Ann Neurol. 2002;52:448–457. doi: 10.1002/ana.10312. [DOI] [PubMed] [Google Scholar]
- Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina M, Futai E, Tanabe C, Sasagawa N, Kiso Y, Ishiura S. Inhibition by KMI-574 leads to dislocalization of BACE1 from lipid rafts. J Neurosci Res. 2009;87:360–368. doi: 10.1002/jnr.21858. [DOI] [PubMed] [Google Scholar]
- Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P, Runz H, Kuhl S, Bertsch T, von Bergmann K, Hennerici M, Beyreuther K, Hartmann T. Simvastatin strongly reduces levels of Alzheimer's disease beta -amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn TD, Stamper GF, Morollo AA, Ringe D. A side reaction of alanine racemase: transamination of cycloserine. Biochemistry. 2003;42:5775–5783. doi: 10.1021/bi027022d. [DOI] [PubMed] [Google Scholar]
- Gable K, Slife H, Bacikova D, Monaghan E, Dunn TM. Tsc3p is an 80-amino acid protein associated with serine palmitoyltransferase and required for optimal enzyme activity. The Journal of biological chemistry. 2000;275:7597–7603. doi: 10.1074/jbc.275.11.7597. [DOI] [PubMed] [Google Scholar]
- Geekiyanage H, Chan C. MicroRNA-137/181c Regulates Serine Palmitoyltransferase and In Turn Amyloid {beta}, Novel Targets in Sporadic Alzheimer's Disease. J Neurosci. 2011;31:14820–14830. doi: 10.1523/JNEUROSCI.3883-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geekiyanage H, Jicha GA, Nelson PT, Chan C. Blood serum miRNA: non-invasive biomarkers for Alzheimer's disease. Exp Neurol. 2012;235:491–496. doi: 10.1016/j.expneurol.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Ramos A, Diaz-Nido J, Smith MA, Perry G, Avila J. Effect of the lipid peroxidation product acrolein on tau phosphorylation in neural cells. J Neurosci Res. 2003;71:863–870. doi: 10.1002/jnr.10525. [DOI] [PubMed] [Google Scholar]
- Graff-Radford NR, Crook JE, Lucas J, Boeve BF, Knopman DS, Ivnik RJ, Smith GE, Younkin LH, Petersen RC, Younkin SG. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007;64:354–362. doi: 10.1001/archneur.64.3.354. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- Han X, D MH, McKeel DW, Jr, Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer's disease: potential role in disease pathogenesis. J Neurochem. 2002;82:809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- Hanada K, Hara T, Nishijima M. Purification of the serine palmitoyltransferase complex responsible for sphingoid base synthesis by using affinity peptide chromatography techniques. The Journal of biological chemistry. 2000;275:8409–8415. doi: 10.1074/jbc.275.12.8409. [DOI] [PubMed] [Google Scholar]
- Hanada K, Hara T, Nishijima M, Kuge O, Dickson RC, Nagiec MM. A mammalian homolog of the yeast LCB1 encodes a component of serine palmitoyltransferase, the enzyme catalyzing the first step in sphingolipid synthesis. The Journal of biological chemistry. 1997;272:32108–32114. doi: 10.1074/jbc.272.51.32108. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Bandaru VV, Bae M, Mattson MP. Roles for dysfunctional sphingolipid metabolism in Alzheimer's disease neuropathogenesis. Biochimica et biophysica acta. 2010;1801:878–886. doi: 10.1016/j.bbalip.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Huang Y, Li B, Gong CX, Schuchman EH. Deregulation of sphingolipid metabolism in Alzheimer's disease. Neurobiol Aging. 2010;31:398–408. doi: 10.1016/j.neurobiolaging.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, Kauppinen S, Delacourte A, De Strooper B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer's disease correlates with increased BACE1/beta-secretase expression. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6415–6420. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry MS, Passmore AP, Todd S, McGuinness B, Craig D, Johnston JA. The development of effective biomarkers for Alzheimer's disease: a review. Int J Geriatr Psychiatry. 2012 doi: 10.1002/gps.3829. [DOI] [PubMed] [Google Scholar]
- Hojjati MR, Li Z, Zhou H, Tang S, Huan C, Ooi E, Lu S, Jiang XC. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. The Journal of biological chemistry. 2005;280:10284–10289. doi: 10.1074/jbc.M412348200. [DOI] [PubMed] [Google Scholar]
- Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Hur JY, Welander H, Behbahani H, Aoki M, Franberg J, Winblad B, Frykman S, Tjernberg LO. Active gamma-secretase is localized to detergent-resistant membranes in human brain. The FEBS journal. 2008;275:1174–1187. doi: 10.1111/j.1742-4658.2008.06278.x. [DOI] [PubMed] [Google Scholar]
- Ichi I, Nakahara K, Kiso K, Kojo S. Effect of dietary cholesterol and high fat on ceramide concentration in rat tissues. Nutrition. 2007;23:570–574. doi: 10.1016/j.nut.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Imbimbo BP, Hutter-Paier B, Villetti G, Facchinetti F, Cenacchi V, Volta R, Lanzillotta A, Pizzi M, Windisch M. CHF5074, a novel gamma-secretase modulator, attenuates brain beta-amyloid pathology and learning deficit in a mouse model of Alzheimer's disease. Br J Pharmacol. 2009;156:982–993. doi: 10.1111/j.1476-5381.2008.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ. Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5819–5824. doi: 10.1073/pnas.1017033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien C, Tremblay C, Phivilay A, Berthiaume L, Emond V, Julien P, Calon F. High-fat diet aggravates amyloid-beta and tau pathologies in the 3xTg-AD mouse model. Neurobiol Aging. 2010;31:1516–1531. doi: 10.1016/j.neurobiolaging.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Kalvodova L, Kahya N, Schwille P, Ehehalt R, Verkade P, Drechsel D, Simons K. Lipids as modulators of proteolytic activity of BACE: involvement of cholesterol, glycosphingolipids, and anionic phospholipids in vitro. The Journal of biological chemistry. 2005;280:36815–36823. doi: 10.1074/jbc.M504484200. [DOI] [PubMed] [Google Scholar]
- Kimura T, Ono T, Takamatsu J, Yamamoto H, Ikegami K, Kondo A, Hasegawa M, Ihara Y, Miyamoto E, Miyakawa T. Sequential changes of tau-site-specific phosphorylation during development of paired helical filaments. Dementia. 1996;7:177–181. doi: 10.1159/000106875. [DOI] [PubMed] [Google Scholar]
- Klatt EC. The internet pathology laboatory for medical eductaion. Savannah: University of Utah eccles health sciences library; 1994–2012. [Google Scholar]
- Kluepfel D, Bagli J, Baker H, Charest MP, Kudelski A. Myriocin, a new antifungal antibiotic from Myriococcum albomyces. J Antibiot (Tokyo) 1972;25:109–115. doi: 10.7164/antibiotics.25.109. [DOI] [PubMed] [Google Scholar]
- Lanzillotta A, Sarnico I, Benarese M, Branca C, Baiguera C, Hutter-Paier B, Windisch M, Spano P, Imbimbo BP, Pizzi M. The gamma-secretase modulator CHF5074 reduces the accumulation of native hyperphosphorylated tau in a transgenic mouse model of Alzheimer's disease. J Mol Neurosci. 45:22–31. doi: 10.1007/s12031-010-9482-2. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Liyanage U, Bickel PE, Xia W, Lansbury PT, Jr, Kosik KS. A detergent-insoluble membrane compartment contains A beta in vivo. Nature medicine. 1998;4:730–734. doi: 10.1038/nm0698-730. [DOI] [PubMed] [Google Scholar]
- Lowther J, Yard BA, Johnson KA, Carter LG, Bhat VT, Raman MC, Clarke DJ, Ramakers B, McMahon SA, Naismith JH, Campopiano DJ. Inhibition of the PLP-dependent enzyme serine palmitoyltransferase by cycloserine: evidence for a novel decarboxylative mechanism of inactivation. Mol Biosyst. 2010;6:1682–1693. doi: 10.1039/c003743e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QL, Yang F, Rosario ER, Ubeda OJ, Beech W, Gant DJ, Chen PP, Hudspeth B, Chen C, Zhao Y, Vinters HV, Frautschy SA, Cole GM. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J Neurosci. 2009;29:9078–9089. doi: 10.1523/JNEUROSCI.1071-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malashkevich VN, Strop P, Keller JW, Jansonius JN, Toney MD. Crystal structures of dialkylglycine decarboxylase inhibitor complexes. J Mol Biol. 1999;294:193–200. doi: 10.1006/jmbi.1999.3254. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cutler RG, Jo DG. Alzheimer peptides perturb lipid-regulating enzymes. Nat Cell Biol. 2005;7:1045–1047. doi: 10.1038/ncb1105-1045. [DOI] [PubMed] [Google Scholar]
- Merrill AH, Jr, Nixon DW, Williams RD. Activities of serine palmitoyltransferase (3-ketosphinganine synthase) in microsomes from different rat tissues. J Lipid Res. 1985;26:617–622. [PubMed] [Google Scholar]
- Myhrer T, Paulsen RE. Infusion of D-cycloserine into temporal-hippocampal areas and restoration of mnemonic function in rats with disrupted glutamatergic temporal systems. Eur J Pharmacol. 1997;328:1–7. doi: 10.1016/s0014-2999(97)83019-1. [DOI] [PubMed] [Google Scholar]
- Noland BW, Newman JM, Hendle J, Badger J, Christopher JA, Tresser J, Buchanan MD, Wright TA, Rutter ME, Sanderson WE, Muller-Dieckmann HJ, Gajiwala KS, Buchanan SG. Structural studies of Salmonella typhimurium ArnB (PmrH) aminotransferase: a 4-amino-4-deoxy-L-arabinose lipopolysaccharide-modifying enzyme. Structure. 2002;10:1569–1580. doi: 10.1016/s0969-2126(02)00879-1. [DOI] [PubMed] [Google Scholar]
- Oksman M, Iivonen H, Hogyes E, Amtul Z, Penke B, Leenders I, Broersen L, Lutjohann D, Hartmann T, Tanila H. Impact of different saturated fatty acid, polyunsaturated fatty acid and cholesterol containing diets on beta-amyloid accumulation in APP/PS1 transgenic mice. Neurobiol Dis. 2006;23:563–572. doi: 10.1016/j.nbd.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Patil S, Melrose J, Chan C. Involvement of astroglial ceramide in palmitic acid-induced Alzheimer-like changes in primary neurons. Eur J Neurosci. 2007;26:2131–2141. doi: 10.1111/j.1460-9568.2007.05797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrini S, Thomas C, Brautigam H, Schmeidler J, Ho L, Fraser P, Westaway D, Hyslop PS, Martins RN, Buxbaum JD, Pasinetti GM, Dickstein DL, Hof PR, Ehrlich ME, Gandy S. Dietary composition modulates brain mass and solubilizable Abeta levels in a mouse model of aggressive Alzheimer's amyloid pathology. Mol Neurodegener. 2009;4:40. doi: 10.1186/1750-1326-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DK, Carton J, Shah AK, Meredith F, Uhlinger DJ, Hannun YA. Serine palmitoyltransferase regulates de novo ceramide generation during etoposide-induced apoptosis. The Journal of biological chemistry. 2000;275:9078–9084. doi: 10.1074/jbc.275.12.9078. [DOI] [PubMed] [Google Scholar]
- Puglielli L, Ellis BC, Saunders AJ, Kovacs DM. Ceramide stabilizes beta-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid beta-peptide biogenesis. The Journal of biological chemistry. 2003;278:19777–19783. doi: 10.1074/jbc.M300466200. [DOI] [PubMed] [Google Scholar]
- Quartermain D, Mower J, Rafferty MF, Herting RL, Lanthorn TH. Acute but not chronic activation of the NMDA-coupled glycine receptor with D-cycloserine facilitates learning and retention. Eur J Pharmacol. 1994;257:7–12. doi: 10.1016/0014-2999(94)90687-4. [DOI] [PubMed] [Google Scholar]
- Resende R, Ferreiro E, Pereira C, Oliveira CR. ER stress is involved in Abeta-induced GSK-3beta activation and tau phosphorylation. J Neurosci Res. 2008;86:2091–2099. doi: 10.1002/jnr.21648. [DOI] [PubMed] [Google Scholar]
- Samad F, Hester KD, Yang G, Hannun YA, Bielawski J. Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes. 2006;55:2579–2587. doi: 10.2337/db06-0330. [DOI] [PubMed] [Google Scholar]
- Satoi H, Tomimoto H, Ohtani R, Kitano T, Kondo T, Watanabe M, Oka N, Akiguchi I, Furuya S, Hirabayashi Y, Okazaki T. Astroglial expression of ceramide in Alzheimer's disease brains: a role during neuronal apoptosis. Neuroscience. 2005;130:657–666. doi: 10.1016/j.neuroscience.2004.08.056. [DOI] [PubMed] [Google Scholar]
- Sawamura N, Ko M, Yu W, Zou K, Hanada K, Suzuki T, Gong JS, Yanagisawa K, Michikawa M. Modulation of amyloid precursor protein cleavage by cellular sphingolipids. The Journal of biological chemistry. 2004;279:11984–11991. doi: 10.1074/jbc.M309832200. [DOI] [PubMed] [Google Scholar]
- Schilling S, Zeitschel U, Hoffmann T, Heiser U, Francke M, Kehlen A, Holzer M, Hutter-Paier B, Prokesch M, Windisch M, Jagla W, Schlenzig D, Lindner C, Rudolph T, Reuter G, Cynis H, Montag D, Demuth HU, Rossner S. Glutaminyl cyclase inhibition attenuates pyroglutamate Abeta and Alzheimer's disease-like pathology. Nature medicine. 2008;14:1106–1111. doi: 10.1038/nm.1872. [DOI] [PubMed] [Google Scholar]
- Schmelz EM, Dombrink-Kurtzman MA, Roberts PC, Kozutsumi Y, Kawasaki T, Merrill AH., Jr Induction of apoptosis by fumonisin B1 in HT29 cells is mediated by the accumulation of endogenous free sphingoid bases. Toxicol Appl Pharmacol. 1998;148:252–260. doi: 10.1006/taap.1997.8356. [DOI] [PubMed] [Google Scholar]
- Schupf N, Tang MX, Fukuyama H, Manly J, Andrews H, Mehta P, Ravetch J, Mayeux R. Peripheral Abeta subspecies as risk biomarkers of Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14052–14057. doi: 10.1073/pnas.0805902105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster GM, Schmidt WJ. D-cycloserine reverses the working memory impairment of hippocampal-lesioned rats in a spatial learning task. Eur J Pharmacol. 1992;224:97–98. doi: 10.1016/0014-2999(92)94825-g. [DOI] [PubMed] [Google Scholar]
- Shah C, Yang G, Lee I, Bielawski J, Hannun YA, Samad F. Protection from high fat diet-induced increase in ceramide in mice lacking plasminogen activator inhibitor 1. The Journal of biological chemistry. 2008;283:13538–13548. doi: 10.1074/jbc.M709950200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DCaH, B B., editors. Theory and practice of histotechnology. 2nd Edition. Battelle press; 1980. [Google Scholar]
- Sisodia SS. Beta-amyloid precursor protein cleavage by a membrane-bound protease. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:6075–6079. doi: 10.1073/pnas.89.13.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Gargini C, Novelli E, Sala G, Piano I, Gasco P, Ghidoni R. Inhibition of ceramide biosynthesis preserves photoreceptor structure and function in a mouse model of retinitis pigmentosa. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18706–18711. doi: 10.1073/pnas.1007644107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromme Johannesen T, Myhrer T. Impaired visual memory in rats reared in isolation is reversed by D-cycloserine in the adult rat. Eur J Pharmacol. 2002;437:73–77. doi: 10.1016/s0014-2999(02)01282-7. [DOI] [PubMed] [Google Scholar]
- Sundaram KS, Lev M. L-cycloserine inhibition of sphingolipid synthesis in the anaerobic bacterium Bacteroides levii. Biochem Biophys Res Commun. 1984a;119:814–819. doi: 10.1016/s0006-291x(84)80323-x. [DOI] [PubMed] [Google Scholar]
- Sundaram KS, Lev M. Inhibition of sphingolipid synthesis by cycloserine in vitro and in vivo. J Neurochem. 1984b;42:577–581. doi: 10.1111/j.1471-4159.1984.tb02716.x. [DOI] [PubMed] [Google Scholar]
- Sundaram KS, Lev M. Inhibition of cerebroside synthesis in the brains of mice treated with L-cycloserine. J Lipid Res. 1985;26:473–477. [PubMed] [Google Scholar]
- Sundaram KS, Lev M. The long-term administration of L-cycloserine to mice: specific reduction of cerebroside level. Neurochem Res. 1989;14:245–248. doi: 10.1007/BF00971318. [DOI] [PubMed] [Google Scholar]
- Tomiyama T, Matsuyama S, Iso H, Umeda T, Takuma H, Ohnishi K, Ishibashi K, Teraoka R, Sakama N, Yamashita T, Nishitsuji K, Ito K, Shimada H, Lambert MP, Klein WL, Mori H. A mouse model of amyloid beta oligomers: their contribution to synaptic alteration, abnormal tau phosphorylation, glial activation, and neuronal loss in vivo. J Neurosci. 2010;30:4845–4856. doi: 10.1523/JNEUROSCI.5825-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai GE, Falk WE, Gunther J, Coyle JT. Improved cognition in Alzheimer's disease with short-term D-cycloserine treatment. Am J Psychiatry. 1999;156:467–469. doi: 10.1176/ajp.156.3.467. [DOI] [PubMed] [Google Scholar]
- Turinsky J, Bayly BP, O'Sullivan DM. 1,2-Diacylglycerol and ceramide levels in rat skeletal muscle and liver in vivo. Studies with insulin, exercise, muscle denervation, and vasopressin. The Journal of biological chemistry. 1990;265:7933–7938. [PubMed] [Google Scholar]
- Unger RH, Orci L. Diseases of liporegulation: new perspective on obesity and related disorders. FASEB J. 2001;15:312–321. doi: 10.1096/fj.00-0590. [DOI] [PubMed] [Google Scholar]
- van Oijen M, Hofman A, Soares HD, Koudstaal PJ, Breteler MM. Plasma Abeta(1–40) and Abeta(1–42) and the risk of dementia: a prospective case-cohort study. Lancet Neurol. 2006;5:655–660. doi: 10.1016/S1474-4422(06)70501-4. [DOI] [PubMed] [Google Scholar]
- Vetrivel KS, Cheng H, Kim SH, Chen Y, Barnes NY, Parent AT, Sisodia SS, Thinakaran G. Spatial segregation of gamma-secretase and substrates in distinct membrane domains. The Journal of biological chemistry. 2005;280:25892–25900. doi: 10.1074/jbc.M503570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrivel KS, Cheng H, Lin W, Sakurai T, Li T, Nukina N, Wong PC, Xu H, Thinakaran G. Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. The Journal of biological chemistry. 2004;279:44945–44954. doi: 10.1074/jbc.M407986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada S, Morishima-Kawashima M, Qi Y, Misono H, Shimada Y, Ohno-Iwashita Y, Ihara Y. Gamma-secretase activity is present in rafts but is not cholesterol-dependent. Biochemistry. 2003;42:13977–13986. doi: 10.1021/bi034904j. [DOI] [PubMed] [Google Scholar]
- Wagner JA. Strategic approach to fit-for-purpose biomarkers in drug development. Annu Rev Pharmacol Toxicol. 2008;48:631–651. doi: 10.1146/annurev.pharmtox.48.113006.094611. [DOI] [PubMed] [Google Scholar]
- Wahrle S, Das P, Nyborg AC, McLendon C, Shoji M, Kawarabayashi T, Younkin LH, Younkin SG, Golde TE. Cholesterol-dependent gamma-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol Dis. 2002;9:11–23. doi: 10.1006/nbdi.2001.0470. [DOI] [PubMed] [Google Scholar]
- Wang G, Dinkins M, He Q, Zhu G, Poirier C, Campbell A, Mayer-Proschel M, Bieberich E. Astrocytes Secrete Exosomes Enriched with Proapoptotic Ceramide and Prostate Apoptosis Response 4 (PAR-4): POTENTIAL MECHANISM OF APOPTOSIS INDUCTION IN ALZHEIMER DISEASE (AD) The Journal of biological chemistry. 2012;287:21384–21395. doi: 10.1074/jbc.M112.340513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RD, Sgoutas DS, Zaatari GS, Santoianni RA. Inhibition of serine palmitoyltransferase activity in rabbit aorta by L-cycloserine. J Lipid Res. 1987;28:1478–1481. [PubMed] [Google Scholar]
- Won JS, Im YB, Khan M, Contreras M, Singh AK, Singh I. Lovastatin inhibits amyloid precursor protein (APP) beta-cleavage through reduction of APP distribution in Lubrol WX extractable low density lipid rafts. J Neurochem. 2008;105:1536–1549. doi: 10.1111/j.1471-4159.2008.05283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Hu T, Zhang L, Chen J, Du J, Ding J, Jiang H, Shen X. Residues Asp164 and Glu165 at the substrate entryway function potently in substrate orientation of alanine racemase from E. coli: Enzymatic characterization with crystal structure analysis. Protein Sci. 2008;17:1066–1076. doi: 10.1110/ps.083495908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S, Nishijima M, Hanada K. Localization, topology, and function of the LCB1 subunit of serine palmitoyltransferase in mammalian cells. The Journal of biological chemistry. 2003;278:4176–4183. doi: 10.1074/jbc.M209602200. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T, Yamashita A, Luo Y. Fumonisin occurrence in corn from high- and low-risk areas for human esophageal cancer in China. Appl Environ Microbiol. 1994;60:1626–1629. doi: 10.1128/aem.60.5.1626-1629.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zendzian-Piotrowska M, Baranowski M, Zabielski P, Gorski J. Effects of pioglitazone and high-fat diet on ceramide metabolism in rat skeletal muscles. J Physiol Pharmacol. 2006;57(Suppl 10):101–114. [PubMed] [Google Scholar]
- Zha Q, Ruan Y, Hartmann T, Beyreuther K, Zhang D. GM1 ganglioside regulates the proteolysis of amyloid precursor protein. Mol Psychiatry. 2004;9:946–952. doi: 10.1038/sj.mp.4001509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.