Abstract
Small leucine rich proteoglycans (SLRPs) are involved in a variety of biological and pathological processes. This review focuses on their regulatory roles in matrix assembly. SLRPs have protein cores and hypervariable glycosylation with multivalent binding abilities. During development, differential interactions of SLRPs with other molecules results in tissue-specific spatial and temporal distributions. The changing expression patterns play a critical role in the regulation of tissue-specific matrix assembly and, therefore, tissue function. SLRPs have significant structural roles within extracellular matrices. In addition, they have instructive roles, regulating collagen fibril growth, fibril organization, and extracellular matrix assembly. Moreover, they are involved in mediating cell-matrix interactions. Abnormal SLRP expression and/or structures result in dysfunctional extracellular matrices and pathophysiology. Altered expression of SLRPs has been found in many disease models, and structural deficiency also causes altered matrix assembly. SLRPs regulate the assembly of the extracellular matrix, which defines the microenvironment, modulating both the extracellular matrix and cellular functions leading to an impact on tissue function.
Keywords: Proteoglycans, Glycosaminoglycans, SLRP, Extracellular Matrix, Development, Pathophysiology
The small leucine rich proteoglycan (SLRP) family has important roles in a range of biological processes and pathophysiologies, including collagen fibrillogenesis (for reviews, see [1, 2]), signal transduction (for reviews, see [3–5]) and tumor growth (for reviews, see [6, 7]). This review is focused on the roles of SLRPs in matrix assembly during development, and their dysfunctional regulation in pathophysiology.
Structure and multivalent binding abilities
The SLRP family has been expanded to five classes based on homologies at both the genomic and protein level (Table 1). At a genomic level, eighteen genes encoding SLRPs are spread over seven chromosomes, with some genes clustered on chromosomes, suggesting duplication to generate the functional redundancy during evolution [5]. At the protein level, SLRPs have a variable number of tandem leucine-rich repeats (LRRs) comprising the major central domain. Each LRR has a conserved hallmark motif LXXLxLXXNxL (L: leucine which can be substituted by isoleucine, valine and other hydrophobic amino acids; x: any amino acids). The stretch of these LRRs in the central domain forms a curved solenoid structure with both convex and concave faces. The central LRRs domain is flanked by cysteine-rich domains on two sides. At the N-terminus, all SLRPs contain four cysteines with class-conserved internal spacing [8]. The N-termini in different SLRPS is variably modified to provide unique functions for each SLRP [9–12]. Among the conserved C-terminal cysteine-rich capping motif, a distinctive feature for the canonical class I, II, and III SLRPs is the “ear repeat“, which is the penultimate LRR that always is the longest LRR extending outwards from the convex face. The ear repeat is proposed to maintain the conformation of the protein core and influence ligand binding ability. A mutation in this position has been found in human congenital stromal corneal dystrophy [13].
Table 1.
Interactions of SLRPs with other matrix molecules
| SLRPs | Matrix assembly | |
|---|---|---|
| Class I | Decorin | Collagen I [17–20]; Collagen II, III [21];Collagen V [22]; Collagen VI [23, 24]; Collagen XII [25]; Collagen XIV [26]; Fibronectin [27, 28]; Tthrombospondin-1 [29]; MAGP-1 and fibrillin-1 [30]; tenascin-X [31] |
| Biglycan | Collagen I [32]; Collagen II [21]; Collagen III [33]; Collagen VI [34]; collagen IX and biglycan [35];Collagen II and VI complex[36]; Tropoelastin and MAGP-1[37] | |
| Asporin | Collagen I [38] | |
| ECM2 | ||
| Class II | Fibromodulin | Collagen I [39]; Collagen II [40]; Collagen VI [41]; Collagen IX[42]; Collagen XII [25]. |
| Lumican | Collagen I [43]; Aggrecan [44]; Beta-1 integrin [45]; Beta-2 integrin ; [46]; Alpha2beta1 integrin[47, 48]. | |
| Keratocan | ||
| Osteoadherin | Alpha v beta 3 integrin [49]; NC domain of Collagen IX[42]. | |
| PRELP | Perlecan and collagen [50] | |
| Class III | Osteoglycin/mimecan | Collagen I [51]. |
| Opticin | Heparan and chondroitin sulfate proteoglycans, collagen [52] | |
| Epiphycan | ||
| Class IV | Chondroadherin | Integrin alpha2beta1 [53]; Collagen II [54] |
| Nyctalopin | ||
| Tsukushi | ||
| Class V | Podocan | Collagen I [55] |
| Podocan-like protein 1 | ||
SLRPs are proteoglycans that have both protein cores and glycosaminoglycan chains (GAGs), although non-canonical class IV and V SLRPs that do not contain any GAGs are also included in this family. The GAGs of SLRPs are differentially processed in development and aging, and are variable with regard to size, number, sulfation and epimerization in different tissues. For instance, lumican is predominantly a highly sulfated proteoglycan that is present in the cornea, but a glycoprotein in other tissues [14]. The GAGs of SLRPs are important in cytokine binding, extracellular matrix assembly and hydration. SLRPs also are modified by N-glycosylation. Together with O-linked GAGs, these polysaccharide modifications affect the conformational stability of the protein core [15] and secretion [16]. Variations in glycosylation modify the binding affinities of SLRPs at different developmental stages. The hypervariable glycosylation and N-terminal variations endow SLRPs with multiple binding abilities that have been demonstrated in interactions with other molecules both in vivo and in vitro.
Spatial and temporal distribution of SLRPs during development
SLRPs are dynamically synthesized, secreted, deposited and degraded in vivo. Their multivalent binding abilities, secretion speed, local concentration and presence/absence of other molecules all affect their existing form and functions. In the dynamic process of metabolism, SLRPs are subject to the alterations of the microenvironments and are regulated at different levels, both intracellularly and extracellularly. At the chromosomal level, the gene clustering balances the expression of different SLRPs during development. For instance, keratocan, which is downstream of lumican on chromosome 12, is regulated by lumican [56]. Differential splicing, alternative polyadenylation and multiple promoter use during transcription all provide tissue-specific regulation at different developmental stages. For instance, the expression of SLRPs and cytokines are regulated bi-directionally through a common regulatory framework [57], providing feedback mechanisms in regulating matrix assembly and remodeling. In the secretory pathway, SLRPs are subject to surveillance chaperones and post-translational modifications in the ER [58]. Dysregulation in these steps may lead to under-glycosylated, misfolded or dislocated SLRPs [58]. In the extracellular matrix, SLRPs are subject to many proteases such as MMPs [59], aggrecanases [60], bone morphogenetic protein-1 [61], and granzyme B [62]. On the other hand, SLRPs are selectively resistant to some MMPs [63] and protect collagen fibrils from cleavage by collagenases [64] when they bind to the collagen fibril surface. Secreted SLRPs can be recycled by endocytosis [65]. Endocytosis not only affects the metabolism of SLRPs, but also modulates other biological processes. For instance, endocytosis of decorin evokes protracted internalization and degradation of the epidermal growth factor receptor [66]; intracellular accumulation and nuclear localization of aberrantly expressed decorin are observed in tumor cells [67]. SLRPs are ubiquitous molecules and are deposited in the extracellular matrix and mainly associated with collagens in interstitial connective tissues such as cornea, bone and tendon. However, SLRPs are expressed in tissues independent of collagen secretion in early developmental stages to regulate cell migration, proliferation and differentiation [68, 69]. In spite of their similar structure and some common functions, the spatial distribution and organization of SLRPs are tissue-specific and dynamic during development. For example, keratocan is mostly restricted to the corneal stroma postnatally [70]; biglycan has a peak levels in tendon and muscle in embryonic stages [71], but its expression levels are low in adult tissues. The cornea provides an excellent example of differential spatial and temporal expression patterns for a number of different SLRPs. The cornea expresses six different SLRPs that have unique expression patterns both spatially and temporally. The corneal class I SLRPs, decorin and biglycan, are distributed across the corneal stroma, but biglycan expression decreases significantly after birth while decorin remains relatively stable. The corneal class II SLRPs also display differential expression patterns. Keratocan has a constant temporal and spatial expression pattern in corneal development and the mature cornea [56]. In contrast, during development, lumican is homogenous in both anterior and posterior stroma, but becomes restricted to the posterior stroma in the adult animal [72]. Fibromodulin is not considered a corneal component, however, it has a narrow window of expression extending into the central cornea during early postnatal development [73]. The class III SLRP, osteoglycin, is only localized to the epithelium and basement membrane zone [72]. The complex expression patterns of SLRPs during corneal development are tightly regulated. This provides tissue-specific regulation of collagen fibrillogenesis and stromal matrix assembly, hydration, and cornea-sclera integration during corneal development, generating the functional attributes of transparency and refraction to the cornea.
The nature of the GAGs attached to the SLRPs also is regulated during development. For instance, the switch of the polylactosamine form to keratan sulfate lumican in cornea is coincident with eye opening and contributes to corneal transparency [74]. During bone formation, dermatan sulfate biglycan is only expressed during the cell proliferation phase, ceases during the early matrix deposition phase and then is repressed with GAGs switched to the chondroitin sulfate at the initiation of mineralization [75]. The spatial and temporal variations of SLRPs also are observed in cartilage, tendon [76], ocular [77] and odontal tissues [78] during development. The tissue-specific temporal and spatial expression of SLRPs plays both instructive and structural roles in matrix assembly during development.
The regulatory and structural roles of SLRPs in matrix assembly
The extracellular matrix provides mechanical strength and support, but also provides a microenvironment that defines the concentration of growth factors, hydration, pH, and electro-chemo gradients that shape tissue-specific function. The extracellular matrix is mainly composed of glycoproteins, proteoglycans and collagens. SLRPs with their multivalent binding abilities regulate matrix assembly during development at different levels.
Instructive roles in collagen fibrillogenesis
Collagen fibrils are the major component of most extracellular matrices. The assembly and deposition of collagen fibrils involves a sequence of events that occurs in both intracellular and extracellular compartments. Procollagen molecules are synthesized, hydroxylated, glycosylated, assembled from three polypeptides, and folded in the rough endoplasmic reticulum. Packaging occurs in the Golgi, and transport is via specialized and elongated intracellular compartments with secretion at the cell surface. The extracellular removal of N-and C-propeptides after procollagen secretion is required for the formation of collagen. Collagen molecules assemble into striated protofibrils with a characteristic 67nm banding pattern. Protofibrils are immature fibrils that have small and uniform diameters as well as short lengths compared to mature fibrils. During development, collagen fibrils are initially assembled as uniform and relatively short protofibrils (diameter ~20 nm, length 4–12 µm) (Fig.1A). The protofibrils are D-periodic with tapered ends [79–82]. In mature tissues, collagen fibrils are functionally continuous, i.e., are long with lengths that have not been measured, and have diameters in the range 20–500 nm depending on the tissue and developmental stage [79, 80, 83]. The mature fibril is assembled by end to end and lateral association of protofibrils (Fig. 1A, B). A model for the multi-step assembly of mature fibrils from preformed intermediates, protofibrils, is presented in Fig. 2. Procollagen is processed into collagen that assembles into protofibrils that are closely associated with the cell surface. Collagen assembly into protofibrils involves the interaction of at least 2 fibril-forming collagens. Collagens V and XI are variants of the same collagen type and have key roles in nucleating protofibril assembly [84–87].The protofibrils are deposited and incorporated into the developing extracellular matrix where they are stabilized via interactions with macromolecules such as small leucine-rich proteoglycans. This stabilization can coincide with assembly, or can occur with changing patterns at the time of and after assembly. Protofibril stabilization is not a single defined interaction, but rather a continuum that varies in a tissue- and developmental-specific manner. The stabilized protofibrils result in a discontinuous extracellular matrix during periods of rapid growth and development. Maturation is generally associated with larger diameter fibrils that become longer and functionally continuous. Initially, this involves linear fibril growth involving end-overlap of the protofibrils. In most tissues, this is followed by lateral fibril growth where the fibrils associate and fuse laterally to generate large diameter fibrils seen in most mature tissues. These associations involve molecular rearrangements necessary to regenerate the cylindrical fibril structure. In this process, some or all components stabilizing the protofibrils are lost or replaced during formation of the mature fibrils. Throughout this process, lysyl oxidase mediates intra- and intermolecular covalent cross-linking of collagen within the fibril. As the number of intermolecular crosslinks increases with fibril maturation, molecular rearrangement is limited and mature fibril structure is stabilized. The growth in length and diameter as well as covalent crosslinking increases the mechanical strength of the connective tissue.
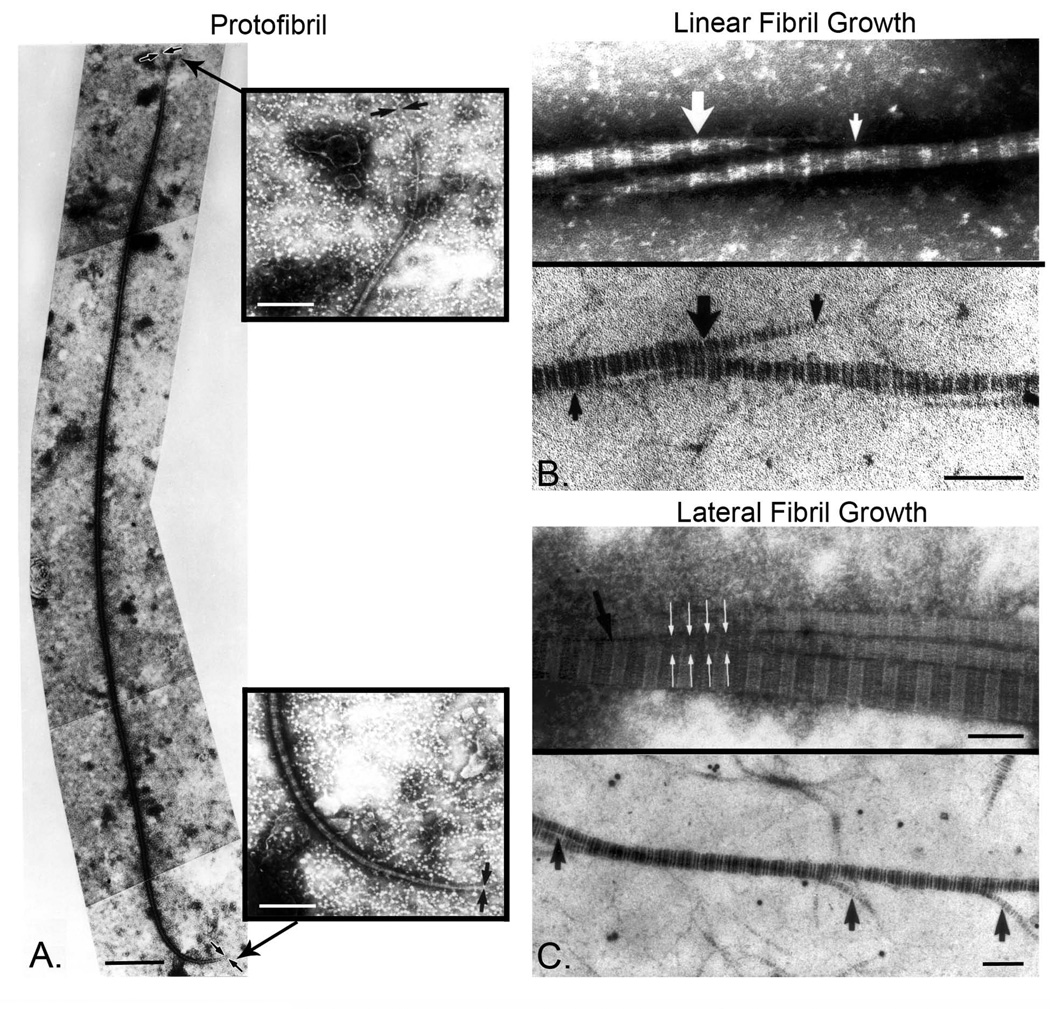
Fig. 1. SLRPs regulate linear and lateral fibril linear.
A. Protofibrils were extracted from 14 day chicken embryo tendons. Tendons were washed, swollen, and homogenized. This procedure almost completely disrupted the 14-day tendons. The suspension was negatively stained and observed by transmission electron microscopy. Intact fibrils of discrete lengths were observed. Bar, 1µm. The ends of extracted protofibrils were asymmetric with long (indicated by arrows in upper inset) and short (indicated by arrows in bottom inset) tapers. Bar, 250nm. B. Collagen fibrils grow by linear (end to end) association of protofibrils. Transmission electron microscopy of fibrils from extracted and cryosectioned tendons, illustrating a linear growth of protofibrils. This mechanism produces fibrils of increasing length without significantly altering fibril diameter. Bar, 100nm. C. Collagen fibrils grow by lateral associations of preformed protofibrils. Transmission electron microscopy of fibrils from extracted and cryosectioned tendons illustrate lateral association. The extensive lateral association or fusion of fibril segments would produce fibrils of increasing length and larger diameter. Bar, 100nm. Modified from Birk et al. [79]
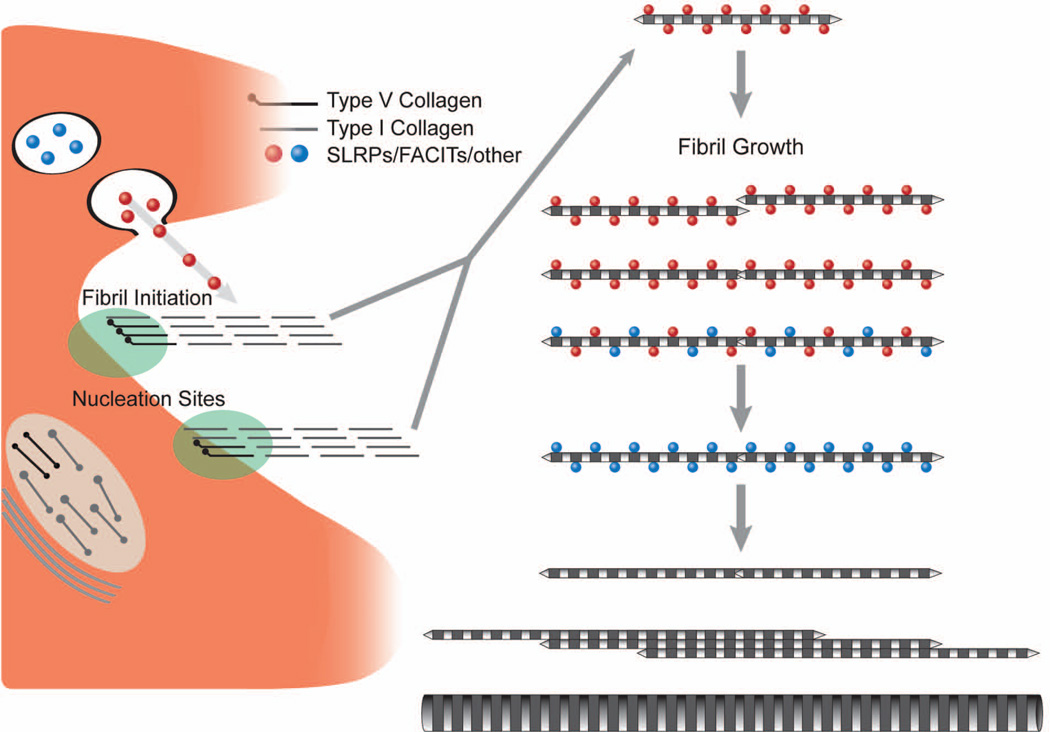
Fig. 2.
Model illustrates the involvement of SLRPs in regulation of linear and lateral fibril growth. Collagen fibrillogenesis is a multiple-step process that is tightly regulated by the interaction of different molecules. The initial step involves heterotypic collagen I/V nucleation at cell surface, SLRPs bind to the protofibril surface, regulating the linear growth and lateral growth of protofibril to mature collagen fibrils. Deficiency of SLRPs leads to dysfunctional linear and lateral fusion with alterations in fibril structure and function. Modified from Birk et al. [84]
Almost all SLRPs bind collagen fibrils (Table 1), and gene-targeted mouse models have demonstrated their critical instructive roles in fibrillogenesis and matrix assembly (for reviews, please see [2]). Decorin also binds to procollagen [88], indicating that SLRPs may bind to collagen before collagen fibril assembly. The binding of SLRPs to collagen has been analyzed by many researchers. Immuno-electron microscopy and in vitro peptide binding assays demonstrate that SLRPs can have different or shared binding sites as well as different binding affinities. This permits the finely-tuned regulation of fibrillogenesis through the cooperation of different SLRPs. Class I SLRPs, decorin and biglycan, bind the “d” and “e” bands of collagen fibrils [17, 18]; class II SLRPs, lumican and fibromodulin bind the “a” and “c” bands of collagen fibrils [89]. Same class SLRPs have the same binding site on the collagen and therefore compete with each other for binding; for instance, asporin competes with decorin [38] and lumican competes with fibromodulin [43, 90]. In contrast, different class SLRPs do not compete because of the different binding sites on the collagen fibrils [91]. During development, the similar binding abilities between SLRPs provide functional redundancy in fibrillogenesis. For instance, both decorin-deficient and biglycan-deficient mice have mild corneal stromal phenotypes [92]. The decorin-deficient mice demonstrated a significant up-regulation of biglycan expression suggesting a functional compensation. When this compensation was prevented in a compound decorin/biglycan-deficient mouse model, a very severe stromal phenotype resulted (Fig. 3). These data support functional redundancy within SLRP classes.
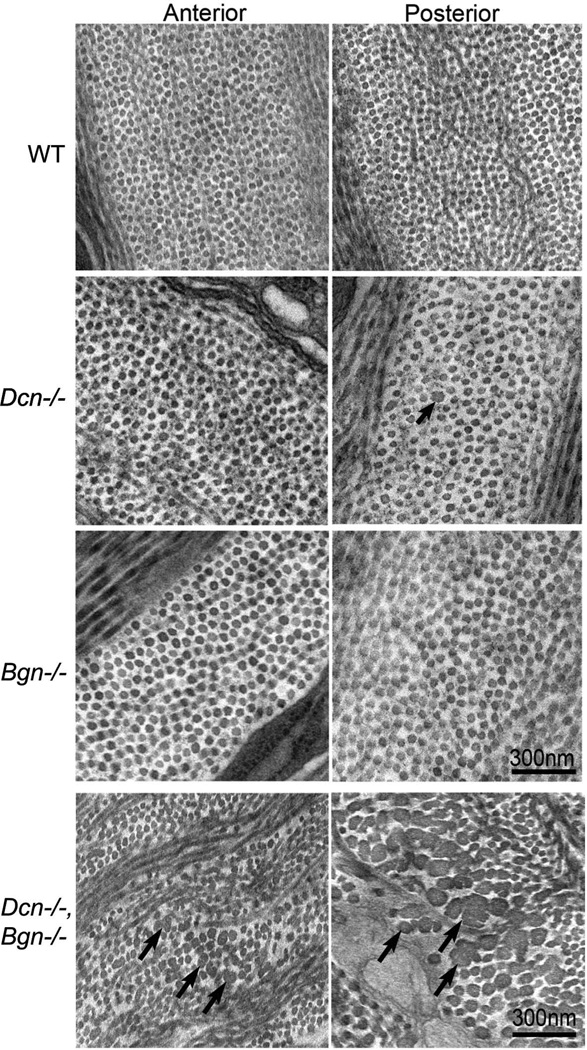
Fig. 3. Collagen fibril structure in Dcn−/−, Bgn−/−, and Dcn−/−/Bgn−/− corneal stromas.
Both fibril and stromal architecture in decorin-deficient (Dcn−/−) and biglycan-deficient (Bgn−/−) corneas were comparable with wild-type controls. Occasional abnormal fibrils were observed in the Dcn−/− stroma (arrow). In contrast, the compound mutants demonstrated an aberrant fibril phenotype (arrows). Large, irregular fibrils were present in both anterior and posterior stroma, but the posterior region had the more severe phenotype. In addition, there were disruptions in fibril packing and organization in the double-null mice. Transmission electron micrographs of the posterior and anterior stroma from: wild type (WT), Dcn−/−, Bgn−/−, and Dcn−/−/Bgn−/− mice at P60. Modified from Zhang et al. [92]
SLRPs bind collagen primarily through their central domains. For instance, decorin binds collagen I via LRR 4–6 [19] which is further located to sequence SYIRIADTNIT [20]; lumican and fibromodulin have a homologous sequence in LRR 5–7 for collagen binding. On the other hand, SLRPs have multiple collagen binding domains; for instance, decorin has multiple binding domains for interaction with collagen I [93]. A crystal structure of the decorin-collagen complex suggests that one decorin monomer can bind multiple collagens via multiple binding domains [94]. Class II fibromodulin also has a second collagen I binding site via Glu-353 and Lys-355 in leucine-rich repeat 11 [39], which facilitates collagen fibril cross-linking [90] and maturation. During tendon development, fibromodulin facilitates the growth of protofibrils into mature fibrils [76]. Binding of SLRPs on the collagen fibril surface regulates fibril growth steps and a common feature in SLRP-null models is dysfunctional regulation of fibril diameter and altered fibril structure [2, 72, 76, 92].
Glycosaminoglycans (GAGs) affect SLRP protein core conformation during biosynthesis [15]. However, in vitro fibrillogenesis assays indicate that SLRP protein cores are critical in regulating fibrillogenesis, which is independent of the GAGs [95]. The SLRP protein cores bind collagen fibril via their concave face with GAGs extending outward into the inter-fibril space. The sulfated GAGs regulate matrix hydration [96] and interact with adjacent collagen fibrils. Periodic interactions between GAGs and collagen fibrils are observed under high-resolution scanning electron microscopy [97]. Three-dimensional electron morphological studies also show that the GAGs can tether two or more collagen fibrils to form a network [98, 99]. In cell culture, mutant decorin without GAGs was associated with larger diameter collagen fibrils in the 3D matrix [100]. Dermatan sulfate epimerase 1-deficient mice also exhibit altered collagen structure in skin [101]. Computational studies suggest that GAGs bridge and transfer force between adjacent fibrils, providing mechanical integrity to the tissue [102]. Therefore, the GAGs of SLRPs can be involved in the regulation of fibrillogenesis, but also influence inter-fibril spacing and organization during matrix assembly. In the absence of decorin, fibrils in the periodontal ligament are randomly organized instead of the normal parallel orientation [103]. On the other hand, GAGs can also modulate the micro-environment during matrix assembly. For instance, decorin and biglycan inhibit HAP-induced crystal growth through GAGs and regulate mineralization process during bone formation [104].
SLRPs not only bind collagen I, but also other fibrillar collagens such as collagen II and III [21]. SLRPs have different affinities for different fibrillar collagens, which contribute to the modulation of their functions within different tissues. For instance, biglycan interacts strongly with collagen II, but has a lower affinity for collagen I; therefore, different or modified roles would be expected in tissues with different collagen compositions, e.g., cartilage versus cornea, bone or tendon.
Constructive roles in matrix assembly
SLRPs have multiple binding domains with elongated sulfated GAGs. PRELP has both a collagen binding domain and a heparin binding domain. It can function in the integration of adjacent extracellular matrices by binding perlecan in the basement membrane and collagen in underlying connective tissues [50]. Collagen VI is a ubiquitous collagen that forms a flexible network that interweaves among collagen fibrils in connective tissues and between fibrils and cells. Immuno-electron localization showed that collagen VI microfilaments cross collagen I at the “d” band where decorin and biglycan also bind [105]. Both decorin and biglycan bind collagen VI [24, 34]. In vitro studies demonstrate that biglycan also can organize collagen VI into hexagonal-like networks [106]. SLRPs can connect collagen VI, collagen II, matrilin-1 and aggrecan, forming a complex in pericellular matrix, as demonstrated in cultured chondrosarcoma cells [107]. SLRPs such as fibromodulin, decorin and biglycan also bind fibril-associated collagens such as collagen XII and collagen XIV via protein core or glycosaminoglycans; therefore, they indirectly regulate collagen fibril organization [25, 26, 108, 109]. Other extracellular matrix molecules that interact with SLRPs include microfibrillar proteins MAGP-1 and fibrillin-1 complex [30], tropoelastin and microfibril-associated glycoprotein-1 complex [37], and aggrecan [44].
Involvement of SLRPs in cell-matrix interaction during matrix assembly
Although collagen fibrils can self-assemble, the cell also participates in the organization of the fibrils through interactions involving integrins, fibronectin, thrombospondins, tenascins, etc. [110]. SLRPs can be involved in cell-matrix interactions by directly interfering with plasma membrane receptors and pericellular matrix molecules. For instance, decorin inhibits cell attachment though fibronectin [111], thrombospondin [112], and tenasin [113]. Lumican [45, 48], osteoadherin [49], and chondroadherin [53] all have high affinity for integrins. Biglycan also regulates muscle cell behavior through binding plasma membrane alpha dystroglycan through its GAGs [114], playing a role in muscular dystrophies. Nyctalopin, a cell membrane-associated SLRP, acts by networking cell receptors and pericellular matrix proteins to modulate cellular behavior [115]. SLRPs are cytokine reservoirs in the extracellular matrix [12], matrix barriers restricting molecular diffusion [116], and matrikines directly interacting with cell surface receptors [5].Therefore, SLRPs influence cell behaviors including differentiation, apoptosis, proliferation and migration through multiple means. SLRPs that can influence both cell behavior and matrix assembly are critical for the structural integrity of tissues.
Altered expression of SLRPs disrupt matrix integrity
Collagen fibrillogenesis is tightly regulated to generate tissue-specific structures and therefore functions. In the corneal stroma, homogenous small diameter fibrils, which are regularly packed and arranged as orthogonal lamellae, are required for corneal transparency. Lumican-deficient mice exhibit progressive corneal opacity with age. This is associated with irregularly packed, large diameter collagen fibrils with irregular, cauliflower-like contours in the posterior stroma. The altered fibril characteristics are consistent with the dysfunctional regulation of lateral fibril growth steps. The requirement for a homogeneous population of small diameter fibrils necessary for transparency is inconsistent with lateral fibril growth in the corneal stroma. This abnormal phenotype is coincident with the spatial restriction of lumican to the posterior stoma, resulting in increased light scattering and opacity in the region [117, 118]. Mice that are deficient in decorin or biglycan, another class I SLRP in the cornea, only have a mild phenotype. However, compound mutant mice deficient for both decorin and biglycan demonstrated a severe phenotype with increased numbers of large diameter fibrils, a very heterogeneous diameter distribution and irregular, cauliflower-like contours in both anterior and posterior stroma. Again, the spatial distribution of the abnormal phenotype was coincident with decorin expression. Both in vivo and in vitro studies demonstrated that biglycan is up-regulated in the absence of decorin and can functionally compensate for the loss of decorin. The data suggest that decorin is a major regulatory SLRP in the cornea [92]. Keratocan-deficient mice have thinner corneal stromas and a narrower cornea-iris angle, indicating that the keratan sulfate containing keratocan regulates stroma hydration and shape during development [96]. Fibromodulin, which is expressed during a very narrow window during development of the corneal stroma, is involved in the regulation of cornea-sclera integration during corneal postnatal development [73]. In humans, cornea plana is associated with missense and frameshift mutations resulting in a single amino acid substitution or a C-terminal truncated keratocan [119, 120]. High myopia is associated with intronic variations and SNPs in fibromodulin, PRELP and opticin genes [121, 122]. Increased keratocan expression is observed in the stroma of keratoconus corneas [123]. Therefore, cornea-specific temporal and spatial expression of SLRPs is critical for precisely-regulated matrix assembly during development.
Tendons are composed of uniaxially arranged collagen fibrils, organized as fibers (fibril bundles), which provide the tensile properties required for transmission of force. As illustrated in the mouse flexor tendon, fibrillogenesis involves sequential steps during development (Fig. 3). First, protofibrils are assembled around P (postnatal) 4 and have a normal diameter distribution with relatively small diameters. Next, there is a transition from the assembly to growth phase where protofibrils begin the linear and lateral growth required for fibril maturation. Around P10, larger diameter fibrils added to the smaller diameter protofibril population. These immature fibrils continue to grow to maturation from one month to 3 month. This phase is characterized by lateral fibril growth generating a broad, heterogeneous distribution of fibril diameters characteristic of the structurally and functionally mature tissue. Abnormal fibril structure was present in decorin-deficient tendons. The fibrils had larger diameters with irregular fibril contours indicating altered regulation of lateral collagen fibril growth in the decorin deficient tendons. There were tendon-specific differences in the severity of the fibril phenotype [124, 125]. The underlying basis of the site-specific differences requires further study, but may result from tissue-specific differences in SLRP expression patterns. Besides decorin, biglycan (class I), fibromodulin and lumican (class II) are the major SLRPs expressed during tendon development. Cooperation between different SLRPs is important in the regulation of tendon fibril growth and maturation [126]. During tendon development, lumican expression has a peak level [76] around P8 and then decreases dramatically. The expression of fibromodulin peaks at P14, is maintained until P30, and then decreases dramatically. In the absence of fibromodulin, the collagen associated lumican level is increased. In lumican-deficient tendons, fibril alterations are observed early, but the mature tendon is nearly normal. Fibromodulin-deficient tendons are comparable with the lumican-deficient mice in early development, but have an increased smaller diameter fibril population in mature tendons [127]. In mature tendons, lumican-deficient tendons were comparable to wild type tendons, while the stiffness was significantly reduced in the fibromodulin-deficient tendons. Interestingly, as lumican was reduced in compound mutant mice deficient in fibromodulin, the stiffness was reduced in proportion to lumican expressed: Fmod−/−/Lum+/+ > Fmod−/−/Lum+/− > Fmod−/−/Lum−/−[128]. We interpret this as a dominant SLRP interacting with a modulatory SLRP in the regulation of tissue-specific fibrillogenesis. The double-deficient mice have an early additive phenotype, but are comparable with the fibromodulin-deficient mice at maturation. Both lumican and fibromodulin inhibit fibril lateral grow during initial assembly of protofibrils, and may influence fibril number, as fewer fibrils are observed in tail tendon in fibromodulin deficient mice [129]. In later stages, fibromodulin facilitates fibril growth through growth steps leading to mature fibrils (Fig. 4). Decorin also regulates tendon fibril development cooperatively with biglycan [130]. Decorin-deficient mice not only exhibit disrupted collagen fibril structure, but also altered viscoelastic properties, indicating that decorin regulates interfibril organization [131].
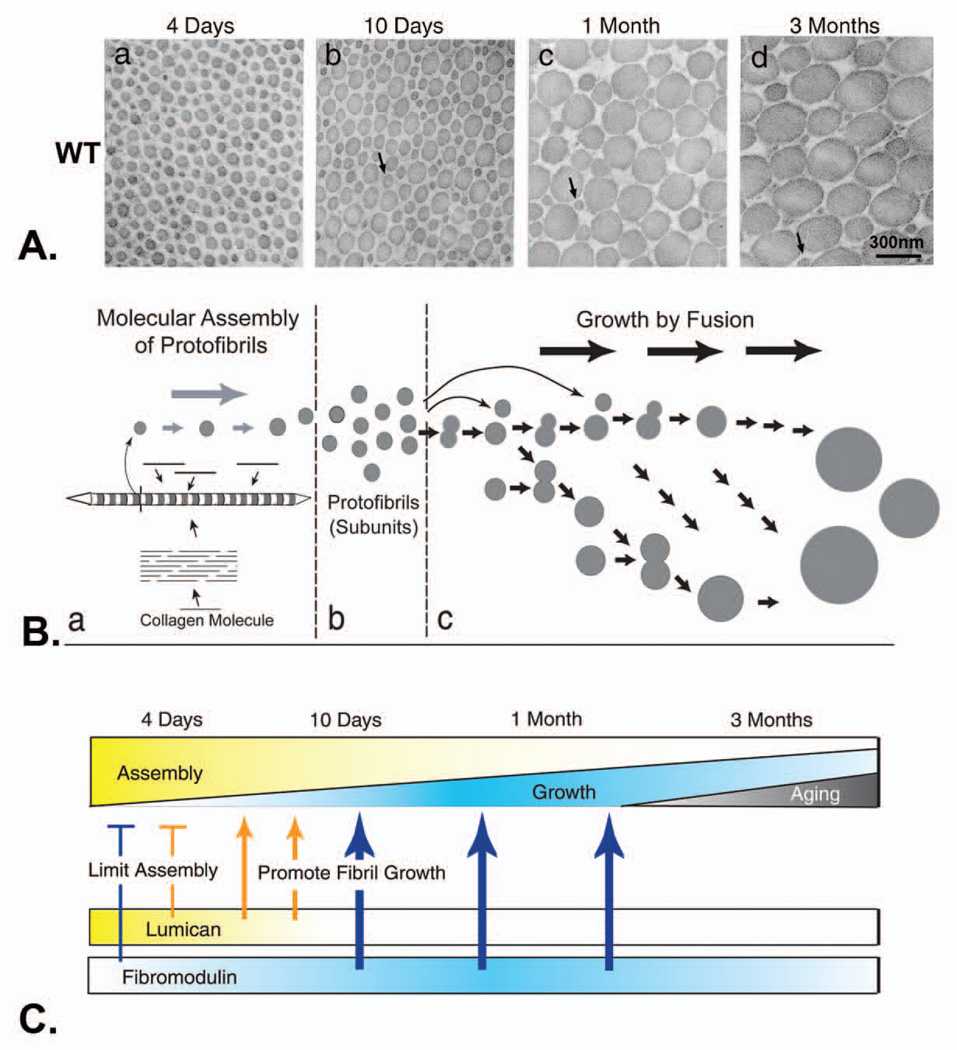
Fig. 4. Lumican and fibromodulin cooperatively regulate collagen fibrillogenesis in developing mouse tendon.
A. Collagen fibril structure during development in wild-type mouse tendons. Transmission electron micrographs of transverse sections from wild type mouse flexor tendons (a–d). Fibril structure was analyzed at different developmental stages, 4 days (a), 10 days (b), 1 month (c), and 3 months (d) postnatal. Bar, 300 nm. Arrows indicate protofibrils. B. Model for regulation of fibril growth by lumican and fibromodulin. The steps in collagen fibrillogenesis during tendon development are presented (a–c). (a) In the early steps of fibril formation, the molecular assembly of collagen monomers into protofibrils occurs in the pericellular space. Collagen molecules (bars) assemble into quarter-staggered arrays forming protofibrils, seen here as striated structures with tapered ends in longitudinal section and in cross section as circular profiles. Growth in length and diameter is by accretion of collagen at this stage. (b) Protofibrils in mouse tendons are stabilized through their interactions with fibril-associated macromolecules such as SLRPs. (c) Fusion of the protofibrils generates the mature fibril in a multistep manner. Progression through this growth process could be both by additive fusion (i.e., protofibrils add to its product, indicated by horizontal arrows) and by like-fusion (i.e., products from different steps can only fuse with like products, indicated by arrows in the oblique direction). C. The proportion of the assembly and growth steps occurring during development is illustrated. During the early stages, assembly is the main event and its relative contribution gradually decreases to the minimum degree necessary for maintenance at maturation. The relative contribution of growth by fusion increases to ~1 month. This is followed by a decrease during maturation (bottom). The expression data for lumican and fibromodulin is illustrated. The phenotypes observed in mutant mice indicate stage-specific regulatory mechanisms. At 4 days, both lumican and fibromodulin limit the assembly of the collagen monomers (bars). Characteristic of 10 days, growth from preformed protofibrils begins, and changes in both lumican and fibromodulin promote the transition from assembly to fibril growth by fusion (thin arrows). At later stages, only fibromodulin promotes the growth steps (thick arrows) Modified from Ezura et al. [127]
SLRP-deficient mice exhibit phenotypes that are consistent with dysfunctional matrix assembly in connective tissues such as skin, bone, cartilage and teeth [132], as well as non-connective tissues such as liver [133] and the pregnant uterus [134, 135]. These phenotypes resemble those observed in many human diseases. For instance, targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice [136]; biglycan/fibromodulin-deficient mice have abnormal collagen fibrils in tendons that leads to gait impairment, ectopic ossification, and osteoarthritis [137]; Ehlers-Danlos-like changes such as skin laxity, and fragility as well as joint laxity are found in in decorin and biglycan-deficient mice [130], as well as lumican and fibromodulin-deficient mice [128]. Indeed, the altered expression of SLRPs has been observed in a broad range of human diseases such as Marfan syndrome [138], localized scleroderma [139], infantile progeroid patients [140], osteogenesis imperfecta [141], systemic sclerosis [142], and carbohydrate-deficient glycoprotein syndrome [143].
The multivalent binding capacity of SLRPs is required for their normal function. However, this advantage could become a disadvantage when SLRPs provide an adhesion site for pathogens or toxic substances. Borrelia burgdorferi, the causative agent of Lyme disease, resides mainly in the extracellular matrix involving binding with decorin via decorin-binding proteins A and B [144]. Indeed, mice that are deficient in the decorin gene are more resistant to Lyme disease [145, 146]. Decorin also plays an important role in forming amyloid plaques in Alzheimer’s disease [147]. In a patient with proximal myopathy, a skeletal muscle-specific form of decorin is found as a target antigen for a serum IgM. SLRPs such as decorin and biglycan can link LDL and apolipoproteins to collagen, resulting in an accumulation of these toxic substances in atherosclerosis [148, 149].
Structural deficiency of SLRPs in altered matrix assembly
Structural deficiency that leads to dysfunctional matrix assembly also is found in human diseases. In a patient with progeroid, only 50% of the decorin protein core molecules are substituted with glycosaminoglycan chains due to two point mutations in beta4 galactosyltransferase I (beta4GalT-7). The heterogeneously glycosylated decorin is thought to be the major mechanistic cause of the defective skin seen in Ehlers-Danlos syndrome [150].
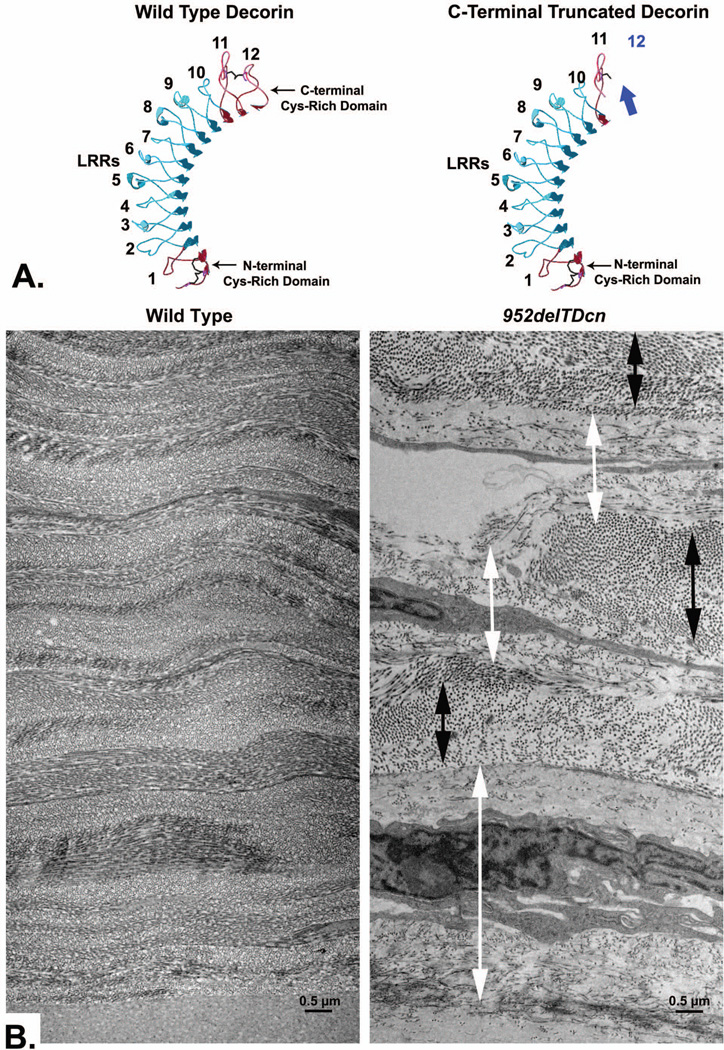
Human congenital stromal corneal dystrophy is the only human disease that has been associated with a mutant decorin gene. Three different frame-shift mutations have been reported, and all of these mutations (c.947delG; c.967delT; c.941delC) are at the C-terminus of decorin [151–154]. These deletion mutations all lead to identical truncations of decorin lacking a 33 amino acid segment that includes the "ear" repeat, which is a feature specific for small leucine-rich proteoglycans (Fig. 5). A recently published decorin-associated CSCD involves a novel nucleotide substitution (c.1036T>G) in decorin [155, 156]. The substitution of the cysteine with glycine within the C-terminus caused a milder phenotype than in the truncated mutations [155, 156]. Presumably, the loss of the cys reside results in a deficiency in disulfide bond formation in this critical region. The resulting altered conformation, rather than a truncation, may explain the milder functional consequences. A novel animal model that recapitulates human CSCD was generated in our lab [157]. Mutant mice expressed both wild-type and mutant decorin. Corneal opacities were found throughout, with increased severity toward the posterior stroma. The architecture of the lamellae was disrupted, with relatively normal lamellae separated by regions of abnormal fibril organization. Within abnormal zones, the interfibrillar spacing and the fibril diameters were increased (Fig. 5). Truncated decorin negatively affected the expression of endogenous decorin, biglycan, lumican, and keratocan and positively affected fibromodulin [157]. In vitro studies demonstrate that the truncation of the decorin C-terminus results in a misfolded protein retained in the ER. The mutant decorin alters cytoplasmic trafficking and induces ER stress (unpublished data). This leads to dysregulated homeostasis of extracellular matrix components and disrupted matrix assembly in cornea stroma.
Fig. 5. A transgenic mouse model of human congenital stromal corneal dystrophy associated with a C-terminal truncated decorin.
A. Swiss-model of mouse decorin and a truncated decorin lacking 33 C-terminal amino acids is presented. LRR domains were numbered 1 to 12 from N-terminus to C-terminus, (blue arrow indicates the truncated C-terminal). B. In the transgenic mouse expressing truncated decorin, the orthogonal lamellar structure is disrupted. Relatively normal lamellae (black double-headed arrow) are separated by abnormal zones (white double-headed arrow). In these abnormal zones, collagen fibrils are irregularly packed and embedded in an electron-lucent substance, with increased inter-fibrillar spacing. Note that the abnormal zones are often adjacent to keratocytes and more pronounced in the posterior stroma. Bar = 0.5 µm. Modified from Chen et al. [13, 157]
Conclusions and future directions
Extracellular matrices provide structural scaffolds that are tissue-specific, developmental stage-specific and are altered in pathophysiological conditions. These scaffolds also define cell function. Cells sense signals within the micro-environment that reside in the extracellular matrix, and are able to respond by altering cellular function. In addition, the response may be to manipulate the microenvironment to benefit their growth or other function. Alternatively, the altered microenvironment may provide signals for cells that migrate in during development or in pathological processes such as inflammation and cancer. Almost all SLRP-deficient mice exhibit a phenotype in connective tissues, suggesting extensive cooperation between SLRPs and other molecules. Dynamic interactions involving SLRPs, cells and their extracellular matrices provide a diversity of specific tissue functions (Fig. 6). The redundancy of SLRPs in tissues supports important roles in maintaining tissue stasis. In contrast, differences in SLRPs are important in fine tuning tissue-specific microenvironments to promote a particular cellular and/or tissue function. For instance, compound decorin and P53-deficient mice provide a permissive condition for tumor growth [158]. In early embryonic development, SLRPs regulate cell migration and differentiation via cytokines and cell receptors. In later stages, they either instruct or regulate matrix assembly, thereby shaping tissue function. SLRPs are indispensable structural components of the extracellular matrix in mature tissues. In pathological conditions, such as inflammation and wound healing, SLRPs facilitate tissue repair and regeneration [159, 160]. Finally, SLRPs may be associated with tissue changes associated with aging [161]. Extracellular matrices are regulated by SLRPs during assembly, but these matrices also regulate the dynamic distribution and function of SLRPs during development and diseases. SLRPs in extracellular matrices may provide prognosis markers, but also may lead to pharmacological targeting in the treatment of a broad range of diseases [162].
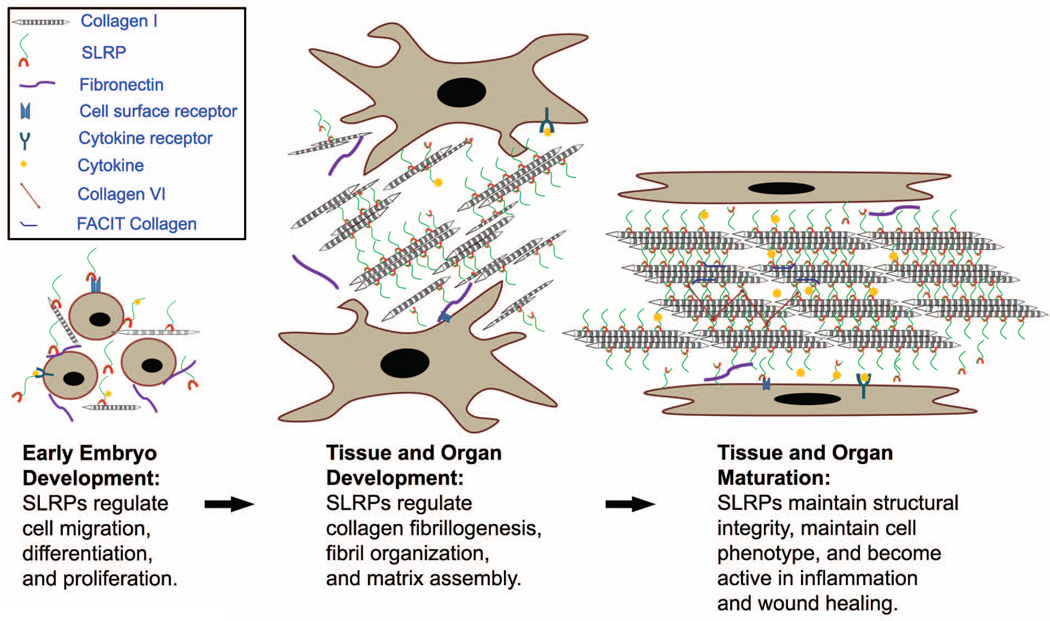
Fig. 6. Roles of SLRPs in extracellular matrix assembly during development and maturation.
Dynamic interactions involving SLRPs, cells and their extracellular matrix result in the diversity and modulation of tissue-specific function. In development, SLRPs regulate cell migration, differentiation and proliferation via cytokines and cell receptors. In later stages, they instruct matrix assembly through the regulation of linear and lateral fibril growth through binding to the collagen fibril surface. They are indispensable constructive components of the matrix in mature tissues, interacting with other extracellular matrix components such as FACIT collagens, and collagen VI. They also modulate the function of cytokines in the extracellular matrix. In pathological conditions, such as inflammation and the injury response to wounding, SLRPs facilitate tissue repair and regeneration.
Acknowledgments
This work was supported by grants from the NIH; NEI EY05129 and NIAMSD AR44745.
Abbreviations
- SLRPs
Small leucine rich proteoglycans
- LRRs
leucine-rich repeats
- GAGs
glycosaminoglycan chains
- ER
endoplasmic reticulum
- CSCD
Congenital stromal corneal dystrophy
- PRELP
Proline/arginine-rich end leucine-rich repeat protein
Footnotes
This study was supported by NIH grants EY05129 and AR44745
References
- 1.Kalamajski S, Oldberg A. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol. 2010;29:248–253. doi: 10.1016/j.matbio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12:107R–116R. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- 3.Moreth K, Iozzo RV, Schaefer L. Small leucine-rich proteoglycans orchestrate receptor crosstalk during inflammation. Cell Cycle. 2012;11:2084–2091. doi: 10.4161/cc.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neill T, Schaefer L, Iozzo RV. Decorin: a guardian from the matrix. Am J Pathol. 2012;181:380–387. doi: 10.1016/j.ajpath.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 2008;283:21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldoni S, Iozzo RV. Tumor microenvironment: Modulation by decorin and related molecules harboring leucine-rich tandem motifs. Int J Cancer. 2008;123:2473–2479. doi: 10.1002/ijc.23930. [DOI] [PubMed] [Google Scholar]
- 7.Schaefer L, Iozzo RV. Small leucine-rich proteoglycans, at the crossroad of cancer growth and inflammation. Curr Opin Genet Dev. 2012;22:56–57. doi: 10.1016/j.gde.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 8.McEwan PA, Scott PG, Bishop PN, Bella J. Structural correlations in the family of small leucine-rich repeat proteins and proteoglycans. J Struct Biol. 2006;155:294–305. doi: 10.1016/j.jsb.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Bengtsson E, Neame PJ, Heinegard D, Sommarin Y. The primary structure of a basic leucine-rich repeat protein, PRELP, found in connective tissues. J Biol Chem. 1995;270:25639–25644. doi: 10.1074/jbc.270.43.25639. [DOI] [PubMed] [Google Scholar]
- 10.Le Goff MM, Bishop PN. Focus on molecules: opticin. Exp Eye Res. 2007;85:303–304. doi: 10.1016/j.exer.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Lorenzo P, Aspberg A, Onnerfjord P, Bayliss MT, Neame PJ, Heinegard D. Identification and characterization of asporin. a novel member of the leucine-rich repeat protein family closely related to decorin and biglycan. J Biol Chem. 2001;276:12201–12211. doi: 10.1074/jbc.M010932200. [DOI] [PubMed] [Google Scholar]
- 12.Tillgren V, Onnerfjord P, Haglund L, Heinegard D. The tyrosine sulfate-rich domains of the LRR proteins fibromodulin and osteoadherin bind motifs of basic clusters in a variety of heparin-binding proteins, including bioactive factors. J Biol Chem. 2009;284:28543–28553. doi: 10.1074/jbc.M109.047076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S, Birk DE. Focus on molecules: decorin. Exp Eye Res. 2011;92:444–445. doi: 10.1016/j.exer.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funderburgh JL, Funderburgh ML, Mann MM, Conrad GW. Arterial lumican. Properties of a corneal-type keratan sulfate proteoglycan from bovine aorta. J Biol Chem. 1991;266:24773–24777. [PubMed] [Google Scholar]
- 15.Krishnan P, Hocking AM, Scholtz JM, Pace CN, Holik KK, McQuillan DJ. Distinct secondary structures of the leucine-rich repeat proteoglycans decorin and biglycan. Glycosylation-dependent conformational stability. J Biol Chem. 1999;274:10945–10950. doi: 10.1074/jbc.274.16.10945. [DOI] [PubMed] [Google Scholar]
- 16.Seo NS, Hocking AM, Hook M, McQuillan DJ. Decorin core protein secretion is regulated by N-linked oligosaccharide and glycosaminoglycan additions. J Biol Chem. 2005;280:42774–42784. doi: 10.1074/jbc.M511531200. [DOI] [PubMed] [Google Scholar]
- 17.Pringle GA, Dodd CM. Immunoelectron microscopic localization of the core protein of decorin near the d and e bands of tendon collagen fibrils by use of monoclonal antibodies. J Histochem Cytochem. 1990;38:1405–1411. doi: 10.1177/38.10.1698203. [DOI] [PubMed] [Google Scholar]
- 18.Fleischmajer R, Fisher LW, MacDonald ED, Jacobs L, Jr, Perlish JS, Termine JD. Decorin interacts with fibrillar collagen of embryonic and adult human skin. J Struct Biol. 1991;106:82–90. doi: 10.1016/1047-8477(91)90065-5. [DOI] [PubMed] [Google Scholar]
- 19.Svensson L, Heinegard D, Oldberg A. Decorin-binding sites for collagen type I are mainly located in leucine-rich repeats 4–5. J Biol Chem. 1995;270:20712–20716. doi: 10.1074/jbc.270.35.20712. [DOI] [PubMed] [Google Scholar]
- 20.Kalamajski S, Aspberg A, Oldberg A. The decorin sequence SYIRIADTNIT binds collagen type I. J Biol Chem. 2007;282:16062–16067. doi: 10.1074/jbc.M700073200. [DOI] [PubMed] [Google Scholar]
- 21.Douglas T, Heinemann S, Bierbaum S, Scharnweber D, Worch H. Fibrillogenesis of collagen types I, II, and III with small leucine-rich proteoglycans decorin and biglycan. Biomacromolecules. 2006;7:2388–2393. doi: 10.1021/bm0603746. [DOI] [PubMed] [Google Scholar]
- 22.Whinna HC, Choi HU, Rosenberg LC, Church FC. Interaction of heparin cofactor II with biglycan and decorin. J Biol Chem. 1993;268:3920–3924. [PubMed] [Google Scholar]
- 23.Corpuz LM, Funderburgh JL, Funderburgh ML, Bottomley GS, Prakash S, Conrad GW. Molecular cloning and tissue distribution of keratocan. Bovine corneal keratan sulfate proteoglycan 37A. J Biol Chem. 1996;271:9759–9763. doi: 10.1074/jbc.271.16.9759. [DOI] [PubMed] [Google Scholar]
- 24.Bidanset DJ, Guidry C, Rosenberg LC, Choi HU, Timpl R, Hook M. Binding of the proteoglycan decorin to collagen type VI. J Biol Chem. 1992;267:5250–5256. [PubMed] [Google Scholar]
- 25.Font B, Eichenberger D, Rosenberg LM, van der Rest M. Characterization of the interactions of type XII collagen with two small proteoglycans from fetal bovine tendon, decorin and fibromodulin. Matrix Biol. 1996;15:341–348. doi: 10.1016/s0945-053x(96)90137-7. [DOI] [PubMed] [Google Scholar]
- 26.Ehnis T, Dieterich W, Bauer M, Kresse H, Schuppan D. Localization of a binding site for the proteoglycan decorin on collagen XIV (undulin) J Biol Chem. 1997;272:20414–20419. doi: 10.1074/jbc.272.33.20414. [DOI] [PubMed] [Google Scholar]
- 27.Winnemoller M, Schmidt G, Kresse H. Influence of decorin on fibroblast adhesion to fibronectin. Eur J Cell Biol. 1991;54:10–17. [PubMed] [Google Scholar]
- 28.Dugan TA, Yang VW, McQuillan DJ, Hook M. Decorin binds fibrinogen in a Zn2+-dependent interaction. J Biol Chem. 2003;278:13655–13662. doi: 10.1074/jbc.M300171200. [DOI] [PubMed] [Google Scholar]
- 29.Merle B, Malaval L, Lawler J, Delmas P, Clezardin P. Decorin inhibits cell attachment to thrombospondin-1 by binding to a KKTR-dependent cell adhesive site present within the N-terminal domain of thrombospondin-1. J Cell Biochem. 1997;67:75–83. [PubMed] [Google Scholar]
- 30.Trask BC, Trask TM, Broekelmann T, Mecham RP. The microfibrillar proteins MAGP-1 and fibrillin-1 form a ternary complex with the chondroitin sulfate proteoglycan decorin. Mol Biol Cell. 2000;11:1499–1507. doi: 10.1091/mbc.11.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elefteriou F, Exposito JY, Garrone R, Lethias C. Binding of tenascin-X to decorin. FEBS Lett. 2001;495:44–47. doi: 10.1016/s0014-5793(01)02361-4. [DOI] [PubMed] [Google Scholar]
- 32.Schonherr E, Witsch-Prehm P, Harrach B, Robenek H, Rauterberg J, Kresse H. Interaction of biglycan with type I collagen. J Biol Chem. 1995;270:2776–2783. doi: 10.1074/jbc.270.6.2776. [DOI] [PubMed] [Google Scholar]
- 33.Vynios DH, Papageorgakopoulou N, Sazakli H, Tsiganos CP. The interactions of cartilage proteoglycans with collagens are determined by their structures. Biochimie. 2001;83:899–906. doi: 10.1016/s0300-9084(01)01332-3. [DOI] [PubMed] [Google Scholar]
- 34.Wiberg C, Hedbom E, Khairullina A, Lamande SR, Oldberg A, Timpl R, Morgelin M, Heinegard D. Biglycan and decorin bind close to the n-terminal region of the collagen VI triple helix. J Biol Chem. 2001;276:18947–18952. doi: 10.1074/jbc.M100625200. [DOI] [PubMed] [Google Scholar]
- 35.Chen CH, Yeh ML, Geyer M, Wang GJ, Huang MH, Heggeness MH, Hook M, Luo ZP. Interactions between collagen IX and biglycan measured by atomic force microscopy. Biochem Biophys Res Commun. 2006;339:204–208. doi: 10.1016/j.bbrc.2005.10.205. [DOI] [PubMed] [Google Scholar]
- 36.Fresquet M, Jowitt TA, Stephen LA, Ylostalo J, Briggs MD. Structural and functional investigations of Matrilin-1 A-domains reveal insights into their role in cartilage ECM assembly. J Biol Chem. 2010;285:34048–34061. doi: 10.1074/jbc.M110.154443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reinboth B, Hanssen E, Cleary EG, Gibson MA. Molecular interactions of biglycan and decorin with elastic fiber components: biglycan forms a ternary complex with tropoelastin and microfibril-associated glycoprotein 1. J Biol Chem. 2002;277:3950–3957. doi: 10.1074/jbc.M109540200. [DOI] [PubMed] [Google Scholar]
- 38.Kalamajski S, Aspberg A, Lindblom K, Heinegard D, Oldberg A. Asporin competes with decorin for collagen binding, binds calcium and promotes osteoblast collagen mineralization. Biochem J. 2009;423:53–59. doi: 10.1042/BJ20090542. [DOI] [PubMed] [Google Scholar]
- 39.Kalamajski S, Oldberg A. Fibromodulin binds collagen type I via Glu-353 and Lys-355 in leucine-rich repeat 11. J Biol Chem. 2007;282:26740–26745. doi: 10.1074/jbc.M704026200. [DOI] [PubMed] [Google Scholar]
- 40.Hedbom E, Heinegard D. Interaction of a 59-kDa connective tissue matrix protein with collagen I and collagen II. J Biol Chem. 1989;264:6898–6905. [PubMed] [Google Scholar]
- 41.Takahashi T, Cho HI, Kublin CL, Cintron C. Keratan sulfate and dermatan sulfate proteoglycans associate with type VI collagen in fetal rabbit cornea. J Histochem Cytochem. 1993;41:1447–1457. doi: 10.1177/41.10.8245404. [DOI] [PubMed] [Google Scholar]
- 42.Kalchishkova N, Furst CM, Heinegard D, Blom AM. NC4 Domain of cartilage-specific collagen IX inhibits complement directly due to attenuation of membrane attack formation and indirectly through binding and enhancing activity of complement inhibitors C4B-binding protein and factor H. J Biol Chem. 2011;286:27915–27926. doi: 10.1074/jbc.M111.242834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Svensson L, Narlid I, Oldberg A. Fibromodulin and lumican bind to the same region on collagen type I fibrils. FEBS Lett. 2000;470:178–182. doi: 10.1016/s0014-5793(00)01314-4. [DOI] [PubMed] [Google Scholar]
- 44.Dunlevy JR, Rada JA. Interaction of lumican with aggrecan in the aging human sclera. Invest Ophthalmol Vis Sci. 2004;45:3849–3856. doi: 10.1167/iovs.04-0496. [DOI] [PubMed] [Google Scholar]
- 45.D'Onofrio MF, Brezillon S, Baranek T, Perreau C, Roughley PJ, Maquart FX, Wegrowski Y. Identification of beta1 integrin as mediator of melanoma cell adhesion to lumican. Biochem Biophys Res Commun. 2008;365:266–272. doi: 10.1016/j.bbrc.2007.10.155. [DOI] [PubMed] [Google Scholar]
- 46.Lee S, Bowrin K, Hamad AR, Chakravarti S. Extracellular matrix lumican deposited on the surface of neutrophils promotes migration by binding to beta2 integrin. J Biol Chem. 2009;284:23662–23669. doi: 10.1074/jbc.M109.026229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niewiarowska J, Brezillon S, Sacewicz-Hofman I, Bednarek R, Maquart F-X, Malinowski M, Wiktorska M, Wegrowski Y, Cierniewski CS. Lumican inhibits angiogenesis by interfering with α2Î21 receptor activity and downregulating MMP-14 expression. Thromb Res. 2011;128:452–457. doi: 10.1016/j.thromres.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 48.Zeltz C, Brezillon S, Kapyla J, Eble JA, Bobichon H, Terryn C, Perreau C, Franz CM, Heino J, Maquart F-X, Wegrowski Y. Lumican inhibits cell migration through α2Î21 integrin. Exp Cell Res. 2010;316:2922–2931. doi: 10.1016/j.yexcr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Lucchini M, Couble ML, Romeas A, Staquet MJ, Bleicher F, Magloire H, Farges JC. Alpha v beta 3 integrin expression in human odontoblasts and co-localization with osteoadherin. J Dent Res. 2004;83:552–556. doi: 10.1177/154405910408300708. [DOI] [PubMed] [Google Scholar]
- 50.Bengtsson E, Morgelin M, Sasaki T, Timpl R, Heinegard D, Aspberg A. The leucine-rich repeat protein PRELP binds perlecan and collagens and may function as a basement membrane anchor. J Biol Chem. 2002;277:15061–15068. doi: 10.1074/jbc.M108285200. [DOI] [PubMed] [Google Scholar]
- 51.Tasheva ES, Koester A, Paulsen AQ, Garrett AS, Boyle DL, Davidson HJ, Song M, Fox N, Conrad GW. Mimecan/osteoglycin-deficient mice have collagen fibril abnormalities. Mol Vis. 2002;8:407–415. [PubMed] [Google Scholar]
- 52.Hindson VJ, Gallagher JT, Halfter W, Bishop PN. Opticin binds to heparan and chondroitin sulfate proteoglycans. Invest Ophthalmol Vis Sci. 2005;46:4417–4423. doi: 10.1167/iovs.05-0883. [DOI] [PubMed] [Google Scholar]
- 53.Camper L, Heinegard D, Lundgren-Akerlund E. Integrin alpha2beta1 is a receptor for the cartilage matrix protein chondroadherin. J Cell Biol. 1997;138:1159–1167. doi: 10.1083/jcb.138.5.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mansson B, Wenglen C, Morgelin M, Saxne T, Heinegard D. Association of chondroadherin with collagen type II. J Biol Chem. 2001;276:32883–32888. doi: 10.1074/jbc.M101680200. [DOI] [PubMed] [Google Scholar]
- 55.Shimizu-Hirota R, Sasamura H, Kuroda M, Kobayashi E, Saruta T. Functional characterization of podocan, a member of a new class in the small leucine-rich repeat protein family. FEBS Lett. 2004;563:69–74. doi: 10.1016/S0014-5793(04)00250-9. [DOI] [PubMed] [Google Scholar]
- 56.Carlson EC, Liu CY, Chikama T, Hayashi Y, Kao CW, Birk DE, Funderburgh JL, Jester JV, Kao WW. Keratocan, a cornea-specific keratan sulfate proteoglycan, is regulated by lumican. J Biol Chem. 2005;280:25541–25547. doi: 10.1074/jbc.M500249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tasheva ES, Klocke B, Conrad GW. Analysis of transcriptional regulation of the small leucine rich proteoglycans. Mol Vis. 2004;10:758–772. [PubMed] [Google Scholar]
- 58.Oldberg A, Antonsson P, Moses J, Fransson LA. Amino-terminal deletions in the decorin core protein leads to the biosynthesis of proteoglycans with shorter glycosaminoglycan chains. FEBS Lett. 1996;386:29–32. doi: 10.1016/0014-5793(96)00407-3. [DOI] [PubMed] [Google Scholar]
- 59.Monfort J, Tardif G, Reboul P, Mineau F, Roughley P, Pelletier JP, Martel-Pelletier J. Degradation of small leucine-rich repeat proteoglycans by matrix metalloprotease-13: identification of a new biglycan cleavage site. Arthritis Res Ther. 2006;8:R26. doi: 10.1186/ar1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Melching LI, Fisher WD, Lee ER, Mort JS, Roughley PJ. The cleavage of biglycan by aggrecanases. Osteoarthritis Cartilage. 2006;14:1147–1154. doi: 10.1016/j.joca.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 61.Scott IC, Imamura Y, Pappano WN, Troedel JM, Recklies AD, Roughley PJ, Greenspan DS. Bone morphogenetic protein-1 processes probiglycan. J Biol Chem. 2000;275:30504–30511. doi: 10.1074/jbc.M004846200. [DOI] [PubMed] [Google Scholar]
- 62.Boivin WA, Shackleford M, Vanden Hoek A, Zhao H, Hackett TL, Knight DA, Granville DJ. Granzyme B cleaves decorin, biglycan and soluble betaglycan, releasing active transforming growth factor-Î21. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Imai K, Hiramatsu A, Fukushima D, Pierschbacher MD, Okada Y. Degradation of decorin by matrix metalloproteinases: identification of the cleavage sites, kinetic analyses and transforming growth factor-beta1 release. Biochem J. 1997;322(Pt 3):809–814. doi: 10.1042/bj3220809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geng Y, McQuillan D, Roughley PJ. SLRP interaction can protect collagen fibrils from cleavage by collagenases. Matrix Biol. 2006;25:484–491. doi: 10.1016/j.matbio.2006.08.259. [DOI] [PubMed] [Google Scholar]
- 65.Hausser H, Schonherr E, Muller M, Liszio C, Bin Z, Fisher LW, Kresse H. Receptor-mediated endocytosis of decorin: involvement of leucine-rich repeat structures. Arch Biochem Biophys. 1998;349:363–370. doi: 10.1006/abbi.1997.0471. [DOI] [PubMed] [Google Scholar]
- 66.Zhu JX, Goldoni S, Bix G, Owens RT, McQuillan DJ, Reed CC, Iozzo RV. Decorin evokes protracted internalization and degradation of the epidermal growth factor receptor via caveolar endocytosis. J Biol Chem. 2005;280:32468–32479. doi: 10.1074/jbc.M503833200. [DOI] [PubMed] [Google Scholar]
- 67.Banerjee AG, Bhattacharyya I, Lydiatt WM, Vishwanatha JK. Aberrant expression and localization of decorin in human oral dysplasia and squamous cell carcinoma. Cancer Res. 2003;63:7769–7776. [PubMed] [Google Scholar]
- 68.Soto-Suazo M, San Martin S, Ferro ES, Zorn TM. Differential expression of glycosaminoglycans and proteoglycans in the migratory pathway of the primordial germ cells of the mouse. Histochem Cell Biol. 2002;118:69–78. doi: 10.1007/s00418-002-0414-2. [DOI] [PubMed] [Google Scholar]
- 69.Wilda M, Bachner D, Just W, Geerkens C, Kraus P, Vogel W, Hameister H. A comparison of the expression pattern of five genes of the family of small leucine-rich proteoglycans during mouse development. J Bone Miner Res. 2000;15:2187–2196. doi: 10.1359/jbmr.2000.15.11.2187. [DOI] [PubMed] [Google Scholar]
- 70.Conrad AH, Conrad GW. The keratocan gene is expressed in both ocular and non-ocular tissues during early chick development. Matrix Biol. 2003;22:323–337. doi: 10.1016/s0945-053x(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 71.Lechner BE, Lim JH, Mercado ML, Fallon JR. Developmental regulation of biglycan expression in muscle and tendon. Muscle Nerve. 2006;34:347–355. doi: 10.1002/mus.20596. [DOI] [PubMed] [Google Scholar]
- 72.Chakravarti S, Zhang G, Chervoneva I, Roberts L, Birk DE. Collagen fibril assembly during postnatal development and dysfunctional regulation in the lumican-deficient murine cornea. Dev Dyn. 2006;235:2493–2506. doi: 10.1002/dvdy.20868. [DOI] [PubMed] [Google Scholar]
- 73.Chen S, Oldberg A, Chakravarti S, Birk DE. Fibromodulin regulates collagen fibrillogenesis during peripheral corneal development. Dev Dyn. 2010;239:844–854. doi: 10.1002/dvdy.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ying S, Shiraishi A, Kao CW, Converse RL, Funderburgh JL, Swiergiel J, Roth MR, Conrad GW, Kao WW. Characterization and expression of the mouse lumican gene. J Biol Chem. 1997;272:30306–3013. doi: 10.1074/jbc.272.48.30306. [DOI] [PubMed] [Google Scholar]
- 75.Waddington RJ, Roberts HC, Sugars RV, Schonherr E. Differential roles for small leucine-rich proteoglycans in bone formation. Eur Cell Mater. 2003;6:12–21. doi: 10.22203/ecm.v006a02. discussion 21. [DOI] [PubMed] [Google Scholar]
- 76.Ezura Y, Chakravarti S, Oldberg A, Chervoneva I, Birk DE. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J Cell Biol. 2000;151:779–788. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doane KJ, Ting WH, McLaughlin JS, Birk DE. Spatial and temporal variations in extracellular matrix of periocular and corneal regions during corneal stromal development. Exp Eye Res. 1996;62:271–283. doi: 10.1006/exer.1996.0033. [DOI] [PubMed] [Google Scholar]
- 78.Matheson S, Larjava H, Hakkinen L. Distinctive localization and function for lumican, fibromodulin and decorin to regulate collagen fibril organization in periodontal tissues. J Periodontal Res. 2005;40:312–324. doi: 10.1111/j.1600-0765.2005.00800.x. [DOI] [PubMed] [Google Scholar]
- 79.Birk DE, Nurminskaya MV, Zycband EI. Collagen fibrillogenesis in situ: fibril segments undergo post-depositional modifications resulting in linear and lateral growth during matrix development. Dev Dyn. 1995;202:229–243. doi: 10.1002/aja.1002020303. [DOI] [PubMed] [Google Scholar]
- 80.Birk DE, Zycband EI, Winkelmann DA, Trelstad RL. Collagen fibrillogenesis in situ: fibril segments are intermediates in matrix assembly. Proc Natl Acad Sci U S A. 1989;86:4549–4553. doi: 10.1073/pnas.86.12.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Graham HK, Holmes DF, Watson RB, Kadler KE. Identification of collagen fibril fusion during vertebrate tendon morphogenesis. The process relies on unipolar fibrils and is regulated by collagen-proteoglycan interaction. J Mol Biol. 2000;295:891–902. doi: 10.1006/jmbi.1999.3384. [DOI] [PubMed] [Google Scholar]
- 82.Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. Biochem J. 1996;316(Pt 1):1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Canty EG, Kadler KE. Collagen fibril biosynthesis in tendon: a review and recent insights. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:979–985. doi: 10.1016/s1095-6433(02)00212-x. [DOI] [PubMed] [Google Scholar]
- 84.Birk DE, Bruckner P. Collagens, Suprastructures, and Collagen Fibril Assembly in. In: Mecham RP, editor. The Extracellular matrix: an overview, Biology of Extracellular Matrix. Springer-Verlag: Berlin Heidelberg; 2011. pp. 77–115. [Google Scholar]
- 85.Blaschke UK, Eikenberry EF, Hulmes DJ, Galla HJ, Bruckner P. Collagen XI nucleates self-assembly and limits lateral growth of cartilage fibrils. J Biol Chem. 2000;275:10370–10378. doi: 10.1074/jbc.275.14.10370. [DOI] [PubMed] [Google Scholar]
- 86.Wenstrup RJ, Florer JB, Brunskill EW, Bell SM, Chervoneva I, Birk DE. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem. 2004;279:53331–53337. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- 87.Wenstrup RJ, Smith SM, Florer JB, Zhang G, Beason DP, Seegmiller RE, Soslowsky LJ, Birk DE. Regulation of collagen fibril nucleation and initial fibril assembly involves coordinate interactions with collagens V and XI in developing tendon. J Biol Chem. 2011;286:20455–20465. doi: 10.1074/jbc.M111.223693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Keene DR, San Antonio JD, Mayne R, McQuillan DJ, Sarris G, Santoro SA, Iozzo RV. Decorin binds near the C terminus of type I collagen. J Biol Chem. 2000;275:21801–21804. doi: 10.1074/jbc.C000278200. [DOI] [PubMed] [Google Scholar]
- 89.Miyagawa A, Kobayashi M, Fujita Y, Hamdy O, Hirano K, Nakamura M, Miyake Y. Surface ultrastructure of collagen fibrils and their association with proteoglycans in human cornea and sclera by atomic force microscopy and energy-filtering transmission electron microscopy. Cornea. 2001;20:651–656. doi: 10.1097/00003226-200108000-00019. [DOI] [PubMed] [Google Scholar]
- 90.Kalamajski S, Oldberg A. Homologous sequence in lumican and fibromodulin leucine-rich repeat 5–7 competes for collagen binding. J Biol Chem. 2009;284:534–539. doi: 10.1074/jbc.M805721200. [DOI] [PubMed] [Google Scholar]
- 91.Neame PJ, Kay CJ, McQuillan DJ, Beales MP, Hassell JR. Independent modulation of collagen fibrillogenesis by decorin and lumican. Cell Mol Life Sci. 2000;57:859–863. doi: 10.1007/s000180050048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang G, Chen S, Goldoni S, Calder BW, Simpson HC, Owens RT, McQuillan DJ, Young MF, Iozzo RV, Birk DE. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J Biol Chem. 2009;284:8888–8897. doi: 10.1074/jbc.M806590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schonherr E, Hausser H, Beavan L, Kresse H. Decorin-type I collagen interaction. Presence of separate core protein-binding domains. J Biol Chem. 1995;270:8877–8883. doi: 10.1074/jbc.270.15.8877. [DOI] [PubMed] [Google Scholar]
- 94.Orgel JP, Eid A, Antipova O, Bella J, Scott JE. Decorin core protein (decoron) shape complements collagen fibril surface structure and mediates its binding. PLoS One. 2009;4:e7028. doi: 10.1371/journal.pone.0007028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rada JA, Cornuet PK, Hassell JR. Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) core proteins. Exp Eye Res. 1993;56:635–648. doi: 10.1006/exer.1993.1081. [DOI] [PubMed] [Google Scholar]
- 96.Liu CY, Birk DE, Hassell JR, Kane B, Kao WW. Keratocan-deficient mice display alterations in corneal structure. J Biol Chem. 2003;278:21672–21677. doi: 10.1074/jbc.M301169200. [DOI] [PubMed] [Google Scholar]
- 97.Raspanti M, Viola M, Forlino A, Tenni R, Gruppi C, Tira ME. Glycosaminoglycans show a specific periodic interaction with type I collagen fibrils. J Struct Biol. 2008;164:134–139. doi: 10.1016/j.jsb.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 98.Henninger HB, Maas SA, Underwood CJ, Whitaker RT, Weiss JA. Spatial distribution and orientation of dermatan sulfate in human medial collateral ligament. J Struct Biol. 2007;158:33–45. doi: 10.1016/j.jsb.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lewis PN, Pinali C, Young RD, Meek KM, Quantock AJ, Knupp C. Structural interactions between collagen and proteoglycans are elucidated by three-dimensional electron tomography of bovine cornea. Structure. 2010;18:239–245. doi: 10.1016/j.str.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 100.Ruhland C, Schonherr E, Robenek H, Hansen U, Iozzo RV, Bruckner P, Seidler DG. The glycosaminoglycan chain of decorin plays an important role in collagen fibril formation at the early stages of fibrillogenesis. FEBS J. 2007;274:4246–4255. doi: 10.1111/j.1742-4658.2007.05951.x. [DOI] [PubMed] [Google Scholar]
- 101.Maccarana M, Kalamajski S, Kongsgaard M, Magnusson SP, Oldberg A, Malmstrom A. Dermatan sulfate epimerase 1-deficient mice have reduced content and changed distribution of iduronic acids in dermatan sulfate and an altered collagen structure in skin. Mol Cell Biol. 2009;29:5517–5528. doi: 10.1128/MCB.00430-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Redaelli A, Vesentini S, Soncini M, Vena P, Mantero S, Montevecchi FM. Possible role of decorin glycosaminoglycans in fibril to fibril force transfer in relative mature tendons--a computational study from molecular to microstructural level. J Biomech. 2003;36:1555–1569. doi: 10.1016/s0021-9290(03)00133-7. [DOI] [PubMed] [Google Scholar]
- 103.Hakkinen L, Strassburger S, Kahari VM, Scott PG, Eichstetter I, Lozzo RV, Larjava H. A role for decorin in the structural organization of periodontal ligament. Lab Invest. 2000;80:1869–1880. doi: 10.1038/labinvest.3780197. [DOI] [PubMed] [Google Scholar]
- 104.Sugars RV, Milan AM, Brown JO, Waddington RJ, Hall RC, Embery G. Molecular interaction of recombinant decorin and biglycan with type I collagen influences crystal growth. Connect Tissue Res. 2003;44(Suppl 1):189–195. doi: 10.1080/713713596. [DOI] [PubMed] [Google Scholar]
- 105.Keene DR, Ridgway CC, Iozzo RV. Type VI microfilaments interact with a specific region of banded collagen fibrils in skin. J Histochem Cytochem. 1998;46:215–220. doi: 10.1177/002215549804600210. [DOI] [PubMed] [Google Scholar]
- 106.Wiberg C, Heinegard D, Wenglen C, Timpl R, Morgelin M. Biglycan organizes collagen VI into hexagonal-like networks resembling tissue structures. J Biol Chem. 2002;277:49120–49126. doi: 10.1074/jbc.M206891200. [DOI] [PubMed] [Google Scholar]
- 107.Wiberg C, Klatt AR, Wagener R, Paulsson M, Bateman JF, Heinegard D, Morgelin M. Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J Biol Chem. 2003;278:37698–37704. doi: 10.1074/jbc.M304638200. [DOI] [PubMed] [Google Scholar]
- 108.Font B, Aubert-Foucher E, Goldschmidt D, Eichenberger D, van der Rest M. Binding of collagen XIV with the dermatan sulfate side chain of decorin. J Biol Chem. 1993;268:25015–25018. [PubMed] [Google Scholar]
- 109.Font B, Eichenberger D, Goldschmidt D, Boutillon MM, Hulmes DJ. Structural requirements for fibromodulin binding to collagen and the control of type I collagen fibrillogenesis--critical roles for disulphide bonding and the C-terminal region. Eur J Biochem. 1998;254:580–587. doi: 10.1046/j.1432-1327.1998.2540580.x. [DOI] [PubMed] [Google Scholar]
- 110.Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schmidt G, Hausser H, Kresse H. Interaction of the small proteoglycan decorin with fibronectin. Involvement of the sequence NKISK of the core protein. Biochem J. 1991;280(Pt 2):411–414. doi: 10.1042/bj2800411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Davies Cde L, Melder RJ, Munn LL, Mouta-Carreira C, Jain RK, Boucher Y. Decorin inhibits endothelial migration and tube-like structure formation: role of thrombospondin-1. Microvasc Res. 2001;62:26–42. doi: 10.1006/mvre.2001.2311. [DOI] [PubMed] [Google Scholar]
- 113.Minamitani T, Ariga H, Matsumoto K. Deficiency of tenascin-X causes a decrease in the level of expression of type VI collagen. Exp Cell Res. 2004;297:49–60. doi: 10.1016/j.yexcr.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 114.Bowe MA, Mendis DB, Fallon JR. The small leucine-rich repeat proteoglycan biglycan binds to alpha-dystroglycan and is upregulated in dystrophic muscle. J Cell Biol. 2000;148:801–810. doi: 10.1083/jcb.148.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cao Y, Posokhova E, Martemyanov KA. TRPM1 forms complexes with nyctalopin in vivo and accumulates in postsynaptic compartment of ON-bipolar neurons in mGluR6-dependent manner. J Neurosci. 2011;31:11521–11526. doi: 10.1523/JNEUROSCI.1682-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Magzoub M, Jin S, Verkman AS. Enhanced macromolecule diffusion deep in tumors after enzymatic digestion of extracellular matrix collagen and its associated proteoglycan decorin. FASEB J. 2008;22:276–284. doi: 10.1096/fj.07-9150com. [DOI] [PubMed] [Google Scholar]
- 117.Chakravarti S, Petroll WM, Hassell JR, Jester JV, Lass JH, Paul J, Birk DE. Corneal opacity in lumican-null mice: defects in collagen fibril structure and packing in the posterior stroma. Invest Ophthalmol Vis Sci. 2000;41:3365–3373. [PMC free article] [PubMed] [Google Scholar]
- 118.Song J, Lee YG, Houston J, Petroll WM, Chakravarti S, Cavanagh HD, Jester JV. Neonatal corneal stromal development in the normal and lumican-deficient mouse. Invest Ophthalmol Vis Sci. 2003;44:548–557. doi: 10.1167/iovs.02-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pellegata NS, Dieguez-Lucena JL, Joensuu T, Lau S, Montgomery KT, Krahe R, Kivela T, Kucherlapati R, Forsius H, de la Chapelle A. Mutations in KERA, encoding keratocan, cause cornea plana. Nat Genet. 2000;25:91–95. doi: 10.1038/75664. [DOI] [PubMed] [Google Scholar]
- 120.Lehmann OJ, El-ashry MF, Ebenezer ND, Ocaka L, Francis PJ, Wilkie SE, Patel RJ, Ficker L, Jordan T, Khaw PT, Bhattacharya SS. A novel keratocan mutation causing autosomal recessive cornea plana. Invest Ophthalmol Vis Sci. 2001;42:3118–3122. [PubMed] [Google Scholar]
- 121.Wang P, Li S, Xiao X, Guo X, Zhang Q. An evaluation of OPTC and EPYC as candidate genes for high myopia. Mol Vis. 2009;15:2045–2049. [PMC free article] [PubMed] [Google Scholar]
- 122.Majava M, Bishop PN, Hagg P, Scott PG, Rice A, Inglehearn C, Hammond CJ, Spector TD, Ala-Kokko L, Mannikko M. Novel mutations in the small leucine-rich repeat protein/proteoglycan (SLRP) genes in high myopia. Hum Mutat. 2007;28:336–344. doi: 10.1002/humu.20444. [DOI] [PubMed] [Google Scholar]
- 123.Wentz-Hunter K, Cheng EL, Ueda J, Sugar J, Yue BY. Keratocan expression is increased in the stroma of keratoconus corneas. Mol Med. 2001;7:470–477. [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- 125.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang G, Young BB, Ezura Y, Favata M, Soslowsky LJ, Chakravarti S, Birk DE. Development of tendon structure and function: regulation of collagen fibrillogenesis. J Musculoskelet Neuronal Interact. 2005;5:5–21. [PubMed] [Google Scholar]
- 127.Ezura Y, Chakravarti S, Oldberg A, Chervoneva I, Birk DE. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J Cell Biol. 2000;151:779–788. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jepsen KJ, Wu F, Peragallo JH, Paul J, Roberts L, Ezura Y, Oldberg A, Birk DE, Chakravarti S. A syndrome of joint laxity and impaired tendon integrity in lumican- and fibromodulin-deficient mice. J Biol Chem. 2002;277:35532–35540. doi: 10.1074/jbc.M205398200. [DOI] [PubMed] [Google Scholar]
- 129.Svensson L, Aszodi A, Reinholt FP, Fassler R, Heinegard D, Oldberg A. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem. 1999;274:9636–9647. doi: 10.1074/jbc.274.14.9636. [DOI] [PubMed] [Google Scholar]
- 130.Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, Bianco P, Young MF. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002;17:1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- 131.Elliott DM, Robinson PS, Gimbel JA, Sarver JJ, Abboud JA, Iozzo RV, Soslowsky LJ. Effect of altered matrix proteins on quasilinear viscoelastic properties in transgenic mouse tail tendons. Ann Biomed Eng. 2003;31:599–605. doi: 10.1114/1.1567282. [DOI] [PubMed] [Google Scholar]
- 132.Haruyama N, Sreenath TL, Suzuki S, Yao X, Wang Z, Wang Y, Honeycutt C, Iozzo RV, Young MF, Kulkarni AB. Genetic evidence for key roles of decorin and biglycan in dentin mineralization. Matrix Biol. 2009;28:129–136. doi: 10.1016/j.matbio.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baghy K, Dezso K, Laszlo V, Fullar A, Peterfia B, Paku S, Nagy P, Schaff Z, Iozzo RV, Kovalszky I. Ablation of the decorin gene enhances experimental hepatic fibrosis and impairs hepatic healing in mice. Lab Invest. 2012;91:439–451. doi: 10.1038/labinvest.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sanches JC, Jones CJ, Aplin JD, Iozzo RV, Zorn TM, Oliveira SF. Collagen fibril organization in the pregnant endometrium of decorin-deficient mice. J Anat. 2009;216:144–155. doi: 10.1111/j.1469-7580.2009.01170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu Z, Aron AW, Macksoud EE, Iozzo RV, Hai CM, Lechner BE. Uterine dysfunction in biglycan and decorin deficient mice leads to dystocia during parturition. PLoS One. 2012;7:e29627. doi: 10.1371/journal.pone.0029627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xu T, Bianco P, Fisher LW, Longenecker G, Smith E, Goldstein S, Bonadio J, Boskey A, Heegaard AM, Sommer B, Satomura K, Dominguez P, Zhao C, Kulkarni AB, Robey PG, Young MF. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet. 1998;20:78–82. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]
- 137.Ameye L, Aria D, Jepsen K, Oldberg A, Xu T, Young MF. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J. 2002;16:673–680. doi: 10.1096/fj.01-0848com. [DOI] [PubMed] [Google Scholar]
- 138.Raghunath M, Superti-Furga A, Godfrey M, Steinmann B. Decreased extracellular deposition of fibrillin and decorin in neonatal Marfan syndrome fibroblasts. Hum Genet. 1993;90:511–515. doi: 10.1007/BF00217450. [DOI] [PubMed] [Google Scholar]
- 139.Izumi T, Tajima S, Nishikawa T. Stimulated expression of decorin and the decorin gene in fibroblasts cultured from patients with localized scleroderma. Arch Dermatol Res. 1995;287:417–420. doi: 10.1007/BF00373421. [DOI] [PubMed] [Google Scholar]
- 140.Beavan LA, Quentin-Hoffmann E, Schonherr E, Snigula F, Leroy JG, Kresse H. Deficient expression of decorin in infantile progeroid patients. J Biol Chem. 1993;268:9856–9862. [PubMed] [Google Scholar]
- 141.Fedarko NS, Robey PG, Vetter UK. Extracellular matrix stoichiometry in osteoblasts from patients with osteogenesis imperfecta. J Bone Miner Res. 1995;10:1122–1129. doi: 10.1002/jbmr.5650100718. [DOI] [PubMed] [Google Scholar]
- 142.Westergren-Thorsson G, Coster L, Akesson A, Wollheim FA. Altered dermatan sulfate proteoglycan synthesis in fibroblast cultures established from skin of patients with systemic sclerosis. J Rheumatol. 1996;23:1398–1406. [PubMed] [Google Scholar]
- 143.Gu J, Wada Y. Aberrant expressions of decorin and biglycan genes in the carbohydrate-deficient glycoprotein syndrome. J Biochem. 1995;117:1276–1279. doi: 10.1093/oxfordjournals.jbchem.a124855. [DOI] [PubMed] [Google Scholar]
- 144.Guo BP, Norris SJ, Rosenberg LC, Hook M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brown EL, Wooten RM, Johnson BJ, Iozzo RV, Smith A, Dolan MC, Guo BP, Weis JJ, Hook M. Resistance to Lyme disease in decorin-deficient mice. J Clin Invest. 2001;107:845–852. doi: 10.1172/JCI11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liang FT, Brown EL, Wang T, Iozzo RV, Fikrig E. Protective niche for Borrelia burgdorferi to evade humoral immunity. Am J Pathol. 2004;165:977–985. doi: 10.1016/S0002-9440(10)63359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Snow AD, Mar H, Nochlin D, Kresse H, Wight TN. Peripheral distribution of dermatan sulfate proteoglycans (decorin) in amyloid-containing plaques and their presence in neurofibrillary tangles of Alzheimer's disease. J Histochem Cytochem. 1992;40:105–113. doi: 10.1177/40.1.1370306. [DOI] [PubMed] [Google Scholar]
- 148.Pentikainen MO, Oorni K, Lassila R, Kovanen PT. The proteoglycan decorin links low density lipoproteins with collagen type I. J Biol Chem. 1997;272:7633–7638. doi: 10.1074/jbc.272.12.7633. [DOI] [PubMed] [Google Scholar]
- 149.Klezovitch O, Edelstein C, Zhu L, Scanu AM. Apolipoprotein(a) binds via its C-terminal domain to the protein core of the proteoglycan decorin. Implications for the retention of lipoprotein(a) in atherosclerotic lesions. J Biol Chem. 1998;273:23856–23865. doi: 10.1074/jbc.273.37.23856. [DOI] [PubMed] [Google Scholar]
- 150.Gotte M, Kresse H. Defective glycosaminoglycan substitution of decorin in a patient with progeroid syndrome is a direct consequence of two point mutations in the galactosyltransferase I (beta4GalT-7) gene. Biochem Genet. 2005;43:65–77. doi: 10.1007/s10528-005-1068-2. [DOI] [PubMed] [Google Scholar]
- 151.Bouhenni RA, Al Shahwan S, Morales J, Wakim BT, Chomyk AM, Alkuraya FS, Edward DP. Identification of differentially expressed proteins in the aqueous humor of primary congenital glaucoma. Exp Eye Res. 2011;92:67–75. doi: 10.1016/j.exer.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 152.Bredrup C, Stang E, Bruland O, Palka BP, Young RD, Haavik J, Knappskog PM, Rodahl E. Decorin accumulation contributes to the stromal opacities found in congenital stromal corneal dystrophy. Invest Ophthalmol Vis Sci. 2010;51:5578–5582. doi: 10.1167/iovs.09-4933. [DOI] [PubMed] [Google Scholar]
- 153.Kim J-h, Ko JM, Lee I, Kim JY, Kim MJ, Tchah H. A novel mutation of the decorin gene identified in a Korean family with congenital hereditary stromal dystrophy. Cornea. 2011;30:1473–1477. doi: 10.1097/ICO.0b013e3182137788. [DOI] [PubMed] [Google Scholar]
- 154.Rodahl E, Van Ginderdeuren R, Knappskog PM, Bredrup C, Boman H. A second decorin frame shift mutation in a family with congenital stromal corneal dystrophy. Am J Ophthalmol. 2006;142:520–521. doi: 10.1016/j.ajo.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 155.Lee JH, Ki C-S, Chung E-S, Chung T-Y. A novel decorin gene mutation in congenital hereditary stromal dystrophy: a Korean family. Korean J Ophthalmol. 2012;26:301–305. doi: 10.3341/kjo.2012.26.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen S, Sun M, Meng X, Iozzo RV, Kao WWY, Birk DE. Pathophysiological mechanisms of autosomal dominant congenital stromal corneal dystrophy: C-terminal-truncated decorin results in abnormal matrix assembly and altered expression of small leucine-rich proteoglycans. Am J Pathol. 2011;179:2409–2419. doi: 10.1016/j.ajpath.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Iozzo RV, Chakrani F, Perrotti D, McQuillan DJ, Skorski T, Calabretta B, Eichstetter I. Cooperative action of germ-line mutations in decorin and p53 accelerates lymphoma tumorigenesis. Proc Natl Acad Sci U S A. 1999;96:3092–3097. doi: 10.1073/pnas.96.6.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ansorge HL, Adams S, Jawad AF, Birk DE, Soslowsky LJ. Mechanical property changes during neonatal development and healing using a multiple regression model. J Biomech. 2011;45:1288–1292. doi: 10.1016/j.jbiomech.2012.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ansorge HL, Hsu JE, Edelstein L, Adams S, Birk DE, Soslowsky LJ. Recapitulation of the Achilles tendon mechanical properties during neonatal development: a study of differential healing during two stages of development in a mouse model. J Orthop Res. 2012;30:448–456. doi: 10.1002/jor.21542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dunkman AA, Buckley MR, Mienaltowski MJ, Adams SM, Thomas SJ, Satchell L, Kumar A, Pathmanathan L, Beason DP, Iozzo RV, Birk DE, Soslowsky LJ. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol. 2012 doi: 10.1016/j.matbio.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Theocharis AD, Skandalis SS, Tzanakakis GN, Karamanos NK. Proteoglycans in health and disease: novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J. 2010;277:3904–3923. doi: 10.1111/j.1742-4658.2010.07800.x. [DOI] [PubMed] [Google Scholar]