Abstract
Decorin, a secreted small leucine-rich proteoglycan, acts as a tumor repressor in a variety of cancers, mainly by blocking the action of several receptor tyrosine kinases such the receptors for hepatocyte, epidermal and insulin-like growth factors. In the present study we investigated the effects of decorin in an experimental model of thioacetamide-induced hepatocarcinogenesis, and its potential role in modulating the signaling of platelet-derived growth factor receptor-α (PDGFRα). Genetic ablation of decorin led to enhanced tumor prevalence and higher tumor count as compared to wild-type animals. These findings correlated with decreased levels of the cyclin-dependent kinase inhibitor p21WAF1/CIP1 and concurrent activation (phosphorylation) of PDGFRα in the hepatocellular carcinomas generated in the decorin-null vis-à-vis wild-type mice. Notably, in normal liver PDGFRα localized primarily to the membrane of non-parenchymal cells, whereas in the malignant counterpart PDGFRα was expressed by the malignant cells at their cell surfaces. This process was facilitated by a genetic background lacking endogenous decorin. Double immunostaining of the proteoglycan and the receptor revealed only minor colocalization leading to the hypothesis that decorin would bind to the natural ligand PDGF rather than the receptor itself. Indeed, we found that decorin binds to PDGF using purified proteins and immune blot assays. Collectively, our findings support the idea that decorin acts as a secreted tumor repressor during hepatocarcinogenesis by hindering the action of another receptor tyrosine kinase such as the PDGFRα, and could be a novel therapeutic agent in the battle against liver cancer.
Keywords: decorin, liver cancer, PDGFRα, proteoglycan, carcinogenesis
Introduction
Hepatocellular carcinoma (HCC) represents one of the most rapidly spreading cancers in the world. In the majority of liver cancers, chronic inflammation-induced fibrosis or cirrhosis precedes the development of the tumor, although this is not essential for tumor formation. At the same time, the process resulting in the overproduction of extracellular matrix favors cancer development at least by two ways; (I) Accelerated hepatocyte regeneration leads to insufficient DNA damage repair, and (II) The pathological matrix impairs the presentation of signals coming from the environment.
The extracellular matrix is a complex, well-organized structure of macromolecules interacting with each other and with the resident cells of the connective tissue. As a result, matrix macromolecules provide structural integrity, influence cell growth regulation, migration and differentiation. Consequently, in the last decade research on carcinogenesis has extended its area of interest focusing not only on tumor cells but also on the surrounding tumor stroma including that of abnormal synthesis and deposition of proteoglycans.
Decorin is a small leucine-rich proteoglycan (SLRP) of the extracellular matrix containing a single chondroitin sulfate or dermatan sulfate chain, and primarily produced by fibroblasts and myofibroblasts [1, 2]. It is present in a low quantity in the normal healthy liver, around the central veins and in the portal tracts. Together with other matrix proteins the amount of decorin significantly increases during fibrogenesis [3, 4]. Previous studies showed that the decorin protein core is able to bind the TGFβ1 [5], directly blocking the bioactivity of the growth factor, thereby functioning as protective endogenous agent against fibrosis [6, 7]. By modulating tumor stroma deposition and cell signaling pathways decorin is recognized as a promising tumor growth and migration inhibitor [8, 9].
Decorin directly binds to the epidermal growth factor receptor (EGFR) and inhibits its activity as well as other members’ activity of the ErbB receptor tyrosine kinase family [10]. These receptors are frequently overexpressed and/or mutated in various cancers accelerating tumor progression [11]. Moreover, decorin targets EGFR to degradation by caveolar-mediated endocytosis [12]. Besides EGFR decorin is a known ligand for hepatocyte growth factor receptor Met [13]. Such binding downregulates the receptor, and blocks its activity. Furthermore, decorin directly binds to IGF-IR [14, 15] and VEGF receptor type 2 [16], suppressing signaling pathways originated from these receptors. In parallel, decorin inhibits the endogenous VEGF (vascular endothelial growth factor) production of tumor cells [17]. This pan-RTK blockage often leads to growth arrest and hinders the tumor growth by keeping tumor cells in quiescence. It has been proven that a functional p21WAF1/CIP1 that causes G1 phase arrest is indispensable for the tumor repressor action of decorin in most tumor cell lines [18]. Decorin typically surrounds proliferating tumor cells in the so-called tumor microenvironment [19]. The elevated concentration of decorin around tumor cells may be a form of paracrine defensive mechanism by stromal cells counteracting the growth of malignant cells on the invasive front of solid tumors [20, 21].
Platelet-derived growth factors and their receptors have crucial roles in the development and maintenance of liver tumors. Both PDGFRα and β levels represent valuable prognostic markers in patients with hepatocellular carcinoma (HCC) [22]. The importance of PDGFRβ is well documented as it represents a target of the multikinase inhibitor Sorafenib used in targeted therapy of HCC [23, 24]. It is known that PDGFRα is involved in tumor angiogenesis and maintenance of the tumor microenvironment and has been implicated in development and metastasis of HCC [25, 26]. Another study reported that about 70% of hepatocellular carcinomas had elevated PDGFRα levels due to diverse mechanisms, suggesting that targeting this receptor may be of therapeutic value [26].
Very little is known on the role of decorin in hepatic tumorigenesis. The few limited reports have shown that decorin inhibits the proliferation of hepatoma cell lines in vitro [27], and that decorin gene expression is significantly downregulated in HCC as shown by gene expression analyses [28, 29]. Furthermore, in an earlier report decorin inhibited PDGF-stimulated vascular smooth muscle cell functions by binding to the ligand PDGF and preventing PDGFR phosphorylation [30]. Based on these observations, we hypothesized that hepatic decorin, primarily expressed by the non-parenchymal liver cells (stellate cells and fibroblasts), would act in a paracrine fashion to hamper the bioactivity of PDGFRα during the course of chemical-induced hepatocarcinogenesis.
Results
Lack of decorin leads to enhanced tumor formation in the liver
By Immunostaining, as expected, decorin was detectable only in wild-type animals where it was deposited in periportal connective tissue and around the central veins confirming the knock out phenotype of the animals (Fig. 1A and B).
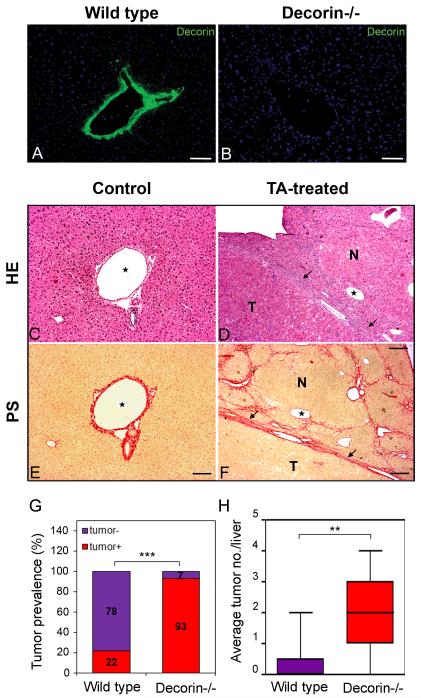
Fig. 1.
Decorin immunostaining and morphology of TA-induced liver tumors and tumor prevalence. Immunostaining for decorin in representative liver samples from wild-type control (A) and decorin-deficient control (B) mice. Nuclei are counterstained with DAPI (blue). Scale bar = 100 μm. Representative pictures of hematoxylin-eosin stained normal (C) and tumor bearing livers (D) induced by thioacetamide. Connective tissue-specific picrosirius staining on untreated control livers (E) and livers with hepatocellular carcinoma (F). TA=thioacetamide treatment. HE=hematoxilin-eosin staining. PS=picrosirius staining specific for connective tissue. N=nodule, T=tumor. Arrows point at tumor borders. Asterisks show the same vein on HE and PS-stained sections. Scale bars=100 μm. (G): diagrams show the ratio of tumor-bearing mice in experimental groups of wild type (Wt) and decorin knock out (Dcn−/−) mice. n=12, ***P<0.001 assessed by χ2-test. (H): columns represent the average tumor count per liver in livers exposed to TA. n=12, ** P <0.01.
Metabolization of thioacetamide (TA) in hepatocytes via cytochrome p450 causes fibrosis, and subsequently hepatic cirrhosis. Thus chronic TA exposure provokes hyperregeneration of hepatocytes initiating hepatocarcinogenesis in the cirrhotic liver [31, 32] (Fig. C-F). Tumors formed after TA exposure were rich in cytoplasm with strong eosinophil staining, and had a connective tissue capsule (Fig. 1D and F). Ninety-three percent of decorin-null animals developed macroscopic tumors in their livers in contrast to 22% found in the wild type ones (n=15, P<0.001) (Fig. 1G). In parallel with the higher tumor prevalence, elevated number of tumors was detected in mice lacking decorin when compared to those with wild-type genetic background. We detected a 7.3 fold increase in tumor number with an average of 2.2 tumors per liver in Dcn−/− mice vs. 0.3 tumors per wild-type liver (P<0.01) (Fig.1H). In conclusion, the lack of decorin sensitized the liver for tumor formation suggesting that decorin acts as a soluble tumor repressor during experimental hepatocarcinogenesis.
The tumor repressor activity of decorin utilizes p21WAF1/CIP1 in liver cancer
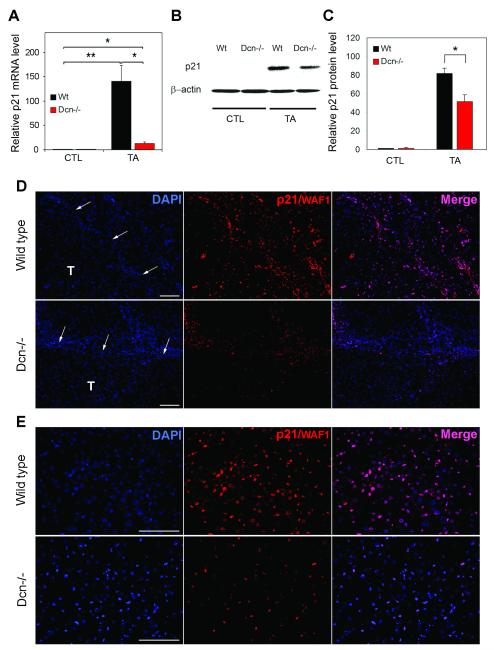
As decorin is known to exert its inhibitor effect on tumor cell proliferation via p21WAF1/CIP1 in most cases, we tested whether p21WAF1/CIP1 would be changed in our experimental hepatocarcinogenesis model. At the mRNA level (Fig. 2A), p21WAF1/CIP1 was induced 140 folds in the wild-type TA-treated livers compared to control samples (P<0.01). In contrast, in the decorin-null livers only a 12.6 fold increase (~9% of wild-type) was detected vis-a-vis Dcn−/− control ones (P<0.05) (Fig. 2A). At the protein level (Fig. 2B,C), p21WAF1/CIP1 was barely detectable in both control groups. In TA-treated livers, wild type samples contained 1.8 times more p21WAF1/CIP1 protein than that of Dcn−/− ones (p<0.05) as seen on Western blots (Fig. 2B) and quantified by densitometric analysis of the bands (Fig. 2C). To determine what cell types express p21WAF1/CIP1 and are affected by the absence or presence of decorin, fluorescent immunostaining specific for p21WAF1/CIP1 was performed (Fig. 2D,E). In wild type TA-treated livers, stromal cells showed strong nuclear staining, and hepatocytes of the non-tumorous tissue also expressed p21WAF1/CIP1(Fig. 2D). In decorin-null sections, stromal cells of the connective tissue also displayed immunopositivity, but to a lesser extent than that of wild type. Beside, hepatocytes outside the tumor were almost completely negative in contrast to livers of wild type animals (Fig. 2D). Within the tumors, both tumor and stromal cells displayed immunopositivity, meanwhile decorin−/− tumor cells appeared to lack the p21WAF1/CIP1, while stromal cells of the tumor express p21WAF1/CIP1(Fig. 2D,E). These results indicate that lack of decorin in the liver reduces the levels of p21WAF1/CIP1, a powerful cyclin-dependent kinase inhibitors and presumably would favor growth of the malignant cells.
Fig. 2.
Alterations in p21WAF1/CIP1 level in wild type and Dcn−/− animals. (A) Columns represent the relative p21 mRNA levels in livers of wild type (Wt) and decorin knock out (Dcn−/−) mice without treatment (control=CTL) or with TA exposure. *P<0.05, **P<0.01. (B) Representative picture of Western blots specific for p21 protein and β-actin loading control. (C) Relative levels of p21 normalized to β-actin obtained by densitometrical analysis of p21 Western blots. *P<0.05. (D) Representative pictures of p21WAF1/CIP1 immunostaining (red) on tumor-bearing liver sections from wild type and decorin-null (Dcn−/−) mice. Nuclei were counterstained with DAPI. T=tumor, arrows point at tumor border. Scale bar=100 μm. (E) P21WAF1/CIP1 immunpositivity (red) captured within the tumors of wild type and decorin knock out animals. Nuclei are shown in blue (DAPI). Scale bar=100 μm.
Ablation of decorin results in elevation of activated PDGFRα
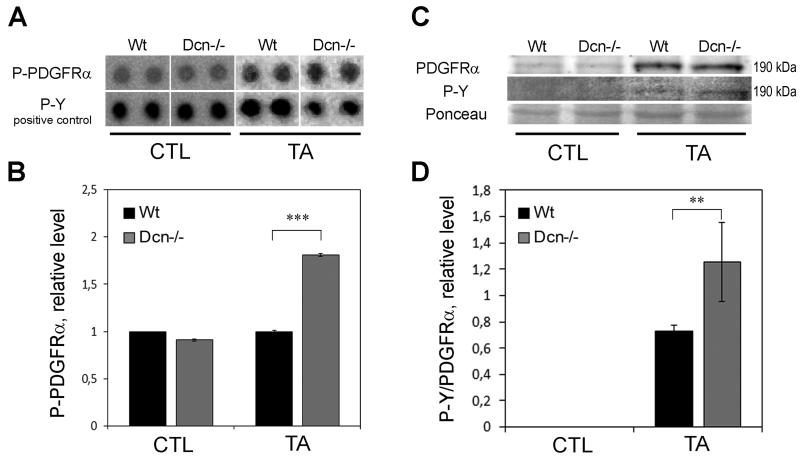
As decorin is known to bind to, and block the activity of several RTKs, we performed a protein array for phospho-RTKs to determine if the lack of the pan-RTK inhibitor decorin would lead to activation of any of these receptors. Four receptors were found to show altered phosphorylation due to the lack of decorin, and we selected PDGFRα for further examination. The protein array results revealed that Dcn−/− TA-treated samples contained 1.8-times more phospho-PDGFRα than that of wild type (P<0.001) (Fig. 3A, B). These data were validated by Western blot analysis followed by densitometry of bands (Fig. 3C, D). As a result, PDGFRα was present in a small quantity both in Dcn−/− and wild-type control samples, showing no difference between the genotypes. Phosphorylation of the receptor was not detected in either of the control groups. TA-treatment increased the PDGFRα level in Wt and Dcn−/− samples, exerting a 1.8-fold higher phosphorylation level of PDGFRα in decorin-null liver homogenates than that of wild type ones (P<0.01, Fig. 3D), confirming results obtained from phospho-RTK array.
Fig. 3.
Changes in PDGFRα and phospho-PDGFRα in TA-induced liver cancer. (A) Representative image of the phospho-RTK array dots of phospho-PDGFRα and phospho-tyrosine (P-Y) positive control in untreated (CTL) and thioacetamide-exposed liver samples (TA) of wild type (Wt) and decorin-null (Dcn−/−) animals. (B) Columns represent the results of densitometry of array dots showing relative levels normalized to P-Y positive control. ***P<0.001. (C) Representative image of Western blot membrane with PDGFRα and phospho-tyrosine (P-Y) immunostaining and Ponceau-staining as loading control. Note that for PDGFRα and P-Y the blots were double-stained, the same band is shown with using chemiluminescent and fluorescent detection. (D) The diagram shows the phosphorylated PDGFRα level relative to the total receptor amount, normalized to Ponceau staining. **P<0.01.
PDGFRα mainly localizes on non-parenchymal cells of the normal liver
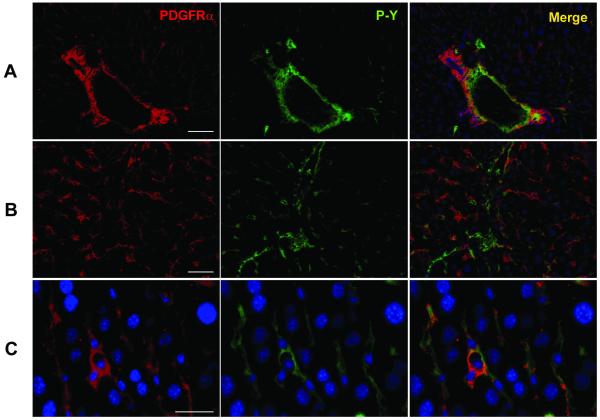
Next we determined the subcellular localization and expression of PDGFRα in normal and experimental livers. In the absence of any experimental challenge, we did not observe any difference by immunofluorescence staining between decorin-null and wild type animals in the location, amount or phosphorylation status of PDGFRα (Fig. 4). The receptor mainly localized in the periportal areas (Fig. 4A) and often in sinusoidal pattern (Fig. 4B). However, there was little co-localization with anti-phospho-PDGFRα in the sinusoid suggesting that under normal conditions this receptor is not significantly activated. Sections of normal livers from either genotype showed no immunopositivity for the receptor on the surface of hepatocytes. The majority of PDGFRα localized on the cell membrane of non-parenchymal cells, such as fibroblasts, myofibroblasts as seen in higher magnification (Fig. 4C).
Fig. 4.
Localization of PDGFRα in the normal liver. PDGFRα and phospho-tyrosine (P-Y) double immunostaining in the periportal area (A) and within a lobule (B) of the liver. Within the parenchyma (C), not the hepatocytes, but the non-parenchymal cells are positive for immunostaining. Scale bar = 100 μm (for A and B); Scale bar = 50 μm (for C).
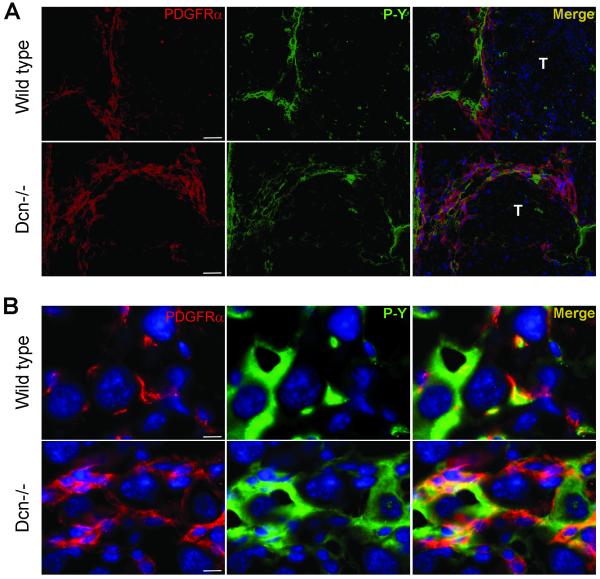
Induced PDGFRα level, and its appearance on hepatocytes in TA-treated livers
Thioacetamide treatment caused elevated levels of PDGFRα and its phosphorylated form as detected by immunostaining. The majority of the receptor was located in cirrhotic septa and connective tissue capsule of the tumors (Fig 5A.). In Dcn−/− livers, we detected more severe cirrhosis than wild-type animals, and this was associated with enhanced expression of both total and phosphorylated PDGFR (Fig. 5A). Within the tumors, the receptor appeared on the surface of tumor cells in a greater extent in decorin-null livers than that of wild-type ones (Fig. 5B). In conclusion, TA-treatment led to the induction of PDGFRα levels and increased its activation state, and likely induced the de novo expression of this receptor in the tumorigenic cells and its localization at the cell surface. Notably, these events were mainly observed in decorin-null liver sections rather than in wild-type samples, suggesting that lack of decorin is permissive for liver carcinogenesis.
Fig. 5.
Localization of PDGFRα in TA-treated livers of wild type and decorin-null animals. (A): PDGFRα and phospho-tyrosine (P-Y) double immunostaining in cirrhotic septa of wild type (Wt) and decorin-null (Dcn−/−) liver sections. Scale bar=100 μm. (B): Tumor cells in wild type (1st row) and Dcn−/− (2nd row) TA-treated livers stained by anti-PDGFRα and phospho-tyrosine (P-Y) antibodies. Scale bar=10 μm.
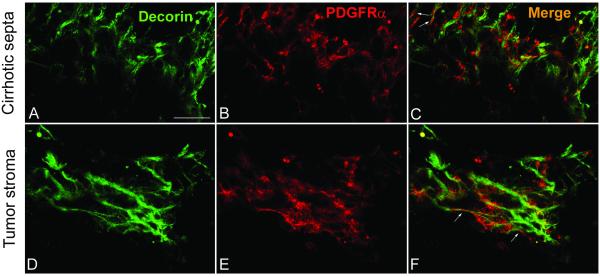
Decorin does not colocalize with PDGFRα but binds directly to its natural ligand PDGF
Next, we wanted to test the possibility that decorin could directly block the activity of we PDGFRα. To this end, we determined whether there was a subcellular co-localization of endogenous decorin and PDGFRα in wild-type livers. Double immunostaining of decorin and PDGFRα revealed that decorin and the receptor did not significantly co-localize in most parts of wild-type TA-treated livers including cirrhotic connective tissue septa (Fig. 6. A-C) and tumor stroma (Fig. 6. D-F). Only a minor fraction the immunofluorescence reaction specific for decorin and the receptor were found in focal overlapping areas (white arrows in Fig. 6F). These observations suggest that decorin hinders the action of the receptor using a mechanism other than direct binding and downregulation of PDGFRα in our experimental animal model of hepatocarcinogenesis.
Fig. 6.
Colocalization of decorin and PDGFR in tumors of cirrhotic liver. Decorin and PDGFR double immunostaining in sections of wild type liver exposed to thioacetamide. Representative pictures of the reactions in cirrhotic septa (A-C) and in tumor stroma (D-F). Scale bar=100 μm.
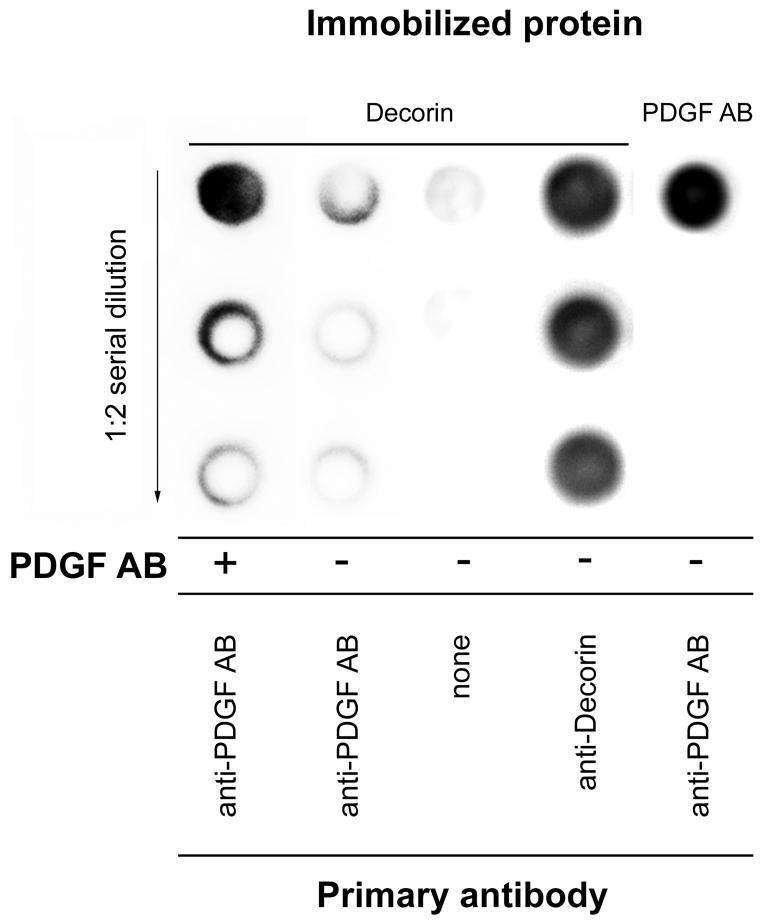
As colocalization experiments of decorin and PDGFRα failed to provide explanation for the higher activated level of PDGFRα in decorin-null TA-treated livers, we tested if decorin were able to directly bind the PDGF ligand. Immobilized recombinant human decorin was incubated with PDGF AB, followed by an immunoreaction using an antibody specific for PDGF AB. Indeed, dots of decorin exposed to PDGF AB were visualized with the PDGF AB antibody, reporting that the proteoglycan-PDGF complex was formed (Fig. 7).
Fig. 7.
Detection of interaction between decorin and PDGF AB. Human recombinant decorin (the four columns on the left) or PDGF AB (right column) was immobilized for dot blot analysis. Dots of 1:2 serial dilution of decorin (left 3 columns) were either incubated with PDGF AB ligand or with TBS, and visualized by PDGF AB specific antibody. Immobilized decorin was visualized by anti-decorin antibody, and PDGF AB by PDGF AB antibody respectively.
Discussion
Today we understand that extracellular matrix macromolecules form not only an inert, space-filling microenvironment but they create a vivid playground for interaction of cells with each other, as well as with the components residing in the matrix. These interactions modulate the utilization of signals that regulate the behavior of cells controlling key cellular events of physiologic and pathological processes, namely adhesion, migration, proliferation, differentiation, survival and angiogenesis [33-38]. A soluble matrix molecule that has been shown to be involved in the regulation of all the aforementioned cellular events, and thereby markedly contributes to health and disease, is decorin, a small leucine-rich extracellular matrix proteoglycan [39-41]. The evidences suggest that decorin among others represents a potent antitumor molecule [42, 43]. Early studies with decorin-deficient mice have indicated that although the lack of decorin does not lead to the development of spontaneous tumors [44], it is permissive for tumorigenesis [45]. More recently, however, it has been shown that decorin-null mice in a pure C57Bl/6 background have the propensity of developing intestinal tumors, especially when fed a western-type high caloric diet [46, 47]. Furthermore, ectopic expression of decorin has been shown to cause generalized growth suppression in neoplastic cells of various histologic origin [18]. As a consequence, several other studies supporting the antitumor and antimetastatic activity for decorin have been published [48-53]. Decorin expression in neoplastic tissues in vivo is relatively unexplored in clinical reports, by contrast to the large amount of studies on decorin’s behavior on in vitro tumor cell and xenograft models [10, 54-56]. Regarding liver tumors, we know that decorin inhibits proliferation of HuH7 [27] and HepG2 [57] hepatoma cell lines. In addition, decorin was found to be suppressed in hepatocellular carcinomas shown by gene expression microarray analyses [28, 29]. In line with these reports, our present study provides the first evidence that decorin acts as a tumor repressor in an experimental animal model of primary hepatocarcinogenesis as its lack is accompanied with significantly higher tumor prevalence and elevated tumor count. In parallel with enhanced tumor formation in decorin-null animals vs. wild type ones, we detected that livers of knock-out animals failed to induce p21WAF1/CIP1 expression both at the mRNA and protein levels when compared to wild-type samples. P21WAF1/CIP1 is a potent cyclin-dependent kinase inhibitor, leading to G1 arrest of the cell cycle [58, 59]. In this way, ablation of decorin in liver cancer leads to impaired cell cycle regulation allowing higher proliferation rate for tumor cells. This observation is in harmony with the well-known fact that decorin utilizes P21WAF1/CIP1 to display its tumor repressor effect in most cases [18, 57, 60].
Decorin is known to modulate the mechanism of cell growth and to induce signal transduction via growth factor receptors at the cell surface. Of note, decorin modulates and induces signal transduction along pathways involving the EGFR [11, 12, 61], the IGF-IR [14, 42], and Met [49]. In this study, we performed a phospho-protein array to test changes in the activation state of RTKs upon decorin deficiency in liver cancer. Our results unveiled a novel player in signaling of epithelial tumors affected by decorin, namely PDGFRα. Elevated amount of total as well as active PDGFRα was found in Dcn−/− livers exposed to the hepatotoxin thioacetamide compared to wild-type TA-treated livers both in the cirrhotic septa and within the tumor stroma. Notably, elevated PDGF signaling has been associated with fibrotic diseases such as pulmonary fibrosis, liver cirrhosis, scleroderma, glomerulosclerosis and cardiac fibrosis [25]. Certainly, in our earlier report the lack of decorin was associated with enhanced fibrosis of the liver [3], providing a fine explanation for higher PDGFRα levels in decorin-null cirrhotic livers. PDGFRα can play important roles not only in tumor angiogenesis, but also in directly stimulating tumor cell proliferation [25, 26]. The receptor is not present in adult normal hepatocytes as its expression substantially declines during development with no significant activation or phosphorylation [26]. Indeed, we did not detect the presence o f t his receptor in both wild-type and in decorin-null normal livers. Unexpectedly, in tumor cells of TA-treated livers, PDGFRα appeared in the cell membrane of tumor cells and became activated to a greater extent in Dcn−/− animals than in ones with a normal genetic background. This observation is in a good agreement with earlier reports demonstrating that HCC cell lines and resected tumors exhibit increased expression of both total and activated phosphorylated PDGFRα, with higher levels in more aggressive cell lines and in tumors with poor prognosis [26]. In addition, inhibition of PDGFRα by neutralizing monoclonal antibody was shown to inhibit survival and proliferation of several human and mouse cell lines, including HCC [26, 62, 63]. Hence, PDGFRα appears to play significant roles not only in tumor angiogenesis but also in cancer proliferation and survival in HCCs and its targeting may be effective in the therapy of this malignancy.
To address a potential mechanism of action of decorin we utilized double immunostaining of decorin and PDGFRα. Surprisingly, there was only focal co-localization of the two proteins in a minor fraction of the sections of cirrhotic areas and in tumor stroma of wild-type livers. This observation suggested that the effects of decorin might not be through a direct protein/receptor interaction as in the case of the other RTKs. It is well established that almost all binding domains for growth factors are located in the leucine-rich region of protein core of decorin, which acts as a docking reservoir for RTKs within the extracellular matrix [64]. Such direct interaction was revealed with e.g. transforming growth factor-β1 (TGFβ1) resulting the blockade of the growth factor receptor [5]. Thus, we hypothesized that decorin would bind the natural ligand PDGF, and by this indirect way would sequester the growth factor and attenuate PDGFRα activity. Cell-free binding studies revealed a binding interaction between human recombinant decorin and PDGF AB, confirming our hypothesis of indirect activity.
Further studies are needed to clarify if decorin is capable to establish a direct interaction with PDGFR. Our observation is in harmony with the report showing that decorin sequester PDGF BB thereby providing a potential mechanism for inhibition of intimal hyperplasia after balloon angioplasty [30]. Furthermore, our earlier observations of oligoarray hybridization (unpublished data) showed that the mRNA levels of PDGF A and B are elevated in Dcn−/− TA-induced tumor samples compared to wild type ones. Beside PDGF AB and BB we do not know yet whether decorin is able to bind other forms of the PDGF ligand such as AA or CC, but it would be of importance to examine as future prospective.
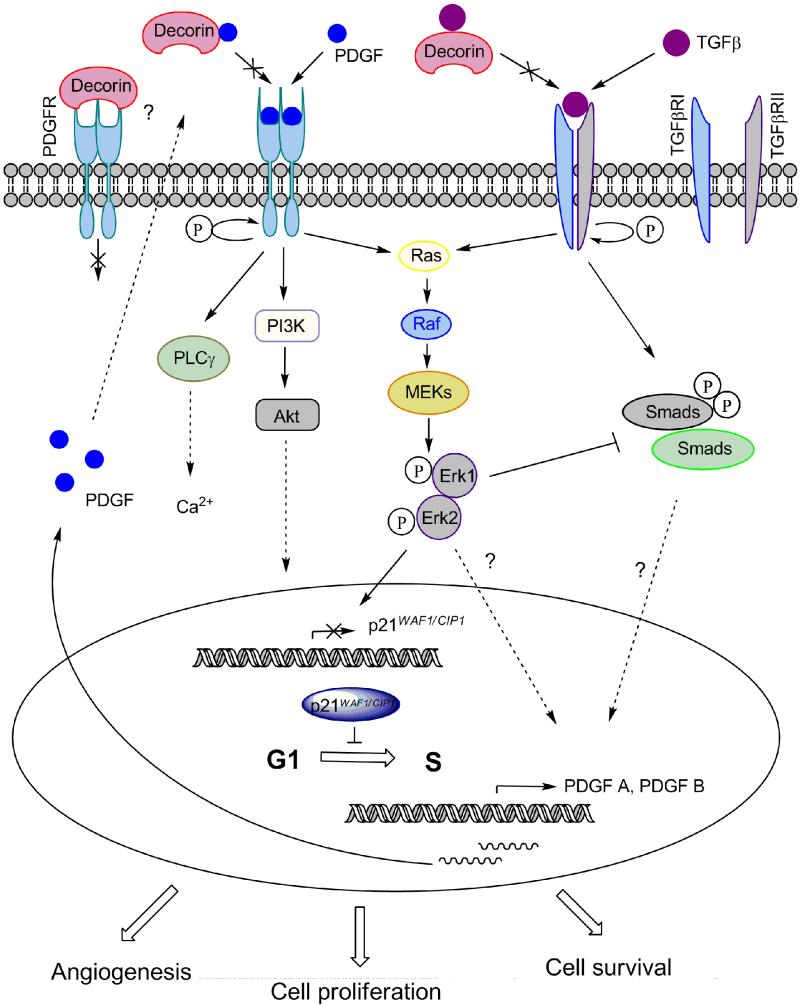
Based on our previous results, lack of decorin leads to enhanced efficiency of TGF-β1 during the development of hepatic fibrosis [3]. In line with this observation, TGF-β1 increases PDGF B mRNA levels in a dose-dependent manner [65], as well as in in vitro experiments on EMT with hepatocytes revealed a marked induction of PDGF A expression and the levels of PDGFRα and β upon TGF-β1 exposure [66]. Moreover, PDGF induces its own expression in an autocrine fashion via ERK1/2 [67]. Our earlier studies revealed elevated amounts of active phospho-ERK1/2 in decorin-null livers during fibrogenesis [3]. Taken together, it is possible that in the absence of decorin the higher activity of TGF-β1 causes overexpression of PDGF which would further trigger its own production (Fig. 8.). Additional mechanisms for decorin in affecting PDGF production may occur. For example, the expression of PDGF B is enhanced by Wnt/β-catenin signaling [68], and decorin is a known inhibitor of this important pathway [20, 69]. In this way, we hypothesize that when decorin is not present, the production of the ligand PDGF increases, as it is not sequestered by decorin. The accumulated PDGF in the extracellular space would then be free to bind to its cognate receptors. These events would ultimately lead to higher activity of the PDGF receptor (Fig. 8.).
Fig. 8.
Action of decorin on PDGF signaling in experimental liver cancer. The action of TGFβ is known to upregulate PDGF ligands. The presence of decorin hinders both Smad-dependent and independent signaling from the TGFβ receptor leading to decreased expression of PDGFs. In the extracellular environment, decorin may prevent PDGF from binding to its receptors resulting in an interference with downstream signaling. PDGF may act in an autocrine or a paracrine fashion. The changes in ERK1/2 upon the presence or absence of decorin, can be an outcome of crosstalk between the different growth factor receptors. Decorin utilizes p21WAF1/CIP1 for blockade of cell cycle to display its tumor repressor effect in most model systems.
It is known that decorin inhibits the production of VEGF by tumor cells [17] and can directly block VEGFR2 receptor at the same time [16]. It is possible, that similarly to this action, decorin inhibits PDGF production and may directly block the PDGFR in tumors causing decreased phosphorylation of the receptor. The cellular origin of PDGF production remains unclear as the ligands may also affect the cancer cells following its secretion from stromal cells [70] via paracrine fashion, in parallel with its synthesis by tumor cells (autocrine mode) [66]. The different isoforms can originate from different cell types of the liver [71]. It has been shown in several studies and in different cancer models that glycosaminoglycans, such as chondroitin sulfate play key partner roles in PDGFR effects [72-74] and can contribute to matrix remodeling [75-77]. Taking this knowledge into account, it is possible that either decorin protein core or its DS/CS chain exerts regulatory effect on PDGF signaling. Further investigations are necessary to identify which constituent of decorin proteoglycan is responsible for its action on PDGF signaling.
In conclusions, we provide novel information regarding a natural stroma-specific product of the liver and its potential role in suppressing tumor growth by hindering the action of PDGFRα in hepatocarcinogenesis, in part by its ability to bind and sequester PDGF AB. Our studies support the potential utilization of decorin as a viable therapeutic agent, either alone or in combination [20, 43, 51]. The fact that decorin is a nontoxic natural biological product, and therefore not immunogenic by itself, provides a rationale for targeted delivery and/or expression of decorin in cancer tissues as a new antioncogenic strategy [43, 64, 78, 79].
Materials and methods
Decorin-null mice
All animal experiments were conducted according to the ethical standards of the Animal Health Care and Control Institute, Csongrád County, Hungary, permit No. XVI/03047-2/2008. Decorin deficient mice were generated as previously described [44]. In brief, the inactivation of the decorin gene was achieved by targeted disruption of the exon 2 inserting a PGK-Neo cassette. Two male and two female C57Bl/6 mice heterozygous for decorin gene (Dcn−/−), which were backcrossed into C57Bl/6 background for nine generations, were bred until homozygosity. The genotype of the offspring was determined by PCR. Tail DNA was isolated by using high salt method. Subsequently 3 primers were applied, sense and antisense specific for the exon 2, and one corresponding to the PGK-Neo cassette. PCR products were analyzed by 2% agarose gel electrophoresis.
Thioacetamide treatment
For induction of liver cancer, we utilized a total of 24 one month-old male mice. Twelve wild-type, and 12 decorin knock-out (Dcn−/−) animals with C57Bl/6 background were exposed to thioacetamide (TA) dissolved in drinking water (150 mg/L). To obtain fully-developed hepatocellular carcinoma, animals were subjected to TA treatment for 7 months. Age-matched untreated animals with identical genetic background served as controls. Mice were terminated after 7 months of thioacetamide treatment by cervical dislocation in ether anesthesia. At termination, body weight and liver weight of the animals were measured and the number of macroscopically detectable tumors was counted. Half of the liver samples were frozen for further processing and the other half was fixed in 10% formaldehyde and embedded in paraffin for histological analysis. Paraffin sections were dewaxed in xylene and stained with hematoxylin and eosin, or processed further for immunohistochemistry. Stained sections were used for histological diagnosis.
Real-time RT-PCR
For RT-PCR, total RNAs were isolated from frozen livers. After homogenization in liquid nitrogen total RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany), according to the protocol provided by the manufacturer. The yield and purity of the isolated RNA were estimated by an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). The integrity and size distribution of the total RNA purified were analyzed using Experion RNA Chips and the Experion Automated Electrophoresis Station (Bio-Rad). Complementary DNAs (cDNAs) were generated from 1 μg of total RNA by M-MLV Reverse Transcriptase kit (Invitrogen by Life technologies Carlsbad, CA, USA) according to the instructions of the supplier. Real-time PCR was performed in an ABI Prism 7000 Sequence Detection System (Applied Biosystems by Life Technologies, Carlsbad, CA, USA), using ABI TaqMan Gene Expression Assays for mouse p21WAF1/CIP1(CDKN1A, Assay ID: Mm00432448_m1) applying 18S rRNA as endogenous control (Part No: 4319413E) according to the manufacturer’s protocol. All samples were run in duplicates in 20 μl total volume containing 50 ng cDNA using TaqMan Universal PCR Master Mix (Part No.:4324018, Applied Biosystems by Life Technologies). The thermal cycle conditions for reactions were as follows: denaturation for 10 min at 95°C, 40 cycles of denaturation at 95°C for 15 sec and annealing at 60°C for 1 min. Results were obtained as threshold cycle (CT) values. Expression levels were calculated by using the 2−ΔΔCT method.
Phospho-RTK array and Western Blot
Total proteins were extracted from frozen liver tissues. After homogenization in liquid nitrogen 1 ml of lysis buffer was added to the samples (20 mM TRIS pH=7.5, 2 mM EDTA, 150 mM NaCl, 1% Triton-X100, 0.5% Protease Inhibitor Cocktail (Sigma, St. Luis, MO) 2 mM Na3 VO4, 10 mM NaF. After incubation for 30 min on ice, samples were centrifuged at 15000 g for 20 min. Supernatants were kept and protein concentrations were measured as described before by Bradford [80]. The activities of phospho-receptor tyrosine kinases (phospho-RTKs) were assessed by their relative levels of phosphorylation using the Proteome Profiler Array (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. The same liver protein samples were used for Western blot. Pooled samples of five livers from the same experimental group were homogenized in lysis buffer (described above) and adjusted to 300 μg protein/250 μl lysate. Signals were developed by incubating the membrane in SuperSignal West Pico Chemiluminescent Substrate Kit (Thermo Fisher Scientific Inc., Waltham, MA USA), and visualized on a Kodak Image Station 4000MM Digital Imaging System.
For Western blot, 30 μg of total proteins were mixed with loading buffer containing β-mercaptoethanol and were incubated at 99°C for 5 min. Denatured samples were loaded onto a 10% polyacrylamide gel and were run for 30 min at 200 V on a Mini Protean vertical electrophoresis equipment (Bio-Rad, Hercules, CA). Proteins were transferred to PVDF membrane (Millipore) by blotting for 1.5 h at 100 V. Ponceau staining was applied to determine blotting efficiency. Membranes were blocked with 3 w/v% non-fat dry milk (Bio-Rad) in TBS for 1 h followed by incubation with the primary antibodies (p21WAF1/CIP1: ab7960 Abcam, Cambridge, UK; PDGFR: RD-AF1062, R&D Systems, Minneapolis, MN, USA; p-Y: RD-MAB1676, R&D Systems) at 4 °C for 16 h. Mouse β-actin or Ponceau staining served as loading control. Membranes were washed 5 times with TBS containing 0.5 v/v% Tween-20, then were incubated with appropriate secondary antibodies for 1 h. For p21, anti-rabbit immunoglobulins/HRP (P 0448) from DakoCytomation Glostrup Denmark was applied. For PDGFRα and phospho-tyrosine double staining, PDGFRα was visualized by anti-goat immunoglobulins/HRP (P0449), and P-Y by anti-mouse immunglobulins/biotin (E0443) with a subsequent incubation with Qdot® 525 streptavidin conjugate (Q10141MP, Invitrogen b Life Technologies) f o r 4 5 m i n . Signals were detected by SuperSignal West Pico Chemiluminescent Substrate Kit (Pierce/Thermo Scientific, Waltham, MA), and visualized by Kodak Image Station 4000MM Digital Imaging System. For PDGFRα and P-Y double staining, membranes were visualized for luminescence, and then exposed to UV light for fluorescent detection of Qdot signals with the same system. The density of the bands was also measured by the Kodak Image Station.
Immunofluorescence
For p21WAF1/CIP1 immunostaining, formalin-fixed paraffin-embedded sections were dewaxed by xylene and ethanol; antigens were retrieved by incubation in target retrieval solution (DakoCytomation) for 20 min using a pressure cooker. For PDGFRα, phospho-tyrosine and decorin reactions, frozen sections of the liver were fixed in ice-cold methanol for 20 min. Next, slides were washed in phosphate-buffered saline (PBS), blocked with 5 w/v% BSA/PBS containing 10% nonimmune serum of secondary antibody at 37 °C for 30 min. After washing, sections were incubated with the primary antibody specific for PDGFRα (antibody 1: RD-AF1062, R&D Systems, Minneapolis, MN, USA and antibody 2: #3174, Cell Signaling Technology, Danvers, MA, USA), phospho-tyrosine (RD-MAB1676, R&D Systems), decorin (AF1060 R&D Systems, Minneapolis, MN, USA) or p21WAF1/CIP1(ab7960, Abcam) diluted in 1:50 in PBS containing 1 w/v% BSA at 37 °C for 1.5 h or at 4 °C for 16 h. Appropriate fluorescent secondary antibodies (for PDGFRα antibody 1: Alexa Fluor 555 donkey anti—goat IgG: cat. no.: A21432; for PDGFRα antibody 2: Alexa Fluor 555 donkey anti—rabbit IgG: cat. no.: A31572; for phospho-tyrosine: Alexa Fluor 488 donkey anti-mouse IgG: cat. no.: A21202; and for decorin: Alexa Fluor 488 donkey anti—goat IgG: cat. no.:A11055, all from Invitrogen by Life Technologies) were applied at room temperature for 30 min. Nuclei were stained with 4′-6′-diamidino-phenylindole (DAPI). Pictures were taken by Nikon Eclipse E600 microscope with the help of Lucia Cytogenetics version 1.5.6 program, or by confocal laser scanning microscope (MRC-1024, Bio-Rad Richmond, CA).
Decorin and PDGF AB interaction
Decorin was purified from the secretions of Chinese hamster ovary (CHO) cells transfected with a full length decorin-expressing pcDNA3.1 vector as described before [81-83]. For dot blot, approximately 2 μg decorin/dot was applied on a nitrocellulose membrane (Millipore, Billerica, MA, USA), in a 1:2 serial dilution in wells for detecting PDGF AB and decorin interacton. Next, the membrane was blocked with 3% non-fat dry milk dissolved in Tris-buffered saline (TBS). Subsequently, membranes were incubated with PDGF AB (ab73228, AbCam Cambridge, UK) in a concentration of 4 μg/mL dissolved in TBS or TBS without PDGF AB for 18 h at 4 °C. Next, membranes were washed and probed with primary antibody specific for PDGF AB (ab50201, Abcam) in a dilution of 1:500 in TBS for 18 h at 4 °C. After washing HRP conjugated secondary antibody (anti-goat immunoglobulins/HRP (P0449), Dako) was applied for 1 h. Dot blot images were visualized by SuperSignal West Pico Chemiluminescent Substrate Kit (Pierce/Thermo Scientific), and visualized by Kodak Image Station 4000MM Digital Imaging System.
Statistical analysis
All statistical analyses were made with Graphpad Prism 4.03 software (Graphpad Software Inc.). Data were tested for normal distribution by D’Agostino & Pearson’s omnibus normality test. Significance of changes were tested by non-parametric tests (Mann-Whitney) or Students’ t-tests depending on the distribution of the data. The difference between wild type and Dcn−/− groups in tumor prevalence was tested for significance by χ2-test. The independent experimental sets were compared for reproducibility. Only reproducible significant changes were considered as significant. Significance was declared at the standard P<0.05 level.
Acknowledgement
This work was supported in part by Hungarian Scientific Research Fund, grants 67925 and 100904 (to IK); grant 105763 (to KB), and by the National Institutes of Health grant RO1 CA39481 (to RVI). The authors would like to thank András Sztodola and Mónika Borza for their help with animal experiments, Dr. Sándor Paku for his assistance with confocal laser scanning microscope, and for Zsuzsa Kaminszky for her technical assistance.
Abbreviations
- PDGFR
platelet-derived growth factor receptor
- HCC
hepatocellular carcinoma
- SLRP
small leucine-rich proteoglycan
- RTK
receptor tyrosine kinase
- EGFR
epidermal growth factors receptor
- TA
thioacetamide
References
- 1.Ferdous Z, Wei VM, Iozzo R, Hook M, Grande-Allen KJ. Decorin-transforming growth factor- interaction regulates matrix organization and mechanical characteristics of three-dimensional collagen matrices. J Biol Chem. 2007;282:35887–98. doi: 10.1074/jbc.M705180200. [DOI] [PubMed] [Google Scholar]
- 2.Hocking AM, Shinomura T, McQuillan DJ. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol. 1998;17:1–19. doi: 10.1016/s0945-053x(98)90121-4. [DOI] [PubMed] [Google Scholar]
- 3.Baghy K, Dezso K, Laszlo V, Fullar A, Peterfia B, Paku S, Nagy P, Schaff Z, Iozzo RV, Kovalszky I. Ablation of the decorin gene enhances experimental hepatic fibrosis and impairs hepatic healing in mice. Lab Invest. 2011;91:439–51. doi: 10.1038/labinvest.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudas J, Kovalszky I, Gallai M, Nagy JO, Schaff Z, Knittel T, Mehde M, Neubauer K, Szalay F, Ramadori G. Expression of decorin, transforming growth factor-beta 1, tissue inhibitor metalloproteinase 1 and 2, and type IV collagenases in chronic hepatitis. Am J Clin Pathol. 2001;115:725–35. doi: 10.1309/J8CD-E9C8-X4NG-GTVG. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–4. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 6.Baghy K, Iozzo RV, Kovalszky I. Decorin-TGFbeta axis in hepatic fibrosis and cirrhosis. J Histochem Cytochem. 2012;60:262–8. doi: 10.1369/0022155412438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaefer L. Small leucine-rich proteoglycans in kidney disease. J Am Soc Nephrol. 2011;22:1200–7. doi: 10.1681/ASN.2010050570. [DOI] [PubMed] [Google Scholar]
- 8.Iozzo RV. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 1997;32:141–74. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- 9.Iozzo RV. The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem. 1999;274:18843–6. doi: 10.1074/jbc.274.27.18843. [DOI] [PubMed] [Google Scholar]
- 10.Santra M, Eichstetter I, Iozzo RV. An anti-oncogenic role for decorin. Down-regulation of ErbB2 leads to growth suppression and cytodifferentiation of mammary carcinoma cells. J Biol Chem. 2000;275:35153–61. doi: 10.1074/jbc.M006821200. [DOI] [PubMed] [Google Scholar]
- 11.Iozzo RV, Moscatello DK, McQuillan DJ, Eichstetter I. Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem. 1999;274:4489–92. doi: 10.1074/jbc.274.8.4489. [DOI] [PubMed] [Google Scholar]
- 12.Zhu JX, Goldoni S, Bix G, Owens RT, McQuillan DJ, Reed CC, Iozzo RV. Decorin evokes protracted internalization and degradation of the epidermal growth factor receptor via caveolar endocytosis. J Biol Chem. 2005;280:32468–79. doi: 10.1074/jbc.M503833200. [DOI] [PubMed] [Google Scholar]
- 13.Goldoni S, Humphries A, Nystrom A, Sattar S, Owens RT, McQuillan DJ, Ireton K, Iozzo RV. Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol. 2009;185:743–54. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schonherr E, Sunderkotter C, Iozzo RV, Schaefer L. Decorin, a novel player in the insulin-like growth factor system. J Biol Chem. 2005;280:15767–72. doi: 10.1074/jbc.M500451200. [DOI] [PubMed] [Google Scholar]
- 15.Iozzo RV, Buraschi S, Genua M, Xu SQ, Solomides CC, Peiper SC, Gomella LG, Owens RC, Morrione A. Decorin antagonizes IGF receptor I (IGF-IR) function by interfering with IGF-IR activity and attenuating downstream signaling. J Biol Chem. 2011;286:34712–21. doi: 10.1074/jbc.M111.262766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan GA, Girish GV, Lala N, Di Guglielmo GM, Lala PK. Decorin is a novel VEGFR-2-binding antagonist for the human extravillous trophoblast. Mol Endocrinol. 2011;25:1431–43. doi: 10.1210/me.2010-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant DS, Yenisey C, Rose RW, Tootell M, Santra M, Iozzo RV. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene. 2002;21:4765–77. doi: 10.1038/sj.onc.1205595. [DOI] [PubMed] [Google Scholar]
- 18.Santra M, Mann DM, Mercer EW, Skorski T, Calabretta B, Iozzo RV. Ectopic expression of decorin protein core causes a generalized growth suppression in neoplastic cells of various histogenetic origin and requires endogenous p21, an inhibitor of cyclin-dependent kinases. J Clin Invest. 1997;100:149–57. doi: 10.1172/JCI119507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iozzo RV. Tumor stroma as a regulator of neoplastic behavior. Agonistic and antagonistic elements embedded in the same connective tissue. Lab Invest. 1995;73:157–60. [PubMed] [Google Scholar]
- 20.Neill T, Schaefer L, Iozzo RV. Decorin: a guardian from the matrix. Am J Pathol. 2012;181:380–7. doi: 10.1016/j.ajpath.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buraschi S, Neill T, Owens RT, Iniguez LA, Purkins G, Vadigepalli R, Evans B, Schaefer L, Peiper SC, Wang ZX, Iozzo RV. Decorin protein core affects the global gene expression profile of the tumor microenvironment in a triple-negative orthotopic breast carcinoma xenograft model. PLoS One. 2012;7:e45559. doi: 10.1371/journal.pone.0045559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Shi Y, Jiang CY, Wei LX, Lv YL, Wang YL, Dai GH. Coexpression of PDGFR-alpha, PDGFR-beta and VEGF as a prognostic factor in patients with hepatocellular carcinoma. Int J Biol Markers. 2011;26:108–16. doi: 10.5301/JBM.2011.8322. [DOI] [PubMed] [Google Scholar]
- 23.Furuse J. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Biologics. 2008;2:779–88. doi: 10.2147/btt.s3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pang RW, Poon RT. From molecular biology to targeted therapies for hepatocellular carcinoma: the future is now. Oncology. 2007;72(Suppl 1):30–44. doi: 10.1159/000111705. [DOI] [PubMed] [Google Scholar]
- 25.Oseini AM, Roberts LR. PDGFRalpha: a new therapeutic target in the treatment of hepatocellular carcinoma? Expert Opin Ther Targets. 2009;13:443–54. doi: 10.1517/14728220902719233. [DOI] [PubMed] [Google Scholar]
- 26.Stock P, Monga D, Tan X, Micsenyi A, Loizos N, Monga SP. Platelet-derived growth factor receptor-alpha: a novel therapeutic target in human hepatocellular cancer. Mol Cancer Ther. 2007;6:1932–41. doi: 10.1158/1535-7163.MCT-06-0720. [DOI] [PubMed] [Google Scholar]
- 27.Shangguan JY, Dou KF, Li X, Hu XJ, Zhang FQ, Yong ZS, Ti ZY. [Effects and mechanism of decorin on the proliferation of HuH7 hepatoma carcinoma cells in vitro] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2009;25:780–2. [PubMed] [Google Scholar]
- 28.Chung EJ, Sung YK, Farooq M, Kim Y, Im S, Tak WY, Hwang YJ, Kim YI, Han HS, Kim JC, Kim MK. Gene expression profile analysis in human hepatocellular carcinoma by cDNA microarray. Mol Cells. 2002;14:382–7. [PubMed] [Google Scholar]
- 29.Miyasaka Y, Enomoto N, Nagayama K, Izumi N, Marumo F, Watanabe M, Sato C. Analysis of differentially expressed genes in human hepatocellular carcinoma using suppression subtractive hybridization. Br J Cancer. 2001;85:228–34. doi: 10.1054/bjoc.2001.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nili N, Cheema AN, Giordano FJ, Barolet AW, Babaei S, Hickey R, Eskandarian MR, Smeets M, Butany J, Pasterkamp G, Strauss BH. Decorin inhibition of PDGF-stimulated vascular smooth muscle cell function: potential mechanism for inhibition of intimal hyperplasia after balloon angioplasty. Am J Pathol. 2003;163:869–78. doi: 10.1016/S0002-9440(10)63447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker FF. Thioacetamide hepatocarcinogenesis. J Natl Cancer Inst. 1983;71:553–8. [PubMed] [Google Scholar]
- 32.Camus-Randon AM, Raffalli F, Bereziat JC, McGregor D, Konstandi M, Lang MA. Liver injury and expression of cytochromes P450: evidence that regulation of CYP2A5 is different from that of other major xenobiotic metabolizing CYP enzymes. Toxicol Appl Pharmacol. 1996;138:140–8. doi: 10.1006/taap.1996.0107. [DOI] [PubMed] [Google Scholar]
- 33.Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255–64. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- 34.Jarvelainen H, Sainio A, Koulu M, Wight TN, Penttinen R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol Rev. 2009;61:198–223. doi: 10.1124/pr.109.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marastoni S, Ligresti G, Lorenzon E, Colombatti A, Mongiat M. Extracellular matrix: a matter of life and death. Connect Tissue Res. 2008;49:203–6. doi: 10.1080/03008200802143190. [DOI] [PubMed] [Google Scholar]
- 36.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341:126–40. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hielscher AC, Qiu C, Gerecht S. Breast cancer cell-derived matrix supports vascular morphogenesis. Am J Physiol Cell Physiol. 2012;302:C1243–56. doi: 10.1152/ajpcell.00011.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferdous Z, Peterson SB, Tseng H, Anderson DK, Iozzo RV, Grande-Allen KJ. A role for decorin in controlling proliferation, adhesion, and migration of murine embryonic fibroblasts. J Biomed Mater Res A. 2010;93:419–28. doi: 10.1002/jbm.a.32545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iozzo RV, Schaefer L. Proteoglycans in health and disease: novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J. 2010;277:3864–75. doi: 10.1111/j.1742-4658.2010.07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seidler DG, Dreier R. Decorin and its galactosaminoglycan chain: extracellular regulator of cellular function? IUBMB Life. 2008;60:729–33. doi: 10.1002/iub.115. [DOI] [PubMed] [Google Scholar]
- 42.Iozzo RV, Sanderson RD. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med. 2011;15:1013–31. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theocharis AD, Skandalis SS, Tzanakakis GN, Karamanos NK. Proteoglycans in health and disease: novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J. 2010;277:3904–23. doi: 10.1111/j.1742-4658.2010.07800.x. [DOI] [PubMed] [Google Scholar]
- 44.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–43. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iozzo RV, Chakrani F, Perrotti D, McQuillan DJ, Skorski T, Calabretta B, Eichstetter I. Cooperative action of germ-line mutations in decorin and p53 accelerates lymphoma tumorigenesis. Proc Natl Acad Sci U S A. 1999;96:3092–7. doi: 10.1073/pnas.96.6.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bi X, Pohl NM, Qian Z, Yang GR, Gou Y, Guzman G, Kajdacsy-Balla A, Iozzo RV, Yang W. Decorin-mediated inhibition of colorectal cancer growth and migration is associated with E-cadherin in vitro and in mice. Carcinogenesis. 2012;33:326–30. doi: 10.1093/carcin/bgr293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bi X, Tong C, Dockendorff A, Bancroft L, Gallagher L, Guzman G, Iozzo RV, Augenlicht LH, Yang W. Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation. Carcinogenesis. 2008;29:1435–40. doi: 10.1093/carcin/bgn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biaoxue R, Xiguang C, Hua L, Hui M, Shuanying Y, Wei Z, Wenli S, Jie D. Decreased expression of decorin and p57(KIP2) correlates with poor survival and lymphatic metastasis in lung cancer patients. Int J Biol Markers. 2011;26:9–21. doi: 10.5301/jbm.2011.6372. [DOI] [PubMed] [Google Scholar]
- 49.Goldoni S, Seidler DG, Heath J, Fassan M, Baffa R, Thakur ML, Owens RT, McQuillan DJ, Iozzo RV. An antimetastatic role for decorin in breast cancer. Am J Pathol. 2008;173:844–55. doi: 10.2353/ajpath.2008.080275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu Y, Sun H, Owens RT, Wu J, Chen YQ, Berquin IM, Perry D, O’Flaherty JT, Edwards IJ. Decorin suppresses prostate tumor growth through inhibition of epidermal growth factor and androgen receptor pathways. Neoplasia. 2009;11:1042–53. doi: 10.1593/neo.09760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reed CC, Waterhouse A, Kirby S, Kay P, Owens RT, McQuillan DJ, Iozzo RV. Decorin prevents metastatic spreading of breast cancer. Oncogene. 2005;24:1104–10. doi: 10.1038/sj.onc.1208329. [DOI] [PubMed] [Google Scholar]
- 52.Shintani K, Matsumine A, Kusuzaki K, Morikawa J, Matsubara T, Wakabayashi T, Araki K, Satonaka H, Wakabayashi H, Iino T, Uchida A. Decorin suppresses lung metastases of murine osteosarcoma. Oncol Rep. 2008;19:1533–9. [PubMed] [Google Scholar]
- 53.Troup S, Njue C, Kliewer EV, Parisien M, Roskelley C, Chakravarti S, Roughley PJ, Murphy LC, Watson PH. Reduced expression of the small leucine-rich proteoglycans, lumican, and decorin is associated with poor outcome in node-negative invasive breast cancer. Clin Cancer Res. 2003;9:207–14. [PubMed] [Google Scholar]
- 54.Reed CC, Gauldie J, Iozzo RV. Suppression of tumorigenicity by adenovirus-mediated gene transfer of decorin. Oncogene. 2002;21:3688–95. doi: 10.1038/sj.onc.1205470. [DOI] [PubMed] [Google Scholar]
- 55.Tralhao JG, Schaefer L, Micegova M, Evaristo C, Schonherr E, Kayal S, Veiga-Fernandes H, Danel C, Iozzo RV, Kresse H, Lemarchand P. In vivo selective and distant killing of cancer cells using adenovirus-mediated decorin gene transfer. Faseb J. 2003;17:464–6. doi: 10.1096/fj.02-0534fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Santra M, Skorski T, Calabretta B, Lattime EC, Iozzo RV. De novo decorin gene expression suppresses the malignant phenotype in human colon cancer cells. Proc Natl Acad Sci U S A. 1995;92:7016–20. doi: 10.1073/pnas.92.15.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Wang Y, Du Z, Wang Q, Wu M, Wang X, Wang L, Cao L, Hamid AS, Zhang G. Recombinant human decorin suppresses liver HepG2 carcinoma cells by p21 upregulation. Onco Targets Ther. 2012;5:143–52. doi: 10.2147/OTT.S32918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–16. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 59.Harper JW, Elledge SJ, Keyomarsi K, Dynlacht B, Tsai LH, Zhang P, Dobrowolski S, Bai C, Connell-Crowley L, Swindell E, et al. Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell. 1995;6:387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Luca A, Santra M, Baldi A, Giordano A, Iozzo RV. Decorin-induced growth suppression is associated with up-regulation of p21, an inhibitor of cyclin-dependent kinases. J Biol Chem. 1996;271:18961–5. doi: 10.1074/jbc.271.31.18961. [DOI] [PubMed] [Google Scholar]
- 61.Csordas G, Santra M, Reed CC, Eichstetter I, McQuillan DJ, Gross D, Nugent MA, Hajnoczky G, Iozzo RV. Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo. J Biol Chem. 2000;275:32879–87. doi: 10.1074/jbc.M005609200. [DOI] [PubMed] [Google Scholar]
- 62.Loizos N, Xu Y, Huber J, Liu M, Lu D, Finnerty B, Rolser R, Malikzay A, Persaud A, Corcoran E, Deevi DS, Balderes P, Bassi R, Jimenez X, Joynes CJ, Mangalampalli VR, Steiner P, Tonra JR, Wu Y, Pereira DS, Zhu Z, Ludwig DL, Hicklin DJ, Bohlen P, Witte L, Kussie P. Targeting the platelet-derived growth factor receptor alpha with a neutralizing human monoclonal antibody inhibits the growth of tumor xenografts: implications as a potential therapeutic target. Mol Cancer Ther. 2005;4:369–79. doi: 10.1158/1535-7163.MCT-04-0114. [DOI] [PubMed] [Google Scholar]
- 63.LaRochelle WJ, Jensen RA, Heidaran MA, May-Siroff M, Wang LM, Aaronson SA, Pierce JH. Inhibition of platelet-derived growth factor autocrine growth stimulation by a monoclonal antibody to the human alpha platelet-derived growth factor receptor. Cell Growth Differ. 1993;4:547–53. [PubMed] [Google Scholar]
- 64.Sofeu Feugaing DD, Gotte M, Viola M. More than matrix: The multifaceted role of decorin in cancer. Eur J Cell Biol. 2012 doi: 10.1016/j.ejcb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 65.Rydziel S, Canalis E. Expression and growth factor regulation of platelet-derived growth factor B transcripts in primary osteoblast cell cultures. Endocrinology. 1996;137:4115–9. doi: 10.1210/endo.137.10.8828465. [DOI] [PubMed] [Google Scholar]
- 66.Gotzmann J, Fischer AN, Zojer M, Mikula M, Proell V, Huber H, Jechlinger M, Waerner T, Weith A, Beug H, Mikulits W. A crucial function of PDGF in TGF-beta-mediated cancer progression of hepatocytes. Oncogene. 2006;25:3170–85. doi: 10.1038/sj.onc.1209083. [DOI] [PubMed] [Google Scholar]
- 67.Finlay GA, Hunter DS, Walker CL, Paulson KE, Fanburg BL. Regulation of PDGF production and ERK activation by estrogen is associated with TSC2 gene expression. Am J Physiol Cell Physiol. 2003;285:C409–18. doi: 10.1152/ajpcell.00482.2002. [DOI] [PubMed] [Google Scholar]
- 68.Reis M, Czupalla CJ, Ziegler N, Devraj K, Zinke J, Seidel S, Heck R, Thom S, Macas J, Bockamp E, Fruttiger M, Taketo MM, Dimmeler S, Plate KH, Liebner S. Endothelial Wnt/beta-catenin signaling inhibits glioma angiogenesis and normalizes tumor blood vessels by inducing PDGF-B expression. J Exp Med. 2012;209:1611–27. doi: 10.1084/jem.20111580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buraschi S, Pal N, Tyler-Rubinstein N, Owens RT, Neill T, Iozzo RV. Decorin antagonizes Met receptor activity and down-regulates {beta}-catenin and Myc levels. J Biol Chem. 2010;285:42075–85. doi: 10.1074/jbc.M110.172841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Breitkopf K, Roeyen C, Sawitza I, Wickert L, Floege J, Gressner AM. Expression patterns of PDGF-A, -B, -C and -D and the PDGF-receptors alpha and beta in activated rat hepatic stellate cells (HSC) Cytokine. 2005;31:349–57. doi: 10.1016/j.cyto.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 71.Borkham-Kamphorst E, Kovalenko E, van Roeyen CR, Gassler N, Bomble M, Ostendorf T, Floege J, Gressner AM, Weiskirchen R. Platelet-derived growth factor isoform expression in carbon tetrachloride-induced chronic liver injury. Lab Invest. 2008;88:1090–100. doi: 10.1038/labinvest.2008.71. [DOI] [PubMed] [Google Scholar]
- 72.Afratis N, Gialeli C, Nikitovic D, Tsegenidis T, Karousou E, Theocharis AD, Pavao MS, Tzanakakis GN, Karamanos NK. Glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J. 2012;279:1177–97. doi: 10.1111/j.1742-4658.2012.08529.x. [DOI] [PubMed] [Google Scholar]
- 73.Fthenou E, Zafiropoulos A, Katonis P, Tsatsakis A, Karamanos NK, Tzanakakis GN. Chondroitin sulfate prevents platelet derived growth factor-mediated phosphorylation of PDGF-Rbeta in normal human fibroblasts severely impairing mitogenic responses. J Cell Biochem. 2008;103:1866–76. doi: 10.1002/jcb.21570. [DOI] [PubMed] [Google Scholar]
- 74.Berdiaki A, Zafiropoulos A, Fthenou E, Katonis P, Tsatsakis A, Karamanos NK, Tzanakakis GN. Regulation of hyaluronan and versican deposition by growth factors in fibrosarcoma cell lines. Biochim Biophys Acta. 2008;1780:194–202. doi: 10.1016/j.bbagen.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Ruhland C, Schonherr E, Robenek H, Hansen U, Iozzo RV, Bruckner P, Seidler DG. The glycosaminoglycan chain of decorin plays an important role in collagen fibril formation at the early stages of fibrillogenesis. FEBS J. 2007;274:4246–55. doi: 10.1111/j.1742-4658.2007.05951.x. [DOI] [PubMed] [Google Scholar]
- 76.Schaefer L, Iozzo RV. Small leucine-rich proteoglycans, at the crossroad of cancer growth and inflammation. Curr Opin Genet Dev. 2012;22:56–7. doi: 10.1016/j.gde.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 77.Merline R, Iozzo RV, Schaefer L. Small leucine-rich proteoglycans: Multifunctional signaling effectors. In: Karamanos N, editor. Extracellular Matrix: Pathobiology and Signaling. De Gruyter; Berlin, Germany: 2012. pp. 185–196. [Google Scholar]
- 78.Seidler DG, Goldoni S, Agnew C, Cardi C, Thakur ML, Owens RT, McQuillan DJ, Iozzo RV. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J Biol Chem. 2006;281:26408–18. doi: 10.1074/jbc.M602853200. [DOI] [PubMed] [Google Scholar]
- 79.Iozzo RV, Karamanos N. Proteoglycans in health and disease: emerging concepts and future directions. FEBS J. 2010;277:3863. doi: 10.1111/j.1742-4658.2010.07796.x. [DOI] [PubMed] [Google Scholar]
- 80.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 81.Moscatello DK, Santra M, Mann DM, McQuillan DJ, Wong AJ, Iozzo RV. Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor. J Clin Invest. 1998;101:406–12. doi: 10.1172/JCI846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamaguchi Y, Ruoslahti E. Expression of human proteoglycan in Chinese hamster ovary cells inhibits cell proliferation. Nature. 1988;336:244–6. doi: 10.1038/336244a0. [DOI] [PubMed] [Google Scholar]
- 83.McBain AL, Mann DM. Purification of recombinant human decorin and its subdomains. Methods Mol Biol. 2001;171:221–9. doi: 10.1385/1-59259-209-0:221. [DOI] [PubMed] [Google Scholar]