Abstract
Sex differences in the structure and organization of the corpus callosum (CC) can be attributed to genetic, hormonal or environmental effects, or a combination of these factors. To address the role of gonadal hormones on axon myelination, functional axon conduction and immunohistochemistry analysis of the CC in intact, gonadectomized and hormone‐replaced gonadectomized animals were used. These groups were subjected to cuprizone diet‐induced demyelination followed by remyelination. The myelinated component of callosal compound action potential was significantly decreased in ovariectomized and castrated animals under normal myelinating condition. Compared to gonadally intact cohorts, both gonadectomized groups displayed more severe demyelination and inhibited remyelination. Castration in males was more deleterious than ovariectomy in females. Callosal conduction in estradiol‐supplemented ovariectomized females was significantly increased during normal myelination, less attenuated during demyelination, and increased beyond placebo‐treated ovariectomized or intact female levels during remyelination. In castrated males, the non‐aromatizing steroid dihydrotestosterone was less efficient than testosterone and estradiol in restoring normal myelination/axon conduction and remyelination to levels of intact males. Furthermore, in both sexes, estradiol supplementation in gonadectomized groups increased the number of oligodendrocytes. These studies suggest an essential role of estradiol to promote efficient CC myelination and axon conduction in both sexes.
Keywords: compound action potential, corpus callosum, demyelination, estrogens, gonadectomy, remyelination, sex difference, testosterone
Introduction
As the brain's largest white matter tract and main interhemispheric commissure, the human corpus callosum (CC) is comprised of approximately 200 million fibers 42. The CC connects the two hemispheres, facilitates the rapid transfer of information between homotopic 4, 20 and heterotopic cortical areas, and integrates sensorimotor and cognitive information 46. It is not surprising that the CC has been investigated extensively for assessing sexual dimorphism. Until recently, the existence of sexual dimorphisms in the CC has been debated. Using advanced imaging techniques, many groups have observed a significant sexual dimorphism in the white matter of healthy human brains, with the CC showing increased fractional anisotropy in women compared to men 23, 45.
Sexual dimorphism of brain structures may be related to genetic, hormonal or environmental effects, or a combination of these factors. Sex steroid hormones include androgens [eg, testosterone (T)], estrogens [of which 17β‐estradiol (E2) is the most potent in mammals] and progesterone, all of which are derived from cholesterol 40. Steroid hormones are synthesized primarily in the gonads, adrenal glands and placenta. Estradiol, progesterone and T are present from birth in both males and females, although circulating levels differ considerably. Sex hormones are mostly known for their role in sex organ development and physical maturation during puberty, but a prime target of sex steroid hormones is the brain 29, 43. Sex steroids operate as trophic factors affecting brain development and plasticity and can stimulate neurite outgrowth, synapse number, dendritic branching and myelination through their direct impact on glial cells (for review, see 8, 10, 18, 37). Steroidogenic enzymes are present in both neurons and glia, and many of these enzymes are region specific 31. Endogenous concentrations of neurosteroids and steroidogenic enzymes can be modulated by the ovarian cycle, stress, hormone manipulations and aging 3. Sex steroid hormones and neurosteroids can affect gene expression by binding to classical nuclear receptors, binding to membrane receptors located on cell membranes or modulating neurotransmitter receptor function. In vitro studies indicate that all brain cell types [neurons, astrocytes, oligodendrocytes (OLs) and microglia] express both estrogen receptors ERα and ERβ [(reviewed in 18, 44] and that astrocytes also express the progesterone receptor 24 and androgen receptor in vitro 17, 21.
To investigate the role of sex hormones on CC axon conduction and effect on myelination, we used the cuprizone mouse model. Cuprizone (biscyclohexanone oxaldihydrazone) ingestion in mice kills mature OLs. This results in axon demyelination of distinct brain regions including the CC, the most frequently investigated white matter tract in this animal model 39. An increase in OLs and axon myelination occurs when cuprizone diet is replaced with normal diet. As demyelination and remyelination is induced in a highly reproducible manner, we were able to compare effects of hormone deficiency and hormone replacement during normal myelination, demyelination, and remyelination 28.
To visualize OLs and myelin after cuprizone diet‐induced demyelination and subsequent normal diet remyelination transgenic C57BL/6 mice expressing enhanced green fluorescent proteins (EGFP) under the proteolipid protein (PLP) promoter, a cell‐specific promoter to OLs (PLP_EGFP) 25 were used. These mice were gonadally intact, gonadectomized or gonadectomized and implanted with a hormone‐releasing pellet. Electrophysiological analysis in this study was performed by recording CC compound action potentials (CAPs) 11, 12. Changes in CAP amplitudes resulting from myelinated callosal fibers were assessed. To assess the status of myelin density and OL lineage cells, recorded slices were subsequently subjected to immunohistochemical analysis 11.
We report that gonadectomy (GDX) in young adult male and female mice negatively impacts normal myelination, cuprizone diet‐induced demyelination and subsequent remyelination as observed by CAP analysis and immunohistochemistry. Sex hormone replacement (E2 in females and either T or E2 in males) reversed some of these GDX‐induced deficits.
Methods
Animals
Breeding pairs of PLP_EGFP mice on the C57BL/6J background were a kind gift from Dr. Wendy Macklin (University of Colorado, Denver). Mice were bred in‐house at the University of California, Los Angeles (UCLA) animal facility. All procedures were conducted in accordance with the National Institutes of Health (NIH) and were approved by the Institutional Guide for the Care and Use of Laboratory Animals Committee of UCLA. All mice were at 8–10 weeks of age prior to the start of cuprizone treatment.
GDX and hormone treatment
GDXies were carried out in 7‐week‐old animals. Ovariectomy (OVX) and castration (CST) were performed as described in 34. Sham animals underwent the same procedure, except sutures were not placed on the fallopian tubes and the ovaries remained intact in females and the testes were neither sutured nor removed in males. Sixty‐day release pellets of 5 mg/kg/day 5α‐dihydrotestosterone (DHT) or 5 mg/kg/day T or 0.025 mg/kg/day E2 as well as placebo (plac) pellets containing the carrier binder (cholesterol‐methyl cellulose‐α‐lactulose) alone were purchased from Innovative Research of America (Sarasota, FL, USA). Following anesthesia via isoflurane inhalation, pellets were implanted subcutaneously in the scapular area of the neck using a trocar 15 4 days after GDX and 3 days before initiation of cuprizone diet.
Diet
Female and male mice were fed 0.2% cuprizone (biscyclohexanone oxaldihydrazone; Sigma‐Aldrich, St. Louis, MO, USA) milled with chow (Harlan Teklad 2918, Madison, WI, USA) as previously described 11. To study cuprizone diet‐induced demyelination, male and female animals were fed cuprizone‐milled chow for 3 weeks (3 weeks; DM). Next, groups of mice were returned to a normal chow diet for 3 weeks to analyze remyelination (3 + 3 weeks; RM). DM and RM conditions were always compared to age‐ and sex‐matched control mice on normal diet (normal myelination; NM). At 3 weeks and 6 weeks time points, NM animals showed no difference as a result, these mice were pooled into one group. A treatment timeline is shown below.
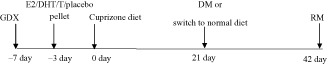
Treatment conditions and animal groups (Tables 1 and 2)
Table 1.
Gonadally intact female mice were subjected to sham (Intact/sham) or ovariectomy (OVX) procedure. OVXed animals were either implanted with placebo (OVX + placebo) or estradiol hormone pellets (OVX + E2). These 3 sets of mice were split into normal myelination (NM), demyelination (DM), and remyelination (RM) groups
| Females | |||
|---|---|---|---|
| NM | Intact/sham | OVX + plac | OVX + E2 |
| DM | Intact/sham | OVX + plac | OVX + E2 |
| RM | Intact/sham | OVX + plac | OVX + E2 |
Table 2.
Gonadally intact male mice were subjected to sham (Intact/sham) or castration (CST) procedure. CST animals were either implanted with placebo (CST + placebo), dihydrotestosterone (CST + DHT), testosterone (CST + T), and estradiol (CST + E2). These 5 sets of mice were split into normal myelination (NM), demyelination (DM), and remyelination (RM) groups
| Males | |||||
|---|---|---|---|---|---|
| NM | Intact/sham | CST + plac | CST + DHT | CST + T | CST + E2 |
| DM | Intact/sham | CST + plac | CST + DHT | CST + T | CST + E2 |
| RM | Intact/sham | CST + plac | CST + DHT | CST + T | CST + E2 |
Number of mice
There were 10 animals per treatment group (4 animals for electrophysiology recording + 4 animals perfused for immunohistochemistry + 2 animals perfused for electron microscopy). Age‐ and sex‐matched controls were used with each experimental DM and RM group. The experiment was repeated at least twice.
Electrophysiological CAP recording procedures
As described previously 11, 12, mice were deeply anesthetized using isoflurane and decapitated. The brain was removed and sectioned into 400‐μm coronal sections while submersed in ice‐cold carbogen (95% O2 and 5% CO2) artificial cerebrospinal fluid (ACSF, in mM NaCl 124, KCl 5, NaH2PO4 1.25, NaHCO3 26, MgSO4 1.3, CaCl2 2, glucose 10; adjusted to pH 7) using a vibratome (Leica, Microsystems Inc., Buffalo Grove, IL, USA). Slices were incubated for at least 1 h in room temperature carbogen ACSF. Two to three slices per animal containing the hippocampus (plates 35–48, Paxinos and Franklin mouse brain atlas) were used for electrophysiology recordings of CAPs 17. The CC was stimulated at 1 mm from the midline using a tungsten bipolar electrode. Responses were measured 2 mm from the stimulating electrode with a 3 M NaCl‐filled glass micropipette recording electrode. Stimulation used for evoked CAPs was constant current stimulus‐isolated square wave pulses. For analyses of CAP amplitude, standardized input–output functions were generated for each slice by varying the intensity of stimulus pulses (200 μs duration, delivered at 0.2 Hz) in steps from approximately threshold level to an asymptotic maximum (0.3–4.0 mA) for the short latency negative CAP component. To enhance the signal‐to‐noise ratio, all quantitative electrophysiological analyses were conducted on waveforms, which were the average of four successive sweeps. Evoked callosal CAP field potentials were amplified and filtered using an Axopatch 200A amplifier (Molecular Devices, Sunnyvale, CA, USA). Waveforms were recorded and analyzed offline with Microcal Origin software (OriginLab Corporation, Northampton, MA, USA). The early component generated predominantly by relatively fast‐conducting myelinated axons (N1) between 1–2 ms, and a later occurring component reflecting mainly slower unmyelinated axons (N2) appeared at 3–6 ms. External application of the potassium blocking agent 4‐Aminopyridine (4‐AP), to the CC, potently increased the duration of N2, but did not significantly alter N1 35, consistent with a submyelin distribution of potassium channels and accessibility of the channels along unmyelinated axons to the 4‐AP. We specifically analyzed the N1 component (reflective of fast‐conducting myelinated axons) of the CAP recording as we were interested in the role of sex hormones on adult myelination and remyelination.
Histopathology, immunohistochemistry and electron microscopy (EM)
Paraformaldehyde‐perfused, cryoprotected and cryostat‐sectioned brain sections and sections from brain slices cut for electrophysiology (postfixed, cryoprotected and cut) were used for immunohistochemistry 11. CC‐containing brain slices corresponding to plates 40 to 50 in the mouse brain atlas of Franklin and Paxinos (2001) were examined by immunohistochemistry 11, 32, and various cell‐type‐specific antibodies were used. White matter immunostaining was enhanced by treating sections with 95% ethanol and 5% acetic acid for 15 minutes prior to permeabilization and blocking. To detect axons: anti‐neurofilament 200kD (NF200) (1:500, Millipore, Billerica, MA, USA, 1:1000, Sigma); astrocytes: Anti‐Glial Fibrillary Acidic Protein (GFAP) (1:1000, Chemicon, Billerica, MA, USA); OL progenitors (OLPs): anti‐oligodendrocyte transcription factor 2 (olig2) (1:500, Millipore); mature OLs: anti‐Pi isoform of glutathione‐S‐transferase (GST‐pi) or anti‐adenomatous polyposis coli (CC1) (1:1000, Chemicon; 1:1000, GenTex, Irvine, CA, USA); overall population of OLPs, pre‐OLs and fluorescent OLs: PLP_EGFP fluorescence; myelin: anti‐myelin basic protein (MBP; 1:1000, Millipore); leukocyte common antigen‐cluster of differentiation‐45 labels all leukocytes, including‐ T cells, microglia/macrophage/monocyte: anti‐CD45 (1:1000, PharMingen, La Jolla, CA, USA) antibodies were used. The second antibody step was performed by labeling with antibodies conjugated to TRITC and Cy5 (1:1000, Millipore). IgG‐control experiments were performed for all primary antibodies, and no staining was observed under these conditions. To assess the number of cells, a nuclear stain 4′, 6‐Diamidino‐2‐phenylindole (DAPI 2 ng/mL; Molecular Probes, Grand Island, NY, USA) was added for 15 minutes prior to final washes after secondary antibody addition. The sections were mounted on slides, allowed to dry and coverslipped in Fluoromount‐G (Fisher Scientific, Pittsburgh, PA, USA). For EM, paraformaldehyde and glutaraldehyde‐perfused brains were cut in half sagittally. The genu area of CC was identified under a dissecting microscope and 4‐mm2 blocks (from the mid CC up to one‐third of the splenium corresponding to the CC area of plates 40–50 from the atlas of Paxinos and Franklin) were carefully dissected, Epon embedded, stained with uranyl acetate‐lead citrate and analyzed as in previous studies 11.
Microscopy and quantification
Brain slices were immunostained, subjected to confocal fluorescence microscopy and quantified as previously described 11, 12. All images with CC1 and olig2/Ki67 staining were counted and graphed as counts/0.37 mm2 where the 0.37 mm2 corresponds to the area of the 40× image. MBP, CD45 and GFAP immunostained images were converted to grayscale and then analyzed by density measurement using ImageJ v1.41 http://rsb.info.nih.gov/ij/ 13.
Statistical analysis
A total of 18–22 sections per treatment group were immunostained and at least 14–16 sections per group were analyzed. To quantify electrophysiology results from each treatment group, recordings from 2–3 caudal slices (n = 4 animals; experiment repeated twice) for a total of 8–16 recordings were analyzed. For EM, 10 random caudal area fields per animal at x4800 and x14 000 were used to quantify the “g‐ratio”. Values are expressed as mean ± standard error of the mean (SEM). All statistical evaluations were performed by analysis of variance (ANOVA) with post hoc, independent pair wise analysis or followed by a Newman–Keuls post hoc analysis (Prism®, GraphPad, San Diego, CA). The group sizes used for all statistical comparisons of morphometric determinations were n = 6–8 subjects per experimental group. For EM analysis, n = 3–4 subjects/experimental group from two separate experiments were used.
Results
Callosal conduction studies: differences between gonadally intact male and female mice
To characterize the functional consequences of cuprizone diet‐induced demyelination and the effectiveness of remyelination during recovery from cuprizone diet‐induced demyelination, CAP electrophysiological measurements of the CC axons were performed 11, 26. Brain slices from gonadally intact male and female mice after 3 weeks of 0.2% cuprizone diet (demyelination condition; DM) and 3 weeks of cuprizone diet followed by 3 weeks of normal diet (remyelination condition; RM) were used to measure CAPs.
The callosal CAP has previously been characterized as a biphasic waveform with an early component generated predominantly by relatively fast‐conducting myelinated axons (N1) and a later occurring component reflecting mainly slower unmyelinated axons (N2; 11, 36). CAP stimulation results in a large stimulus artifact (Figure 1A av; 11). The stimulus artifact results from the generation of an electrical field which passes current directly through the bath solution instead of through the tissue slice. The artifact generally lasts less than 0.2 ms and is blocked beyond the dashed line and disregarded for the purpose of CAP analysis (Figure 1A ai,ii, and v). Normally, myelinated (NM) control recordings of male and female mice show distinct N1 and N2 (Figure 1A ai,ii). Peak N1 amplitude at 4 mA current stimulus was 0.80 ± 0.05 mV for males and 0.89 ± 0.03 mV for females. Similar to published results 11, 12, after 3 weeks of cuprizone diet a significant decrease in the fast‐conducting N1 amplitudes was observed (*P < 0.05, n = 18). There was a marked delay and an equal decrease in N2 peak amplitudes of males and females under the DM condition (Figure 1A ai,ii; quantification previously shown in Crawford et al 11, 12.
Figure 1.
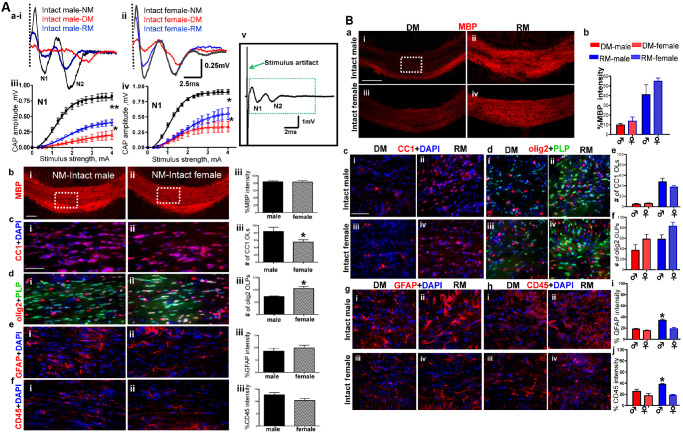
A. Corpus callosum (CC) compound action potential (CAP) recordings and immunohistochemistry reveal sex differences during normal myelination, demyelination and remyelination. (ai,ii) CAP responses were recorded from slices with midline‐crossing segments of the CC overlying the mid‐dorsal hippocampus. Typical CC CAPs from male and female C57BL/6 mice on a normal diet (NM: black), cuprizone diet (DM: red) and cuprizone diet followed by normal diet (RM: blue) evoked at the highest stimulus of 4 mA. A near complete loss of N1 in male and female DM groups followed by a significant recovery under RM was observed. (aiii,iv) Quantification of N1 amplitudes showed a significant decrease in all animals under DM and RM condition compared to NM. (av) Shown here is a typical evoked callosal CAP field potential showing the size of the stimulus artifact. It is generally blocked after 0.2 ms and is hidden to enhance the relatively fast‐conducting (N1) component that appears between 1–2 ms, and a later occurring component (N2) that appears at ∼3–6 ms. (bi,ii) Brain slices with caudal CC were immunostained with myelin basic protein (MBP‐red) and imaged at 10× magnification (scale bar = 100 μm). Consecutive brain sections were immunostained for various markers and imaged at 40× magnification in an area similar to the ones shown by the dashed squares in bi,ii (scale bar = 50 μm). (biii) Quantification of MBP fluorescence intensity normalized to 100% shows no sex difference. (ci–iii) There were more mature OLs (CC1‐red and counterstained for nuclei DAPI‐blue) in males than females. Females showed significantly fewer CC1+ OLs. (di–iii) Number of OLPs (olig2‐red and PLP_EGFP‐green) in the CC was significantly increased in female CC compared to males. Females show significantly more olig2+ OLPs. (ei–iii and fi–iii) The number of reactive astrocytes (GFAP‐red) and microglia (CD45‐red counterstained with DAPI‐blue) did not change between males and females (*P < 0.05; **P < 0.001 compared to NM; 2–4 mA stimulus strength; n = 4 to 8). B. Cuprizone diet‐induced demyelination and remyelination show similar pathologies in the CC of gonadally intact males and females. (ai–iv) Immunohistochemistry images of the CC from males and females during DM and RM condition show no difference in myelin basic protein (MBP‐red) immunostained CC (imaged at 10× magnification, scale bar = 100 μm). (b) MBP fluorescence intensity decreases during DM and recovers during RM comparably in males and females. Quantification of MBP fluorescence intensity normalized to 100% showed no sex difference. (ci–iv, di–iv, e, f) Consecutive brain sections were immunostained and imaged at 40× magnification in an area similar to that of the dashed square in c–i. OL cell (CC1‐red, DAPI‐blue) counts show severe loss of OLs during DM and similar degree of recovery during RM in both males and females. Further, males and females show comparable loss of OLPs (olig2‐red and PLP_EGFP‐green) during DM and recovery during RM (gi–iv and fi–iv). Increased fluorescence intensity of GFAP (red) and CD45 (red) indicate significantly more reactive astrocyte and activated microglia in both sexes during the DM vs. NM condition. These values continue to increase during RM in males, but remain at DM levels in females, indicating a continually elevated secondary immune response in males during RM (*P < 0.05; n = 8).
When animals were switched from the cuprizone diet to a normal diet, they underwent significant remyelination (Figure 1A ai,ii). The male N1 CAP component under RM conditions recovered to only 50% of peak N1 obtained under NM conditions (**P < 0.001, n = 12). The female N1 CAP component under RM conditions recovered to nearly 70% of N1 obtained under NM conditions (*P < 0.05, n = 12). N2 peak delay was reversed with significant improvement of amplitude in both male and female mice (Figure 1A ai,ii).
Overall, the CAP amplitude of myelinated callosal axons during normal myelination was similar in male and female CC. Demyelination‐induced decreases in N1 component of callosal CAPs were more pronounced in the male group as compared to the female group. Similarly, remyelination‐induced recovery in males was less efficient than in females.
Immunohistochemical differences between male and female mice
To identify the causes of potential sex differences in callosal conduction, CC‐containing brain slices were immunostained to identify the causes of potential sex differences in callosal conduction. Brain slices from all groups were examined for MBP, mature OLs, OLPs, astrocytes and microglia (Figure 1A b–f, B a–j). Callosal myelin integrity, as assessed by MBP staining intensity, showed no significant sex difference under the NM condition (Figure 1A b). Counting OL lineage cell numbers revealed a significant sex difference in the number of OL cells. Male CC had significantly more OLs (P < 0.5; n = 12) and significantly fewer OLPs (P < 0.5; n = 12) compared to female CC (Figure 1A b,c). Astrocyte and microglia immunoreactivity were similar in both male and female CC (Figure 1A e,f).
Three weeks of cuprizone diet induced a significant level of demyelination in the CC. Male and female DM groups showed significant demyelination‐induced decreases in MBP immunoreactivity (Figure 1B). A significant decrease in number of OLs and a slight increase in number of OLPs were observed under DM conditions for both sexes. In addition, a sustained increase in GFAP+ reactivity in astrocytes with larger processes and enlarged cell body was observed as compared to normal group, in which the GFAP+ cells were smaller and had less GFAP+ immunoreactivity. Similarly, CD45+ cells in the normal group had weaker immunoreactivity as compared to DM and RM condition where CD45+ cells had increased immunoreactivity and a globoid‐macrophagic appearance indicative of activation (Figure 1B). An increase in reactive astrocytes and activated microglia was observed during demyelination, but with no significant sex difference. When animals were switched from cuprizone diet to normal diet, a significant increase in MBP fluorescence intensity was observed. The increase in MBP staining intensity was due to an increase in axon myelination, which was due to an increase in mature OL population after cuprizone withdrawal (Figure 1B). No significant sex difference was observed in MBP intensity and number of OL lineage cells during RM condition (Figure 1B c–f). Males under the remyelinating condition had an even larger increase in astrocyte and microglia immunoreactivity staining intensity as compared to females under normal and demyelinating conditions (P < 0.05; Figure 1B g–j).
Circulating sex hormones and myelination: effect of OVX
To determine if the circulating hormones could influence the sex differences observed in CC CAPs and cell numbers, age‐matched female and male mice were gonadectomized (GDX) and subjected to the cuprizone diet‐induced demyelination and subsequent normal diet paradigm. Ovariectomized (OVX) females will control for estrogen production by ovaries in females and the castrated (CST) males will control for the effects of endogenous androgen production by the testes.
A small but significant decrease in callosal conduction (both N1 and N2 peaks) was observed in ovariectomized females as compared to intact females during NM conditions (compare Figures 1A aiii,iv and 2A ai,ii). Specifically, peak N1 amplitude at 4 mA current stimulus was 0.72 ± 0.03 mV for the ovariectomized females and 0.89 ± 0.05 mV for the gonadally intact females. Demyelination induced a significant decrease in N1 amplitude: 0.11 ± 0.01 mV for ovariectomized group as compared to 0.32 ± 0.01 mV for gonadally intact females. RM‐induced N1 amplitude recovery was significantly attenuated in ovariectomized females (0.30 ± 0.02 mV) compared to gonadally intact group of 0.60 ± 0.10 mV (compare Figures 1A aiii, iv and 2A ai, ii).
Figure 2.
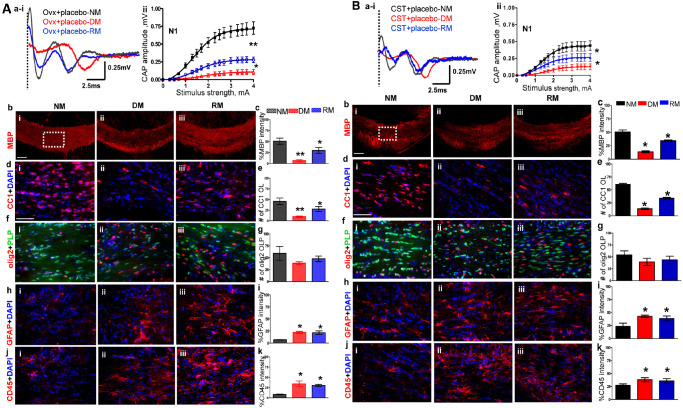
A. Ovariectomy (OVX) increases severity of demyelination and reduces efficiency of remyelination in females. (ai,ii) CAP responses for OVX females during NM (black), DM (red) and RM (blue). Representative traces for CAP response to 4.0 mA stimulus in OVX females induced a significant decrease in N1 peak amplitude during NM, DM and RM as compared to gonadally intact female mice. Quantification of N1 CAP amplitude shows a significant decrease in peak N1 CAP amplitude during DM with incomplete recovery during RM. DM is more severe, and RM is less efficient in OVX females compared with gonadally intact females. (bi–iii, c) MBP immunoreactivity imaged at 10× from the CC under NM, DM and RM condition showed a significant decrease during DM and a significant recovery during RM. (di–iii, e) Consecutive brain sections were immunostained and imaged at 40× magnification from various groups in an area similar to that of the dashed square in bi. OLs stained with CC1 (red) and counterstained for nuclei with DAPI (blue). Cell loss is significant during DM with partial but significant recovery during RM. (fi–iii, g) OLPs expressing PLP_EGFP and stained with olig2 (red) show nonsignificant trends of decrease during DM and recovery during RM. Cell counts are significantly lower than gonadally intact counterparts. (hi–iii, i) Reactive astrocytes stained with GFAP (red) and counterstained for nuclei with DAPI (blue) show a significant increase in intensity that persists in DM and RM. (ji–iii, k) Microglia stained with CD45 (red) and counterstained with DAPI (blue) show a significant increase in immunoreactivity that persists during DM and RM (*P < 0.05; n = 6). B. Castration increases the severity of demyelination and reduces efficiency of remyelination in males. (ai–iii) CAP responses for castrated (CSTed) males during NM (black), DM (red) and RM (blue). Representative traces for CAP response to 4.0 mA stimulus in CSTed mice induced a significant decrease in N1 peak amplitude during NM, DM and RM as compared to gonadally intact mice. Quantification of N1 CAP amplitude shows severe N1 signal loss during DM with incomplete recovery during RM. CSTed mice showed a more severe DM‐induced decrease and less efficient RM‐induced increase in N1 amplitude as compared to gonadally intact males. (bi–iii, c) MBP levels were assessed by intensity changes in MBP (red) immunoreactivity during NM, DM and RM. MBP immunoreactivity imaged at 10× from the CC under NM, DM and RM. Quantification of MBP intensity showed a significant decrease during DM in CST males, with significantly less efficient recovery during RM as compared to gonadally intact males. (di–iii, e) Consecutive brain sections were immunostained and imaged at 40× magnification from various groups in an area similar to that of the dashed square in bi. Mature OLs stained with CC1 (red) and counterstained with DAPI (blue) showed a significant loss of cells during DM with partial but significant recovery during RM. (fi–iii, g) OLPs stained with olig2 (red) and PLP_EGFP (green) cells show nonsignificant trends of decrease during DM and recovery during RM. (hi–iii, i) GFAP+ astrocytes (red) counterstained with DAPI (blue) show a significant increase in intensity that persists in DM and RM conditions. (ji–iii, k) CD45+ microglia (red) counterstained with DAPI (blue) showed a significant increase in fluorescence intensity during DM that persisted during RM. in CST + placebo males as compared to gonadally intact mice (*P < 0.05; n = 8).
Callosal myelin staining intensity in ovariectomized females as compared to gonadally intact females was significantly decreased during NM, DM and RM conditions as compared to gonadally intact females (Figures 1A b,c, B a,b and 2A b,c). Callosal OL numbers in ovariectomized females during NM and DM were not significantly different as compared to gonadally intact females. Under the RM conditions, the ovariectomized group had significantly fewer CC1+ OLs as compared to gonadally intact animals (Figure 2A d,e). The numbers of olig2+ cells from the ovariectomized CC were significantly decreased under NM, DM and RM conditions as compared to gonadally intact females (Figure 2A f,g). Similar to gonadally intact females, microglia and astrocyte staining intensity were upregulated under RM and DM conditions in ovariectomized females (Figure 2A h–k).
Circulating sex hormones and myelination: effect of CST
Callosal conduction in CSTed mice during normal myelination was significantly decreased compared to gonadally intact male groups (compare Figures 1A i–iii and 2B ai,ii). Specifically, peak N1 amplitude at 4 mA current stimulus was 0.50 ± 0.03 mV for castrated vs. 0.80 ± 0.02 mV for gonadally intact males. Demyelination induced a significant decrease in N1 amplitude: 0.13 ± 0.01 mV for CSTed males as compared to 0.23 ± 0.02 mV for gonadally intact males. The CSTed group's remyelination‐induced N1 amplitude recovery of 0.27 ± 0.02 mV was also significantly attenuated compared to the gonadally intact group's N1 of 0.40 ± 0.03 mV.
Callosal myelin staining intensity was significantly decreased in CSTed animals under NM, DM and RM condition as compared to gonadally intact animals (Figures 1B a,b and 2B b,c). Callosal CC1+ OL numbers in NM, DM and RM groups of CSTed animals were significantly decreased compared to corresponding gonadally intact animals. In addition, olig2+ OLPs did not recover in the RM condition and were significantly decreased as compared to gonadally intact males (Figures 1B c–f and 2B d,e). Microglia and astrocyte immunostaining intensity of CSTed animals were significantly upregulated under NM, RM and DM conditions compared to gonadally intact males (Figures 1B g–j and 2B h–k).
Circulating sex hormones and myelination: OVX plus estradiol treatment
To investigate the role of circulating estrogen on callosal conduction estrogen in the form of a time release estradiol (E2) pellet was replaced in OVX females (OVX + E2). Callosal conduction of OVX + E2 mice during normal myelination and remyelination was significantly increased compared to OVX + placebo and gonadally intact females (compare Figures 1A aii–iv, 2A ai,ii and 3ai,ii). Peak N1 amplitude at 4 mA current stimulus was 1.20 ± 0.10 mV for OVX + E2 animals, 0.72 ± 0.03 mV for OVX + placebo animals and 0.89 ± 0.13 mV for gonadally intact females. Three‐week cuprizone diet in OVX + E2 animals resulted in significantly less demyelination‐induced decrease in N1 CAPs. Peak N1 amplitude was 0.50 ± 0.13 mV, nearly twice as much as for the OVX + placebo group, under DM conditions (Figure 3ai,ii). RM conditions in the OVX + E2 group induced a robust N1 peak amplitude of 1.0 ± 0.12 mV, which was significantly more than those of the OVX + placebo (0.30 ± 0.02 mV) and gonadally intact groups (0.60 ± 0.10 mV; P < 0.05, n = 6–12). Immunohistochemical analysis of callosal MBP staining intensity and OL and OLP numbers in OVX + E2 animals were significantly increased compared to OVX + placebo animals under NM, DM and RM conditions (Figure 3b–g). Presence of estradiol in ovariectomized animals did not change the activation or numbers of microglia and astrocyte staining compared to placebo‐treated ovariectomized animals (Figure 3h–k).
Figure 3.
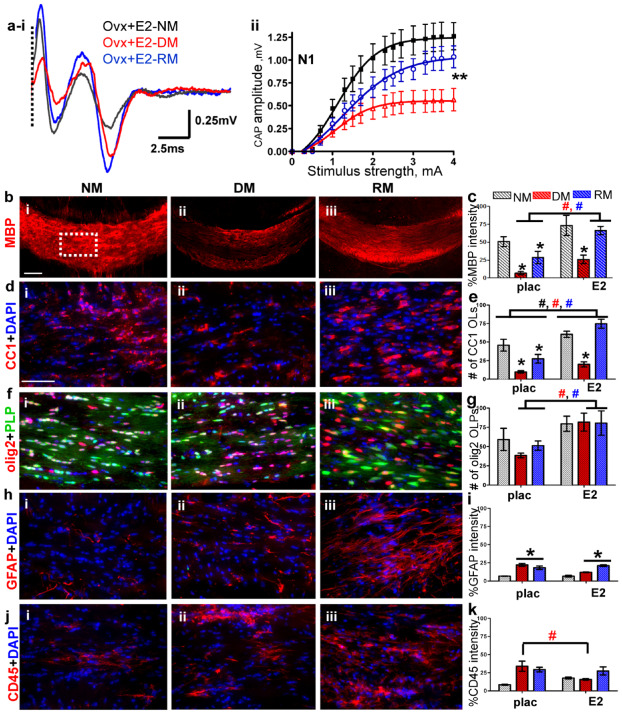
Estradiol (E2) hormone supplementation improves normal myelination, partially ameliorates demyelination and accelerates remyelination. (ai–ii) CAP responses for E2‐administered ovariectomized (OVX) females under NM (black), DM (red) and RM (blue) conditions. Representative traces for CAP response to 4.0 mA stimulus induced a significant increase in N1 peak amplitude during NM, DM and RM. Quantification of N1 CAP amplitudes reveals a significant increase in N1 peak amplitudes during NM, DM and RM as compared to placebo‐administered OVX and gonadally intact females. (bi–iii, c) Increased MBP (red) staining intensity during NM, DM and RM condition in OVX + E2 was observed as compared to OVX + placebo mice. (di–iii, e) Consecutive brain sections were immunostained for various markers and imaged at 40× magnification in an area similar to the one shown by the dashed squares in bi. Increased numbers of CC1+ OLs (red) counterstained with DAPI (blue) were present in CC of OVX + E2 mice compared to OVX + placebo mice. (fi–iii, g) Cells expressing PLP_EGFP and co‐stained with olig2 (red) had a sustained increase under NM, DM and RM condition as compared to OVX + E2 mice. (hi–iii, i) GFAP+ astrocytes (red) and counterstained with DAPI (blue) expressed increased fluorescence during DM and RM condition, but were similar to OVX + placebo group. (ji–iii, k) CD45+ (red) microglia counterstained with DAPI (blue) revealed similar levels of fluorescence intensity during DM and RM in OVX + E2 and OVX + placebo groups (*P < 0.05; n = 8–12); (ANOVA; #P < 0.5, n = 6–8; colors represent significance for NM, DM or RM).
Circulating sex hormones and myelination: CST plus DHT, T and estradiol treatment
To address the effect of circulating testosterone (T) on callosal myelination without the potential confounding effect of its conversion to estradiol, non‐aromatizing dihydrotestosterone (DHT) was used in CSTed animals. A small, but significant improvement was observed in the N1 component of callosal CAP conduction of the CST + DHT group as compared to the CST + placebo group during NM and RM conditions. No effect of DHT was observed during DM conditions (compare Figures 2B ai,ii and 4A ai,ii). Peak N1 amplitude during the NM condition at 4 mA current stimulus was 0.56 ± 0.03 mV for the CST + DHT vs. 0.50 ± 0.02 mV for the CST + placebo group. Remyelination‐induced N1 amplitude in CST + DHT group under the RM condition was 0.40 ± 0.02 mV compared to 0.30 ± 0.03 mV in the CST + placebo group.
Figure 4.
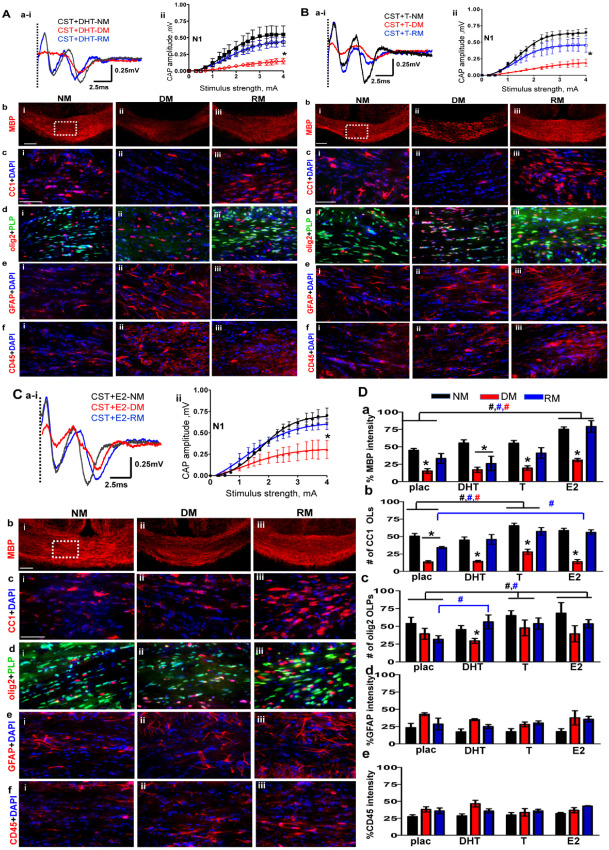
A. Non‐aromatizing hormone dihydrotestosterone (DHT) supplementation in castrated (CST) males does not affect normal myelination, demyelination or remyelination. (ai–ii) CAP responses for DHT‐administered CST males under NM (black), DM (red) and RM (blue) condition. Representative traces for CAP response to 4.0 mA stimulus induced a small increase in N1 peak amplitude during NM and RM condition as compared to placebo‐treated CST animals. N1 amplitude was significantly attenuated during DM in CST + DHT similar to CST + placebo groups. Quantification of N1 CAP amplitudes reveals small but significant increase in N1 peak amplitudes during NM and RM compared to CST + placebo mice. (bi–iii) MBP (red) immunostaining during NM, DM and RM in the CST + DHT group showed a significant decrease during DM and an increase during RM, but these changes were similar to those seen in the CST + placebo group. (ci–iii) Consecutive brain sections were immunostained and imaged at 40× magnification from various groups in an area similar to that of the dashed square in bi. During DM condition, a significant decrease and during RM, a significant recovery in CC1+ OLs‐red counterstained with DAPI‐blue was observed in both CST + DHT and CST + placebo groups. (di–iii) CST + DHT group showed an increase in OLPs (PLP_EGFP‐green + olig2‐red) during RM condition as compared to CST + placebo group. (ei–iii, fi–iii) GFAP+ astrocyte (red) and CD45+ microglia (red) counterstained with DAPI (blue) showed similar fluorescence intensity increases in DM and RM condition and were similar to CST + placebo mice (*P < 0.05; n = 10). B. Hormone replacement with testosterone (T) partially reverses oligodendrocyte cell loss and callosal conduction associated with demyelination and accelerates recovery during remyelination of CST males. (ai,ii) CAP responses for T‐administered CST males under NM (black), DM (red) and RM (blue) conditions. Representative traces for CAP response to 4.0 mA stimulus induced a significant increase in N1 peak amplitude during NM and RM condition as compared to placebo‐treated CST animals. Quantification of N1 CAP amplitudes reveals a significant increase in N1 peak amplitudes of T‐administered group under NM and RM condition as compared to CST + placebo males. N1 amplitude was significantly attenuated during DM in CST + T similar to CST + placebo groups. (bi–iii) MBP immunostaining intensity in CST + T mice was significantly decreased during DM but recovered during RM condition similar to CST + placebo mice. (ci–iii) Consecutive brain sections were immunostained and imaged at 40× magnification from various groups in an area similar to that of the dashed square in bi. A significant increase in OLs (CC1‐red+DAPI‐blue) under NM, DM and RM in the CST + T group was observed. The number of OLs was significantly increased as compared to CST + placebo group. (di–iii) There was a significant increase in OLPs (PLP_EGFP‐green + olig2‐red) in CST + E2 group under NM, DM and RM conditions as compared to CST + placebo mice. (ei–iii, fi–iii) GFAP+ astrocytes (red) and CD45+ microglia (red) counterstained for nuclei with DAPI (blue) showed similar increases in fluorescence intensity during DM and RM conditions similar to CST + placebo mice (*P < 0.05; n = 8). C. Hormone replacement with estradiol (E2) partially reverses OL cell loss associated with demyelination and accelerates recovery during remyelination of CST males. (ai–ii) CAP responses for E2‐administered CST males under NM (black), DM (red) and RM (blue) condition. Representative traces for CAP response to 4.0 mA stimulus induced a significant increase in N1 peak amplitude during NM and RM condition as compared to placebo‐treated CST animals. N1 amplitude was significantly attenuated during DM in CST + E2 similar to CST + placebo groups. Quantification of N1 CAP amplitudes reveals a significant increase in N1 peak amplitudes of E2‐administered group under NM, DM and RM condition as compared to CST + placebo males. (bi–iii) MBP (red) immunostaining intensity was significantly increased for CST + E2 under NM, DM and RM condition as compared to CST + placebo. (ci–iii) OLs stained with CC1 (red) and counterstained with DAPI (blue) showed significant cell loss during DM and significant recovery during RM. CST + E2 group had a significant increase in CC1+ OLs under NM and RM condition as compared to CST + placebo group. (di–iii) There was a significant increase in olig2+ OLPs (PLP_EGFP‐green + olig2‐red) with E2 supplementation under NM, DM and RM condition as compared to CST + placebo groups. (ei–iii) GFAP+ astrocytes (red) and CD45+ microglia (red) showed increased staining intensity under DM and RM condition that was similar to CST + placebo mice. (*P < 0.05; n = 8). D. Summary of results of (from left to right) placebo‐, DHT‐, T‐ and E2‐ pellet implanted castrated animals during normal myelination, demyelination and remyelination. (a) MBP fluorescence intensity showing similar decreases during DM and increases during RM conditions within placebo‐, DHT‐, T‐ and E2‐supplemented CST groups. E2 supplement was the only hormone to increase fluorescence intensity significantly for NM, DM and RM condition in CST + E2 as compared to CST + placebo. E2 supplementation circumvents the negative aspect of castration on MBP staining intensity under all conditions. (b) More so, under RM conditions, CC1+ OL cell count per area was increased in T‐ and E2‐implanted but not DHT‐implanted castrated animals. (c) Similarly, under RM conditions, olig2+ OLPs showed a significant increase in cell count per area under all hormone replacement paradigms as compared to CST + placebo. Moreover, T and E2 supplement supported elevated OLP and OL cell counts during NM, pointing to a direct hormonal role in OLP proliferation. (d,e) GFAP fluorescence intensity and CD45 fluorescence intensity, normalized to 100%, shows equal reactivity and did not change with hormone replacement (t‐test *P < 0.05 n = 8–12; ANOVA, #P < 0.5, n = 6–8; colors represent significance for NM, DM or RM).
Under the NM condition, the CST + DHT group showed a small, but nonsignificant improvement in callosal myelin staining by MBP as compared to the CST + placebo group. No significant difference was observed in DM and RM conditions between DHT and placebo‐treated castrated animals (Figure 4A,D). In addition, DHT supplementation did not lead to significant changes in callosal OL numbers under NM, DM and RM conditions (Figure 4A,D). There was a significant improvement in olig2+ OLPs only during the RM condition (Figure 4A,D). No significant difference was observed in microglia and astrocyte staining intensity in NM, RM and DM conditions in CST + DHT compared to CST + placebo groups (Figure 4A,D).
Next, the effect of T on callosal conduction in CSTed male mice was tested. The CST + T group showed a significant improvement in the N1 component of callosal CAP conduction during the NM condition as compared to the CST + placebo group (compare Figures 2B ai,ii and 4B ai,ii). Peak N1 amplitude during the NM condition at 4 mA current stimulus was 0.65 ± 0.02 mV for CST + T vs. 0.50 ± 0.02 mV for CST + placebo males. Demyelination in the CST + T group was similar to that of CST + placebo animals. N1 CAP amplitudes during DM in CST + T and CST + placebo groups were similar. Remyelination‐induced N1 amplitude recovery was 0.40 ± 0.01 mV, which was more than the CST + placebo group's peak amplitude of 0.30 ± 0.03 mV.
Callosal myelin staining of CST + T animals showed a small improvement compared to CST + placebo animals during NM. No significant difference was observed in DM and RM conditions between T‐ and placebo‐treated CSTed animals (Figures 2B and 4B). Significant improvement in callosal OL numbers under DM and RM condition was observed (Figure 4B,D). There was a significant improvement in olig2+ OLPs during NM, DM and RM condition with T supplementation compared to the placebo alone (Figure 4B,D). No significant difference was observed in microglia and astrocyte staining intensity in NM, RM and DM groups of CST + T groups compared to CST + placebo groups (Figure 4B,D).
The more robust improvement of callosal conduction with T could be due to its aromatization to estradiol. To test the effect of circulating estradiol hormone, male CSTed animals were implanted with estradiol pellets (CST + E2). CST + E2 animals under NM, DM and RM conditions showed a significant improvement in the N1 CAP as compared to the CST + placebo group (compare Figures 2B ai,ii and 4C ai,ii). Peak N1 amplitude during the NM condition at 4 mA current stimulus was 0.70 ± 0.02 mV for the CST + E2 group vs. 0.50 ± 0.02 mV for the CST + placebo group. N1 CAP amplitudes under the DM condition for the CST + E2 group were 0.32 ± 0.06 mV vs. 0.20 ± 0.02 mV for the CST + placebo group. Remyelination‐induced N1 amplitude recovery for the CST + E2 group was 0.62 ± 0.04 mV, which was more than the CST + placebo group's peak amplitude of 0.30 ± 0.03 mV.
Callosal myelin staining of CST + E2 animals was significantly increased in NM, DM and RM conditions as compared to CST + placebo groups (Figures 2B and 4C,D). Significant improvement in callosal OL numbers under the RM condition was observed (Figures 2B and 4C,D). There was a significant improvement in olig2+ OLPs during NM, DM and RM conditions in the CST + E2 as compared to the CST + placebo group (Figures 2B and 4C,D). No significant difference was observed in microglia and astrocyte staining intensity under NM, DM and RM conditions for the CST + E2 groups compared to the CST + placebo groups (Figure 4D d,e).
Ultrastructure analysis of callosal axons
Finally, to quantify the effect of sex hormones on normal myelination, demyelination and remyelination, g‐ratios (diameter of axon/diameter of axon plus myelin sheath) of callosal axons were calculated. High magnification electron micrographs of intact females and males under the normal condition did not show significant differences in numbers of myelinated and non‐myelinated axons (Figure 5A a). Quantification of g‐ratio under NM (0.79 ± 0.01), DM (0.91 ± 0.01) and RM (0.81 ± 0.01) conditions for intact females were similar to those of intact males under NM (0.79 ± 0.01), DM (0.93 ± 0.02) and RM (0.81 ± 0.02) conditions (Figure 5A b).
Figure 5.

A. Similar g‐ratios in callosal axons of male and female groups. To quantify effects of sex hormones on normal myelination, demyelination and remyelination, g‐ratios (diameter of axon/diameter of axon + myelin sheath) were measured. Electron micrographs of CC axons from normal myelination condition of gonadally intact females (ai) and males (aii) are shown. Calculated g‐ratio of intact male and female from NM, DM and RM groups show a significant increase in g‐ratio during the demyelination phase and a significant recovery during the remyelinating phase (b). B. Effect of OVX and estradiol (E2) replacement on callosal axon myelination. The effect of OVX and estradiol add‐back is represented by electron micrographs of CC axons from normal myelination groups. OVX significantly increased the number of thinly myelinated and non‐myelinated axons as compared to gonadally intact groups (ai,ii). OVX animals implanted with estradiol significantly increased the number of myelinated axons (bi,ii). Calculated g‐ratio of OVX alone and OVX + E2 callosal axons female from NM, DM and RM, group show a significant increase in g‐ratio during the demyelination phase and a significant recovery during the remyelination phase (c). Callosal axons from OVX + E2 groups during normal myelination and remyelination showed a significant decrease in g‐ratios as compared to OVX + placebo. C. Effect of castration and hormone replacement on callosal axon myelination. Castration also induced an increase in unmyelinated axons (ai,ii). Dihydrotestosterone (DHT), testosterone (T) and E2 pellets implanted in castrated males showed variable effects on recovery of axon myelination (a–d). There were less unmyelinated axons in castrated groups treated with either T or E2, but not DHT, which were similar to castrated animals implanted with placebo. Quantification of myelinated and non‐myelinated axons during cuprizone diet‐induced demyelination and subsequent remyelination showed a significant difference between groups. T and E2 implanted animals had more myelinated axons compared to placebo‐ and DHT‐implanted groups (t‐test *P < 0.05; n = 3, ANOVA; #P < 0.5; colors represent significance for NM, DM or RM. Images are at taken at 4800× (i) and 7200× (ii) and the scale bar is 1 μm.
OVX increased the number of thinly myelinated and demyelinated callosal axons, but this was reversed in OVX + E2 animals (Figure 5B a,b). The calculated g‐ratio of the OVX + placebo group under NM (0.83 ± 0.01) and RM (0.85 ± 0.01) conditions were significantly different from OVX + E2 callosal axons under NM (0.80 ± 0.02) and RM (0.83 ± 0.01) conditions, but this was not the case under the DM condition (0.91 ± 0.02 and 0.90 ± 0.03 respectively; Figure 5B c).
CST in males also induced an increase in unmyelinated axons (Figure 5C ai, aii). DHT, T, estradiol (E2) pellet implantation in castrated males showed variable effects (Figure 5C a–d). There were less unmyelinated axons in castrated groups treated with either T or E2 but not DHT. CST + DHT males were similar to placebo‐administered CSTed animals. Quantification of myelinated and non‐myelinated axons during cuprizone diet‐induced demyelination and subsequent normal diet remyelination showed a significant difference between groups. Placebo‐implanted NM (0.84 ± 0.01), DM (0.93 ± 0.05), and RM (0.86 ± 0.02) and DHT‐implanted NM (0.83 ± 0.01), DM (0.93 ± 0.05), and RM (0.84 ± 0.01)‐castrated groups had similar g‐ratios. However, T‐implanted NM (0.81 ± 0.02), and RM (0.82 ± 0.03) groups and E2‐implanted NM (0.80 ± 0.01 and RM (0.81 ± 0.001) CSTed groups were significantly different from the placebo‐treated CSTed groups (t‐test *P < 0.05, n = 3, ANOVA, #P < 0.05).
Discussion
Though sex hormones are mostly known for their role in sex organ development and physical maturation during puberty, the brain is a prominent target for sex steroid hormones. Sex steroids operate as trophic factors affecting brain development and plasticity. The role of sex hormones in myelination has been speculated but not established. Direct impact of sex hormones on glial cells can influence axon myelination (for review, see 18, 30). In 1966, Curry and Heim reported that estradiol administration causes an increase in myelination of neonatal rat brain 14. Many studies performed with OLP/OL cell culture have shown increased yields of OLPs in female cultures 27. Progesterone presence also increases the number of OLs, formation of myelin sheaths and synthesis of myelin proteins 1. T can induce OL differentiation and amplify excitotoxic damage of OLs acting via activation of AMPA/kainate receptors 7, 27. In vivo analysis of rodent brain and spinal cord has shown an increase in number of OL in males compared to females 9. Overall, sex steroids have been shown to influence OL development and axon myelination, and they have the potential to affect the processes of demyelination, remyelination and axonal damage.
Similar to previous studies 8, we also found sex differences in number of OLP/OLs, where increased number of OLs in the male CC and proliferating OLPs in the female CC were observed. We hypothesized that the differential OL/OLP numbers in males and females will contribute to differences in axon myelination and axon conduction. Instead, neither increases in myelinated callosal axons nor increase in callosal conduction were observed in males compared to females. The potential reason for different number of OL and OLPs in male and females could be strictly due to a difference in the environment (including hormone differences) and/or the rate of proliferation and differentiation of the cells as speculated earlier 8. These differences could be pivotal in establishing the differences in the rates of demyelination during disease and remyelination during repair. In support of this conclusion, cuprizone diet‐induced demyelination caused a more severe decrease of callosal conduction in males as compared to females with no significant differences in myelin density. Similarly, the remyelination paradigm induced less callosal conduction recovery in males as compared to females, without changes in myelin density. The difference in number of OLPs and OLs in male and females during demyelination and remyelination were not significant. Whether differentiating OLPs in the female brain are responsible for more efficient remyelination than in males, or whether fewer differentiating OLPs in the male brain lead to inefficient remyelination is unclear. We did not find a correlation to support the hypothesis. Surprisingly, the only difference in immunoreactivity between males and females was increased number of activated microglia and astrocytes in males vs. females during remyelination. These activated astrocytes and microglia may be responsible for increased production of cytotoxic factors, including proinflammatory cytokines and reactive oxygen intermediates, and this could be one of the reasons for decreased remyelination in males vs. females.
GDX induced a significant decrease in OLP/OL numbers, number of myelinated axons and axon conduction during all myelinating conditions confirming the positive role of sex hormones in axon myelination. GDX effects on normal myelination, demyelination and remyelination were more severe in males as compared to females.
Hormone replacement by estradiol administration in ovariectomized females caused an overall increase in OLP/OL numbers, axon myelination and axon conduction as compared to placebo‐administered ovariectomized females under all myelinating conditions. E2 pellets at 0.025 mg/kg/day are known to induce a continuous ∼10–25 pg/mL E2 serum level 2, 6, 33 compared to 2–5 pg in ovariectomized mice implanted a with placebo pellet 33. The E2 concentration of 20–30 pg/mL serum level with the 0.025 mg pellet in OVX females is near the diestrus levels and is not “>100 pg/mL E2 neuroprotective serum levels” that has been used in various disease models 2, 32. It is to be noted that with the implanted E2 pellet, E2 release is continuous and not cyclical, in contrast to gonadally intact females that undergo regular estrous cycle. Steady E2 levels may have a more potent and dramatic effect on OL lineage cell and myelination as the OVX + E2 group showed greater myelination, and conduction than gonadally intact females during NM, DM and RM. Similar increases in OLPs and number of myelinated axons were observed in pregnant mice with a sustained increase in estrogens 19.
Hormone replacement studies in CSTed males were more complex as CST decreases circulating T levels. T can influence myelination by activating androgen receptors, and estradiol receptors following aromatization. Unlike OVXed females, which were only implanted with estradiol, CST males were implanted with DHT, T or estradiol pellets. First, to assess the effect of T on myelination and axon conduction not mediated by estradiol, DHT was favored as it originates via irreversible reduction of T by 5alpha‐reductase enzyme. DHT pellets at 5 mg/kg/day induce a continuous ∼1.4 ng/mL 5 serum level compared to 0.2 ng/mL in castrated mice with placebo pellet and ∼1.5 ng/mL in intact or sham surgery males 6. Second, we used T to mimic more physiological conditions. T pellets at 5 mg induce a continuous ∼2 ng/mL 6 compared to less than 0.1 ng/mL in placebo pellet‐implanted CST mice and ∼2 ng/mL serum level in intact or sham surgery males 6. Lastly, estradiol was used to understand the extent of estradiol effect in males because of aromatization of T to estradiol. Estradiol pellets at 0.025 mg/kg/day potentially induce a continuous 20–30 pg/mL 16, 22 serum level compared to 10–20 pg/mL in placebo‐implanted CST mice and 10–20 pg/mL in intact or sham surgery males 5, 22.
The effect of hormone replacement in castrated males was surprising. Instead of finding increased callosal myelination and conduction with DHT, compared to other hormones, estradiol = T > DHT improved callosal conduction during normal myelination. The same order also decreased the demyelination extent and significantly improved remyelination as compared to placebo‐administered castrated groups. Even though, estradiol > T induced a significant increase in myelin staining intensity during remyelination, T > estradiol > DHT induced an increase in number of mature OLs during NM and RM, whereas estradiol > T > DHT induced a dramatic increase in number of OLPs under all conditions. Replacement of castrated males with DHT, T and estradiol had no effect on the extent of astrocyte and microglia activation. Thus, circulating estradiol (and T) seems to play an important role in maintaining the normal integrity of callosal myelination and axon conduction, while GDX has a significant negative impact on this integrity. This is not surprising given that sex hormone surges during the prenatal period, and later phases of life are known to affect brain organization 9, 38.
The results presented in this report are a starting point to understanding the role of sex hormones on axon myelination during development and disease. These experiments only address the effect of circulating serum hormone effects on callosal axon conduction because of potential changes in myelination and not axon health or hormone effects on related neurons (future study). Cuprizone treatment disrupts estrous cyclicity in female mice and leads to diminished weights of sex organs in male and female mice 41. In gonadally intact animals, this could interfere with potential hormone influences on demyelination and remyelination. Additional studies should be performed in another demyelinating mouse model (e.g., the lysolecithin model) to support and confirm the above effects. Androgens are produced in testes and adrenals, but in the present study, CST was done by only removing testes‐dependent T. In addition, brain regions may still convert locally produced T to estradiol; hence, a completely steroid‐free environment is not achieved. In future studies, to confirm the effects of lack of gonadal hormones on OLs and axon myelination, use of developmental and OL conditional aromatase knockout and estrogen receptor knockout mice will have to be performed.
In conclusion, the use of GDX and hormone manipulations using the cuprizone diet‐induced demyelination and subsequent normal diet remyelination model provides evidence for a significant role of circulating sex hormones, especially estrogen, on callosal myelination. These types of animal studies address the influence of sex steroids on animal brain structure giving valuable insights into the etiology of healthy human brain maturation, and they also serve as a model for the development of neuropsychiatric illnesses and neurodegenerative disorders that present a skewed sex ratio.
Disclosure Statement
The authors have nothing to disclose.
Acknowledgments
This work was generously supported by NMSS grant RG 4538‐A‐2 and NIH grant R21NS075198 to STW. Importantly, we wish to express our deep appreciation to Drs. Rhonda Voskuhl and Art Arnold for their insightful suggestions. We also acknowledge Ms. Sienmi Du, Ms. Elizabeth Umeda and Ms. Anna Khalaj for additional gonadectomies, hormone pellet implantations and paper editing.
References
- 1. Baulieu EE, Schumacher M (2000) Progesterone as a neuroactive neurosteroid, with special reference to the effect of progesterone on myelination. Hum Reprod 15(Suppl. 1):1–13. [DOI] [PubMed] [Google Scholar]
- 2. Bebo BF, Jr , Fyfe‐Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H (2001) Low‐dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol 166:2080–2089. [DOI] [PubMed] [Google Scholar]
- 3. Behan M, Wenninger JM (2008) Sex steroidal hormones and respiratory control. Respir Physiol Neurobiol 164:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bloom JS, Hynd GW (2005) The role of the corpus callosum in interhemispheric transfer of information: excitation or inhibition? Neuropsychol Rev 15:59–71. [DOI] [PubMed] [Google Scholar]
- 5. Brouillette J, Rivard K, Lizotte E, Fiset C (2005) Sex and strain differences in adult mouse cardiac repolarization: importance of androgens. Cardiovasc Res 65:148–157. [DOI] [PubMed] [Google Scholar]
- 6. Carswell HV, Macrae IM, Gallagher L, Harrop E, Horsburgh KJ (2004) Neuroprotection by a selective estrogen receptor beta agonist in a mouse model of global ischemia. Am J Physiol Heart Circ Physiol 287:H1501–H1504. [DOI] [PubMed] [Google Scholar]
- 7. Caruso A, Di Giorgi Gerevini V, Castiglione M, Marinelli F, Tomassini V, Pozzilli C et al (2004) Testosterone amplifies excitotoxic damage of cultured oligodendrocytes. J Neurochem 88:1179–1185. [DOI] [PubMed] [Google Scholar]
- 8. Cerghet M, Skoff RP, Bessert D, Zhang Z, Mullins C, Ghandour MS (2006) Proliferation and death of oligodendrocytes and myelin proteins are differentially regulated in male and female rodents. J Neurosci 26:1439–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Collaer ML, Hines M (1995) Human behavioral sex differences: a role for gonadal hormones during early development? Psychol Bull 118:55–107. [DOI] [PubMed] [Google Scholar]
- 10. Cooke BM, Woolley CS (2005) Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol 64:34–46. [DOI] [PubMed] [Google Scholar]
- 11. Crawford DK, Mangiardi M, Tiwari‐Woodruff SK (2009) Assaying the functional effects of demyelination and remyelination: revisiting field potential recordings. J Neurosci Methods 182:25–33. [DOI] [PubMed] [Google Scholar]
- 12. Crawford DK, Mangiardi M, Xia X, Lopez‐Valdes HE, Tiwari‐Woodruff SK (2009) Functional recovery of callosal axons following demyelination: a critical window. Neuroscience 164:1407–1421. [DOI] [PubMed] [Google Scholar]
- 13. Crawford DK, Mangiardi M, Song B, Patel R, Du S, Sofroniew MV et al (2010) Oestrogen receptor {beta} ligand: a novel treatment to enhance endogenous functional remyelination. Brain 133:2999–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Curry J (1966) J, 3rd, and Heim LM. Brain myelination after neonatal administration of oestradiol. Nature 209:915–916. [DOI] [PubMed] [Google Scholar]
- 15. Dalal M, Kim S, Voskuhl RR (1997) Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen‐specific T lymphocyte response. J Immunol 159:3–6. [PubMed] [Google Scholar]
- 16. Elhage R, Arnal JF, Pieraggi MT, Duverger N, Fievet C, Faye JC, Bayard F (1997) 17 beta‐estradiol prevents fatty streak formation in apolipoprotein E‐deficient mice. Arterioscler Thromb Vasc Biol 17:2679–2684. [DOI] [PubMed] [Google Scholar]
- 17. Garcia‐Ovejero D, Veiga S, Garcia‐Segura LM, Doncarlos LL (2002) Glial expression of estrogen and androgen receptors after rat brain injury. J Comp Neurol 450:256–271. [DOI] [PubMed] [Google Scholar]
- 18. Garcia‐Segura LM, Melcangi RC (2006) Steroids and glial cell function. Glia 54:485–498. [DOI] [PubMed] [Google Scholar]
- 19. Gregg C, Shikar V, Larsen P, Mak G, Chojnacki A, Yong VW, Weiss S (2007) White matter plasticity and enhanced remyelination in the maternal CNS. J Neurosci 27:1812–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hofer S, Frahm J (2006) Topography of the human corpus callosum revisited—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 32:989–994. [DOI] [PubMed] [Google Scholar]
- 21. Jung‐Testas I, Baulieu EE (1998) Steroid hormone receptors and steroid action in rat glial cells of the central and peripheral nervous system. J Steroid Biochem Mol Biol 65:243–251. [DOI] [PubMed] [Google Scholar]
- 22. Kachadroka S, Hall AM, Niedzielko TL, Chongthammakun S, Floyd CL (2010) Effect of endogenous androgens on 17beta‐estradiol‐mediated protection after spinal cord injury in male rats. J Neurotrauma 27:611–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kanaan RA, Allin M, Picchioni M, Barker GJ, Daly E, Shergill SS et al (2012) Gender differences in white matter microstructure. Plos ONE 7:e38272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Labombarda F, Meffre D, Delespierre B, Krivokapic‐Blondiaux S, Chastre A, Thomas P et al (2010) Membrane progesterone receptors localization in the mouse spinal cord. Neuroscience 166:94–106. [DOI] [PubMed] [Google Scholar]
- 25. Mallon BS, Macklin WB (2002) Overexpression of the 3′‐untranslated region of myelin proteolipid protein mRNA leads to reduced expression of endogenous proteolipid mRNA. Neurochem Res 27:1349–1360. [DOI] [PubMed] [Google Scholar]
- 26. Mangiardi M, Crawford DK, Xia X, Du S, Simon‐Freeman R, Voskuhl RR, Tiwari‐Woodruff SK (2011) An animal model of cortical and callosal pathology in multiple sclerosis. Brain Pathol 21:263–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marin‐Husstege M, Muggironi M, Raban D, Skoff RP, Casaccia‐Bonnefil P (2004) Oligodendrocyte progenitor proliferation and maturation is differentially regulated by male and female sex steroid hormones. Dev Neurosci 26:245–254. [DOI] [PubMed] [Google Scholar]
- 28. Matsushima GK, Morell P (2001) The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol 11:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McEwen B (2002) Estrogen actions throughout the brain. Recent Prog Horm Res 57:357–384. [DOI] [PubMed] [Google Scholar]
- 30. Melcangi RC, Magnaghi V, Galbiati M, Martini L (2001) Steroid effects on the gene expression of peripheral myelin proteins. Horm Behav 40:210–214. [DOI] [PubMed] [Google Scholar]
- 31. Mellon SH, Griffin LD (2002) Synthesis, regulation, and function of neurosteroids. Endocr Res 28:463. [DOI] [PubMed] [Google Scholar]
- 32. Morales LB, Loo KK, Liu HB, Peterson C, Tiwari‐Woodruff S, Voskuhl RR (2006) Treatment with an estrogen receptor alpha ligand is neuroprotective in experimental autoimmune encephalomyelitis. J Neurosci 26:6823–6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moran AL, Nelson SA, Landisch RM, Warren GL, Lowe DA (2007) Estradiol replacement reverses ovariectomy‐induced muscle contractile and myosin dysfunction in mature female mice. J Appl Physiol 102:1387–1393. [DOI] [PubMed] [Google Scholar]
- 34. Palaszynski KM, Loo KK, Ashouri JF, Liu HB, Voskuhl RR (2004) Androgens are protective in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J Neuroimmunol 146:144–152. [DOI] [PubMed] [Google Scholar]
- 35. Preston RJ, Waxman SG, Kocsis JD (1983) Effects of 4‐aminopyridine on rapidly and slowly conducting axons of rat corpus callosum. Exp Neurol 79:808–820. [DOI] [PubMed] [Google Scholar]
- 36. Reeves TM, Phillips LL, Povlishock JT (2005) Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp Neurol 196:126–137. [DOI] [PubMed] [Google Scholar]
- 37. Romeo RD, Waters EM, McEwen BS (2004) Steroid‐induced hippocampal synaptic plasticity: sex differences and similarities. Neuron Glia Biol 1:219–229. [DOI] [PubMed] [Google Scholar]
- 38. Schulz KM, Molenda‐Figueira HA, Sisk CL (2009) Back to the future: the organizational‐activational hypothesis adapted to puberty and adolescence. Horm Behav 55:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stidworthy MF, Genoud S, Suter U, Mantei N, Franklin RJ (2003) Quantifying the early stages of remyelination following cuprizone‐induced demyelination. Brain Pathol 13:329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stoffel‐Wagner B (2001) Neurosteroid metabolism in the human brain. Eur J Endocrinol 145:669–679. [DOI] [PubMed] [Google Scholar]
- 41. Taylor LC, Gilmore W, Ting JP, Matsushima GK (2009) Cuprizone induces similar demyelination in male and female C57BL/6 mice and results in disruption of the estrous cycle. J Neurosci Res 88:391–402. [DOI] [PubMed] [Google Scholar]
- 42. Tomasch J (1954) Size, distribution, and number of fibres in the human corpus callosum. Anat Rec 119:119–135. [DOI] [PubMed] [Google Scholar]
- 43. van Honk J, Pruessner JC (2010) Psychoneuroendocrine imaging: a special issue of psychoneuroendocrinology. Psychoneuroendocrinology 35:1–4. [DOI] [PubMed] [Google Scholar]
- 44. Vanderhorst VG, Gustafsson JA, Ulfhake B (2005) Estrogen receptor‐alpha and ‐beta immunoreactive neurons in the brainstem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J Comp Neurol 488:152–179. [DOI] [PubMed] [Google Scholar]
- 45. Whitford TJ, Savadjiev P, Kubicki M, O'Donnell LJ, Terry DP, Bouix S et al (2011) Fiber geometry in the corpus callosum in schizophrenia: evidence for transcallosal misconnection. Schizophr Res 132:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Witelson SF (1989) Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain 112(Pt 3):799–835. [DOI] [PubMed] [Google Scholar]


