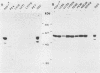
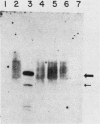
Abstract
Ornithine decarboxylase (ODC) (EC 4.1.1.17) is an early enzyme of polyamine synthesis, and its activity rises quickly at the onset of growth and differentiation in most eucaryotes. Some have speculated that the enzyme protein may have a role in the synthesis of rRNA in addition to its role in catalyzing the decarboxylation of ornithine (G. D. Kuehn and V. J. Atmar, Fed. Proc. 41:3078-3083, 1982; D. H. Russell, Proc. Natl. Acad. Sci. USA 80:1318-1321, 1983). To test this possibility, we sought mutational evidence for the indispensability of the ODC protein for normal growth of Neurospora crassa. We found three new, ODC-deficient mutants that lacked ODC protein. Among these and by reversion analysis of an earlier set of mutants, we found that two ODC-deficient mutants carried nonsense mutations in the ODC structural gene, spe-1. Allele LV10 imparted a complete deficiency for enzyme activity (less than 0.006% of normal) and had no detectable ODC antigen. Allele PE4 imparted a weak activity to cells (0.1% of derepressed spe+ cultures) and encoded a lower-molecular-weight ODC subunit (Mr = 43,000) in comparison to that of the wild-type strain (Mr = 53,000). Strains carrying either mutation, like other spe-1 mutants, grew at a normal rate in exponential culture if the medium was supplemented with spermidine, the main end product of the polyamine pathway in N. crassa. Unless an antigenically silent, N-terminal fragment with an indispensable role persists in the LV10-bearing mutant, we conclude that the ODC protein has no role in the vegetative growth of this organism other than the synthesis of polyamines. The data extend earlier evidence that spe-1 is the structural gene for ODC in N. crassa. The activity found in mutants bearing allele PE4 suggests that the amino acids nearest the carboxy terminus do not contribute to the active site of the enzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERTON J. I., LOCKE D. J. Extraction of pigment from cooked cured-meat products. Nature. 1955 May 7;175(4462):818–819. doi: 10.1038/175818b0. [DOI] [PubMed] [Google Scholar]
- Algranati I. D., Goldemberg S. H. Initiation, elongation and termination of polypeptide synthesis in cell-free systems from polyamine-deficient bacteria. Biochem Biophys Res Commun. 1981 Nov 16;103(1):8–15. doi: 10.1016/0006-291x(81)91653-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Case M. E., Giles N. H. Revertants and secondary arom-2 mutants induced in non-complementing mutants in the arom gene cluster of Neurospora crassa. Genetics. 1974 Aug;77(4):613–626. doi: 10.1093/genetics/77.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H. Regulatory mutations affecting ornithine decarboxylase activity in Saccharomyces cerevisiae. J Bacteriol. 1980 Jun;142(3):791–799. doi: 10.1128/jb.142.3.791-799.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Krasner G. N., DiGangi J. J., Ristow J. L. Distinct roles of putrescine and spermidine in the regulation of ornithine decarboxylase in Neurospora crassa. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4105–4109. doi: 10.1073/pnas.82.12.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Ristow J. L., Ginsburgh C. L. Independent localization and regulation of carbamyl phosphate synthetase A polypeptides of Neurospora crassa. Mol Gen Genet. 1981;181(2):215–221. doi: 10.1007/BF00268429. [DOI] [PubMed] [Google Scholar]
- Eversole P., DiGangi J. J., Menees T., Davis R. H. Structural gene for ornithine decarboxylase in Neurospora crassa. Mol Cell Biol. 1985 Jun;5(6):1301–1306. doi: 10.1128/mcb.5.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K., Kashiwagi K., Kishida K., Kakegawa T., Hirose S. Decrease in the S1 protein of 30-S ribosomal subunits in polyamine-requiring mutants of Escherichia coli grown in the absence of polyamines. Eur J Biochem. 1981;114(1):127–131. doi: 10.1111/j.1432-1033.1981.tb06182.x. [DOI] [PubMed] [Google Scholar]
- Jorstad C. M., Morris D. R. Polyamine limitation of growth slows the rate of polypeptide chain elongation in Escherichia coli. J Bacteriol. 1974 Sep;119(3):857–860. doi: 10.1128/jb.119.3.857-860.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn G. D., Atmar V. J. Posttranslational control of ornithine decarboxylase by polyamine-dependent protein kinase. Fed Proc. 1982 Dec;41(14):3078–3083. [PubMed] [Google Scholar]
- LESTER H. E., GROSS S. R. Efficient method for selection of auxotrophic mutants of Neurospora. Science. 1959 Feb 27;129(3348):572–572. doi: 10.1126/science.129.3348.572. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McDougall K. J., Deters J., Miskimen J. Isolation of putrescine-requiring mutants of Neurospora crassa. Antonie Van Leeuwenhoek. 1977;43(2):143–151. doi: 10.1007/BF00395669. [DOI] [PubMed] [Google Scholar]
- Paulus T. J., Davis R. H. Regulation of polyamine synthesis in relation to putrescine and spermidine pools in Neurospora crassa. J Bacteriol. 1981 Jan;145(1):14–20. doi: 10.1128/jb.145.1.14-20.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus T. J., Kiyono P., Davis R. H. Polyamine-deficient Neurospora crassa mutants and synthesis of cadaverine. J Bacteriol. 1982 Oct;152(1):291–297. doi: 10.1128/jb.152.1.291-297.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. H. Microinjection of purified ornithine decarboxylase into Xenopus oocytes selectively stimulates ribosomal RNA synthesis. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1318–1321. doi: 10.1073/pnas.80.5.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steglich C., Grens A., Scheffler I. E. Chinese hamster cells deficient in ornithine decarboxylase activity: reversion by gene amplification and by azacytidine treatment. Somat Cell Mol Genet. 1985 Jan;11(1):11–23. doi: 10.1007/BF01534730. [DOI] [PubMed] [Google Scholar]
- Steglich C., Scheffler I. E. An ornithine decarboxylase-deficient mutant of Chinese hamster ovary cells. J Biol Chem. 1982 Apr 25;257(8):4603–4609. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W., Cohn M. S., Hafner E. W. Streptomycin resistance (rpsL) produces an absolute requirement for polyamines for growth of an Escherichia coli strain unable to synthesize putrescine and spermidine [delta(speA-speB) delta specC]. J Bacteriol. 1981 Aug;147(2):702–704. doi: 10.1128/jb.147.2.702-704.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Polyamine requirement for efficient translation of amber codons in vivo. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7087–7091. doi: 10.1073/pnas.79.23.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney P. A., Morris D. R. Polyamine auxotrophs of Saccharomyces cerevisiae. J Bacteriol. 1978 Apr;134(1):214–220. doi: 10.1128/jb.134.1.214-220.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]