Abstract
Background
Despite controversy over the benefit of prostate specific antigen (PSA) screening, little is known about risk profiles and treatment patterns in men diagnosed with prostate cancer who have a PSA value less than or equal to 4 ng/mL.
Methods
We utilized data from the Surveillance, Epidemiology, End Results system to describe patient characteristics and treatment patterns of 123,934 men with newly diagnosed prostate cancer in 2004–2006. Age-standardized treatment rates were calculated in five-year age strata. Logistic regression was used to quantify the odds ratios (OR) of men with low– and high–risk disease and the use of radical prostatectomy (RP) or radiation therapy (RT).
Results
Men with a PSA of 4.0 ng/ml or less represent 14% of incident prostate cancer cases. Fifty-four percent of men diagnosed with prostate cancer and PSA ≤ 4.0 ng/mL harbor low-risk disease (stage ≤ T2a, PSA level ≤ 10 ng/mL, and Gleason score ≤ 6), but over 75% of them received RP or RT. Men with screen-detected prostate cancer and PSA values ≤ 4 ng/mL were 1.49 (CI 1.38–1.62) and 1.39 (CI 1.30–1.49) times more likely to receive RP and RT, respectively, and were less likely to have high-grade disease than men who had non-screen detected prostate cancer (OR=0.67; 95% CI:0.60–0.76).
Conclusions
Most men diagnosed with prostate cancer with a PSA threshold ≤ 4.0 ng/mL had low-risk disease but underwent aggressive local therapy. Lowering biopsy threshold, while lacking the ability to distinguish indolent cancers from aggressive cancers, may increase overdiagnosis and overtreatment.
Introduction
More than 90% of all prostate cancers are diagnosed at a localized stage, and the five- year relative survival rate for patients who are diagnosed with localized disease is almost 100%.1–2 The relative five-year survival for all stages combined increased from 69% to almost 99% during the period 1975 to 2003.1 The tremendous improvement in survival has been attributed to early detection and treatment. However, there have been concerns about the potential overdiagnosis and overtreatment of localized prostate cancer.3–6 Despite these concerns, some researchers argue that PSA level is associated with a continuum of cancer risk and recommend lowering the 4 ng/mL threshold for biopsy.7–10 Due to our inability to distinguish indolent cancers from aggressive cancers at the present time, lowering the biopsy threshold may increase overdiagnosis and overtreatment. For instance, a recent study estimated that overdiagnosis ranged from 23% to 42% of all screen–detected prostate cancer in the United States.11 To date, relatively little is known about the risk profile and factors associated with treatment of men who were diagnosed with prostate cancer at a PSA level lower than 4 ng/mL. We undertook a nationwide study using 2004–2006 data from the Surveillance, Epidemiology, End Results (SEER) Program, which contains the first available population-based prostate specific antigen (PSA) and Gleason scores, to describe the risk profile and treatment patterns of prostate cancer patients with PSA below 4 ng/ml at diagnosis.
Methods
The SEER 2004–2006 database from 16 SEER tumor registries was used to identify patients for this study. The SEER program covers approximately 26% of the US population and has a 98% completeness in case ascertainment.12 We used ICD-O-3 site code C619 to identify newly diagnosed prostate cancer cases. Men were excluded from this study if they were 24 years of age or younger (n=96), had missing PSA values (n=24,679), or had missing Gleason Score and clinical stage or could not be classified into one of the three risk strata (n=2,635). The final study cohort consists of 123,934 subjects.
PSA documented in the SEER data is the highest lab value prior to diagnostic biopsy or treatment. Demographic and clinical features included age at prostate cancer diagnosis, race, cancer stage, cancer grade, localized/regional/distant stage and tumor size were stratified by PSA level. Distant stage was defined as having a neoplasm that has spread to parts of the body remote from the primary tumor either by direct extension or by discontinuous metastasis to distant organs, issues, or via the lymphatic system to distant lymph nodes. The sizes of tumor were calculated from the diameter (d) of the primary tumor (Volume of a sphere is V = (π/6)*d3), and this estimation came from a subgroup of the SEER population (n=15,137). Men were categorized into three risk groups on the basis of the American Joint Committee on Cancer clinical stage, PSA level, and Gleason score13: low risk (stage T2a or lower, a PSA level ≤ 10 ng/mL, and a Gleason score of 6 or lower), intermediate risk (stage T2b or a PSA level from 10.1 to 20 ng/mL or a Gleason score of 7), and high risk (stage T2c or higher or a PSA level >20 ng/mL or a Gleason score of 8 or higher). Treatment following initial diagnosis for prostate cancer was categorized into attempted curative treatment and conservative management. Attempted curative treatment consisted of radical prostatectomy (RP) and radiation therapy (RT) (i.e. external beam radiation therapy and/or brachytherapy). The conservative management group was composed of men who were not treated with either radical prostatectomy or radiation therapy.
Treatment rates were calculated by age and risk stratification. Additionally, age-standardized treatment rates were calculated by PSA level, Gleason score and cancer stage. Treatment rates were age standardized in five year age strata according to the direct method14 and using prostate cancer patients in SEER 2004–2006 as the standard population. The association between PSA level at diagnosis and age at diagnosis was examined by one-way analysis of variance using linear contrast. The independent distribution in Gleason score, cancer stage and risk categories between different levels of PSA at diagnosis were evaluated by the Mantel–Haneszel statistic for ordinal or cardinal outcomes. The distribution of attempted curative treatment stratified by the level of PSA, Gleason score and cancer stage was evaluated using the Cochran-Mantel–Hanszel statistic.
We used logistic regression to quantify the odds ratio (OR) of men with tumor that was identified by needle biopsy due to elevated PSA to have clinical characteristics of high grade cancer. We used multinomial logistic regression to measure the relative propensity for attempted curative treatment among men. Age, race and year of diagnosis were included in these analyses as covariates. All analyses were performed using SAS statistical software (version 9.1, SAS institute, Cary, NC).
Results
Characteristics and risk profile of this cohort of 123,934 patients are summarized in Table 1 and stratified into four categories based on the pre-biopsy PSA value. Of these patients, 14% had a PSA value of ≤ 4 ng/ml, 73.5% had values between 4.1 and 20 ng/ml and 12.5% had values greater than 20 ng/ml. Compared to men in higher PSA groups, men with PSA values 4 ng/ml or less were younger and had lower Gleason scores. Of these patients with PSA values 4 ng/mL or less, 38% were screen detected. Approximately 54% of patients with PSA 4.0 ng/ml or less at diagnosis had low risk cancers compared to only 48% of men with PSA values between 4 and 10 (p<0.001). The percentage of patients who had intermediate or high risk cancer was positively associated with PSA level. Among patients who had PSA values greater than 20 ng/mL, 70% had tumor sizes larger than 0.5cm3, in contrast to 52%, 65% and 71% among patients who had PSA levels 0–4, 4.1–10 and 10.1–20 ng/ml respectively.
Table 1.
Clinical characteristics and primary therapy among prostate cancer patients by Prostate Specific Antigen Value, SEER (2004–2005)
| Prostate Specific Antigen Value, ng/mL | ||||
|---|---|---|---|---|
| 0–4 (n=17,343) | 4.1–10 (n=71,352) | 10.1–20 (n=19,695) | 20+ (n=15,544) | |
| Cases % of all | 14.0 | 57.6 | 15.9 | 12.5 |
| Mean age, years (SD) | 63.9 (9.6) | 65.8 (8.8) | 69.6 (9.6) | 70.8 (10.6) |
| 25–54, % | 16.7 | 10.0 | 6.9 | 6.8 |
| 55–64, % | 36.5 | 34.8 | 23.2 | 22.1 |
| 65–74, % | 32.2 | 38.3 | 36.8 | 32.1 |
| 75 and above, % | 14.7 | 17.0 | 33.1 | 39.0 |
| Race | ||||
| White, % | 83.3 | 80.6 | 76.1 | 74.0 |
| Black, % | 10.6 | 11.5 | 13.6 | 16.4 |
| Others,% | 6.1 | 7.9 | 10.3 | 9.6 |
| Gleason Score, % | ||||
| Gleason 2–5 | 3.5 | 2.2 | 2.2 | 1.5 |
| Gleason 6 | 56.4 | 50.5 | 35.5 | 19.1 |
| Gleason 7 | 30.4 | 36.9 | 39.5 | 34.5 |
| 3+4 | 23.1 | 27.0 | 25.9 | 20.2 |
| 4+3 | 6.9 | 9.4 | 12.9 | 13.8 |
| Gleason 8–10 | 8.5 | 9.9 | 20.9 | 39.6 |
| Unknown | 1.1 | 0.5 | 1.9 | 5.3 |
| Clinical Stage, % | ||||
| T1 (T1c) | 43.3 (37.8) | 62.6 (61.6) | 54.4 (52.9) | 38.3 (36.3) |
| T2 | 54.0 | 35.5 | 40.6 | 43.9 |
| T3/T4 | 2.4 | 1.7 | 4.2 | 13.0 |
| Unknown | 0.3 | 0.2 | 0.8 | 4.8 |
| Distant*, % | 1.0 | 0.6 | 3.1 | 20.5 |
| Risk Stratification, % | ||||
| Low | 53.7 | 48.2 | – | – |
| Intermediate | 26.0 | 32.7 | 67.3 | – |
| High | 20.3 | 19.1 | 32.7 | 100.0 |
| Tumor Size**, % | n=956 | n=5656 | n=1200 | n=625 |
| <=0.2 cm3 | 29.0 | 19.6 | 17.1 | 17.4 |
| 0.21–0.5 cm3 | 19.5 | 16.0 | 12.1 | 12.7 |
| 0.5+ cm3 | 51.5 | 64.5 | 70.8 | 69.9 |
| Treatment, % | ||||
| Conservative Management 23.1 | 21.6 | 34.7 | 56.0 | |
| Radical Prostatectomy | 43.9 | 38.0 | 24.0 | 12.5 |
| Radiation | 33.0 | 40.1 | 41.3 | 31.5 |
| EBRT | 16.5 | 22.1 | 29.5 | 25.1 |
| Brachytherapy | 12.4 | 13.4 | 6.3 | 3.1 |
| Both | 4.0 | 4.7 | 5.4 | 3.3 |
defined by historic stage and metastasis at diagnosis.
Tumor size was provided by a sub–sample in SEER.
Difference across the level of PSA values was statistically significant for all characteristics due to our big sample size.
EBRT: External Beam Radiation Therapy
Approximately 13% of the study cohort had PSA values greater than 20 ng/ml at diagnosis. These men were older and had higher tumor grades and stages of disease when compared to the remainder of the cohort. Their chance of having disease outside the prostate was 21% compared to only 1% for men with PSA values less than 4.0 ng/ml and 1.1% for men with PSA values between 4 and 20 ng/ml.
More than 70% of men with PSA values less than 20 ng/ml received either RP or RT. RP was performed on 44% of men with PSA values 4.0 ng/ml or less, 38% of men with PSA values 4.1–10 ng/ml and 24% of men with PSA values 10.1–20 ng/ml. Radiation was performed on 33% of men with PSA values 4.0 ng/ml or less, 40% of men with PSA values 4.1–10 ng/ml and 41.3% of men with PSA values 10–20 ng/ml.
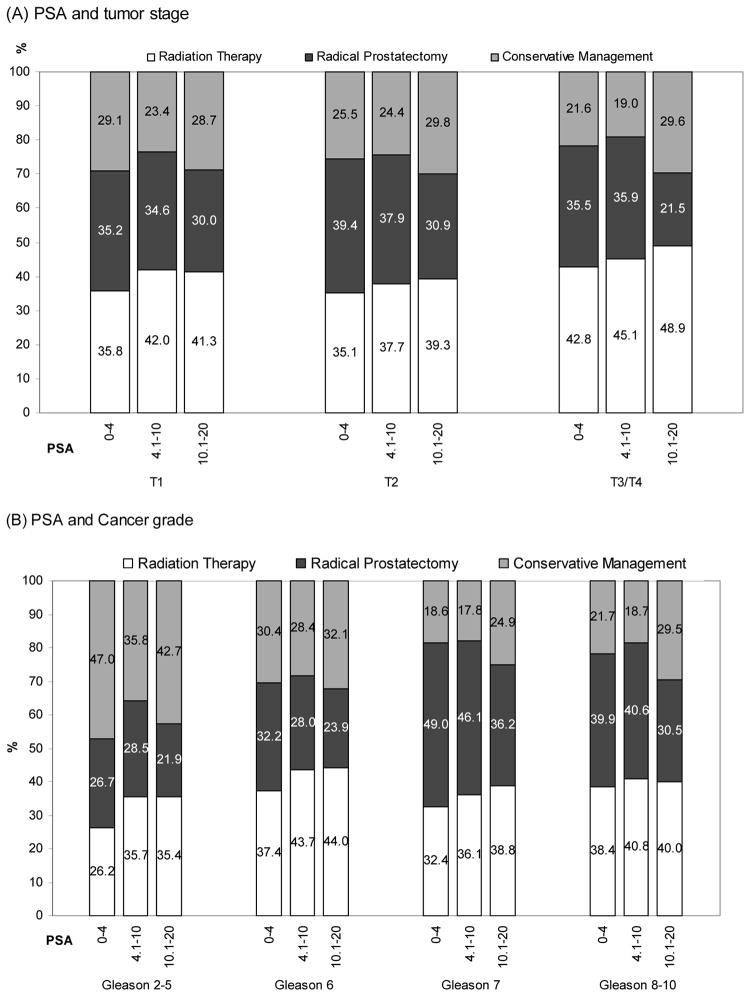
Figure 1 presents the age standardized percentage distribution of treatments stratified by cancer stage, Gleason score and PSA level. Treatment patterns were similar across tumor stages with a slightly lower rate of intervention with either RT or RP in the group of men with T1 disease and a PSA value of 0 – 4 ng/ml (Figure 1A). Rates of attempted curative treatment were comparable among men with Gleason score 7 or 8–10 disease, but RP was slightly less common among men with Gleason 6 disease. Over half of the men with Gleason 2–5 disease received either RT or RP.
Figure 1.
Age standardized percentage distribution of treatment according to tumor stage, cancer grade and prostate specific antigen (PSA) (A) PSA and tumor stage, (B) PSA and cancer grade, SEER 2004–2006.
*Age standardization of treatment rate was performed according to the direct method using five– year age strata and the prostate cancer patients population in SEER 2004–2006 as the standard population.
**Differences in receiving attempted curative therapy across the level of PSA value was statistically significant (p<.001) for all tumor stages and cancer grades.
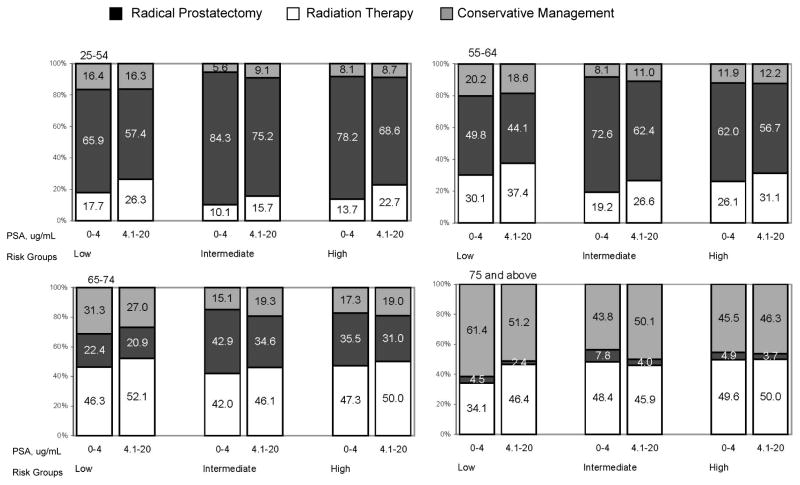
The percentage of men who did not receive any attempted curative treatment was 27%, 22% and 36% respectively for men with low, intermediate and high risk disease. The results by age groupings are presented in Figure 2. Conservative treatment strategies increased with patient age, especially for those men over age 75, while RP dominated among men age 65 years or less. RT was performed on approximately half of the men age 65–74, while RP was performed on approximately 1/3 of men with intermediate or high risk disease and much less frequently on men with low-risk disease.
Figure 2.
Percentage distribution of therapy stratified by age, prostate specific antigen level and risk group, SEER 2004–2006
* Patients were categorized into three risk groups on the basis of clinical stage, PSA level and Gleason score: low–risk (≤ T2a, PSA level ≤ 10 ng/mL, and a Gleason score ≤ 6), intermediate–risk (T2b or Gleason score 7 or a PSA level from 10.1 to 20 ng/mL) and high–risk (≥ T2c or PSA level >20ng/mL or Gleason score ≥ 8)
Table 2 presents cancer features and uses of treatments among men with PSA value 4 ng/mL or less. Men with screen detected cancer were less likely to have high grade tumors (OR=0.67, CI=0.60–0.76), have disease outside the prostate (OR=0.30, CI=0.20–0.47) or have tumor size > 0.5 cm3 (OR=0.75, CI=0.68–0.83). However, these men were 1.49 times (CI 1.38–1.62) more likely to receive RP and 1.39 times (CI 1.30–1.49) to receive RT, compared to those with non–screen detected cancer.
Table 2.
High risk cancer features and use of radical prostatectomy or radiation by screen status among men with PSA value of 4 ug/mL or less.
| High-Risk Features | Treatments** | ||||
|---|---|---|---|---|---|
| Gleason Score 8–10 | M1 (Distant) | Tumor size >0.5 cm3 | Radical Prostatectomy | Radiation Therapy | |
| Screen detected | 2.4% | 0.4% | 9.6% | 82.5% | 35.6% |
| Non Screen detected | 6.2% | 1.4% | 11.3% | 73.5% | 31.5% |
| Odds Ratio (Screen detected vs not) (95% CI) | 0.67 (0.60–0.76) | 0.30 (0.20–0.47) | 0.75 (0.68–0.83) | 1.49 (1.38–1.62) | 1.39 (1.30–1.49) |
Adjusted for age, race, year of diagnosis
Multinomial logistic regression
Discussion
This is the first large–scale US population–based study to document the risk profile and treatment patterns among men diagnosed with prostate cancer who had PSA levels of 4 ng/mL or less. In 2004–2006, 14% of prostate cancer patients were diagnosed at PSA level of 4 ng/mL or less. These patients were less likely to have high–grade cancer and more than half were classified as having low–risk cancer. Despite their lower risk of having clinically significant disease, treatment rates for men with PSA values of 4.0 ng/ml or less were comparable to men presenting with PSA values of 4–20 ng/ml. Treatments were especially common among men with screen detected cancer. The finding that men in low–risk groups were treated intensively raises the concern of overtreatment, especially among older patients.
Approximately a quarter of patients received conservative management as their primary treatment in SEER between 2004 and 2006. However, SEER registries lack explicit data regarding initial and longitudinal use of hormonal therapy. Based on our earlier study, 41% of patients aged over 65 without surgery or radiation received androgen deprivation therapy within 6 months of diagnosis.15 We performed a subgroup analysis among men aged 65 and older in SEER-Medicare and found 5.8% of men aged between 65 and 74 years and 19.8% of men aged 75 years and above received androgen deprivation therapy (ADT). The use of ADT is comparable between patients with PSA levels under 4 and above 4 ug/mL. The use of ADT is suspected to be lower among patients below age 65. Therefore, the percentage of patients without any initial therapy would be even lower among older patients. Two previous studies reported treatment patterns before 2002 from SEER.3, 16 Their results showed that the proportion of men who select attempted curative treatment is strongly associated with patient age and tumor characteristics. However, our study demonstrates that Gleason score, PSA level and risk stratification does not appear to substantially influence the decision to have attempted curative therapy.
Recently publicized results from the European Randomized Study of Screening for Prostate Cancer showed that 1410 men would need to be screened and 48 additional cases of prostate cancer would need to be treated to prevent one death from prostate cancer.17 Given that US patients are in general diagnosed at earlier stages and are more likely to receive attempted curative therapy, the number needed to treat in order to save one patient is likely to be higher in the US than in Europe.
Based on the recent update of the Scandinavian Prostate Cancer Group–4 trial, men aged 65 years and older treated with radical prostatectomy fared no better than men treated with conservative management.18 Our results demonstrate that 66% of men aged 65–74 years with low risk disease and a PSA value 4.0 ng/ml or less received either RP or RT. These findings suggest that many contemporary men receiving treatment for localized prostate cancer are unlikely to benefit from the intervention. Furthermore, it has been documented that men who receive any treatment have increased risk of treatment-related side effects.19–21 Therefore, it is critical that patients be counseled about treatment-associated side effects and benefits when deciding about therapy.22–24
The number of people being defined as having abnormal PSA would double to approximately six million if the threshold decreased from 4 to 2.5 ug/mL.25 Estimates suggest that 32% of men with abnormal PSA would be diagnosed with prostate cancer in their needle biopsy.26 Based on results in this study, among these 1.9 million men, 82.5% of them would receive attempted curative treatments while only 2.4% have high grade cancer (Table 2). However, no evidence suggests that delaying biopsy until the PSA level increased to 4 ng/mL would result in an excessive number of potentially non curable disease. Although abandoning an upper limit of normal for PSA would allow physicians to detect more cancer, the benefits of diagnosing prostate cancer would be likely to be a tradeoff for treatment complications related to cancers that may never have caused harm,.
Our analysis was limited by the nature of the data source. The SEER system collects information from all patients in 16 registries. The Gleason Scores and PSA values recorded by the SEER system reflect the information that was used to make clinical decisions. The SEER system does not record information such as percent free PSA or the number of cores positive on biopsy. The major strength of our analysis derives from a large sample size that is population based and includes patients from defined geographic areas in all clinical settings, rather than selected medical institutes.
Our study found that aggressive local therapy was provided to the majority of patients diagnosed with prostate cancer. These results underscore the fact that PSA, the current biomarker, is not sufficient for treatment decisions. Without the ability to distinguish indolent cancers from aggressive cancers, lowering the biopsy threshold may increase the risk of overdiagnosis and overtreatment.
Acknowledgments
We thank Thanusha Puvananayagam, MPH, Cancer Institute of New Jersey assistant staff, for outstanding administrative and technical assistance.
Funding/Support: The study was supported by the following grants and awards: National Cancer Institute grant # RO1 CA 116399, Cancer Institute of New Jersey core grant NCI CA–72720–10 and Robert Wood Johnson foundation grant # 60624.
Footnotes
Financial Disclosure: None reported.
Disclaimer: This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The content of the information does not necessarily reflect the position or the policy of the Government or the employers, and no official endorsement should be inferred.
Authors Contributions: Dr. Lu-Yao had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Shao, Albertsen, Roberts, Lin, Mehta, Stein, DiPaola and Lu-Yao. Acquisition of data: Shao, Lin and Lu-Yao. Analysis and interpretation of data: Shao, Albertsen, Roberts, Lin, Mehta, Stein, DiPaola and Lu-Yao. Drafting of the manuscript: Shao, Albertsen, Roberts, Lin, Mehta, Stein, DiPaola and Lu-Yao. Critical revision of the manuscript for important intellectual content: Shao, Albertsen, Roberts, Lin, Mehta, Stein, DiPaola and Lu-Yao. Statistical analysis: Shao and Lin. Administrative, technical, and material support: DiPaola and Lu-Yao. Study supervision: DiPaola and Lu-Yao.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Ries L, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2004. 2007 based on November 2006 SEER data submission. http://seer.cancer.gov/csr/1975_2004/
- 3.Miller DC, Gruber SB, Hollenbeck BK, Montie JE, Wei JT. Incidence of initial local therapy among men with lower-risk prostate cancer in the United States. J Natl Cancer Inst. 2006;98(16):1134–1141. doi: 10.1093/jnci/djj308. [DOI] [PubMed] [Google Scholar]
- 4.Cooperberg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004;22(11):2141–2149. doi: 10.1200/JCO.2004.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll PR. Early stage prostate cancer--do we have a problem with over-detection, overtreatment or both? J Urol. 2005;173(4):1061–1062. doi: 10.1097/01.ju.0000156838.67623.10. [DOI] [PubMed] [Google Scholar]
- 6.Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94(13):981–990. doi: 10.1093/jnci/94.13.981. [DOI] [PubMed] [Google Scholar]
- 7.Antenor JA, Han M, Roehl KA, Nadler RB, Catalona WJ. Relationship between initial prostate specific antigen level and subsequent prostate cancer detection in a longitudinal screening study. J Urol. 2004;172(1):90–93. doi: 10.1097/01.ju.0000132133.10470.bb. [DOI] [PubMed] [Google Scholar]
- 8.Krumholtz JS, Carvalhal GF, Ramos CG, et al. Prostate-specific antigen cutoff of 2.6 ng/mL for prostate cancer screening is associated with favorable pathologic tumor features. Urology. 2002;60(3):469–473. doi: 10.1016/s0090-4295(02)01875-7. discussion 473–464. [DOI] [PubMed] [Google Scholar]
- 9.Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 10.Thompson IM, Ankerst DP, Etzioni R, Wang T. It’s time to abandon an upper limit of normal for prostate specific antigen: assessing the risk of prostate cancer. J Urol. 2008;180(4):1219–1222. doi: 10.1016/j.juro.2008.07.089. [DOI] [PubMed] [Google Scholar]
- 11.Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101(6):374–383. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV–3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 13.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy or external beam radiation therapy for patients with clinically localized prostate carcinoma in the prostate specific antigen era. Cancer. 2002;95(2):281–286. doi: 10.1002/cncr.10657. [DOI] [PubMed] [Google Scholar]
- 14.Rothman KJ, Greenland S. Modern Epidemiology. 2. Philadelphia: Lippincott Williams and Wilkins; 1998. pp. 7–12.pp. 253–260.pp. 329–342. [Google Scholar]
- 15.Lu-Yao GL, Albertsen PC, Moore DF, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300(2):173–181. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong YN, Mitra N, Hudes G, et al. Survival associated with treatment vs observation of localized prostate cancer in elderly men. JAMA. 2006;296(22):2683–2693. doi: 10.1001/jama.296.22.2683. [DOI] [PubMed] [Google Scholar]
- 17.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 18.Bill-Axelson A, Holmberg L, Filen F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian prostate cancer group-4 randomized trial. J Natl Cancer Inst. 2008;100(16):1144–1154. doi: 10.1093/jnci/djn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steineck G, Helgesen F, Adolfsson J, et al. Quality of life after radical prostatectomy or watchful waiting. N Engl J Med. 2002;347(11):790–796. doi: 10.1056/NEJMoa021483. [DOI] [PubMed] [Google Scholar]
- 20.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 21.Nieder AM, Porter MP, Soloway MS. Radiation therapy for prostate cancer increases subsequent risk of bladder and rectal cancer: a population based cohort study. J Urol. 2008;180(5):2005–2009. doi: 10.1016/j.juro.2008.07.038. discussion 2009–2010. [DOI] [PubMed] [Google Scholar]
- 22.Madalinska JB, Essink-Bot ML, de Koning HJ, et al. Health-related quality-of-life effects of radical prostatectomy and primary radiotherapy for screen-detected or clinically diagnosed localized prostate cancer. J Clin Oncol. 2001;19(6):1619–1628. doi: 10.1200/JCO.2001.19.6.1619. [DOI] [PubMed] [Google Scholar]
- 23.Shah NL, Sanda M. Health-related quality of life in treatment for prostate cancer: looking beyond survival. Support Cancer Ther. 2004;1(4):230–236. doi: 10.3816/SCT.2004.n.015. [DOI] [PubMed] [Google Scholar]
- 24.Ferrer M, Suarez JF, Guedea F, et al. Health-related quality of life 2 years after treatment with radical prostatectomy, prostate brachytherapy, or external beam radiotherapy in patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72(2):421–432. doi: 10.1016/j.ijrobp.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 25.Welch HG, Schwartz LM, Woloshin S. Prostate-specific antigen levels in the United States: implications of various definitions for abnormal. J Natl Cancer Inst. 2005;97(15):1132–1137. doi: 10.1093/jnci/dji205. [DOI] [PubMed] [Google Scholar]
- 26.Welch HG, Fisher ES, Gottlieb DJ, Barry MJ. Detection of prostate cancer via biopsy in the Medicare-SEER population during the PSA era. J Natl Cancer Inst. 2007;99(18):1395–1400. doi: 10.1093/jnci/djm119. [DOI] [PubMed] [Google Scholar]