Abstract
Polyphenols are compounds found in foods such as tea, coffee, cocoa, olive oil, and red wine and have been studied to determine if their intake may modify cardiovascular disease (CVD) risk. Historically, biologic actions of polyphenols have been attributed to antioxidant activities, but recent evidence suggests that immunomodulatory and vasodilatory properties of polyphenols may also contribute to CVD risk reduction. These properties will be discussed, and recent epidemiological evidence and intervention trials will be reviewed. Further identification of polyphenols in foods and accurate assessment of exposures through measurement of biomarkers (i.e., polyphenol metabolites) could provide the needed impetus to examine the impact of polyphenol-rich foods on CVD intermediate outcomes (especially those signifying chronic inflammation) and hard endpoints among high risk patients. Although we have mechanistic insight into how polyphenols may function in CVD risk reduction, further research is needed before definitive recommendations for consumption can be made.
Keywords: Polyphenols, inflammation, olive oil, flavonoid, phenolic acids, flow-mediated vasodilation, endothelial dysfunction, cell signaling, adhesion markers
Introduction
Atherosclerosis, the pathological condition often underlying cardiovascular disease (CVD), is a chronic inflammatory condition involved in the initiation and perpetuation of atherosclerotic lesions, which may erode or rupture leading to clinical events such as angina, myocardial infarction, or cerebrovascular attack. Because a poor quality diet, smoking, and physical inactivity account for much of modifiable CVD risk, the role of diets rich in bioactive compounds in maintaining or improving cardiovascular health is of utmost interest. As this underlying chronic inflammation plays a key role in development and progression of CVD, bioactive compounds with anti-inflammatory properties such as polyphenols (PPs) is the focus of this review.
Polyphenol Classification and Food Sources
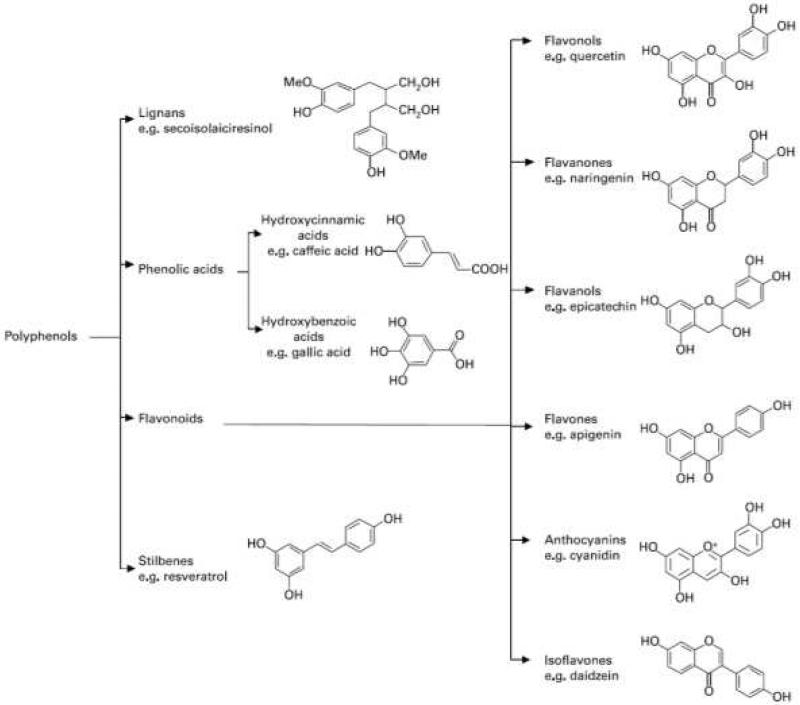
Polyphenols naturally exist in plants and plant products, including fruits, vegetables, nuts, herbs, cocoa, and tea. Over 500 different PPs exist and are classified based on structure, with the phenolic hydroxyl groups as the common structural feature. Differences in primary aromatic rings, oxidation status, and functional groups delineate the individual PPs. Four classes of PPs comprise the majority of the PPs in foods: flavonoids, lignans, phenolic acids, and stilbenes. Other PPs also exist in foods and are combined into a separate class (“other PPs”) [1]. Polyphenol classes, example compounds, and their presence in select foods are detailed in Table 1 and Fig.1. Quantification of individual PPs in these foods is not without difficulty as individual foods often contain a wide variety of phenolic compounds, and a combination of various techniques such as high-performance liquid chromatography, gas chromatography, and gas chromatography-mass spectrometry are necessary for complete PP analysis; these factors should be taken into consideration when examining PPs in foods and the contribution of specific compounds or foods to improving CVD outcomes.
Table 1.
Polyphenol classes, compounds and content in common foods.1
| Polyphenol Class |
Polyphenol Subclass2 |
Example Compounds |
Dietary Sources (mg/serving) | |||
|---|---|---|---|---|---|---|
| <25 | 25-50 | 50-100 | >100 | |||
| Flavonoids | Flavonols | Kaempferol, Myricetin, Quercetin |
Black Tea (22) | Spinach (36) | Capers (56) | |
| Walnuts (19) | Plum (31) | |||||
| Black Beans (10) | Onion (29) | |||||
| Dark Chocolate (7) | Blueberry (29) |
|||||
| Red Wine (6) | ||||||
| Almonds (1) | ||||||
| White Wine (1) | ||||||
|
|
||||||
| Flavanones | Naringenin, Hesperetin |
Red Wine (1) | Grapefruit (67) | |||
| Orange (63) | ||||||
|
|
||||||
| Flavan-3-ols | Catechin, Epigallocatechin Gallate, Procyanidin |
Grape Juice (5) | Red Wine (41) |
Dark Chocolate (60) | Black Tea (176) |
|
| Plum (4) | Cocoa (28) | Green Tea (171) |
||||
| White Wine (2) | ||||||
| Almonds (1) | ||||||
| Blueberry (1) | ||||||
|
|
||||||
| Flavones | Apigenin, Luteolin |
Oregano (1) | ||||
|
|
||||||
| Anthocyanins | Anthocyanidin, Anthocyanin |
Red Wine (20) | Black Beans (35) |
Blueberry (99) | ||
| Plum (32) | ||||||
|
|
||||||
| Isoflavones | Diadzein, Genistein |
Tofu (35) | ||||
|
| ||||||
| Lignans | Lignans | Lariciresinol, Secoisolariciresinol |
Flaxseed (61) | Sesame Oil (217) |
||
|
| ||||||
| Phenolic Acids |
Hydroxybenzoic Acid; Hydroxycinnamic Acids |
Egallic Acid, Vanillic Acid, Caffeic Acid, Ferulic Acid |
Grape Juice (15) | Flaxseed (47) |
Plum (59) | Coffee (509) |
| Red Wine (15) | Black Tea (45) |
Walnut (127) | ||||
| Rosemary (12) | Green Olives (38) |
Blueberry (101) |
||||
| Grapefruit (10) | Black Beans (32) |
|||||
| Dark Chocolate (7) | ||||||
| White Wine (5) | ||||||
| Cocoa (2) | ||||||
| Oregano (2) | ||||||
| Rolled Oats (1) | ||||||
|
| ||||||
| Stilbenes | Stilbenes | Resveratrol, Resveratrol 3-O- glycoside |
Red Wine (3) | |||
| White Wine (1) | ||||||
|
| ||||||
| Other Polyphenols |
Tyrosol, Curcuminoids |
Hydroxytyrosol, Curcumin |
Olive Oil (8) | Green Olives (59) |
||
| Coffee (6) | Turmeric (54) | |||||
| Red Wine (4) | ||||||
| White Wine (1) | ||||||
These foods are not comprehensive, but rather were selected based on quantity of polyphenols in the food item and common rate of consumption. Polyphenol content of foods was obtained from Phenol Explorer [5].
Polyphenol subclasses displayed are examples and are not comprehensive; individual foods are divided by polyphenol subclass for only the flavonoid class
Figure 1.
Major classes of dietary polyphenols and example chemical structures. The Figure does not include polyphenols that are categorized as “other polyphenols.” Adapted from Spencer et al. [10].
Research on the impact of PPs on inflammation and CVD has primarily focused on the flavonoid class. Flavonoids are composed of a three-ring structure and can be subdivided according to the presence of an oxy group at position 4, a double bond between carbon atoms 2 and 3, or a hydroxyl group in position 3 of the C (middle) ring [2]. This class is divided into the following primary sub-classes: flavonols, flavanones, flavan-3-ols, flavones, anthocyanins, and isoflavones (Fig. 1). Flavonols such as kaempferol, myricetin, quercetin, and isorhamnetin are ubiquitous in foods and are most concentrated in spices, berries and cocoa. Flavanones are found in citrus fruits, and major compounds are naringenin and hesperetin. Flavan-3-ols include epicatechin, gallocatechin, epigallocatechin, epicatechingallate, epigallocatechingallate, and procyanidin (polymer). The most commonly associated food with the flavan-3-ol compounds is both black and green tea, containing 176 mg and 171 mg/8 oz, respectively. As with most foods, green tea contains a wide variety of PPs within various subclasses; green tea contains 14 different polyphenolic compounds within the flavan-3-ol subclass, as well as four compounds within both the flavonol subclass and phenolic acid class. Polyphenols in the anthocyanin class primarily consist of anthocyanidin and anthocyanin and are prominent in foods that are red/purple in color, such as berries and red wine. Isoflavones are unique in that they resemble estrogen in structure and, therefore, are classified as phytoestrogens; these foods are found in soy products such as tofu, roasted soy nuts, and miso.
Another class of polyphenols is lignans, which are characterized by their 1,4-diarylbutane structure (Fig.1) and are found in the highest concentration (lariciresinol, matairesinol, secoisolariciresinol) in seeds such as flax and sesame seeds (Table 1). The primary PPs in the phenolic acid class are hydroxybenzoic acid and hydroxycinnamic acid and can be found in appreciable quantities in coffee, walnuts, plums, and blueberries. The stilbene class is primarily defined by resveratrol, a 1,2-diarylethene structure found in red wine that is thought to contain anti-inflammatory properties. The final class of PPs consists of polyphenolics unable to be categorized into the other classes (termed Other Polyphenols, Table 1). These PPs are subcategorized into 14 subclasses, and include such compounds as tyrosol and curcuminoids; polyphenolics within the tyrosol subclass are high in olive oil and are thought to contribute to the health benefits seen with olive oil consumption. While specific PPs are highest in certain foods, no food contains only one class of PPs, and it is likely the complementary or synergistic nature of these compounds in foods is what results in cardioprotection. Olive oil is a prime example, containing flavones, lignans, phenolic acids (hydroxybenzoic and hydroxycinnamic acids), and an appreciable quantity of other phenols (e.g., tyrosol, hydroxytyrosol). In addition, foods may contain other anti-inflammatory components such as tocopherol and monounsaturated fatty acids as found in olive oil [1], and; therefore, the anti-inflammatory properties of whole foods used in studies (as discussed in this review) may not solely be attributed to the phenolic content. In addition, varieties of the same food differ, with virgin olive oils (produced by direct-press/centrifugation) containing 150-350 mg PP/kg olive oil [3] and refined olive oil not containing an appreciable quantity of phenolic compounds. While PP content in foods is fairly diverse and individualized to the particular food, a PP supplement often contains a very limited variety of compounds (e.g., resveratrol). As a result, supplementation of individual PPs may have a different impact on CVD outcomes than consumption of whole foods; the impact of supplements on CVD in human trials will not be discussed.
Dietary Intake
Because of the poor standardization of assays used for PP separation and quantification, accurate quantification of PP in foods has been difficult, resulting in an incomplete resource for PP quantities in foods. Recently, release of USDA databases including flavonoid, isoflavone and proanthocyanidin content [4] has aided in determining PP content in foods. Also, an additional database (Phenol Explorer [http://www.phenol-explorer.eu]) is now available and provides the most comprehensive means by which to determine PP intake [5, 6]. PP intakes in the USA have not been well characterized, but reports for other populations exist. The usual intake of PPs in a Finnish population has been estimated at approximately 900 mg/day [7]. Quantification of PP intake through the SU.VI.MAX study conducted in Europe indicates that PP intake originates primarily from hydroxycinnamic acids (coffee), proanthocyanidins (apples, cocoa products), and catechins (tea, red wine) [8]. When categorized by foods, the greatest PP intake was from nonalcoholic beverages (coffee, tea), fruit (apples, strawberries), and alcoholic beverages (red wine, white wine) [8]. In addition, in the Spanish-conducted “PREvención con DIeta MEDiterránea” (PREDIMED) study, mean PP intake was 820 mg/day, flavonoids 443 mg/day, and phenolic acids 304 mg/day, with hydroxycinnamic acids as the highest PP consumed (276 mg/day). This PP intake was attributed to fruit, coffee and olives/olive oil [9]. Because of the low intake of these aforementioned foods in the USA (except for coffee), it is likely that PP intake is lower in the USA as compared to Europe.
Bioavailability, Metabolism, and Metabolites
Not only does the wide variety of phenolic compounds in individual foods make the objective determination of the impact of each compound on health complex, but the understanding of PP metabolism and metabolite production is lacking. Polyphenols are largely metabolized following ingestion, with PPs being metabolized in the stomach, small and large intestine, and liver. Assessment of dietary PP intake has been suggested through measurement in serum and urine metabolites, but, as indicated, it has been challenging due to incomplete understanding of the absorption and metabolism of all PPs; the knowledge regarding specific metabolites produced, time to appearance in biological fluids, metabolite-intake dose relationship, and the impact of environmental factors on metabolism is necessary in order to effectively utilize PP metabolites as biomarkers for intake [10]. Despite this, investigators have measured metabolites as a marker of PP intake; PREDIMED used both tyrosol and its metabolite, hydroxytyrosol, as markers of olive oil intake [11, 12]. In another randomized crossover trial contrasting virgin olive oil versus refined olive oil intakes, the presence of phenols in the plasma LDL fraction reflected sustained, daily ingestion of virgin olive oil [13]. Further understanding of PP metabolism is warranted and will be critical for determining the impact of foods on inflammation and related CVD.
Mechanisms Underlying Cardioprotection
The beneficial impact of PPs on CVD is diverse, but critical to their beneficial impact is their role in countering chronic and acute inflammation [25,26]. Several mechanisms explaining the anti-inflammatory activity of PPs (especially the flavonoids) will be described, including antioxidant and radical scavenging activities and regulation of cellular activities of inflammation-related cells and their molecular targets, including improvements in endothelial structure and function (Table 2).
Table 2.
Major anti-inflammatory mechanisms and example phenolic compounds that elicit these effects1
| Antioxidant Activity |
| Inhibition of Reactive Oxygen and Nitrogen Species |
| Direct scavenging of peroxynitrite, hydroxyl radicals, superoxide anion, nitric oxide |
| Modifies signaling cascades and transcriptional networks 2 |
| Inhibition of Pro-oxidant Enzymes |
| Suppress activity and expression of endothelial NAPDH oxidase, lipoxygenase, myeloperoxidase, cyclooxygenase-2, phospholipase, inducible nitric oxide synthase (quercetin, apigenin, kaempferol) |
| Blocks NFκB signaling and expression (resveratrol, quercetin) |
| Downregulates TNFα, IL1β, IL-6 synthesis (resveratrol, olive oil) |
| Reduces expression of VCAM-1 and ICAM-1 (resveratrol, olive oil) |
| Reduces MCP-1, MIP-1α expression (quercetin, resveratrol) |
| Upregulation of NrF2 genes and thioredoxin to maintain redox state (epicatechin, tea) |
| Reduces adhesion of immune cells (T lymphocytes, monocytes) to endothelium |
| Suppression of adhesion markers on endothelium, vascular smooth muscle cells: CD40. VCAM, ICAM, E-selectin, P-selectin (red wine, nuts, olive oil) |
| Inhibit expression of MMP1 (resveratrol) |
| Counters Endothelial dysfunction |
| Downregulates endothelial contractility factor, decreases endothelin-1 expression and release (resveratrol) |
| Increases nitric oxide availability because of increased activity of endothelial nitric oxide synthase (delphinidin, quercetin, resveratrol, red wine) |
| Upregulates endothelial derived hyperpolarizing factor (red wine, grapes) |
MAPK, Mitogen-activated protein kinase; JNK, Jun N-terminal kinase; NADPH, nicotinamide adenine dinucleotide phosphate; NF-kB, nuclear factor-kappa B; TNF-α, tumor necrosis factor-α, IL-1B, interleukin-1 beta; IL-6, interleukin 6; VCAM, vascular cell adhesion molecule; ICAM, intercellular adhesion molecule; MCP-1, monocyte chemoattractant protein-1; MIP, macrophage inflammatory protein; CD40, Cluster of differentiation 40; Nrf2, nuclear transcription factor erythroid-2 related factor- 2; MMP1, metalloproteinase I.
Modulates tyrosine and serine-threonine protein kinases (MAPKs [i.e., p38, JNK]) involved in signal transduction and cell activation
Antioxidant and Radical Scavenging Activities
Many reports have described the antioxidant properties of PPs, a function largely attributable to their phenolic hydroxy groups [14]. Antioxidant capacity is mediated by the ability to scavenge free oxygen and nitrogen species, abrogating the pro-inflammatory activity of reactive oxygen species (ROS)-generating enzymes such as cyclooxygenase (COX), lipoxygenase (LOX), and inducible nitric oxide synthase (iNOS). Polyphenols also contribute to the antioxidant defense of endothelial cells by reducing NADPH oxidase expression and its activity directly. Finally, PPs stimulate antioxidant activities of other enzymes such as catalase [15]. Production of ROS such as the potent LDL oxidant peroxynitrite from superoxide and nitric oxide (NO) in the vessel lumen is a primary trigger for endothelial injury, an event that drives a marked inflammatory response [16].
The evidence for these antioxidant properties is largely based on in vitro models. For example, in vitro, resveratrol, the stilbene found in red wine, and other phenols protect LDL against peroxynitrite-mediated oxidation [16]. Resveratrol also has been shown to up-regulate thioredoxin, a cellular protein reductase responsible for maintenance of redox environment within myocytes. Resveratrol protects against oxidative stress not solely because of a direct antioxidant capacity, but also by up-regulating other endogenous antioxidant pathways [17]; resveratrol enhances the expression of superoxide dismutase, catalase, and glutathione peroxidase in cardiac and aortic smooth muscle cells [18, 19]. Further work suggests that these events result from changes in transcriptional activity of nuclear transcription factor erythroid-2 related factor-2 (Nrf2) and upregulation of the expression of several Nrf2 genes---NAD(P)H:quinone oxidoreductase I, γ-glutamylcysteine synthetase, and heme oxygenase [20]. Changes in the expression of these genes along with those for thioredoxin may be responsible for the purported antioxidant activity of resveratrol and epicatechin. Other phenolic compounds may function as antioxidants. Quercetin reduces LDL oxidation at physiologic concentrations through inhibition of myeloperoxidase [21]. Other flavonoids (specifically, fisetin and quercetin) modulate macrophage-stimulated LDL oxidation, possibly through inhibition of generation of lipid hydroperoxides. Proanthocyanidins have a concentration-dependent scavenging ability and are more potent than vitamins C or E and resveratrol [22].
There are few large trials that demonstrate the benefits of PP exposure as has been seen in in vitro studies. To date, the best examples are derived from the Spanish primary prevention trial PREDIMED. In a substudy, Covas and colleagues examined the impact of virgin olive oil, refined olive oil, and a mixture of both in two equal parts in a crossover trial of three, 3-week periods [23]. Virgin olive oil had the greatest benefit with respect to favorable lipid changes and reductions in oxidative stress markers. Plasma oxidized LDL concentrations, those of conjugated dienes and hydroxyl fatty acids, decreased in parallel with higher phenolic content. However, the recent International Life Sciences Institute Europe working group questioned the biologic relevance of the antioxidant effects of PPs for cardiovascular health on the grounds that there is limited evidence for the impact of these dietary PPs on circulating biomarkers such as F2-isoprostanes, oxidized LDL, and conjugated dienes [24]. In addition, circulating levels of PPs are low in comparison to those of other antioxidants such as vitamin C, urate, and tocopherols. This group stated that the evidence for the antioxidant mechanism of dietary PPs in cardioprotection continues to accumulate but is still not yet conclusive [24].
Inflammatory Mechanisms
Many of the effects that dietary PPs exhibit are likely through impacts on transcriptional networks or signaling cascades that modulate gene expression, promoting anti-inflammatory mediators, and NO production. These counter the adhesive nature of the vascular-immune cellular interplay, thereby abrogating or limiting endothelial dysfunction. Dietary PPs act on both of these processes (inflammation and endothelial dysfunction) implicated in the development of CVD by in part altering the recruitment or “homing” of inflammatory cells from the circulation and down-regulating the production of adhesion molecules by the endothelium, thereby hindering cellular migration into the subendothelial space and reducing atherosclerotic plaque formation. In addition, foods rich in PPs, in particular, flavonoids, have been shown to 1) modify endothelial formation of NO and EDHF, as shown in isolated blood vessels, and 2) improve endothelial function as shown in experimental models of CVD and with functional tests such as flow-mediated vasodilation (FMD) in humans.
Soluble pro-inflammatory molecules such as tumor necrosis factor α (TNFα), interleukin-6 (IL-6), and C-reactive protein (CRP) secreted by circulating immune cells interact with adhesion markers expressed on T lymphocytes (CD49d) and monocytes (CD40); the latter is critical to leucocyte homing. These circulating cells interact with molecules produced by the endothelium, such as E-selectin and the intercellular adhesion molecule-1 (ICAM-1). In vitro studies demonstrate that resveratrol moderates the overexpression of adhesion molecules (VCAM-1 and ICAM-1) through an inhibition of the NF-κB pathway in endothelial cells [28]. Resveratrol also abrogates the angiotensin II-induced adhesion of leukocytes by reducing cellular adhesion molecule expression and reducing serum monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein 1α (MIP-1α) [29]. This PP also has been shown to reduce pro-inflammatory cytokine secretion (IL-6 and TNFα) through downregulation of the NF-κB pathway [30] as well as promote adiponectin expression and release from human adipose tissue. The anti-inflammatory properties of quercetin are also similar to that observed for resveratrol in that these PP target signaling pathways are related to the NF-κB pathway, thereby decreasing mRNA and protein levels of TNFα, IL-6, MIP1α and P-selectin [32]. The interactions between circulating inflammatory cells and endothelial cells along the luminal surface of blood vessels modulate vascular function, in part by changes in release of nitric oxide (NO), endothelium derived hyperpolarizing factor (EDHF), and prostacyclin. Endothelial dysfunction is often due to an imbalance between vasorelaxation and vasoconstriction. The latter results from reduced availability of NO and EDHF and increased NADPH oxidase-dependent oxidative stress with the resultant formation of vasoconstrictive factors (such as endothelin-1) in the arterial wall [27]. Quercetin possesses vasodilatory properties through a reduction in endothelin-1 mRNA expression [31]. Delphinidin, quercetin and resveratrol, as well as PP extracts from red wine, activate endothelial nitric oxide synthase (eNOS), the enzyme responsible for NO production and resultant endothelium-dependent vasorelaxation [33]. The role PPs have on eNOS is suggestive of an intracellular redox-sensitive mechanism [34-36]. These PPs (resveratrol, quercetin, and delphinidin) may also activate eNOS through changes in calcium signaling and estrogen receptor function.
Direct translation of the impact of individual compounds in cell culture systems or animal models into human application is difficult because of multiple PPs in individual foods and overall dietary patterns. Thus, clinical studies utilizing foods or beverages provide a more realistic assessment of clinical impact. In a randomized crossover trial designed to examine the alcohol-independent effects of phenolic compounds of red wine on expression of soluble and leukocyte adhesion molecules and proinflammatory cytokines, 67 high CV risk male subjects received each of three treatments in random order: 30 g red wine, 30 g dealcoholized red wine or 30 g gin (containing no PP) daily for 30 days [37]. The red wine and dealcoholized red wine beverage treatments decreased serum concentrations of ICAM-I and IL-6 as well as the expression of T-lymphocyte and monocyte markers when compared to gin, indicating that the PP fraction was responsible for reduced inflammation. On the other hand, both alcohol and PPs may influence atherosclerosis as evidenced by downregulation of CD40a, CD40L, and MCP-1, VCAM-1, and E-selectin levels only in the red wine group.
Polyphenols in olive oils and nuts as part of a Mediterranean diet (MedDiet) have also been shown to affect changes in adhesion molecules and pro-inflammatory cytokines in over 772 subjects on a 3-month long dietary intervention that was part of the PREDIMED trial. This three-arm, parallel-group trial tested two Mediterranean dietary interventions on cardiovascular endpoints through the daily consumption of a MedDiet supplemented with either virgin olive oil (1L per week per family) or mixed nuts (30 g per week per individual). The control group consumed a low-fat diet devoid of these foods. The MedDiet treatment with PP-rich olive oil reduced circulating CRP levels, while both MedDiet treatments (virgin olive oil; nuts) lowered IL-6, ICAM-1, and VCAM-1[38]. In another PREDIMED substudy of 112 subjects, IL-6, ICAM-1, CD49d, and CD40 were reduced following 3 months of both the MedDiet interventions in comparison to the low fat diet [39], while VCAM-1 and CRP were reduced by the virgin olive oil treatment only. Other groups have supported these findings; virgin olive oil lowered plasma CRP and IL-6 concentrations and downregulated expression of VCAM-1, ICAM-1 and E-selectin in the endothelium, an effect attributable to the phenolic extract from olive oil [40]. Further work by these researchers suggests olive oil phenolic extract, in particular oleuropein aglycone, disrupts TNFα signaling [41] and, thus, reduces the self-perpetuating inflammatory process in the vascular wall that ultimately leads to plaque formation and vulnerability.
Fitzpatrick and coworkers were among the first to show that select foods and extracts of PP-rich foods (i.e., wines, grape juice, grape skin extracts) induce endothelium relaxation in aortic rings [42]. Because such foods cause endothelium-dependent relaxation that is abrogated by competitive inhibitors of eNOS and guanylcyclase; it may be inferred that these PPs modify NO formation; evidence suggests that EDHF is involved as well [35, 43]. In addition, endothelium-dependent relaxation of porcine coronary artery rings induced by red wine PPs has been observed at concentrations of 3 μg/mL or higher [35], which approximates the plasma concentrations of PP monomers following intake of 100 mL red wine [44].
Functional changes in the endothelium as a result of this inflammatory process have been assessed by flow mediated vasodilation (FMD) in subjects [45]. At doses approximating two glasses of red wine [46] or 46 g of dark chocolate every day for 2 weeks [47], FMD improved in healthy subjects. In a recent meta-analysis, ingestion of chocolate or cocoa flavan-3-ols was associated with a 3.2 % increase in FMD (95 % CI: 2.0, 4.3; 11 studies, 373 participants, I2=84 %) for acute effects (within 2 hours of ingestion), and after chronic intake, FMD increased by 1.3% (95% CI: 1.0, 1.7; 11 studies, 382 participants, I2=0 %) [48]. Among those with coronary heart disease, chronic black tea consumption [49] or red grape extracts [50] was also shown to improve FMD. Based on a recent meta-analysis, researchers found that each 1 % increase in FMD was associated with a 13 % reduction in risk for CV events (95 % CI: 0.83, 0.91) [51].
Impact on Cardiovascular Disease Endpoints
Evidence from cross-sectional and prospective cohort studies suggests that intakes of certain PPs (flavonoid classes, in particular) reduce CVD risk. In a recent review of 12 cohort studies, a decrease in age-adjusted CHD mortality was observed with increasing intakes of flavonoids [52]. Four USA cohorts were included: the Health Professionals Follow-up study, Iowa Women’s Health study, the Women’s Health study, and the Nurses’ Health Study (NHS). In the NHS cohort, the highest quintile of (>63 mg/d) flavanone intake was associated with reduced ischemic strokes (RR=0.81, 95 % CI, 0.66, 0.99, p=0.04) [53]. In the Cancer Prevention Study II Nutrition Cohort, those with the highest total flavonoid intakes (highest quintile, median=512.5 mg/d) had 18 % fewer deaths from cardiovascular disease (multivariate RR=0.82, 95 % CI, 0.73, 0.92, p=0.01) compared to the lowest flavonoid intakes (median=94.5 mg/d). Similar cardiovascular risk reductions were noted for five flavonoid subclasses: anthocyanidins, flavan-3-ols, flavones, flavonols, and proanthocyanidins. No change is risk was seen with flavanone intake. Among men, fewer deaths from stroke were associated with flavonoid intakes (RR=0.63, 95 % CI, 0.44, 0.89, p trend=0.04) as compared to no impact of flavonoids on deaths from ischemic heart disease (RR=0.90, 95 %CI, 0.72, 1.13). As in prior cohort studies, intakes were assessed by the 152-item FFQ, and nutrient data were derived from three USDA databases [54]. In yet another meta-analysis that included six cohort studies in three countries, flavonol intakes (>21mg/d) reduced incidence of strokes by 20 % (pooled RR=0.80 (95 % CI: 0.65, 0.98)[55]. There is little evidence for cardioprotection with higher isoflavone or lignin intake. The observed risk reduction in incident strokes is consistent with risk reductions observed for hypertension. Risk of incident hypertension was reduced by 5-8 % among those with higher intakes (>16 mg/d) of anthocyanins (mostly blueberries and strawberries) [56]. The authors suggest that some of the structural similarities, the B-ring hydroxylation and methyoxylation pattern, may explain the vasodilatory properties of these compounds. In addition, PP in olive oil have also been associated with better CVD outcomes, in particular, stroke. The Three-City study in French adults reported a 41 % reduction in stroke incidence among those using olive oil for cooking and salad dressing as compared to those who had never used olive oil (RR=0.59, 95 % CI: 6, 63, p=0.03) after 5.3 years of follow-up [57].
Particularly key to an evaluation of the evidence from observational studies is dietary assessment of PP intakes. All too often only a limited number of PPs are considered in food analyses used in food composition databases [58, 59]. Some groups have relied on the recent USDA database which contains 38 flavonoid aglycones [4, 60, 61], but others who have examined cardiovascular endpoints and dietary flavonoids are based on analyses derived from older, less complete USDA databases. More recently, a new Phenol-Explorer database [5] mentioned previously is being adopted by more research groups [24, 62]. However, food composition data for many PPs (in particular phenolic acid content) are still incomplete.
While evidence continues to accumulate from observational and other studies, one cannot forget the ongoing primary prevention trial, PREDIMED, of 7,447 participants (55-80 years, 57 % women) in Spain. At baseline, trial participants had to be free of cardiovascular disease, but could have type 2 diabetes or ≥3 cardiovascular risk factors (www.predimed.es, www.predimed.org). The two MedDiet interventions (MedDiet supplemented with either olive oil or nuts) reflect consumption of a broader spectrum of PPs---the contribution of not only flavonoids, but also phenolic acids and some lignans. Lower rates of incident diabetes [63], metabolic syndrome [64], and hypertension [65] have been reported for those assigned to the MedDiet with olive oil or nuts when compared to those on the low-fat diet intervention. The reductions in incident diabetes were particularly compelling; the multivariable adjusted hazard ratios were 0.49 (95 % CI: 0.25, 0.97, p=0.047) and 0.48 (95 % CI: 0.24, 0.96, p= 0.053) in the MedDiet supplemented with olive oil and nut groups, respectively, compared with the control group. Diabetes risk reduction occurred in the absence of significant changes in body weight or physical activity. While these findings clearly identify a MedDiet as beneficial for diabetes, an important risk equivalent for CVD, the primary outcomes are not yet published, and we do not yet know if CVD incidence will be reduced in those consuming a dietary pattern high in PPs (MedDiet). To the best of these authors’ knowledge, there are no other large clinical trials in which the effects of PP intakes on cardiovascular morbidity or mortality have been investigated.
Conclusion & Future Directions
There are several challenges to our current understanding of the role that PPs play in the inflammatory process and related inflammatory diseases. These include: 1) a paucity of information on the content of several PP compounds in foods, 2) the use of incomplete and outdated databases by researchers, 3) a limited number of studies regarding the utility, kinetics, choice of fluid or tissue, and choice of assay for biomarkers of PP intake, and 4) the dearth of adequately powered food-based trials in the USA similar to PREDIMED. However, recent mechanistic insights provide support for the potential role of PPs in the inflammatory process including reduced ROS, cytokine expression, and endothelial inflammatory markers, as well as increased NO production. However, one cannot extrapolate the benefits of PP intake solely through examination of these mechanisms. Intervention trials are needed using PP-rich foods to assess whether typical intakes of such provided foods will result in cardioprotection. Efforts similar to what PREDIMED investigators have fostered might be fruitful here in the USA. To the best of our knowledge, no such long-term food based trial with cardiovascular hard endpoints has been conducted in the USA. As in PREDIMED, several translational, mechanistic studies as well as short-term clinical studies with immediate CV markers (such as pro-inflammatory adhesion markers of measures of endothelial function (i.e., FMD) could be planned within the bigger trial. In addition, potential biomarkers of dietary exposures (i.e., plasma or urinary tyrosol, etc.) could be evaluated in this trial in high CVD risk American adults. Finally, coordination of PP determinations in foods is needed at an international level so food composition data can be shared for both Phenol Explorer and USDA databases.
Footnotes
Conflicts of Interest:
Christy Tangney declares that she has no conflicts of interest.
Heather E. Rasmussen declares that she has no conflicts of interest.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.García-Villalba R, Carrasco-Pancorbo A, Nevedomskaya E, et al. Exploratory analysis of human urine by LC-ESI-TOF MS after high intake of olive oil: Understanding the metabolism of polyphenols. Anal Bioanal Chem. 2010;398:463–75. doi: 10.1007/s00216-010-3899-x. [DOI] [PubMed] [Google Scholar]
- 2.Marzocchella L, Fantini M, Benvenuto M, et al. Dietary flavonoids: Molecular mechanisms of action as anti-inflammatory agents. Recent Pat Inflamm Allergy Drug Discov. 2011;5:200–20. doi: 10.2174/187221311797264937. [DOI] [PubMed] [Google Scholar]
- 3.Gimeno E, Fito M, Lamuela-Raventos RM, et al. Effect of ingestion of virgin olive oil on human low-density lipoprotein composition. Eur J Clin Nutr. 2002;56:114–20. doi: 10.1038/sj.ejcn.1601293. [DOI] [PubMed] [Google Scholar]
- 4.USDA Nutrient Data Laboratory [accessed December 2012];USDA database for flavonoids content of selected foods. Release 3.0. 2011. Available from: http://www.ars.usda.gov/nutrientdata.
- 5.Neveu V, Perez-Jimenez J, Vos F, et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford) 2010;2010:ba024. doi: 10.1093/database/bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothwell JA, Urpi-Sarda M, Boto-Ordoñez M, et al. Phenol-Explorer 2.0: a major update of the Phenol-Explorer database integrating data on polyphenol metabolism and pharmacokinetics in humans and experimental animals. Database (Oxford) 2012:2012. doi: 10.1093/database/bas031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ovaskainen ML, Torronen R, Koponen JM, et al. Dietary intake and major food sources of polyphenols in Finnish adults. J Nutr. 2008;138:562–66. doi: 10.1093/jn/138.3.562. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Jimenez J, Fezeu L, Touvier M, et al. Dietary intake of 337 polyphenols in French adults. Am J Clin Nutr. 2011;93:1220–28. doi: 10.3945/ajcn.110.007096. [DOI] [PubMed] [Google Scholar]
- 9•.Tresserra-Rimbau A, Medina-Remón A, Pérez-Jiménez J, et al. Dietary intake and major food sources of polyphenols in a Spanish population at high cardiovascular risk: The PREDIMED study. Nutr Metab Cardiovasc Dis. 2013 doi: 10.1016/j.numecd.2012.10.008. in press. This group examines the total polyphenols and its classes including flavonoids and phenolic acids in diets of Spanish subjects in the PREDIMED trial. The distribution of polyphenols (from olives, and olive oils) is contrasted with those from the Su.VI.MAX (French) population sample. Both reports rely on composition data from the Phenol Explorer database.
- 10.Spencer JP, Abd El Mohsen MM, Minihane AM, Mathers JC. Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br J Nutr. 2008;99:12–22. doi: 10.1017/S0007114507798938. [DOI] [PubMed] [Google Scholar]
- 11.Caruso D, Visioli F, Patelli R, et al. Urinary excretion of olive oil phenols and their metabolites in humans. Metabolism. 2001;50:1426–28. doi: 10.1053/meta.2001.28073. [DOI] [PubMed] [Google Scholar]
- 12.Orozco-Solano MI, Ferreiro-Vera C, Priego-Capote F, Luque de Castro MD. Automated method for determination of olive oil phenols and metabolites in human plasma and application in intervention studies. J Chromatogr. 2012;1258:108–16. doi: 10.1016/j.chroma.2012.08.057. [DOI] [PubMed] [Google Scholar]
- 13.de la Torre-Carbot K, Chavez-Servin JL, Jauregui O, et al. Elevated circulating LDL phenol levels in men who consumed virgin rather than refined olive oil are associated with less oxidation of plasma LDL. J Nutr. 2010;140:501–08. doi: 10.3945/jn.109.112912. [DOI] [PubMed] [Google Scholar]
- 14.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–56. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 15.Steffen Y, Gruber C, Schewe T, Sies H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch Biochem Biophys. 2008;469:209–19. doi: 10.1016/j.abb.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Borriello A, Cucciolla V, Della Ragione F, Galletti P. Dietary polyphenols: focus on resveratrol, a promising agent in the prevention of cardiovascular diseases and control of glucose homeostasis. Nutr Metab Cardiovasc Dis. 2010;20:618–25. doi: 10.1016/j.numecd.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Ahsan MK, Lekli I, Ray D, et al. Redox regulation of cell survival by the thioredoxin superfamily: an implication of redox gene therapy in the heart. Antioxid Redox Signal. 2009;11:2741–758. doi: 10.1089/ars.2009.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia N, Daiber A, Habermeier A, et al. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. J Pharmacol Exp Ther. 2010;335:149–54. doi: 10.1124/jpet.110.168724. [DOI] [PubMed] [Google Scholar]
- 19.Spanier G, Xu H, Xia N, et al. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4) J Physiol Pharmacol. 2009;60:111–16. [PubMed] [Google Scholar]
- 20.Ungvari Z, Bagi Z, Feher A, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Kostyuk VA, Potapovich AI, Suhan TO, et al. Antioxidant and signal modulation properties of plant polyphenols in controlling vascular inflammation. Eur J Pharmacol. 2011;658:248–56. doi: 10.1016/j.ejphar.2011.02.022. These researchers present endothelial cell culture studies that support the role of plant polyphenols in vascular inflammation as not only antioxidants but also as modulators of inflammatory redox signaling pathways.
- 22.Al-Awwadi NA, Araiz C, Bornet A, et al. Extracts enriched in different polyphenolic families normalize increased cardiac NADPH oxidase expression while having differential effects on insulin resistance, hypertension, and cardiac hypertrophy in high-fructose-fed rats. J Agric Food Chem. 2005;53:151–57. doi: 10.1021/jf048919f. [DOI] [PubMed] [Google Scholar]
- 23.Covas MI, Nyyssonen K, Poulsen HE, et al. The effect of polyphenols in olive oil on heart disease risk factors: a randomized trial. Ann Intern Med. 2006;145:333–41. doi: 10.7326/0003-4819-145-5-200609050-00006. [DOI] [PubMed] [Google Scholar]
- 24••.Hollman PC, Cassidy A, Comte B, et al. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J Nutr. 2011;141:989S–1009S. doi: 10.3945/jn.110.131490. This report reflects the consensus among international experts regarding the biological relevance of antioxidant effects of polyphenols in cardiovascular health and suggests a broader view of these bioactive compounds in modulation of inflammation balance.
- 25.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 26.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 27.Andriantsitohaina R, Auger C, Chataigneau T, et al. Molecular mechanisms of the cardiovascular protective effects of polyphenols. Br J Nutr. 2012;108:1532–49. doi: 10.1017/S0007114512003406. [DOI] [PubMed] [Google Scholar]
- 28.Deng YH, Alex D, Huang HQ, et al. Inhibition of TNF--mediated endothelial cell-monocyte cell adhesion and adhesion molecules expression by the resveratrol derivative, trans-3,5,4′-trimethoxystilbene. Phytother Res. 2011;25:451–57. doi: 10.1002/ptr.3279. [DOI] [PubMed] [Google Scholar]
- 29.Rius C, Abu-Taha M, Hermenegildo C, et al. Trans- but not cis-resveratrol impairs angiotensin-II-mediated vascular inflammation through inhibition of NF-kB activation and peroxisome proliferator-activated receptor-gamma upregulation. J Immunol. 2010;185:3718–27. doi: 10.4049/jimmunol.1001043. [DOI] [PubMed] [Google Scholar]
- 30.Olholm J, Paulsen SK, Cullberg KB, et al. Anti-inflammatory effect of resveratrol on adipokine expression and secretion in human adipose tissue explants. Int J Obes (Lond) 2010;34:1546–53. doi: 10.1038/ijo.2010.98. [DOI] [PubMed] [Google Scholar]
- 31.Romero M, Jimenez R, Sanchez M, et al. Quercetin inhibits vascular superoxide production induced by endothelin-1: Role of NADPH oxidase, uncoupled eNOS and PKC. Atherosclerosis. 2009;202:58–67. doi: 10.1016/j.atherosclerosis.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Boesch-Saadatmandi C, Loboda A, Wagner AE, et al. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: role of miR-155. J Nutr Biochem. 2011;22:293–99. doi: 10.1016/j.jnutbio.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Chalopin M, Tesse A, Martinez MC, et al. Estrogen receptor alpha as a key target of red wine polyphenols action on the endothelium. PLoS ONE. 2010;5:e8554. doi: 10.1371/journal.pone.0008554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duarte J, Andriambeloson E, Diebolt M, Andriantsitohaina R. Wine polyphenols stimulate superoxide anion production to promote calcium signaling and endothelial-dependent vasodilatation. Physiol Res. 2004;53:595–602. [PubMed] [Google Scholar]
- 35.Ndiaye M, Chataigneau M, Lobysheva I, et al. Red wine polyphenol-induced, endothelium-dependent NO-mediated relaxation is due to the redox-sensitive PI3-kinase/Akt-dependent phosphorylation of endothelial NO-synthase in the isolated porcine coronary artery. FASEB J. 2005;19:455–57. doi: 10.1096/fj.04-2146fje. [DOI] [PubMed] [Google Scholar]
- 36.Anselm E, Chataigneau M, Ndiaye M, et al. Grape juice causes endothelium-dependent relaxation via a redox-sensitive Src- and Akt-dependent activation of eNOS. Cardiovasc Res. 2007;73:404–13. doi: 10.1016/j.cardiores.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 37•.Chiva-Blanch G, Urpi-Sarda M, Llorach R, et al. Differential effects of polyphenols and alcohol of red wine on the expression of adhesion molecules and inflammatory cytokines related to atherosclerosis: A randomized clinical trial (American Journal of Clinical Nutrition (2012) 95; (326-34)) Am J Clin Nutr. 2012;95:1506. doi: 10.3945/ajcn.111.022889. The report describes the findings from a randomized trial in which subjects of high cardiovascular risk were presented in crossover design: 30g daily of red wine for 30 day, 30 g gin daily for the same period and 30 g daily of dealcoholized red wine to ascertain what constituents are responsible for observed changes in adhesion molecules and cytokines.
- 38.Estruch R, Martinez-Gonzalez MA, Corella D, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 39.Mena MP, Sacanella E, Vazquez-Agell M, et al. Inhibition of circulating immune cell activation: a molecular antiinflammatory effect of the Mediterranean diet. Am J Clin Nutr. 2009;89:248–56. doi: 10.3945/ajcn.2008.26094. [DOI] [PubMed] [Google Scholar]
- 40.Dell’Agli M, Fagnani R, Mitro N, et al. Minor components of olive oil modulate proatherogenic adhesion molecules involved in endothelial activation. J Agric Food Chem. 2006;54:3259–64. doi: 10.1021/jf0529161. [DOI] [PubMed] [Google Scholar]
- 41.Dell’Agli M, Fagnani R, Galli GV, et al. Olive oil phenols modulate the expression of metalloproteinase 9 in THP-1 cells by acting on nuclear factor-kappaB signaling. J Agric Food Chem. 2010;58:2246–52. doi: 10.1021/jf9042503. [DOI] [PubMed] [Google Scholar]
- 42.Fitzpatrick DF, Hirschfield SL, Coffey RG. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am J Physiol. 1993;265:H774–8. doi: 10.1152/ajpheart.1993.265.2.H774. [DOI] [PubMed] [Google Scholar]
- 43.Schini-Kerth VB, Auger C, Kim JH, et al. Nutritional improvement of the endothelial control of vascular tone by polyphenols: role of NO and EDHF. Pflugers Arch. 2010;459:853–62. doi: 10.1007/s00424-010-0806-4. [DOI] [PubMed] [Google Scholar]
- 44.Duthie GG, Pedersen MW, Gardner PT, et al. The effect of whisky and wine consumption on total phenol content and antioxidant capacity of plasma from healthy volunteers. Eur J Clin Nutr. 1998;52:733–36. doi: 10.1038/sj.ejcn.1600635. [DOI] [PubMed] [Google Scholar]
- 45.Iwamoto Y, Maruhashi T, Fujii Y, et al. Intima-media thickness of brachial artery, vascular function, and cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2012;32:2295–2303. doi: 10.1161/ATVBAHA.112.249680. [DOI] [PubMed] [Google Scholar]
- 46.Agewall S, Wright S, Doughty RN, et al. Does a glass of red wine improve endothelial function? Eur Heart J. 2000;21:74–8. doi: 10.1053/euhj.1999.1759. [DOI] [PubMed] [Google Scholar]
- 47.Engler MB, Engler MM, Chen CY, et al. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J Am Coll Nutr. 2004;23:197–204. doi: 10.1080/07315724.2004.10719361. [DOI] [PubMed] [Google Scholar]
- 48•.Hooper L, Kay C, Abdelhamid A, et al. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr. 2012;95:740–51. doi: 10.3945/ajcn.111.023457. This paper is a thorough review of recent studies examining these polyphenol-rich foods on cardiovascular outcomes.
- 49.Duffy SJ, Keaney JF, Jr, Holbrook M, et al. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation. 2001;104:151–56. doi: 10.1161/01.cir.104.2.151. [DOI] [PubMed] [Google Scholar]
- 50.Lekakis J, Rallidis LS, Andreadou I, et al. Polyphenolic compounds from red grapes acutely improve endothelial function in patients with coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2005;12:596–600. doi: 10.1097/00149831-200512000-00013. [DOI] [PubMed] [Google Scholar]
- 51.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;26:631–40. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 52.Peterson JJ, Dwyer JT, Jacques PF, McCullough ML. Associations between flavonoids and cardiovascular disease incidence or mortality in European and US populations. Nutr Rev. 2012;70:491–508. doi: 10.1111/j.1753-4887.2012.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cassidy A, Rimm EB, O’Reilly EJ, et al. Dietary flavonoids and risk of stroke in women. Stroke. 2012;43:946–51. doi: 10.1161/STROKEAHA.111.637835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCullough ML, Peterson JJ, Patel R, et al. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am J Clin Nutr. 2012;95:454–64. doi: 10.3945/ajcn.111.016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hollman PC, Geelen A, Kromhout D. Dietary flavonol intake may lower stroke risk in men and women. J Nutr. 2010;140:600–04. doi: 10.3945/jn.109.116632. [DOI] [PubMed] [Google Scholar]
- 56.Cassidy A, O’Reilly EJ, Kay C, et al. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am J Clin Nutr. 2011;93:338–47. doi: 10.3945/ajcn.110.006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Samieri C, Feart C, Proust-Lima C, et al. Olive oil consumption, plasma oleic acid, and stroke incidence: the Three-City Study. Neurology. 2011;77:418–25. doi: 10.1212/WNL.0b013e318220abeb. [DOI] [PubMed] [Google Scholar]
- 58.Erdman JW, Jr, Balentine D, Arab L, et al. Flavonoids and heart health: proceedings of the ILSI North America Flavonoids Workshop, May 31-June 1, 2005, Washington, DC. J Nutr. 2007;137:718S–737S. doi: 10.1093/jn/137.3.718S. [DOI] [PubMed] [Google Scholar]
- 59.Peterson J, Dwyer J, Adlercreutz H, et al. Dietary lignans: Physiology and potential for cardiovascular disease risk reduction. Nutr Rev. 2010;68:571–603. doi: 10.1111/j.1753-4887.2010.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.USDA Nutrient Data Laboratory [accessed December 2012];USDA database for the isoflavone content of selected foods. 2008 Release 2.0. Available from: http://www.ars.usda.gov/nutrientdata.
- 61.USDA [accessed December 2012];Nutrient Data Laboratory:USDA database for proanthocyanidin content of selected foods. 2004 Available from: http://www.ars.usda.gov/nutrientdata.
- 62.Pérez-Jiménez J, Fezeu L, Touvier M, et al. Dietary intake of 337 polyphenols in French adults. Am J Clin Nutr. 2011;93:1220–28. doi: 10.3945/ajcn.110.007096. [DOI] [PubMed] [Google Scholar]
- 63.Salas-Salvado J, Bullo M, Babio N, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. 2011;34:14–19. doi: 10.2337/dc10-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salas-Salvado J, Fernandez-Ballart J, Ros E, et al. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: one-year results of the PREDIMED randomized trial. Arch Intern Med. 2008;168:2449–58. doi: 10.1001/archinte.168.22.2449. [DOI] [PubMed] [Google Scholar]
- 65.Medina-Remon A, Zamora-Ros R, Rotches-Ribalta M, et al. Total polyphenol excretion and blood pressure in subjects at high cardiovascular risk. Nutr Metab Cardiovasc Dis. 2011;21:323–31. doi: 10.1016/j.numecd.2009.10.019. [DOI] [PubMed] [Google Scholar]